Abstract
Aims
We hypothesized that carvedilol can effectively suppress autonomic nerve activity (ANA) in ambulatory dogs during sinus rhythm and atrial fibrillation (AF), and that carvedilol withdrawal can lead to rebound elevation of ANA. Carvedilol is known to block pre-junctional β2-adrenoceptor responsible for norepinephrine release.
Methods and results
We implanted radiotransmitters to record stellate ganglion nerve activity (SGNA), vagal nerve activity (VNA), and superior left ganglionated plexi nerve activity (SLGPNA) in 12 ambulatory dogs. Carvedilol (12.5 mg orally twice a day) was given for 7 days during sinus rhythm (n = 8). Four of the eight dogs and an additional four dogs were paced into persistent AF. Carvedilol reduced heart rate [from 103 b.p.m. (95% confidence interval (CI), 100–105) to 100 b.p.m. (95% CI, 98–102), P = 0.044], suppressed integrated nerve activities (Int-NAs, SGNA by 17%, VNA by 19%, and SLGPNA by 12%; all P < 0.05 vs. the baseline), and significantly reduced the incidence (from 8 ± 6 to 3 ± 3 episodes/day, P < 0.05) and total duration (from 68 ± 64 to 16 ± 21 s/day, P < 0.05) of paroxysmal atrial tachycardia (PAT). Following the development of persistent AF, carvedilol loading was associated with AF termination in three dogs. In the remaining five dogs, Int-NAs were not significantly suppressed by carvedilol, but SGNA significantly increased by 16% after carvedilol withdrawal (P < 0.001).
Conclusion
Carvedilol suppresses ANA and PAT in ambulatory dogs during sinus rhythm.
Keywords: Autonomic nervous system, Atrium, Arrhythmia, Atrial fibrillation, Carvedilol
What's new?
Carvedilol effectively suppressed vagal nerve activity (VNA) and superior left ganglionated plexi nerve activity (SLGPNA) in addition to stellate ganglion nerve activity (SGNA) in the basal state.
The incidence and duration of paroxysmal atrial tachyarrhythmia decreased during carvedilol loading and increased after carvedilol withdrawal.
Carvedilol impacted the SGNA–VNA correlation and circardian patterns.
After the development of persistent atrial fibrillation (AF), carvedilol administration did not suppress SGNA, VNA, and SLGPNA, while carvedilol withdrawal during AF was followed by increased SGNA.
Introduction
β-Blockers are commonly used in the treatment of heart failure and cardiac arrhythmias. Large clinical trials have documented the efficacy of β-blockers in improving the outcomes of heart failure.1,2 However, not all β-blockers are equally effective in these regards. The Carvedilol Or Metoprolol European Trial (COMET) compared the effects of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure.3 The investigators found that carvedilol is more effective than metoprolol in prolonging survival. Carvedilol is also more effective than placebo in reducing the incidence of atrial fibrillation (AF) and atrial flutter after myocardial infarction.4 Because carvedilol is a non-selective β-blocker that blocks α1-, β1-, and, β2-adrenergic receptors, whereas metoprolol is a selective β1-adrenegic blocker, it is possible that the additional post-junctional blocking effects of carvedilol explain its superior clinical efficacy over metoprolol. However, on further analyses of the COMET, Bristow et al.5 concluded that post-junctional adrenoceptor blockade, that is, in addition to β1-receptor antagonism will likely produce only minimal or no incremental benefit in chronic heart failure. An alternative explanation of the COMET results is that carvedilol, but not metoprolol, blocks pre-junctional β2-adrenergic receptors (facilitating norepinephrine release) and thereby reduce cardiac sympathetic outflow.6 The latter hypothesis was supported by a clinical study that showed carvedilol, but not metoprolol, reduces systemic and cardiac norepinephrine spillover, an indirect measure of norepinephrine release.7 However, no study has directly documented the effects of carvedilol on cardiac sympathetic nerve activity in ambulatory animals. We have developed a method to simultaneously measure stellate ganglion nerve activity (SGNA), vagal nerve activity (VNA), and superior left ganglionated plexi nerve activity (SLGPNA) in ambulatory dogs at baseline and during pacing-induced AF.8 The purpose of the present study was to test the hypothesis that carvedilol can effectively suppress autonomic nerve activity (ANA) in ambulatory dogs during sinus rhythm and pacing-induced AF, and that carvedilol withdrawal can lead to rebound elevation of ANA. The results of this study may be helpful in explaining the beneficial effects of carvedilol in the management of patients with heart failure and cardiac arrhythmias.
Methods
The animal research protocol was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine; the Methodist Research Institute, Indianapolis, USA; and the Seoul National University Hospital, Seoul, Republic of Korea. The protocol conforms to the best practices as defined by the Guide for the Care and Use of Laboratory Animals.
Cardiac autonomic nerve recordings in an ambulatory canine model
Twelve mongrel dogs (24 ± 5 kg, all male) were used for chronic autonomic nerve recordings. Nerve recording protocols were described in detail elsewhere.8 Briefly, a Data Sciences International radiotransmitter (D70-EEE) was used to record three ANAs, including left SGNA, left VNA from the superior cardiac branch of the left thoracic vagus nerve, and SLGPNA from the superior left ganglionated plexi located between the left superior pulmonary vein and left atrium (LA). Pacing lead for rapid atrial pacing was implanted at the bottom of the left atrial appendage and connected with a Kappa pacemaker (Medtronic) or a Current DR pacemaker (St Jude Medical). After 2 weeks of recovery, carvedilol loading test (12.5 mg orally twice a day) was performed while the dogs were in sinus rhythm (phase 1 study, n = 8). Three ANAs were recorded before, during, and after carvedilol withdrawal (Figure 1). Rapid atrial pacing (640 b.p.m. for Medtronic pacemaker or 600 b.p.m. for St Jude Medical pacemaker) was performed continuously for 6 days followed by 1 day of post-pacing recording. Rapid atrial pacing was restarted if AF did not sustain for >48 h. The total pacing duration preceding the induction of persistent AF (>48 h) was 5 ± 3 weeks. Four dogs did not develop persistent AF after 8–10 weeks of pacing. Therefore, eight of the eight dogs were used to study the effect of carvedilol during sinus rhythm, whereas only four dogs were available for the study of carvedilol in persistent AF. To bolster the number of dogs in the persistent AF group, we added four dogs that developed persistent AF after rapid atrial pacing. In these latter four dogs, the effects of carvedilol were studied only during persistent AF. All eight dogs that developed persistent AF were observed for an additional 7 days, and all of them were found to be in AF throughout this additional 7-day observational period. After documenting the presence of AF for >9 days in all the dogs, we performed a second carvedilol loading test (phase 2, N = 8). Stellate ganglion nerve activity, VNA, and SLGPNA were recorded before, during, and after carvedilol withdrawal. During carvedilol loading, three of the eight dogs converted spontaneously from AF to sinus rhythm. The remaining five dogs were in persistent AF before, during, and after carvedilol therapy.
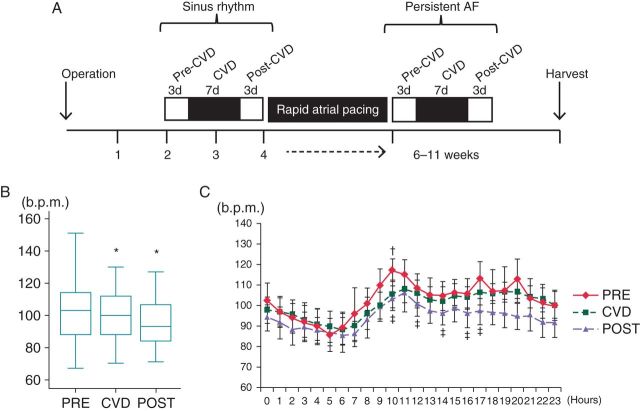
Figure 1.
Effects of carvedilol on heart rate. (A) Diagram of the study protocol. Two weeks after the operation, a carvedilol loading study was performed during sinus rhythm. A rapid atrial pacing protocol was then initiated and maintained until the development of persistent (>48 h) AF. Afterwards, the carvedilol loading study was repeated. (B) Heart rate during sinus rhythm before carvedilol (PRE), during carvedilol (CVD), and after carvedilol withdrawal (POST) in phase 1 (sinus rhythm) of the study. (C) Circadian variation of heart rate before, during, and after the administration of carvedilol. b.p.m., beats per minute; CVD, carvedilol. *P < 0.05 vs. PRE; †P < 0.05 PRE vs. CVD; ‡P < 0.05 PRE vs. POST.
Data analyses
A three-fold increase in amplitude over baseline noise was considered an indication of nerve activity.8 We used custom-designed software to calculate the integrated nerve activities (Int-NAs) relative to carvedilol loading. Stellate ganglion nerve activity, VNA, and SLGPNA were high-pass filtered at 100 Hz. The filtered signals were rectified, integrated with a 100 ms time constant, and summed at 3 s segments over 24 h recordings to quantify the Int-NAs. The 24 h nerve activities of each dog were averaged before, during, and after carvedilol loading and compared. We also counted the number of paroxysmal atrial tachycardia (PAT) episodes in the phase 1 study, defined as an abrupt (>50 b.p.m./s) increase in the atrial rate to >200 b.p.m. that persisted for at least 5 s.9 We integrated the nerve activity at 1 min intervals to obtain 1440 points over a 24 h period. We then constructed plots of integrated SGNA vs. integrated VNA and integrated VNA vs. integrated SLGPNA, similar to a method that described elsewhere.10,11 Because rapid atrial activation during persistent AF may significantly contaminate the SLGPNA recording, we did not analyse the SLGPNA recording during persistent AF.
Statistical analysis
Data were presented as mean values and 95% confidence intervals (CIs). Repeated-measure analyses of variance or mixed analysis were used to compare the Int-NAs, correlation coefficients, and the incidence and duration of PAT before, during, and after carvedilol withdrawal. Pearson correlation coefficients were used to analyse the associations between nerve activities. A P ≤ 0.05 was considered statistically significant.
Results
Carvedilol suppresses heart rate and nerve activities in sinus rhythm
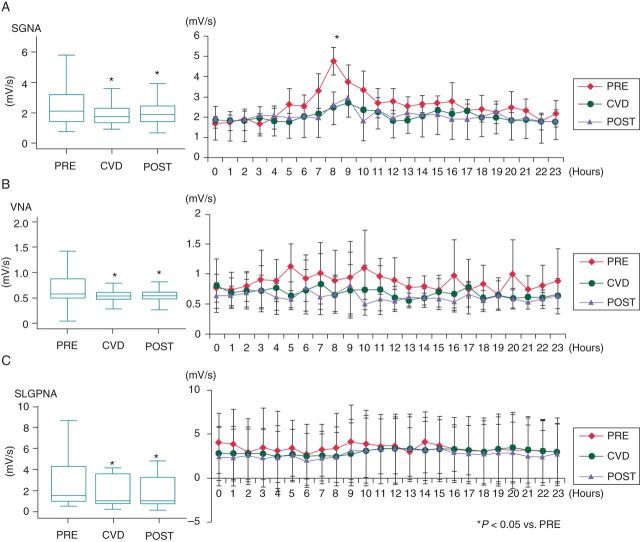
Heart rate decreased during carvedilol loading [from 102.8 b.p.m. (95% CI, 100.4–105.2) to 100.3 b.p.m. (95% CI, 98.4–102.1)], P = 0.044). After carvedilol withdrawal, a further significant decrease in heart rate was observed compared with values recorded at baseline and during carvedilol [94.4 b.p.m. (95% CI, 92.5–96.4), P < 0.001 compared with baseline, P < 0.001 compared with carvedilol loading period, Figure 1B]. We analysed the hourly change in heart rate at baseline, during carvedilol loading, and after carvedilol withdrawal. Before carvedilol loading, there was a morning surge of heart rate starting at 6 AM. This circadian variation was attenuated after carvedilol loading and withdrawal (Figure 1C). Integrated nerve activities were suppressed during carvedilol loading in sinus rhythm, resulting in reduction in SGNA by 17% [from 2.4 mV/s (95% CI, 2.2–2.6) to 2.0 mV/s (95% CI, 1.9–2.2), P < 0.001], VNA by 19% [from 0.9 mV/s (95% CI, 0.8–1.0) to 0.7 mV/s (95% CI, 0.6–0.8), P < 0.001], and SLGPNA by 12% [from 3.8 mV/s (95% CI, 3.0–4.5) to 3.3 mV/s (95% CI, 2.6–4.0), P = 0.005, Figure 2]. Furthermore, these effects persisted for up to 3 days after carvedilol withdrawal. On Day 3 after withdrawal, SGNA was reduced by 16% [from 2.4 mV/s (95% CI, 2.2–2.6) to 2.1 mV/s (95% CI, 1.9–2.2), P < 0.001], VNA by 29% [from 0.9 mV/s (95% CI, 0.8–1.0) to 0.6 mV/s (95% CI, 0.6–0.7), P < 0.001], and SLGPNA by 19% [from 3.8 mV/s (95% CI, 3.0–4.5) to 3.0 mV/s (95% CI, 2.4–3.7), P < 0.001] compared with the baseline. As shown in Figure 2, there was a significant reduction in SGNA at 8 AM during and after carvedilol loading compared with the baseline.
Figure 2.
Change of Int-NAs before (PRE), during (CVD), and after (POST) carvedilol loading during sinus rhythm. Stellate ganglion nerve activity (SGNA) (A), VNA (B), and SLGPNA (C) decreased after carvedilol loading compared with the baseline. All three nerve activities remain suppressed after carvedilol withdrawal. Among these nerve activities, only SGNA showed a morning surge (asterisk). The morning surge of SGNA disappeared after carvedilol loading and remained suppressed after carvedilol withdrawal. *P < 0.05 vs. PRE.
Carvedilol suppresses vagal nerve activity
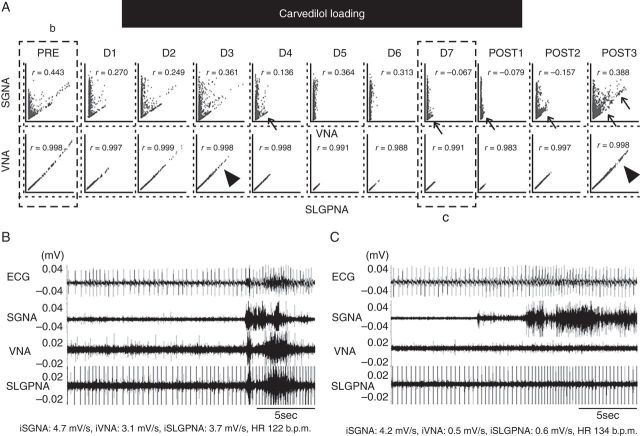
Two dogs in the phase 1 study showed significant changes in SGNA–VNA correlation during carvedilol loading (Figure 3). Figure 3A shows the effects of carvedilol on the SGNA–VNA and VNA–SLGPNA correlations. In this example, the baseline SGNA–VNA correlation was L-shaped, but the lower arm of the ‘L’ disappeared on Days 5–6 (between the first two arrows), and reappeared after carvedilol withdrawal. Actual nerve activity recorded before and during carvedilol loading is seen in Figure 3B and C. Figure 3C show the suppression of VNA and SLGPNA by carvedilol in comparison with Figure 3B. This finding indicates a highly selective suppression of VNA by carvedilol in this dog. The SGNA–VNA correlation coefficient was reduced at the end of carvedilol loading and just after carvedilol withdrawal (from 0.443 to −0.079), but recovered to its baseline value 3 days after carvedilol withdrawal. While the VNA–SLGPNA correlation coefficient did not change significantly during carvedilol loading, significant suppression of VNA was observed. The arrowheads point to the high VNA and SLGPNA portion of the graph. That portion was suppressed after Day 4 and returned 3 days after carvedilol withdrawal.
Figure 3.
Carvedilol selectively suppressed VNA and SLGPNA. (A) L-shaped correlation between SGNA and VNA at baseline (b). Carvedilol loading eliminated the lower arm of the L-shape on Days 5 (D5) and 6 (D6), resulting in isolated SGNA without VNA on those two days. The VNA–SLGPNA correlation remains linear during D5 and D6, but the high output portion of the graph (arrowhead, D3) was eliminated. That portion of the graph recovered 3 days after carvedilol withdrawal (POST3). (B, C) Representative examples of ANA. (B) Simultaneous discharges of SGNA, VNA, and SLGPNA. (C) Carvedilol selectively suppressed VNA and SLGPNA, but not SGNA.
Circadian variations of carvedilol effects
The circadian variation of the SGNA–VNA correlation was analysed. The SGNA–VNA correlation increased during daytime and decreased during night-time (Figure 4A). There was a significant difference between the lowest correlation coefficient of SGNA–VNA (8 PM to 12 AM) and the daytime coefficient (Figure 4C). However, after carvedilol loading, this difference in the SGNA–VNA correlation coefficient was attenuated, resulting in the disappearance of significant circadian variation (Figure 4B and D). Figure 4B shows a major reduction in SGNA from 8 AM to 12 PM, which is consistent with the absence of circadian variation in Figure 4D.
Figure 4.
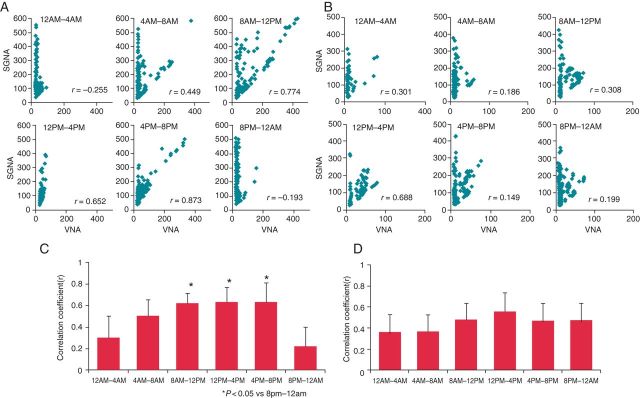
Effects of carvedilol on circadian variation of SGNA–VNA correlation. The correlation between SGNA and VNA was significantly better during daytime than during night-time (A, C). After carvedilol loading, this difference was no longer present (B, D). *P < 0.05 vs. 8 PM–12 AM.
Effect of carvedilol on paroxysmal atrial tachyarrhythmia
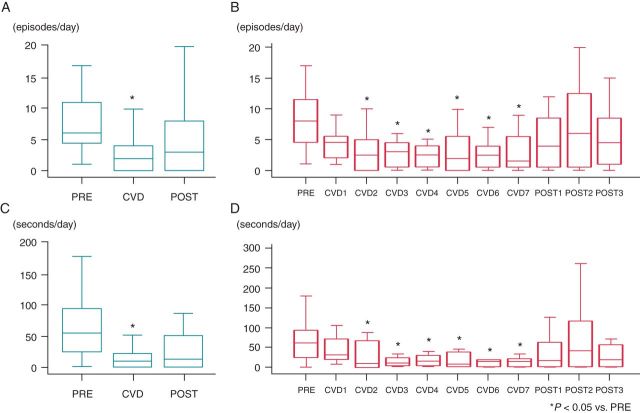
We detected 226 episodes of PAT during 2088 h of pre-pacing recording in eight dogs. Carvedilol loading significantly reduced the incidence of PAT [7.9/day (95% CI, 2.8–13.0) vs. 2.5/day (95% CI, 1.8–3.2), P < 0.001, Figure 5A]. After withdrawal of carvedilol, the number of PAT episodes increased [from 2.5/day (95% CI, 1.8–3.2] to 5.0/day (95% CI, 2.4–7.6), P = 0.002]. The number of PAT episodes decreased significantly between carvedilol loading Days 2 and 7 (Figure 5B). In addition, the duration of PAT decreased significantly after carvedilol loading [67.5 s/day (95% CI, 8.2–126.8) vs. 16.5 s/day (95% CI, 10.3–22.8), P = 0.003], and increased after carvedilol withdrawal [16.5 s/day (95% CI, 10.3–22.8) vs. 36.9 s/day (95% CI, 8.9–62.8), P = 0.162, Figure 5C]. Figure 5D shows a significant reduction in PAT duration between carvedilol loading Days 2 and 7.
Figure 5.
Incidence and duration of PAT before (PRE), during (CVD), and after (POST) carvedilol loading. Carvedilol loading significantly reduced the incidence (A, B) and duration (C, D) of PAT between Days 2 and 7. After withdrawal of carvedilol, the incidence and duration of PAT returned to baseline level. *P < 0.05 vs. PRE.
Carvedilol converts persistent atrial fibrillation to sinus rhythm
Three of the eight dogs with persistent AF converted to sinus rhythm during carvedilol loading. Atrial fibrillation was converted to sinus rhythm after 2, 4, and 6 days of carvedilol loading, respectively. There were no significant changes in SGNA [2.2 mV/s (95% CI, 1.9–2.5) in AF vs. 2.1 mV/s (95% CI, 1.9–2.4) during carvedilol loading vs. 2.2 mV/s (95% CI, 1.9–2.5) after sinus conversion, all P > 0.05]. Vagal nerve activity tended to increase during carvedilol loading and after sinus conversion, but the changes were statistically insignificant [0.9 mV/s (95% CI, 0.8–1.1) in AF vs. 1.0 mV/s (95% CI, 0.9–1.1) during carvedilol loading vs. 1.0 mV/s (95% CI, 0.9–1.1) after conversion to sinus rhythm, all P > 0.05].
Carvedilol had effects on nerve activity or heart rate during persistent atrial fibrillation
Carvedilol did not suppress Int-NA in the five dogs that did not convert to sinus rhythm during the loading period. No significant changes were observed in SGNA [from 2.3 mV/s (95% CI, 2.1–2.5) to 2.4 mV/s (95% CI, 2.2–2.6)], VNA [from 0.8 mV/s (95% CI, 0.8–0.8) to 0.8 mV/s (95% CI, 0.8–0.8), or SLGPNA [from 17.0 mV/s (95% CI, 15.6–18.5) to 16.9 mV/s (95% CI, 15.6–18.3)] during carvedilol loading (all P > 0.05, Figure 6). However, after carvedilol withdrawal, SGNA increased by 16% [from 2.4 mV/s (95% CI, 2.2–2.6) to 2.7 mV/s (95% CI, 2.6–3.0), P = 0.027], whereas VNA and SLGPNA remained unchanged [VNA from 0.8 mV/s (95% CI, 0.8–0.8) to 0.9 mV/s (95% CI, 0.8–0.9); SLGPNA from 16.9 mV/s (95% CI, 15.6–18.3) to 18.4 mV/s (95% CI, 17.3–19.6), P > 0.05]. After the development of persistent AF, the average heart rate was increased from 102.8 b.p.m. (95% CI, 100.4–105.2) to 123.3 b.p.m. (95% CI, 121.5–125.0) (P < 0.05). Carvedilol significantly reduced the ventricular rate [from 123.3 b.p.m. (95% CI, 121.5–125.0) to 120.3 b.p.m. (95% CI, 119.5–121.2), P < 0.001]. Furthermore, ventricular rate was also reduced after carvedilol withdrawal [123.3 b.p.m. (95% CI, 121.5–125.0) vs. 120.2 b.p.m. (95% CI, 119.0–121.5), P < 0.001 compared with before carvedilol loading]. Prior to carvedilol loading, the ventricular response rate during AF was higher from 8 AM to 4 PM (124.7 ± 11.4 b.p.m.) than from midnight to 8 AM (120.3 ± 5.6 b.p.m., P = 0.025). However, this daytime heart rate elevation was not significantly affected by carvedilol loading or withdrawal.
Figure 6.
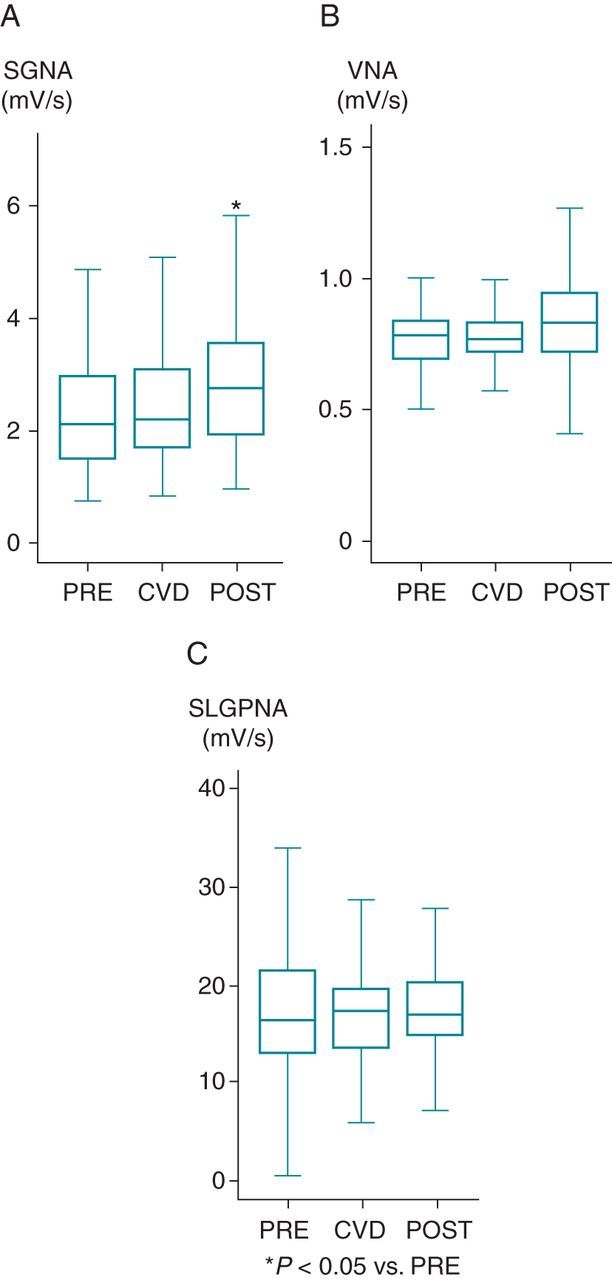
The change in Int-NA before (PRE), during (CVD), and after (POST) carvedilol loading during AF rhythm (n = 5 dogs). Stellate ganglion nerve activity (SGNA) (A), VNA (B), and SLGPAN (C) did not decrease during carvedilol loading. Interestingly, all three nerve activities increased after carvedilol withdrawal. *P < 0.05 vs. PRE.
Discussion
This study demonstrated that (i) at baseline, carvedilol effectively suppressed SGNA, VNA, SLGPNA, heart rate, and spontaneous PAT episodes; (ii) carvedilol had an effect on the SGNA–VNA correlation and circardian patterns; and (iii) during AF, carvedilol administration did not suppress ANA, but carvedilol withdrawal was followed by increased ANA.
Neural modulation by carvedilol
Carvedilol is known to have multiple actions. By affecting a variety of ion channels and currents, it exerts a more potent antiarrhythmic effect than those exerted by other β-blocking agents. Carvedilol is a potent blocker of the Ca2+ channels, Na+ channels, and various native K+ channels, that is the rapidly activating component of the delayed rectifier K+ current (Ikr), the transient outward K+ current (Ito), and the slowly activating component of the delayed rectifier K+ current (Iks).12,13 Sympathetic nerve activity is known to be important in cardiac arrhythmogenesis and sudden death.14 Therefore, drugs acting on the nervous system may have antiarrhythmic and antifibrillatory properties that potentially provide a therapeutic approach to the prevention of sudden cardiac death.15 A major finding of the present study is that carvedilol suppressed ANA outflow in ambulatory dogs during sinus rhythm. We also demonstrated that carvedilol suppressed the baseline heart rate and reduced the number of PAT episodes, consistent with the hypothesis that reduction of sympathetic outflow is an effective therapeutic approach to cardiac arrhythmia. Our finding is consistent with a clinical study that showed carvedilol reduces systemic and cardiac norepinephrine spillover, an indirect measure of norepinephrine release.7 The authors of the latter manuscript attributed their findings to the blocking effect of carvedilol on pre-junctional β2-adrenergic receptors that are responsible for the release of norepinephrine from the nerve terminals. However, a more recent finding showed that carvedilol is the only β-blocker that directly reduces the open duration of the cardiac ryanodine receptor.16 Because there is a tight functional coupling between ryanodine receptors and L-type calcium channels in the neurons,17 it is possible that the ryanodine receptor-suppressing effects of carvedilol contributed to the reduced ANA discharges observed in the present study.
Vagus nerves are mainly composed of choline acetyltransferase positive-cholinergic nerves, but a small portion of the nerves stained positively for thyroxine hydroxylase.18 Vagal nerve activity may include sympathetic and parasympathetic nerve activities. In addition, adrenergic and cholinergic nerves are distributed in the pulmonary vein (PV) and LA junction,19 suggesting that SLGPNA may include both nerve activities. Therefore, the effects of carvedilol are not specific to SGNA, as VNA and SLGPNA are also suppressed during carvedilol loading. Because simultaneous sympathovagal discharges and SLGPNA discharges are known to trigger PAT in this model,8,20,21 simultaneous reduction of ANA outflow from all these structures may in part underlie the antiarrhythmic efficacy of carvedilol.
Carvedilol withdrawal in sinus rhythm
Abrupt β-blocker discontinuation has been reported to increase ischaemic events in patients with coronary heart disease.22,23 Heart rate variability measurements indicate that β-blocker withdrawal in congestive heart failure shifts the autonomic balance in favour of increased sympathetic tone.24 However, there is no study that directly evaluated the change of cardiac ANAs after β-blocker withdrawal. A second major finding of this study is that there is no ANA rebound after carvedilol withdrawal in sinus rhythm, whereas significant rebound was observed after carvedilol withdrawal during persistent AF. There is significant neural remodelling, including nerve sprouting and atrial sympathetic hyperinnervation, in canine models of pacing-induced AF and in patients with AF.21,25 The anatomical evidence of cardiac nerve sprouting, coupled with increased SGNA and VNA,8 contribute to the spontaneous PAT in this canine model. However, in addition to suppressing sympathetic outflow, carvedilol also suppresses cardiac β-receptors. The effects of carvedilol on ANA outflow and on the myocardium may not be parallel to each other. Therefore, carvedilol withdrawal during sinus rhythm is associated with a further reduction of ANA but a rebound increase of PAT episodes.
Neural modulation for atrial fibrillation
Neural modulation has been used by multiple authors to control AF. Fat pads were innervated by sympathetic and parasympathetic nerve fibres.19 Radiofrequency catheter ablation aimed at the ganlionated plexi in the fat pad may reduce AF recurrence in human patients.26 Ganglionated plexi ablation as an adjunt to PV isolation showed comparable results with standard PV isolation.27 Oh et al.28 recently reported that botulinum toxin injection into fat pads temporarily blocked autonomic activity, resulting in the suppression of vagally mediated AF. However, the invasiveness of botox injection and the short period of its effect may limit its application in AF patients. Yu et al.29 reported a novel drug delivery method using magnetic nanoparticles targeting fat pads. An intravascular drug delivery system combined with a magnetic field could be used to target epicardial fat pads. However, because it is difficult to eliminate fat pads completely by any method, the above techniques may lead to only partial denervation and increased vulnerability to vagally induced AF.30 Similarly, dissection of a single fat pad may paradoxically increase the incidence of post-operative AF.31 Therefore, drugs that effectively suppress sympathetic outflow hold significant promise in treatment of AF.
Study limitations
Because three of the eight dogs converted to sinus rhythm during carvedilol loading, the data on AF are limited to the animals that failed to convert. The mechanism of AF termination during carvedilol loading remains unclear. Our study did not compare the effects of carvedilol on ANA with those of other β-blockers. Therefore, it cannot conclude whether the observed autonomic modulation effects are specific to carvedilol or represent class effects.
Conclusions
This study demonstrates that carvedilol suppresses ANA during sinus rhythm but not during AF. Carvedilol withdrawal is associated with continued ANA suppression in sinus rhythm, but with ANA rebound in AF. These findings suggest that β-blockers can directly suppress ANA in ambulatory dogs during sinus rhythm, and that the ANA response to β-blockers is altered by the induction of AF.
Funding
This study was supported in part by NIH Grants P01 HL78931, R01 HL78932, 71140, R21HL106554; a Korea National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (357-2008-1-E00028, 2010-0023262), funded by the Korean Government and grant nos. 03-2011-0370, 04-2010-0260, 04-2010-1140 from the SNUH research fund (E.-K.C.); an AHA Established Investigator Award (S.-F.L.); a Medtronic-Zipes Endowment (P.-S.C.) and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Acknowledgements
We thank Moo-Kang Kim, Hyun-Jung Lee, Lei Lin, Erica Foster, and Stephanie Plummer for their support.
Conflict of interest: Medtronic Inc. and St Jude Medical donated equipment used in these studies. P.-S.C. is a consultant to Cyberonic Inc. All other authors have no conflicts to declare.
References
- 1.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 2.Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 3.Poole-Wilson PA, Swedberg K, Cleland JG, Di LA, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J, Kober L, Robertson M, Dargie H, Colucci W, Lopez-Sendon J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005;45:525–30. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Feldman AM, Adams KF, Jr, Goldstein S. Selective versus nonselective beta-blockade for heart failure therapy: are there lessons to be learned from the COMET trial? J Card Fail. 2003;9:444–53. doi: 10.1016/j.cardfail.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Rump LC, Majewski H. Beta adrenoceptor facilitation of norepinephrine release is not dependent on local angiotensin II formation in the rat isolated kidney. J Pharmacol Exp Ther. 1987;243:1107–12. [PubMed] [Google Scholar]
- 7.Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, et al. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation. 2001;104:2194–9. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 8.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swissa M, Zhou S, Paz O, Fishbein MC, Chen LS, Chen PS. A canine model of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1851–7. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 10.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, et al. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–9. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara T, Shen MJ, Han S, Maruyama M, Park HW, Fishbein MC, et al. Heart failure decreases nerve activity in the right atrial ganglionated plexus. J Cardiovasc Electrophysiol. 2012;23:404–12. doi: 10.1111/j.1540-8167.2011.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Niwa R, Kamiya K, Toyama J, Kodama I. Carvedilol blocks the repolarizing K+ currents and the L-type Ca2+ current in rabbit ventricular myocytes. Eur J Pharmacol. 1999;376:189–201. doi: 10.1016/s0014-2999(99)00368-4. [DOI] [PubMed] [Google Scholar]
- 13.Karle CA, Kreye VA, Thomas D, Rockl K, Kathofer S, Zhang W, et al. Antiarrhythmic drug carvedilol inhibits HERG potassium channels. Cardiovasc Res. 2001;49:361–70. doi: 10.1016/s0008-6363(00)00265-0. [DOI] [PubMed] [Google Scholar]
- 14.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–15. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucchesi BR, Kniffen FJ. Pharmacological modification of arrhythmias after experimentally induced acute myocardial infarction. Drugs acting on the nervous system. Circulation. 1975;52:III241–7. [PubMed] [Google Scholar]
- 16.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–9. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–22. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- 18.Park HW, Shen MJ, Han S, Shinohara T, Maruyama M, Lee YS, et al. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–80. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–43. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–43. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–25. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizgala HF, Counsell J. Acute coronary syndromes following abrupt cessation of oral propranolol therapy. Can Med Assoc J. 1976;114:1123–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Egstrup K. Transient myocardial ischemia after abrupt withdrawal of antianginal therapy in chronic stable angina. Am J Cardiol. 1988;61:1219–22. doi: 10.1016/0002-9149(88)91158-7. [DOI] [PubMed] [Google Scholar]
- 24.Tygesen H, Andersson B, Di Lenarda A, Rundqvist B, Sinagra G, Hjalmarson A, et al. Potential risk of beta-blockade withdrawal in congestive heart failure due to abrupt autonomic changes. Int J Cardiol. 1999;68:171–7. doi: 10.1016/s0167-5273(98)00356-8. [DOI] [PubMed] [Google Scholar]
- 25.Gould PA, Yii M, McLean C, Finch S, Marshall T, Lambert GW, et al. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:821–9. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 26.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Wang Z, Wang W, Wang J, Gao M, Hou Y. Efficacy of cardiac autonomic denervation for atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2012;23:592–600. doi: 10.1111/j.1540-8167.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Choi EK, Zhang Y, Mazgalev TN. Botulinum toxin injection in epicardial autonomic ganglia temporarily suppresses vagally mediated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:560–5. doi: 10.1161/CIRCEP.111.961854. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Scherlag BJ, Dormer K, Nguyen KT, Pope C, Fung KM, et al. Autonomic denervation with magnetic nanoparticles. Circulation. 2010;122:2653–9. doi: 10.1161/CIRCULATIONAHA.110.940288. [DOI] [PubMed] [Google Scholar]
- 30.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:1272–9. doi: 10.1046/j.1540-8167.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 31.Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. J Am Coll Cardiol. 2004;43:994–1000. doi: 10.1016/j.jacc.2003.07.055. [DOI] [PubMed] [Google Scholar]