Summary
Although cardiac sarcomas are rare in comparison to their soft tissue counterparts, they are the second most common type of primary cardiac neoplasm. Of the few hundred cases reported, most has been based on autopsy series. A series of 27 cardiac sarcomas removed at surgery for curative and diagnostic intent were reviewed for clinicopathologic features with correlation to available postoperative follow-up data in 17 patients. There were 6 angiosarcomas, 6 myxofibrosarcomas, 3 malignant peripheral nerve sheath tumors, 3 leiomyosarcomas, 2 synovial sarcomas, 1 epithelioid hemangioendothelioma, 1 chondrosarcoma, 1 osteosarcoma, and 4 poorly differentiated sarcomas. There was a wide age and size range with slight female predilection. There were 20 cases that arose in the atria/pulmonary vessels, 4 in the ventricles, 1 in mitral valve, and 2 in epi/pericardium. There was a slight left predilection. The histologic grade was low in 4, moderate in 3, and high in 20 cases. Six high-grade and 1 low-grade tumors were also treated with adjuvant chemotherapy and/or radiation. In 17 patients with follow-up data, 6 of 12 patients with high-grade tumor died (4 within 5 days of the initial surgery, 1 in 21 months, and 1 in 131 months), and 1 patient with moderate-grade tumor and all 4 patients with low-grade tumor were alive without evidence of disease at the end of follow-up. Tumor grade appeared to be prognostically important in cardiac sarcoma. Long survival was achieved in patients who survived the initial surgery well.
Keywords: Cardiac, Pathology, Surgery, Sarcoma, Long survival
1. Introduction
Primary tumors of the heart are rare. About 75% of primary cardiac tumors are benign mesenchymal tumors, most of which are myxomas. Primary sarcomas of the heart, although very rare, are the second most common type of primary cardiac neoplasm and account for most of the malignant primary cardiac tumors [1]. At present, only a few hundred primary cardiac sarcomas have been reported, most of which are based on autopsy series [2]. Similar to their soft tissue counterparts, cardiac sarcomas constitute a variety of histologic types [3]. Primary cardiac sarcomas can occur at any age but more frequently between the third and fifth decades with a mean age of 41 years [4]. Left-sided cardiac involvement is more common than right-sided involvement [5]. The clinical presentation of patients with cardiac sarcoma depends on the size as well as the location of the tumor. Patients usually present with dyspnea and symptoms due to embolic phenomena and pericardial effusion and, rarely, metastasis to other sites [6–10]. Depending on the tumor location in the heart, complete excision is often difficult at the time of surgery; and adjuvant chemotherapy and radiation have been used in treating cardiac sarcomas [10,11]. If localized to the heart, orthotopic heart transplantation has been shown to provide long-term survival in some patients without recurrence despite immunosuppression [12]. However, the prognosis of primary cardiac sarcomas in general remains poor in current medical literature [10]. The median survival in patients with cardiac sarcomas has been reported to be about 6 months with a mean of 11 months [4]. In this study, we identified 27 primary cardiac sarcomas removed at surgery mainly for curative intent and analyzed the clinicopathologic characteristics of the tumors and survival in 17 cases with the available clinical information and follow-up.
2. Material and methods
All cases of sarcomas involving the heart were retrieved from the files of Surgical Pathology at the University of Pennsylvania, Children’s Hospital of Philadelphia, Cleveland Clinic, University of Pittsburgh Medical Center, and the consultation file of one of the authors (J. S. B.) between 1975 and 2006. Twenty-nine cases were identified. Of the 29 cases, 2 were the result of metastatic lesions to the heart and were eliminated from the study.
ll histologic materials, including the original immunohistochemically stained slides, were retrieved and reviewed to ensure the accuracy of the histologic diagnosis in all cases by one of the coauthors (P. J. Z.). The tumors were graded according to the National Cancer Institute (NCI) criteria [13]. Briefly, the tumors were categorized to low, moderate, and high grade based on the histologic type, cellularity, cellular pleomorphism, mitotic count, and presence of absence of tumor necrosis. In selected difficult cases, the diagnosis and grading were also consulted with 2 other coauthors (J. S. B. and J. R. G.). Additional immunohistochemical staining was performed in selected cases. Standard immunohistochemical methods were used with the EnVision Plus system and a DAKO autostainer (DAKO, Carpenteria, CA). Frozen tissue was submitted for molecular analysis (reverse transcriptase polymerase chain reaction) for the SYT-SSX1/SSX2 gene fusion product resulting from the t(X;18) translocation in one case as previously described [14]. Fluorescent in situ hybridization analysis for SYT translocation using LSI SYT Dual Color Break Apart Rearrangement Probe (Vysis, Abbott Laboratories, Downers Grove, IL) was also performed on paraffin section of another case. Clinical information including age, sex, clinical presentation, imaging studies, treatments, and follow-up was collected and reviewed.
3. Results
Patients’ ages ranged from 9 weeks to 84 years (mean, 41.6 years). There were 11 males, 15 females, and in one case, the sex was unknown. There were 20 tumors that arose in the atria/pulmonary vessels (11 left, 8 right, 1 side unknown), 4 in the ventricles (2 left, 2 right), 1 in mitral valve, and 2 in epi/pericardium. The tumor size was known in 17 cases and ranged from 0.9 to 10.0 cm (mean, 5.3 cm). Outcome information or clinical follow-up was available in 17 cases (Tables 1 and 2) and absent or incomplete in 10 cases (Table 3).
Table 1.
Clinicopathologic data of alive patients with cardiac sarcomas
| Case | Age (y) | Sex | Diagnosis | Grade | Clinical presentation | Size/Site | Treatment | Outcome and follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 wk | F | Angiosarcoma | Low | Pericardial effusion during intrauterine life | 3 × 3 cm/RA | Excision with RA augmented with autologous pericardium | Alive and well, NED in 96 mo |
| 2 | 66 y | M | EHE | Low | CHF, TIA, CAD, diffuse ischemic cardiomyopathy | 0.9 cm/LA | Heart transplant | Postoperative complicated acute cellular rejections, NED in 5 mo |
| 3 | 66 y | F | Myxofibrosarcoma | Low | Increasing DOE, palpitations, and status post incomplete “myxoma” resection 1 year before current admission for having a recurrent tumor in LA | 3 × 3 cm/LA, mitral valve and the base of the LV | Resection of recurrent tumor with no gross tumor left behind | Recovered well after surgery for recurrence, NED in 12 mo |
| 4 | 14 y | F | MPNST | Low | 2 months of tachycardia, chest pain, dyspnea; echo: left atrial mass | 5.0 × 4.5 × 4.3 cm/LA | Partial resection followed by chemo, recurred in 36 mo and treated with reexcision | Done well following resection of this recurrent tumor; no additional chemotherapy, NED in 36 mo |
| 5 | 21 y | M | Sarcoma, NOS | High | Elevated WBC count and a left atrial mass | 6.0 cm/superior aspect of LA | Chemo followed by excision via a left atriotomy | Metastases to skin, adrenal, and frontal cortex; alive in 15 mo |
| 6 | 28 y | F | Sarcoma, NOS | High | NA | 7 × 4 × 3 cm/LA appendage tumor | Excision and heart transplant with positive margin | Complicated by acute cellular rejection initially, NED in 2 mo |
| 7 | 29 y | M | Synovial sarcoma, monophasic | High | Malaise, cardiac mass, pericardial effusion | 8.1 cm/posterior aspect of LV, pericardium | Incomplete excision; chemo postoperatively | Tolerated surgery and chemo well and discharged to home; alive NED in 13 mo |
| 8 | 34 y | F | Myxofibrosarcoma | High | A left atrial mass | 6.8 cm/LA | Resection followed by radiation | Developed metastases in paraspinal region in 2mo, alive in 19 mo with PET scan consistent with radiation changes and NED |
| 9 | 6 y | F | Myxofibrosarcoma | Mod | Left coronary embolism and myocardial infarction | 1.2 cm papillary mass/mitral valve | Embolectomy, resection of tumor, allograft placement followed by heart transplantation | No residual tumor in native heart; NED in 112 mo with annual heart biopsy monitoring rejection |
| 10 | 3 mo | M | Leiomyosarcoma | High | Irritability and tachypnea; echo: large pericardial effusion and RA mass | 5.3 cm/free wall of RA | Resection of tumor, with pulmonary artery homograft | Alive and NED in 96 mo |
| 11 | 23 y | M | Myxofibrosarcoma | High | History of asthma; progressive dyspnea for 12 mo, originally attributed to asthma; CT scan showed a left atrial mass | Primary: 7 × 5 × 3cm/roof and septum of LA; recur: 4 × 4 cm/entire roof of LA, right superior PV and the annulus of MV with dropped satellites at the confluence of right PV | Primary: excision with positive margin; recurrent: reexcision followed by chemo | Tolerated both surgeries well; recurrent in 36 mo |
Abbreviations: y, year; wk, week; mo, month; M, male; F, female; RA, right atrium; LA, left atrium; LV, left ventricle; DOE, dyspnea on exertion; NA, not available; CHF, congestive heart failure; TIA, transient ischemic attack; CAD, coronary artery disease; WBC, white blood cell count; NED, no evidence of disease; NOS, not otherwise specific; PV, pulmonary vein; chemo, chemotherapy; echo, echocardiogram; M, male; F, female; Mod, moderate.
Table 2.
Clinicopathologic data of patients who died of cardiac sarcoma
| Case | Age (y) | Sex | Diagnosis | Grade | Clinical presentation | Size/Site | Treatment | Outcome and follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | Angiosarcoma | High | Twitching and tingling in wrist; workup revealed right frontal lobe angiosarcoma with no primary lesion found until 6 y later presented with ventricular arrhythmias and a cardiac mass | 2 × 2 cm/posterior apex of LV | Apical resection with patch reconstruction | Died postoperatively of pulmonary embolism |
| 2 | 41 | F | Angiosarcoma (epithelioid) | High | Increasing pedal edema, DOE, orthopnea | 5 × 4 cm/RA | Resection with positive margin, postoperative chemo | Died in 21 mo |
| 3 | 4 | F | Sarcoma, NOS | High | Stroke and bilateral occipital hemorrhagic lesions and a large left atrial mass seen on echo | 3 × 3.5 cm/encroaching on orifices of both PVs; 0.6 × 0.3 cm/apex of LV | Resection with pericardial patch reconstruction | Died 2 d after surgery, multiple metastases found in brain, kidney, adrenal, and left ovary |
| 4 | 50 | M | Sarcoma, NOS | High | DM, HTN, and EtOH with CHF resistant to medical management; pulmonic valve lesion found on echo | Size unknown/PA | Unresectable and only biopsy taken | Died 5 d postoperatively secondary to complete AV block; seeding of pericardium and multiple tumor emboli in lungs |
| 5 | 45 | F | Leiomyosarcoma | High | Sudden onset of lower extremity edema, ascites with inferior vena cava occlusion on MRI | 10.0 cm/RA occluding inferior vena cava | Debulking only | Died 5 d postoperatively from multisystem organ failure |
| 6 | 50 | F | Osteosarcoma | High | Dyspnea, dizziness for 6 wk, myxoma on echo | 6 cm polypoid mass/LA | Excision with positive margin, postoperative chemo; reexcision with chemo and radiation; gastrectomy for metastasis | Local recurrence at 53 mo; metastasis to stomach at 93 mo; died shortly after her second local recurrence at 131 mo |
Abbreviations: RA, right atrium; LA, left atrium; LV, left ventricle; CHF, congestive heart failure; DM, diabetes mellitus; echo, echocardiogram; DOE, dyspnea on exertion; HTN, hypertension; EtOH, alcoholic; PV, pulmonary vein; M, male; F, female; NOS, not otherwise specific; PET, positron emission tomography; MV, mitral valve; PA, pulmonary artery; AV, atrial-ventricular; MRI, magnetic resonance imaging; y, year; M, male; F, female.
Table 3.
Cases without clinical follow-up
| Case | Age (y) | Sex | Diagnosis | Grade | Clinical presentation | Size/Site | Treatment | Outcome and follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | NA | NA | Angiosarcoma | High | NA | Unknown size/epicardia | NA | NA |
| 2 | 60 | M | Angiosarcoma | High | Shortness of breath, effusion | Unknown size/RA | NA | NA |
| 3 | 35 | M | Angiosarcoma | High | Effusion | Unknown size/pericardium | NA | NA |
| 4 | 66 | M | Synovial sarcoma | High | NA | Unknown size/RA | NA | NA |
| 5 | 60 | F | Leiomyosarcoma | High | Shortness of breath, 4 mo history of cardiac murmur | Unknown size/RV | NA | NA |
| 6 | 10 | M | MPNST | High | RA mass | Unknown size/RA | NA | NA |
| 7 | 48 | F | Myxofibrosarcoma | High | Unknown size/atrium | NA | NA | |
| 8 | 64 | F | MPNST | High | Scheduled for pacemaker insertion; echo: RV mass and pericardial effusion; CT: RLL and azygos pulmonary lesions | Unknown size/RV | Excision with patch reconstruction | Metastasis to lung, but lost to follow-up |
| 9 | 84 | F | Dedifferentiated chondrosarcoma | Mod | Thought to have LA myxoma | 3.5 cm pedunculated mass/LA | Excision with negative stalk base | Recent case |
| 10 | 42 | M | Myxofibrosarcoma | Mod | Progressive dyspnea | 2.5 cm/dome of LA; 5.5 cm/anterior LA | Resection with positive margin | Recover well 3 wk after surgery and lost to follow-up |
Abbreviations: y, year; wk, weeks; RA, right atrium; RV, right ventricle; LA, left atrium; NA, not available; RLL, right lower lobe of lung; echo, echocardiogram; M, male; F, female; Mod, moderate.
3.1. Cases
Histologically, the tumors were classified as 6 angiosarcomas, 1 epithelioid hemangioendothelioma (EHE), 6 myxofibrosarcomas, 3 malignant peripheral nerve sheath tumors (MPNST), 3 leiomyosarcomas, 2 synovial sarcomas, 1 dedifferentiated chondrosarcoma, 1 osteosarcoma, and 4 poorly differentiated sarcomas (Figs. 1–3).
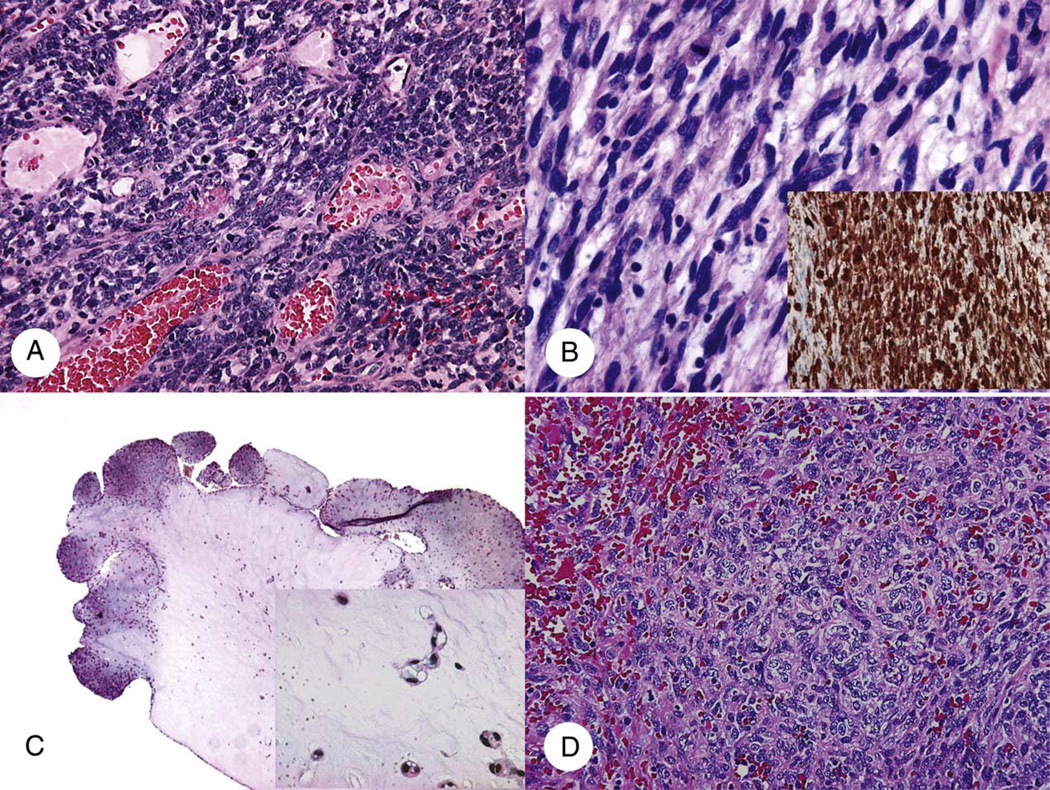
Fig. 1.
Primary cardiac soft tissue sarcomas. A, Monophasic synovial sarcoma with uniform spindle cell morphology. B, MPNST with S100 immunostaining (insert). C, EHE with polypoid growth and bland epithelioid tumor cells with vacuolated cytoplasms in cords or nests in hyaline myxoid stroma (insert). D, High-grade angiosarcoma with poorly formed vascular spaces (A, B, and D: hematoxylin-eosin [H&E] ×200; C: H&E ×25; B inset, anti-S100 antibody, immunoperoxidase ×200; C inset, H&E, ×400).
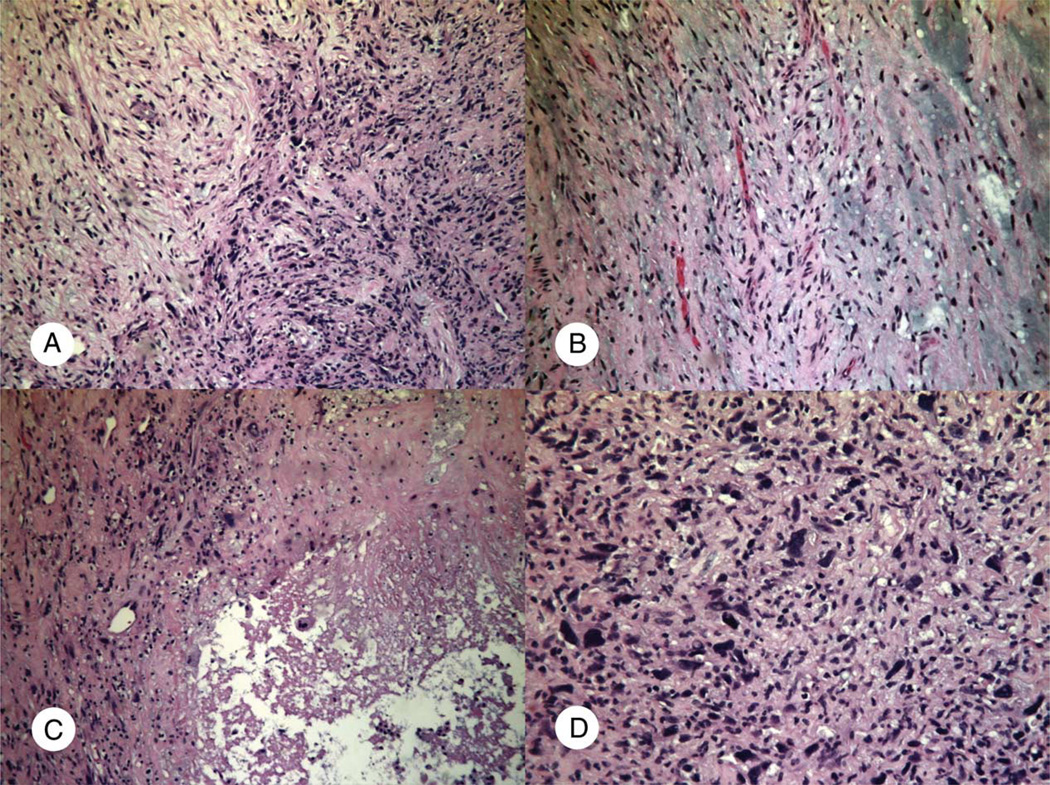
Fig. 3.
High-grade myxofibrosarcoma (case 4; Table 1). A, Interface of the high-grade and low-grade areas to show difference in cellularity and nuclear pleomorphism. B, Hypocellular area with curvilinear vasculature and myxoid stroma. C and D, High-grade areas with necrosis (C) and higher cellularity and marked nuclear pleomorphism (D) (A, B, and C: H&E ×100; D: H&E ×200).
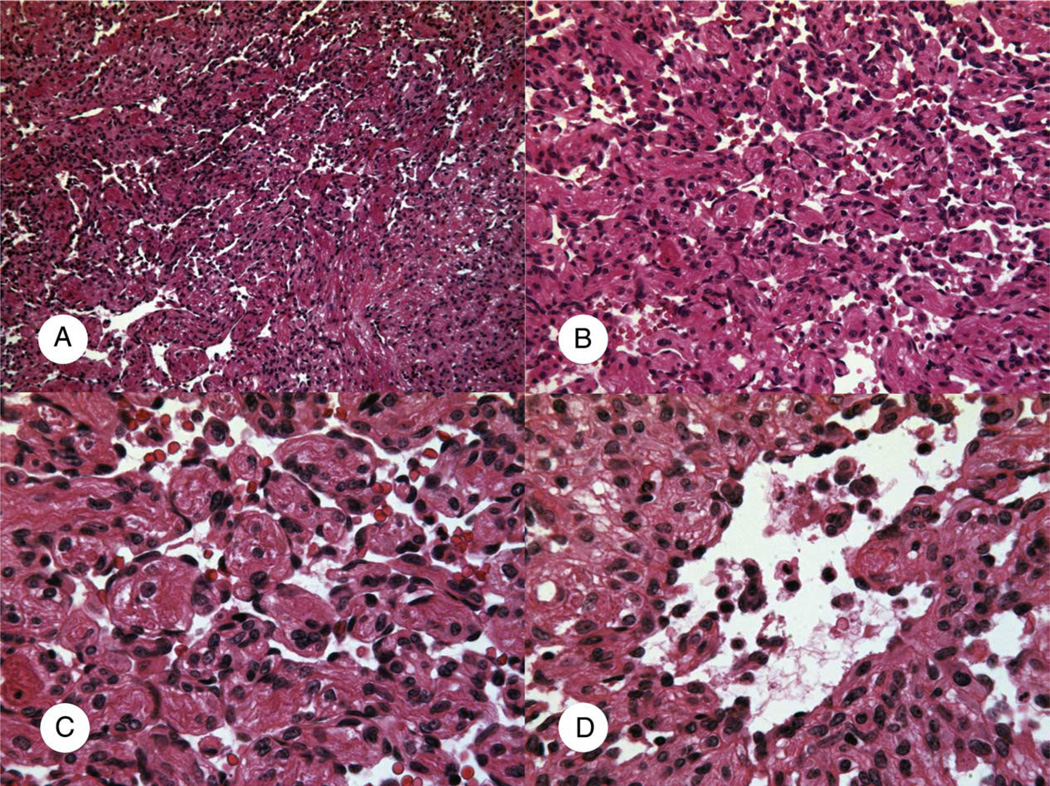
At the time of initial diagnosis, all vascular tumors were positive for at least one endothelial marker (factor VIII–related antigen, CD31, and/or CD34). The case of well-differentiated angiosarcoma arose in the right atrium of a 9-week-old infant who presented with intrauterine epicardial effusion (case 1; Table 1). At surgery, the tumor occupied 2 third of the right atrium. Histologically, it showed collapsed, complex anastomosing vascular channels lined by flat or tufted bland endothelial cells (Fig. 4). No mitotic figure or necrosis was identified.
Fig. 4.
Cardiac well-differentiated angiosarcoma (case 1; Table 1). A and B, Complex anastomosing vascular channels. C, The bland and flat endothelial cell lining. D, Tufted endothelial proliferation (A: H&E ×50; B: H&E ×100; C and D: H&E ×200).
The case of EHE was 0.9 cm mural polypoid mass incidentally found in the left atrium of a 66-year-old male patient (case 2, Table 1) who underwent cardiac transplantation for ischemic cardiomyopathy (Fig. 1C). Tumor cells were positive for all 3 endothelial markers (factor VIII, CD31, and CD34).
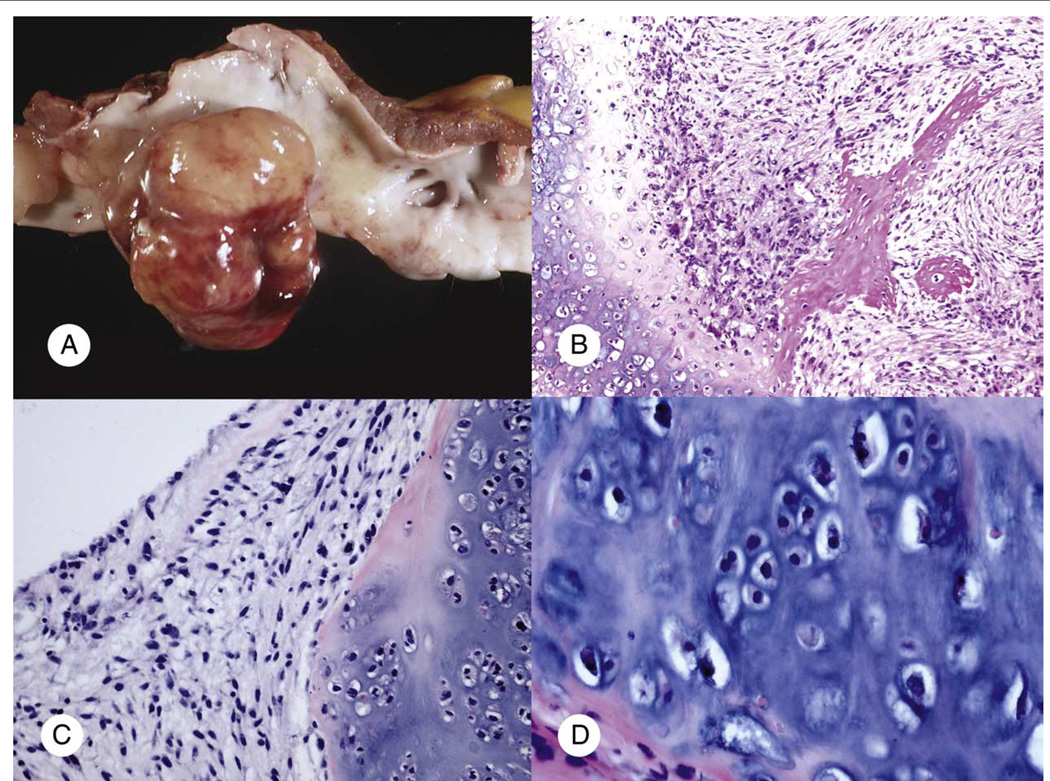
Unequivocal chondroid or osteoid formation was seen, respectively, in the chondrosarcoma and osteosarcoma case (Fig. 2). In either case, no past or current history of primary bone tumor was identified. Both tumors arose in the left atrium and clinically were felt to be suggestive of myxoma. The chondrosarcoma (case 9; Table 3) presented as a pedunculated 3.5-cm mass in the left atrium of an 84-year-old woman. Histologically, the tumor is composed of well-differentiated chondrosarcoma and focal nonchondroblastic spindle cell element consistent with dedifferentiation (Fig. 2). The osteosarcoma (case 6; Table 2) was a 6-cm polypoid mass involving the left atrium of a 50-year-old woman. Histologically, it also showed chondroblastic differentiation (Fig. 2).
Fig. 2.
Primary cardiac bone sarcomas. A and B, Cardiac osteosarcoma (case 6, Table 2): multilobulated polypoid mass grossly invaded to the resected left atrial wall (A) and osteoid formation and chondroblastic differentiation were seen microscopically (B). C and D, Cardiac chondrosarcoma (case 9; Table 3) with well-differentiated chondroblastic element and nonchondroblastic spindle cell area (A: gross image; B: H&E ×100; C: H&E ×200; D: H&E ×400).
Both synovial sarcomas were monophasic type (Fig. 1A) with cytokeratin immunoreactivity and positive SYT-SSX1/SSX2 gene fusion product by reverse transcriptase polymerase chain reaction (case 7; Table 1) or positive SYT breakapart signal by fluorescent in situ hybridization corresponding to the t(X;18) translocation (case 4; Table 3). All 3 MPNSTs showed at least focal S100 immunoreactivity (Fig. 1B).
The tumors were low grade in 4 (15%), moderate grade in 3 (11%), and high grade in 20 cases (74%). For myxofibrosarcoma, focal areas of low-grade histology were present in 1 high-grade and 1 moderate-grade cases (Fig. 3).
3.2. Initial surgery
Information for initial surgery was available in 20 patients, 18 of which underwent surgery for curative intent, 1 had a diagnostic biopsy only, and 1 had debulking only. Although complete excision of the tumors was considered impossible in 9 (52%) of 18 patients at the time of surgery, an attempt was made to achieve negative margins in these cases. Three patients received heart transplantation in the series. In case 6 in Table 1, heart transplant was used as a primary surgical mean to excise a large (7 cm) poorly differentiated sarcoma in the left atrial appendage. In the second case (case 9; Table 1), heart transplant was used to treat massive myocardial infarction due to tumor embolism in left coronary artery after initial surgery. For the third case (case 2; Table 1), the patient had an orthotopic heart transplant for ischemic cardiomyopathy, and a 0.9-cm exophytic EHE was found incidentally in the left atrium of the native heart.
3.3. Clinical outcome or follow-up information
Of the 17 patients with clinical outcome or follow-up information, 12 (71%) had high-grade tumor, 1 (6%) moderate-grade tumor, and 4 (23%) low-grade tumor; 12 (71%) had left-sided tumor, and 5 (29%) had right-sided tumor. Four patients died within 5 days of initial surgery. For the 13 patients who survived the initial surgery, the follow-up period ranges from 2 to 131 months (mean, 45.7 months). The mean length of follow-up was 27.8 months for patients with high-grade tumor including those who died within 5 days of surgery (n = 12) and 37.3 months for patients with low-grade tumor (n = 4).
3.3.1. Moderate- and high-grade tumors
Of 12 patients with high-grade tumor, 6 (50%) died, 4 within 5 days of the initial surgery (Table 2). Two additional patients died in the follow-up period, one (case 2; Table 2) died in 21 months after incomplete excision of the tumor with postoperative chemotherapy and the other (case 6; Table 2) in 131 months with multiple recurrences and metastasis treated with reexcision, chemotherapy, and radiation. Five patients developed metastasis. Of the 5 patients with metastatic disease, 3 died, 2 within 5 days of the surgery (cases 1 and 3; Table 2) and 1 in 131 months (case 6; Table 2). The remaining 2 patients (cases 5 and 8; Table 1) were alive at the end of the 15 and 19 months of follow-up, respectively. One additional patient with right ventricular MPNST developed lung metastasis at the time of surgery but was lost to follow-up (Table 3; case 8). Six patients with high-grade tumor were alive with 2 to 96 months of follow-up (mean, 30.2 months). Of these 6 patients, 4 had no evidence of disease, 1 had metastasis, and 1 had positive margin at reexcision at the end of follow-up (Table 1). One patient with moderate-grade tumor was alive with no evidence of disease at the end of 121 months of follow-up.
3.3.2. Low-grade tumors
Of the 4 patients with low-grade sarcoma, 2 had local recurrence, and both were treated with reexcision. All the patients with low-grade tumor did not develop metastasis and were alive with no evidence of disease at the end of 5 to 96 months of follow-up (mean, 37.3 months).
3.3.3. Tumor location and size
Of the 6 patients who died of disease, 3 had left-sided tumor and 3 had right-sided tumor (Table 2). Of the 6 patients who developed metastatic lesions, 5 had left-sided tumor. In one case (case 1; Table 2), the patient developed an intracranial metastasis from an occult left ventricular angiosarcoma. The mean size of the tumors in patients who died of a disease, who developed metastasis, or who were alive without metastasis was 5.2, 4.86, and 4.54 cm, respectively.
3.3.4. Adjuvant chemotherapy and radiation
In addition to the 2 fatal cases (cases 2 and 6 in Table 2), 3 other patients were treated with postoperative adjuvant chemotherapy (cases 4, 7, and 11; Table 1), one with preoperative chemotherapy (case 5; Table 1) and one with radiation only (case 8; Table 1). In patients (n = 7) who received additional chemotherapy and/or radiation, the tumors were high grade in 6 patients and low grade in 1 patient.
3.3.5. Survival analysis
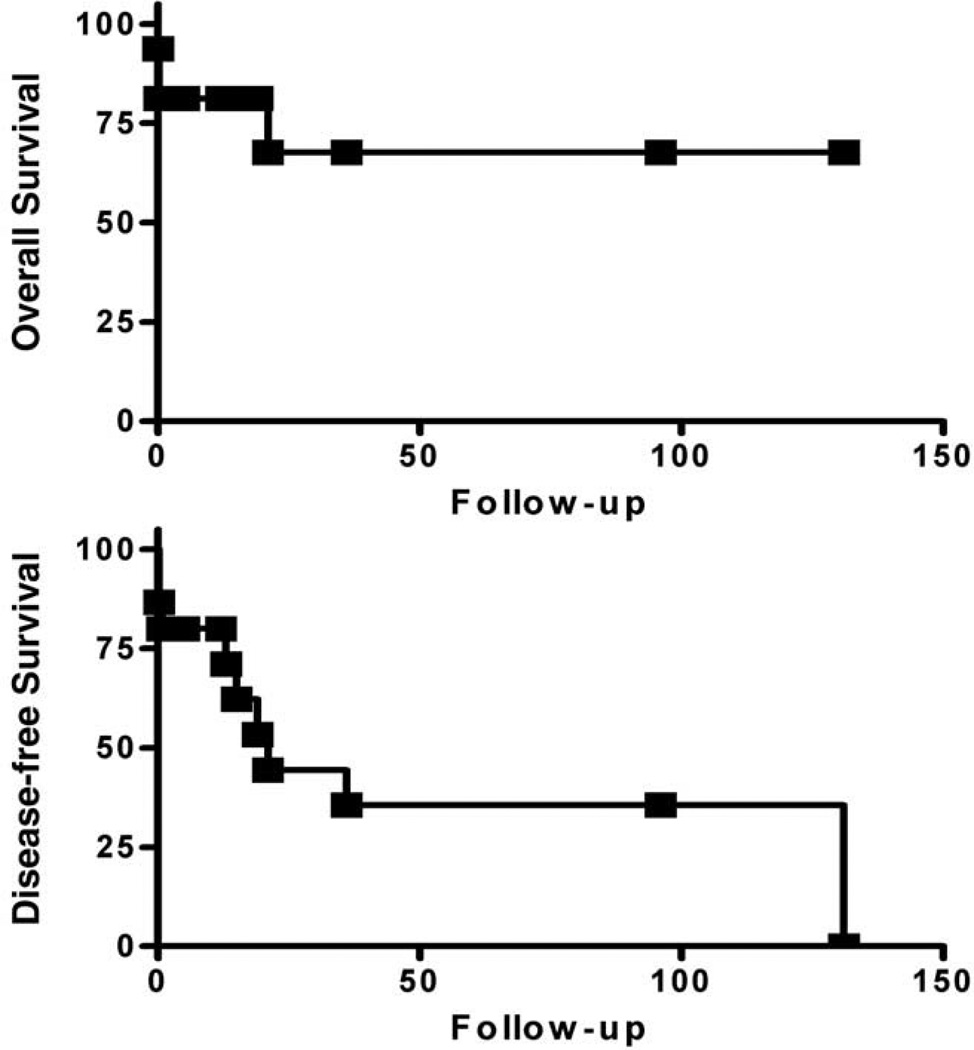
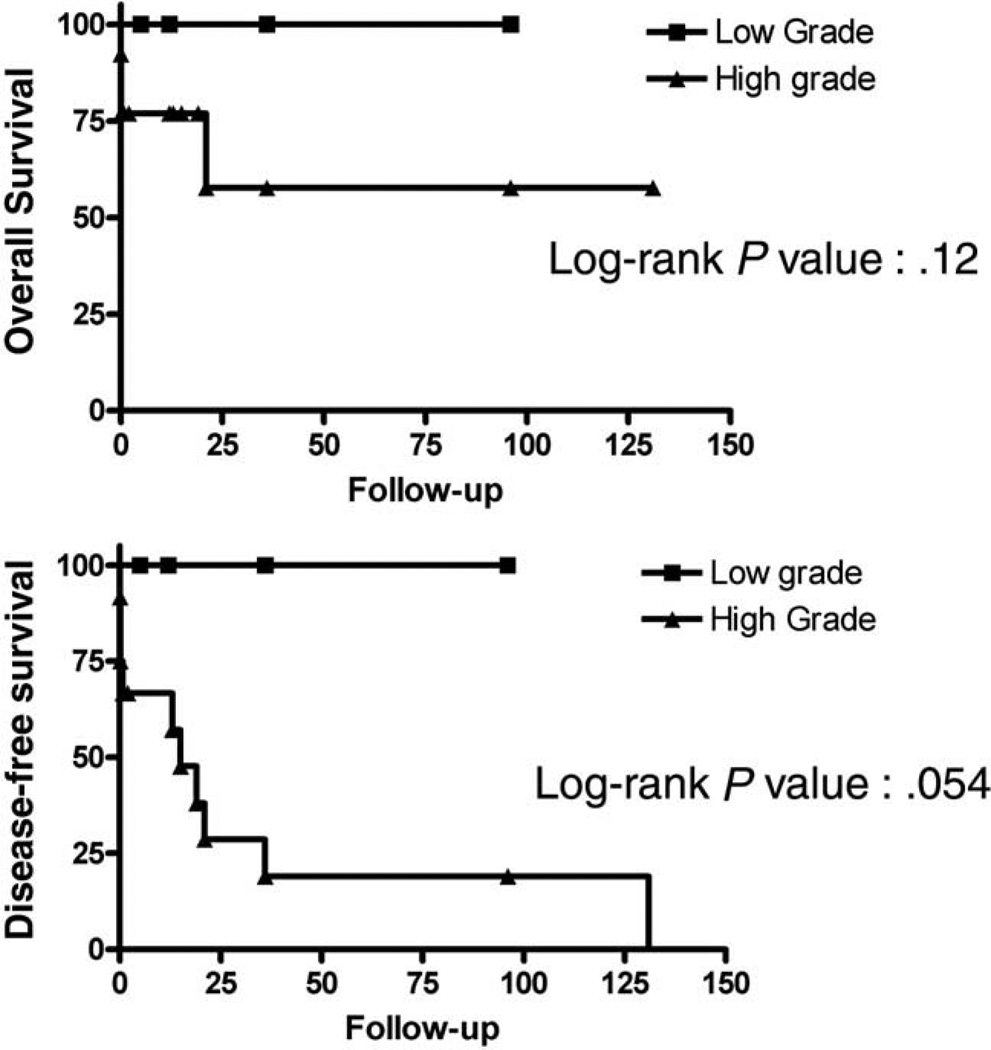
The curves of overall survival and disease-free survival for all patients with clinical outcome and follow-up are shown in Figs. 5 and 6. Kaplan-Meier plots and statistical analyses were performed using Prism 4 software (GraphPad Software Inc, San Diego, CA). The disease-free survival was 21 months for all cases (n = 17), 15 months for high-grade tumors (n = 12), and 21 months for high-grade tumor after removing the 4 patients who died shortly after surgery. The difference in overall survival between high-grade and low-grade tumors was not statistically significant (P = .12), but the difference in disease-free survival approached statistical significance (P = .054).
Fig. 5.
Kaplan-Meier plots for overall survival (top) and disease-free survival (bottom) of all cardiac sarcoma cases. Months of clinical follow-up are plotted on the x-axes and percentage of surviving cases plotted on the y-axes.
Fig. 6.
Kaplan-Meier plots for overall survival (top) and disease-free survival (bottom) of high-grade (triangles) and low-grade (squares) cardiac sarcoma cases. Months of clinical follow-up are plotted on the x-axes and percentage of surviving cases plotted on the y-axes.
4. Discussion
Most (74%) of the cardiac sarcomas in our series were high grade. As a group, high-grade cardiac sarcomas tended to behave more aggressively, and 4 (33%) of the 12 patients with high-grade tumor died within 5 days of the initial surgery. However, not all high-grade cardiac sarcomas pursued a rapid fatal clinical course. For the 8 patients who survived the initial surgery, even with incomplete excision of the tumor, only 2 (25%) patients died in 21 and 131 months of follow-up, respectively. The remaining 6 patients with high-grade tumor, who survived the initial surgery with follow-up data, were alive with 2 to 96 months of follow-up (mean, 30.2 months). Although a small series, our findings show that for patients who survived the initial surgery, the outlook for a long-term survival is very promising. However, it is unclear if adjuvant chemotherapy and radiation offered additional survival benefit in this group of patients due to small sampling size. In contrast, despite the local recurrence in 2 cases, all 4 patients with low-grade sarcoma tolerated surgery well for the primary and recurrent tumors and were alive and disease-free in 5 to 96 months (mean, 37.3 months) of follow-up. The difference in disease-free survival between patients with low-grade tumor and those with high-grade tumor approaches statistical significance. The lack of statistical significance in overall survival is likely due to the small number of cases in the series. Because only 1 moderate-grade tumor had follow-up data, it is not sufficient to assess the biologic behavior of the moderate-grade tumors.
In agreement with previously published series [11,15], angiosarcoma was one of the most common cardiac sarcomas. Almost all the angiosarcomas reported previously were high grade or moderate grade. In this series, we reported a very unusual case of well-differentiated angiosarcoma in a 9 week-old infant. The tumor was resected with a portion of right atrium, which was augmented with autologous pericardium. The patient was alive and well at the end of 96 months of follow-up. We also reported a case of EHE, a low-grade vascular tumor that has only been previously reported in the heart fewer than 5 incidences [16–18].
In this series, we also identified myxofibrosarcoma being another very common histologic type for cardiac sarcoma. The presence of areas of low-grade histology in some cases of high-grade and moderate-grade myxofibrosarcoma suggests a possibility of a high-grade transformation in a low-grade lesion in these cases (Fig. 3). Although both myxofibrosarcoma and myxoma, the most commonly seen primary cardiac tumor, share histologic features of myxoid matrix and fibroblastic stromal cell element, it remains unclear if there is any relation between the two.
Cardiac synovial sarcoma has been reported more commonly in the biphasic type arising in the right atrium [19–22]. Both cases in this series were spindle cell monophasic type with molecular confirmation, one arose in the left ventricle and the other in right atrium.
In approximately 80% of the cases, the leiomyosarcoma arose in the left atrium with only few cases reported from other chambers of the heart [23,24]. In our series, all 3 leiomyosarcomas were right sided, 2 from the atrium and 1 from the ventricle.
Although there have been case reports of primary pericardial MPNSTs [25], many reported cases lacked the ultrastructural or immunohistochemical evidence of neural differentiation [26]. All 3 MPNSTs in our series showed at least focal S100 immunoreactivity, supporting nerve sheath differentiation (Fig. 1B).
In addition to soft tissue sarcomas, bone sarcomas can also arise in heart [27–30]. In this series, we reported 1 dedifferentiated chondrosarcoma and 1 osteosarcoma of the heart. Because of the predunculated growth, the dedifferentiated chondrosarcoma (case 9; Table 3) was completely excised with negative stalk margin. Because it was a recent case, no clinical follow-up is available. Chondroblastic differentiation has been previously reported in cardiac osteosarcoma (Fig. 2) [28]. The 6-cm left atrial osteosarcoma was excised with positive margin followed by adjuvant chemotherapy. The tumor recurred in 53 months and treated with reexcision and adjuvant chemotherapy and radiation. At 93 months, the patient developed metastasis to the stomach, which subsequently was treated with partial gastrectomy. The patient died of the disease shortly after the second local recurrence 131 months after the initial surgery.
Four (15%) primary cardiac sarcomas in our series were poorly differentiated, and their histologic subtype could not be precisely classified. The incidence of unclassifiable sarcomas varies from 0% to 50%, depending on the series [8,26,31]. The mean survival of patients with undifferentiated sarcoma has been reported to be about 3 months [32]. Of 4 cases of undifferentiated sarcoma in this series, 3 (75%) had metastatic disease at the time of diagnosis. There were 2 (50%) patients who died of the disease shortly after surgery: 1 patient was alive with metastatic disease at 15 months of follow-up after being treated with surgery and chemotherapy, and the other was alive with no evidence of disease at the end of 2 months of follow-up after being treated with heart transplant with positive margin.
In summary, similar to the previous reports, our series show that cardiac sarcomas occurred in a wide age range and more commonly involved the atrium than other parts of the heart with predilection for the left side. In addition to angiosarcoma, we have also identified that myxofibrosarcoma is another common histology type for primary cardiac sarcomas. Primary cardiac sarcomas appear to behave more aggressively than those arising in somatic soft tissue, likely due in part to the difficulty in completely excising the tumor in this location [10]. In addition, cardiac surgery itself tends to subject the patients to more surgically related risk factors than other surgical procedures done in somatic tissue as operative mortality for surgical resection of cardiac sarcomas has been reported as 8.3% [33]. Despite the high incidence of unresectable cases and other risk factors, similar to sarcomas at other sites, wide surgical resection remains the cornerstone for treatment of cardiac sarcomas with variable outcomes in the literature [10,34]. Heart transplantation has recently been used to treat cardiac sarcoma, but its role is still controversial and has not been widely accepted as a main surgical mean for treating cardiac sarcoma [12,35,36]. In our series, 3 patients were treated with heart transplant, but only 1 was primarily for resecting the tumor and the other 2 were for ischemic heart disease either related or unrelated to the tumor. As in treating high-grade and advanced-stage soft tissue sarcomas, adjuvant chemotherapy and radiation have been used in cardiac sarcoma with prolonged remission after a combined modality approach in some patients [10,37,38]. However, their role in our series is unclear due to sampling size. The high metastatic incidence from left-sided tumors in this series likely resulted from the high proportion of left-sided diseases in the cases with follow-up information. It is unclear that the left-sided diseases have higher metastatic potential. There is no correlation between tumor size or location and patient survival. Histologic grading appears to have prognostic significance because none of the patients with low-grade sarcoma died of disease, whereas 6 (50%) of the 12 patients with high-grade sarcoma and outcome information died. However, in contrast to the dismal prognosis reported in some series with a median survival of only 6 months [10,21,38], our series showed a 15-month median disease-free survival for patients with high-grade cardiac sarcoma, which is further improved to 21 months after eliminating the 4 perioperative deaths. However, our data are not conclusive in regard to which factors were attributing to such survival improvement in this group of patients. In general, the fitness of patients’ overall physical condition to endure cardiac surgery, general improvement of cardiac surgical equipment and technology, a more balanced surgical approach in achieving optimal preservation and reconstruction of the cardiac anatomy and function, and improved postoperative care might be the attributing factors to minimize the operative mortality of cardiac surgery. Surgery remains to be the treatment of choice for cardiac sarcoma. In addition to histologic grade, the survival of the initial surgery is another important prognostic indicator for patients with high-grade cardiac sarcoma.
References
- 1.Silverman NA. Primary cardiac tumor. Ann Surg. 1980;191:127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcallister HA, Fenoglio JJ., Jr . Atlas of tumor pathology, 2nd series, fascicle 15. Washington, D.C.: Armed Forces Institute of Pathology, ARP Press; 1978. Tumors of the cardiovascular system; pp. 81–102. [Google Scholar]
- 3.Weiss SW, Goldblum JR. General considerations. In: Weiss SW, Goldblum JR, editors. Soft tissue tumors. 4th ed. St. Louis: C.V. Mosby Co; 2001. pp. 1–18. [Google Scholar]
- 4.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Hausheer FH, Josephson RA, Grochow LB, Weissman D, Brinker JA, Weisman HF. Intracardiac sarcoma diagnosed by left ventricular endomyocardial biopsy. Chest. 1987;92:177–179. doi: 10.1378/chest.92.1.177. [DOI] [PubMed] [Google Scholar]
- 6.Shechter M, Glikson M, Agranat O, Motro M. Echocardiographic demonstration of mitral block caused by left atrial spindle cell sarcoma. Am Heart J. 1992;1234:232–234. doi: 10.1016/0002-8703(92)90776-r. [DOI] [PubMed] [Google Scholar]
- 7.Miralles A, Brarcamonte L, Soncul H, et al. Cardiac tumors: clinical experience and surgical results in 74 patients. Ann Thorac Surg. 1991;52:886–895. doi: 10.1016/0003-4975(91)91241-m. [DOI] [PubMed] [Google Scholar]
- 8.Bear PA, Moodie DS. Malignant primary cardiac tumors. The Cleveland clinic experience, 1956 to 1986. Chest. 1987;92:860–862. doi: 10.1378/chest.92.5.860. [DOI] [PubMed] [Google Scholar]
- 9.Herhusky MJ, Gregg SB, Virmani R, Chun PK, Bender H, Gray GF., Jr Cardiac sarcomas presenting as metastatic disease. Arch Pathol Lab Med. 1985;109:943–945. [PubMed] [Google Scholar]
- 10.Shanmugam S. Primary cardiac sarcoma. Euro J Cardio-Thoracic Surg. 2006;29:925–932. doi: 10.1016/j.ejcts.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624–1628. doi: 10.1038/bjc.1998.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein DJ, Oz MC, Rose EA, Fisher P, Michler RE. Experience with heart transplantation for cardiac tumors. J Heart Lung Transpl. 1995;14:382–386. [PubMed] [Google Scholar]
- 13.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas: results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53:530–541. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.De Leeuw B, Balemans M, Olde Weghuis D, et al. Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18) (p11.2;q11.2)-positive synovial sarcomas. Hum Mol Genet. 1995;4:1097. doi: 10.1093/hmg/4.6.1097. [DOI] [PubMed] [Google Scholar]
- 15.Raaf HN, Raaf JH. Sarcomas related to the heart and vasculature. Semin Surg Oncol. 1994;10:374–382. doi: 10.1002/ssu.2980100511. [DOI] [PubMed] [Google Scholar]
- 16.Marchiano D, Fisher F, Hofstetter S. Epithelioid hemangioendothelioma of the heart with distant metastases, A case report and literature review. J Cardiovasc Surg. 34:529–533. [PubMed] [Google Scholar]
- 17.Agaimy A, Kaiser A, Wunsch PH. Epithelioid hemangioendothelioma of the heart in association with myelodysplastic syndrome. Z Kardiol. 91:352–356. doi: 10.1007/s003920200038. [DOI] [PubMed] [Google Scholar]
- 18.Moulai N, Chavanon O, Guillou L, et al. Atypical primary epithelioid hemanigoendothelioma of the heart. J Thorac Oncol. 2006;1:188–189. [PubMed] [Google Scholar]
- 19.Nicholson AG, Rigby M, Lincoln C, Meller S, Fisher C. Synovial sarcoma of the heart. Histopathology. 1997;30:349–352. doi: 10.1046/j.1365-2559.1997.d01-616.x. [DOI] [PubMed] [Google Scholar]
- 20.Bittira B, Tsang J, Huynh T, Morin JF, Huttner I. Primary right atrial synovial sarcoma manifesting as transient ischemic attacks. Ann Thorac Surg. 2000;69:1949–1951. doi: 10.1016/s0003-4975(00)01283-2. [DOI] [PubMed] [Google Scholar]
- 21.Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up in 24 cases. Histopathology. 1999;34:295–304. doi: 10.1046/j.1365-2559.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Rajhi N, Husain S, Coupland R, McNamee C, Jha N. Primary pericardial synovial sarcoma: a case report and literature review. J Surg Oncol. 1999;70:194–198. doi: 10.1002/(sici)1096-9098(199903)70:3<194::aid-jso10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Antunes MJ, Vanderonck KM, Andrade CM, Rebelo LS. Primary cardiac leiomyosarcomas. Ann Thorac Surg. 1991;51:999–1001. doi: 10.1016/0003-4975(91)91031-p. [DOI] [PubMed] [Google Scholar]
- 24.Fox JP, Freitas E, McGiffin DC, Firouz-Abadi AA, West MJ. Primary leiomyosarcoma of the heart: a rare cause of obstruction of the left ventricular outflow tract. Aust N Z J Med. 1991;21:881–883. doi: 10.1111/j.1445-5994.1991.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 25.Ursell PC, Albala A, Fenoglio JJ., Jr Malignant neurogenic tumor of the heart. Hum Pathol. 1982;13:640–645. doi: 10.1016/s0046-8177(82)80007-5. [DOI] [PubMed] [Google Scholar]
- 26.Molina JE, Edwards JE, Ward HB. Primary cardiac tumors: experience at the University of Minnesota. Thorac Cardiovasc Surg. 1990;38:183–191. doi: 10.1055/s-2007-1014064. [DOI] [PubMed] [Google Scholar]
- 27.Miwa S, Konishi Y, Matsumoto M, Minakata K. Primary cardiac chondrosarcoma: a case report. Jpn Circ J. 1997;61:795–797. doi: 10.1253/jcj.61.795. [DOI] [PubMed] [Google Scholar]
- 28.Seidal T, Wandt B, Lundin SE. Primary chondroblastic osteogenic sarcoma of the left atrium. Case report. Scand J Thorac Cardiovasc Surg. 1992;26:223–236. doi: 10.3109/14017439209099084. [DOI] [PubMed] [Google Scholar]
- 29.Zanella M, Falconieri G, Bussani R, Sinagra G, Della Libera D. Polypoid osteosarcoma of the left atrium: report of a new case with autopsy confirmation and review of the literature. Ann Diagn Pathol. 1998;2:167–172. doi: 10.1016/s1092-9134(98)80004-x. [DOI] [PubMed] [Google Scholar]
- 30.Sogabe O, Ohya T. Right ventricular failure due to primary right ventricle osteosarcoma. Gen Thorac Cardiovasc Surg. 2007;55:19–22. doi: 10.1007/s11748-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 31.Blondeau P. Primary cardiac tumors—French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38:192–195. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 32.Burke AP, Virmani R. Atlas of tumor pathology. 3rd ed. Washington: Armed Forces Institute of Pathology; 1995. [Google Scholar]
- 33.Putnam JB, Sweeny MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcoma. Ann Thorac Surg. 1991;51:906–910. doi: 10.1016/0003-4975(91)91003-e. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmeier A, Deiters S, Schmidt C, et al. Radical resection of cardiac sarcoma. Thorac Cardiovasc Surg. 2004;52:77–81. doi: 10.1055/s-2004-817809. [DOI] [PubMed] [Google Scholar]
- 35.Jimenes Mazuecos JMJ, Manso RF, Cubero JS, Ramos JT, Oteo Domingues JF, Rivera LAP. Is heart transplantation for primary cardiac sarcoma a useful therapeutic option? Rev Esp Cardiol. 2003;56:408–411. doi: 10.1016/s0300-8932(03)76886-9. [DOI] [PubMed] [Google Scholar]
- 36.Uberfuhr P, Meiser B, Fuchs A, et al. Heart transplantation: an approach to treating primary cardiac sarcoma? J Heart Lung Transplant. 2002;21:1135–1139. doi: 10.1016/s1053-2498(02)00409-6. [DOI] [PubMed] [Google Scholar]
- 37.Merry GM, Reardon MJ, Haas J, Lazar J, Hindenburg A. A combined modality approach to recurrent cardiac sarcoma resulting in a prolonged remission. Chest. 2003;123:1766–1768. doi: 10.1378/chest.123.5.1766. [DOI] [PubMed] [Google Scholar]
- 38.Mayer F, Aebert H, Rudert M, et al. Primary malignant sarcomas of the heart and great vessels in adult patients—a single-center experience. Oncologist. 2007;12:1134–1142. doi: 10.1634/theoncologist.12-9-1134. [DOI] [PubMed] [Google Scholar]