Abstract
Background
Vascular complications are a known risk of catheter-based pulmonary vein antral isolation (PVAI). Procedure-related thromboembolic events necessitate full-dose anticoagulation, which worsens outcomes in the event of vascular access injury.
Objective
Real-time ultrasound allows direct visualization of vascular structures. We hypothesized that ultrasound use with venipuncture reduces vascular complications associated with PVAI.
Methods
Retrospective analysis of all adverse events occurring with PVAI was performed during two periods: 2005– 2006 when ultrasound was not used and 2008–2010 when ultrasound was routinely employed. All patients received full-dose IV heparin during PVAI. In the no ultrasound cohort, only 14 % underwent PVAI without stopping warfarin, while 91 % of patients in the ultrasound cohort were on continued warfarin. Only patients deemed at high risk for thromboembolism with a periprocedural international normalized ratio (INR) less than 2 were bridged with subcutaneous low-molecular-weight heparin.
Results
Ultrasound reduced total vascular complications (1.7 vs. 0.5 %, p<0.01) and decreased the incidence of major vascular complications by sevenfold. Warfarin with INR≥1.2 on the day of PVAI was associated with more vascular complications (4.3 vs. 1.2 %, p<0.01). Ultrasound guidance overcame the risk associated with warfarin therapy. Vascular complications in anticoagulated patients with INR≥1.2 using ultrasound guidance were two- and ninefold lower than those in patients not using ultrasound with an INR<1.2 (0.5 vs. 1.2 %, p<0.05) and INR≥1.2 (0.5 vs. 4.3 %, p<0.01), respectively.
Conclusion
Ultrasound-guided venipuncture improves the safety profile of PVAI, reducing vascular complications in patients on warfarin to levels below those with no ultra-sound and off warfarin.
Keywords: Vascular complications, Ultrasound guidance, Pulmonary vein antral isolation, Periprocedural anticoagulation, Procedure-related thromboembolic events
1 Introduction
Catheter-directed pulmonary vein antral isolation (PVAI) has emerged as a widely accepted therapy for paroxysmal and persistent forms of drug-refractory atrial fibrillation [1]. Since the inception of PVAI, the rate of major procedure-related complications has decreased with initial reports ranging from 3 to 6 % [2, 3]. In particular, the risk of thromboembolic complications has been significantly attenuated with recent improvements in peri- and intra-procedural anticoagulation management. However, vascular-related injury, resulting in hematoma, pseudoaneurysm, and arteriovenous fistula, remains among the most common complications and is exacerbated by the effects of anticoagulation. Such clinical sequelae directly impact patient morbidity and can prolong hospitalization. Moreover, vascular complications can necessitate interruption of post-procedural anticoagulation and indirectly increase thromboembolic risk during the post-procedure period.
Ultrasound imaging allows direct visualization of peripheral arterial and venous anatomy. Ultrasound guidance is frequently used for cannulation of the internal jugular and subclavian vessels, as it has been shown to reduce such vascular complications [4, 5]. No prior studies have evaluated the clinical utility of ultrasound guidance for femoral venipuncture, particularly in the setting of anticoagulation. The objective of this study was to determine if the use of ultrasound during femoral venous cannulation reduces vascular complications in a large cohort of patients undergoing PVAI. In this report, we compared the vascular complication rates of patients undergoing PVAI with routine use of ultra-sound to visually guide venipuncture to rates in historical controls undergoing PVAI prior to the initiation of routine ultrasound-guided venipuncture.
2 Methods
We report the results of a retrospective analysis of data which were prospectively collected in our electrophysiology lab database between two sequential time periods (January 2005–December 2006 and July 2008–May 2010). All patients who underwent catheter-based PVAI at the Cleveland Clinic during these periods were included. Patients in the earlier cohort underwent anatomic based venipuncture [nonultrasound (US) group, n=1,909]. Ultrasound guidance was initially tested in the early 2007 and gradually phased into practice through the early part of 2008; this period was not included. All patients in the later cohort (July 2008–May 2010) underwent ultrasound-guided venipuncture (US group, n=1,511).
Venous access procedures
An 18-gauge, 7-cm length Cook bevel-tipped needle was used to access the bilateral femoral veins in all PVAI cases and, based upon operator preference, the right internal jugular vein in a small minority of cases. Two 8-French (F) sheaths were inserted using an over-the-wire technique into the right femoral vein. One 9- or 11-F sheath (depending on operator preference of intracardiac echocardiogram catheter size) and an additional 8-F sheath (if a coronary sinus catheter was used) were inserted into the left femoral vein. The right internal jugular vein was used for coronary sinus catheter placement by some operators, using similar venipuncture techniques.
Venipuncture was performed by an electrophysiology fellow under staff supervision. Venous access was acquired using surface anatomic landmarks and palpation of the femoral artery in patients in the non-US group.
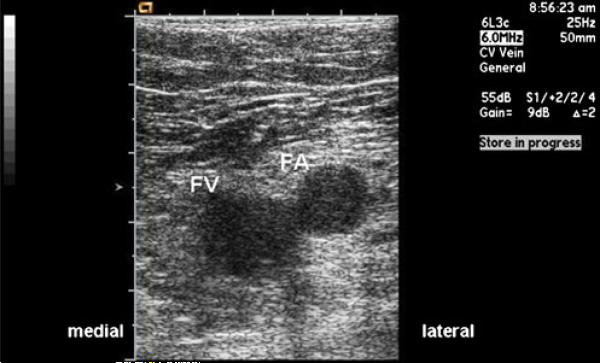
In the US group, a surface vascular ultrasound probe was inserted into a sterile plastic sleeve and used to image the femoral vasculature. Real-time ultrasound imaging of the spatial relationship of the artery and vein and of the course of the access needle visually guided venipuncture in the US group (Fig. 1).
Fig. 1.
Vascular ultrasound image showing visualization of the left femoral vein. FA femoral artery, FV femoral vein
Pulmonary vein antral isolation procedures
Details of the PVAI technique used in the present study have been published previously [6]. In brief, the left atrium was accessed via two transseptal punctures, guided by intracardiac echo-cardiography and fluoroscopy, for mapping and ablation. A heparin bolus (80–100 mg/kg) was given prior to the first transseptal puncture. Continuous heparin infusion was administered during the procedure with the rate adjusted to maintain activated clotting times in the range of 350–400 s. Complete PVAI and substrate modification were performed with radiofrequency energy delivered either via an 8-mm tip non-irrigated catheter or a 3.5-mm tip irrigated catheter. Heparin was discontinued at the end of the procedure and reversed with protamine. Vascular sheaths were removed when the activated clotting time was <250 s. Hemostasis was achieved with manual compression.
Perioperative anticoagulation
Reflective of evolving practice at our institution, starting in November 2006 pre-procedural anticoagulation with warfarin was not interrupted. As such, all patients were instructed to continue their usual maintenance doses of warfarin with a target international normalized ratio (INR) on the day of the procedure between 2.0 and 3.0. Prior to November 2006, with the exception of patients deemed at high risk for thromboembolic complication, warfarin was routinely stopped before planned PVAI and restarted the evening of the procedure. In both groups, only those patients considered to be at significant risk for thromboembolic complication and who had a subtherapeutic INR were bridged with low-molecular-weight heparin (LMWH, 1 mg/kg, administered subcutaneously twice daily) post-PVAI until their INR was at least 2.0. The decision to use bridging anticoagulation was made by the primary operator on a case-by-case basis. Post-procedure bridging with LMWH was quantified by searching the hospital inpatient pharmacy database, which records dispensed medications. For the purposes of analyses, a normal prothrombin international normalized ratio was defined as an INR of less than 1.2.
Follow-up and data collection
Groin access site checks were performed serially throughout the PVAI procedure, upon completion of the procedure and on the following day prior to discharge. Physical exam findings of groin swelling, pain, or bruit necessitated further evaluation with duplex ultrasound. Vascular access complications, including hematoma, pseudoaneurysm, and arteriovenous fistula, were categorized as major if they resulted in prolongation of hospitalization, repeat hospitalization, blood transfusion, percutaneous thrombin injection, or surgical intervention. All other complications were recorded and further cataloged as nonvascular access bleeding (pericardial effusion/ tamponade) or non-bleeding (transient ischemia attack, stroke, pulmonary vein stenosis, and phrenic nerve injury) complications.
All patients were instructed to notify us with any complications related to the procedure and to call with concerns regarding symptoms possibly related to a procedural complication. All patients were urged to return for follow-up 3–4 months post-procedure with a cardiac computed tomography (CT) scan to evaluate for pulmonary vein stenosis and a clinic visit with the electrophysiologist who performed the PVAI. For those who could not return readily, due to the extended travel distance, a report of the local follow-up visit with their physician and images of their CT scan were obtained for our records. All procedure-related complications were entered in our electrophysiology laboratory database.
Statistical analyses
Categorical variables are expressed as percentages, and continuous variables are reported as means ± standard error of the mean, unless otherwise stated. Differences between groups were determined with chi-square tests for categorical variables and Student's t tests for continuous variables. Results were considered statistically significant at p values of <0.05.
3 Results
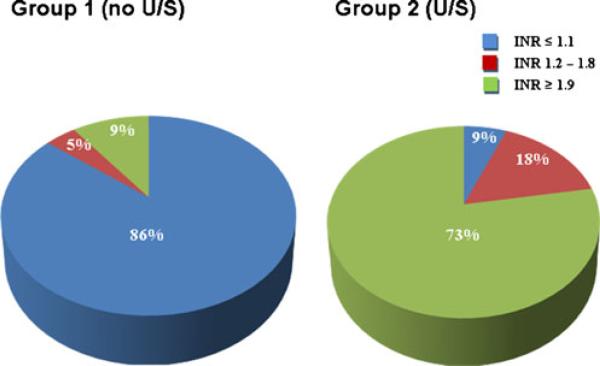
In a total of 3,420 patients who underwent PVAI, ultrasound guidance was used for femoral access in 1,511 patients and not used in 1,909 patients. Patient characteristics are shown in Table 1. In the US group, 91 % of subjects underwent PVAI with active warfarin therapy and had an INR≥1.2 on the day of the procedure. Prior to November 2006, warfarin therapy was uninterrupted only in subjects deemed to be at increased risk of thromboembolic complication. Only 14 % of the non-US group had an INR≥1.2 on the day of the procedure. This difference was statistically significant, p<0.01 (Fig. 2).
Table 1.
Baseline characteristics
| Non-US n = 1,909 (%) | US n = 1,511 (%) | p value | |
|---|---|---|---|
| Age of >75 years | 84 (4) | 82 (5) | NS |
| Female gender | 410 (21) | 355 (23) | NS |
| Creatinine of >2 mg/dl | 3 (0.2) | 3 (0.2) | NS |
| Platelet count of <100,000 | 8 (0.4) | 12 (0.8) | NS |
| Aspirin | 395 (21) | 604 (40) | <0.001 |
| Clopidogrel | 38 (2) | 60 (4) | <0.001 |
| INRof ≥1.2 day of PVAI | 279 (14) | 1,382 (91) | <0.001 |
| LMWH bridging post-PVAI | 238 (12) | 158 (10) | NS |
Non-US non-ultrasound guidance puncture, US ultrasound guidance puncture, INR international normalized ratio, LMWH low-molecular-weight heparin (1 mg/kg subcutaneous twice daily), PVAI pulmonary vein antral isolation
Fig. 2.
Procedural warfarin therapy was significantly more prevalent in the ultrasound-guided puncture group. Of the patients, 91 % in the ultrasound guidance group had an INR≥1.2 on the day of the procedure, and 14 % of patients in the non-ultrasound guidance group had an INR≥1.2 on the day of the procedure (p<0.01)
High-risk subjects in both groups received post-procedure LMWH, 1 mg/kg subcutaneously twice daily, if the INR on the day of the PVAI was subtherapeutic. The decision for bridging anticoagulation was made by the primary operator on a case-by-case basis. LMWH bridging was employed as a strategy in 12 % of subjects in the non-US group and 10 % in the US group; p is not significant (NS).
Use of ultrasound guidance resulted in significant reductions in total and major vascular access-related bleeding complications (Table 2). Despite the more prevalent use of warfarin in the US group compared to the non-US group, reflected in the higher mean INR (2.3±0.02 vs. 1.3±0.02, p<0.01) and greater fraction of subjects with an INR≥1.2 (91 vs. 14 %, p<0.01), total vascular complications were reduced. Overall, eight patients (0.5 %) in the US group and 32 patients (1.7 %) in the non-US group experienced a vascular complication, p≤0.01. A major vascular complication, requiring additional hospitalization or intervention, occurred in only two patients (0.1 %) in the US group, compared to 13 patients (0.7 %) in the non-US group, p≤0.01.
Table 2.
Clinical outcomes
| Non-US n = 1,909 (%) | US n = 1,511 (%) | p value | |
|---|---|---|---|
| Vascular complications | 32 (1.7) | 8 (0.5) | <0.01 |
| Major | 13 (0.7) | 2 (0.1) | <0.01 |
| Hematoma | 6 | 1 | |
| Arteriovenous fistula | 4 | 0 | |
| Pseudoaneurysm | 3 | 0 | |
| Retroperitoneal bleed | 0 | 1 | |
| Minor | 19 (1.0) | 6 (0.4) | <0.05 |
| Hematoma | 17 | 3 | |
| Arteriovenous fistula | 1 | 1 | |
| Pseudoaneurysm | 1 | 2 | |
| Nonvascular bleeding complications | 18 (0.9) | 15 (1.0) | NS |
| Pericardial effusion/tamponade | 17 | 15 | |
| Hemothorax | 1 | 0 | |
| Non-bleeding complications | 13 (0.7) | 4 (0.3) | NS |
| Embolic stroke | 8 | 0 | |
| Pulmonary vein stenosis | 5 | 2 | |
| Other | 0 | 2 |
Non-US non-ultrasound guidance puncture, US ultrasound guidance puncture
The incidence of nonvascular bleeding complications was not different between the groups (1.0 vs. 0.9 %, p=NS). Moreover, non-bleeding complications were also similar between the groups (0.3 vs. 0.7 %, p=NS).
Of the 279 patients in the non-US group who were receiving warfarin with INR≥1.2, there were 12 vascular complications (4.3 %). Of the 1,630 patients in the non-US group who were not on warfarin (INR<1.2), there were 20 vascular complications (1.2 %). Warfarin use was associated with a 3.5-fold increase in vascular complications (p<0.01).
Ultrasound guidance in patients on warfarin significantly reduced the incidence of a vascular complication. There were 12 vascular complications (4.3 %) among the 279 non-US patients with INR≥1.2. In contrast, there were only seven vascular complications (0.5 %) among the 1,382 US-guided patients with an INR≥1.2. This ninefold reduction in vascular complication rate suggests a benefit of ultrasound that overcomes the increased risk of warfarin use (p<0.01). Moreover, patients who were maintained on warfarin and who underwent ultrasound-guided venipuncture were still two times less likely to have a vascular complication than those in whom warfarin was stopped and ultrasound was not used (0.5 vs. 1.2 %, p<0.05).
4 Discussion
In our single-center experience of a large group of patients who underwent PVAI, real-time ultrasound to guide femoral vein cannulation resulted in a significant threefold reduction in the total number of vascular complications and a sevenfold reduction in the number of major vascular complications. The use of ultrasound allows the operator to appreciate anatomic variations in arterial and venous spatial relationships, as well as of the vessels themselves. Such imaging provides visualization of the access needle tip course and trajectory. As a result of our experience with vascular complications reported here, currently, all operators at the Cleveland Clinic routinely use ultrasound guidance to gain femoral venous access in all electrophysiology procedures.
Since November 2006, pre-procedure warfarin therapy has routinely been maintained with a target INR of 2.0 to 3.0 on the day of the procedure day. This practice is supported by data from Wazni et al. [7] and Hussein et al. [9], demonstrating that periprocedural continuation of warfarin not only confers protection against thromboembolic events but is also safe, as evidenced by the overall low rates of bleeding complications. Ultrasound guidance was being phased in at our institution, beginning in 2007. As such, the subset of patients from the Cleveland Clinic in those reports who underwent PVAI and in whom anticoagulation was uninterrupted were likely to have undergone the procedure with US-guided venipuncture. The relatively low rates of vascular complications reported in that study could, in part, be due to obviating the need for bridging with LMWH. Our data, however, indicate that the use of ultrasound guidance likely played a more important role in reducing vascular complications.
In the present study, patients on warfarin (INR≥1.2 on the day of the PVAI) who underwent venipuncture without ultra-sound guidance had the highest vascular complication risk. The addition of ultrasound guidance in such anticoagulated patients reduced vascular complications by ninefold. Strikingly, these high-risk anticoagulated patients, in whom ultra-sound was used, had an even lower vascular complication rate than patients not taking warfarin (INR<1.2) and not undergoing ultrasound guided venipuncture.
A comprehensive survey of 181 electrophysiology centers worldwide and a multicenter Italian registry reported 4 to 6 % combined rates of major complications [2, 3]. Among the most serious of all complications is cerebral embolism, the occurrence of which has significantly decreased with advances in pre-, intra-, and post-procedure anticoagulation management [7–10]. However, with the need for maintaining anticoagulation, bleeding risks inevitably remained considerable. The incidence of clinically relevant peripheral vascular complications causing pain, excessive blood loss, intervention, and/or prolonged hospitalization has been reported to be 1.5–8.4 % [2, 3, 7–12]. In the current study, the incidence of vascular complications of 1.7 % when ultrasound guidance was not utilized is consistent with these previously published reports. The incidence of vascular complications was reduced to 0.5 % when ultra-sound guidance was used.
The clinical utility of real-time ultrasound has been described previously by the Agency of Healthcare Research and Quality in the recent Analysis of Patient Safety Practices report [13]. Though systematic evaluation of the use of ultrasound guidance for femoral venous cannulation was limited, its benefit was noted predominantly in high-risk patients [14]. Recently, ultrasound guidance was shown to facilitate femoral artery cannulation and decrease bleeding complications in patients undergoing left heart catheterization and coronary angiography [15]. In light of the fact that catheter-based ablation for atrial fibrillation necessitates anticoagulation both intra- and post-procedure, it is logical that ultrasound can minimize the risk of inadvertent arterial and venous trauma and thus reduce vascular complications. In our experience, use of ultrasound allows visualization of closely proximate venous branching points and arterial branches. Avoidance of these structures appears to minimize the bleeding risks associated with insertion of sheaths. Moreover, visualization of the vein during the puncture allows more reliable insertion of the needle tip into the center of the vein and avoidance of a glancing approach towards the periphery of the vessel.
Other approaches might also reduce femoral venous access-related vascular complications. Abhishek et al. [16] reported a significant drop in such when they avoided arterial puncture and used a micropuncture technique for venous access. They reported no major vascular complication in 162 patients utilizing this modified technique. Conceivably, a micropuncture technique combined with ultrasound visualization could minimize vascular access complications in a complimentary fashion, especially in the high-risk anticoagulated patient.
4.1 Study limitations
A limitation of our study is the nonrandomized design and the use of historical controls. However, the use of ultrasound at our institution resulted in significant safety improvements that became evident early in the evolution of our use of this technique, rendering a randomized trial unacceptable to us. Thus, we used sequential enrollment of patients over two time periods during which ultrasound was not used and had routinely become incorporated as a standard practice for the PVAI procedure at our institution. During these periods, anticoagulation practice also evolved, such that there was a significantly greater fraction of patients in the later ultra-sound cohort receiving warfarin on the day of the PVAI. Despite this potential confounding effect, which could have negated the beneficial effects of ultrasound, the merit of ultrasound remained evident in our results.
Routine anticoagulation bridging with LMWH is not the standard practice at our institution. It is employed only in those patients perceived to be at high risk for thromboembolism with low INR. Data acquired from the inpatient pharmacy database revealed that a similar ratio of patients in each cohort were administered LMWH bridging. While experience in using heparin or LMWH post-device implant or extraction has been noted to increase bleeding complications, univariate analysis of our data did not show this to be a significant variable contributing to the difference in vascular complications between the two groups. This lack of statistical significance may, in part, be due to the comparably smaller number of patients who received this type of post-procedural anticoagulation.
Though operator experience in the performance of PVAI was likely gained over time, we do not believe that it contributed to the improved vascular outcomes in the later cohort. The occurrence of technique-mediated complications, both bleeding (nonvascular) and non-bleeding type, was similar between the groups. Improvement in catheter skills and transseptal cannulation is unlikely to directly affect vascular complications from standard femoral sheath insertion. Veni-puncture is a well-established, basic procedural skill that is already acquired by the majority of advanced cardiovascular trainees that enter our electrophysiology fellowship program. As such, the learning curve is typically not steep. Though we cannot quantify variation in technique and skill level between individuals in the two study periods, we minimized any potential difference in our comparison groups by choosing similar time periods of fellowship training.
Lastly, we cannot exclude the possibility that operators used greater care, perhaps subconsciously, in “high-risk” anticoagulated patients. Yet, the fact that the non-US group with INR≥1.2 had the highest vascular complication rate argues against this premise and also demonstrates that any such added attention failed to mitigate this risk.
4.2 Clinical implications
Real-time ultrasound guidance for central venous access significantly improves the safety profile of PVAI by reducing the risk of overall and major vascular complications. In conjunction with our current protocol in which patients undergo the procedure with a therapeutic INR, ultrasound significantly minimizes the risk of vascular trauma. In turn, the prevention of post-procedure vascular complications allows for safe continuation of anticoagulation without interruption.
Abbreviations
- PVAI
Pulmonary vein antral isolation
- AF
Atrial fibrillation
- AV
Arteriovenous
- F
French
- mg/kg
Milligram per kilogram
- mg
Milligram
- s
Seconds
- mm
Millimeter
- INR
International normalized ratio
- CT
Computed tomography
- OR
Odds ratio
- mg/dl
Milligram per deciliter
- US
Ultrasound
Footnotes
Conflict of interest Dr. Christine C. Tanaka-Esposito, Dr. Patrick J. Tchou, and Dr. Daniel J. Cantillon have received modest honorarium from Medtronic, Inc.
References
- 1.ACC/AHA/EHS 2006 Guidelines for management of patients with atrial fibrillation. JACC Cardiovasc Interv. 2006;48(4):e149–246. [Google Scholar]
- 2.Cappato R, Calkins H, Chen S, et al. Worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 3.Bertaglia E, Zoppo F, Tondo C, et al. Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multi-center prospective registry on procedural safety. Heart Rhythm. 2007;4:1265–1271. doi: 10.1016/j.hrthm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Ortega R, Song M, Hansen C, et al. Ultrasound-guided internal jugular vein cannulation. The New England Journal of Medicine. 2010;362:e57. doi: 10.1056/NEJMvcm0810156. [DOI] [PubMed] [Google Scholar]
- 5.Braner D, Lai S, Eman S, et al. Central venous catheterization—subclavian vein. The New England Journal of Medicine. 2007;357:e26. doi: 10.1056/NEJMvcm074357. [DOI] [PubMed] [Google Scholar]
- 6.Kanj M, Wazni O, Natale A. How to do circular mapping catheter-guided pulmonary vein antrum isolation: the Cleveland Clinic approach. Heart Rhythm. 2006;3:866–869. doi: 10.1016/j.hrthm.2006.02.1033. [DOI] [PubMed] [Google Scholar]
- 7.Wazni O, Beheiry S, Fahmy T, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio. Circulation. 2007;116:2531–2534. doi: 10.1161/CIRCULATIONAHA.107.727784. [DOI] [PubMed] [Google Scholar]
- 8.Ren J, Marchlinski F, Callans D, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast. Journal of Cardiovascular Electrophysiology. 2005;16:474–477. doi: 10.1046/j.1540-8167.2005.40465.x. [DOI] [PubMed] [Google Scholar]
- 9.Hussein A, Martin D, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm. 2009;6:1425–1429. doi: 10.1016/j.hrthm.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Segerson N, Marschang H, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratios. Pacing Clin Electrophysiol. 2009;32:995–999. doi: 10.1111/j.1540-8159.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 11.Spragg D, Dalal D, Cheema A, et al. Complications of catheter ablation for atrial fibrillation incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627–631. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 12.Prudente L, Moorman R, Lake D, et al. Femoral vascular complications following catheter ablation of atrial fibrillation. J Inter Card Electro. 2009;26:59–64. doi: 10.1007/s10840-009-9402-y. [DOI] [PubMed] [Google Scholar]
- 13.Agency of Healthcare Research and Quality Making heath care safer: a critical analysis of patient safety practices. 2001 http:// www.ahcpr.gov/clinic/ptsafety/chap21.htm.
- 14.Hilty W, Hudson P, Levitt M, et al. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Annals of Emergency Medicine. 1997;29:331–336. doi: 10.1016/s0196-0644(97)70344-5. [DOI] [PubMed] [Google Scholar]
- 15.Seto A, Abu-Fadel M, Sparling J, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access with Ultrasound Trial). JACC Cardiovasc Interv. 2010;7:751–758. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Abhishek F, Heist E, Barrett C, et al. Effectiveness of a strategy to reduce major vascular complications from catheter ablation of atrial fibrillation. Journal of Interventional Cardiac Electrophysiology. 2011;30:211–215. doi: 10.1007/s10840-010-9539-8. [DOI] [PubMed] [Google Scholar]