Abstract
The ε4 allele of apolipoprotein E (apo E) is associated with an increased risk for developing Alzheimer's disease (AD). This may be due to interactions between apo E and the amyloid-β protein (Aβ). To assess the effects of human apo E isoforms on Aβ deposition in vivo, we bred apo E3 and apo E4 hemizygous (+/–) transgenic mice expressing apo E by astrocytes to mice homozygous (+/+) for a mutant amyloid precursor protein (APPV717F) transgene that develop age-dependent AD neuropathology. All mice were on a mouse apo E null (–/–) background. By nine months of age, APPV717F+/–, apo E–/– mice had developed Aβ deposition, and, as reported previously, the quantity of Aβ deposits was significantly less than that seen in APPV717F+/– mice expressing mouse apo E. In contrast to effects of mouse apo E, similar levels of human apo E3 and apo E4 markedly suppressed early Aβ deposition at nine months of age in APPV717F+/– transgenic mice, even when compared with mice lacking apo E. These findings suggest that human apo E isoforms decrease Aβ aggregation or increase Aβ clearance relative to an environment in which mouse apo E or no apo E is present. The results may have important implications for understanding mechanisms underlying the link between apo E and AD.
Introduction
Apolipoprotein E (apo E), a 299–amino acid lipid transport protein, plays an important role in plasma cholesterol homeostasis (1, 2). In humans, there are three common alleles of apo E: ε2, ε3, and ε4. The protein isoforms produced by these alleles differ by only a single amino acid: E2 (cys112, cys158), E3 (cys112, arg158), which is the most common, and E4 (arg112, arg158) (1, 2). In addition to prominent expression in the liver, apo E is also highly expressed in the brain (3), where it is synthesized predominantly by astrocytes and some microglia (4–6). Genetic epidemiologic studies have shown that the ε4 allele of apo E is a risk factor for Alzheimer's disease (AD), as well as for a poor outcome following different forms of central nervous system (CNS) injury (7, 8). The mechanisms underlying these associations remain to be defined; however, substantial evidence suggests that interactions between apo E and the amyloid-β protein (Aβ) are in some way critical in regard to AD. Both in vitro (9–14) and in vivo (15–18) data suggest that the ability of apo E to interact with Aβ influences deposition of the Aβ peptide. An early hallmark of AD neuropathology is deposition of Aβ in diffuse, neuritic, and cerebrovascular plaques. While Aβ (Aβ40 and Aβ42) is normally found in body fluids, including cerebrospinal fluid (CSF) in a soluble form, in AD and in preclinical AD it is converted in the brain and in cerebral vasculature to a fibrillar form with a characteristic β-sheet configuration. An accumulation of insoluble Aβ is felt to play an important role in the pathophysiology of AD, since all mutations in genes known to cause familial AD as well as Down's syndrome result in either an increase in total Aβ or an increase in Aβ42 (19). Thus, understanding how factors such as apo E influence Aβ aggregation and clearance is likely to provide important insights into AD pathogenesis.
Recent studies have provided compelling evidence that mouse apo E can influence Aβ deposition in vivo. It was found that transgenic mice that overexpress a mutant amyloid precursor protein (APP) gene that causes familial AD (APPV717F) develop an age- and region-dependent Aβ deposition similar to that seen in AD (20). When these mice were bred onto a mouse apo E–/– background, there was a significant decrease in Aβ deposition and thioflavine S–positive (grossly fibrillar) deposits compared with animals expressing endogenous mouse apo E (18). Thus, the expression of mouse apo E in some way influences Aβ metabolism to promote Aβ deposition. To determine the influence of human apo E isoforms on Aβ deposition in vivo, we have bred APPV717F+/+, apo E–/– mice to transgenic glial fibrillary acidic protein (GFAP)–apo E mice whose astrocytes express physiological levels of human apo E in the absence of mouse apo E (GFAP–apo E3 and apo E4+/–) (21). Surprisingly, in contrast to effects of mouse apo E, expression of human apo E isoforms dramatically suppressed early Aβ deposition in vivo, even when compared with mice lacking apo E.
Methods
Animals and tissue preparation.
APPV717F+/+, apo E–/– mice (18) on an outbred background [(Swiss Webster × C57BL/6 × DBA/2) × C57BL/6] were bred with GFAP–apo E3+/– (line 37) and GFAP–apo E4+/– (lines 22 and 11) transgenic mice (21). F1 progeny of this cross were compared with each other. The GFAP–apo E founder transgenic mice were originally on a C57BL/CBA background, and for these experiments had been backcrossed four generations to apo E–/– mice on a C57BL/6 background (The Jackson Laboratory, Bar Harbor, Maine, USA). APPV717F+/–, mouse apo E+/+ mice were generated by crossing APPV717F+/+, apo E+/+ mice [(Swiss Webster × C57BL/6 × DBA/2) × C57BL/6] with C57BL/6 mice. APPV717F+/–, mouse apo E+/– mice were generated by crossing APPV717F+/+, apo E–/– mice [(Swiss Webster × C57BL/6 × DBA/2) × C57BL/6] with apo E–/– mice on a C57BL/6 background. Animals were screened for the presence of the APPV717F and GFAP–apo E transgenes by PCR as described (18, 21). At 12, 24, and 39 weeks of age, mice were anesthetized with intraperitoneal pentobarbital (150 mg/kg) and were perfused transcardially with 0.1 M PBS (pH 7.4) at 4°C. Brains were divided into left and right hemispheres. The right hemisphere was immersion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C overnight. After fixation, the brain was cryoprotected in 30% sucrose in PBS at 4°C and frozen in powdered dry ice. Brain regions from the left hemisphere were dissected and frozen in powdered dry ice before analysis.
Histological analysis.
Tissue sections were cut in the coronal plane at 40 μm on a freezing sliding microtome from the genu of the corpus callosum through the caudal extent of the hippocampus. For qualitative analysis of Aβ immunoreactivity, sections were immunostained as described (21) with the following Aβ antibodies: 1282 (gift of D. Selkoe, Brigham and Women's Hospital, Boston, Massachusetts, USA), 10D5, 6E10, and 4G8 (Senetek Inc., St. Louis, Missouri, USA). Thioflavine S staining was performed as described (18). For quantitative analysis of hippocampal size and Aβ-immunoreactive (Aβ-IR) deposits, every sixth section throughout the extent of the hippocampus was immunostained with antibody 1282. Utilizing these sections, the total volume of both the right hippocampus as well as Aβ-IR deposits were then assessed in each animal using unbiased stereology as described (22, 23).
Biochemical analysis.
Nondenaturing gradient gel electrophoresis, SDS-PAGE, and Western blotting were performed as described (21, 24). Antibodies used were 6E10 to human APP (Senetek Inc.); goat anti–apo E (Calbiochem-Novabiochem Corp., San Diego, California, USA, and Chemicon International, Temecula, California, USA); and rabbit anti-LRP (gift of J. Herz, University of Texas–Southwestern, Dallas, Texas, USA). Apo E levels in brain tissue were determined by quantitative Western blotting using as standards recombinant human and mouse apo E produced as described (25). Aβ ELISA was performed as described (18, 26).
Statistical analysis.
Quantitative data are presented as mean ± SEM. Data were analyzed by ANOVA followed by Student-Newman-Keuls test with significance set at P < 0.05.
Results
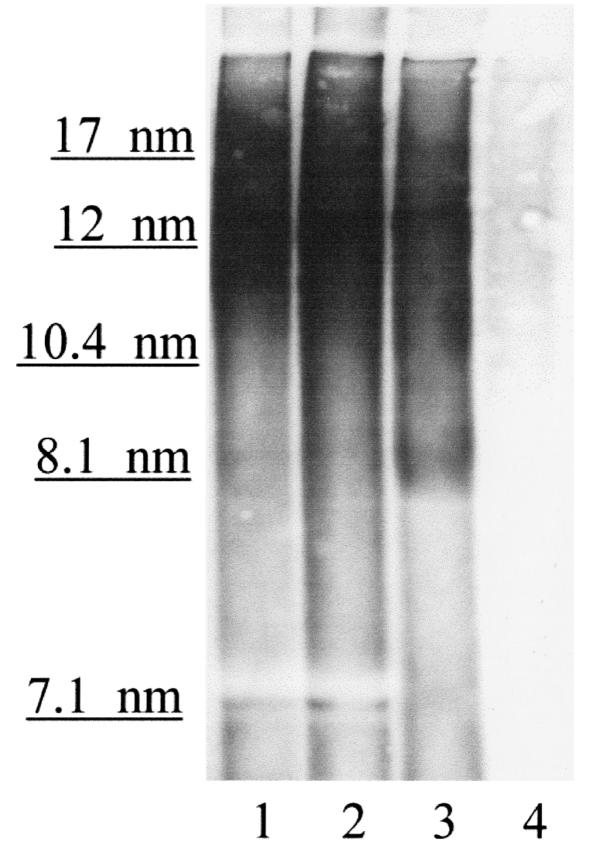
It was shown previously (18) that homozygous (+/+) APPV717F transgenic mice on an apo E–/– background had significantly less Aβ deposition than in the presence of mouse apo E. This finding has been recently replicated in an older cohort of APPV717F+/– mice (27). This effect was dependent on the gene dosage of mouse apo E. While mouse apo E is homologous to human apo E (∼70% identical at the amino acid level; ref. 2), it has been shown to have differential effects on plasma lipoprotein metabolism when compared with human apo E isoforms in vivo (28). Since apo E in the CNS is produced within the blood–brain barrier and is not derived from plasma (29), we have begun to explore the effects of human apo E isoforms produced at physiological levels in the brain on Aβ deposition in vivo. We recently produced human apo E isoform–specific transgenic mice in which human apo E isoforms are expressed in the brain under the control of the astrocyte-specific GFAP promoter (21). These mice were then bred onto a mouse apo E–/– background. Expression levels of human apo E in the brain of different lines of these mice are ver y similar to those found in normal human brains (21), as well as to levels of mouse apo E in normal mouse brains. Astrocytes from these animals secrete apo E, which is present in lipoprotein particles that are high-density lipoprotein–like (HDL-like) in size (21). As assessed by nondenaturing gradient gel electrophoresis, these astrocyte-secreted lipoprotein particles are similar in size to apo E–containing HDL-like particles found in human CSF (Figure 1).
Figure 1.
Nondenaturing gradient gel electrophoresis followed by Western blot analysis with goat anti–apo E antibody reveals that apo E in human CSF and apo E secreted by astrocytes are in HDL-like particles of overlapping size, mostly between 10 and 17 nm. Lane 1, pooled human CSF (20 μl) containing 100 ng apo E. Lane 2, pooled human CSF (30 μl) containing 150 ng apo E. Lane 3, astrocyte-conditioned media (20 μl) containing 100 ng apo E4 derived from GFAP–apo E4+/– astrocytes after 3 days in culture and collected as described (21). Lane 4, astrocyte-conditioned media (20 μl) from GFAP–apo E–/– cells. Size markers (Pharmacia Biotech Inc., Piscataway, New Jersey, USA) are expressed in nanometers. CSF, cerebrospinal fluid; GFAP, glial fibrillary acidic protein; HDL, high-density lipoprotein.
To assess the effects of human apo E isoforms on Aβ deposition in vivo, we bred APPV717F+/+, apo E–/– mice to GFAP–apo E4+/– line 22 mice and compared F1 animals (all of which were APPV717F+/– and either apo E4+/– or apo E–/–) at 13, 26, and 39 weeks of age. We also compared Aβ deposition in these mice with APPV717F+/–, mouse apo E+/+ mice on the same genetic background. It is important to note that APPV717F+/–, mouse apo E+/+ mice have been shown to develop age- and region-dependent Aβ deposition by ∼34–35 weeks of age independent of genetic background (20, 26). At 13 and 26 weeks of age, there was no evidence of Aβ deposition in apo E–/– or apo E4+/– mice. However, at 39 weeks of age, we found that apo E–/– mice had developed significant amounts of extracellular Aβ immunoreactivity in the hippocampus (Figures 2 and 3). While the quantity of Aβ-IR deposits in the absence of apo E was variable at this age, all (n = 8) apo E–/– mice assessed from this cross had hippocampal Aβ deposition that in some animals was similar in extent to that seen in the presence of mouse apo E. Similar to previous observations, however, we did find that hippocampal Aβ deposition in 39-week-old APPV717F+/–, apo E–/– mice was significantly less than that found in APPV717F+/–, mouse apo E+/+ animals (P < 0.05) (Figures 2 and 3). In addition, mouse apo E+/+ animals had cortical Aβ deposition (Figure 2) as well as thioflavine S–fluorescent (grossly fibrillar) deposits that were not present in apo E–/– mice (data not shown). It is interesting to note that APPV717F+/– mice (n = 4), which were heterozygous for mouse apo E (+/–), had levels of Aβ deposition that were qualitatively intermediate between mouse apo E+/+ and apo E–/– animals; also, some of the Aβ deposits in these animals were thioflavine S–positive (data not shown). These results are similar to those reported previously (18).
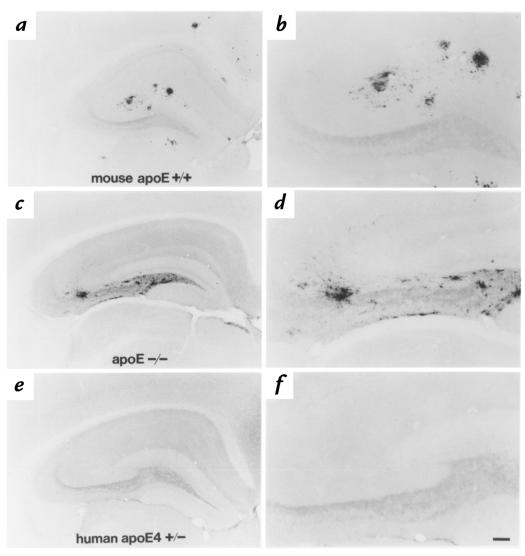
Figure 2.
Human apo E4 expression by astrocytes suppresses Aβ deposition, as assessed by anti-Aβ immunostaining in APPV717F+/– mice at 39 weeks of age. APPV717F+/–, mouse apo E+/+ animals had numerous hippocampal and some cortical Aβ-IR deposits by 39 weeks of age (a and b). APPV717F+/–, apo E–/– animals had less Aβ-IR deposits than those expressing mouse apo E; however, there was still a significant amount of deposition in all animals assessed. In addition, the hippocampal Aβ that was present in apo E–/– mice was in a different distribution, with more Aβ immunoreactivity in the hilus of the dentate gyrus and none in the cortex (c and d). In APPV717F+/–, apo E4+/– line 22 animals, hippocampal Aβ immunoreactivity was completely absent in most animals (e and f). Scale bar: 60 μm for b, d, and f; 150 μm for a, c, and e. Aβ, amyloid β; APP, amyloid precursor protein; IR, immunoreactive.
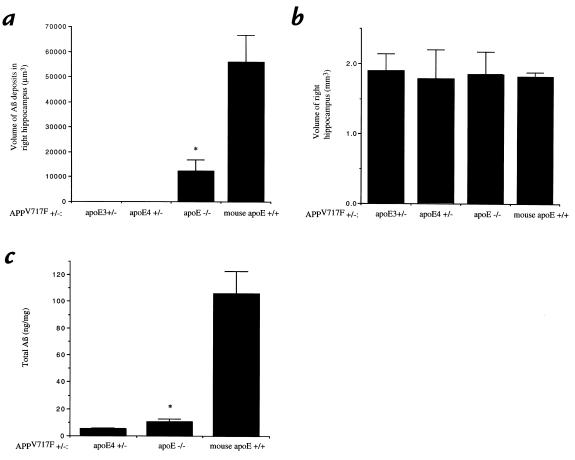
Figure 3.
Quantitation of Aβ deposition in APPV717F+/– mice. The total volume of Aβ-IR deposits in the right hippocampus (a) as well as the volume of the right hippocampus itself (b) was determined in each group of APPV717F+/– mice: apo E3+/– line 37 (n = 4), apo E4+/– line 22 (n = 7), apo E–/– (n = 8), and mouse apo E+/+ (n = 8). There was a significantly smaller volume of Aβ-IR deposits (P < 0.05) in the mice expressing human apo E3 compared with mice expressing no apo E or mouse apo E. The volume of the hippocampus was not statistically different between the different groups. Aβ ELISA for total Aβ in the left hippocampus of APPV717F+/– mice that were apo E4+/–, apo E–/–, and mouse apo E+/+ (c) revealed the same pattern of results as was found for Aβ-IR deposits histologically. *P < 0.05 comparing apo E–/– mice with apo E3+/– and apo E4+/– mice.
Surprisingly, in contrast to animals expressing mouse apo E or no apo E, APPV717F+/–, apo E4+/– line 22 mice (n = 7) had almost no detectable Aβ deposition at 39 weeks of age (Figures 2 and 3). Of seven apo E4+/– line 22 mice, six had no detectable Aβ deposition and one animal had one Aβ-IR deposit present in a total of 13 sections assessed throughout the extent of the hippocampus. Thus, while all the APPV717F+/–, apo E–/– mice at 39 weeks of age had Aβ-IR deposits, most apo E4+/– mice had none. We quantified the total volume of Aβ immunoreactivity in the right hippocampus in the presence of apo E4, no apo E, and mouse apo E, using stereological methods. There was significantly less Aβ deposition in APPV717F+/–, apo E4+/– mice than in APPV717F+/–, apo E–/– littermates (P < 0.05) (Figures 2 and 3). In addition, both groups of mice had significantly less Aβ deposition than was observed in APPV717F+/–, mouse apo E+/+ mice (P < 0.05). The amount of Aβ deposition detected in APPV717F+/–, mouse apo E+/+ mice at 39 weeks of age is almost identical to that described previously by others (20, 26). To rule out the possibility that the effect of decreased Aβ deposition observed in animals expressing human apo E4 was due to a nonspecific effect seen only in this particular apo E4 transgenic line (line 22), we also bred APPV717F+/+, apo E–/– mice to another apo E4 transgenic line (GFAP–apo E4+/– line 11) that expresses similar levels of apo E4 (Figure 4). When we compared F1 littermates that were apo E–/– (n = 5) with apo E4+/– line 11 (n = 5) at 39–42 weeks of age, we found that all five apo E–/– mice had Aβ deposition in the hippocampus, while four apo E4–expressing mice had no Aβ deposition and one animal had two Aβ-IR deposits in a total of 13 hippocampal sections assessed. To assess the effect of apo E3, we also examined the F1 offspring of APPV717F+/+, apo E–/– mice crossed with GFAP–apo E3+/– line 37animals that express indistinguishable levels of apo E compared with GFAP–apo E4+/– line 22 (Figure 4) (21). When we examined apo E3+/– mice (n = 4) along with their age-matched apo E–/– littermates (n = 4) at 39 weeks of age, the results were identical to those seen with the mice expressing apo E4 (Figure 3).
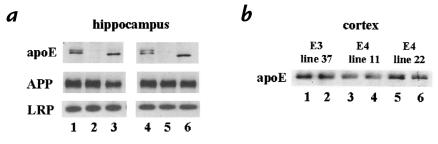
Figure 4.
Western blot analysis of apo E, APP, and LRP in hippocampal and cortical tissue lysates in APPV717F+/– mice. (a) Apo E levels in mouse apo E+/+ mice were similar to that seen in APPV717F+/–, apo E4+/– line 22 mice, and there were no significant differences in human APP or LRP levels between the groups. For hippocampal samples, 30 μg of detergent-soluble protein from individual 39-week-old mice was loaded per lane. Lanes 1 and 4, apo E4+/– line 22. Lanes 2 and 5, apo E–/–. Lanes 3 and 6, mouse apo E+/+. Human apo E is seen as a doublet (∼35 and 37 kDa) due to a sialylated form; a single band is observed for mouse apoE (∼34 kDa). Human APP is ∼120 kDa, and the transmembrane subunit of LRP is ∼85 kDa. (b) Apo E levels are similar in the different human apo E transgenic lines. Detergent-soluble protein (50 μg) from the cortex of individual mice was loaded to each lane. Lanes 1 and 2, apo E3+/– line 37. Lanes 3 and 4, apo E4+/– line 11. Lanes 5 and 6, apo E4+/– line 22. There was no significant difference in the level of human APP or of LRP between any of the groups of mice either qualitatively as shown here or quantitatively as assessed by densitometric analysis (data not shown). LRP, low-density lipoprotein receptor–related protein.
There were no Aβ-IR deposits in three apo E3–expressing mice, while one animal had a single Aβ-IR deposit in a total of 13 sections assessed. In contrast, all four of the apo E–/– littermates had numerous Aβ-IR deposits.
The effects of mouse and human apo E on histological Aβ deposition paralleled results obtained by Aβ ELISA (Figure 3c). Total Aβ levels were significantly higher in hippocampal tissue from APPV717F+/–, mouse apo E+/+ mice than in APPV717F+/–, apo E–/– and APPV717F+/–, apo E4+/– animals. In addition, total Aβ levels were higher in APPV717F+/–, apo E–/– tissue than in APPV717F+/–, apo E4+/– tissue (P < 0.05), although the difference in Aβ deposition observed by immunohistochemistry between these two groups was more dramatic than that observed by ELISA at this age.
It is interesting to note that the effect of human apo E on suppression of early Aβ deposition occurred with levels of apo E in the brain very similar to those seen in wild-type (apo E+/+) mice. We compared apo E protein levels in the hippocampus from animals that were APPV717F+/–, mouse apo E+/+ and APPV717F+/–, apo E4+/– (line 22). Levels of apo E protein in the hippocampus as determined by quantitative Western blotting (ng per 50 μg detergent-soluble protein) were 23.1 ± 2.4 for apo E4 and 33.5 ± 2.1 for mouse apo E (Figure 4a). Apo E levels in cortex and hippocampus were almost always the same within each animal studied. In addition, cortical apo E levels in the apo E3 and apo E4 transgenic animals were very similar to each other (Figure 4b). In some previous experiments, there was no evidence that apo E could directly interact with amyloid precursor protein (APP) in vitro (30), and the presence or absence of expression of mouse apo E in vivo did not alter levels of APP present in APPV717F mice (18). To further address the possibility that the expression of apo E could in some way influence the level of APP and secondarily influence Aβ levels, we assessed whether expression of apo E4 affected the level of human APP. There was no difference in the level of APP in the hippocampus (Figure 4a) or cortex (data not shown) when comparing animals expressing apo E4, mouse apo E, or no apo E (Figure 4a). Similar data was obtained with apo E3–expressing mice. We also investigated whether or not the expression of apo E influenced the level of the low-density lipoprotein receptor–related protein (LRP), a receptor for apo E that is expressed at high levels in the brain. Similar to results obtained for APP, there was no clear difference in LRP levels in the hippocampus when comparing mice expressing apo E4, mouse apo E, or no apo E (Figure 4). While the absolute level of functional LRP could still be involved in modulating Aβ clearance, it seems unlikely that the expression of apo E somehow alters its level of expression.
Discussion
The development of mouse models in which there is age- and region-dependent Aβ deposition has allowed researchers to study the effects that genes modifying Alzheimer's disease (AD) have on Aβ metabolism. An association between apo E4 and AD was first identified in 1993 (7). Unlike mutations in APP or presenilins that cause rare autosomal-dominant, early-onset forms of familial AD, apo E isoforms appear to act as risk modifiers for the most common form of late-onset AD, with apo E4 being a risk factor and apo E2 possibly being protective (7). Several hypotheses have been put forward as to why apo E4 may serve as an AD risk factor. One hypothesis is that apo E modifies conversion of Aβ from a soluble to a fibrillar form and that the conversion by apo E4 is more efficient compared with the other apo E isoforms, leading to earlier and greater Aβ deposition with its secondary consequences. Our results demonstrate that expression of human apo E3 and E4 in the brain at physiologically relevant levels by astroglial cells, which normally produce the bulk of apo E in the CNS, suppresses early Aβ deposition in a mouse model of AD. This result is striking given that the presence of mouse apo E in this model has the opposite effect (18) and that more Aβ deposition is observed in apo E–/– mice than in animals expressing human apo E3 or E4. While the mechanism(s) by which human apo E isoforms reduces Aβ deposition remains unknown, human and mouse apo E appear to modify early Aβ deposition in vivo in fundamentally different ways. Since human apo E isoforms suppress early Aβ deposition, it will be interesting to determine the effects of apo E isoform expression on AD pathology over even longer periods of time in both hemizygous and homozygous APPV717F transgenic mice as well as in other mouse models that develop Aβ deposition. Although human apo E retards early Aβ deposition in APPV717F mice, with increasing age the ability of apo E to suppress Aβ deposition may be reduced or eliminated.
From what is known about apo E and Aβ, two possibilities seem likely to account for the differential effects of mouse and human apo E on early Aβ deposition. First, it maybe that at certain concentrations of Aβ, mouse apo E promotes while human apo E inhibits Aβ fibrillogenesis. In regard to this property, mouse apo E and human apo E isoforms have not been compared previously. Such studies will be important and may require study of CNS-like apo E/lipoproteins and their interactions with Aβ. It has been suggested (31) that the conversion of Aβ from a soluble to fibrillar form may occur in vivo via “seeded” polymerization, in which Aβ42 forms a very small aggregated seed (nucleation step) followed by polymerization (fibril elongation and fiber–fiber association) of Aβ40 and Aβ42. While some in vitro studies (11–13) suggest that conversion of Aβ to amyloid fibrils is facilitated by apo E, two reports (14, 32) have found that apo E decreased fibrillogenesis by inhibiting Aβ seeding or nucleation. The reason for the differing results found in these studies is not clear, but possibilities include differences in methods or the Aβ and apo E preparations used. It is also possible that at low concentrations of Aβ, apo E inhibits Aβ nucleation, but that once a critical concentration of Aβ is reached, apo E promotes fibrillogenesis. In either case, an isoform-specific difference in inhibiting (e.g., E2 > E3 > E4) or promoting (e.g., E2 < E3 < E4) fibril formation could account for the differences observed in AD. If so, this would predict that at later time points, APPV717F, human apo E transgenic mice will develop fibrillar Aβ deposition, but that the age of onset would be apo E isoform–dependent. It is important to note that most in vitro studies on Aβ aggregation have not used apo E preparations in which apo E is present in lipoprotein particles, a form in which it is found in vivo. Lipid-free and lipid-associated apo E interact differently with other molecules, including Aβ (10). Since apo E in the CSF, and likely in the brain parenchyma, is in HDL-like lipoprotein particles (33, 34), determining interactions of physiologically relevant apo E preparations with Aβ may be critical to understanding the relevant effects of apo E on Aβ fibrillogenesis.
A second possibility as to why expression of human apo E suppresses and mouse apo E promotes early Aβ deposition is that human apo E may promote clearance of Aβ better than mouse apo E. It is has been shown that different forms of apo E can associate with Aβ in vitro and in vivo (7, 10, 12, 35, 36). It is also appears that under physiological conditions, a significant amount of soluble Aβ in plasma (37) and CSF (38) is found associated with apo E–containing lipoprotein particles. When Aβ is complexed with such particles, apo E receptors may be capable of modulating the amount of soluble or fibrillar Aβ in brain parenchyma through cellular clearance mechanisms. If similar amounts of Aβ associate with mouse and human apo E/lipoproteins, then there may be differential binding, uptake, or degradation of the different forms of apo E via apo E receptors. Several apo E receptors, including the low-density lipoprotein receptor, LRP, the very-low-density lipoprotein receptor, LR8/apo ER2, LRP-2/gp330, and LR11/SorLA-1, are expressed in the brain (17, 39–44). To date, studies have not quantitatively addressed whether there are differences between CNS-produced mouse and human apo E/lipoproteins and interactions with these receptors. Of particular interest in the brain is LRP since it (a) is expressed at high levels by all major neural cell types (17); (b) is a receptor for several other AD-associated proteins such as α2-macroglobulin and APP (45, 46); and (c) along with α2-macroglobulin, has itself been genetically linked with AD (47, 48). It has recently been shown that α2-macroglobulin binds Aβ with high affinity (49) and facilitates its clearance via the LRP (50). While it has been shown that both human and mouse apo E can interact with human and mouse LRP (51, 52), it is not known if there is differential binding. Another reason for apo E clearance could be differential interactions of mouse and human apo E with heparin sulfate proteoglycans (HSPG). In some cells, apo E appears to bind initially to HSPG, some of which is transferred to LRP before internalization (53). There also appears to be an HSPG-independent means of apo E cellular uptake (54). It is important to note that there is evidence that mouse and human apo E have differing biological effects that may involve clearance mechanisms. Human apo E3, when present in mouse plasma, was found to markedly delay clearance of large remnant lipoprotein particles, and did not protect mice nearly as well against an atherogenic diet when compared with mouse apo E (28). One possibility raised is that this was due to species differences in affinities of apo E–containing remnant particles for receptors such as the low-density lipoprotein receptor.
Even if mouse and human apo E are quantitatively cleared to a similar extent via cellular receptor systems, if Aβ differentially associates with mouse and human apo E/lipoproteins, Aβ clearance could be secondarily affected via clearance mechanisms. For example, more soluble Aβ may associate with human apo E than mouse apo E lipoproteins. Furthermore, in the absence of apo E, Aβ may associate with the other major apolipoprotein known to be expressed in the brain and which binds to Aβ, apo J (55). If more Aβ is associated with brain lipoprotein particles in the presence of human apo E and apo J (e.g., apo E3 and E4+/– mice), or apo J alone (e.g., apo E–/– mice), than in the presence of mouse apo E and apo J (e.g., mouse apo E+/+ mice), this could potentially facilitate Aβ clearance via LRP as well as via gp330/LRP-2 (56), a receptor for apo J expressed in the choroid plexus and ependyma (40). The effect of human apo E on suppression of early Aβ deposition may be analogous to its effects on suppression of atherosclerosis. For example, apo E expression by bone marrow macrophages (57) and cells of the arterial wall (58) suppressed atherosclerosis in mice. Furthermore, effects of macrophage-derived human apo E suppressed atherosclerosis independent of any effect on plasma cholesterol (59). The latter findings suggest that macrophage-derived apo E/lipoproteins may enhance removal or efflux of cholesterol from cells of the arterial wall. Recent data also suggest that apo E/lipoproteins in the brain may perform a similar function after injury (60). Thus, as apo E/lipoproteins secreted by macrophages may be protective against atherosclerosis by promoting reverse cholesterol transport, apo E–containing, HDL-like particles in the brain may, in an analogous way, be able to promote reverse Aβ transport and delay Aβ deposition by targeting it for removal and clearance.
Acknowledgments
This work was supported by grants from the Alzheimer's Association (RG3-96-26), the Ruth K. Broad Foundation, a Paul Beeson Faculty Scholar Award from AFAR, and NIH-AG13956 (D.M. Holtzman). L.K. Chang was supported by a Glenn-AFAR scholarship, and A.M. Fagan was supported by NIH-AG00861.
Footnotes
D.M. Holtzman and K.R. Bales contributed equally to this study.
References
- 1.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 2.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 3.Newman TC, Dawson PA, Rudel LL, Williams DL. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985;260:2452–2457. [PubMed] [Google Scholar]
- 4.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakai M, Kawamata T, Maeda K, Tanaka C. Expression of apoE mRNA in rat microglia. Neurosci Lett. 1996;211:41–44. doi: 10.1016/0304-3940(96)12716-6. [DOI] [PubMed] [Google Scholar]
- 6.Stone DJ, et al. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143:313–318. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- 7.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 8.Roses AD. Apolipoprotein E, a gene with complex biological interactions in the aging brain. Neurobiol Dis. 1997;4:170–186. doi: 10.1006/nbdi.1997.0161. [DOI] [PubMed] [Google Scholar]
- 9.Strittmatter WJ, et al. Binding of human apolipoprotein E to synthetic amyloid β peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaDu MJ, et al. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23404–23406. [PubMed] [Google Scholar]
- 11.Ma J, Yee A, Brewer HB, Das S, Potter H. Amyloid-associated proteins alpha-1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 12.Sanan DA, et al. Apolipoprotein E associates with β amyloid peptide of Alzheimer's disease to form novel monofibrils. J Clin Invest. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castano EM, et al. Fibrillogenesis in Alzheimer's disease of amyloid beta peptides and apolipoprotein E. Biochem J. 1995;306:599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans KC, Berger EP, Cho C-G, Weisgraber KH, Lansbury PT. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer's disease. Proc Natl Acad Sci USA. 1994;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 16.Schmechel DE, et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 18.Bales KR, et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 19.Selkoe DJ. Alzheimer's disease: genotypes, phenotype, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 20.Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, et al. GFAP-apoE transgenic mice: Astrocyte specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalieri, B. 1966. Geometria degli indivisibili. Unione Tipografico. Torino, Italy.
- 23.Fagan AM, Garber M, Barbacid M, Silos-Santiago I, Holtzman DM. A role for TrkA during maturation of striatal and basal forebrain cholinergic neurons in vivo. J Neurosci. 1997;17:7644–7654. doi: 10.1523/JNEUROSCI.17-20-07644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCall MR, Forte TM, Shore VG. Heterogeneity of nascent high density lipoproteins secreted by the hepatoma-derived cell line, Hep G2. J Lipid Res. 1988;29:1127–1137. [PubMed] [Google Scholar]
- 25.Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM. Apolipoprotein E–containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- 26.Johnson-Wood K, et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bales KR, et al. Apolipoprotein E and amyloid deposition in transgenic mice. Soc Neurosci Abstr. 1998;24:1502. (Abstr.) [Google Scholar]
- 28.Sullivan PM, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 29.Linton MF, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biere AL, et al. Co-expression of beta-amyloid precursor protein (betaAPP) and apolipoprotein E in cell culture: analysis of betaAPP processing. Neurobiol Dis. 1995;2:177–187. doi: 10.1006/nbdi.1995.0019. [DOI] [PubMed] [Google Scholar]
- 31.Lansbury PTJ. Stuctural neurology: are seeds at the root of neuronal degeneration? Neuron. 1997;19:1151–1154. doi: 10.1016/s0896-6273(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 32.Wood SJ, Chan W, Wetzel R. Seeding of Aβ fibril formation is inhibited by all three isotypes of apolipoprotein E. Biochemistry. 1996;35:12623–12628. doi: 10.1021/bi961074j. [DOI] [PubMed] [Google Scholar]
- 33.Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci USA. 1979;76:4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaDu MJ, et al. Nascent astroctye particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 35.Naslund J, et al. Characterization of stable complexes involving apolipoprotein E and the amyloid β peptide in Alzheimer's disease brain. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 36.Wisniewski T, Lalowski M, Golabek AA, Vogel T, Frangione B. Is Alzheimer's disease an apolipoprotein E amyloidosis? Lancet. 1995;345:956–958. doi: 10.1016/s0140-6736(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 37.Biere AL, et al. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- 38.Koudinov AR, Koudinova NV, Kumar A, Beavis RC, Ghiso J. Biochemical characterization of Alzheimer's soluble beta protein in human cerebrospinal fluid — association with high density lipoprotein. Biochem Biophys Res Commun. 1996;223:592–597. doi: 10.1006/bbrc.1996.0940. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW, Simmons DM, Hofmann SL, Goldstein JL, Brown MS. Localization of mRNA for low density lipoprotein receptor and a cholesterol synthetic enzyme in rabbit nervous system by in situ hybridization. Proc Natl Acad Sci USA. 1988;85:9821–9825. doi: 10.1073/pnas.85.24.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kounnas MZ, Haudenschild CC, Strickland DK, Argraves WS. Immunological localization of glycoprotein 330, low density lipoprotein receptor related protein and 39 kDa receptor associated protein in embryonic mouse tissues. In Vivo. 1994;8:343–351. [PubMed] [Google Scholar]
- 41.Christie RH, Chung H, Rebeck GW, Strickland D, Hyman BT. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:491–498. doi: 10.1097/00005072-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, et al. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoportein receptor family predominantly expressed in brain. J Biol Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki H, et al. Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J Biol Chem. 1996;271:24761–24768. doi: 10.1074/jbc.271.40.24761. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen L, et al. Molecular characterization of a novel human hybrid-type receptor that binds the α2-macroglobulin receptor-associated protein. J Biol Chem. 1996;271:31379–31383. doi: 10.1074/jbc.271.49.31379. [DOI] [PubMed] [Google Scholar]
- 45.Strickland DK, et al. Sequence identity between the α2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- 46.Kounnas MZ, et al. LDL-receptor-related protein, a multifunctional apoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 47.Kang DE, et al. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer's disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- 48.Blacker D, et al. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 49.Du Y, et al. α2 macroglobulin as a β-amyloid peptide-binding plasma protein. J Neurochem. 1997;69:299–305. [PubMed] [Google Scholar]
- 50.Narita N, Holtzman DM, Schwartz AL, Bu G. α2-macroglobulin complexes with and mediates the endocytosis of β-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 51.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci USA. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holtzman DM, Fagan AM. Potential role of apoE in structural plasticity in the nervous system: Implications for diseases of the central nervous system. Trends Cardiovasc Med. 1998;8:250–255. doi: 10.1016/s1050-1738(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 53.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer's disease connection. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 54.Ji ZS, Pitas RE, Mahley RW. Differential cellular accumulation/retention of apolipoprotein E mediated by cell surface heparan sulfate proteoglycans. Apolipoproteins E3 and E2 greater than E4. J Biol Chem. 1998;273:13452–13460. doi: 10.1074/jbc.273.22.13452. [DOI] [PubMed] [Google Scholar]
- 55.Matsubara E, Frangione B, Ghiso J. Characterization of apolipoprotein J–Alzheimer's Aβ interaction. J Biol Chem. 1995;270:7563–7567. doi: 10.1074/jbc.270.13.7563. [DOI] [PubMed] [Google Scholar]
- 56.Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J–amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta peptide. J Biol Chem. 1997;272:18644–18649. doi: 10.1074/jbc.272.30.18644. [DOI] [PubMed] [Google Scholar]
- 57.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 58.Shimano H, et al. Inhibition of diet-induced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J Clin Invest. 1995;95:469–476. doi: 10.1172/JCI117687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellosta S, et al. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagan AM, et al. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (–/–) mice but impaired clearance of axonal degeneration products following injury. Exp Neurol. 1998;151:314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]