Abstract
Background
Extracellular matrix (ECM) is a dynamic and complex environment characterized by biophysical, mechanical and biochemical properties specific for each tissue and able to regulate cell behavior. Stem cells have a key role in the maintenance and regeneration of tissues and they are located in a specific microenvironment, defined as niche.
Scope of review
We overview the progresses that have been made in elucidating stem cell niches and discuss the mechanisms by which ECM affects stem cell behavior. We also summarize the current tools and experimental models for studying ECM–stem cell interactions.
Major conclusions
ECM represents an essential player in stem cell niche, since it can directly or indirectly modulate the maintenance, proliferation, self-renewal and differentiation of stem cells. Several ECM molecules play regulatory functions for different types of stem cells, and based on its molecular composition the ECM can be deposited and finely tuned for providing the most appropriate niche for stem cells in the various tissues. Engineered biomaterials able to mimic the in vivo characteristics of stem cell niche provide suitable in vitro tools for dissecting the different roles exerted by the ECM and its molecular components on stem cell behavior.
General significance
ECM is a key component of stem cell niches and is involved in various aspects of stem cell behavior, thus having a major impact on tissue homeostasis and regeneration under physiological and pathological conditions. This article is part of a Special Issue entitled Matrix-mediated cell behaviour and properties.
Abbreviations: ECM, extracellular matrix; CBCs, crypt base columnar cells; HFSCs, hair follicle stem cells; HSCs, hematopoietic stem cells; ISCs, intestinal stem cells; NSCs, neural stem cells; SGZ, subgranular zone; SVZ, subventricular zone
Keywords: Extracellular matrix, Stem cell, Stem cell niche, Cell receptor, Growth factor, Tissue engineering
Highlights
-
•
Stem cells have a key role in the maintenance and regeneration of tissues.
-
•
The extracellular matrix is a critical regulator of stem cell function.
-
•
Stem cells reside in a dynamic and specialized microenvironment denoted as niche.
-
•
The extracellular matrix represents an essential component of stem cell niches.
-
•
Bioengineered niches can be used for investigating stem cell–matrix interactions.
1. Introduction
All cell types are in contact with the ECM, a complex and dynamic network of macromolecules with different physical and biochemical properties [1], [2]. Although the ECM was once considered an inert supportive scaffold, the fundamental role of ECM in key aspects of cell biology became increasingly evident in last two decades. By either direct or indirect action, ECM regulates cell behavior and plays essential roles during development [3]. Indeed, the ECM is a dynamic and versatile compartment and by modulating the production, degradation, and remodeling of its components, it can support organ development, function and repairing [4], [5]. On the basis of the relative amounts and organization of the different ECM components, this molecular scaffold is peculiar for each tissue and reflects the specific functions required for the cells present in that tissue. Moreover, the structural, biochemical and functional diversity of ECM components confers well-defined physical, biochemical and biomechanical properties to the ECM. Physical properties such as rigidity, porosity, topography and insolubility are able to influence various anchorage-related biological functions, like cell division, tissue polarity and cell migration [6]. From a biochemical point of view, the ECM displays both direct and indirect signaling properties, since it can act directly by binding cell surface receptors or by non-canonical growth factor presentation [3]. A key concept in ECM biology regards how the biomechanical properties of the ECM can influence cell behavior. Indeed, ECM stiffness is an essential property by which cells sense the external forces and respond to the environment in an appropriate manner, a process known as mechanotransduction [7], [8], [9], [10], [11], [12], [13] (Fig. 1). Importantly, all these characteristics and properties are strongly interconnected and one can influence the others. This becomes even more evident when considering that cell–ECM connection is a reciprocal interaction in which cells continually remodel the ECM present in their microenvironment, and these dynamic modifications of the ECM direct cell behavior [3]. It is therefore not surprising that alterations in a specific ECM component or in a player of its regulation can have a remarkable impact on the biochemical, biomechanical and physical properties of the ECM, leading to disorganized network and ultimately failure of organ homeostasis and function.
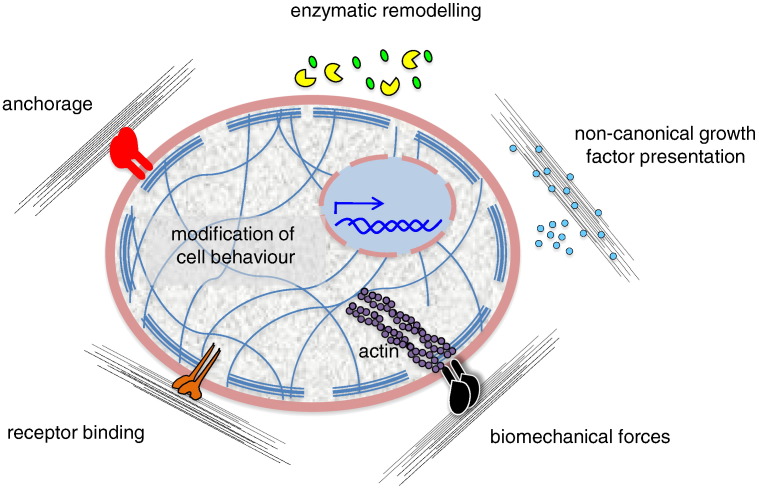
Fig. 1.
Regulation of cell behavior by ECM. The effects exerted on cells by ECM can be differently mediated. The ECM can directly bind different types of cell surface receptors or co-receptors (red, orange, black), thus mediating cell anchorage and regulating several pathways involved in intracellular signaling and mechanotransduction. Moreover, the ECM can act by non-canonical growth factor (cyan) presentation and be remodeled by the action of enzymes (yellow pie), which can release functional fragments (green).
Stem cells are defined by three essential features, as they are i) undifferentiated cells able to give rise both to ii) differentiated daughter cells and iii) cells that retain their stemness by self-renewal [14]. Embryonic and adult stem cells have different capabilities to produce differentiated cells, a property known as potency. Cells present in the early embryo until the blastocyst stage are pluripotent, since they produce all differentiated cell types present in the body, whereas fetal and adult stem cells are able to produce multiple cell lineages (multipotent) or a single differentiated cell lineage (unipotent). In adult tissues, stem cells are usually in a quiescent state and in order to undergo self-renewal they have to enter the cell cycle, divide and generate a progeny of undifferentiated cells [15], [16]. With this mechanism, the stem cell pool is maintained and the long-term homeostasis and regeneration of tissues can be preserved throughout the entire lifespan [17]. The choice of a stem cell to undergo self-renewal is carried out by two cell division mechanisms, which fulfill two different requests by the tissue [18]: i) asymmetric self-renewal, in which each stem cell divides into one stem and one differentiated cell, allows maintaining a constant number of stem cells, which is generally sufficient under physiological conditions; ii) symmetric self-renewal, in which each stem cell originates two daughter stem cells, leads to an expansion of the stem cell pool, a condition required after tissue injury or in diseased conditions causing loss of differentiated cells [19]. In the asymmetric cell division, the mitotic process leads to polarization and asymmetric segregation of components essential for the cell fate determination so that, once cell division is completed, one daughter cell has received RNAs, proteins and other molecules that maintain the undifferentiated program, whereas the other cell receives lineage commitment factors. In the symmetric cell division, the two daughter cells receive the same factors and the decision for commitment and differentiation is not linked to mitosis, rather it is a later event that can involve the newly formed cells [17]. Symmetric or asymmetric divisions are not mutually exclusive, and a mixture of these two mechanisms can be used on subsequent divisions. During mid to late gestation, some mammalian progenitor cells are able to make a developmentally regulated transition from largely symmetric to predominantly asymmetric divisions. Similarly, adult stem cells dividing asymmetrically under steady-state conditions retain the capability to divide symmetrically to restore stem cell pools depleted by injury or disease [19].
Stem cells reside in a dynamic, specialized microenvironment, denoted as ‘niche’, which provides extracellular cues to allow stem cell survival and identity. Moreover, the niche dynamically regulates stem cell behavior, maintaining a balance between quiescence, self-renewal and differentiation [20], [21]. Despite their high potential to proliferate, the niche maintains stem cells in a quiescent and low metabolic state to prevent stem cell exhaustion [22]. Moreover, the niche is thought to protect stem cells from the accumulation of gene mutations that may lead to their malignant transformation into cancer cells [23]. Increasing evidence indicates that deregulation of the stem cell niche plays a key pathogenic role in a number of diseases associated with tissue degeneration, aging and tumorigenesis [24]. Both quiescent and active stem cell subpopulations coexist in several tissues, in separate yet adjoining locations [15], [23]. In these niches, the precise regulation of the balance between symmetric and asymmetric divisions is critical for maintaining proper stem cell number and for fulfilling the needs for differentiated cells within the surrounding tissue [20]. The ability of a stem cell to seed in its niche represents one of the most important features of the niche itself, and the proper binding between stem cells and their niche is essential to maintain the stem cell pool for long-term self-renewal. Thus, the niche establishes a sort of crosstalk between the state and necessity of the tissue and the proper functioning of the stem cell pool [25], [26]. Since its first definition originally proposed in 1978 for the hematopoietic microenvironment [27], the concept of the niche has increased in complexity (Fig. 2). Niches are highly specialized for each type of stem cell, with a defined anatomical localization, and they are composed by stem cells and by supportive stromal cells (which interact each other through cell surface receptors, gap junctions and soluble factors), together with the ECM in which they are located. Moreover, blood vessels carry systemic signals and provide a conduit for the recruitment of inflammatory and other circulating cells into the niche, whereas neural inputs transmit distant physiological cues to the stem cell microenvironment. The diverse and dynamic composition of the ECM provides controlled biochemical, physical, structural, and mechanical properties to the different niches. In addition, secreted or cell surface factors, signaling cascades and gradients, as well as physical factors, such as shear stress, oxygen tension and temperature, contribute to control stem cell behavior in a well-orchestrated manner [25], [26]. Not only the niche components influence stem cell behavior, but also the interactions between stem cells and their niche are reciprocal, since stem cells are able to remodel the niche and secrete ECM components in response to the signals they receive from it [28], [29], [30].
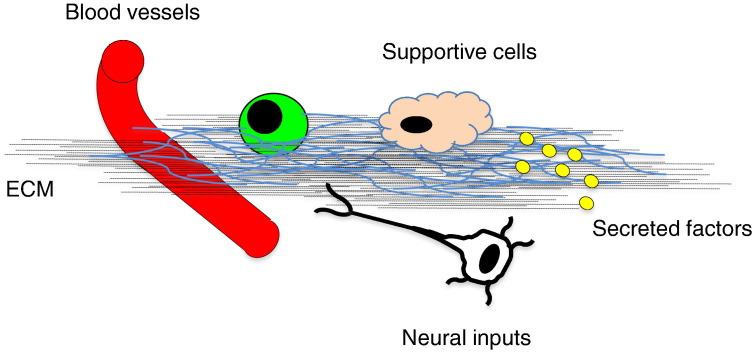
Fig. 2.
Players in stem cell niche. The stem cell niche is a specialized and dynamic microenvironment in which a number of inputs regulate stem cell (green) behavior. These include signals departing from blood vessels, neural and supportive cells, as well as secreted factors and ECM components.
In this review, we will discuss the role of the niche in stem cell homeostasis, highlighting the functions of the ECM in determining niche properties. We focus on how ECM is able to mediate its effects on stem cells, starting from describing the players involved in ECM–stem cell interaction and the role of ECM in non-canonical growth factor presentation, and then examining the role of mechanotransduction and stiffness on cell fate determination. We also summarize the tools used for investigating ECM–stem cell interaction, discuss the properties of engineered niches, and conclude by recapitulating the current knowledge on specific stem cell niches.
2. Role of ECM in stem cell niche
As a constitutive part of the niche, ECM components are key players of the niche instructive power. These extracellular macromolecules, by their assembly and three-dimensional organization, supply a microenvironment in which the signals deriving from cell–ECM interaction, as well as soluble and ECM-bound factors, are integrated in a functional manner to permit the maintenance of stem cell homeostasis [31], [32], [33], [34]. An in vivo evidence supporting the relevance of ECM in stem cell behavior is provided by the reduced ability of altered or aged niches in maintaining stem cell properties [30]. Experiments performed with decellularized tissues, in which the ECM is preserved, represent a further and direct demonstration of the primary role of ECM in the regulation of stem cell properties. These studies demonstrated that natural ECM scaffolds, derived from decellularized tissues, guide stem cell differentiation into the cell types residing in the tissue from which the ECM was derived [35]. On the basis of these properties, decellularized organs have been used in tissue engineering and for developing cell therapy approaches [36], [37].
2.1. ECM–stem cell interaction
Interactions between ECM and stem cells can be directly mediated by a number of cell receptors, including integrins and other receptors. Despite the wide range of putative receptors involved in ECM–stem cell communication, only relatively few studies were focused on the role of non-integrin receptors, as for CD44, which was found to play an important role in the homing of hematopoietic stem cells (HSCs) during transplantation [38], and for Robo4, an axon guidance receptor which was shown to play a role in HSC adhesion to the niche during competitive repopulation assays [39]. On the other hand, an increasing number of studies demonstrated that integrins are key receptors involved in ECM–stem cell interactions and in the adhesion, anchorage and homing of stem cells. Integrins represent a large family of heterodimeric transmembrane receptors that connect the extracellular environment to the intracellular cytoskeleton, thus mediating cell migration, proliferation, survival and differentiation [40]. Different types of integrins are involved in the direct binding to a number of ECM components or to other cell surface adhesion molecules and receptors [41], [42], [43]. Integrins can directly activate downstream signaling via focal adhesion kinase (FAK) and phosphoinositide 3-kinase (PI3K), thus regulating the self-renewal and proliferation of a large number of different stem cells [6], [40], [44]. Follicular stem cells of Drosophila ovary require integrin-mediated interaction for their anchorage to the niche and for their proper self-renewal and asymmetric cell division [45]. The α6β1 integrin, which binds the ECM protein laminin, is essential to home spermatogonial stem cells in the testicular niche [46], and is required for the adhesion of neural stem cells (NSCs) to their vascular niche [47]. The α9 integrin chain, which binds the ECM protein tenascin-C, plays a role in HSC proliferation [48] and in the NSC niche [49]. Moreover, the α4, α6, α9 and β1 integrin chains play essential roles in homing HSCs to the bone marrow niche of irradiated recipient mice [50], [51], [52], [53]. In addition, also αvβ3 integrin regulates HSC homing and proliferation and its expression is mediated by the cytokine ligand thrombopoietin [54], [55], [56]. In the hair follicle, bulge stem cells produce the ECM protein nephronectin, which by interacting with the α8β1 integrin receptor present on the arrector pili muscle maintains the correct position and function of hair follicle stem cells (HFSCs) [57]. In the skeletal muscle, muscle stem cells (also known as satellite cells) interact on one side of their niche with the basal lamina through α7β1 integrin, and on the other side with myofiber plasma membrane through M-cadherin [58]. Furthermore, β1 integrins were found to be essential in preserving the pool of different types of stem cells, by controlling the balance between symmetric and asymmetric divisions (like in skin and brain), as well as stem cell self-renewal and differentiation [59], [60], [61], [62]. Since integrins can also regulate signaling pathways in response to growth factors and cytokines, such as IL-3 and TGF-β [40], [63], [64], [65], [66], and conversely signaling pathways can regulate integrin expression [43], the specific activities of these ECM receptors need to be set within each niche for a particular type of stem cell. Some examples of the interplay between integrins and other signaling molecules were demonstrated in NSCs and in mammary stem cells, where β1 integrins were shown to regulate self-renewal and differentiation by controlling the activity of Notch and of EGF receptor [67], [68]. Moreover, β1 integrins were also found to be essential in regulating the proliferation of intestinal stem cells (ISCs), by mediating Hedgehog signaling [69].
2.2. Non-canonical growth factor presentation
Besides its ability to directly interact with stem cells, the ECM is also able to regulate stem cell activity by non-canonical growth factor presentation. Indeed, several ECM components are able to avidly bind growth factors, regulating their local availability and establishing a biochemical gradient [3]. On the one hand, the ECM can function as reservoir of growth factors, by making them insoluble, unavailable and not bioactive. Examples of this action are represented by fibronectin, vitronectin, collagens and proteoglycans, which bind FGFs, HGFs, VEGFs, BMPs and TGF-β. On the other hand, proteins and proteoglycans of the ECM can function as distributors of growth factors following the action of enzymes, such as metalloproteinases, which induce the remodeling of ECM components and permit the release of factors that were otherwise in an insoluble state [3]. One example of the non-canonical growth factor presentation exerted by the ECM in the niche regards NSCs. Indeed, the ECM that composes the neural fractones was found to promote growth factor activity in the NSC niche by capturing FGF-2 from the milieu, thus favoring NSC function [70], [71]. The same happens for the regulation of muscle satellite cells, where a number of secreted growth factors are bound to the proteoglycan components of the basal lamina or to the surface of satellite cells where they can be locally sequestered in an inactive zymogen form or presented in an active signaling state [18]. Thus, the action of ECM as ‘dispenser on demand’ of soluble biofactors well represents one of the most important features of the ECM, its dynamism.
3. Biophysical properties of the ECM: mechanotransduction, stiffness and cell fate determination
A growing body of evidence in ECM biology points at biophysical properties of the ECM as an important determinant of stem cell behavior. Indeed, every cell in its anatomical localization has to balance the external forces dictated by the mechanical properties of its environment, that results from the compression exerted by neighboring cells as well as the stiffness of the surrounding ECM. To do this, cells regulate their cytoskeleton tension, generating internal forces that are transmitted to the environment by adhesion sites [72]. The focal adhesion complexes, which include integrins, adaptors and signaling proteins, physically link the actomyosin cytoskeleton with the ECM. Together with cytoskeleton, nuclear matrix, nuclear envelope and chromatin, the focal adhesion complexes constitute a complex mechanosensing machinery that determines how cells react to forces generated from the ECM [13]. Therefore, mechanical forces are exerted on and from each single cell, and this interplay between cells and microenvironment generates an isometric tension within the cytoskeleton that allows the maintenance of cell shape and the dynamic response to the external forces, which in the end leads to the fine regulation of cell behavior [11], [72], [73]. The overall cellular response to mechanical stimuli is defined as mechanotransduction. Among a number of mechanotransduction pathways that were proposed (including Ras/MAPK, PI3K/Akt, RhoA/ROCK, Wnt/β-catenin, and TGF-β pathways), the YAP/TAZ transcriptional factors recently emerged as downstream key mediators of the biological effects of ECM elasticity, cell geometry and cytoskeletal organization [72], [74], [75].
It is a matter of fact that stem cell behavior is dependent on tissue stiffness, which is in part regulated by ECM organization and composition [11], [13], and that tissue elasticity is altered during aging, disease and injury [18], [76]. The stiffness of the extracellular microenvironment, mainly expressed by the elastic modulus (or Young's modulus), is several orders of magnitude lower than that sensed by cells cultured onto a plastic dish or glass. Because of the difficulties in manipulating tissue stiffness in vivo, studies on the role of elasticity in regulating stem cell behavior were started together with the development of in vitro technologies able to mimic tissue elasticity (discussed in detail in the next paragraph). Notably, a recent study demonstrated that subtle in vivo modifications of muscle stiffness, by means of fibroblast-mediated collagen VI deposition, are able to affect muscle satellite cell self-renewal and maintenance [77]. Moreover, substrate elasticity was found to regulate muscle satellite cell self-renewal in culture [77], [78]. A number of studies demonstrated that matrices characterized by a stiffness similar to that found in the brain drive cultured stem cells into the neurogenic lineage [79]. Notably, when human mesenchymal stem cells are cultured on ECMs characterized by stiffness values that mimic the elastic moduli of brain, muscle or bone, they start expressing organ-specific transcription factors and undergo tissue-specific cell fate switches into neurons, myoblasts and osteoblasts, respectively [80]. Adult NSCs cultured on hydrogel containing fibronectin show a maximal neuronal differentiation potential when the stiffness of the biomaterials corresponds to that displayed by brain tissue, whereas stiffer gels promote their differentiation into glial cells [81]. The relevance of ECM and its biomechanical properties for in vivo NSC behavior was confirmed by the finding that there are stiffness gradients in the hippocampus. Moreover, in the presence of glial scars and brain tumors, as well as in aging, the mechanical properties change together with the behavior of neurons, NSCs and glioblastoma cells [76]. Human mesenchymal stem cells cultured on hydrogel characterized by an elastic modulus comparable to bone marrow increase their ability to self-renew and maintain multipotency, compared to mesenchymal stem cells grown on stiffer substrates [82]. The osteogenic differentiation of rat mesenchymal stem cells is strongly regulated by substrate stiffness and by the ECM macromolecules pre-adsorbed onto the biomaterials [83]. An additional example of cell behavior regulated by mechanical stiffness is cardiomyocyte commitment. These cells have been shown to correctly differentiate only on matrices that recapitulate the mechanical properties of developing cardiac microenvironment, while they are not able to develop spontaneous beating when grown on structures that mirror the property of a fibrotic scar [84]. Despite a large number of literature data pointing at substrate stiffness as an important feature in regulating stem cell fate, a recent work suggested that cells can sense ECM tethering, rather than the overall stiffness of the material on which they are located [85]. The ability of stem cells to respond to the stiffness of the collagen fibers themselves opens new insights about the role of ECM mechanical properties on the stem cell responses within tissues and niches.
4. Tools for investigating ECM–stem cell interaction and engineered stem cell niches
Ideally, the best way to investigate the function of specific ECM components in stem cell niches should be done by in vivo studies, and some recent works demonstrated the role of definite ECM components in regulating the in vivo activity of stem cells [77], [86]. However, the complexity of the niche makes these studies quite challenging and difficult. Moreover, it should be considered that the lack or a defective function of one specific ECM component may not directly affect stem cell behavior, as on the one hand it may generate side effects acting indirectly on stem cells and on the other hand such effects could be masked by compensatory mechanisms. To overcome such difficulties, scientists have started developing in vitro engineered stem cell niches, with the aim to mimic the in vivo niche more closely than traditional cell culture methods. Currently, these tools are considered the best choice for investigating ECM–stem cell interactions in vitro, since they can not only deconstruct the effects of specific ECM molecules on the single-cell level but also reproduce the complexity of the niche environment. Those bioengineering tools include synthesizing novel biomaterials for stem cell culture, fabricating scaffolds in three dimensions with microscale or nanoscale topography, micropatterning ECM in two dimensions, and performing high-throughput ECM microarrays [32] (Fig. 3). Together with the contribution of sophisticated analysis methods and with modeling approach, these tools allow us to increase our knowledge on stem cell physiology [87]. As discussed above, one mechanism by which the ECM niche influences stem cell fate is through the realization of a bulk stiffness [88]. Hydrogels are a class of materials with high water content, similar to the ECM [89]. The stickiness of these polymers can be chemically tuned and used to reproduce the stiffness of healthy and pathological tissue and thus control differentiation and self-renewal of stem cells [77], [78], [80], [81], [90]. Importantly, in the in vivo conditions stem cells are subjected to gradients of stiffness depending on the ECM present in the niche, and such gradient can be recreated by hybrid hydrogels [91]. High-throughput ECM microarrays are used to screen the role of single ECM components, or a combination of them, in stem cell niche. This approach allows the screening of cell–ECM interactions with greater efficiency compared to conventional coating of a typical microplate covered by a biopolymer [32]. Automatic pipetting allows one to mix together chosen stem cells, ECM and soluble niche components in nanoliter volumes, and dispense the mixture onto a substrate, thus generating spots with a wide range of defined properties; up to one thousand printed artificial niches can be then analyzed in a single experiment [92]. Now such technologies can be also applied to three-dimensional (3D) formats [93]. Such high-throughput assays have begun to be used for analyzing the effects that ECM molecules and regulatory factors, or combination of them, exert on stem cell self-renewal and differentiation [94], [95], [96]. For example, PEG hydrogel substrates were functionalized with integrins and Notch ligands, and their effects evaluated on the clonogenic potential of NSCs, thus allowing a better understanding of the molecular signals that control NSC fate [97].
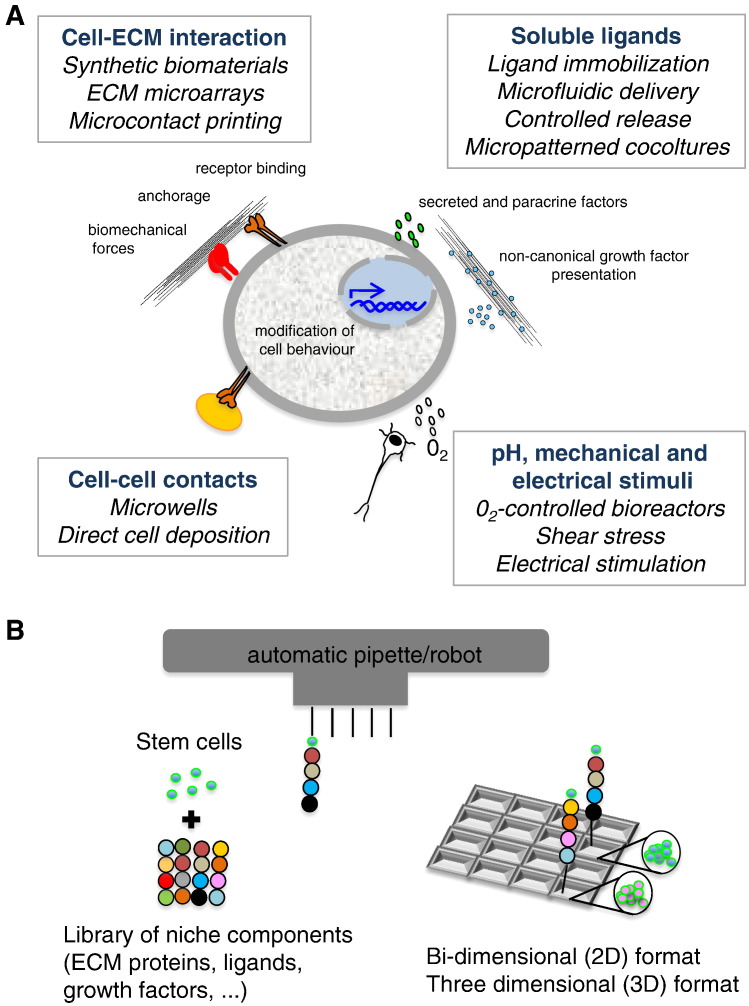
Fig. 3.
Strategies for engineering stem cell niches. (A) Schematic representation of the engineering techniques used to reproduce the chemical, physical and mechanical microenvironment of stem cell niche. (B) Schematic diagram of high-throughput ECM microarrays. Hundreds of thousands of printed artificial ECM niches can be created in one single experiment by the use of automatic pipettes that mix together stem cells and different combinations of niche components.
Another useful approach to understand the effect of ECM on stem cells is the use of micropatterned islands. Through this technique, it is possible to pattern ECM onto glass coverslip with defined shape and dimension and make cells selectively adhere in that region and not onto unpatterned glass. Those micropatterned islands can accommodate single cells and are used to study how specific shapes control the fate of stem cells. Epidermal stem cells cultured on the smallest islands are more likely to differentiate than cells with greater freedom to spread, showing an effect of cell shape on the decision to differentiate [98]. Differently, niche shape can modulate the differentiation program of mesenchymal stem cells, where cell spreading favors osteogenesis and cell rounding promotes adipogenesis [99]. With the same approach, it is possible to generate libraries with thousands of different topographies (circles, triangles, rectangles) and investigate their effect on stem cell behavior. For example, topographical cues can instruct mesenchymal stem cell fate and direct their differentiation toward the osteoblast lineage [100]. Applications for micropatterned islands include also the study of asymmetric division, by controlling the position of the axis of the cell division [101]. In addition, the same patterning approach can be used to study cell–cell interaction at single level [102]. Mesenchymal stem cell differentiation toward the osteogenic or adipogenic fate is dependent on cell–cell contacts, whereas isolated cells are less prone to differentiate [103]. To better recapitulate the niche and the exposure to growth factors, scientists have realized microfluidic systems reproducing the spatio-temporal influence of secreted factors on cells [104]. Moreover, since in the tissues these soluble factors are often bound to ECM, biomimicking approaches are used to conjugate soluble factors to the surface of biomaterials and make them released by cell-mediated proteolysis or external physical stimulation [32].
Although bidimensional systems represent a helpful mean to analyze ECM–stem cell interplay in vitro, the great challenge is the recreation of 3D systems recapitulating the in vivo condition [105], [106]. In fact, in a 3D environment cells naturally polarize with the basal compartment facing the gel, growth factors are highly concentrated compared to bidimensional systems, and cells sense the ECM rigidity and the transport of soluble components in a different way [107] (Fig. 4). With this aim, bioprinting approaches use the printing technology to deposit living cells alone or together with ECM components and soluble factors on a receiving solid or gel substrate. These technologies are able to create spatially defined gradients of immobilized biomolecules to control stem cell fate or can be patterned in a high-throughput manner to study stem cell behavior [108]. Alternatively, stem cells can directly be embedded inside hydrogels to recreate a 3D microenvironment [108], [109]. Notably, for the study of ECM–stem cell interplay, “bioclick and bioclip reactions” into 3D culture systems can be used to covalently couple and selectively remove functional molecules from the ECM biomimetics, with a full spatial and temporal control, thus allowing to test how extracellular signals dynamically control cell behavior over time (the so-called four-dimensional biology) [110]. One representative example of the potentialities of stem cell niche engineering is the possibility to recreate in vitro 3D intestinal epithelial organoids, named “epithelial mini-guts”, from a single Lgr5-positive crypt base columnar cell (CBC) for periods greater than 1.5 years, with the use of ECM and specific growth factors [111]. This and other studies open the perspective to the four-dimensional stem cell biology, where manipulating stem cells through the fine-tuning of cellular interactions, pattern, shape, and size will allow to synthesize or de novo design mini-organs [112], [113].
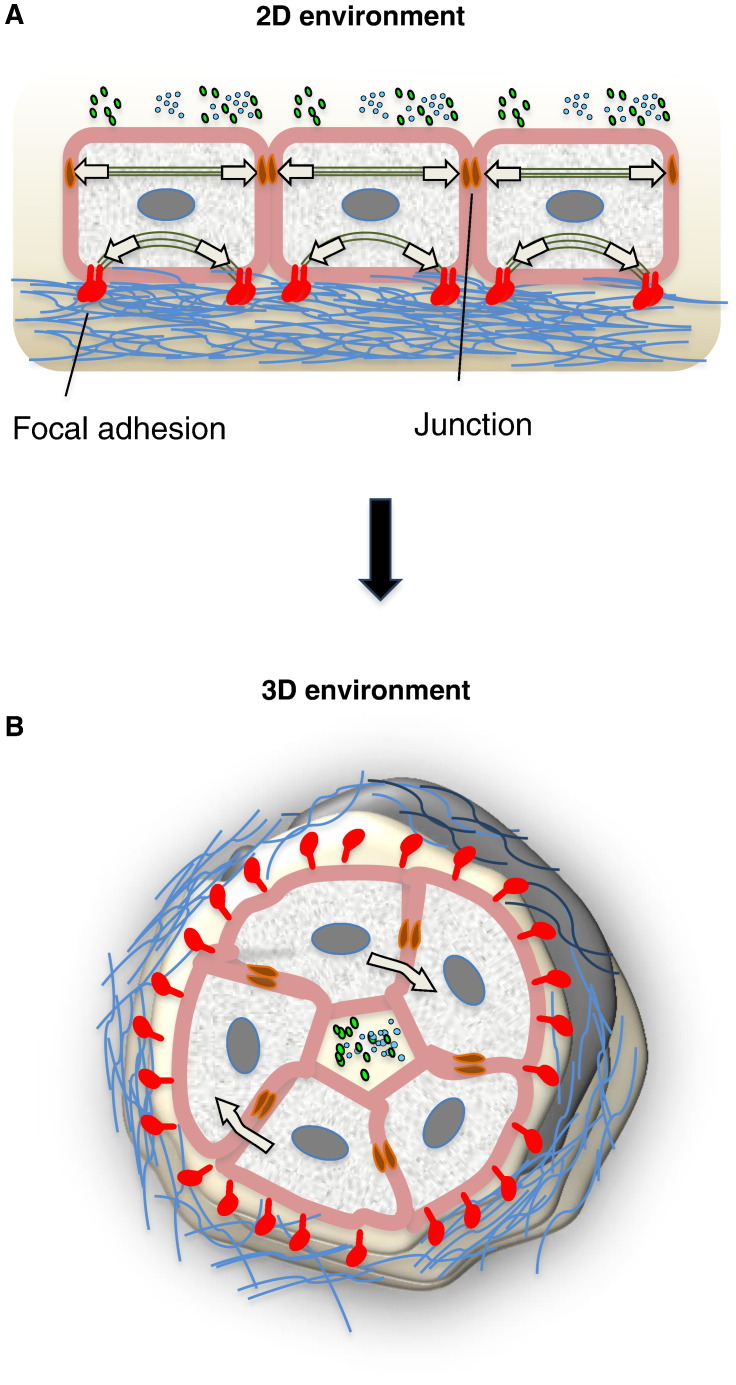
Fig. 4.
3D environment recapitulates in vivo niches. (A) Cells on a rigid planar surface organize focal adhesions and actin stress fibers at the basal surface of the cell and transfer contractile forces to their surface and to other cells. With their apical side, cells interface with secreted factors present in the medium, whereas with their basal side they interact with the ECM, which confers mechanical properties. (B) Inside a 3D microenvironment, the curvature and the softness of matrix materials limit the formation of actin stress fibers. Cells inside a 3D environment experience stress around the whole structure, both in planar and perpendicular directions to the cell basal surface. Secreted factors can be highly concentrated in the inner compartment. In addition, ECM displays a non-linear behavior and gradient of mechanical stiffness.
5. Composition and function of stem cell niches in different tissues
The complexity of the different niches rises from the necessity to protect the unique stem cell capabilities. Identifying and characterizing stem cell niches have been complicated by the fact that in most tissues stem cells are extremely rare and, in many cases, specific markers allowing the precise in vivo identification of a stem cell are lacking. Nonetheless, much progress has recently been made in identifying stem cell niches in several mammalian tissues, including the hematopoietic, epidermal, intestinal, muscular and neural stem cell compartments [24].
5.1. Hematopoietic stem cell niche
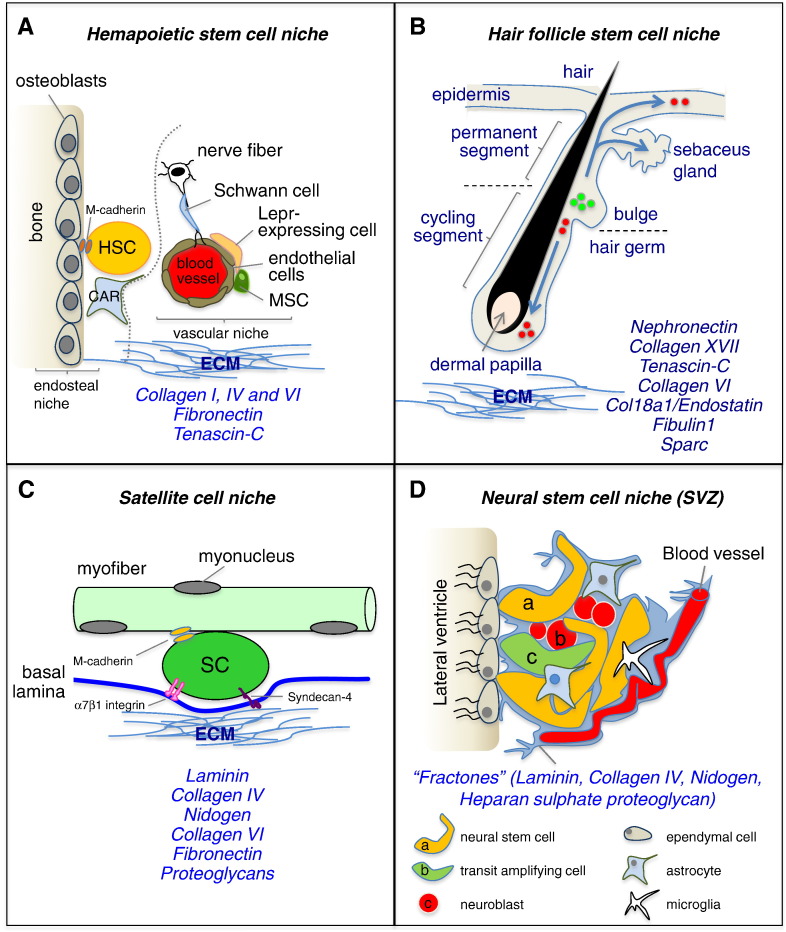
HSCs are multipotent progenitor cells that give rise to all types of mature blood cells [114], and are defined by their ability to self-renew and support long-term multi-lineage hematopoietic engraftment in lethally irradiated mice [115], [116]. They reside along the endosteal surface of trabecular bone, in close proximity to both boneforming osteoblasts and the endothelial cells that line blood vessels [117], and this anatomical localization represents their niche (Fig. 5A). The adult HSC niche consists of two anatomically distinctive cellular entities: i) the “endosteal niche”, populated by osteoblasts; and ii) the “vasculature niche”, located in the perivascular space. These two niches are proposed to be associated with the dormant and activated HSCs, respectively [118], [119], [120]. Despite being anatomically distinct, in the last year the concept of separated niches has been superseded. In fact, sophisticated three-dimensional imaging allowed revealing that there are intimate contacts between these two types of niche elements [121], [122] and that HSCs can move among these niches quite readily and receive inputs from both of them simultaneously [123]. HSCs are anchored to the endosteal niche through tight cell–cell interactions, in particular thanks to N-cadherin. Overexpression of N-cadherin promotes quiescence and preserves HSC activity during serial bone marrow transplantation [124], [125]. In this endosteal niche, osteoblasts have been shown to influence HSC pool by regulating stem cell number [117], [126], and to maintain HSC quiescence by releasing several signals, such as angiopoietin, thrombopoietin, and stromal cell-derived factor-1 [54], [127], as well as HSC self-renewal via Notch activation [128]. On the other side, the vascular niche is located around small sinusoidal blood vessels associated with various stromal and neuronal elements, which regulate HSC differentiation and ultimately mobilization to the peripheral circulation [128], [129]. Several studies demonstrated that a large number of cell types are active component of the HSC niche, including osteoclasts, stromal cells, bone marrow adipocytes, osteal macrophages (osteomacs), CXCL12-abundant reticular (CAR) cells, nestin-positive mesenchymal stem cells, sympathetic nerves including nestin-positive Schwann cells, and endothelial cells associated with leptin receptor-expressing perivascular stromal cells [129], [130], [131], [132], [133]. Moreover, it was recently found that also megakaryocytes can be considered as active components of the HSC niche [134], [135]. HSC self-renewal and differentiation are regulated either through contact-dependent signals, such as VCAM-1 [136], or via soluble factors such as SCF [137]. Only recently several other factors, including calcium ions, oxygen tension and reactive oxygen species, were shown to be critical for proper HSC regulation and have been integrated into the concept of the bone marrow niche [123], [138]. Despite the well-investigated cellular niche, the role of ECM components in the HSC niche has been poorly investigated. Collagen VI, collagen IV, fibronectin and tenascin-C represent some of the ECM proteins that are found in the bone marrow microenvironment [139], [140], [141]. In functional studies, collagen VI was shown to be a strong cytoadhesive substrate for various hematopoietic cell types, thus suggesting that this ECM component may play an important role within the bone marrow microenvironment [140]. In a recent work, it was demonstrated that tenascin-C is necessary for proper hematopoietic regeneration by promoting the in vivo and in vitro proliferation of hematopoietic stem and progenitor cells [48].
Fig. 5.
Stem cell niches and their ECM. The diagrams show four different stem cell niches, together with their cellular and ECM components. ECM molecules playing major roles in the different niches are indicated. (A) HSC niche consists of two anatomically distinct (dotted line) cellular entities, the “endosteal niche”, populated mainly by osteoblasts, and the “vasculature niche”, located in the perivascular space. HSCs can move through those two niches and interact with ECM molecules. A variety of cells, including osteoblasts, Cxcl12-abundant reticular (CAR) cells, nestin-positive mesenchymal stem cells (MSC), Lepr-expressing perivascular cells, and endothelial cells, were shown to be active components of the niche. (B) Diagram of the hair follicle. Multipotent stem cells are located in the bulge, which lies in the outer root sheath just below the sebaceous gland, and contribute to the lineages of the hair follicle, sebaceous gland, and the epidermis (arrows). The ECM surrounds the dermal papilla, a cluster of specialized mesenchymal cells in the hair bulb. (C) In skeletal muscle, satellite cells reside in the niche between myofiber plasma membrane and basal lamina. The myofiber basal lamina is a network of ECM components, including collagen IV, laminin, collagen VI, fibronectin and proteoglycans, which facilitate satellite cell adhesion via binding to receptors such as α7β1 integrin and syndecan-4. (D) In the adult brain, the SVZ niche is composed of three cell populations that lie immediately beneath a monolayer of ependymal cells lining the lateral ventricle and corresponding to the relatively quiescent NSCs (a), mitotically active transit amplifying cells (b), and neuroblasts (c). NSCs are intercalated into the ependymal layer and are also closely associated with the vasculature. NSCs within the SVZ are in contact with endothelial cells, microglia and astrocytes. NSCs are also in contact with fractones, structures rich in ECM molecules and continuous with the basal lamina of blood vessels.
5.2. Hair follicle stem cell niche
HFSCs continually cycle to regenerate the hair follicle, but during wound healing they can also form the sebaceous glands and restore the epidermis [142], [143]. Throughout hair formation, hair follicles undergo dynamic, synchronized cycles of growth (anagen), regression (catagen), and rest (telogen) [144], [145]. During telogen, which can last for months, HFSCs are quiescent and reside within a specialized microenvironment called the bulge, surrounding the hair shaft produced in the previous cycle [146] (Fig. 5B). Throughout telogen, the underlying dermal papilla cells, a population of mesenchymal cells, act as a niche for regulating HFSC activation and are responsible for hair follicle formation and maintenance [142], [147], [148], [149]. Importantly, the arrector pili muscle, a smooth muscle responsible for pulling the hair upright, is also a component of the hair follicle niche, connecting the bulge to the adjacent mesenchyme [57]. Several other non-epithelial cell types, including blood vessels, nerves, adipocytes and mesenchymal cells, participate to the control of HFSC activation and are active component of the dermal environment that surrounds the hair follicle [150], [151], [152]. Moreover, during the anagen phase, in the bulge HFSCs coordinately activate with melanocyte stem cells, needed for hair pigmentation [153], [154]. At distinct stages of the hair cycle, HFSCs receive either inhibitory or activating signals from both the micro- and macro-environments to either remain quiescent or become proliferative [155]. Besides various cell types, also the ECM plays an important role in the HFSC niche. It was demonstrated that several genes encoding for ECM proteins, such as tenascin-C, collagen VI, collagen XVIII/endostatin, fibulin1 and SPARC, display higher expression levels in mouse bulge cells than in differentiated keratinocytes [20], [156]. A seminal work showed that HFSCs in the bulge deposit nephronectin in the underlying basement membrane, thus regulating the adhesion to the bulge of mesenchymal cells expressing α8β1 integrin. Interestingly, nephronectin null mice display fewer arrector pili muscles, and ablation of nephronectin or of α8 integrin affects the anchorage of arrector pili muscle to the bulge. These data led the authors to conclude that bulge stem cells create a smooth muscle cell niche via nephronectin expression, thus revealing a key functional role for a specific ECM component in HFSC niche [57]. HFSCs share their location with another skin stem cell population, the melanocyte stem cells, and it was shown that TGF-β signaling is one of the key niche factors regulating the maintenance of melanocyte stem cells [157]. In another study, it was shown that collagen XVII, a hemidesmosomal transmembrane collagen highly expressed by HFSCs, is required for the physical interaction between HFSCs and melanocyte stem cell and maintains the self-renewal capacity of both stem cell populations. In agreement with this, Col17a1 null mice display premature hair loss, with premature differentiation of HFSCs and melanocyte stem cells, thus demonstrating that this anchoring collagen is a critical component of the HFSC/melanocyte stem cell niche [153].
5.3. Intestinal crypt niche
The mammalian intestine is one of the tissues that renews most rapidly since its epithelium is constantly subjected to insults, such as exposure to digestive enzymes and mechanical erosion. This renewal process requires a constant activation of stem cells located at the base of the crypts. The first identified ISCs were found to be located immediately above Paneth cells in the crypts, at an average position of four cells from the crypt base, and named label-retaining cells (LRC) or + 4 cells [158]. Those cells are marked by the expression of Bmi1 and are believed to generate distinct types of differentiated epithelial cells of the crypts and villi [159]. Lineage tracing studies allowed one to identify CBCs, a distinct population of ISCs interspersed between Paneth cells and marked by the expression of Lgr5 [160]. These cells can self-renew continuously and produce transit-amplifying daughter cells that differentiate in all cell types of the small intestinal epithelium, such as enterocytes, goblet cells, enteroendocrine cells and Paneth cells [160], [161]. Lineage tracing experiments have shown that ISCs, rather than dividing asymmetrically to generate daughter cells of different fates, divide symmetrically and adopt stem or transit-amplifying fates in a stochastic manner [162], [163]. Nevertheless, the precise lineage and hierarchy of the two types of ISCs in the intestinal epithelium are still under debate [164], [165], [166]. Paneth cells are the main component of the ISC niche and release not only bactericidal products [167] but also EGF, TGF-α, Wnt3 and the Notch ligand Dll4 [168]. The role of Paneth cells in ISC maintenance was confirmed by their in vivo genetic removal, resulting in the concomitant loss of CBCs [169]. The ISC niche comprises also other cell types such as enteric neurons, intraepithelial lymphocytes, smooth muscle cells of the muscularis mucosae, lymph and vascular endothelial cells, and a variety of bone-marrow derived stromal cells [170], [171]. Among those cells, pericryptal myofibroblasts reside adjacent to ISCs and regulate their survival through Wnt signaling [170]. Intestinal homeostasis is tightly controlled by four well-characterized signaling pathways, namely Wnt, Notch, EGF and BMP [111]. In addition, the visceral musculature forms a regulatory niche for ISCs by producing several secreted factors that activate signaling pathway to promote maintenance and proliferation of ISCs [172]. The function of the niche in the intestinal crypt requires the presence of ECM molecules in the basement membrane and of humoral factors derived from mesenchymal stem cells and subepithelial myofibroblasts surrounding the epithelium and from epithelial cells proliferating within the crypt [173]. The importance of the basement membrane for ISCs was provided by in vitro experiments showing that, in the presence of appropriate ECM proteins, single Lrg5-positive cells are able to generate autonomously organoids similar to intestinal crypts [111], [168]. ECM proteins and integrin receptors interact to keep ISCs within the niche, as the intestinal epithelium constantly undergoes physical compression and stretch enforced by the surrounding muscles [172]. Both positive and negative signals are finely tuned to regulate ISC behavior during tissue homeostasis and regeneration and protect them from dysfunction and disease [174], [175].
5.4. Muscle satellite cell niche
In adult skeletal muscles, a population of muscle stem cells, also called satellite cells, is located on the surface of myofibers in close contact with the myofiber plasma membrane and beneath the basal lamina, and this well-defined anatomic localization represents their niche [176] (Fig. 5C). In physiological conditions, satellite cells are in quiescent state, but upon damage or following muscle exercise satellite cells become activated, thus leading to proliferation and terminal differentiation in order to regenerate the damaged myofibers. Satellite cells are able to fuse both with the existing damaged myofibers and with each other to form de novo myofibers. Once activated, a subset of satellite cells returns to the quiescent state in their original niche, thus providing the necessary replenishment for self-renewal [177]. Given their anatomical localization, satellite cells are exposed to an asymmetric distribution of niche components, with myofiber signals on their apical surface and basal lamina signals on their basal surface [58], [178] (Fig 5C). The myofiber basal lamina contains a network of ECM components that include collagen IV, laminin, perlecan, nidogen, collagen VI, fibronectin and other proteoglycans and glycoproteins [179], [180]. The location of satellite cells is often in the proximity of blood vessels, which release endocrine factors to regulate the homeostasis of myofibers and their associated myogenic progenitors [181]. Innervation also plays a critical role in muscle function, and in its absence myofibers become atrophic and the satellite cell pool decreases due to apoptosis and loss of proliferative capacity [182]. In addition, several other cell types, including fibroblasts, fibro-adipogenic progenitors, immune cells, endothelial cells and osteoblasts, contribute to the satellite cell niche via paracrine mechanisms [183], [184], [185], [186]. Functional perturbations of skeletal muscle by chemical, electrical and mechanical stress also affect the muscle stem cell niche [187], [188], [189], [190]. Interestingly, oxygen concentration was shown to modulate the function of satellite cells, by regulating their differentiation toward myogenic and adipogenic fates [191] or by controlling their quiescent state and self-renewal capability [192]. A number of other extrinsic factors are known to modulate satellite cells quiescence, activation, expansion, self-renewal and differentiation, including EGF, HGF, FGF, IGF, angiopoietin-1, nitric oxide, and members of the Wnt and TGF-β signaling molecules [18], [193]. Similarly, myofibers generate numerous factors that, via secretion or presentation on the myofiber plasma membrane, influence satellite cell behavior, such as SDF-1, which binds the CXCR4 receptor present on satellite cells and activates a migratory response [194], [195]. Additionally, satellite cells themselves express ligands that regulate their own cell fates through autocrine and juxtacrine signaling, such as Notch, the main regulator of their quiescence and self-renewal [196], [197]. Satellite cells also express the Tie2 receptor, which interacts with the angiopoietin-1 ligand and contributes to the maintenance of quiescence [181].
The ECM plays a major role in skeletal muscles, not only by stabilizing myofiber during contraction and by transmitting forces along adjacent myofibers, but also by regulating myogenesis and muscle regeneration. Although the role of the ECM within muscle stem cell niche is still under investigation, recent studies have clearly demonstrated the influence of specific ECM components in regulating satellite cell activity and how their modifications alter the satellite cell function and the regenerative properties of skeletal muscle [77], [86]. Reciprocally, also satellite cells transcribe in a regulated manner a number of genes coding for ECM proteins, such as versican, glypicans and fibrillin-2, based on the necessity to bind growth factors [198]. Satellite cells can dynamically remodel their niche based on their state of activation. For example, collagen VI, expressed not only by interstitial fibroblasts but also by quiescent satellite cells, represents a key component of muscle stem cell niche required for preserving satellite cell self-renewal and muscle regeneration, and its expression is down-regulated in activated satellite cells [77]. Conversely, activated satellite cells have a transient high-level expression of fibronectin, which in turn stimulates Wnt7a signaling through the Fzd7/Sdc4 co-receptor complex to induce the symmetric expansion of satellite cells [86]. At a more general level, increased ECM deposition and changes in ECM composition were shown to negatively affect satellite cell–myofiber interactions. Such global ECM changes may affect the muscle stem cell niche through multiple mechanisms, by changing the ability of the basal lamina to serve as a reservoir for ECM-bound growth factors, modifying satellite cell adhesion to the basal lamina, and altering the elastic properties of muscle tissue [18].
5.5. Neural stem cell niche
The brain contains two major locations for NSCs, corresponding to the subventricular zone (SVZ) of the lateral ventricles and to the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. NSCs in the SVZ generate neuroblasts that migrate to the olfactory bulb, where they generate interneurons, cells essential for the maintenance of the olfactory bulb. In contrast, neurons produced in the hippocampal SGZ integrate into the immediately adjacent granule cell layer, where they are important for learning and memory. In addition, adult NSCs generate oligodendrocytes and astrocytes [199], [200] (Fig. 5D). These two types of adult NSCs reside in different niches. In the SVZ, NSCs lie next to the ventricle, and possess processes that contact the cerebrospinal fluid and blood vessels. NSCs in the dentate radial gyrus are found deeper in the brain parenchyma, away from the walls of the ventricle and surrounded by neurons and other glial cells [201]. Both cell types have long processes that contact the vasculature. The intrinsic properties of NSCs present in the SGZ, such as self-renewal and multipotency, are still a matter of debate despite the fact that it was demonstrated that those cells can be isolated and cultured, generating self-renewing cells that can differentiate into neurons [202], [203], [204], [205]. Several signaling molecules, such as Wnt, Notch, Shh and BMP, as well as growth and neurotrophic factors and neurotransmitters, were found to regulate hippocampal neurogenesis [206], [207], [208], [209], [210]. Other molecules implicated in axon guidance and synapse formation, such as Wnt7a, have been shown to regulate the early steps of neurogenesis, for example NSC self-renewal and the cell cycle progression of neural progenitors [211].
Independently from the niche they occupy, NSCs are closely associated with the vasculature and with basal lamina components. They reside adjacent to a variety of neighboring cells, including their own neuronal progeny, resident mature astrocytes and microglia, and endothelial and smooth muscle cells of blood vessels [200], [212], [213]. Among those, the perivascular niche has a key influence on progenitor cells, regulating their mobilization and differentiation [214]. Astrocytes were also found to regulate NSC self-renewal and differentiation [215], [216]. Adding further complexity, the presence or absence of mature neurons can influence NSC fate through a feedback loop [200]. Additional factors, such as oxygen tension, regulate NSC activities; for example, mild hypoxia strongly enhances both self-renewal and neurogenic abilities [217]. Moreover, different physiological stimuli, such as learning, exposure to environmental enrichment, running and stress, can affect the rate of proliferation, differentiation, and survival of newborn neurons in the hippocampus [209]. Besides these different cells and factors, the ECM is also an important component of the neurogenic microenvironment. Both SVZ and SGZ niches express various ECM molecules, such as tenascin-C, netrins, laminin and various proteoglycans [49], [70], [218], [219]. In the SVZ, NSCs are in association with projections of the vascular basement membrane, referred to as fractones and composed mainly of laminin, collagen IV, nidogen and proteoglycans [70]. Those ECM molecules modulate NSC maintenance and differentiation, and influence the migration of their progeny [220]. One example is represented by heparan and chondroitin sulfate proteoglycans, which regulate the proliferation and neurogenic differentiation of NSCs, mainly by presenting growth factors, such as EGF and FGF, to NSC receptors [221], [222]. Some ECM molecules, such as netrin-1, Slit-1 and Slit-2, are involved in providing the direction for migration of neurogenic precursors [223]. Another ECM protein, reelin, plays a key role in controlling the migration of neurogenic precursors to the target olfactory bulb [224], and this process is abolished in reeler mutant mice, where the protein is missing [225]. It was also shown that decreased reelin expression in the adult brain leads to defective brain plasticity, suggesting that reelin deficiency may be implicated in some brain disorders [226]. Other studies demonstrated that the tenascin family of ECM protein plays key roles in NSC niche. Tenascin-C is highly expressed in the SVZ, and tenascin-C deficient mice have altered numbers of NSCs due to defective response to FGF2 and BMP4, thus suggesting a role for this ECM molecule in the regulation of growth factor signaling in NSC niche [227]. Tenascin-R is expressed in the olfactory bulb, where it promotes the detachment and radial migration of neurogenic precursors. Interestingly, grafting of cells expressing tenascin-R into non-neurogenic regions redirects neurogenic precursors toward these regions, indicating that tenascin-R mediates the recruitment of neurogenic precursors in the adult forebrain [228].
6. Conclusions
In recent years, progressions in our knowledge of cell biology demonstrated that the study of cell behavior cannot exclude signals deriving from the environment in which cells are located. The key role of ECM in regulating cell behavior now represents a well-established fact and this concept is especially critical for stem cells, which are defined by a unique and specialized niche in which ECM represents one essential player. The instructive cue of ECM on stem cells is a result of a number of different characteristics of the extracellular environment, starting from its biophysical and biomechanical properties up to its biochemical activity. All these aspects are dynamically orchestrated in vivo in order to retain stem cell identity, guide cell fate determination and maintain stem cell pool. Further in vivo studies, together with the application of in vitro engineered niches, will allow us to increase our understanding about which specific components and characteristics of the ECM play a role in the behavior of stem cells in the different tissues. These studies will likely open promising new scenarios, as the more accurate mimicking of some essential features of in vivo niches using in vitro systems can not only improve the knowledge of stem cell biology, but also develop new application for cell-based therapies in humans [229]. The possibility to synthesize well-defined extracellular environments as scaffolds for implanting stem cells in the body represents a fascinating and promising venue for allowing the proper maintenance and activity of stem cells in a broad range of degenerating diseases [230], [231].
Acknowledgements
We apologize to colleagues whose studies were not cited owing to space limitations. The authors wish to thank Telethon-Italy (GGP10225 and GGP11082), the Italian Ministry of Education, University and Research (FIRB Strategic Project RBAP11Z3YA_003), and the University of Padova for financial support. The authors declare no conflict of interest.
Footnotes
This article is part of a Special Issue entitled Matrix-mediated cell behaviour and properties.
Contributor Information
Anna Urciuolo, Email: annaurciolo@hotmail.com.
Paolo Bonaldo, Email: bonaldo@bio.unipd.it.
References
- 1.Ozbek S., Balasubramanian P.G., Chiquet-Ehrismann R., Tucker R.P., Adams J.C. The evolution of extracellular matrix. Mol. Biol. Cell. 2010;21:4300–4305. doi: 10.1091/mbc.E10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt F.M., Huck W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 3.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011;3:1–24. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kölsch V., Seher T., Fernandez-Ballester G.J., Serrano L., Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- 8.Montell D.J. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 9.Puklin-Faucher E., Sheetz M.P. The mechanical integrin cycle. J. Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reilly G.C., Engler A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Mammoto T., Ingber D.E. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore S.W., Roca-Cusachs P., Sheetz M.P. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuFort C.C., Paszek M.J., Weaver V.M. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman I. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung T.H., Rando T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs E., Chen T. A matter of life and death: self-renewal in stem cells. EMBO Rep. 2013;14:39–48. doi: 10.1038/embor.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosgrove B.D., Sacco A., Gilbert P.M., Blau H.M. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2010;78:185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison S.J., Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 21.Morrison S.J., Spradling A.C. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life; pp. 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orford K.W., Scadden D.T. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 23.Greco V., Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones D.L., Wagers A.J. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 25.Lander A.D., Kimble J., Clevers H., Fuchs E., Montarras D., Buckingham M., Calof A.L., Trumpp A., Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagers A.J. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362–369. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 28.Watt F.M., Driskell R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trumpp A., Essers M., Wilson A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz A., Oh S. Age related changes of the extracellular matrix and stem cell maintenance. Prev. Med. (Baltim) 2012;54:S50–S56. doi: 10.1016/j.ypmed.2012.01.003. (Suppl.) [DOI] [PubMed] [Google Scholar]
- 31.Discher D.E., Mooney D.J., Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peerani R., Zandstra P.W. Enabling stem cell therapies through synthetic stem cell-niche engineering. J. Clin. Invest. 2010;120:60–70. doi: 10.1172/JCI41158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pera M.F., Tam P.P.L. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- 34.Watt F.M., Fujiwara H. Cell–extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005124. (pii: a005124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama K.H., Batchelder C.A., Lee C.I., Tarantal A.F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng. Part A. 2010;16:2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Gutierrez A., Yagi H., Uygun B.E., Navarro-Alvarez N., Uygun K., Kobayashi N., Yang Y., Yarmush M.L. Cell delivery: from cell transplantation to organ engineering. Cell Transplant. 2010;19:655–665. doi: 10.3727/096368910X508753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J.J., Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Avigdor A., Goichberg P., Shivtiel S., Dar A., Peled A., Samira S., Kollet O., Hershkoviz R., Alon R., Hardan I., Ben-Hur H., Naor D., Nagler A., Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34 + stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 39.Smith-Berdan S., Nguyen A., Hassanein D., Zimmer M., Ugarte F., Ciriza J., Li D., García-Ojeda M.E., Hinck L., Forsberg E.C. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legate K.R., Wickström S.A., Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 41.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 42.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brizzi M.F., Tarone G., Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Buitenhuis M. The role of PI3K/protein kinase B (PKB/c-akt) in migration and homing of hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2011;18:226–230. doi: 10.1097/MOH.0b013e32834760e5. [DOI] [PubMed] [Google Scholar]
- 45.O'Reilly A.M., Lee H.-H., Simon M.A. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol. 2008;182:801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanatsu-Shinohara M., Takehashi M., Takashima S., Lee J., Morimoto H., Chuma S., Raducanu A., Nakatsuji N., Fässler R., Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Shen Q., Wang Y., Kokovay E., Lin G., Chuang S., Goderie S.K., Roysam B., Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell–cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura-Ishizu A., Okuno Y., Omatsu Y., Okabe K., Morimoto J., Uede T., Nagasawa T., Suda T., Kubota Y. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119:5429–5437. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kazanis I., Belhadi A., Faissner A., Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. J. Neurosci. 2007;27:13991–13996. doi: 10.1523/JNEUROSCI.3279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potocnik A., Brakebusch C., Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 51.Qian H., Tryggvason K., Jacobsen S.E., Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 52.Grassinger J., Haylock D.N., Storan M.J., Haines G.O., Williams B., Whitty G.A., Vinson A.R., Be C.L., Li S., Sørensen E.S., Tam P.P.L., Denhardt D.T., Sheppard D., Choong P.F., Nilsson S.K. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood. 2009;114:49–59. doi: 10.1182/blood-2009-01-197988. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber T.D., Steinl C., Essl M., Abele H., Geiger K., Müller C.A., Aicher W.K., Klein G. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshihara H., Arai F., Hosokawa K., Hagiwara T., Takubo K., Nakamura Y., Gomei Y., Iwasaki H., Matsuoka S., Miyamoto K., Miyazaki H., Takahashi T., Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Li G., Tse W., Bunting K.D. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113:4856–4865. doi: 10.1182/blood-2008-09-181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umemoto T., Yamato M., Ishihara J., Shiratsuchi Y., Utsumi M., Morita Y., Tsukui H., Terasawa M., Shibata T., Nishida K., Kobayashi Y., Petrich B.G., Nakauchi H., Eto K., Okano T. Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara H., Ferreira M., Donati G., Marciano D.K., Linton J.M., Sato Y., Hartner A., Sekiguchi K., Reichardt L.F., Watt F.M. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuang S., Gillespie M.A., Rudnicki M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Raymond K., Deugnier M.-A., Faraldo M.M., Glukhova M.A. Adhesion within the stem cell niches. Curr. Opin. Cell Biol. 2009;21:623–629. doi: 10.1016/j.ceb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Marthiens V., Kazanis I., Moss L., Long K., Ffrench-Constant C. Adhesion molecules in the stem cell niche — more than just staying in shape? J. Cell Sci. 2010;123:1613–1622. doi: 10.1242/jcs.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suh H.N., Han H.J. Collagen I regulates the self-renewal of mouse embryonic stem cells through α2β1 integrin- and DDR1-dependent Bmi-1. J. Cell. Physiol. 2011;226:3422–3432. doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- 62.Chen S., Lewallen M., Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Defilippi P., Rosso A., Dentelli P., Calvi C., Garbarino G., Tarone G., Pegoraro L., Brizzi M.F. beta1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J. Cell Biol. 2005;168:1099–1108. doi: 10.1083/jcb.200405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uberti B., Dentelli P., Rosso A., Defilippi P., Brizzi M.F. Inhibition of β1 integrin and IL-3Rβ common subunit interaction hinders tumour angiogenesis. Oncogene. 2010;29:6581–6590. doi: 10.1038/onc.2010.384. [DOI] [PubMed] [Google Scholar]
- 65.Margadant C., Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivaska J., Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- 67.Campos L.S., Decker L., Taylor V., Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J. Biol. Chem. 2006;281:5300–5309. doi: 10.1074/jbc.M511886200. [DOI] [PubMed] [Google Scholar]
- 68.Brisken C., Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 69.Jones R.G., Li X., Gray P.D., Kuang J., Clayton F., Samowitz W.S., Madison B.B., Gumucio D.L., Kuwada S.K. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J. Cell Biol. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerever A., Schnack J., Vellinga D., Ichikawa N., Moon C., Arikawa-Hirasawa E., Efird J.T., Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 71.Douet V., Kerever A., Arikawa-Hirasawa E., Mercier F. Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif. 2013;46:137–145. doi: 10.1111/cpr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halder G., Dupont S., Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 73.Mammoto A., Mammoto T., Ingber D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y., Chen C.S., Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012;41:519–542. doi: 10.1146/annurev-biophys-042910-155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keung A.J., de Juan-Pardo E.M., Schaffer D.V., Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urciuolo A., Quarta M., Morbidoni V., Gattazzo F., Molon S., Grumati P., Montemurro F., Tedesco F.S., Blaauw B., Cossu G., Vozzi G., Rando T.A., Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilbert P.M., Havenstrite K.L., Magnusson K.E.G., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Georges P.C., Miller W.J., Meaney D.F., Sawyer E.S., Janmey P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 81.Saha K., Keung A.J., Irwin E.F., Li Y., Little L., Schaffer D.V., Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winer J.P., Janmey P.A., McCormick M.E., Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 83.Wang P.-Y., Tsai W.-B., Voelcker N.H. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater. 2012;8:519–530. doi: 10.1016/j.actbio.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 84.Engler A.J., Carag-Krieger C., Johnson C.P., Raab M., Tang H.-Y., Speicher D.W., Sanger J.W., Sanger J.M., Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trappmann B., Gautrot J.E., Connelly J.T., Strange D.G.T., Li Y., Oyen M.L., Cohen Stuart M.A., Boehm H., Li B., Vogel V., Spatz J.P., Watt F.M., Huck W.T.S. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 86.Bentzinger C.F., Wang Y.X., von Maltzahn J., Soleimani V.D., Yin H., Rudnicki M.A. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J., Tzanakakis E.S. Deconstructing stem cell population heterogeneity: single-cell analysis and modeling approaches. Biotechnol. Adv. 2013:1–15. doi: 10.1016/j.biotechadv.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., Chen C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 90.Gilbert P.M., Corbel S., Doyonnas R., Havenstrite K., Magnusson K.E.G., Blau H.M. A single cell bioengineering approach to elucidate mechanisms of adult stem cell self-renewal. Integr. Biol. (Camb) 2012;4:360–367. doi: 10.1039/c2ib00148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tse J.R., Engler A.J. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6:e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flaim C.J., Chien S., Bhatia S.N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 93.Gobaa S., Hoehnel S., Roccio M., Negro A., Kobel S., Lutolf M.P. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 94.Soen Y., Mori A., Palmer T.D., Brown P.O. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol. Syst. Biol. 2006;2:37. doi: 10.1038/msb4100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flaim C.J., Teng D., Chien S., Bhatia S.N. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 96.LaBarge M.A., Nelson C.M., Villadsen R., Fridriksdottir A., Ruth J.R., Stampfer M.R., Petersen O.W., Bissell M.J. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr. Biol. (Camb) 2009;1:70–79. doi: 10.1039/b816472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roccio M., Gobaa S., Lutolf M.P. High-throughput clonal analysis of neural stem cells in microarrayed artificial niches. Integr. Biol. 2012;4:391–400. doi: 10.1039/c2ib00070a. [DOI] [PubMed] [Google Scholar]
- 98.Connelly J.T., Gautrot J.E., Trappmann B., Tan D.W.-M., Donati G., Huck W.T.S., Watt F.M. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 99.Kilian K.A., Bugarija B., Lahn B.T., Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unadkat H.V., Hulsman M., Cornelissen K., Papenburg B.J., Truckenmüller R.K., Carpenter A.E., Wessling M., Post G.F., Uetz M., Reinders M.J.T., Stamatialis D., van Blitterswijk C.A., de Boer J. An algorithm-based topographical biomaterials library to instruct cell fate. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16565–16570. doi: 10.1073/pnas.1109861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Théry M., Racine V., Piel M., Pépin A., Dimitrov A., Chen Y., Sibarita J.-B., Bornens M. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19771–19776. doi: 10.1073/pnas.0609267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukuda J., Khademhosseini A., Yeh J., Eng G., Cheng J., Farokhzad O.C., Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 103.Tang J., Peng R., Ding J. The regulation of stem cell differentiation by cell–cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Chung B.G., Flanagan L.A., Rhee S.W., Schwartz P.H., Lee A.P., Monuki E.S., Jeon N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 105.Lund A.W., Yener B., Stegemann J.P., Plopper G.E. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng. Part B Rev. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang H., Dai S., Bi J., Liu K. Biomimetic three-dimensional microenvironment for controlling stem cell fate. Interface Focus. 2011;1:792–803. doi: 10.1098/rsfs.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adler S., Basketter D., Creton S., Pelkonen O., van Benthem J., Zuang V., Andersen K.E., Angers-Loustau A., Aptula A., Bal-Price A., Benfenati E., Bernauer U., Bessems J., Bois F.Y., Boobis A., Brandon E., Bremer S., Broschard T., Casati S., Coecke S. Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch. Toxicol. 2011;85:367–485. doi: 10.1007/s00204-011-0693-2. [DOI] [PubMed] [Google Scholar]
- 108.Tasoglu S., Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013;31:10–19. doi: 10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J., Sachdev P., Sidhu K. Alginate microcapsule as a 3D platform for the efficient differentiation of human embryonic stem cells to dopamine neurons. Stem Cell Res. 2013;11:978–989. doi: 10.1016/j.scr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Tibbitt M.W., Anseth K.S. Dynamic microenvironments: the fourth dimension. Sci. Transl. Med. 2012;4:160ps24. doi: 10.1126/scitranslmed.3004804. [DOI] [PubMed] [Google Scholar]
- 111.Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 112.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikkola H.K.A., Orkin S.H. The journey of developing hematopoietic stem cells. 2005;3744:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 115.Wilson A., Laurenti E., Oser G., Van Der Wath R.C., Blanco-bose W., Jaworski M., Macdonald H.R., Offner S., Dunant C.F., Eshkind L., Bockamp E., Lio P. 2008. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair; pp. 1118–1129. [DOI] [PubMed] [Google Scholar]
- 116.Essers M.A.G., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A., Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J., Niu C., Ye L., Huang H., He X., Tong W.-G., Ross J., Haug J., Johnson T., Feng J.Q., Harris S., Wiedemann L.M., Mishina Y., Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 118.Kopp H., Avecilla S.T., Hooper A.T., Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 119.Scadden D.T. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 120.Wilson A., Oser G.M., Jaworski M., Blanco-Bose W.E., Laurenti E., Adolphe C., Essers M.A., Macdonald H.R., Trumpp A. Dormant and self-renewing hematopoietic stem cells and their niches. Ann. N. Y. Acad. Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 121.Lo Celso C., Fleming H.E., Wu J.W., Zhao C.X., Miake-Lye S., Fujisaki J., Côté D., Rowe D.W., Lin C.P., Scadden D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie Y., Yin T., Wiegraebe W., He X.C., Miller D., Stark D., Perko K., Alexander R., Schwartz J., Grindley J.C., Park J., Haug J.S., Wunderlich J.P., Li H., Zhang S., Johnson T., Feldman R.A., Li L. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 123.Wang L.D., Wagers A.J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hosokawa K., Arai F., Yoshihara H., Iwasaki H., Nakamura Y., Gomei Y., Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 125.Arai F., Hosokawa K., Toyama H., Matsumoto Y., Suda T. Role of N-cadherin in the regulation of hematopoietic stem cells in the bone marrow niche. Ann. N. Y. Acad. Sci. 2012;1266:72–77. doi: 10.1111/j.1749-6632.2012.06576.x. [DOI] [PubMed] [Google Scholar]
- 126.Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., Milner L.A., Kronenberg H.M., Scadden D.T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 127.Arai F., Hirao A., Ohmura M., Sato H. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Weber J.M., Calvi L.M. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone. 2010;46:281–285. doi: 10.1016/j.bone.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakamura-Ishizu A., Suda T. Hematopoietic stem cell niche: an interplay among a repertoire of multiple functional niches. Biochim. Biophys. Acta. 2013;1830:2404–2409. doi: 10.1016/j.bbagen.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 130.Kunisaki Y., Frenette P.S. The secrets of the bone marrow niche: enigmatic niche brings challenge for HSC expansion. Nat. Med. 2012;18:864–865. doi: 10.1038/nm.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen Y., Nilsson S.K. Bone, microenvironment and hematopoiesis. Curr. Opin. Hematol. 2012;19:250–255. doi: 10.1097/MOH.0b013e328353c714. [DOI] [PubMed] [Google Scholar]
- 132.Greenbaum A., Hsu Y.-M.S., Day R.B., Schuettpelz L.G., Christopher M.J., Borgerding J.N., Nagasawa T., Link D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith J.N.P., Calvi L.M. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31:1044–1050. doi: 10.1002/stem.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Olson T.S., Caselli A., Otsuru S., Hofmann T.J., Williams R., Paolucci P., Dominici M., Horwitz E.M. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heazlewood S.Y., Neaves R.J., Williams B., Haylock D.N., Adams T.E., Nilsson S.K. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 2013;11:782–792. doi: 10.1016/j.scr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 136.Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsai J.J., Dudakov J.A., Takahashi K., Shieh J.-H., Velardi E., Holland A.M., Singer N.V., West M.L., Smith O.M., Young L.F., Shono Y., Ghosh A., Hanash A.M., Tran H.T., Moore M.A.S., van den Brink M.R.M. Nrf2 regulates haematopoietic stem cell function. Nat. Cell Biol. 2013;15:309–316. doi: 10.1038/ncb2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klein G., Beck S., Müller C.A. Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J. Cell Biol. 1993;123:1027–1035. doi: 10.1083/jcb.123.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Klein G., Muller C., Tillet E. Collagen type VI in the human bone marrow microenvironment: a strong cytoadhesive component. Blood. 1995;86:1740–1748. [PubMed] [Google Scholar]
- 141.Nilsson S.K., Debatis M.E., Dooner M.S., Madri J.a., Quesenberry P.J., Becker P.S. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 142.Yang C.-C., Cotsarelis G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plikus M.V., Gay D.L., Treffeisen E., Wang A., Supapannachart R.J., Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin. Cell Dev. Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blanpain C., Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Myung P., Ito M. Dissecting the bulge in hair regeneration. J. Clin. Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Enshell-Seijffers D., Lindon C., Kashiwagi M., Morgan B.A. Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Driskell R.R., Clavel C., Rendl M., Watt F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chi W., Wu E., Morgan B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brownell I., Guevara E., Bai C.B., Loomis C.A., Joyner A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Festa E., Fretz J., Berry R., Schmidt B., Rodeheffer M., Horowitz M. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goldstein J., Horsley V. Home sweet home: skin stem cell niches. Cell. Mol. Life Sci. 2012;69:2573–2582. doi: 10.1007/s00018-012-0943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanimura S., Tadokoro Y., Inomata K., Binh N.T., Nishie W., Yamazaki S., Nakauchi H., Tanaka Y., McMillan J.R., Sawamura D., Yancey K., Shimizu H., Nishimura E.K. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 154.Chang C.-Y., Pasolli H.A., Giannopoulou E.G., Guasch G., Gronostajski R.M., Elemento O., Fuchs E. NFIB is a governor of epithelial–melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee J., Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Semin. Cell Dev. Biol. 2012;23:906–916. doi: 10.1016/j.semcdb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morris R.J., Liu Y., Marles L., Yang Z., Trempus C., Li S., Lin J.S., Sawicki J.A., Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 157.Nishimura E.K. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 158.Gordon J., Schmidt G., Roth K. Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J. 1992;6:3039–3050. doi: 10.1096/fasebj.6.12.1521737. [DOI] [PubMed] [Google Scholar]
- 159.Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 161.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 162.Lopez-Garcia C., Klein A.M., Simons B.D., Winton D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 163.Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 164.Buczacki S.J.A., Zecchini H.I., Nicholson A.M., Russell R., Vermeulen L., Kemp R., Winton D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 165.Nusse Y.M., Klein O.D. If a stem cell dies in the crypt, and no one is around to see it…. Cell Stem Cell. 2013;12:389–390. doi: 10.1016/j.stem.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 166.Zhu Y., Huang Y.-F., Kek C., Bulavin D.V. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12:298–303. doi: 10.1016/j.stem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 167.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 168.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 169.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yeung T.M., Chia L.A., Kosinski C.M., Kuo C.J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell. Mol. Life Sci. 2011;68:2513–2523. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Powell D.W., Pinchuk I.V., Saada J.I., Chen X., Mifflin R.C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin G., Zhang X., Ren J., Pang Z., Wang C., Xu N., Xi R. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev. Biol. 2013;377:177–187. doi: 10.1016/j.ydbio.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 173.Smith N.R., Davies P.S., Silk A.D., Wong M.H. Epithelial and mesenchymal contribution to the niche: a safeguard for intestinal stem cell homeostasis. Gastroenterology. 2012;143:1426–1430. doi: 10.1053/j.gastro.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Medema J.P., Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 175.Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. doi: 10.1053/j.gastro.2012.08.031. (e7) [DOI] [PubMed] [Google Scholar]
- 176.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tedesco F.S., Dellavalle A., Diaz-Manera J., Messina G., Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Collins C.A., Partridge T.A. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341. doi: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- 179.Sanes J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 180.Grounds M. In: Skeletal Muscle Repair and Regeneration. Schiaffino S., Partridge T., editors. Springer; New York: 2008. Complexity of extracellular matrix and skeletal muscle regeneration; pp. 269–302. [Google Scholar]
- 181.Christov C., Chrétien F. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ciciliot S., Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 2010;16:906–914. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- 183.Bentzinger C.F., von Maltzahn J., Rudnicki M.A. Extrinsic regulation of satellite cell specification. Stem Cell Res. Ther. 2010;1:27. doi: 10.1186/scrt27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Murphy M.M., Lawson J.A., Mathew S.J., Hutcheson D.A., Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saclier M., Cuvellier S., Magnan M., Mounier R., Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013;280:4118–4130. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 187.Molgó J., Colasante C., Adams D.S., Jaimovich E. IP3 receptors and Ca2 + signals in adult skeletal muscle satellite cells in situ. Biol. Res. 2004;37:635–639. doi: 10.4067/s0716-97602004000400019. [DOI] [PubMed] [Google Scholar]
- 188.Tatsumi R., Liu X., Pulido A., Morales M., Sakata T., Dial S., Hattori A., Ikeuchi Y., Allen R.E. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am. J. Physiol. Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 189.Guo B.S., Cheung K.K., Yeung S.S., Zhang B.T., Yeung E.W. Electrical stimulation influences satellite cell proliferation and apoptosis in unloading-induced muscle atrophy in mice. PLoS One. 2012;7:e30348. doi: 10.1371/journal.pone.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hara M., Tabata K., Suzuki T., Do M.-K.Q., Mizunoya W., Nakamura M., Nishimura S., Tabata S., Ikeuchi Y., Sunagawa K., Anderson J.E., Allen R.E., Tatsumi R. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am. J. Physiol. Cell Physiol. 2012;302:C1741–C1750. doi: 10.1152/ajpcell.00068.2012. [DOI] [PubMed] [Google Scholar]
- 191.Redshaw Z., Loughna P.T. Oxygen concentration modulates the differentiation of muscle stem cells toward myogenic and adipogenic fates. Differentiation. 2012;84:193–202. doi: 10.1016/j.diff.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 192.Liu W., Wen Y., Bi P., Lai X., Liu X.S., Liu X., Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012;139:2857–2865. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Montarras D., L'honoré A., Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- 194.Ratajczak M., Majka M., Kucia M. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 195.Sherwood R.I., Christensen J.L., Conboy I.M., Conboy M.J., Rando T.A., Weissman I.L., Wagers A.J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 196.Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pisconti A., Cornelison D.D.W., Olguín H.C., Antwine T.L., Olwin B.B. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J. Cell Biol. 2010;190:427–441. doi: 10.1083/jcb.201003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pallafacchina G., François S., Regnault B., Czarny B., Dive V., Cumano A., Montarras D., Buckingham M. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2010;4:77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 199.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 200.Miller F.D., Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 201.Fuentealba L.C., Obernier K., Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peltier J., Agrawal S., Robertson M.J., Schaffer D.V. In vitro culture and analysis of adult hippocampal neural progenitors. Methods Mol. Biol. 2010;621:65–87. doi: 10.1007/978-1-60761-063-2_5. [DOI] [PubMed] [Google Scholar]
- 203.Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G., Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Encinas J.M., Michurina T.V., Peunova N., Park J.-H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lugert S., Vogt M., Tchorz J.S., Müller M., Giachino C., Taylor V. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat. Commun. 2012;3:670. doi: 10.1038/ncomms1670. [DOI] [PubMed] [Google Scholar]
- 206.Suh H., Deng W., Gage F.H. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 207.Schwarz T.J., Ebert B., Lie D.C. Stem cell maintenance in the adult mammalian hippocampus: a matter of signal integration? Dev. Neurobiol. 2012;72:1006–1015. doi: 10.1002/dneu.22026. [DOI] [PubMed] [Google Scholar]
- 208.Faigle R., Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Varela-Nallar L., Inestrosa N.C. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013;7:100. doi: 10.3389/fncel.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Piccin D., Yu F., Morshead C.M. Notch signaling imparts and preserves neural stem characteristics in the adult brain. Stem Cells Dev. 2013;22:1541–1550. doi: 10.1089/scd.2012.0390. [DOI] [PubMed] [Google Scholar]
- 211.Qu Q., Sun G., Murai K., Ye P., Li W., Asuelime G., Cheung Y.-T., Shi Y. Wnt7a regulates multiple steps of neurogenesis. Mol. Cell. Biol. 2013;33:2551–2559. doi: 10.1128/MCB.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ferrón S.R., Charalambous M., Radford E., McEwen K., Wildner H., Hind E., Morante-Redolat J.M., Laborda J., Guillemot F., Bauer S.R., Fariñas I., Ferguson-Smith A.C. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Porlan E., Perez-Villalba A., Delgado A.C., Ferrón S.R. Paracrine regulation of neural stem cells in the subependymal zone. Arch. Biochem. Biophys. 2013;534:11–19. doi: 10.1016/j.abb.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 214.Goldman S.A., Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat. Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Song H., Stevens C.F., Gage F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 216.Shen Q., Goderie S.K., Jin L., Karanth N., Sun Y., Abramova N., Vincent P., Pumiglia K., Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 217.De Filippis L., Delia D. Hypoxia in the regulation of neural stem cells. Cell. Mol. Life Sci. 2011;68:2831–2844. doi: 10.1007/s00018-011-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kazanis I., Lathia J.D., Vadakkan T.J., Raborn E., Wan R., Mughal M.R., Eckley D.M., Sasaki T., Patton B., Mattson M.P., Hirschi K.K., Dickinson M.E., ffrench-Constant C. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J. Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kazanis I., ffrench-Constant C. Extracellular matrix and the neural stem cell niche. Dev. Neurobiol. 2011;71:1006–1017. doi: 10.1002/dneu.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barros C.S., Franco S.J., Müller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb. Perspect. Biol. 2011;3:a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sirko S., von Holst A., Wizenmann A., Götz M., Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–2738. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 222.Akita K., von Holst A., Furukawa Y., Mikami T., Sugahara K., Faissner A. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells. 2008;26:798–809. doi: 10.1634/stemcells.2007-0448. [DOI] [PubMed] [Google Scholar]
- 223.de Wit J., Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv. Exp. Med. Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- 224.Hack I., Bancila M., Loulier K., Carroll P., Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat. Neurosci. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- 225.D'Arcangelo G., Miao G., Chen S. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 226.Frotscher M. Role for Reelin in stabilizing cortical architecture. Trends Neurosci. 2010;33:407–414. doi: 10.1016/j.tins.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 227.Garcion E., Halilagic A., Faissner A., Ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–3432. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 228.Saghatelyan A., de Chevigny A., Schachner M., Lledo P.M. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat. Neurosci. 2004;7:347–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- 229.Naderi H., Matin M.M., Bahrami A.R. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J. Biomater. Appl. 2011;26:383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 230.Chen F.M., Wu L.A., Zhang M., Zhang R., Sun H.H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189–3209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 231.Orlando G., Baptista P., Birchall M., De Coppi P., Farney A., Guimaraes-Souza N.K., Opara E., Rogers J., Seliktar D., Shapira-Schweitzer K., Stratta R.J., Atala A., Wood K.J., Soker S. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl. Int. 2011;24:223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]