Abstract
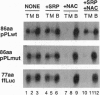
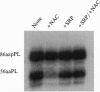
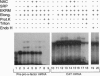
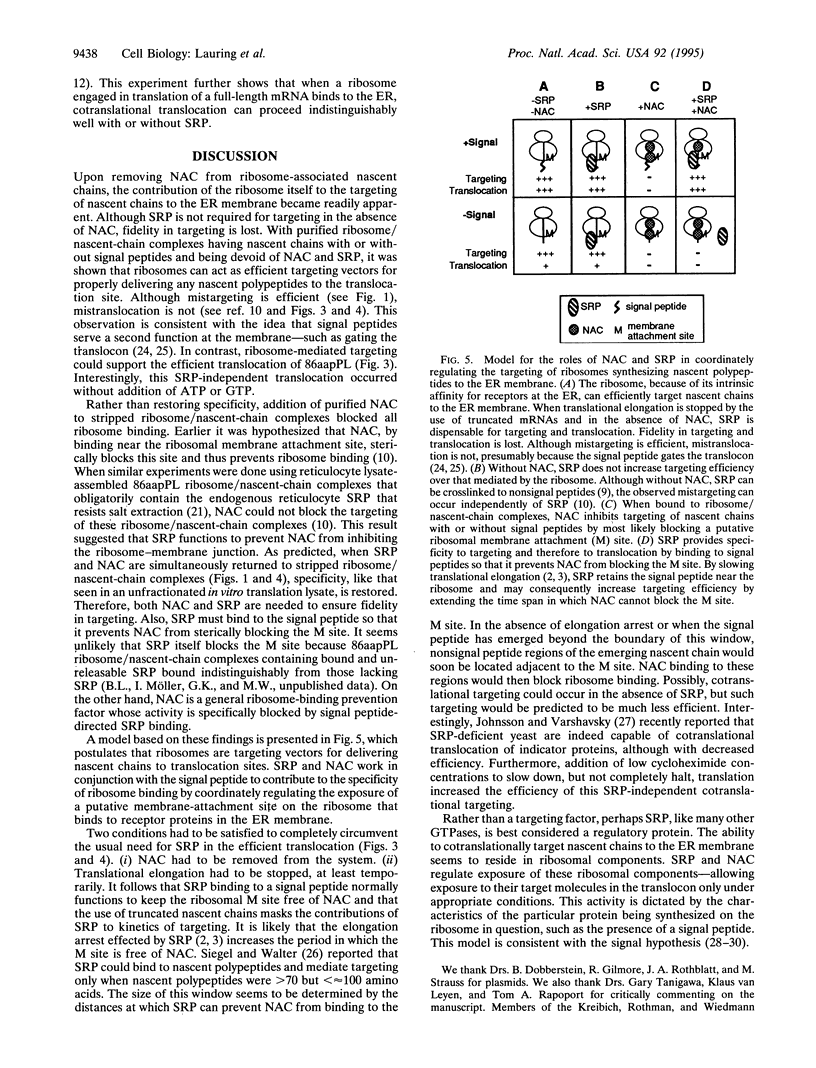
Signal peptides direct the cotranslational targeting of nascent polypeptides to the endoplasmic reticulum (ER). It is currently believed that the signal recognition particle (SRP) mediates this targeting by first binding to signal peptides and then by directing the ribosome/nascent chain/SRP complex to the SRP receptor at the ER. We show that ribosomes can mediate targeting by directly binding to translocation sites. When purified away from cytosolic factors, including SRP and nascent-polypeptide-associated complex (NAC), in vitro assembled translation intermediates representing ribosome/nascent-chain complexes efficiently bound to microsomal membranes, and their nascent polypeptides could subsequently be efficiently translocated. Because removal of cytosolic factors from the ribosome/nascent-chain complexes also resulted in mistargeting of signalless nascent polypeptides, we previously investigated whether readdition of cytosolic factors, such as NAC and SRP, could restore fidelity to targeting. Without SRP, NAC prevented all nascent-chain-containing ribosomes from binding to the ER membrane. Furthermore, SRP prevented NAC from blocking ribosome-membrane association only when the nascent polypeptide contained a signal. Thus, NAC is a global ribosome-binding prevention factor regulated in activity by signal-peptide-directed SRP binding. A model presents ribosomes as the targeting vectors for delivering nascent polypeptides to translocation sites. In conjunction with signal peptides, SRP and NAC contribute to this specificity of ribosomal function by regulating exposure of a ribosomal membrane attachment site that binds to receptors in the ER membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Mok W., Kreibich G., Sabatini D. D. Ribosomal-membrane interaction: in vitro binding of ribosomes to microsomal membranes. J Mol Biol. 1974 Sep 25;88(3):559–580. doi: 10.1016/0022-2836(74)90408-2. [DOI] [PubMed] [Google Scholar]
- Collins P. G., Gilmore R. Ribosome binding to the endoplasmic reticulum: a 180-kD protein identified by crosslinking to membrane-bound ribosomes is not required for ribosome binding activity. J Cell Biol. 1991 Aug;114(4):639–649. doi: 10.1083/jcb.114.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Crowley K. S., Liao S., Worrell V. E., Reinhart G. D., Johnson A. E. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994 Aug 12;78(3):461–471. doi: 10.1016/0092-8674(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Collins P., Johnson J., Kellaris K., Rapiejko P. Transcription of full-length and truncated mRNA transcripts to study protein translocation across the endoplasmic reticulum. Methods Cell Biol. 1991;34:223–239. doi: 10.1016/s0091-679x(08)61683-0. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Flint N., Dobberstein B. Requirements for the membrane insertion of signal-anchor type proteins. J Cell Biol. 1991 Apr;113(1):25–34. doi: 10.1083/jcb.113.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N., Varshavsky A. Ubiquitin-assisted dissection of protein transport across membranes. EMBO J. 1994 Jun 1;13(11):2686–2698. doi: 10.1002/j.1460-2075.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalies K. U., Görlich D., Rapoport T. A. Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J Cell Biol. 1994 Aug;126(4):925–934. doi: 10.1083/jcb.126.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Marcantonio E. E., Sabatini D. D. Ribophorins I and II: membrane proteins characteristic of the rough endoplasmic reticulum. Methods Enzymol. 1983;96:520–530. doi: 10.1016/s0076-6879(83)96045-7. [DOI] [PubMed] [Google Scholar]
- Lauring B., Sakai H., Kreibich G., Wiedmann M. Nascent polypeptide-associated complex protein prevents mistargeting of nascent chains to the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5411–5415. doi: 10.1073/pnas.92.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., High S., Wood H., Giner A., Tollervey D., Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992 Oct 22;359(6397):741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Nunnari J. M., Zimmerman D. L., Ogg S. C., Walter P. Characterization of the rough endoplasmic reticulum ribosome-binding activity. Nature. 1991 Aug 15;352(6336):638–640. doi: 10.1038/352638a0. [DOI] [PubMed] [Google Scholar]
- Prehn S., Wiedmann M., Rapoport T. A., Zwieb C. Protein translocation across wheat germ microsomal membranes requires an SRP-like component. EMBO J. 1987 Jul;6(7):2093–2097. doi: 10.1002/j.1460-2075.1987.tb02475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz A. J., Meyer D. I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature. 1990 Aug 9;346(6284):540–544. doi: 10.1038/346540a0. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. The affinity of signal recognition particle for presecretory proteins is dependent on nascent chain length. EMBO J. 1988 Jun;7(6):1769–1775. doi: 10.1002/j.1460-2075.1988.tb03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. Signal peptides open protein-conducting channels in E. coli. Cell. 1992 May 15;69(4):677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Tazawa S., Unuma M., Tondokoro N., Asano Y., Ohsumi T., Ichimura T., Sugano H. Identification of a membrane protein responsible for ribosome binding in rough microsomal membranes. J Biochem. 1991 Jan;109(1):89–98. [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Johnson A. E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Wiedmann B., Sakai H., Davis T. A., Wiedmann M. A protein complex required for signal-sequence-specific sorting and translocation. Nature. 1994 Aug 11;370(6489):434–440. doi: 10.1038/370434a0. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Signal recognition particle mediates a transient elongation arrest of preprolactin in reticulocyte lysate. J Cell Biol. 1989 Dec;109(6 Pt 1):2617–2622. doi: 10.1083/jcb.109.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]