Abstract
The current study (i) determined whether NeuroQuant® volumetrics are reflective of differences in medial temporal lobe (MTL) volumes between healthy older adults and those with mild cognitive impairment (MCI) and (ii) examined the relationship between RBANS indices and MTL volumes. Forty-three healthy older adults and 57 MCI patients completed the RBANS and underwent structural MRI. Hippocampal and inferior lateral ventricle (ILV) volumes were obtained using NeuroQuant®. Results revealed significantly smaller hippocampal and larger ILV volumes in MCI patients. MTL volumes were significantly related to the RBANS Immediate and Delayed Memory and Language indices but not the Attention or Visuoconstruction indices; findings that demonstrate anatomical specificity. Following discriminant function analysis, we calculated a cutpoint that may prove clinically useful for integrating MTL volumes into the diagnosis of MCI. These findings demonstrate the potential clinical utility of NeuroQuant® and are the first to document the relationship between RBANS indices and MTL volumes.
Keywords: Aging, Alzheimer's disease, Atrophy, Dementia, Hippocampus, Inferior lateral ventricles
Introduction
Mild cognitive impairment (MCI) is a diagnosis meant to capture the transitional “gray area” between normal aging and dementia. This clinical diagnosis is characterized by subjective memory complaints and objective memory impairment with relatively preserved general cognition and everyday functioning (Albert et al., 2011; formally referred to as amnestic MCI—Petersen, 2004). Although other cognitive deficits can be present in MCI, the current report focuses on learning and memory deficits.
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, Tierney, Mohr, & Chase, 1998) was specifically developed to aid in the detection of dementia. Most relevant to the current study are the Immediate Memory Index (IMI), which assesses the learning of a word list and a short story, and the Delayed Memory Index (DMI), which assesses recall of the word list (and recognition thereof), story, and a figure after ∼15 min. The utility of the IMI and DMI for diagnosing MCI and Alzheimer's disease (AD) has been documented (Duff et al., 2008; Duff, Hobson, Beglinger, & O'Bryant, 2010). However, it is currently unknown whether performances on these indices reflect volumetric measures of medial temporal lobe (MTL) integrity.
Consistent with the learning and memory deficits in MCI, there is substantial evidence of MTL atrophy in MCI (for a review, see Jack, 2012). However, the clinical use of volumetric data has been limited by a number of methodological factors such as the labor-intensive nature of this approach and the lack of standardization across sites (see Hampstead & Brown, 2013 for a review). This disconnect is unfortunate since structural MRI is often acquired during the diagnostic workup. This leaves a critical gap in translation of research findings to clinical practice.
NeuroQuant® is a commercially available, fully automated analysis service that provides a limited set of volumetric data in ∼15 min. In previous research, the MTL (i.e., inferior lateral ventricles (ILVs), hippocampus) volumes provided by NeuroQuant® were comparable with those obtained from a computer-aided manual segmentation procedure in patients with early AD (Brewer, Magda, Airriess, & Smith, 2009). The ease with which clinicians and researchers can use this tool and the fact that providers can bill for its use with existing CPT codes suggests that NeuroQuant® may ultimately gain traction in clinical practice. However, it is currently unknown whether these volumes are reflective of reduced volume in MCI relative to healthy older adults and whether they reflect objective memory deficits.
The current study had two primary aims. The first aim was to determine whether MTL volumetrics provided by NeuroQuant® are able to replicate the established differences between cognitively intact (“healthy”) older adults and those with MCI. Based on the literature above, these measures should show that MCI patients have reduced hippocampal volumes and greater ILV volumes relative to healthy older adults. Despite the utility of the RBANS in detecting MCI and AD (Duff et al., 2008, 2010), we are not aware of any studies that have examined the relationship between the memory indices and MTL integrity. Thus, the second aim was to examine the relationship between the RBANS IMI and DMI and MTL volumes relative to theoretically unrelated RBANS indices (e.g., Attention, Visuoconstruction). Within this aim, we sought to further describe the nature of the relationship between the subtests comprising these memory indices and MTL volumes. An exploratory third aim was to examine the utility of these MTL volumes for diagnosing MCI.
Methods
Participants
Data from a total of 100 older adults, age 53–86, were available for this study. These participants came from a larger research program investigating structural and functional neuroimaging changes associated with the cognitive rehabilitation of memory in healthy aging and MCI (e.g., Hampstead et al., 2011, 2012). Fifty-seven patients were recruited from memory disorder clinics. All had received a clinical diagnosis of (amnestic) MCI according to Petersen's (2004) criteria during a consensus conference of neuropsychologists, neurologists, and/or geriatricians in which all relevant clinical data were considered. Specifically, patients (or an informant) reported subjective memory difficulty and demonstrated objective evidence of decline via neuropsychological testing within the context of preserved everyday functioning. Forty-three healthy older controls (HOC) were recruited from the community and the same clinics (e.g., patient spouses). All were free of subjective complaints and objective evidence of memory impairment, as all performances on RBANS memory indices were within normal limits (i.e., within 1 SD of the mean).
General exclusion criteria included a history of neurologic injury or disease (e.g., stroke, moderate or severe traumatic brain injury, epilepsy), psychiatric disorders (e.g., severe depression, bipolar disorder, schizophrenia), current or past alcohol or drug abuse. The Institutional Review Board of Emory University and the Research and Development Committee of the Atlanta VAMC approved the study. All participants provided written informed consent.
Materials
Neuropsychological tests
After consenting to our larger research program, each participant completed a brief neuropsychological screening protocol to ensure that (i) participants with MCI had not progressed to AD or reverted to normal and (ii) control participants were, in fact, cognitively intact. This protocol was completely independent of the clinical diagnostic process and included the RBANS (Randolph et al., 1998).
Structural MRI and volumetric measurements
All participants underwent high-resolution anatomic MRI scanning as part of a series of cognitive rehabilitation studies (e.g., Hampstead et al., 2011, 2012). The scans were performed using a Siemens Trio 3T MRI Scanner (Siemens Medical Solutions, Malvern, PA) with a 12-channel head coil. High-resolution anatomic images were acquired using a 3D MPRAGE sequence (TR 2300 ms, TE 3.9 ms, FA 8°) with 176, 1 mm thick, sagittal slices (FOV 256 mm, in-plane resolution 1 × 1 mm, in-plane matrix 256 × 256). All data were visually inspected by a senior MR technologist at the time of acquisition; suboptimal scans were repeated to ensure usable data.
Volumetric data were obtained via neuroQuant®. The segmentation process uses a proprietary automated pipeline that performs image filtering, artifact correction, segmentation, error measurement, and report generation (personal communication with Dr. Sebastian Magda January 2, 2014). Details of the analysis process are provided in Brewer and colleagues (2009). Briefly, NeuroQuant® performs two initial quality control checks (personal communication with Dr. Sebastian Magda January 2, 2014). First, it examines the data to ensure that the MR sequence adheres to the automatic segmentation specifications. During this step, NeuroQuant® calculates a measurement index number that quantifies the deviation between the target brain and a normalized anatomic atlas. An extreme value aborts the segmentation process. Second, NeuroQuant® corrects for gradient nonlinearities and field inhomogeneities and uses the same methods as the Alzheimer's Disease Neuroimaging Initiative to minimize between-scanner distortions. The skull is then stripped and data are transformed using discrete cosine nonlinear registration to a probabilistic atlas (see Brewer et al., 2009). During the segmentation process, each voxel is given a neuroanatomical label based on its location within the atlas and then iteratively checked to maximize the probability that it belongs to the labeled structure (see Brewer et al., 2009 for additional details). Following segmentation, NeuroQuant® generates a report with volumes of structures as a percent of intracranial volume (ICV), which accounts for head size thereby allowing for interindividual comparisons. The program calculates the ICV by summing all of the segmented brain structures, brainstem, meninges, and cerebrospinal fluid external to the brain surface (personal communication with Dr. Sebastian Magda February 1, 2014). Volumes based on the percent of ICV have previously been shown to increase reliability relative to other methods, thereby making it more amenable to between-site/sample comparisons (Gold & Squire, 2005). Therefore, we used the percent ICV values for the MTL regions listed subsequently.
NeuroQuant® provides the volumes of three MTL regions: the hippocampus, inferior lateral ventricles, and the amygdala. We included the hippocampus given its long-standing association with learning and memory and evidence of change in MCI/AD. We also included the ILVs since it can be conceptualized as a general measure of MTL integrity that presumably reflects changes in structures that are not measured by NeuroQuant® (e.g., entorhinal cortex, parahippocampal gyrus). Total volumes for these regions were calculated by combining the left and right hemisphere volumes. There was no a priori justification to include the amygdala since the RBANS stimuli are not likely to elicit emotional processing; this decision also reduced the probability of a Type I error by reducing the number of statistical comparisons.
Statistical analyses
All analyses were performed using IBM SPSS Statistics 20. Between-group differences on demographic variables were assessed with multiple independent samples χ2 or t-tests, as appropriate. These results showed a significant difference in gender distribution between groups. Therefore, between-group differences for neuropsychological data and brain volumes were analyzed using a multivariate analysis of covariance (MANCOVA), with post hoc one-way analysis of covariance (ANCOVA) comparisons with account for effect of sex. Given between group differences in sex distribution, we used partial correlations to control for sex when examining the relationship between the RBANS indices and MTL volumes across the entire sample as well as within each group. Additional partial correlations examined the relationship between RBANS memory subtest performances and MTL total (i.e., bilateral) volumes and within each hemisphere independently. We used the false discovery rate (FDR) to correct for multiple comparisons. The FDR minimizes both type I and type II error (Storey, 2003) and is calculated as follows: computed P (#tests+1))/(2*(#tests)). The FDR corrected significance thresholds for each series of tests are provided subsequently. Finally, we performed a discriminant function analysis to determine the sensitivity and specificity of the total hippocampal and ILV volumes for group classification as well as to identify a cutpoint that may be useful in clinical practice.
Results
Aim 1: MTL Volume Differences
Demographic, neuropsychological, and volumetric between group comparisons can be seen in Table 1. The FDR corrected p-value for this series of 20 comparisons was p ≤ .026. There were no significant differences in ethnicity, age, or education between the groups. As noted, there was a significant difference in the distribution of males and females across groups. Within the MCI group the distribution of males and females was similar, whereas females outnumbered males in in the HOC group. Although comparable cognitive profiles are evident between the sexes in healthy aging (Barnes et al., 2003), there is evidence that healthy older males show greater volume loss relative to females (Alexander et al., 2006). Therefore, we used sex as a covariate in subsequent analyses. Results of the MANCOVA revealed a significant effect of group on neuropsychological performance and MTL volumes (F(11,87) = 12.55, p < .001, ηp2 = .61). As expected, the RBANS Index scores, as well as all the individual subtests comprising the memory scales, were significantly higher in the healthy controls relative to the MCI patients. Regarding Aim 1, MCI patients demonstrated significantly smaller hippocampal and larger ILV volumes compared with the healthy controls, thereby showing that volumes obtained via NeuroQuant® replicate previous findings (see Jack, 2012).
Table 1.
Demographic, RBANS, and brain volumes for HOC and patients with MCI
| All participants (n = 100) | HOC (n = 43) | MCI (n = 57) | χ2 (1, n = 100) | p-value | |
|---|---|---|---|---|---|
| Frequency | Frequency | Frequency | |||
| Gender | |||||
| Male | 37 | 9 | 28 | 8.357 | .004 |
| Female | 63 | 34 | 29 | ||
| Frequency | Frequency | Frequency | χ2 (2, n = 100) | – | |
| Ethnicity | |||||
| Caucasian | 71 | 31 | 40 | 2.381 | .304 |
| African American | 26 | 12 | 14 | ||
| Latino | 3 | 0 | 3 | ||
| M(SD) | M(SD) | M(SD) | t1,98 | ||
| Age (years) | 70.72 (7.71) | 70.26 (7.29) | 71.07 (8.06) | .521 | .604 |
| Education (years) | 16.4 (2.27) | 16.33 (2.06) | 16.46 (2.43) | .284 | .777 |
| F(1,97) | – | ||||
| RBANS Memory Indices (Std. score) | |||||
| Immediate Memory | 96.17 (17.19) | 110.51 (8.39) | 85.35 (13.87) | 100.24 | <.001 |
| Visuospatial/Constructional Index | 97.18 (16.14) | 101.35 (14.65) | 94.04 (16.62) | 8.26 | .005 |
| Language Index | 98.32 (12.43) | 105.16 (12.58) | 93.16 (9.57) | 24.34 | <.001 |
| Attention Index | 103.14 (14.84) | 108.63 (13.74) | 99.00 (14.40) | 15.33 | .001 |
| Delayed Memory | 89.71 (20.21) | 106.88 (7.91) | 76.75 (16.68) | 110.37 | <.001 |
| RBANS memory subtests (z-score) | |||||
| List learning | −0.38 (1.24) | 0.55 (0.77) | −1.08 (1.05) | 62.05 | <.001 |
| Story learning | −0.29 (1.12) | 0.49 (0.61) | −0.88 (1.05) | 58.37 | <.001 |
| List recall | −0.46 (1.24) | 0.65 (0.76) | −1.3 (0.79) | 137.01 | <.001 |
| List recognition | −0.70 (1.69) | 0.34 (0.50) | −1.49 (1.84) | 32.32 | <.001 |
| Story recall | −0.54 (1.48) | 0.54 (0.63) | −1.35 (1.42) | 65.73 | <.001 |
| Figure recall | −0.83 (1.31) | 0.07 (0.62) | −1.51 (1.29) | 60.37 | <.001 |
| Brain volumes (% of total ICV)† | |||||
| Left inferior lateral ventricle | 0.09 (0.03) | 0.08 (0.02) | 0.10 (0.04) | 6.745 | .011 |
| Right inferior lateral ventricle | 0.09 (0.04) | 0.07 (0.03) | 0.10 (0.05) | 5.434 | .022 |
| Total inferior lateral ventricle | 0.18 (0.07) | 0.15 (0.05) | 0.20 (0.08) | 6.57 | .012 |
| Left hippocampus | 0.24 (0.03) | 0.25 (0.03) | 0.23 (0.03) | 7.80 | .006 |
| Right hippocampus | 0.25 (0.04) | 0.26 (0.03) | 0.24 (0.04) | 5.31 | .023 |
| Total hippocampus | 0.48 (0.07) | 0.51 (0.06) | 0.46 (0.07) | 9.90 | .002 |
Notes: Bold p-values represent those surviving the FDR correction procedure (FDR p ≤ .026).
†Percent of total ICV provided by NeuroQuant®.
Aim 2: Relationship Between RBANS Performances and MTL Volumes
The FDR corrected p-values for this series of correlations are provided in Tables 2 and 3. Across the entire sample, ILV volume was significantly and inversely related to performance on the IMI, DMI, and Language Index. Hippocampal volume was positively related to only the DMI. The relationship between the ILV and Language Index was driven by performance on the semantic fluency (r = −.291, p = .003) but not the naming subtest (r = .016, p = .873). Importantly, MTL volumes were not related to the Attention or Visuospatial/Constructional Index. Within the individual groups, only the MCI group demonstrated a significant (positive) relationship between the DMI and hippocampal volume; no other results reached statistical significance.
Table 2.
Partial correlations (p-values) between RBANS indices and total ILV and HP volumes (in percent of total ICV − ICV)
| All participants |
HOC |
MCI |
||||
|---|---|---|---|---|---|---|
| Percent ICV |
Percent ICV |
Percent ICV |
||||
| ILV | HP | ILV | HP | ILV | HP | |
| IMI | −0.246* | 0.159 | −0.105 | −0.180 | −0.093 | −0.047 |
| (0.014) | (0.115) | (0.509) | (0.254) | (0.494) | (0.731) | |
| DMI | −0.258* | 0.417* | −0.145 | 0.138 | −0.106 | 0.367* |
| (0.010) | (<0.001) | (0.359) | (0.383) | (0.436) | (0.005) | |
| LI | −0.281* | 0.167 | −0.197 | 0.083 | −0.218 | −0.006 |
| (0.005) | (0.098) | (0.211) | (0.602) | (0.107) | (0.966) | |
| VI | −0.06 | −0.002 | −0.076 | −0.111 | 0.034 | −0.101 |
| (0.552) | (0.984) | (0.634) | (0.484) | (0.805) | (0.457) | |
| AI | −0.169 | 0.147 | 0.003 | 0.151 | −0.129 | −0.037 |
| (0.094) | (0.148) | (0.987) | (0.341) | (0.345) | (0.787) | |
Notes: AI = Attention Index; DMI = Delayed Memory Index; HOC = healthy older controls; IMI = Immediate Memory Index; LI = Language Index; MCI = mild cognitive impairment; VI = Visuospatial/Constructional Index.
*p ≤ .026 (FDR corrected p-value).
Table 3.
Partial correlations (p-values) between individual RBANS learning/memory subtests and MTL volumes (in percent of ICV)
| All participants |
||||||
|---|---|---|---|---|---|---|
| ILV (% ICV) |
HP (% ICV) |
|||||
| Left | Right | Total | Left | Right | Total | |
| List learning | −0.214 | −0.223 | −0.228* | 0.228* | 0.189 | 0.190 |
| (0.034) | (0.027) | (0.023) | (0.023) | (0.061) | (0.060) | |
| Story learning | −0.242* | −0.113 | −0.178 | 0.181 | 0.038 | 0.109 |
| (0.016) | (0.266) | (0.079) | (0.073) | (0.710) | (0.284) | |
| List recall | −0.214 | −0.212 | −0.222 | 0.257* | 0.213 | 0.300* |
| (0.033) | (0.035) | (0.027) | (0.010) | (0.035) | (0.003) | |
| List recognition | −0.103 | −0.121 | −0.118 | 0.242* | 0.271* | 0.334* |
| (0.309) | (0.233) | (0.245) | (0.016) | (0.007) | (0.001) | |
| Story recall | −0.133 | −0.135 | −0.140 | 0.270* | 0.243* | 0.259* |
| (0.189) | (0.182) | (0.167) | (0.007) | (0.015) | (0.010) | |
| Figure recall | −0.217 | −0.243* | −0.242* | 0.338* | 0.286* | 0.349* |
| (0.031) | (0.015) | (0.016) | (0.001) | (0.004) | (<0.001) | |
*p ≤ .025 (FDR corrected p-value).
Results for the individual memory subtests are shown in Table 3, though the individual group data (i.e., HOC and MCI) are omitted because there were no statistically significant relationships. In the entire sample, there were significant inverse correlations between total ILV volume and list learning and figure recall. Lateralized inverse relationships were evident between story learning and left ILV volume, figure recall and right ILV volume, and list learning and left hippocampal volume. Virtually, all measures of delayed recall (including List Recognition) were related to hippocampal volumes bilaterally; the sole exception was List Recall, which was related to left and total hippocampal volume.
Aim 3: Discriminant Function Analyses
The exploratory discriminant function analysis yielded a significant Wilks' Lambda of .831, p < .001, where the volumes of the ILV and hippocampus collectively yielded 72.1% sensitivity and 61.4% specificity for diagnostic group. To increase the clinical relevance of this analysis, we calculated a cutpoint using the group centroids (HOC = 0.514; MCI = −0.387). This cutpoint ((HOC + MCI)/2 = 0.064) is based on the discriminant function value and is calculated as follows. First, ILV and hippocampal volumes (in %ICV) are converted to z-scores using the means and SDs from our entire sample (values provided in Table 1). The resulting z-scores are entered into the following discriminant function equation:
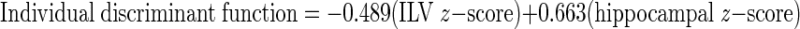 |
Resulting values over the cutoff of 0.064 indicate that the individual is more likely cognitively healthy, whereas values below the cutoff indicate the individual is more likely to have MCI.
Discussion
The current study addressed three clinically relevant questions. First, our primary aim was to evaluate whether NeuroQuant® is useful for detecting differences in MTL volumes between cognitively healthy older adults and patients with MCI. Our data clearly answer in the affirmative given the significant between-group differences in both hippocampal and ILV volumes. These findings replicate previous literature (Jack, 2012) and reinforce the potential clinical utility of NeuroQuant®, especially since it provides a rapid and standardized method of obtaining brain volumes.
Regarding our second aim, the results revealed significant correlations between the ILV and both the IMI and DMI across all participants. These findings complement previous research showing relationships between memory test performances and MTL integrity in older adults across the spectrum from healthy to AD (Arlt et al., 2013; Mungas et al., 2005). The observed relationships presumably reflect the general integrity of the MTL since the ILV volume may reflect atrophy that is known to occur in other MTL regions not measured by NeuroQuant® (e.g., entorhinal cortex, parahippocampal gyrus) (McDonald et al., 2012; Rodrigue & Raz, 2004). Considering the individual subtests of the memory indices reinforces this conclusion since measures that were related to ILV were infrequently also related to hippocampal volumes. We previously reported that the ILV volume was related to cognitive rehabilitation success in patients with MCI (Hampstead et al., 2012); a finding suggests that this measure may be useful in both diagnosing and determining effective treatments for MCI.
The significant relationship between the ILV volume and the Language Index was driven by semantic fluency performance. This relationship is perhaps not surprising since semantic fluency is known to be impaired in those with AD (for review, see Henry, Crawford, & Phillips, 2004), and the MTL plays a role in semantic retrieval (Sheldon & Moscovitch, 2012). Similarly, temporal lobe atrophy was associated with semantic fluency decline over a 2-year follow-up in patients with MCI (McDonald et al., 2012).
Only the DMI was related to hippocampal volume, both in the entire sample and in the MCI group alone. These findings are consonant with the known role of the hippocampus in memory retention and previous research demonstrating that smaller hippocampal volume is associated with worse memory test performance (Arlt et al., 2013; Apostolova et al., 2010; Mungas et al., 2005). They also reinforce previous conclusions that the DMI, relative to the IMI, is more sensitive to memory impairment in those with AD (Duff et al., 2008) as well as our earlier report that MCI patients experience relatively intact encoding but impaired retention (Gillis, Quinn, Phillips, & Hampstead, 2013). The fact that virtually all of the memory subtests (i.e., those occurring after a time delay) were related to hippocampal volumes bilaterally likely reflects the bilateral nature of the presumed disease process (Apostolova et al., 2012).
Critically, the patterns of correlations demonstrate meaningful anatomical specificity since there were no significant relationships between either MTL volume and theoretically unrelated RBANS indices (i.e., attention, visuoconstruction). That said, our results generally did not reveal significant correlations between the RBANS Indices and MTL volumes within the individual participant groups. This outcome is likely due to the limited range of RBANS scores within each group, especially considering we required the healthy older group to perform better than −1 SD on all tests whereas MCI patients, by definition, demonstrated memory deficits. The sole exception of the DMI in patients with MCI reinforces the notion of a continuum in MCI where memory and hippocampal volumes range from near normal to AD like.
The discriminant function analysis from Aim 3 adds to the clinical relevance of the above results since we were able to determine a cutoff that may ultimately help translate research findings into the clinic by allowing providers to integrate MRI data into the diagnostic process. This is, to our knowledge, the first such attempt and will undoubtedly need to be revised as larger datasets become available in the future. Our observed sensitivity and specificity are similar to previous attempts at classifying HOC and MCI using manual segmentation and specialized automated segmentation techniques (Westman et al., 2011), yet our MTL volumes were rapidly acquired using a widely available technique. In addition, this is the first known study to offer a cutoff score, which can also be useful for clinicians when considering a diagnosis of MCI.
In conclusion, the current findings demonstrate the potential clinical utility of NeuroQuant®, validate the RBANS as a predictor of MTL integrity across the “normal” to MCI spectrum, and provide methods for integrating MTL volumes into clinical practice. Future research could investigate the predictive ability of NeuroQuant's® MTL volumes for progression of “healthy” to MCI and MCI to AD.
Conflict of Interest
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. There are no conflicts of interest for any of the authors.
Funding
The research described here was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service—Award B6366W to BMH, Atlanta VAMC RR&D Center of Excellence. Additional support from the Emory University Alzheimer's Disease Research Center is also acknowledged (NIA: 2P50AG025688).
References
- Alexander G. E., Chen K., Merkley T. L., Reiman E. M., Caselli R. J., Aschenbrenner M., et al. Regional network of magnetic resonance imaging gray matter volume in healthy aging. NeuroReport. 2006;17(10):951–956. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Apostolova L. G., Green A. E., Babakchanian S., Hwang K. S., Chou Y., Toga A. W., et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment and Alzheimer's disease. Alzheimer Disease and Associated Disorders. 2012;26(1):17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova L. G., Morra J. H., Green A. E., Hwang K. S., Avedissian C., Woo E. The Alzheimer's Disease Neuroimaging Initiative. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. NeuroImage. 2010;51(1):488–499. doi: 10.1016/j.neuroimage.2009.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendation from the National Institute on Aging – Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt S., Buchert R., Spies L., Eichenlaub M., Lehmbeck J. T., Jahn H. Association between fully automated MRI-based volumetry of different brain regions and neuropsychological test performance in patients with amnestic mild cognitive impairment and Alzheimer's disease. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(4):335–344. doi: 10.1007/s00406-012-0350-7. [DOI] [PubMed] [Google Scholar]
- Barnes L. L., Wilson R. S., Schneider J. A., Bienias J. L., Evans D. A., Bennett D. A. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60(11):1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a. [DOI] [PubMed] [Google Scholar]
- Brewer J. B., Magda S., Airriess C., Smith M. E. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer's disease. American Journal of Neuroradiology. 2009;30:578–580. doi: 10.3174/ajnr.A1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Hobson V. L., Beglinger L. J., O'Bryant S. E. Diagnostic accuracy of the RBANS in mild cognitive impairment: Limitations on assessing milder impairments. Archives of Clinical Neuropsychology. 2010;25:429–441. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Humphreys Clark J. D., O'Bryant S. E., Mold J. W., Schiffer R. B., Sutker P. B. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: Sensitivity, specificity, and positive and negative predictive powers. Archives of Clinical Neuropsychology. 2008;23:603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M. M., Quinn K. M., Phillips P. A. T., Hampstead B. M. Impaired retention is responsible for temporal order memory deficits in mild cognitive impairment. Acta Psychologica. 2013;143:88–95. doi: 10.1016/j.actpsy.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J. J., Squire L. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead B. M., Brown G. S. Using neuroimaging to inform clinical practice for the diagnosis and treatment of mild cognitive impairment. Clinics in Geriatric Medicine. 2013;29(4):829–845. doi: 10.1016/j.cger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Hampstead B. M., Sathian K., Phillips P. A., Amaraneni A., Delaune W. R., Stringer A. Y. Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: A randomized, single-blind study. Neuropsychology. 2012;26(3):385–399. doi: 10.1037/a0027545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead B. M., Stringer A. Y., Stilla R. F., Deshpande G., Hu X., Moore A. B., et al. Activation and effective connectivity changes following explicit-memory training for face-name pairs in patients with mild cognitive impairment: A pilot study. Neurorehabilitation and Neural Repair. 2011;25(3):210–222. doi: 10.1177/1545968310382424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. D., Crawford J. R., Phillips L. H. Verbal fluency performance in dementia of the Alzheimer's type: A meta-analysis. Neuropsychologia. 2004;42(9):1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jack C. R. Alzheimer's disease: New concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263(3):344–361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C. Mild cognitive impairment as a diagnostic entity. Journal of International Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- McDonald C. R., Gharapetian L., McEvoy L. K., Fennema-Notestine C., Hagler D. J., Jr, Holland D. Alzheimer's Disease Neuroimaging Initiative. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiology of Aging. 2012;33(2):242–253. doi: 10.1016/j.neurobiolaging.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D., Harvey D., Reed B. R., Jajust W. J., DeCarli C., Beckett L., et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65(4):565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C., Tierney M. C., Mohr E., Chase T. N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rodrigue K. M., Raz M. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. Journal of Neuroscience. 2004;24(4):956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S., Moscovitch M. The nature and time-course of medial temporal lobe contributions to semantic retrieval: An fMRI study on verbal fluency. Hippocampus. 2012;22(6):1451–1466. doi: 10.1002/hipo.20985. [DOI] [PubMed] [Google Scholar]
- Storey J. D. The false discovery rate: A Bayesian interpretation and the q-value. The Annals of Statistics. 2003;31:2013–2035. [Google Scholar]
- Westman E., Simmons A., Zhang Y., Muehlboeck J.-S., Tunnard C., Liu Y., et al. Multivariate analysis of MRI data for Alzheimer's disease, mild cognitive impairment and healthy controls. NeuroImage. 2011;54:1178–1187. doi: 10.1016/j.neuroimage.2010.08.044. [DOI] [PubMed] [Google Scholar]


