Abstract
This review will cover the roles of neurotrophins in inner ear development, neuronal maintenance, neuronal process regeneration, and clinical applications including possible augmentation of cochlear implant function. Severe to profound deafness is most often secondary to a loss of or injury to cochlear mechanosensory cells, and there is often an associated loss of the peripheral auditory neural structures, specifically the spiral ganglion neurons and peripheral auditory fibers. Cochlear implantation is currently our best hearing rehabilitation strategy for severe to profound deafness. These implants work by directly electrically stimulating the remnant auditory neural structures within the deafened cochlea. When administered to the deafened cochlea in animal models, neurotrophins, specifically BDNF and NT3, have been shown to dramatically improve spiral ganglion neuron survival and stimulate peripheral auditory fiber regrowth. In animal models, neurotrophins administered in combination with cochlear implantation has resulted in significant improvements in the electrophysiological and psychophysical measures of outcome. While further research must be done before these therapies can be applied clinically, neurotrophin therapies for the inner ear show great promise in enhancing cochlear implant outcomes and the treatment of hearing loss.
Keywords: neurotrophin, BDNF, NT3, cochlear implant
INTRODUCTION
Deafness occurs in 0.1-0.2% of newborns (Morton, 1991), and hearing loss affects up to 70% of the population over the age of 75 years (Campbell et al., 1999; Sprinzl and Riechelmann, 2010). Hearing loss may be genetic or acquired, and it is the end result of a variety of pathologies, including genetic mutations, congenital ear malformations, infection, ototoxic insult, aging and noise exposure. Regardless of the underlying etiology, the majority of deafness is related primarily to loss of or damage to cochlear mechanosensory cells, and a secondary lesion in the neural network. The neural lesion may include regression of peripheral auditory fibers (PAF) and loss of spiral ganglion neurons (SGN) may also occur to a variable degree (Spoendlin, 1971a; Wright, 1976; Terayama et al., 1977; Bichler et al., 1983; Nadol et al., 1989). Cochlear implant (CI) devices are currently the best hearing rehabilitation strategy for the severely and profoundly deaf as the inner ear mechanosensory and neural elements do not spontaneously regenerate. The CI electrode array is surgically inserted into the scala tympani of the cochlea, and sounds are converted to electrical signals that are then delivered to the implanted electrodes. Cochlear implants function by directly stimulating the residual auditory neuronal structures in the deafened ear, thereby bypassing the defective or missing mechanosensory cells, and the status of these remnant neural structures likely directly impacts cochlear implant outcomes. Neurotrophin molecules play a critical role in the development and maintenance of many neural systems, and their application to the deafened inner ear has been found to promote SGN health and survival (Staecker et al., 1996b; Gillespie et al., 2003; Agterberg et al., 2008), as well as PAF regrowth towards the source of the neurotrophins (Wise et al., 2005; Shibata et al., 2010; Wise et al., 2010). Inner ear neurotrophin therapy shows promise in the augmentation of cochlear implantation outcomes by improving the health of the cochlear neural substrates and bringing neurons in closer proximity to the CI electrode array.
ANATOMY OF THE COCHLEA
The human cochlea is an anatomically and functionally complex structure that allows us to hear and distinguish sounds of many different frequencies and intensities, thereby allowing us to interact and communicate with our surrounding world. The membranous cochlea is a small spiral structure contained within the dense otic capsule of the temporal bone, and it is composed of 3 interrelated compartments: the scala vestibuli, scala tympani and scala media. The organ of Corti, the sensory organ of hearing, resides on the basilar membrane (BM). The organ of Corti is composed of supporting cells and specialized mechanosensory cells, called hair cells due to the bundle of stereocilia located on their apical surface. It is the hair cells that initiate a neural signal in response to sound. The mechanical energy of sound causes movement and deflection of the BM in a tonotopic fashion, with high frequency sounds preferentially moving the basal BM and low frequency sounds moving the apical BM. This in turn causes a deflection of the stereocilia of the hair cells, leading to depolarization of the cells and release of neurotransmitter that stimulates the nerve endings. The hair cells are innervated by the peripheral auditory fibers of SGNs, bipolar cells that provide afferent input to the cochlear nucleus (Merchan-Perez and Liberman, 1996). For more in-depth reviews of cochlear anatomy and histology please see these reviews: (Santi and Mancini, 1998; Raphael and Altschuler, 2003; Davis and Liu, 2011; Nayagam et al., 2011).
THE COCHLEAR PATHOLOGY OF HEARING LOSS
Various animal models have been used to simulate and investigate the morphologic and physiologic effects of insults to the inner ear. Many animal studies have utilized aminoglycoside antibiotics administered by various methods to induce severe ototoxic lesions. When administered directly to the cochlea, there is a loss of both cochlear sensory and supporting cells associated with a regression of the PAFs and a progressive loss of the majority of SGNs to the point where there are few remaining fibers and neurons within months of sustaining the lesion (Terayama et al., 1977; Terayama et al., 1979; Bichler et al., 1983). Similarly, neomycin administered topically to the round window membrane has been demonstrated to induce degeneration of SGNs over a period of months, though the effect is not as severe as that seen with direct cochlear infusion (Zappia and Altschuler, 1989). Other studies have utilized noise exposure to induce the loss of cochlear hair cells and the formation of a phalangeal scar (Spoendlin, 1971b; Hawkins, 1973; Johnsson and Hawkins, 1976; Abrashkin et al., 2006), and PAF degeneration is associated with and proportional to the damage seen in the organ of Corti (Wright, 1976; Bohne and Harding, 1992).
In mammalian animal models of deafness, spontaneous regeneration of hair cells has not been seen. However, occasional spontaneous re-sprouting of PAFs has been seen following the administration of various otologic lesions. Months after severing the eighth cranial nerve and causing a near total loss of SGNs in Rosenthal’s canal, Spoendlin and Suter (1976) found many large new fibers in the region between the habenula perforata and the inner hair cells (Spoendlin and Suter, 1976). Similarly, spontaneously regenerated PAFs have been noted following noise induced hearing loss (Wright, 1976; Bohne and Harding, 1992; Strominger et al., 1995; Lawner et al., 1997), as well as following aminoglycoside induced ototoxicity (Johnsson and Hawkins, 1972; Terayama et al., 1977; Terayama et al., 1979; Santi et al., 1982). However, this spontaneous re-sprouting of PAFs has not been accompanied by a concomitant increase in the number of SGNs in Rosenthal’s canal (Spoendlin and Suter, 1976) and has primarily been seen in cochlea demonstrating only focal lesions with intact adjacent sensory and supporting cells in the organ of Corti (Bohne and Harding, 1992; Strominger et al., 1995). These findings have led to the conclusion that any spontaneous peripheral fiber regeneration that occurs arises from residual surviving SGNs and requires intact supporting and sensory cells to provide support and guidance to the re-growing fibers. Unfortunately, in human deafness, as well as in severe ototoxic lesions induced in animals, there is often a widespread loss of both sensory and supporting cells within the organ of Corti leading to the formation of a flat epithelium (Merchant et al., 2005; Kim and Raphael, 2007). Without surviving supporting and sensory cells to provide support to the surviving SGNs, no significant spontaneous fiber regrowth into the basilar membrane area has been seen in mammalian animal models following the induction of a severe ototoxic lesion (Shibata et al., 2010; Wise et al., 2010).
People with bilateral severe to profound deafness that cannot be adequately rehabilitated with conventional hearing aid amplification are candidates for cochlear implantation. CI candidates may be deaf for a variable duration of time and due to a variety of etiologies, and as such the histological and functional status of the remnant auditory neuronal structures within the cochlea is quite variable (Hinojosa and Lindsay, 1980; Nadol et al., 1989; Nadol, 1997; Pfingst et al., 2011). While the extent of auditory neural degeneration varies between species, and humans generally demonstrate a less severe loss of auditory neural structures following deafness as compared to animal models (Teufert et al., 2006; Linthicum and Fayad, 2009), human temporal bone studies have shown a decrease in SGNs in deaf individuals as compared to normal hearing controls (Kerr and Schuknecht, 1968; Hinojosa and Lindsay, 1980). Furthermore, human studies have demonstrated that particular etiologies of deafness, such as post-natal infectious labyrinthitis, are correlated with a severe loss of SGNs (Kerr and Schuknecht, 1968; Nadol et al., 1989). The extent of degeneration of inner ear sensorineural structures following deafness in humans may have implications for the success of cochlear implantation.
NEUROTROPHINS IN THE INNER EAR
Role of Neurotrophins in Inner Ear Development
Neurotrophins are a family of molecules critical to the development and maintenance of neural systems. Thus far four neurotrophins have been identified in mammals: nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT3) and neurotrophin-4 (NT4). These four molecules share a similar sequence and structure as they all descend from a common ancestral gene, and all four are active in directing how neurons grow and differentiate during development and beyond (Huang and Reichardt, 2001). Each neurotrophin binds to a specific subtype of Trk receptor with high affinity (Lu et al., 2005), and some low affinity cross binding to other Trk receptors and the p75 receptor also occurs (see Chapter by Davis and Green for more details). Within the auditory system, BDNF and NT3 are the two neurotrophins essential for normal auditory neural development (Pirvola et al., 1992). Both BDNF and NT3 are expressed at high levels in the cochlear sensory epithelium during development (Ylikoski et al., 1993; Sugawara et al., 2007), and in vitro studies of cochlear explants have demonstrated that BDNF and NT3 at physiologic levels promote both SGN survival and neurite outgrowth (Pirvola et al., 1992). Null mutant mouse studies have shown that NT3 is critical for the development of the majority of SGNs and cochlear afferent neurons, initially presumed to be the type I afferent fibers to the inner hair cells (Fariñas et al., 1994; Ernfors et al., 1995). In contrast, BDNF is critical for the development of vestibular ganglion neurons and a minority of cochlear afferent neurons, initially presumed to be the type II afferent fibers to the outer hair cells (Ernfors et al., 1995). Although these early studies suggested that NT3 and BDNF specifically corresponded to the development of type I and type II afferent auditory fibers respectively, additional studies utilizing immunocytochemistry to study the distribution of neurotrophin receptors during development have actually shown that all afferent cochlear neurons, both type I and II, express receptors for both BDNF and NT3 (Mou et al., 1997; Fariñas et al., 2001).
Further null mutant mouse studies have suggested that there is a gradient of expression within the cochlea for each neurotrophin during development. Through in situ hybridization techniques on developing, post natal and adult rats, NT3 and BDNF have been found to be robustly expressed throughout the cochlear inner and outer hair cells during embryonic development (Pirvola et al., 1992). During early embryonic development BDNF is expressed in the apical turns, and expression progresses towards the base later in development (Fariñas et al., 2001). In contrast, NT3 expression is more pronounced in the cochlear base in the early stages of development, and the expression gradient progresses more apically until it is primarily expressed in the apex of post natal and adult mice (Fariñas et al., 2001; Sugawara et al., 2007). NT3 continues to be expressed in the inner hair cells and adjacent supporting cells of the post natal and adult cochlea (Sugawara et al., 2007), and BDNF is primarily expressed within the vestibular system of the adult cochleovestibular apparatus (Pirvola et al., 1992).
This progression in the gradient of each neurotrophin’s expression over the course of cochlear embryologic development explains the phenotypic appearance of null mutant mice. NT3 knockout mice have demonstrated an almost complete absence of innervation in the basal turns with a more moderate decrease in nerve fibers in the middle and apical turns (Fritzsch et al., 1997), whereas BDNF deficient mice demonstrate a more subtle loss of neurons primarily in the apical turns (Ernfors et al., 1995). The critical time for development of basal innervation occurs early when NT3 is expressed in the organ of Corti while BDNF expression is absent in the base. Therefore the normal endogenous expression of BDNF does not rescue the NT3 null mutation in the basal cochlea. However, the NT3 null mutation is completely rescued in the transgenic mouse expressing BDNF under the control of the NT3 promoter, thereby inducing expression of BDNF in the basal cochlea earlier in development (Fariñas et al., 2001). These findings suggest that each neurotrophin has a distinctive gradient of expression that evolves over the course of cochlear development, and that this gradient is critical to the normal development of cochlear innervation. Mice deficient for both neurotrophins do not develop either vestibular or spiral ganglion neurons (Ernfors et al., 1995), further demonstrating that both BDNF and NT3 are essential for normal inner ear neural development.
In maturing spiral ganglion explants, each neurotrophin has been shown to direct the physiologic firing patterns of the neurons on which it acts, with NT3 promoting an apical firing pattern and BDNF promoting a firing pattern consistent with basal neurons (Adamson et al., 2002; Zhou et al., 2005). In this fashion, neurotrophins appear to continue to exert critical effects in the developing and mature cochlea through their influence on the tonotopic tuning of the neural elements within the cochlea.
Role of Neurotrophins in Inner Ear Maintenance
Neurotrophins may have primary effects on cell physiology at one time point and greater effects on survival and proliferation at another time point depending on the time of exposure to their target cell population (Adamson et al., 2002). In the mature mammal both BDNF and NT3 continue to play an important role in the survival and maintenance of the auditory neurons. Following the loss of cochlear hair cells in the adult mammalian ear there is a dramatic loss of both PAFs and SGNs, presumably secondary to the loss of neurotrophic support from the hair cells (Staecker et al., 1998). The remaining SGNs appear unhealthy with smaller soma (Kanzaki et al., 2002) and pyknotic, misshapen nuclei (Ernfors et al., 1996; Staecker et al., 1996b; Gillespie et al., 2003). Many studies have now demonstrated that when the deafened ear is treated with exogenous BDNF or NT3, there is a significant enhancement of SGN survival to near normal levels (Ernfors et al., 1996; Staecker et al., 1996b; Miller et al., 1997; Staecker et al., 1998; Altschuler et al., 1999; Nakaizumi et al., 2004) and the surviving SGNs appear larger and healthier with well-defined nuclei and nucleoli and abundant Nissl substance in the cytoplasm (Staecker et al., 1996a; Kanzaki et al., 2002). Furthermore, it is important to note that despite the limited endogenous expression of BDNF in the mature adult cochlea, it has comparable effects to NT3 in promoting auditory neural survival when administered exogenously (Staecker et al., 1996b; Staecker et al., 1998). In addition to neurotrophins, other molecules, such as fibroblast growth factor and glial cell line-derived neurotrophic factor, have been shown to influence the maintenance and growth of the auditory nerve (Altschuler et al., 1999; Kanzaki et al., 2002; Glueckert et al., 2008).
Regenerative Effects of Neurotrophins
In addition to the positive effects of exogenous neurotrophins on SGN survival, many studies have also demonstrated neuritogenesis both in vitro and in vivo following exogenous neurotrophin administration. Limited neurite outgrowth has been seen following exogenous neurotrophin administration to spiral ganglion neuronal cultures (Pirvola et al., 1992). More impressive neurite outgrowth has been seen in vivo. Following aminoglycoside deafening and neurotrophin administration into the guinea pig perilymph via a mini osmotic pump, peripheral neuronal fibers were seen growing in a robust yet diffuse fashion due to the elevated levels of the growth factors (Ernfors et al., 1996; Staecker et al., 1996b; Wise et al., 2005; Miller et al., 2007; Glueckert et al., 2008). Similarly, following aminoglycoside deafening and neurotrophin administration into the scala tympani via a viral vector, there was significant regrowth of PAFs into the cochlea (Shibata et al., 2010; Wise et al., 2010). The peripheral fiber regrowth seen with both administration methods was generally disorganized; however, administration of neurotrophin via viral vector promoted more directed growth of the fibers into the basilar membrane area towards transfected cells (Shibata et al., 2010; Wise et al., 2010). Such targeted growth is potentially important for enhancing the outcome of CI therapy because the nerve endings are more likely to be in proximity to the electrode array. Similar growth of PAFs into the basilar membrane area has been seen with inoculation by either adenovirus or adeno-associated viral vectors, as well as with either scala tympani or scala media inoculations (Shibata et al., 2010).
Neurotrophin Concentrations During Development and Beyond
NT3 and BDNF, administered at 1 to 5 ng/ml to developing rat ganglion explants in culture, are sufficient to elicit improved SGN survival and limited neurite outgrowth, and the more robust effect is seen in response to BDNF (Pirvola et al., 1992). However, maximal SGN survival in vitro is seen at higher concentrations of 50 ng/ml for BDNF and 10ng/ml for NT3 (Lefebvre et al., 1994). Many groups have demonstrated improved SGN survival and neurite outgrowth when neurotrophins have been administered in vivo as well; however, the concentrations of neurotrophin required to elicit these effects appeared to be even higher based on early work using mini osmotic pumps for administration. These studies showed improved SGN survival with limited neuritogenesis when either BDNF or NT3 were delivered to the scala tympani in supra-physiologic concentrations ranging from 50 to 100 µg/ml (Ernfors et al., 1996; Yamagata et al., 2004). In the work by Shinohara et al. improved eABR thresholds following CI were seen with neurotrophins administered by mini osmotic pump at a concentration of 100 µg/ml (Shinohara et al., 2002).
More recent in vivo work has demonstrated that improved SGN survival and neurite outgrowth can also be achieved with lower, more physiologic levels of neurotrophins administered via alternative strategies. Drug eluting CI devices are being developed. Physiologic concentrations of neurotrophins have been delivered subacutely over a period of weeks to the inner ear via NT3-loaded alginate beads and have led to improved SGN survival following otologic insult (Noushi et al., 2005). Similarly, physiologic levels of BDNF have been delivered to the scala tympani over a period of two weeks through a CI coated in a hydrogel soaked with BDNF (Chikar et al., 2011). Spiral ganglion survival has also been improved through the ex vivo gene transfer of BDNF to allogenic fibroblasts that were subsequently incorporated into agarose gel coated electrode arrays, thereby delivering BDNF at low levels over a period of weeks (Rejali et al., 2007). While drug eluting devices are attractive in their relative ease and safety of administration, potential disadvantages of this administration method include the lack of control over the rate of drug delivery and the relatively short time over which drug is eluted. Gene therapy holds the potential for the more directed and chronic administration of neurotrophins. Gene therapy with either BDNF or NT3 delivered via an adenoviral vector has achieved concentrations comparable to physiologic levels of neurotrophins when measured in cell culture and in vivo, and these levels have been sufficient to translate into improved SGN survival, neurite outgrowth (Shibata et al., 2010; Wise et al., 2010), and even decreased eABR thresholds elicited by CI stimulation (Chikar et al., 2008).
The Potential Role of Neurotrophins in Cochlear Implantation
Cochlear implantation has provided a highly effective means to rehabilitate deafness due to defects in the peripheral auditory system. Cochlear implants overcome most forms of deafness by bypassing the sensory cells of the cochlea and directly stimulating the remaining auditory neural structures. Although significant benefit is derived from our current CI technology in terms of restoring speech perception abilities in quiet, there continue to be major limitations when listening to complex sounds that are commonly encountered in day to day life, such as speech in noise and music. Current cochlear implants have between 8 and 22 electrodes; however, likely due to current spread and diminished spiral ganglion neuronal populations, these configurations yield closer to 4 to 8 perceptual channels (Dorman et al., 1998; Friesen et al., 2001). In quiet only 4 channels are necessary to achieve adequate sentence understanding, but in noisy environments, as the signal to noise ratio becomes poorer, up to 20 perceptual channels are needed to achieve comparable speech understanding (Dorman et al., 1998). For music appreciation and perception of lexical tone as many as 32 or more perceptual channels are needed (Xu et al., 2002; Kong et al., 2004).
In order to diminish current spread and improve the number of perceptual channels achieved with cochlear implant electrode arrays, various strategies have been developed, including perimodiolar electrode arrays and concomitant neurotrophin treatment, to bring the electrode and auditory neural elements into closer proximity. Perimodiolar electrode arrays which are designed to lie on the medial side of the scala tympani in close proximity to the modiolar wall and thus closer to the SGNs within the cochlear modiolus, were developed in an effort to decrease the electrical threshold required to stimulate the cochlear neural substrates. In computer modeling of the human cochlea, perimodiolar implants indeed appear to reduce current thresholds and improve frequency specificity in the basal turn of the cochlea (Frijns et al., 2001). In clinical studies examining the current thresholds required in individuals implanted with a perimodiolar array as compared to those implanted with a straight electrode array, which may lie more laterally in the scala tympani, it was found that people implanted with a perimodiolar array had lower stimulation thresholds (Cohen et al., 2005; Cohen et al., 2006). Furthermore, individuals implanted with a perimodiolar array had a narrower forward masking profile, indicating that each perimodiolar electrode stimulates a more specific subpopulation of neurons (Cohen et al., 2006). However, unfortunately thus far these electrophysiological benefits of the perimodiolar array have not been translated into improved speech perception outcomes in the clinical population (Fitzgerald et al., 2007).
Only partial viability of the auditory nerve is required to derive some benefit from implantation and human temporal bone studies have not shown a link between SGN counts and CI speech recognition (Nadol et al., 2001; Khan et al., 2005; Fayad and Linthicum, 2006). However, studies have shown a correlation between psychophysical and electrophysiological outcomes and the histological status of the cochlear neural structures. Likely related to the degeneration seen in the PAFs and SGNs, electrically evoked auditory brainstem responses (eABR) are abnormal in the deafened inner ear (Shepherd and Javel, 1997). More damaged cochleae typically require a higher current threshold for stimulation with a cochlear implant and have a decreased dynamic range (Pfingst and Sutton, 1983; Kawano et al., 1998; Kang et al., 2010).
Although chronic electrical stimulation alone improves the survival of SGNs (Altschuler et al., 1999; Kanzaki et al., 2002), it does not promote regeneration or growth of PAFs toward the electrode array. However, in deafened animals treated with CI combined with an intracochlear administration of neurotrophins, there is both morphologic evidence of improved SGN survival beyond that seen with either electrical stimulation or neurotrophins alone (Kanzaki et al., 2002), as well as decreased post implantation eABR thresholds (Shinohara et al., 2002). It is thought that an increase in frequency selectivity and the number of perceptual channels will be seen in connection with the diminished current thresholds associated with neurotrophin treatment.
Clinical Application of Neurotrophin Treatment
One obstacle to translating neurotrophin treatments to the clinical arena is in determining how to optimally deliver the molecules to the inner ear. The human auditory system poses unique advantages and disadvantages regarding the delivery of molecular therapies. Delivery of molecular therapies to the inner ear is made more difficult based on the fact that it is well protected from the systemic circulation by the blood-perilymphatic barrier and it is anatomically situated deep within the dense otic capsule of the temporal bone, making direct access more difficult. However, these characteristics also present some interesting advantages, namely that therapies delivered to the inner ear will likely remain within the auditory system with little or no leakage into the systemic circulation. This limits the risk of inducing adverse side effects in other organ systems. Furthermore, the inner ear fluids allow distribution of neurotrophin treatment throughout the cochlea.
Various methods of administering molecular therapies to the inner ear have been used with success in animal models. For short-term effects, molecules can be delivered on a onetime basis to the inner ear by either diffusion across the round window membrane from the middle ear space or direct infusion into the perilymph through a surgical cochleostomy (Richardson et al., 2004; Noushi et al., 2005; Richardson et al., 2005; Richardson et al., 2006; Havenith et al., 2011; Pfingst et al., 2011). Conflicting results have been published regarding the duration of the effects of neurotrophin treatment after administration has been ceased. While one study found durable benefits of BDNF treatment even after administration has ceased, both in terms of SGN counts and eABR thresholds (Agterberg et al., 2009), another found a severe decrease in SGN density following the removal of neurotrophin support (Gillespie et al., 2003). Given these conflicting findings it is unclear at this time whether neurotrophins need to be administered continuously to maintain their beneficial effects, but it is possible that longer-term administration of neurotrophins will be necessary.
In animal models the two most commonly utilized methods for chronic neurotrophin administration have been: 1) surgical implantation of a mini osmotic pump, and 2) inoculation with a replication deficient viral vector carrying the gene for neurotrophin production. Each method of chronic administration has its own set of advantages and disadvantages. Mini osmotic pumps have been shown to be effective in the chronic administration of molecules directly into the scala tympani of small animals without inducing secondary cochlear damage as assessed by auditory brainstem response (ABR) testing (Brown et al., 1993). However, their application in the clinical world is limited by the facts that one must periodically replenish the reservoir of the agent being administered and that the therapeutic agent is delivered in a nonspecific fashion into the perilymphatic space. Viral vectors have also been used with success in the inner ear. When inoculated directly into the cochlear perilymphatic space, replication deficient adenovirus has been shown to effectively transfect neural, epithelial and connective tissue cells within the cochlea without inducing significant cytotoxic affects (Raphael et al., 1996; Weiss et al., 1997). While adenoviral vectors have been shown to effectively transfect inner ear cells, significant expression of the transgene is typically limited to a period of a few weeks as it is not incorporated into the target cell’s genome (Lalwani and Mhatre, 2003). Adeno-associated viral (AAV) vectors have also been used with success in the inner ear. AAV has the advantage of being unable to replicate without the assistance of a helper virus and it is not associated with human disease. Furthermore, optimal expression is seen at approximately 2 weeks following transfection and significant levels of expression in the inner ear are seen up to 24 weeks following inoculation (Lalwani et al., 1998), and transgene expression has been seen as long as 6 years following AAV administration into the primate brain (Bankiewicz et al., 2006).
While molecular therapies using various administration methods in animal models have shown promise with regard to their potential for clinical application, as with any therapy, the risks and benefits of a treatment must be carefully weighed before being offered to patients. We must be particularly careful in the application of these therapies to the treatment of hearing loss, as hearing loss is not a life threatening condition and therefore only the slightest treatment-associated risks can be tolerated. Currently there are many unanswered questions regarding the effects of neurotrophins in the inner ear, and these will need to be addressed before proceeding with the development of clinical applications. Future work will need to be directed towards further characterizing the type and physiologic properties of these re-grown PAFs, as well as developing strategies to better direct the fibers to grow toward specific CI electrodes in a tonotopic fashion. Ultimately we must prove that neurotrophin treatment in fact has a beneficial effect when administered in concert with cochlear implantation, and that there are no major long term sequelae of treatment, such as the formation of neuromas or other tumors in the inner ear. Still, neurotrophin therapies for the inner ear, particularly in combination with CI, show great early promise and will hopefully come to clinical fruition after further research has been completed.
The Future of Neurotrophin Therapy
The development of the cochlear implant has revolutionized the world of hearing rehabilitation; however, severe limitations remain with this technology and it is likely CI will never be able to fully restore normal hearing. The ultimate goal of hearing research is to develop the means by which normal hearing can indeed be restored through the regeneration of an anatomically and physiologically functional inner ear. In the past this idea of rebuilding the inner ear seemed like an unrealistic dream; however, with our rapidly expanding knowledge and understanding of inner ear physiology and molecular biology this goal has for the first time appeared within reach. As discussed above, neurotrophin administration to the inner ear has been demonstrated on multiple occasions to induce regrowth of PAFs into the basilar membrane area. However, neurotrophins do not regenerate sensory hair cells, and alternative means for regenerating these mechanosensory cells are under development. Atoh1 (formerly known as Math1) is a basic helix-loop-helix transcription factor involved in the Notch signaling pathway and is expressed specifically by cochlear sensory cells during inner ear development. Studies of Atoh1 null mice have demonstrated that Atoh1 is both necessary and sufficient for the development of cochlear hair cells (Bermingham et al., 1999; Fritzsch et al., 2011), and more recent work utilizing viral vectors carrying the Atoh1 transgene have shown that Atoh1 expression can be induced in non-sensory cells of the mature mammalian cochlea (Kawamoto et al., 2003). Furthermore, immature hair cells have been found in the region of the organ of Corti following the forced expression of Atoh1 in these non-sensory cochlear supporting cells (Kawamoto et al., 2003; Izumikawa et al., 2005). Unfortunately, as of yet, efforts to regenerate hair cells in ears that have undergone a severe ototoxic lesion and have a flat epithelium have not been successful, and it is hypothesized that differentiated supporting cells may be necessary for successful transdifferentiation to the hair cell phenotype to occur (Izumikawa et al., 2008). Furthermore, it remains unclear how to induce regeneration of specifically inner and outer hair cells in the normal regimented tonotopic pattern of the cochlea, and the molecular signaling that occurs during normal inner ear development clearly involves more complex pathways than the Notch signaling pathway alone (Fritzsch et al., 2011). Despite these current limitations, as our understanding of inner ear development improves it is foreseeable in the years to come that we will be able to induce the regeneration of functional cochlear mechanosensory cells. These regenerated hair cells cannot restore hearing in isolation, but rather must be restored within the framework of an intact auditory neural circuit. The co-administration of neurotrophin therapy along with the regenerative treatment will be critical to preserving the SGN population until new hair cells are regenerated and connected to the neural circuit.
Supplementary Material
In deaf individuals there is a loss of the mechanosensory and supporting cells within the organ of Corti often leading to a flat epithelium, and a wide variability in the number of remaining viable spiral ganglion neurons within Rosenthal’s canal, the neurons responsible for carrying auditory information from the periphery to the central nervous system. This may impact cochlear implant outcomes. As shown in the beginning of this clip, when there are few remaining spiral ganglion neurons and peripheral auditory fibers in proximity to a cochlear implant electrode array, there is the potential for significant spread of the electrical signal prior to reaching its neural target, resulting in a non-specific excitation of neurons and a decreased ability to distinguish sounds of different frequencies. As illustrated in the latter segment of this clip, replication deficient viral vectors have been used in animals to deliver neurotrophins to the inner ear, resulting in increased survival of spiral ganglion neurons and regrowth of peripheral fibers into the basilar membrane area, in close proximity to the cochlear implant electrode array. As a result of this proximity between the individual cochlear implant electrodes and their neural target, there is the potential to diminish current spread and improve frequency selectivity, thereby improving cochlear implant recipients’ perception of simple and complex sounds.
The mastoid bone and facial recess have already been drilled away to gain exposure to the middle ear space and cochlea. The cochlear implant is seen in position for implantation, with the internal device embedded in the skull (bottom right) and the electrode array placed within the mastoid cavity. At higher magnification, the basal turn of the cochlea is well visualized. A small diamond burr is used to create a basal turn cochleostomy, an opening into the scala tympani of the cochlea. Subsequently the cochlear implant electrode array is fully inserted into the cochlea (Video clips provided by and used with permission from Dr. Alexander Arts, Department of Otolaryngology, University of Michigan, Ann Arbor, MI and Dr. Shin-Ichi Usami, Department of Otolaryngology, Shinshu University School of Medicine, Matsumoto, Japan).
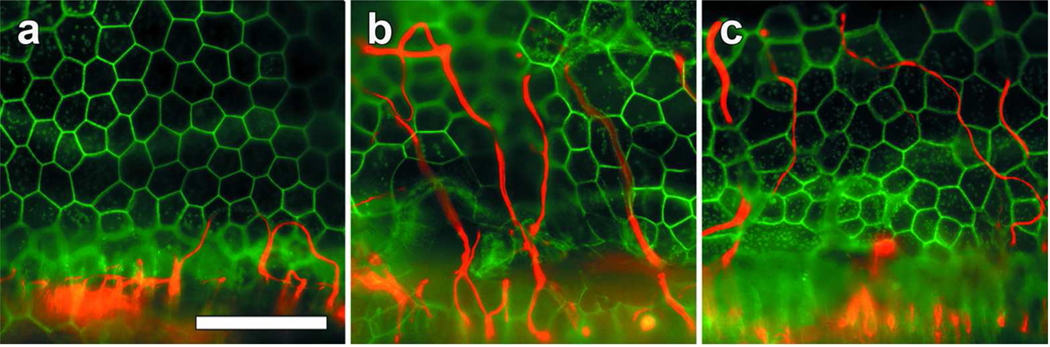
Figure 1.
Whole-mounts of the basal cochlear auditory epithelium stained for neurofilament (red) and actin (green), viewed with epifluorescence microscopy. (a) 90 days following deafening via administration of 5% neomycin directly into the scala tympani of the cochlea, all hair cells and supporting cells are absent and replaced by a flat epithelium. Neurofilament positive peripheral auditory fibers are absent with the exception of a few looping fibers extending into the basilar membrane area. (b and c) Three months after inoculation of a deafened ear with AAV.NT3 (b) and AAV.BDNF (c), there is a persistent flat epithelium with no regeneration of supporting cells or hair cells. However, there are copious neurofilament positive fibers extending in a radial fashion into the basilar membrane area. Most of the fibers weave between the junctions of the flat epithelial cells. Scale Bar=50 µm.
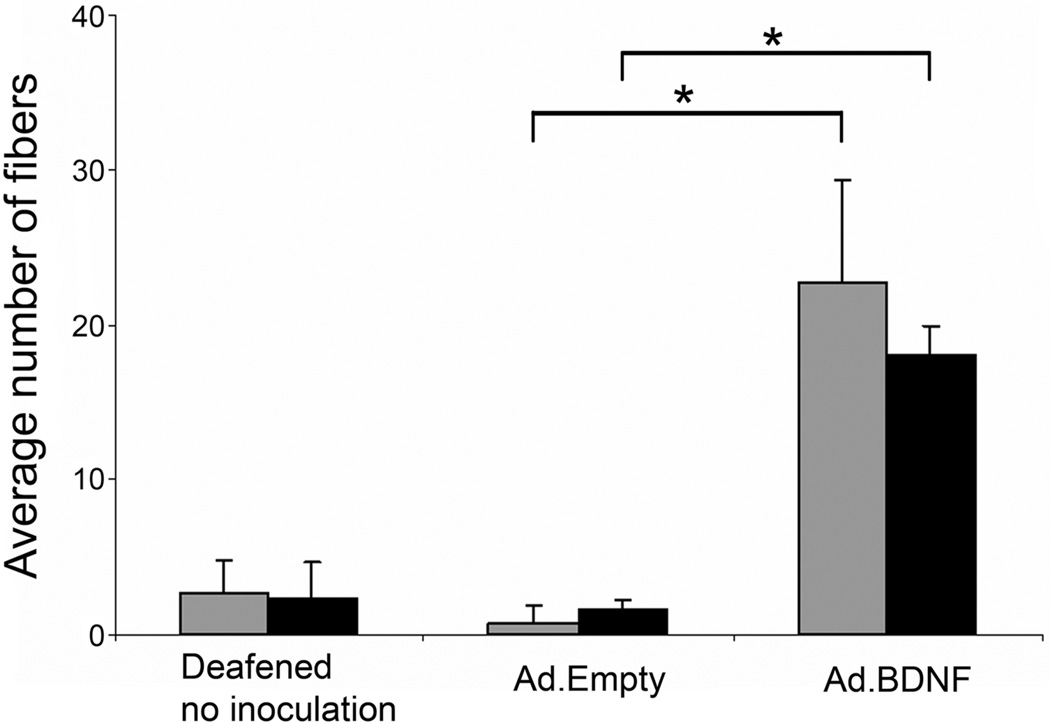
Figure 2.
Quantitative analysis of nerve fibers projecting into the basilar membrane area in deafened ears that have undergone either no treatment, or treatment with Ad.Empty or Ad.BDNF. Deafened animals inoculated with Ad.Empty had similar counts to untreated animals, whereas animals inoculated with Ad.BDNF had a significantly greater average nerve fiber count in the 1st and 2nd turns at 14 (gray bars) and 30 (black bars) days after deafening. (*) indicates p<0.01. Brackets are standard deviation and statistical comparison was done by Student t-test. (Reprinted with permission from Shibata et al., 2010)
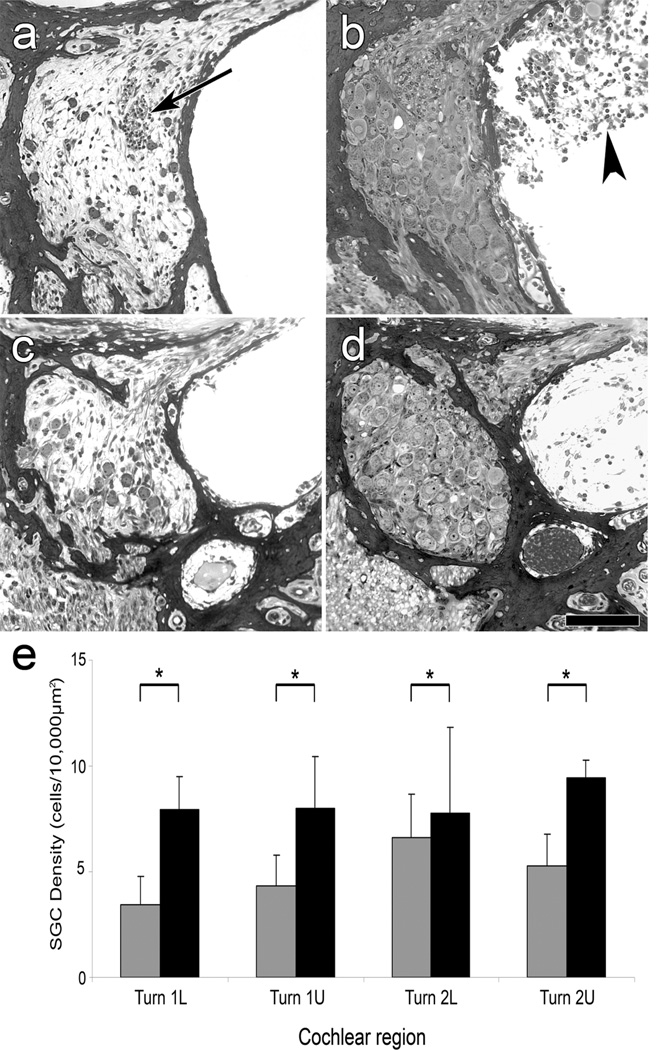
Figure 3.
Light microscope micrographs of plastic sections through Rosenthal’s canal in the 1st (a and b) and 2nd (c and d) cochlear turns 14 days after inoculation of Ad.Empty (a and c), or Ad.BDNF (b and d), and quantification of SGNs (e). (a and c) SGNs are scarce. A presumed efferent fiber bundle is present (arrow in a). (b and d) Rosenthal’s canal in Ad.BDNF inoculated ears exhibits a confluent population of neurons and no gliotic areas. In some of the Ad.BDNF inoculated ears, connective tissue could be observed in the scala tympani (arrow head in b). Scale bar=100 µm. (e) Mean SGN density among the cochlear regions of basal and 2nd turn of Ad.Empty and Ad.BDNF inoculated animals. Comparison of SGN density in Ad.Empty (gray bars) and Ad.BDNF (black bars) inoculated groups in all regions showed significantly larger numbers of surviving SGN in the Ad.BDNF groups. Error bars are standard deviations. Statistical comparison was by one-way ANOVA. (*) indicates p<0.01; L=Lower; U=Upper. (Reprinted with permission from Shibata et al., 2010)
Table 1.
Effects of BDNF and NT3 in the Mammalian Inner Ear-Literature Review Highlights.
| Authors | Year of Pub. |
NTF Used |
Animal Model |
Control | Method of Admin. |
Timing of Admin. |
Effects of Neurotrophin Treatment |
|---|---|---|---|---|---|---|---|
| Staecker et al. | 1996 | BDNF, NT3 | Guinea pig | -Deafened, untreated -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -8 week infusion |
-SGN: Increased SGN survival rate with BDNF, NT3, or both |
| Ernfors et al. | 1996 | NT3 | Guinea pig | -Deafened, untreated -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -2 week infusion |
-SGN: Increased SGN survival rate -PAF: Re-growth of neurofibers -ABR: No significant change in eABR threshold |
| Miller et al. | 1997 | BDNF, NT3 | Guinea pig | -Deafened, untreated -Deafened AP -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -2 week infusion |
-SGN: Complete rescue of SGNs with BDNF; No statistically significant effect induced by NT3 |
| Staecker et al. | 1998 | BDNF | Mouse | -Deafened, untreated -Normal hearing |
-Viral vector (HSV-1) -Scala Tympani |
-Post-deafening -4 week casette expression |
-SGN: Almost complete rescue of SGNs |
| Ruan et al. | 1999 | BDNF, NT3 | Guinea pig | -Deafened, AP | -Mini-osmotic pump -Scala Tympani |
-Pre-deafening -15, 30 or 60 days infusion |
-IHC: Protected from ototoxic lesion with 30 days BDNF treatment or with NT3 treatment -OHC: Protected from ototoxic lesion with NT3 treatment |
| Shoji et al. | 2000 | BDNF, NT3 | Guinea pig | -Deafened, AP | -Mini-osmotic pump -Scala Tympani |
-Pre-deafening×4 days -----OR----- -Post-deafening×1 week |
-OHC: Protected from noise induced lesion with NT3 treatment; Not protected with BDNF treatment -ABR: eABR threshold lowered significantly for ototoxin exposed ear after neurotrophin treatment in a concentration dependent manner |
| Shinohara et al. | 2002 | BDNF, analog of CNTF | Guinea pig | -Deafened, AP | -Mini-osmotic pump -Scala Tympani |
-Post-deafening -26 day infusion |
-SGN: Increased SGN survival rate after treatment with BDNF and CNTF analog -ABR: Decrease in eABR threshold with BDNF + CTNF analog treatment |
| Gillespie et al. | 2003 | BDNF | Guinea pig | -Deafened, untreated -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -4 week infusion |
-SGN: Increased SGN survival rate with continued therapy; 2 weeks following cessation of treatment led to decrease in SGN survival rates |
| Nakaizumi et al. | 2004 | CNTF, BDNF | Guinea pig | — Deafened, untreated -Normal hearing |
-Viral vector (AV) -Scala Tympani |
-Post-deafening -4 week casette expression |
-SGN: Increased SGN survival rate after treatment with BDNF or BDNF + CNTF (CTNF did not significantly enhance protective effect of BDNF) |
| Yamagata et al. | 2004 | CTNF, BDNF | Guinea pig | -Deafened, empty vector | -Mini-osmotic pump -Scala Tympani |
-Post-deafening* -2 week infusion *Neurotrop hin treatment initiated 2 or 6 weeks following deafening |
-SGN: Increased SGN density -ABR: Decreased eABR threshold starting 7 days into treatment; Significant decrease in eABR threshold 14 days after BDNF treatment; eABR threshold for treated ears after 6 week delay is significantly higher than after 2 week delay |
| Shepherd et al. | 2005 | BDNF | Guinea pig | -Deafened AP -Deafened electrode implant |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -4 week infusion |
-SGN: Increased SGN survival rate; Electrical stimulation enhances protective effects of BDNF, but produces no significant trophic support alone -ABR: Significant decrease in eABR threshold with BDNF treatment |
| Wise et al. | 2005 | BDNF, NT3 | Guinea pig | -Deafened untreated* -Normal hearing *5 or 33 days post-deafening |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening* -4 week infusion *Neurotrop hin treatment initiated 5 or 33 days following deafening |
-SGN: Increased SGN survival rate in ear 5 or 33 days after ototoxin exposure with BDNF or NT3 treatment -PAF: Variable regrowth of peripheral processes with BDNF or NT3 |
| Rejali et al. | 2007 | BDNF | Guinea pig | -Deafened, electrode + empty vector transfected fibroblast | -Viral vector (AV) treated fibroblast implant | -Post-deafening -48 days casette expression |
-SGN: Increased SGN survival rate in basal turn adjacent to coated electrode |
| Miller et al. | 2007 | BDNF, FGF | Guinea pig | -Deafened, AP + guinea pig serum albumin -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening* -26 day infusion *Neurotrop hin treatment initiated 4 days, 3 or 6 weeks following deafening |
-SGN: Increased SGN survival rate regardless of treatment delays, but longer delay led to decreased overall SGN density -PAF: Re-growth of peripheral processes with either neurotrophin, decreased fiber re-growth with increased treatment delay |
| Agterberg et al. | 2008 | BDNF | Guinea pig | -Deafened, untreated -Normal hearing, PBS-Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -4 week infusion |
-SGN: Increased SGN survival rate and density; SGNs in treated ears are larger than normal-hearing control ears -PAF: Surrounded by thinner myelin sheath compared to normal hearing ears -ABR: No change in aABR response |
| Chikar et al. | 2008 | BDNF | Guinea pig | -Deafened, electrode implant + empty vector | -Viral vector (AV) -Scala Tympani |
-Post-deafening -80 day expression |
-SGN: Increased SGN survival rate -ABR: Decrease in eABR threshold; Correlation of eABR threshold and SGN density; No significant correlation between SGN density and psychophysical detection thresholds |
| Agterberg et al. | 2009 | BDNF | Guinea pig | -Deafened untreated -Normal hearing |
-Mini-osmotic pump -Scala Tympani |
-Post-deafening -4 week infusion |
-SGN: Increased SGN survival rate; Preserved SGN shape -ABR: eABR response comparable to normal-hearing control, and did not decrease significantly after cessation of treatment; eABR latency in treated ears are comparable to deafened control animal |
| Wise et al. | 2010 | BDNF, NT3 | Guinea pig | -Deafened untreated -Normal hearing, vector-GFP |
-Viral vector (AV) -Scala Tympani or Scala Media |
-Post-deafening -3 week expression |
Inoculation into scala media produced gene expression better localized to organ of Corti than into scala tympani -SGN: Increased SGN survival rate and -PAF: Re-growth of peripheral processes in basal turn |
| Shibata et al. | 2010 | BDNF | Guinea pig | -Deafened untreated -Deafened, empty vector (AV) |
-Viral vector (AV or AAV) -Scala Tympani |
-Post-deafening -14 or 30 day expression |
-SGN: Increased SGN survival rate -PAF: Re-growth of peripheral processes beyond the habenula perforate towards BDNF expressing cells; Insignificant decline in number of peripheral process between 14 and 30 days post-inoculation |
| Havenith et al. | 2011 | BDNF | Guinea pig | -Deafened untreated -Deafened, gelfoam + PBS -Normal hearing -Normal hearing, gelfoam + PBS |
-Neurotrophin soaked gelfoam on intact round window membrane | -Post-deafening -4 week exposure |
-SGN: Increased SGN survival rate in basal turn; No significant effect on SGN shape and size -ABR: No significant effect on eABR peak-to-peak amplitudes |
aABR--acoustically evoked auditory brainstem response; AAV—adeno-associated virus; AP—artificial perilymph; AV—adenovirus; BDNF--brain derived neurotrophic factor; CNTF--ciliary neurotrophic factor; eABR--electrically evoked auditory brainstem response; FGF--fibroblast growth factor; HSV-1—Herpes simplex virus-1; IHC--inner hair cell; NGF--nerve growth factor; NTF—neurotrophin factor; NT3--neurotrophin-3; OHC--outer hair cell; SGN--spiral ganglion neuron; PAF—peripheral auditory fiber; AP—artificial perilymph; GFP—green fluorescent protein; PBS—phosphate buffered saline
ACKNOWLEDGEMENTS
We especially would like to thank Hiu Tung Wong for her significant contributions to the table and animated movie. We also thank Seiji Shibata for providing micrographs from his published work, as well as Drs. Alexander Arts and Shin-Ichi Usami for providing video clips from their live cochlear implant surgeries. Our work is funded by the R. Jamison and Betty Williams Professorship, the Hirschfield Foundation and NIH/NIDCD Grants: R01-DC010412, R01-DC007634, T32-DC005356 and P30-DC05188.
LITERATURE CITED
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang C-H, Crumling MA, Swiderski DL, Beyer LA, Gong T-WL, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218:20–29. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJH, Versnel H, de Groot JCMJ, Smoorenburg GF, Albers FWJ, Klis SFL. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Agterberg MJH, Versnel H, van Dijk LM, de Groot JCMJ, Klis SFL. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10:355–367. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann N Y Acad Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bichler E, Spoendlin H, Rauchegger H. Degeneration of cochlear neurons after amikacin intoxication in the rat. Arch Otorhinolaryngol. 1983;237:201–208. doi: 10.1007/BF00453725. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. The Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults-- United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131–156. [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Di Polo A, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikar JA, Hendricks JL, Richardson-Burns SM, Raphael Y, Pfingst BE, Martin DC. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials. 2011:1–35. doi: 10.1016/j.biomaterials.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LT, Lenarz T, Battmer R-D, Bender von Saebelkampf C, Busby PA, Cowan RSC. A psychophysical forward masking comparison of longitudinal spread of neural excitation in the Contour and straight Nucleus electrode arrays. Int J Audiol. 2005;44:559–566. doi: 10.1080/14992020500258743. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Knight MR, Cowan RSC. Psychophysical measures in patients fitted with Contour and straight Nucleus electrode arrays. Hear Res. 2006;212:160–175. doi: 10.1016/j.heares.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Davis RL, Liu Q. Complex primary afferents: What the distribution of electrophysiologically-relevant phenotypes within the spiral ganglion tells us about peripheral neural coding. Hear Res. 2011;276:34–43. doi: 10.1016/j.heares.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normalhearing listeners using simulations of cochlear-implant signal processors with 6-20 channels. J Acoust Soc Am. 1998;104:3583–3585. doi: 10.1121/1.423940. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH. Multichannel cochlear implants: relation of histopathology to performance. The Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MB, Shapiro WH, McDonald PD, Neuburger HS, Ashburn-Reed S, Immerman S, Jethanamest D, Roland JT, Svirsky MA. The effect of perimodiolar placement on speech perception and frequency discrimination by cochlear implant users. Acta Otolaryngol. 2007;127:378–383. doi: 10.1080/00016480701258671. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Briaire JJ, Grote JJ. The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol. 2001;22:340–349. doi: 10.1097/00129492-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Fariñas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hear Res. 2011;276:16–26. doi: 10.1016/j.heares.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Havenith S, Versnel H, Agterberg MJH, de Groot JCMJ, Sedee R-J, Grolman W, Klis SFL. Spiral ganglion cell survival after round window membrane application of brainderived neurotrophic factor using gelfoam as carrier. Hear Res. 2011;272:168–177. doi: 10.1016/j.heares.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Hawkins JE. Comparative otopathology: aging, noise, and ototoxic drugs. Adv Otorhinolaryngol. 1973;20:125–141. doi: 10.1159/000393093. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Lindsay JR. Profound deafness. Associated sensory and neural degeneration. Arch Otolaryngol. 1980;106:193–209. doi: 10.1001/archotol.1980.00790280001001. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE. Strial atrophy in clinical and experimental deafness. The Laryngoscope. 1972;82:1105–1125. doi: 10.1288/00005537-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE. Degeneration patterns in human ears exposed to noise. Ann Otol Rhinol Laryngol. 1976;85:725–739. doi: 10.1177/000348947608500603. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S, Stöver T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S-I, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Kerr A, Schuknecht HF. The spiral ganglion in profound deafness. Acta Otolaryngol. 1968;65:586–598. doi: 10.3109/00016486809119293. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? The Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6:612–619. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Kong Y-Y, Cruz R, Jones JA, Zeng F-G. Music Perception with Temporal Cues in Acoustic and Electric Hearing. Ear Hear. 2004;25:173–185. doi: 10.1097/01.aud.0000120365.97792.2f. [DOI] [PubMed] [Google Scholar]
- Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, Mhatre A. Longterm in vivo cochlear transgene expression mediated by recombinant adenoassociated virus. Gene Ther. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Mhatre AN. Cochlear gene therapy. Ear Hear. 2003;24:342–348. doi: 10.1097/01.AUD.0000079798.24346.35. [DOI] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997;15:601–617. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5:865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–221. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Adams JC, Nadol JB. Pathology and pathophysiology of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2005;26:151–160. doi: 10.1097/00129492-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85:1959–1969. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Morton NE. Genetic epidemiology of hearing impairment. Ann N Y Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–539. [PubMed] [Google Scholar]
- Nadol JB. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res. 2011;278:2–20. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushi F, Richardson RT, Hardman J, Clark G, O'leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Bowling SA, Colesa DJ, Garadat SN, Raphael Y, Shibata SB, Strahl SB, Su GL, Zhou N. Cochlear infrastructure for electrical hearing. Hear Res. 2011;281:65–73. doi: 10.1016/j.heares.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D. Relation of cochlear implant function to histopathology in monkeys. Ann N Y Acad Sci. 1983;405:224–239. doi: 10.1111/j.1749-6632.1983.tb31635.x. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumäe U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–141. doi: 10.1016/0304-3940(96)12499-x. [DOI] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Noushi F, O'leary S. Inner ear therapy for neural preservation. Audiol Neurootol. 2006;11:343–356. doi: 10.1159/000095896. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O'leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Wise A, O'leary S, Hardman J, Casley D, Clark G. Tracing neurotrophin-3 diffusion and uptake in the guinea pig cochlea. Hear Res. 2004;198:25–35. doi: 10.1016/j.heares.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Santi PA, Mancini P. Cochlear Anatomy and Central Auditory Pathways. In: Cummings C, editor. Otolaryngology Head and Neck Surgery. 3rd Ed. 1998. pp. 2803–2830. [Google Scholar]
- Santi PA, Ruggero MA, Nelson DA, Turner CW. Kanamycin and bumetanide ototoxicity: anatomical, physiological and behavioral correlates. Hear Res. 1982;7:261–279. doi: 10.1016/0378-5955(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerveICorrelation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, Raphael Y. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol. 2010;223:464–472. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, Lindström B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Degeneration behaviour of the cochlear nerve. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1971a;200:275–291. doi: 10.1007/BF00373310. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971b;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Suter R. Regeneration in the VIII nerve. Acta Otolaryngol. 1976;81:228–236. doi: 10.3109/00016487609119954. [DOI] [PubMed] [Google Scholar]
- Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010;56:351–358. doi: 10.1159/000275062. [DOI] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H, Van de Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B, Moonen G, Van de Water TR. The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am J Otol. 1996a;17:486–492. [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996b;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Strominger RN, Bohne BA, Harding GW. Regenerated nerve fibers in the noise-damaged chinchilla cochlea are not efferent. Hear Res. 1995;92:52–62. doi: 10.1016/0378-5955(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501:30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Terayama Y, Kaneko K, Tanaka K, Kawamoto K. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. II. Nerve elements outside the organ of Corti. Acta Otolaryngol. 1979;88:27–36. doi: 10.3109/00016487909137136. [DOI] [PubMed] [Google Scholar]
- Terayama Y, Kaneko Y, Kawamoto K, Sakai N. Ultrastructural changes of the nerve elements following disruption of the organ of CortiINerve elements in the organ of Corti. Acta Otolaryngol. 1977;83:291–302. doi: 10.3109/00016487709128848. [DOI] [PubMed] [Google Scholar]
- Teufert KB, Linthicum FH, Connell SS. The effect of organ of corti loss on ganglion cell survival in humans. Otol Neurotol. 2006;27:1146–1151. doi: 10.1097/01.mao.0000232006.16363.44. [DOI] [PubMed] [Google Scholar]
- Weiss MA, Frisancho JC, Roessler BJ, Raphael Y. Viral-mediated gene transfer in the cochlea. Int J Dev Neurosci. 1997;15:577–583. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Wright CG. Neural damage in the guinea pig cochlea after noise exposure. A light microscopic study. Acta Otolaryngol. 1976;82:82–94. doi: 10.3109/00016487609120865. [DOI] [PubMed] [Google Scholar]
- Xu L, Tsai Y, Pfingst BE. Features of stimulation affecting tonal-speech perception: implications for cochlear prostheses. J Acoust Soc Am. 2002;112:247–258. doi: 10.1121/1.1487843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykkö I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumäe U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Zappia JJ, Altschuler RA. Evaluation of the effect of ototopical neomycin on spiral ganglion cell density in the guinea pig. Hear Res. 1989;40:29–37. doi: 10.1016/0378-5955(89)90096-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Q, Davis RL. Complex regulation of spiral ganglion neuron firing patterns by neurotrophin-3. J Neurosci. 2005;25:7558–7566. doi: 10.1523/JNEUROSCI.1735-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In deaf individuals there is a loss of the mechanosensory and supporting cells within the organ of Corti often leading to a flat epithelium, and a wide variability in the number of remaining viable spiral ganglion neurons within Rosenthal’s canal, the neurons responsible for carrying auditory information from the periphery to the central nervous system. This may impact cochlear implant outcomes. As shown in the beginning of this clip, when there are few remaining spiral ganglion neurons and peripheral auditory fibers in proximity to a cochlear implant electrode array, there is the potential for significant spread of the electrical signal prior to reaching its neural target, resulting in a non-specific excitation of neurons and a decreased ability to distinguish sounds of different frequencies. As illustrated in the latter segment of this clip, replication deficient viral vectors have been used in animals to deliver neurotrophins to the inner ear, resulting in increased survival of spiral ganglion neurons and regrowth of peripheral fibers into the basilar membrane area, in close proximity to the cochlear implant electrode array. As a result of this proximity between the individual cochlear implant electrodes and their neural target, there is the potential to diminish current spread and improve frequency selectivity, thereby improving cochlear implant recipients’ perception of simple and complex sounds.
The mastoid bone and facial recess have already been drilled away to gain exposure to the middle ear space and cochlea. The cochlear implant is seen in position for implantation, with the internal device embedded in the skull (bottom right) and the electrode array placed within the mastoid cavity. At higher magnification, the basal turn of the cochlea is well visualized. A small diamond burr is used to create a basal turn cochleostomy, an opening into the scala tympani of the cochlea. Subsequently the cochlear implant electrode array is fully inserted into the cochlea (Video clips provided by and used with permission from Dr. Alexander Arts, Department of Otolaryngology, University of Michigan, Ann Arbor, MI and Dr. Shin-Ichi Usami, Department of Otolaryngology, Shinshu University School of Medicine, Matsumoto, Japan).