Introduction
Polypharmacy is a major concern in older adults, yet a simple cross-sectional count of medications does not capture the potential complications (and benefits) that occur when medications are started, stopped, and changed over time. The increased complexity that arises from multiple medication changes may lead to problems with adherence and confusion about proper medication use.[Van Wijk, Choudhry] In addition, because adverse drug reactions often occur relatively soon after a medication is started, patients with recent medication changes may be at increased risk of adverse drug events.[Forster] In this study, we sought to longitudinally measure medication changes in a national sample of older veterans as a measure of prescribing complexity in older adults.
Methods
We used Veteran Affairs (VA) outpatient pharmacy data combined with VA and Medicare outpatient and inpatient claims data from fiscal year 2007 and 2008 to identify persons enrolled in the VA who were age 65 or older, received one or more chronic medications from VA as of April 1, 2007, and were alive and continuing to receive medications from VA more than one year later. We restricted the sample to patients who obtained 80% or more of their outpatient primary care and medicine subspecialty visits in the VA health system. We focused on medications intended for chronic, regular use, defined as medications dispensed with a supply of 25 or more days and no indication to use “as needed.”
We defined six types of medication changes. Additions were medications that were not present at study baseline and were started over the following year. Restarts were similar to additions, but comprised medications that had previously been used, were not present at baseline, but which were subsequently restarted after an extended period of non-use. Discontinuations were medications that were present during or beyond baseline but were subsequently not refilled up through 6 months beyond the study period. Disruptions were interruptions of medication refills for ≥ 6 months that were subsequently refilled. Dose changes reflected changes in the daily dose of a medication, and intraclass substitutions comprised replacement of a medication with another drug in the same drug class.
Results
Among 350,415 veterans, mean age was 74 ± 6 years, 98% were male, and 14% were hospitalized over the year of follow-up. At baseline, patients were using a mean of 4.5 ± 2.6 chronic medications. One year later, patients were using a mean of 4.6 ± 2.8 medications, with 77% of patients were taking no greater than 1 more or 1 fewer drugs than one year prior.
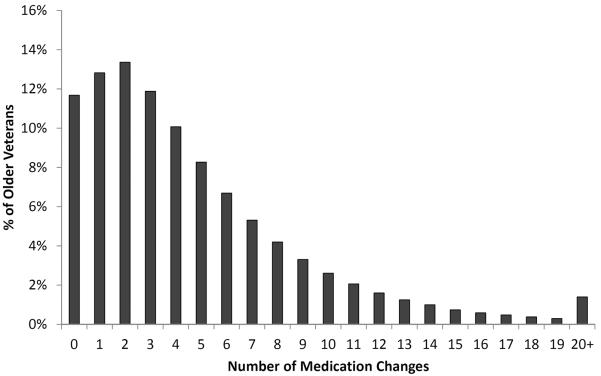
Despite little change in the total number of medications, patients had a mean of 4.7 ± 4.6 medication changes over the one-year study period. Overall, 88% of patients had at least one medication change and 12% of patients had ten or more medication changes (Figure). Medication additions were the most common type of change, being observed in 61% of patients, but other types of changes were common as well (Table).
Figure 3. Frequency of medication changes in older veterans over 1 year of follow-up.
12% of patients had 10 or more medication changes over one year.
Table 1.
Cross-sectional and cumulative changes in medication use over one year of follow-up
| Serial cross sectional characteristics | |
| Number of medications at baseline (mean, SD) | 4.5 ± 2.6 |
| Number of medications at 1-year follow-up (mean, SD) | 4.6 ± 2.8 |
| Cumulative changes over one year | |
| Mean number of medication changes (mean, SD) | 4.7 ± 4.6 |
| Patients with any medication changes | 309,496 (88%) |
| Additions | 213,506 (61%) |
| Restarts | 74,744 (21%) |
| Discontinuations | 202,567 (58%) |
| Disruptions | 97,912 (28%) |
| Dose changes | 178,291 (51%) |
| Intraclass substitution s | 88,471 (25%) |
The number of medications used at baseline was associated with the number of medications changed over the following year (incidence rate ratio 1.13, 95% CI 1.13–1.13). However, this association was not sufficiently robust that baseline medication use could strongly predict the subsequent rate of medication changes. For example, among patients taking 2 medications at baseline, the median number of changes was 2, but one-quarter of these patients had 5 or more medication changes. In contrast, among patients taking 10 medications at baseline, the median number of medication changes was 8, but one-quarter of these patients had 4 or fewer medication changes.
Comment
In this nationwide study, older adults often had multiple drugs started, stopped, or otherwise changed, with a mean of 4.7 medication changes per patient over a one-period. In contrast, cross sectional counts of the number of medications taken at baseline and one year were highly similar.
In older adults, a simple count of medications is often used to identify patients at elevated risk of medication-related problems (“polypharmacy”). However, this approach does not fully capture the true complexity of medication regimens and the potential for adverse events, patient and clinician confusion, and medication non-adherence that can arise when medications are started, stopped, and changed.[MRCI paper; Solem] It is important to note that multiple mediation changes may often benefit patients as appropriate responses to changing patient circumstances, and that future validation of our work is needed. Nonetheless, the number of medication changes may serve as a novel, valuable, and readily measurable marker of patients at high risk of medication-related problems, and help identify patients who should be targeted for close attention and follow-up.
Acknowledgements
The authors thank Kathy Z. Fung, MS, for her assistance compiling data for this manuscript and the residents of the Primary Medical Education Program (PRIME) program at the University of California, San Francisco and the San Francisco VA Medical Center for their feedback on manuscript drafts.
Funding/Support: This research was supported by grants from the National Institute on Aging (RC1-AG036377) and from the National Institute on Aging and the American Federation for Aging Research (1K23-AG030999).
Role of sponsors: The funders had no role in the design, conduct, analyses, authorship, or decision to publish this manuscript.
Footnotes
Author contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest disclosures: none
The authors have no financial conflicts of interest with the topics discussed in this manuscript.
References
- 1.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171(9):814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 2.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349. [PMC free article] [PubMed] [Google Scholar]
- 4.Van Wijk BLG, Klungel OH, Heerdink ER, de Boer A. The association between compliance with antihypertensive drugs and modification of antihypertensive drug regimen. J Hypertens. 2004;22(9):1831–1837. doi: 10.1097/00004872-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Solem CT, Lee TA, Joo MJ, Lambert BL, Walton SM, Pickard AS. Complexity of medication use in newly diagnosed chronic obstructive pulmonary disease patients. AJGP. 2012;10(2):110–122. doi: 10.1016/j.amjopharm.2011.12.003. [DOI] [PubMed] [Google Scholar]