Abstract
Evidence implicates anandamide in dopamine-related cocaine function. In the present study, we investigated the effect of methanandamide (5 mg/kg, i.p.), a stable anandamide analog, on the hyperthermia and hyperactivity induced by a fixed dose of cocaine (15 mg/kg, i.p.). Cocaine administered to rats produced hyperthermia and hyperactivity whereas methanandamide was ineffective. For combined administration, methanandamide attenuated the hyperthermia, but not hyperactivity, induced by cocaine. The effect of methanandamide was abolished by pretreatment with a cannabinoid CB1 receptor antagonist, SR141716A (5 mg/kg, i.p.), or dopamine D2 receptor antagonist, S(−)-raclopride (5 mg/kg, i.p.) but not by capsazepine (40 mg/kg, i.p.), a transient receptor potential vanilloid 1 cation channel antagonist. Methanandamide also attenuated the hyperthermia caused by a dopamine D1 receptor agonist, SKF 38393 (10 mg/kg, s.c.), indicating that it reduces hyperthermia produced by dopamine D1 receptor activation. URB597 (0.25 mg/kg, i.p.), an inhibitor of anandamide metabolism, did not alter cocaine-induced hyperthermia. Our results demonstrate that methanandamide activates cannabinoid CB1 receptors to attenuate cocaine-induced hyperthermia, and that dopamine D2 receptor activation plays a permissive role in the thermoregulatory effects of methanandamide.
Keywords: Anandamide, Cannabinoid, Cocaine, Psychostimulant, Dopamine, Hyperthermia, Methanandamide, D2, D1, SKF 38393, URB597, Raclopride, CB1
1. Introduction
It is well established that cocaine increases locomotor activity and produces stereotyped behavior in animals (Kelly and Iversen, 1976). A lesser known effect of cocaine is the hyperthermia that it causes in humans and animals (Marzuk et al., 1998; Kalant, 2001; Wiechman and Spratto, 1982; Gonzalez, 1993; Lomax and Daniel, 1990; Hamida et al., 2008; Ansah et al., 1996). Increased dopaminergic transmission mediates cocaine-induced hyperthermia and hyperactivity (Hurd and Ungerstedt, 1989; Rockhold et al., 1991; Faunt and Crocker, 1987; Zarrindast and Tabatabai, 1992; Nagashima et al., 1992; Verma and Kulkarni, 1993), but anandamide may also play a role because its brain concentration is elevated following acute psychostimulant exposure (Thiemann et al., 2008; Centonze et al., 2004; Masserano et al., 1999). Anandamide is part of the endocannabinoid system, which consists of three components: receptors, cannabinoid CB1 and CB2; endogenous constituents, such as anandamide and 2-arachidonoyl-glycerol (2-AG), which mimic the pharmacological effects of marijuana by activating cannabinoid receptors; and enzymes which metabolize endogenous cannabinoids (Fride and Mechoulam, 1993; Devane et al., 1992). Anandamide activates both cannabinoid and transient receptor potential vanilloid 1 cation (TRPV1) channels (Zygmunt et al., 1999; Di Marzo et al., 2001), but relatively high doses (20 mg/kg) of the compound produce hypothermia, catalepsy and hypoactivity that is abolished by a cannabinoid CB1 receptor antagonist SR141716A (Costa et al., 1999).
The present study examined the effects of the long-lasting anandamide analog, methanandamide, on the hyperthermia and hyperactivity caused by cocaine and SKF 38393, a dopamine D1 receptor agonist (Abadji et al., 1994). We also determined if the effects of methanandamide were mediated by cannabinoid CB1, TRPV1 and/or dopamine receptors and were similar to the actions of URB597, an inhibitor of the enzyme fatty-acid amide hydrolase (FAAH) that catalyzes anandamide hydrolysis.
2. Results
2.1. Effect of methanandamide on body temperature
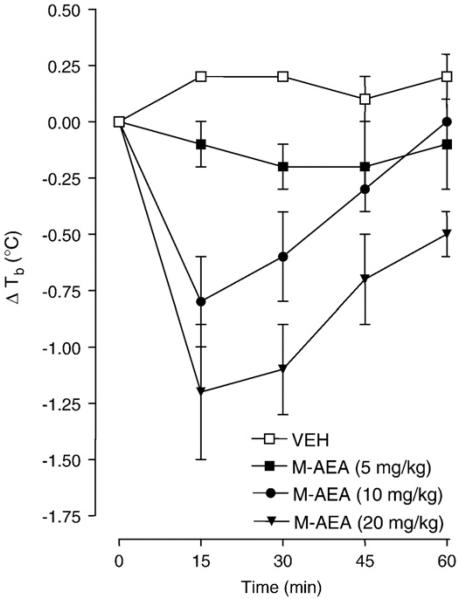
The effects of methanandamide (5, 10 or 20 mg/kg, i.p.) on body temperature are presented in Fig. 1. Repeated-measures ANOVA revealed a significant main effect [F (3, 19)=9.122, P = 0.0020]. Post-hoc analysis revealed that 20 mg/kg of methanandamide produced significant hypothermia compared to vehicle 15, 30, 45 and 60 min post-administration (P<0.05). A dose of 10 mg/kg of methanandamide produced significant hypothermia compared to vehicle 15 and 30 min post-injection (P<0.05). A maximal hypothermia of 1.21±0.5 °C was caused by 20 mg/kg of methanandamide 15 min following administration. Since 5 mg/kg of methanandamide did not affect body temperature compared to vehicle (P>0.05), we selected this dose for our drug combination experiments.
Fig. 1.
Methanandamide (M-AEA) causes dose-related hypothermia. Rats were injected with methanandamide (5, 10 or 20 mg/kg) or vehicle (VEH) and body temperatures were recorded 15, 30, 45 and 60 min post-administration. Data are expressed as the mean±S.E.M. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05 compared to VEH.
2.2. Effect of methanandamide and URB597 on cocaine-evoked hyperthermia: a role for cannabinoid CB1 receptors
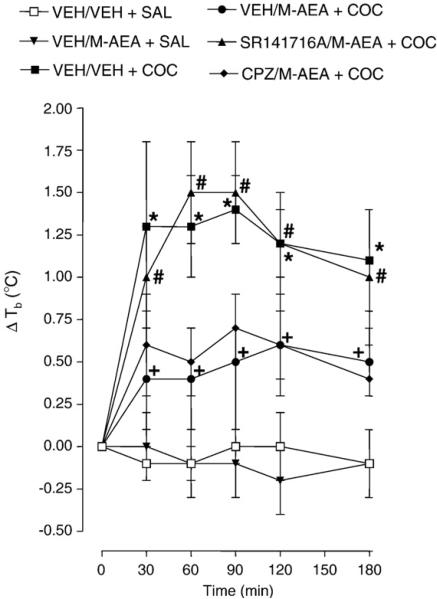
The effects on body temperature of methanandamide (5 mg/kg, i.p.), cocaine (15 mg/kg, i.p.), SR 141716A (5 mg/kg, i.p.) and capsazepine (40 mg/kg, i.p.) are presented in Fig. 2. Repeated-measures ANOVA revealed a significant main effect [F (5, 35)=20.62, P<0.0001]. Compared to control (vehicle+ saline), cocaine caused significant hyperthermia 30, 60, 90, 120, 150 and 180 min post-administration (P<0.05). A maximal hypothermia of 1.50±0.4 °C was observed 30 and 60 min following cocaine administration. Methanandamide did not affect body temperature compared to control (vehicle+saline) (P>0.05). For combined administration, methanandamide inhibited a significant proportion of cocaine (15 mg/kg)-induced hyperthermia 30, 60, 90, 120, 150 and 180 min post-cocaine administration (P<0.05). In rats injected with cocaine following methanandamide pretreatment, body temperatures were significantly lower than rats pretreated with a SR 141716A/methanandamide combination (P<0.05) and not significantly different from rats pretreated with a capsazepine/methanandamide combination (P>0.05). Rats injected with cocaine following pretreatment with a SR 141716A/methanandamide combination displayed body temperatures that did not differ significantly from drug-naïve rats injected with cocaine (P >0.05). SR 141716A or capsazepine did not alter body temperature (P>0.05) (data not shown) (Rawls et al., 2002, 2006).
Fig. 2.
Methanandamide (M-AEA) attenuates cocaine (COC)-induced hyperthermia by activating cannabinoid CB1 receptors. Rats were injected with vehicle (VEH); (M-AEA) (5 mg/kg); SR 141716A (5 mg/kg) plus M-AEA (5 mg/kg); or capsazepine (CPZ) (5 mg/kg) plus M-AEA (5 mg/kg). Twenty min later, rats were injected with COC (15 mg/kg) or saline (SAL) and body temperatures were recorded 30, 90, 90 120 and 180 min post-administration. Data are expressed as the mean±S.E.M. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05 compared to VEH+SAL; +P<0.05 compared to VEH+COC; and #P<0.05 compared to M-AEA+COC.
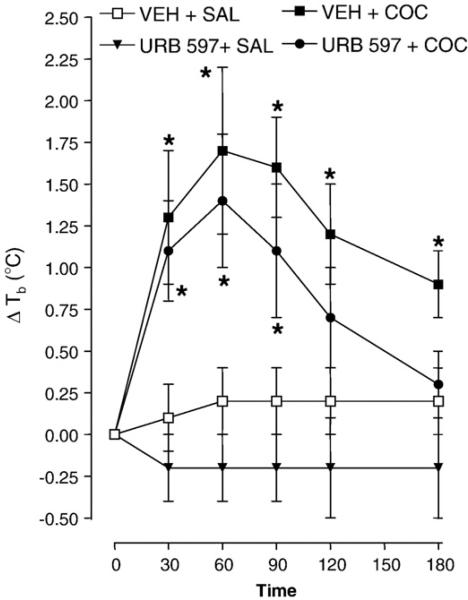
The effect of URB597 (0.25 mg/kg, i.p.) on the hyperthermic response to cocaine (15 mg/kg, i.p.) is presented in Fig. 3. Repeated-measures ANOVA revealed a significant main effect [F (3, 16)=11.89, P=0.0001]. In drug-naïve rats, cocaine produced its normal hyperthermic effect (P < 0.05) whereas URB597did not alter body temperature (P>0.05). For combined administration, the hyperthermia induced by cocaine did not differ in rats pretreated with URB597 or vehicle (P>0.05).
Fig. 3.
URB597 does not affect cocaine (COC)-induced hyperthermia. Rats pretreated with vehicle (VEH) or URB597 (0.25 mg/kg) were injected 20 min later with COC (15 mg/kg) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-injection. Data are expressed as the mean±S.E.M. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05 compared to VEH+SAL.
2.3. Effect of methanandamide on cocaine-evoked hyperthermia: a role for dopamine D2 receptors
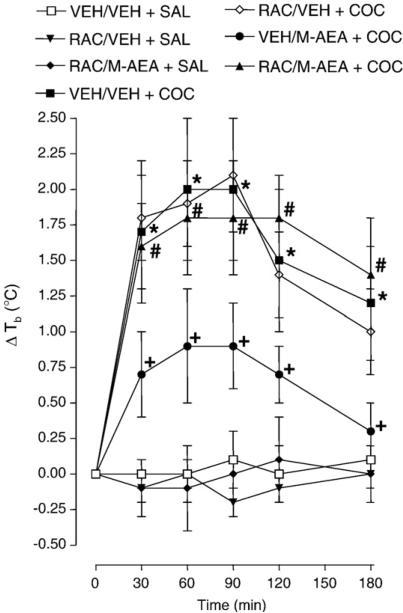
The effect of raclopride (5 mg/kg), a selective dopamine D2 receptor antagonist, on the attenuation of cocaine (15 mg/kg)-induced hyperthermia by methanandamide (5 mg/kg) is presented in Fig. 4. Repeated-measures ANOVA revealed a significant main effect [F (6, 30)=19.76, P<0.0001]. Compared to control (vehicle + saline), cocaine caused significant hyperthermia 30, 60, 90, 120, 150 and 180 min post-administration (P<0.05). Methanandamide again inhibited a significant proportion of cocaine-induced hyperthermia 30, 60, 90, 120, 150 and 180 min post-cocaine administration (P<0.05). Raclopride prevented the effects of methanandamide as rats injected with cocaine following methanandamide pretreatment displayed body temperatures that were significantly lower than rats pretreated with a raclopride/methanandamide combination (P<0.05). Furthermore, cocaine-injected rats pretreated with a raclopride/methanandamide combination displayed body temperatures that were not significantly different from cocaine-injected rats pretreated with vehicle (P>0.05). In cocaine-naïve rats, raclopride did not alter body temperature when given by itself or with methanandamide (P>0.05).
Fig. 4.
Dopamine D2 receptor activation is required for methanandamide (M-AEA) to attenuate cocaine (COC)-induced hyperthermia. Rats were injected with vehicle (VEH); raclopride (RAC) (5 mg/kg); M-AEA (5 mg/kg); or RAC (5 mg/kg) plus M-AEA (5 mg/kg). Twenty min later, rats were injected with COC (15 mg/kg) or saline (SAL) and body temperatures were recorded 30, 90, 90 120 and 180 min post-administration. Data are expressed as the mean±S.E.M. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05 compared to VEH+SAL; +P<0.05 compared to VEH+COC; and #P<0.05 compared to M-AEA+COC.
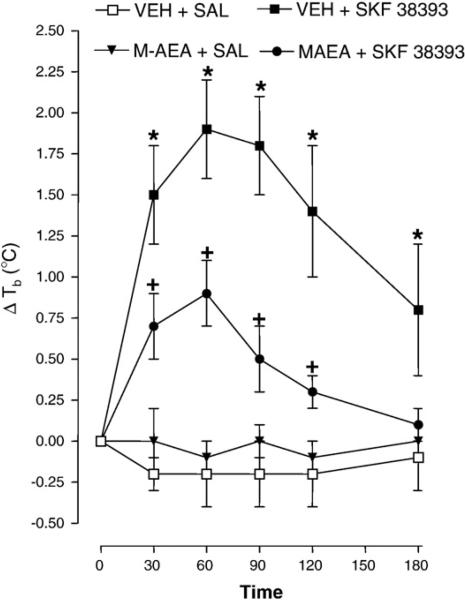
2.4. Effect of methanandamide on the hyperthermia produced by dopamine D1 receptor activation
The effect of methanandamide (5 mg/kg) on hyperthermia caused by SKF 38393 (10 mg/kg) is presented in Fig. 5. Repeated-measures ANOVA revealed a significant main effect [F (3, 21)=16.91, P<0.0001]. Compared to control (vehicle +saline), SKF 38393 caused significant hyperthermia 30, 60, 90, 120, and 180 min post-administration (P<0.05). Pretreatment of rats with methanandamide inhibited a significant proportion of the SKF 38393-evoked hyperthermia at each time point except 180 min (P<0.05). The body temperature of rats injected with vehicle and saline did not differ significantly from rats treated with methanandamide and saline (P>0.05).
Fig. 5.
Methanandamide (M-AEA) does not alter SKF 38393-induced hyperthermia. Rats pretreated with vehicle (VEH) or M-AEA (5 mg/kg) were injected 20 min later with COC (15 mg/kg) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-injection. Data are expressed as the mean±S.E.M. of the change in body temperature (ΔTb) from baseline (time 0). *P<0.05 compared to VEH+SAL and +P<0.05 compared to VEH+SKF 38393.
2.5. Effect of methanandamide on cocaine-induced hyperactivity
The time-course data showing the effects of methanandamide (5 mg/kg) and cocaine (15 mg/kg) for stereotypy are presented in Fig. 6a. Repeated-measures ANOVA revealed a significant main effect [F (3, 30)=19.36, P<0.0001]. The vehicle/cocaine group displayed stereotypy that was significantly different from the vehicle/saline control group (P<0.001). The stereo-typy displayed by the methanandamide/saline group did not differ significantly from the vehicle/saline group (P>0.05). For combined administration, the methanandamide/cocaine group displayed stereotypy that was not significantly different from the stereotypy produced by the vehicle/cocaine group (P>0.05). The corresponding data for ambulatory activity are presented in Fig. 6b, and significance testing produced results similar to those obtained for stereotypy. The main effect was significant [F (3, 30)= 27.07, P <0.0001]. Post-hoc analysis revealed that the vehicle/cocaine group displayed ambulatory activity that was significantly different from the vehicle/saline group (P<0.001). Again, however, the ambulatory activity displayed by the vehicle/cocaine group was not significantly different from the methanandamide/cocaine group (P>0.05).
Fig. 6.
Effects of methanandamide (M-AEA) and cocaine (COC) on stereotypy (A) and ambulation (B). Rats were injected with M-AEA (5 mg/kg) or vehicle (VEH) and placed into test chambers. Twenty min later rats were injected with COC (15 mg/kg) or saline (SAL). Data are expressed as activity counts in 10 min intervals (means±S.E.M.). *P<0.05 compared to VEH+SAL.
3. Discussion
Methanandamide produced dose-related hypothermia that was similar in onset and longer in duration than anandamide-induced hypothermia (Smith et al., 1994; Costa et al., 1999; Stein et al., 1996). The persistent hypothermia was likely attributable to the increased resistance of methanandamide to aminopeptidase hydrolysis, a property that increases its half-life relative to anandamide (Abadji et al., 1994). A dose (5 mg/kg) of methanandamide that by itself did not alter body temperature produced a sustained attenuation of cocaine-induced hyperthermia (Gonzalez, 1993; Lomax and Daniel, 1990; Hamida et al., 2008; Ansah et al., 1996). Anandamide activates two receptors, cannabinoid CB1 and TRPV1, which mediate hypothermia (Devane et al., 1992; Zygmunt et al., 1999; Di Marzo et al., 1994, 2001; Malone and Taylor, 1998; Rawls et al., 2002; Dogan et al., 2004; Swanson et al., 2005). Thus, we hypothesized that methanandamide must have activated one of those two receptors to attenuate cocaine-induced hyperthermia. Experiments revealed that cannabinoid CB1 receptor antagonism by SR 141716A blocked the effect of methanandamide but that TRPV1 receptor antagonism by capsazepine was ineffective. These data indicate that methanandamide activates cannabinoid CB1 receptors to reduce cocaine-induced hyperthermia, a finding that is consistent with evidence that cannabinoid CB1 receptors play a more significant role in anandamide-induced hypothermia than TRPV1 receptors (Costa et al., 1999; Rawls et al., 2006; Wise et al., 2007).
Methanandamide attenuated hyperthermia induced by a dopamine D1 receptor agonist (SKF 38393). Dopamine D1 and D2 receptors are activated by cocaine-evoked extracellular dopamine, but D1 receptors mediate the hyperthermic effect of cocaine whereas D2 receptor activation is associated with hypothermia (Hurd and Ungerstedt, 1989; Rockhold et al., 1991; Faunt and Crocker, 1987; Zarrindast and Tabatabai, 1992; Nagashima et al., 1992; Verma and Kulkarni, 1993; Boulay et al., 1999; Collins et al., 2007). Since both cocaine-and SKF 38393-induced hyperthermia were attenuated by methanandamide in the present study, it is unlikely that inhibition of cocaine-induced extracellular dopamine by methanandamide accounted for its ability to lower the hyperthermic efficacy of cocaine. A more probable explanation is that methanandamide, by activating cannabinoid CB1 receptors, disrupted dopamine D1 receptor signaling in one or more thermoregulatory substrates. Cannabinoid CB1 and dopamine D1 receptors are colocalized in forebrain regions that regulate body temperature and exert opposing actions on the G-protein/adenylyl cyclase signal transduction cascade, with CB1 receptor activation decreasing cyclic AMP levels and D1 receptor activation increasing cyclic AMP levels (Meschler and Howlett, 2001). Increased PKA activity in hypothalamic temperature centers is also associated with increased body temperature (Zhou et al., 2006). Hence, methanandamide may have suppressed the G-protein/adenylyl cyclase signal transduction cascade, thereby inhibiting the normal increase in dopamine D1 receptor signaling that mediates cocaine-induced hyperthermia.
Dopamine D2 receptor antagonism abolished the methanandamide attenuation of cocaine-induced hyperthermia. This suggests that cannabinoid CB1 receptor activation by methanandamide causes downstream activation of dopamine D2 receptors and that activated D2 receptors are required for methanandamide to attenuate cocaine-induced hyperthermia. Cannabinoid CB1 receptor-induced hypothermia is dependent on dopamine D2 receptor activation (Nava et al., 2000) and functional cross-talk between cannabinoid CB1 and dopamine D2 receptors has been demonstrated (Giuffrida et al., 1999; Kearn et al., 2005; Pickel et al., 2006). Dopamine D2 receptors, like cannabinoid CB1 receptors, are negatively coupled to cyclic AMP production (Sibley and Monsma, 1992). Thus, it is possible that concomitant CB1 and D2 receptor activation following methanandamide administration produces an exaggerated inhibition of cyclic AMP production which disrupts the dopamine D1 receptor signaling responsible for cocaine-induced hyperthermia. Future experiments will delineate a mechanism and locus for the cannabinoid CB1-dopamine interaction.
URB597, a FAAH inhibitor that prevents anandamide hydrolysis, did not affect cocaine-induced hyperthermia (Piomelli et al., 2006). URB597 elevates brain anandamide levels and produces analgesic, anti-depressant-like and anxiolytic-like effects that are dependent on cannabinoid CB1 receptor activation (Fegley et al., 2005; Kathuria et al., 2003; Piomelli et al., 2006). It does not produce classical cannabimimetic effects, such as hypothermia, catalepsy and sedation. We confirmed that URB597 lacks hypothermic efficacy and demonstrated that it fails to alter cocaine-induced hyperthermia. Because methanandamide attenuated cocaine-evoked hyperthermia, it may be surprising that the capacity of URB597 to enhance brain anandamide signaling did not translate into a similar effect. One explanation for the ineffectiveness of URB597 is that the kinetics of cannabinoid CB1 receptor activation may differ between methanandamide and URB597. Methanandamide is likely to produce a more rapid recruitment of cannabinoid CB1 receptors than URB597, and this may have contributed to the dissimilar effects of methanandamide and URB 597. Another possibility is that exogenous anandamide (i.e., methanandamide) and endogenous anandamide (i.e., the anandamide elevated following URB597 administration) might access different subpopulations of cannabiniod CB1 receptors (Svízenská et al., 2008; Mackie and Stella, 2006). The inability of URB597 to alter cocaine-induced hyperthermia in our experiments is consistent with its lack of effect on cocaine self-administration in squirrel monkeys (Justinova et al., 2008).
Unlike cocaine-induced hyperthermia, stereotypy and ambulation caused by cocaine were not affected by methanandamide. This finding indicates that a dose of methanandamide which is ineffective when given by itself exerts differential effects on cocaine-induced hyperthermia and hyperactivity. Numerous approaches investigating a role for the endocannabinoid system in acute hyperactivity caused by psychostimulant administration have yielded results that are not entirely consistent. For example, cannabinoid CB1 receptor gene deletion reduces acute hyperactivity following psychostimulant exposure but cannabinoid CB1 receptor blockade is ineffective (Corbillé et al., 2007; Poncelet et al., 1999). The ineffectiveness of methanandamide in our activity experiments does not indicate a minor role for endogenous cannabinoids and cannabinoid CB1 receptors in acute hyper-activity induced by cocaine. In fact, higher doses of methanandamide, which reduce ambulation and stereotypy, would be expected to counteract cocaine-induced hyperactivity (Järbe et al., 2003). Anandamide and the endocannabinoid system are also thought to play a critical role in cocaine-induced behavioral sensitization (Thiemann et al., 2008).
In conclusion, this is the first study to demonstrate that methanandamide reduces the hyperthermic response to a psychostimulant. Our findings provide pharmacological evidence that methanandamide activates cannabinoid CB1 receptors to attenuate cocaine-induced hyperthermia, and that downstream activation of dopamine D2 receptors plays a permissive role in the effects of methanandamide. Although hyperthermic doses of psychostimulants increase the level of anandamide in the brain (Centonze et al., 2004; Thiemann et al., 2008), the exact role of endogenous anandamide in the hyperthermic response has not been fully elucidated.
4. Experimental procedures
4.1. Animals
Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and experimental protocols were approved by the Institutional Animal Care and Use Committee of Temple University. Male Sprague-Dawley rats (Zivic-Miller, Pittsburgh, PA, USA) weighing 275–300 g were housed 2 per cage for 5 days before experimental use. Rats were maintained on a 12-h light/dark cycle (lights on at 7:00 a.m. and off at 7 p.m.).
4.2. Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Bethesda, MD, USA). It was dissolved in 0.9% saline and injected intraperitoneally (i.p.). R-methanandamide (Tocris Bioscience, St. Louis, MO, USA) was dissolved in Tocrisolve at a concentration of 10 mg/ml and then diluted in 0.9% saline to a concentration of 5 mg/ml. Methanandamide was injected i.p. The fatty acid amide hydrolase inhibitor, cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester (URB597) was purchased from Sigma-Aldrich (St. Louis, MO, USA). URB597 was dissolved in a vehicle of 5% Tween 80, 5% polyethylene glycol, and 90% saline and injected i.p. Capsazepine, a TRPV1 receptor antagonist, and [N-(Piper-idin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride] (SR 141716A, Rimonabant), a cannabinoid CB1 receptor antagonist, were purchased from Tocris and dissolved in a vehicle of 20% ethanol, 20% cremophor and 60% saline. Both drugs were injected i.p. S(−)-raclopride, a dopamine D2 receptor antagonist, and (±)-1-Phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide (SKF 38393 hydrobromide), a dopamine D1 receptor agonist, were purchased from Tocris and dissolved in normal saline. Raclopride and SKF 38393 were injected subcutaneously (s.c.). All drugs were administered in a volume of 1 ml/kg.
4.3. Body temperature experiments
Experiments were started between 8:00 and 9:00 a.m. to minimize effects of circadian variation. Rats were placed singly into cages in an environmental room maintained at a constant temperature of 24±0.3 °C and relative humidity of 52±2%. Animals were allowed to acclimate for 60 min before taking the first temperature reading. Prior to drug administration, baseline temperatures were taken every 30 min for 90 min using a thermistor probe (YSI series 400, Yellow Springs Instrument Co., Yellow Springs, OH; sensitivity of 0.10 °C), which was lubricated and inserted approximately 7 cm into the colon. Rats were unrestrained throughout the experiment, with only the tail being held gently between two fingers. Three consecutive body temperature readings were recorded in the baseline interval, prior to drug administration, to establish a baseline temperature. Data were expressed as the mean±S.E.M. of body temperature. Five sets of body temperature experiments were conducted. In all cases, body temperature was recorded every 30 min during a 90-min baseline interval prior to drug administration. The following experiments were conducted.
4.3.1. Experiment 1: Does methanandamide reduce body temperature?
Methanandamide produces cannabimimetic effects such as hypothermia, catalepsy and hypolocomotion when administered to rats and mice at sufficiently high doses (Costa et al., 1999; Smith et al., 1994; Stein et al., 1996; Abadji et al., 1994). For our drug combination experiments, the optimal dose of methanandamide is one that does not by itself alter body temperature or activity. To identify this dose, methanandamide (5, 10 or 20 mg/kg, i.p.) was injected and body temperature was recorded 15, 30, 45 and 60 min post-administration.
4.3.2. Experiment 2: Does methanandamide inhibit cocaine-induced hyperthermia by activating cannabinoid CB1 or TRPV1 receptors?
Rats were pretreated with one of the following drug combinations: vehicle/vehicle; vehicle/methanandamide (5 mg/kg, i.p.); capsazepine (40 mg/kg, i.p.)/vehicle; capsazepine (40 mg/kg, i.p.)/methanandamide (5 mg/kg, i.p.); SR 141716A (5 mg/kg, i.p.)/vehicle; or SR 141716A (5 mg/kg, i.p.)/methanandamide (5 mg/kg, i.p.). Twenty min later, rats were injected with cocaine (15 mg/kg) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-cocaine injection.
4.3.3. Experiment 3: Does the FAAH inhibitor URB597 inhibit cocaine-induced hyperthermia?
Rats pretreated with vehicle or URB597 (0.25 mg/kg, i.p.) were injected 20 min later with cocaine (15 mg/kg, i.p.) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-cocaine injection.
4.3.4. Experiment 4: Is dopamine D2 receptor activation required for methanandamide to attenuate cocaine-induced hyperthermia?
Rats were pretreated with one of the following drug combinations: vehicle/vehicle; raclopride (5 mg/kg, i.p.)/vehicle; raclo-pride (5 mg/kg, i.p.)/methanandamide (5 mg/kg, i.p.); or vehicle/methanandamide (5 mg/kg, i.p.). Twenty min later, rats were injected with cocaine (15 mg/kg) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-cocaine injection.
4.3.5. Experiment 5: Does methanandamide attenuate hyperthermia induced by dopamine D1 receptor activation?
Rats pretreated with vehicle or methanandamide (5 mg/kg, i.p.) were injected 20 min later with SKF 38393 (10 mg/kg, s.c.) or saline and body temperature was measured 30, 60, 90, 120 and 180 min post-SKF 38393 injection.
4.4. Activity measurement
Activity was measured using a Digiscan D Micro System (Accuscan, Columbus, OH, USA) as previously described (Soderman and Unterwald, 2008; Niculescu et al., 2008). Each activity monitor consists of an aluminum frame equipped with 16 infrared light beams and detectors. As the animal moves about the chamber, the beams are broken and recorded by a computer interfaced to the monitors. Ambulatory activity was registered when consecutive light beams were interrupted, and stereotypy was detected when the same light beam was broken repeatedly. In actual experiments, rats were injected with either methanandamide (5 mg/kg) or vehicle and placed into test chambers. Twenty min later rats were injected with cocaine (15 mg/kg) or saline. Data collection began as soon as the animals were placed in the test chambers and activity data are presented as total mean counts in 10-min bins.
4.5. Data analysis
Data were analyzed by one-way repeated measures ANOVA. Differences between groups were determined by Tukey's post-hoc analysis after significance was determined by ANOVA. Values of P<0.05 were considered statistically significant.
Acknowledgments
This work is supported by NIH grants DA022694 (SMR) and T32 DA09580 (EMU).
REFERENCES
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Ansah TA, Wade LH, Shockley DC. Changes in locomotor activity, core temperature, and heart rate in response to repeated cocaine administration. Physiol. Behav. 1996;60:1261–1267. doi: 10.1016/s0031-9384(96)00250-8. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J. Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Vailati S, Colleoni M. SR 141716A, a cannabinoid receptor antagonist, reverses the behavioural effects of anandamide-treated rats. Behav. Pharmacol. 1999;10:327–331. doi: 10.1097/00008877-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol. Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dogan MD, Patel S, Rudaya AY, Steiner AA, Székely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br. J. Pharmacol. 2004;143:1023–1032. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunt JE, Crocker AD. The effects of selective dopamine receptor agonists and antagonists on body temperature in rats. Eur. J. Pharmacol. 1987;133:243–247. doi: 10.1016/0014-2999(87)90019-7. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur. J. Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP. Cocaine alters body temperature and behavioral thermoregulatory responses. Neuroreport. 1993;4:106–108. doi: 10.1097/00001756-199301000-00028. [DOI] [PubMed] [Google Scholar]
- Hamida SB, Plute E, Cosquer B, Kelche C, Jones BC, Cassel JC. Interactions between ethanol and cocaine, amphetamine, or MDMA in the rat: thermoregulatory and locomotor effects. Psychopharmacology (Berl) 2008;197:67–82. doi: 10.1007/s00213-007-1007-5. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. (R)-Methanandamide and delta9-tetrahydrocannabinol-induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur. J. Pharmacol. 2003;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol. Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165:917–928. [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol. Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur. J. Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Lomax P, Daniel KA. Cocaine and body temperature in the rat: effects of ambient temperature. Pharmacology. 1990;40:103–109. doi: 10.1159/000138648. [DOI] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation of delta9-tetrahydrocannabinol-induced hypothermia by fluoxetine in the rat. Br. J. Pharmacol. 1998;124:1419–1424. doi: 10.1038/sj.bjp.0701980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuk PM, Tardiff K, Leon AC, Hirsch CS, Portera L, Iqbal MI, Nock MK, Hartwell N. Ambient temperature and mortality from unintentional cocaine overdose. JAMA. 1998;279:1795–1800. doi: 10.1001/jama.279.22.1795. [DOI] [PubMed] [Google Scholar]
- Masserano JM, Karoum F, Wyatt RJ. SR 141716A, a CB1 cannabinoid receptor antagonist, potentiates the locomotor stimulant effects of amphetamine and apomorphine. Behav. Pharmacol. 1999;10:429–432. doi: 10.1097/00008877-199907000-00010. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Nagashima M, Yamada K, Kimura H, Matsumoto S, Furukawa T. Hyperthermia induced by the dopamine D1 receptor agonist SK&F38393 in combination with the dopamine D2 receptor agonist talipexole in the rat. Pharmacol. Biochem. Behav. 1992;43:993–997. doi: 10.1016/0091-3057(92)90472-r. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G, Gessa GL. Permissive role of dopamine D (2) receptors in the hypothermia induced by delta(9)-tetrahydrocannabinol in rats. Pharmacol. Biochem. Behav. 2000;66:183–187. doi: 10.1016/s0091-3057(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Niculescu M, Perrine SA, Miller JS, Ehrlich ME, Unterwald EM. Trk: a neuromodulator of age-specific behavioral and neurochemical responses to cocaine in mice. J. Neurosci. 2008;28:1198–1207. doi: 10.1523/JNEUROSCI.0988-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J. Comp. Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet M, Barnouin MC, Brelière JC, Le Fur G, Soubrié P. Blockade of cannabinoid (CB1) receptors by 141716 selectively antagonizes drug-induced reinstatement of exploratory behaviour in gerbils. Psychopharmacology. 1999;144:144–150. doi: 10.1007/s002130050987. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J. Pharmacol. Exp. Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Ding Z, Cowan A. Role of TRPV1 and cannabinoid CB1 receptors in AM 404-evoked hypothermia in rats. Pharmacol. Biochem. Behav. 2006;83:508–516. doi: 10.1016/j.pbb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Rockhold RW, Carver ES, Ishizuka Y, Hoskins B, Ho IK. Dopamine receptors mediate cocaine-induced temperature responses in spontaneously hypertensive and Wistar-Kyoto rats. Pharmacol. Biochem. Behav. 1991;40:157–162. doi: 10.1016/0091-3057(91)90337-2. [DOI] [PubMed] [Google Scholar]
- Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol. Biochem. Behav. 2008;90:501–511. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr. Molecular biology of dopamine receptors. Trends Pharmacol. Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J. Pharmacol. Exp. Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: Sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br. J. Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J. Med. Chem. 2005;48:1857–1872. doi: 10.1021/jm0495071. [DOI] [PubMed] [Google Scholar]
- Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenöhrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization Behav. Brain Res. 2008;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Verma A, Kulkarni SK. Differential role of dopamine receptor subtypes in thermoregulation and stereotypic behavior in naive and reserpinized rats. Arch. Int. Pharmacodyn. Ther. 1993;324:17–32. [PubMed] [Google Scholar]
- Wiechman BE, Spratto GR. Body temperature response to cocaine and diazepam in morphine-treated rats. Pharmacology. 1982;25:308–319. doi: 10.1159/000137757. [DOI] [PubMed] [Google Scholar]
- Wise LE, Shelton CC, Cravatt BF, Martin BR, Lichtman AH. Assessment of anandamide's pharmacological effects in mice deficient of both fatty acid amide hydrolase and cannabinoid CB1 receptors. Eur. J. Pharmacol. 2007;557:44–48. doi: 10.1016/j.ejphar.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Tabatabai SA. Involvement of dopamine receptor subtypes in mouse thermoregulation. Psychopharmacology (Berl) 1992;107:341–346. doi: 10.1007/BF02245159. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li CH, Huo HR, Kang XL, Li LF, Jiang N, Jiang TL. Effect of guizhi decoction on PKA and PKC activities of hypothalamus in fever rats. Zhongguo Zhong Yao Za Zhi. 2006;31:66–69. [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]