Abstract
Variations in spatio-temporal patterns of Human Monocytic Ehrlichiosis (HME) infection in the state of Kansas, USA were examined and the relationship between HME relative risk and various environmental, climatic and socio-economic variables were evaluated. HME data used in the study was reported to the Kansas Department of Health and Environment between years 2005–2012, and geospatial variables representing the physical environment [National Land cover/Land use, NASA Moderate Resolution Imaging Spectroradiometer (MODIS)], climate [NASA MODIS, Prediction of Worldwide Renewable Energy (POWER)], and socio-economic conditions (US Census Bureau) were derived from publicly available sources. Following univariate screening of candidate variables using logistic regressions, two Bayesian hierarchical models were fit; a partial spatio-temporal model with random effects and a spatio-temporal interaction term, and a second model that included additional covariate terms. The best fitting model revealed that spatio-temporal autocorrelation in Kansas increased steadily from 2005–2012, and identified poverty status, relative humidity, and an interactive factor, ‘diurnal temperature range x mixed forest area’ as significant county-level risk factors for HME. The identification of significant spatio-temporal pattern and new risk factors are important in the context of HME prevention, for future research in the areas of ecology and evolution of HME, and as well as climate change impacts on tick-borne diseases.
Introduction
Human Monocytic Ehrlichiosis (HME) is a frequently reported tick-borne disease in the south central and southeastern USA. The severity of the disease ranges from a mild non-symptomatic infection to death in humans. Although most known HME cases are not fatal, approximately 3–5% of all ehrlichial infections in the USA result in deaths despite patients receiving appropriate care [1]. Prevention almost entirely relies upon how well humans can avoid ticks; vaccines are not available and managing ticks or quantifying management effectiveness can be difficult. Ehrlichia chaffeensis, an obligate intercellular bacterium affecting the monocytes and macrophages is responsible for HME, and infections are vectored by the Lone Star tick, Amblyomma americanum, a widespread tick species in the central Midwest and southern regions of the USA. A second bacterium E. ewingii, that target the granulocytes also results in similar clinical symptoms but is relatively less frequently encountered [2].
The number of reported cases and the spatial distribution of HME have steadily increased over the past decade since the Centers for Disease Control and Prevention, USA (CDC) designated HME as a reportable disease [2], and per Kansas Administrative Regulations, Ehrlichiosis was first reportable in Kansas in the year 2000. State epidemiologists believe during this period in Kansas, the focus area of this study, HME has gone from a disease that was reported only from a few counties in the southeast corner of the state to being more numerous and widespread across the eastern part of the state. Spatio-temporal dynamics of tick-borne diseases could be influenced by geospatial factors including climate, land cover/land use and socio-economic conditions, which are currently not known for HME in Kansas but could facilitate our understanding the mechanisms of HME transmission and guide disease control strategies under the current social, climatic and environmental changes.
Prior research on HME using GIS and remote-sensing methods has established some very useful hypotheses. Yabsley et al., (2005) [3], identified the relationship between E. chaffeensis and several geospatial variables (land cover/land use, forest fragmentation) using kriging surfaces and logistic regressions. Wimberly et al., (2008) [4] later showed the importance of including model parameters to account for spatial autocorrelation and spatial randomness in geostatistical studies using E. chaffeensis and Anaplasma phagocytophilum examples. This study also identified similar meteorological and land cover/land use variables as important predictors for E. chaffensis. The spatial distributions of the same pathogens were predicted using habitat-level ecological variables in the Mississippi valley by Manangan et al., (2007) [5] wherein the relative importance of variables (soil moisture and forest cover) at different spatial scales was shown. The former two studies considered relatively large regions for the spatial extent, and all had used seropositivity rates among deer populations as indicators of county or public land-level ehrlichial pathogen prevalence.
Influential factors affecting the prevalence and distribution of diseases could differ from one region to another due to natural changes in the geography, and they are quite scale-dependent [6], [7]. In addition, the relevance of certain spatial determinants often becomes evident only in the presence of others. Particularly, climatic variables are often confounded or interactive with other influential factors viz., land cover/land use and socio-economic characteristics and can be difficult to detect [8], [9]. Further, much of the prior work on HME spatial epidemiology is based on conventional statistical approaches that have limitations when epidemiologic datasets are analyzed. Bayesian hierarchical modeling has been recognized as a powerful analytical technique to provide more robust posterior estimates, since they allow for incorporating errors that may arise from mean or median estimates of the independent covariates and observed data through the use of prior probability distributions [10], [11].
The purpose of this study was to evaluate the presence of spatio-temporal autocorrelation and the relationship between key environmental, climatic and socio-economic factors with HME case reports in Kansas where this and other tick-borne diseases are a growing concern. We used annual county-level prevalence data for the period 2005–2012 and modeled the spatio-temporal autocorrelation and effects of independent covariates using Bayesian hierarchical modeling approach.
Materials and Methods
Data
Ethics statement
No patient names were recorded to maintain patient confidentiality and to adhere to the International Ethical Guidelines for Biomedical Research Involving Human Subjects. All patient records/information was anonymized and de-identified prior to analysis. The use of HME data was approved by the Internal Review Boards at Kansas State University and Kansas Department of Health and Environment (IRB # 6733).
HME data
Records of Kansas residents whose HME diagnosis or HME-related laboratory results were reported to the Kansas Department of Health and Environment (KDHE) from 2005 through 2012 were used in the study. Records that were classified as confirmed, probable or suspect as indicated by the Council of State and Territorial Epidemiologists' (CSTE) case definition were considered to be cases [12]. Further detail on case definitions is provided in File S1.
Cases were associated with their county of residence, due to the difficulty in determining where each infection originated, and because this was not routinely assessed or recorded.
Covariates
The publicly available 2006 National Land Cover Dataset [13] for the study region was obtained from the United States Geological Survey (USGS) in a raster grid format. Land cover grids within each county were extracted from the raster dataset and the percentage area they occupy were estimated. A list of land cover variables evaluated in the study is present in Table 1. Among climate variables, the maximum normalized vegetation index (NDVI); minimum land surface temperature (LST); mean LST; diurnal temperature range (DTR) (the difference between daily maximum and minimum temperature averaged over a thirty day period); precipitation and humidity were extracted for each county in the study area. The LST and NDVI estimates were derived from MODIS (Moderate Resolution Imaging Spectroradiometer) imagery [14]. DTR, precipitation and relative humidity were derived from the Prediction of Worldwide Renewable Energy (POWER) web portal of the NASA Langley Research Center [15].
Table 1. Land cover types found in NLCD.
| Land cover land use data | Land cover types |
| National Land Cover Dataset (source: MRLC (2011); years1: 1992–2001; resolution2: 30 m; spatial scale3: 1∶100,000). | Open water, developed—open space, developed—low intensity, developed—medium intensity, developed—high intensity, barren land, deciduous forest, evergreen forest, mixed forest, scrub/shrub, grassland/herbaceous, pasture/hay, cultivated crops, woody wetlands, emergent herbaceous wetland. |
Years represent the time period during which satellite images of land cover were captured for creating the data set, including multiple images within a year.
Resolution indicates the fineness of ground data as captured by a satellite image, shorter resolution meaning higher clarity;
Spatial scale indicates the scale for which interpretations are appropriate.
U.S. Census 2010 data on population and housing were obtained from the National Historical Geographic Information System (NHGIS), a publicly available online resource for U.S. Census Bureau's historical and current population data [16]. Identical census attribute information for Kansas was gathered at the county level. Geographic boundary files for counties were also obtained from the NHGIS. From the tables, 20 housing and 23 population related variables (Table 2) were extracted for each county by spatial query and joined to the census shapefiles using the common GIS codes.
Table 2. Population and housing variables evaluated in the study.
| Census category | Independent variablesa |
| Housing | Housing units (total housing units), Tenure (owner occupied, renter occupied), Tenure (Historic or Latino Householder) (owner occupied, renter occupied), Race of householder (white alone, Black or African American alone, Asian alone), Household size (1-person, 2-person, 3-person, 4-person, 5-person, 6-person, or 7-more person household), Year structure built (Built 2005 or later, 2000 to 2004, 1990 to 1999, 1980 or earlierb). (20 variables). |
| Population | Population (total population), Race (White alone, Black or African American alone, Asian alone), Household income in the past 12 months (Less than $10,000, $10,000 to $14,999, and thirteen other variables that represented $49,999 incremental income thereof up to $199,999, and $200,000 or more), Poverty status in the previous 12 months (income in the past 12 months below poverty level, income in the past 12 months at or above poverty level). (23 variables). |
Definitions of different census variables can be found from their source (NHGIS) website at: https://www.nhgis.org/.
Observations for all the independent variables are counts, in continuous form, and recorded per areal unit (block group, tract or county). Items in italics are Census Table names, and items within parenthesis are independent variables.
The variable 1980 or earlier was derived by summing all the number of houses built prior to 1980 originally available in five-year increments in census.
Statistical analysis and model specification
Let  be the observed number of HME cases among
be the observed number of HME cases among 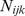 individuals at risk in the population of county
individuals at risk in the population of county  , diagnosed with HME in year
, diagnosed with HME in year  and of gender
and of gender  . We modeled
. We modeled  to follow a Poisson approximation,
to follow a Poisson approximation,  , where
, where  is the expected number of the population at risk for HME and
is the expected number of the population at risk for HME and  is the relative risk. Since HME prevalence is disproportionate among different age groups and gender [17], [18], standardized rates were calculated assuming 9 10-year age classes
is the relative risk. Since HME prevalence is disproportionate among different age groups and gender [17], [18], standardized rates were calculated assuming 9 10-year age classes  Thus, the expected number of HME cases was calculated by
Thus, the expected number of HME cases was calculated by
We used a logit link function in an extended generalized linear model (GLM) structure that incorporated stochastic spatial and temporal functions and as well as different covariate effects. Several models that allowed us to evaluate random and covariate effects on HME prevalence were fitted individually. First, a partial spatio-temporal model (partial ST) was fitted that was notated as following,
Where,  represents the mean prevalence of HME in all counties in all years, and
represents the mean prevalence of HME in all counties in all years, and  and
and  are random terms accounting for spatially structured variation in HME prevalence and unstructured heterogeneity, respectively. No interaction was assumed to exist between
are random terms accounting for spatially structured variation in HME prevalence and unstructured heterogeneity, respectively. No interaction was assumed to exist between  and
and  and were assigned
and were assigned  and
and  priors. Spatial dependence in
priors. Spatial dependence in was applied by assuming a conditional autoregressive model
was applied by assuming a conditional autoregressive model  with a Gaussian distribution, which implies that each
with a Gaussian distribution, which implies that each  is conditional on the neighbor
is conditional on the neighbor  with variance
with variance 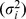 dependent on the number of neighboring counties
dependent on the number of neighboring counties 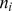 of county
of county  , i.e.,
, i.e.,
A random effect 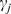 to account for the temporal component of the data was included and was assigned a random walk prior
to account for the temporal component of the data was included and was assigned a random walk prior  [19]. In order to detect potential spatio-temporal interaction effects in HME prevalence,
[19]. In order to detect potential spatio-temporal interaction effects in HME prevalence,  term was included in the partial ST model, which had a
term was included in the partial ST model, which had a  prior [20].
prior [20].
For the covariate model, different covariates were included to the partial ST model in several steps, starting with a model that included all covariates followed by removal of one variable at each step. Covariates were retained in the model unless their removal resulted in the increase of DIC value by 5 units or more. Several two-way interaction effects between covariates were also included in these steps. Candidate explanatory variables to be included in the Bayesian hierarchical models were screened a priori in order to avoid model fitting issues. Several frequentist bivariate logistic regression models evaluated each variable independently and only variables that were significant at p<0.2 were kept. A logistic regression takes the form,
Where  is HME relative risk,
is HME relative risk,  the intercept coefficient, and
the intercept coefficient, and  the coefficient for the explanatory variable
the coefficient for the explanatory variable  Care was taken not to remove candidate variables that were deemed clinically relevant [19]. Multicollinearity among screened variables was tested by estimating the variance inflation factor (VIF) and all variables with a VIF≥10 were considered to indicate multicollinearity [21], in which case, one of the variables was dropped at a time until multicollinearity was absent. Non-linearity among independent variables was evaluated at the screening stage with logistic regressions. Significant variables with non-linearity were categorized using cutoffs based on scatter-plots.
Care was taken not to remove candidate variables that were deemed clinically relevant [19]. Multicollinearity among screened variables was tested by estimating the variance inflation factor (VIF) and all variables with a VIF≥10 were considered to indicate multicollinearity [21], in which case, one of the variables was dropped at a time until multicollinearity was absent. Non-linearity among independent variables was evaluated at the screening stage with logistic regressions. Significant variables with non-linearity were categorized using cutoffs based on scatter-plots.
Model posterior parameters were estimated using a Bayesian framework implemented using R-INLA software [22] on a Linux Beocat cluster computing environment [23]. Distributions of covariate effects on HME prevalence are seldom available for the region; therefore non-informative, uniform priors were selected for the regression parameters,  and their variance components,
and their variance components,  This allows the observed data to have the greatest influence on posterior distributions without being constrained by the choice of prior [24]. The mean estimates from the posterior distribution and their 95% credible intervals (CrI) were calculated and exponentiated to provide odds ratios (ORs) and their corresponding uncertainty measures.
This allows the observed data to have the greatest influence on posterior distributions without being constrained by the choice of prior [24]. The mean estimates from the posterior distribution and their 95% credible intervals (CrI) were calculated and exponentiated to provide odds ratios (ORs) and their corresponding uncertainty measures.
Models were validated by randomly partitioning the county-level relative risk estimates into five subsets and by running the models using only four of the five subsets, while validating model prediction with the fifth subset. The models were run for five times to allow each validation with subset. Each time, the model's performance (prediction accuracy) was measured using area under the receiver-operator's curve (AUC) values with the observed prevalence (dichotomized as 0 or ≥0)”. The mean error and mean absolute error were calculated to quantify prediction bias and overall precision respectively.
Results
There were 347 HME cases reported to KDHE between the years 2005–2012 predominantly distributed in the south and eastern counties of Kansas. A HME case in the dataset was under one of three categories: confirmed (n = 38), probable (n = 63), and suspect (n = 246). Table 3 shows a list of all candidate variables that were significant at p<0.2 level that were evaluated in this study, and Table 4 has the odds ratios and 95% CrIs for significant variables from the Bayesian spatio-temporal models. Non-linearity among significant variables was not observed. The best fitting model indicated significant spatio-temporal effect, income in the past 12 months below poverty level (henceforth, poverty), relative humidity, and an interaction term, ‘DTR x % mixed forest vegetation’ to be significantly associated with HME.
Table 3. Results of univariate logistic regression analysis of candidate covariates evaluated in the study.
| Covariate | Odds ratio (95% CI) | P-value |
| Income in the past 12 months below poverty level | 2.45 (2.02, 2.99) | 0.04 |
| Household size (>5 person) | 1.61 (1.09, 2.39) | 0.17 |
| Relative humidity | 2.77 (2.27, 3.37) | 0.01 |
| Minimum land surface temperature | 1.34 (1.02, 1.77) | 0.12 |
| Diurnal temperature range | 2.74 (1.03, 7.31) | 0.17 |
| % Mixed forest area | 1.49 (1.00, 2.20) | 0.18 |
| Diurnal temperature range x % mixed forest area | 1.82 (1.49, 2.21) | 0.04 |
Table 4. Odds ratios and 95% Credible Intervals (CrI) from two spatio-temporal models evaluating county-level Human Monocytic Ehrlichiosis (HME) prevalence data in Kansas, USA.
| Covariate | Partial ST model [Odds ratio (95% CrI)] | Covariate model [Odds ratio (95% CrI)] |
| Income in the past 12 months below poverty level | 1.82 (1.49, 2.21) | 2.22 (1.82, 2.70) |
| Relative humidity | 3.38 (2.73, 4.20) | 3.49 (2.81, 4.33) |
| Diurnal temperature range x % mixed forest area | 3.00 (1.37, 6.57) | 3.25 (1.48, 7.12) |
Deviance information criterion (DIC) obtained by fitting a Bayesian equivalent was recorded as 3,754, and 2,472, and the σ2 (variance component) was 3.41 (1.31–4.81), and 2.33 (1.14–3.42) for the partial ST model and covariate models, respectively.
The covariate model which incorporated terms for individual covariates in addition spatio-temporal interaction effect performed relatively better than the partial ST model for all years (Table 5), and all further interpretations were based on this model alone. The spatio-temporal autocorrelation parameter  and their 95% CrI estimates quantified the infection risk between the counties over the study period and are plotted in Figure 1. The plot reveals positive and increasing autocorrelation between counties every year with a slight decrease in 2010–2011 period and only moderate increase during the latter part of the study period. The crude rate ratio of reported HME infections per county standardized by county population, and a smoothed map of posterior relative risk of counties between years 2005–2012 is shown in Figs. 2, 3, respectively. The posterior relative risk estimates are based on the final model with environmental, climatic and socio-economic predictors and correspond to the median of the posterior predictive distribution.
and their 95% CrI estimates quantified the infection risk between the counties over the study period and are plotted in Figure 1. The plot reveals positive and increasing autocorrelation between counties every year with a slight decrease in 2010–2011 period and only moderate increase during the latter part of the study period. The crude rate ratio of reported HME infections per county standardized by county population, and a smoothed map of posterior relative risk of counties between years 2005–2012 is shown in Figs. 2, 3, respectively. The posterior relative risk estimates are based on the final model with environmental, climatic and socio-economic predictors and correspond to the median of the posterior predictive distribution.
Table 5. Model validation summary for HME relative risk,  in Kansas between years 2005–2012.
in Kansas between years 2005–2012.
| Year | Model | AUC* | Mean error† | Mean absolute error (%)‡ |
| 2005 | Partial ST | 0.66 (0.61–0.71) | −0.14 | 6.12 |
| Covariate | 0.69 (0.65–0.72) | −0.16 | 5.08 | |
| 2006 | Partial ST | 0.66 (0.62–0.71) | 0.11 | 6.27 |
| Covariate | 0.72 (0.68–0.74) | 0.09 | 5.14 | |
| 2007 | Partial ST | 0.69 (0.59–0.72) | 0.12 | 3.94 |
| Covariate | 0.72 (0.69–0.74) | 0.11 | 4.01 | |
| 2008 | Partial ST | 0.72 (0.65–0.73) | 0.14 | 5.21 |
| Covariate | 0.74 (0.68–0.77) | 0.12 | 4.88 | |
| 2009 | Partial ST | 0.71 (0.58–0.72) | 0.12 | 6.21 |
| Covariate | 0.73 (0.70–0.75) | 0.11 | 5.47 | |
| 2010 | Partial ST | 0.69 (0.61–0.72) | 0.10 | 4.22 |
| Covariate | 0.76 (0.71–0.80) | 0.09 | 4.18 | |
| 2011 | Partial ST | 0.70 (0.65–0.71) | 0.11 | 3.28 |
| Covariate | 0.74 (0.68–0.75) | 0.11 | 3.08 | |
| 2012 | Partial ST | 0.71 (0.67–0.72) | 0.08 | 4.88 |
| Covariate | 0.73 (0.71–0.74) | 0.09 | 3.97 |
*AUC values in the range of 0.5–0.7 indicates poor discriminative capacity, 0.7–0.9 is considered reasonable and >0.9 to be very good.
Overall tendency to over or under-predict relative risk.
Overall precision of models estimated using magnitude of error in predictions.
Figure 1. Spatial autocorrelation parameter (ψij) and 95% CrI for county level human monocytic ehrlichiosis (HME) relative risk between years 2005–2012 in Kansas.
Figure 2. Kansas county level crude rate ratios for human monocytic ehrlichiosis (HME) in relation to total population (normalized by 10,000) between years 2005–2012.
Figure 3. Smoothed relative risk maps for human monocytic ehrlichiosis (HME) in Kansas from 2005–2012.
Discussion
Results from this study shows that between years 2005–2012 there has been a general northwestwardly progression of HME prevalence in Kansas (Fig. 1), and a combination of climatic, environmental and socio-economic factors are important determinants of HME. It is notable that our results are potentially affected by our assumption that infections occurred within the county of residence even though the disease may have been contracted elsewhere. Only a fraction of cases were interviewed to determine where their infections originated, and their responses are subject to recall bias, another potential source of error. Another limitation in the study is the likelihood for clinicians in southeast Kansas to order more tests for HME than others where tick related illnesses are less of a concern. These limitations are typical for many retrospective spatial epidemiological studies, which can only be mitigated by conducting carefully designed prospective studies. Our study however underscores the need for such efforts in Kansas and the general region.
The overall spatial distribution of high-risk counties found in this study conforms to the approximate A. americanum tick distribution in Kansas, the primary vector for this disease, currently estimated by the CDC [25]. Although, CDC estimate of A. americanum distribution in Kansas could be an underestimate since their predictions were based on an acarologic survey conducted around the year 1945 [26]. It is likely for environmental and anthropogenic factors to have altered distribution of this species over these decades. The spatial pattern found in the present study is different from Wimberley et al., (2008) [4], which were mostly discontinuous and sparse within Kansas. This study considered a much larger spatial extent and had used deer serology results for prevalence data. Among all the geospatial variables evaluated in the present study, relative humidity, poverty status, and the combined effect of diurnal temperature range and mixed forest area appear to have played an important role in the spatial and temporal aspects of HME prevalence and in the vector/pathogen transmission cycle. While previous studies have evaluated various geospatial factors as determinants for ehrlichia ‘endemnicity’ [3]–[5], to our knowledge, this is the first time socio-economic factors were included and later identified as risk factors for HME using human incidence rates, and our Bayesian models provide some fresh information on HME spatial epidemiology in Kansas that are perhaps applicable to the broader region of the USA. This hypothesis, however, remains to be tested by conducting similar studies spanning various geographical regions of the USA. The current study lays the foundation for initiating such studies.
Humidity, the amount of water present in the atmosphere has been associated with tick survival in North America [27]–[29] and it is considered to be an important climate related delimiter to the spatial distribution of ticks [30]. There are large variations in the yearly precipitation received across the state of Kansas, with eastern Kansas receiving up to three times more rainfall than west [31]. As a result, climate and vegetation are transitional between the humid east and semi-arid western portion of Kansas that may explain the noted geographic pattern for HME in the present study. Humidity can often be seen associated with the survival and abundance of ticks in the literature, with higher humidity conditions often favoring the long-term survival of some ticks species' life stages through dry seasons [32], [33] among other reasons. Also, higher humidity conditions that are typically recorded during late spring and summer months correlate with higher human outdoor activities, which may increase exposure to infected ticks.
The interaction effect between diurnal temperature range and % mixed forest area towards HME prevalence is similar to our previous finding of the same association with feline cytauxzoonosis in the region [9]. Like HME, this disease is also transmitted by A. americanum ticks, but to felids and several wildlife hosts. One interpretation of the noted interaction effect could be that it is indicative of ticks' biological response to changes in DTR, but that is only specific to mixed forest areas and no other land cover types. Although previous studies have found that the host-seeking behavior of ticks [34] and the survival of tick-borne parasites [35] to be strongly influenced by DTR, the reason for interactive effect with mixed forest area is not clearly known. Mixed forest areas in the study region are likely suitable deer habitats, and are defined by the EPA as areas dominated by trees generally greater than 5 meters tall, and greater than 20% of total vegetation cover. Neither deciduous nor evergreen species are greater than 75 percent of total tree cover in mixed forests [13]. We suspect that in addition to changes in DTR, factors such as deer density, E. chaffeensis prevalence among deer and mixed forest wildlife hosts, and human interactions with mixed forest areas for recreation and hunting activities could be reasons behind this finding. A. americanum is an important vector for many pathogens in the region, and they appear to be influenced by DTR based on our previous and present studies. Laboratory examinations of DTR effects on the phenology and host-seeking behavior of A. americanum and the pathogenicity of ehrlichial pathogens the ticks carry are warranted.
The mechanistic basis for humidity-HME linkage and DTR-mixed forest interactive association with HME at the organismal level (for both A. americanum and E. chaffeensis) is likely to involve multiple pathways and their understanding is important in the context of ecology and evolution of HME, and also in the context of climate change effects on vector-borne diseases. Schwartz (1995) [36] documented an increase in more humid air masses in the later part of the 20th century for eastern Kansas and Missouri and attributed this increase to climate change. The number of reported HME cases in Kansas has increased during the 2005–2012 period, and expanded from extreme southeastern Kansas to a large area of eastern Kansas. This spatio-temporal expansion and changing climate could be related and the linkages is worthy of investigation. Similar suggestions for other tick-borne diseases can be found in the literature [37]–[39]. Also, DTR is considered to be an important climate-change index [40], [41] which has been steadily decreasing since the 1950s due to daily temperature changes [40], [42]. Identifying consistent associations of relative humidity and DTR (either directly or as an interactive factor) with tick-borne diseases from other geographic regions will be useful in our efforts to quantify climate change effects on tick-borne diseases.
Socio-economic status is not frequently associated with tick-borne diseases in the US. Recent studies in Europe however have found mainly poverty but also political and other socio-economic factors with tick-borne encephalitis (TBE) [43], [44]. Also, principal components analyses have revealed lower socio-economic conditions to be stronger predictors compared to climate related factors for the TBE outbreak in Europe during the past decade [45]. Although the diseases that are being discussed here are caused and transmitted by different species, the role of socio-economic factors on HME is worth considering in future investigations. It is noteworthy that in Kansas, the spatial pattern for HME found in the present study and the distribution of poorly ranked counties for health is remarkably similar with only rare exceptions [46]. Poverty association with HME could be related to individuals engaging in outdoor occupations and/or living in close proximity to areas that favor tick habitats. Poverty status could also be a proxy for weakened immune system among individuals, lower literacy levels, and less awareness towards tick-borne diseases and their prevention methods. Studies to quantify individual level socio-economic status on HME incidence and any disparities in access to health care among high risk counties would be useful in preventing this disease.
Conclusions
This study has shown a steady spatio-temporal progression of HME prevalence in Kansas as indicated by the strong autocorrelation estimates and the smoothed maps, and has also found some previously unknown risk factors for this disease. HME incidence in Kansas and much likely other endemic regions in the USA are affected by a combination of factors including climate, land cover/land use and socio-economic conditions. Quantifying the linkages between meteorological factors and the distribution and phenology of A. americanum and E. chaffeensis is needed for both prevention of HME and also for understanding the ecology and evolution of this disease and climate-change impacts. Bayesian spatio-temporal modeling approaches have advantages over traditional Frequentist inference methods and hold promise for disease monitoring and evaluation purposes.
Supporting Information
HME case selection criteria.
(DOCX)
Acknowledgments
We thank Bryanna Pockrandt and Mal Hoover, College of Veterinary Medicine, Kansas State University for excellent technical assistance.
Funding Statement
This work is supported by the National Institutes of Health, USA grant # AI070908 and Kansas State Veterinary Diagnostic Laboratory. This manuscript is contribution number 14-205-J from the Kansas Agricultural Experiment Station. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pavelites JJ, Prahlow JA (2011) Fatal human monocytic ehrlichiosis: a case study. Forensic Science Medicine and Pathology 7: 287–293. [DOI] [PubMed] [Google Scholar]
- 2.CDC Ehrlichiosis Website. Available: http://www.cdc.gov/ehrlichiosis/stats/. Accessed 2014 Jun 12.
- 3. Yabsley MJ, Wimberly MC, Stallknecht DE, Little SE, Davidson WR (2005) Spatial analysis of the distribution of Ehrlichia chaffeensis, causative agent of human monocytotropic ehrlichiosis, across a multi-state region. American Journal of Tropical Medicine and Hygiene 72: 840–850. [PubMed] [Google Scholar]
- 4. Wimberly MC, Baer AD, Yabsley MJ (2008) Enhanced spatial models for predicting the geographic distributions of tick-borne pathogens. International Journal of Health Geographics 7: 15 doi:10.1186/1476-072X-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manangan JS, Schweitzer SH, Nibbelink N, Yabsley MJ, Gibbs SEJ, et al. (2007) Habitat factors influencing distributions of Anaplasma phagocytophilum and Ehrlichia chaffeensis in the Mississippi alluvial valley. Vector-Borne and Zoonotic Diseases 7: 563–573. [DOI] [PubMed] [Google Scholar]
- 6. Fotheringham AS, Wong DWS (1991) The modifiable areal unit problem in multivariate statistical-analysis. Environment and Planning A 23: 1025–1044. [Google Scholar]
- 7. Raghavan RK, Brenner KM, Harrington JA Jr, Higgins JJ, Harkin KR (2013) Spatial scale effects in environmental risk-factor modelling for diseases. Geospatial health 7: 169–182. [DOI] [PubMed] [Google Scholar]
- 8. Patz JA, Graczyk TK, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. International Journal for Parasitology 30: 1395–1405. [DOI] [PubMed] [Google Scholar]
- 9.Raghavan RK, Almes K, Goodin DG, Harrington JA, Stackhouse PW, (In press) Spatially heterogeneous land-cover/land-use and climatic risk factors of tick-borne feline cytauxzoonosis. Vector-Borne and Zoonotic Diseases. [DOI] [PubMed]
- 10. Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL, et al. (2004) A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. International Journal for Parasitology 34: 569–576. [DOI] [PubMed] [Google Scholar]
- 11.Lawson AB (2013) Bayesian disease mapping: hierarchical modeling in spatial epidemiology. Vol. 32 . CRC Press. [Google Scholar]
- 12.CDC National Notifiable Diseases Surveillance System (NNDSS) Website. Available: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=667&DatePub=1/1/2008%2012:00:00%20AM. Accessed 2014 Jun 12.
- 13.Multi-Resolution Land Characteristics Consortium (MRLC) Website. Available: http://www.mrlc.gov/nlcd2001.php. Accessed 2014 Jun 12.
- 14.USGS Land Processes Distributed Active Archive Center (LP DACC) Website. Available: https://lpdaac.usgs.gov/products/modis_products_table. Accessed 2014 Jun 12.
- 15. Eckman RS, Stackhouse PW Jr (2012) CEOS contributions to informing energy management and policy decision making using space-based Earth observations. Applied Energy 90: 206–210. [Google Scholar]
- 16.National Historical Geographic Information System (NHGIS) Website. Available: https://www.nhgis.org/. Accessed 2014 Jun 12.
- 17. Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL (2005) Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001–2002. American Journal of Tropical Medicine and Hygiene 73: 400–409. [PubMed] [Google Scholar]
- 18.CDC Ehrlichiosis Website. Available: http://www.cdc.gov/ehrlichiosis/stats/. Accessed 2014 Jun 2.
- 19.R-INLA Random Walk Model of Order 1 Website. Available: http://www.math.ntnu.no/inla/r-inla.org/doc/latent/rw1.pdf. Accessed 2014 Jun 12.
- 20.Lawson AB (2013) Bayesian disease mapping. Hierarchical modeling in spatial epidemiology. Second Edition. CRC Press New York.
- 21.Hosmer DW, Lemeshow S (1990) Model-Building Strategies and Methods for Logistic Regression. Applied Logistic Regression. Second Edition ed. pp. 91–142.
- 22.R-INLA Project Website. Available: http://www.r-inla.org/. Accessed 2014 Jun 12.
- 23.Beocat Documentation Website. Available: http://www.cis.ksu.edu/beocat/documentation. Accessed 2014 Jun 2.
- 24.Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Posterior simulation: In: Bayesian Data Analysis. Boca Raton, FL: Chapmann and Hall/CRC. pp 283–310.
- 25.CDC Approximate Distribution of the Lone Star Tick Website. Available: http://www.cdc.gov/ticks/maps/lone_star_tick.html. Accessed 2014 Jun 12.
- 26. Bishopp FC, Trembley HL (1945) Distribution and hosts of certain North American ticks. Journal of Parasitology 31: 1–54. [Google Scholar]
- 27. Hair JA, Sauer JR, Durham KA (1975) Water-balance and humidity preference in 3 species of ticks. Journal of Medical Entomology 12: 37–47. [DOI] [PubMed] [Google Scholar]
- 28. Rodgers SE, Zolnik CP, Mather TN (2007) Duration of exposure to suboptimal atmospheric moisture affects nymphal blacklegged tick survival. Journal of Medical Entomology 44: 372–375. [DOI] [PubMed] [Google Scholar]
- 29. Yoder JA, Hedges BZ, Benoit JB (2012) Water balance of the American dog tick, Dermacentor variabilis, throughout its development with comparative observations between field-collected and laboratory-reared ticks. International Journal of Acarology 38: 334–343. [Google Scholar]
- 30. Wimberly MC, Yabsley MJ, Baer AD, Dugan VG, Davidson WR (2008) Spatial heterogeneity of climate and land-cover constraints on distributions of tick-borne pathogens. Global Ecology and Biogeography 17: 189–202. [Google Scholar]
- 31.Goodin DG, Mitchell JE, Knapp MC, Bivens RE (2004) Climate and weather atlas of Kansas. An Introduction. http://www.k-state.edu/ksclimate/documents/kgsed.pdf Accessed: 2014 Jun 12.
- 32. Suess J, Gerstengarbe F-W (2008) What makes ticks tick? Climate change, ticks and tick-borne diseases. Parasitology Research 103: S157–S158. [DOI] [PubMed] [Google Scholar]
- 33. Berger KA, Wang Y, Mather TN (2013) MODIS-derived land surface moisture conditions for monitoring blacklegged tick habitat in southern New England. International Journal of Remote Sensing 34: 73–85. [Google Scholar]
- 34. Randolph SE, Storey K (1999) Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): Implications for parasite transmission. Journal of Medical Entomology 36: 741–748. [DOI] [PubMed] [Google Scholar]
- 35. Ochanda H (2006) Comparison of the survival of Theileria parva-infected adult Rhipicephalus appendiculatus (Acari: Ixodidae) and their infection under simulated climate conditions in the laboratory and in the field. International Journal of Tropical Insect Science 26: 101–107. [Google Scholar]
- 36. Schwartz MD (1995) Detecting structural climate-change - an air mass-based approach in the north central united-states, 1958-1992. Annals of the Association of American Geographers 85: 553–568. [Google Scholar]
- 37. Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E (2009) Effects of climate change on ticks and tick-borne diseases in Europe. Interdisciplinary perspectives on infectious diseases 2009: 593232–593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gilbert L (2010) Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia 162: 217–225. [DOI] [PubMed] [Google Scholar]
- 39. Randolph SE (2010) To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Veterinary Parasitology 167: 92–94. [DOI] [PubMed] [Google Scholar]
- 40. Karl TR, Kukla G, Razuvayev VN, Changery MJ, Quayle RG, et al. (1991) Global warming - evidence for asymmetric diurnal temperature-change. Geophysical Research Letters 18: 2253–2256. [Google Scholar]
- 41.Braganza K, Karoly DJ, Arblaster JM (2004) Diurnal temperature range as an index of global climate change during the twentieth century. Geophysical Research Letters 31.. [Google Scholar]
- 42. Karl TR, Jones PD, Knight RW, Kukla G, Plummer N, et al. (1993) A new perspective on recent global warming - asymmetric trends of daily maximum and minimum temperature. Bulletin of the American Meteorological Society 74: 1007–1023. [Google Scholar]
- 43. Sumilo D, Bormane A, Asokliene L, Vasilenko V, Golovljova I, et al. (2008) Socio-economic factors in the differential upsurge of tick-borne encephalitis in Central and Eastern Europe. Reviews in Medical Virology 18: 81–95. [DOI] [PubMed] [Google Scholar]
- 44.Stefanoff P, Rosinska M, Samuels S, White DJ, Morse DL, et al. (2012) A National Case-Control Study Identifies Human Socio-Economic Status and Activities as Risk Factors for Tick-Borne Encephalitis in Poland. Plos One 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godfrey ER, Randolph SE (2011) Economic downturn results in tick-borne disease upsurge. Parasites & Vectors 4. [DOI] [PMC free article] [PubMed]
- 46.County Health Ranking Website. Available: http://www.countyhealthrankings.org/app/kansas/2013/rankings/outcomes/overall/by-rank. Accessed 2014 Jun 12.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HME case selection criteria.
(DOCX)





