Abstract
Background
The aims of this study were: (a) to determine whether African-Americans (AA) with Mycosis Fungoides (MF) have a worse prognosis despite accounting for varying clinical factors at presentation, and (b) to assess whether a racial disparity exists regarding utilization of radiation therapy (RT) as an initial treatment modality.
Materials and Methods
The SEER 1988–2008 public use database was investigated. Univariate and multivariate analysis was used to assess for factors significantly associated with disease-specific survival (DSS), overall survival (OS) and RT utilization survival.
Results
A total of 4892 patients with MF were identified with a median follow-up of 58 months. On multivariate analysis including tumor registry, age, gender, marital status and tumor stage, AA race was significantly correlated with a worse OS (HR 1.58, p < 0.001) and DSS (HR 1.78, p < 0.001). In regards to RT utilization, more advanced age (OR 1.0057, p < 0.001) and higher stage (OR 3.03, p < 0.001) were associated with a higher likelihood of receiving RT, whereas female gender (OR 0.81, p = 0.03) was associated with a lower likelihood of receiving RT. AA race was not significantly associated with RT utilization (p = 0.58).
Conclusions
AA race was associated with poorer survival despite accounting for demographic factors and tumor stage. Differences in RT utilization by AA race were not found, however RT was less utilized for females. The etiology of this poorer prognosis is unclear and may be related to access to medical care, socioeconomic factors or undetermined biological differences.
Keywords: mycosis fungoides, survival, race, African-Americans, radiation utilization
Introduction
Mycosis Fungoides (MF), the most common form of cutaneous T-cell lymphoma, is a relatively rare disorder that is often clinically challenging to diagnose, as it can be difficult to discriminate from benign disorders1,2. For most patients, a normal lifespan can be expected as the disease usually takes the form of a chronic condition2. Optimal care of patients with MF therefore requires ongoing management and access to medical resources, which creates the potential for racial or socioeconomic disparities among underserved populations.
Although MF is classically seen in middle-aged white males, the incidence rate among African-Americans (AA) is substantially higher than among whites with reported black-to-white incidence rate ratios ranging from 1.5–1.61,3. Recent population-based data has shown that AA with MF are diagnosed at a younger age and present at more advanced stages when compared to whites4. Outcomes related to survival based on large population based registries have not been extensively studied.
Radiation therapy (RT) is one of the most effective therapeutic tools for treating cutaneous T-cell lymphomas based on multiple clinical studies2,5 and has been used for the management of MF since 19026. As RT requires multiple weekly visits and daily transportation over a period of 4–6 weeks, implementation of RT can be challenging. A racial discrepancy in RT utilization could have important implications for patient outcomes, as well as policy changes to our healthcare referral system. At present, it is not known whether or not the utilization of RT differs by race or other demographic factors.
The purpose of this study was to better elucidate the impact of race on clinical outcomes, as well as the utilization of radiation. More specifically, our aims were: (a) to determine if AA with MF have poorer overall and disease-specific survival despite accounting for varying demographic and clinical factors based on the 1988–2008 public use data, and (b) to assess what factors influence the utilization of RT using a large, modern population-based registry.
Methods
The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) public use database was queried, and patients with a diagnosis of MF (International Classification of Disease for Oncology-Third Edition topography code of 9700) were identified. Since cases identified prior to 1988 had poorer staging, patients from 1998–2008 were selected for study. Patient and tumor characteristics were identified, including gender, race, marital status, age, stage and use of RT. Statistical analyses were performed using the SEER*STAT Program (National Cancer Institute, Bethesda, MD), adjusting for registry. Patients over the age of 100 years were excluded from analysis. Death by non-Hodgkins lymphoma was used as a surrogate for disease-specific survival (DSS). Factors associated with overall survival (OS) and DSS were determined using Cox proportional hazards. Univariate and multivariate logistic regression was used to identify variables significantly associated with the use of RT as part of initial treatment (within 6 months of diagnosis). The Yale University School of Medicine Human Investigation Committee determined that the study was exempt from full IRB Committee Review.
Results
Patient Population
A total of 4892 patients were diagnosed with MF between 1988 and 2008. The median follow-up for all patients was 58 months. Patient characteristics are summarized in Table 1. Patients were 74.4% white, 13.4% AA, 6.2% Asian, and 6.0% other.
Table 1.
Patient characteristics
| Factor | Number |
|---|---|
| Gender | |
| Male | 2818 |
| Female | 2074 |
| Race | |
| White | 3640 |
| Black | 657 |
| Asian | 301 |
| Other | 294 |
| Marital Status | |
| Not married | 1384 |
| Married | 2472 |
| Unknown | 1036 |
| Age , median (years) | 60 |
| Stage | |
| T0–T2a | 3386 |
| T3–T4 | 652 |
| Unknown | 854 |
AJCC stage T0–T2 or MFCG stage I–II
Survival Analysis
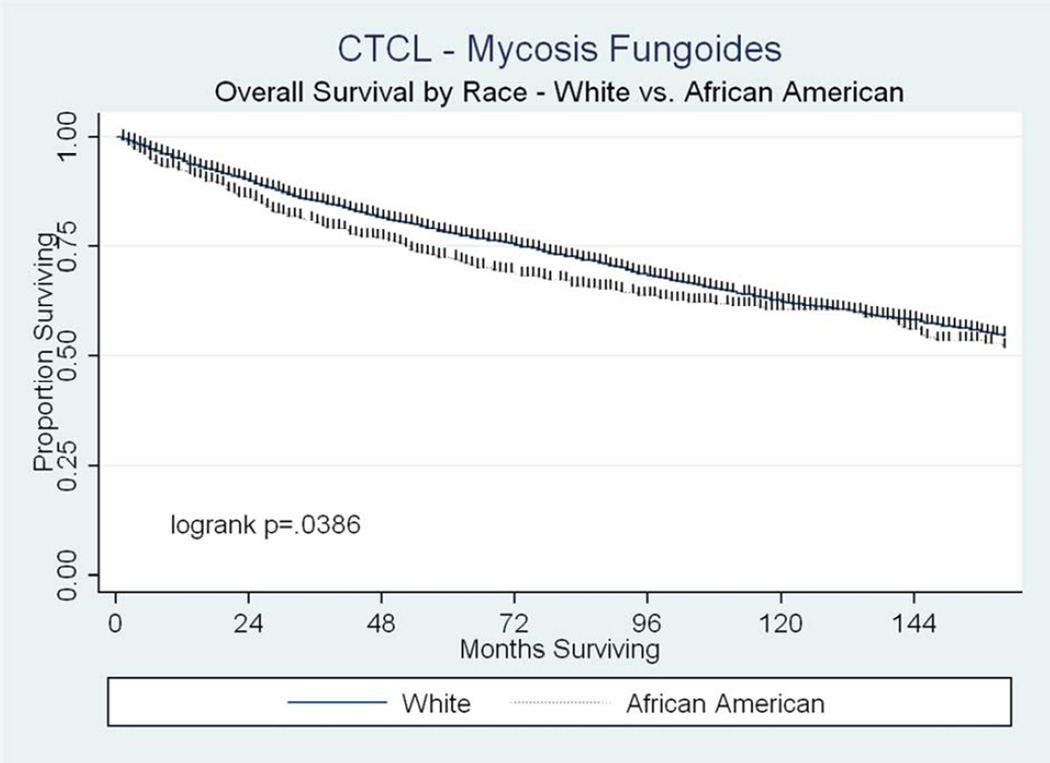
At the time of analysis, 3492 patients were alive. Median OS was 181 months (95% confidence intervals [C.I] 173–191) for all patients. On univariate analysis, OS was significantly lower for patients of AA race, increasing age or higher stage, whereas it was higher for females and married patients (Table 2). On multivariate analysis including tumor registry, these variables remained significantly associated with OS (Table 2). A survival curve comparing AA and white patients is shown in Figure 1. DSS was also significantly worse for patients of AA race, increasing age and higher stages on both univariate and multivariate analysis (Table 3). In contrast, DSS was significantly improved for married patients, and there was no association found between DSS and female gender (Table 3).
Table 2.
Predictors of overall survival on univariate and multivariate analysis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.77 (0.69–0.86) | <0.001 | 0.76 (0.68–0.85) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| AA | 1.17 (1.008–1.35) | 0.039 | 1.58 (1.35–1.86 | <0.001 |
| Asian | 0.85 (0.66–1.08) | 0.18 | 1.05 (0.81–1.38) | 0.70 |
| Other | 0.48 (0.34–0.67) | <0.001 | 0.64 (0.44–0.93) | 0.02 |
| Marital Status | ||||
| Not married | Reference | Reference | ||
| Married | 0.67 (0.60–0.76) | <0.001 | 0.71 (0.62–0.80) | <0.001 |
| Unknown | 0.51 (0.43–0.60) | <0.001 | 0.56 (0.47–0.67) | <0.001 |
| Agea | 1.06 (1.06–1.07) | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| Stage | ||||
| T0–T2b | Reference | Reference | ||
| T3–T4 | 2.86 (2.52–3.26) | <0.001 | 2.32 (2.04–2.65) | <0.001 |
| Unknown | 1.55 (1.35–1.78) | <0.001 | 1.50 (1.30–1.73) | <0.001 |
Continuous variable
AJCC T stage 0–2 or MFCG stage I–II
Figure 1.
Overall survival by race.
Table 3.
Predictors of disease-specific survival on univariate and multivariate analysis
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.91 (0.76–1.09) | 0.32 | 0.92 (0.77–1.12) | 0.41 |
| Race | ||||
| White | Reference | Reference | ||
| AA | 1.74 (1.40–2.16) | <0.001 | 1.78 (1.40–2.27) | <0.001 |
| Asian | 0.65 (0.40–1.04) | 0.07 | 0.84 (0.50–1.41) | 0.50 |
| Other | 0.16 (0.06–0.44) | <0.001 | 0.25 (0.09–0.66) | 0.006 |
| Marital Status | ||||
| Not married | Reference | Reference | ||
| Married | 0.65 (0.53–0.78) | <0.001 | 0.71 (0.58–0.87) | 0.001 |
| Unknown | 0.34 (0.25–0.46) | <0.001 | 0.40 (0.29–0.56) | <0.001 |
| Agea | 1.04 (1.04–1.05) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| Stage | ||||
| T0–T2b | Reference | Reference | ||
| T3–T4 | 5.13 (4.19–6.29) | <0.001 | 4.13 (3.35–5.09) | <0.001 |
| Unknown | 1.96 (1.54–2.49) | <0.001 | 1.86 (1.45–2.39) | <0.001 |
Continuous variable
AJCC T stage 0–2 or MFCG stage I–II
Radiotherapy Utilization
Of the 4892 patients identified, radiation status was unknown in 71 patients (1.5%). In total, 11.7% of patients were recorded as having received RT as initial treatment, whereas 86.8% did not. On univariate analysis, patients of more advanced age or higher stage were more likely to receive RT, whereas patients of female gender and Asian race were significantly less likely (Table 4). Multivariate logistic regression confirmed that more advanced age or higher stage was associated with a higher likelihood of receiving RT and female gender was associated with a lower likelihood of receiving RT (Table 4).
Table 4.
Predictors of RT utilization on univariate and multivariate analysis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | OR (95% C.I.) | P-value | OR (95% C.I.) | P-value |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.74 (0.61–0.88) | <0.001 | 0.81 (0.67–0.98) | 0.03 |
| Race | ||||
| White | Reference | Reference | ||
| AA | 0.99 (0.71–1.19) | 0.53 | 0.92 (0.69–1.23) | 0.58 |
| Asian | 0.56 (0.36–0.86) | 0.008 | 0.66 (0.41–1.07) | 0.10 |
| Other | 0.12 (0.05–0.29) | <0.001 | 0.17 (0.06–0.47) | 0.001 |
| Marital Status | ||||
| Not married | Reference | Reference | ||
| Married | 0.99 (0.82–1.20) | 0.92 | 0.96 (0.79–1.18) | 0.72 |
| Unknown | 0.26 (0.19–0.37) | <0.001 | 0.32 (0.22–0.45) | <0.001 |
| Agea | 1.009 (1.00–1.01) | <0.001 | 1.005 (1.00–1.01) | <0.001 |
| Stage | ||||
| T0–T2b | Reference | Reference | ||
| T3–T4 | 3.27 (2.65–4.02) | <0.001 | 3.03 (2.43–3.77) | <0.001 |
| Unknown | 0.84 (0.64–1.10) | 0.20 | 0.99 (0.75–1.32) | 0.96 |
Continuous variable
AJCC T stage 0–2 or MFCG stage I–II
Discussion
Owing to the rarity of cutaneous lymphomas, they have been difficult to study extensively. The use of large single-institution studies and multicenter databases has greatly improved our understanding of the epidemiological patterns and other details of these malignancies. The SEER registry represents greater than 26% of the US population and has been instrumental in studying rare malignancies. Previous work from these authors examining patients with cutaneous lymphomas in the SEER database found that AA patients with MF were diagnosed at an earlier mean age (51 vs. 59 years) and a higher T-stage (T3-T4) in comparison to Caucasians4. However, the clinical significance of these differences was unknown. In the current investigation, we present novel data demonstrating significantly worse disease-specific and overall survival for AA with MF despite accounting for varying patient and disease characteristics at diagnosis. These findings suggest that this patient population may require more vigilant disease surveillance and consideration of more intensified therapeutic management strategies.
There are limited studies examining the impact of race on the prognosis of MF patients. Weinstock and Gardstein analyzed the patterns of MF incidence and associated mortality using the SEER dataset from 1973–1992. They reported an overall higher mortality rate among blacks than whites (RR = 2.4), and a lower mortality rate among Asians (RR = 0.5). In addition, the male-to-female mortality rate was lowest among AA (1.2), intermediate among whites (2.1) and highest among Asians (2.7). Of note, mortality rates decreased by 22% during the study period and was similar among blacks and whites7. In this report, we analyze a more modern cohort of patients and demonstrate both poorer DSS and OS using multivariate analysis accounting for varying clinical factors at presentation. In contrast, improved survival outcomes for Asians was not confirmed in this study.
A poorer prognosis for certain subgroups of AA patients with MF has also been previously reported. A large single-institution retrospective study of patients between 1989–2007 found that AAs and Hispanics were more than twice as likely to present with early-stage MF (before the age of 40 years) than Caucasian patients. When stratified by gender, this difference was only statistically significant for women and not for men. In pairwise comparisons, AA women with early onset MF were noted to have higher rates of disease progression and worse disease-specific survival than Caucasian females with early-onset disease. The authors observed that among women who presented with early-stage MF, 80% of the Caucasian women presented as stage IA or IB, versus only 52% of AA women, perhaps partially accounting for the differences in outcomes. Given the poor prognosis for this patient population, the authors recommended consideration of more aggressive therapy for AA women8.
The underlying etiology for this racial disparity in outcome is not clear. Socioeconomic differences and/or underlying biological differences may play a role. Response to treatment may also vary by skin color. Phototherapy is safe and commonly used skin-directed therapy. However, studies analyzing patients with psoriasis and sclerotic disorders suggest that phototherapy may be less efficacious in patients with heavily pigmented skin9,10. In contrast, total skin electron beam (TSEB) therapy is independent of baseline skin pigmentation. An investigation to determine whether the clinical response (CR) to and relapse after TSEB differ by ethnicity and gender found that the odds of achieving a CR to TSEB was decreased in patients with more advanced disease but increased in women. In particular, this enhanced response rate was most pronounced in AA women. Given the more favorable response rate and generally poorer prognosis for AA women, the authors suggested that TSEB should be considered earlier in the disease course of these patients11. However, a recent report on patient perspectives found that TSEB therapy was felt to be a more difficult therapy to endure than other topical and systemic treatments12.
In this study, a significant association between AA race and recorded RT utilization was not detected. However, our analysis did find that RT was used less often for other races, whereas RT was utilized more often for older patients and those with more advanced T-stages. Importantly, this study did find that RT was less often utilized for females as initial therapy, which is concerning in light of the previously cited data indicating better response rates for females. The reason for these associations is unclear and may reflect differences in regional treatment patterns or underlying disease characteristics. Regardless, physicians should be aware of these treatment patterns and outcomes in order to best care for these patients.
There are several limitations to our study design that should be acknowledged. Multiple clinical factors are not consistently reported in the SEER registry13. The SEER database does not provide information regarding radiotherapy daily dose or total dose, but does provide information regarding generalized radiotherapy use in any given clinical case. Furthermore, there may be underascertainment of radiotherapy use. Though this has not been shown for CTCL as it has for breast cancer14, we suspect the underlying reasons for underascertainment may be similar. Details regarding systemic and medical therapies are not provided and/or well documented in the registry. The data entered into the registry is also by nature retrospective and subject to the clinicians and institutions ability to accurately record clinical information. Finally, Human T-lymphotropic virus type-1 (HTLV-1) status is not reported, and therefore there is a potential for a small number of HTVL-related lymphomas to be misclassified as MF.
Conclusion
In conclusion, this study demonstrates that AA race is associated with a poorer overall and disease-specific survival despite accounting for demographic factors and other variables. The etiology of this association is unclear and may be related to access to medical care, socioeconomic factors or biological differences. Regardless, physicians should be aware of this difference as AA may require closer follow-up and/or more aggressive therapy. This study did not find any evidence of a racial disparity regarding utilization of RT, but did find that RT was less likely to be utilized for females and more likely to be utilized for older patients and those with a more advanced stage. Further studies investigating the underlying etiology of these associations are warranted.
Clinical Practice Points.
Mycosis fungoides (MF) is a relatively rare disorder but is the most common form of cutaneous T-cell lymphoma
Given the need for chronic disease management, a potential for a racial disparity in outcomes may exist due to varying access to care or underlying biological differences
The clinical course for patients with MF is varied, and depends on the disease burden at presentation
African-American (AA) patients have been shown to present at an earlier age (mean 51 vs 59 years) and higher T-stage (T3-T4) than Caucasians patients
AA patients are associated with a worse disease-specific and overall survival in comparison to Caucasian patients despite accounting for varying clinical factors at presentation
Radiation therapy was found to be more commonly utilized for patients with advanced age and higher stage at presentation, and less commonly utilized for females. AA race was not significantly associated with RT utilization
The etiology of this poorer survival may be multifactorial and further research is warranted
These findings have significant implications for disease surveillance and management in AA patients
Acknowledgment
Supported by NCI grant 1R03CA151153-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of this research was accepted for poster presentation at the annual meeting of the American Radium Society, Las Vegas, NV, April 2012.
Conflicts of Interest Notification:
No potential or actual conflicts of interest
References
- 1.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States 1973–2002. Archives of dermatology. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 2.Galper SL, Smith BD, Wilson LD. Diagnosis and management of mycosis fungoides. Oncology (Williston Park) 2010;24:491–501. [PubMed] [Google Scholar]
- 3.Weinstock MA, Reynes JF. The changing survival of patients with mycosis fungoides: a population-based assessment of trends in the United States. Cancer. 1999;85:208–212. [PubMed] [Google Scholar]
- 4.Wilson LD, Hinds GA, Yu JB. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin Lymphoma Myeloma Leuk. 2012;12:291–296. doi: 10.1016/j.clml.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD, Wilson LD. Management of mycosis fungoides: Part 2. Treatment. Oncology (Williston Park) 2003;17:1419–1428. discussion 30, 33. [PubMed] [Google Scholar]
- 6.Scholtz W. Uber den einfluss der Roentgenstrohlen auf die haut in gesundem und krankem zustande. Arch Dermatol Syph. 1902;59:421–446. [Google Scholar]
- 7.Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999;89:1240–1244. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun G, Berthelot C, Li Y, et al. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. J Am Acad Dermatol. 2009;60:231–235. doi: 10.1016/j.jaad.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Garza LA, Cho S, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Arch Dermatol. 2008;144:851–858. doi: 10.1001/archderm.144.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yones SS, Palmer RA, Garibaldinos TT, Hawk JL. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836–842. doi: 10.1001/archderm.142.7.836. [DOI] [PubMed] [Google Scholar]
- 11.Hinds GA, Alhariri J, Klein RQ, Wilson LD. Treatment of Mycosis Fungoides With Total Skin Electron Beam: Response and Relapse by Ethnicity and Sex. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e31825494d3. [DOI] [PubMed] [Google Scholar]
- 12.Yu JB, Khan AM, Jones GW, Reavely MM, Wilson LD. Patient perspectives regarding the value of total skin electron beam therapy for cutaneous T-cell lymphoma/mycosis fungoides: a pilot study. Am J Clin Oncol. 2009;32:142–144. doi: 10.1097/COC.0b013e3181841f5c. [DOI] [PubMed] [Google Scholar]
- 13.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288–295. [PubMed] [Google Scholar]
- 14.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118:333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]