Abstract
The brain appears to be a target of air pollution. This study aimed to further ascertain behavioral and neurobiological mechanisms of our previously observed preference for immediate reward (Allen, J. L., Conrad, K., Oberdorster, G., Johnston, C. J., Sleezer, B., and Cory-Slechta, D. A. (2013). Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ. Health Perspect. 121, 32–38), a phenotype consistent with impulsivity, in mice developmentally exposed to inhaled ultrafine particles. It examined the impact of postnatal and/or adult concentrated ambient ultrafine particles (CAPS) or filtered air on another behavior thought to reflect impulsivity, Fixed interval (FI) schedule-controlled performance, and extended the assessment to learning/memory (novel object recognition (NOR)), and locomotor activity to assist in understanding behavioral mechanisms of action. In addition, levels of brain monoamines and amino acids, and markers of glial presence and activation (GFAP, IBA-1) were assessed in mesocorticolimbic brain regions mediating these cognitive functions. This design produced four treatment groups/sex of postnatal/adult exposure: Air/Air, Air/CAPS, CAPS/Air, and CAPS/CAPS. FI performance was adversely influenced by CAPS/Air in males, but by Air/CAPS in females, effects that appeared to reflect corresponding changes in brain mesocorticolimbic dopamine/glutamate systems that mediate FI performance. Both sexes showed impaired short-term memory on the NOR. Mechanistically, cortical and hippocampal changes in amino acids raised the potential for excitotoxicity, and persistent glial activation was seen in frontal cortex and corpus callosum of both sexes. Collectively, neurodevelopment and/or adulthood CAPS can produce enduring and sex-dependent neurotoxicity. Although mechanisms of these effects remain to be fully elucidated, findings suggest that neurodevelopment and/or adulthood air pollution exposure may represent a significant underexplored risk factor for central nervous system diseases/disorders and thus a significant public health threat even beyond current appreciation.
Associations between air pollution and adverse cardiopulmonary heath effects are well documented. Ultrafine particles (UFP; <100 nm in diameter), found ubiquitously in ambient indoor and outdoor air (Oberdorster et al., 2004), are considered the more toxic component of ambient air pollution (Oberdorster, 2000). Increasing evidence suggests that air pollutants may also adversely affect the central nervous system (CNS). Epidemiological studies have identified associations between exposure to diverse ambient air pollutants such as particulate matter (PM), ozone, carbon monoxide, and nitrogen dioxide with ischemic cerebrovascular events (Hong et al., 2002; Lisabeth et al., 2008; Lokken et al., 2009), with one of the first studies reporting increased risk of stroke due to exposure to indoor coal fumes (Zhang et al., 1988).
Behavioral function also appears to be a target of the toxic effects of ambient air pollutants. A positive association between diagnosed attention deficit hyperactivity disorder (ADHD) and ambient particulate matter level was reported in school-aged children living in rural or urban India after controlling for potential confounders (Siddique et al., 2011). Correspondingly, we reported that postnatal exposures of mice to concentrated ambient ultrafine particles (CAPS) increased preference for immediate reward, a type of impulsivity using a fixed-ratio waiting-for-reward paradigm (Allen et al., 2013). Ambient black carbon, a marker of traffic-generated PM, was associated with reduced neurocognitive function in urban school-aged children (Suglia et al., 2008). Furthermore, long-term black carbon exposure was also associated with neurocognitive decline in a cohort of men aged 71 ± 7 years (Power et al., 2011) and ambient air pollution with deficits in short-term memory in male rats (Zanchi et al., 2010).
Air pollution causes inflammation and oxidative stress in pulmonary tissue (Alessandrini et al., 2009; Delfino et al., 2009; Fujii et al., 2001; Ishii et al., 2004; Nemmar et al., 2009; van Eeden et al., 2001) with similar effects seen in the CNS (Campbell et al., 2009; Gerlofs-Nijland et al., 2010; Kleinman et al., 2008). In vitro, brain microglia were found to be primed in response to diesel exhaust exposure when later challenged with lipopolysaccharide (Levesque et al., 2011). In vivo studies in rats indicated that subchronic diesel exhaust exposure caused neuroinflammation and elevated early markers of neurodegenerative disease (Levesque et al., 2011). Although experimental data suggests that UFP can directly translocate into the CNS (Oberdorster et al., 2002, 2004), it would not be necessary for this to occur to produce neurotoxicity, because peripheral inflammation itself can adversely affect the CNS (Banks et al., 2002; Delfino et al., 2009; Hagberg and Mallard, 2005; Konsman et al., 2002; Perry, 2004).
To further understand the nature of behavioral deficits produced by ambient air pollutants and their corresponding behavioral and neurobiological mechanisms, this study further examined our previously reported CAPS-induced preference for immediate reward (Allen et al., 2013). To do so, it used a fixed interval (FI) 60 and 120 s (FI60 and FI120, respectively) schedule of food reward, as performance on this schedule has been demonstrated to be a surrogate for impulsivity in both infants and children (Darcheville et al., 1992, 1993). Impulsivity is a component of attention deficit disorder and consistent with a preference for immediate reward. On the FI schedule, delivery of a reinforcer follows the first occurrence of a response (here, a lever press) after an FI of time (in this case, 60 s) has elapsed because the previous reinforcement delivery. The “scalloped” pattern of FI behavior, characterized by periods of little or no responding early in the interval followed by a gradual increase in rate of responding as the opportunity for reinforcer delivery approaches (Ferster and Skinner, 1957), has been observed over a wide range of species, which attests to the generality of these underlying behavioral processes (Kelleher and Morse, 1968). Our studies in rats have confirmed a critical role of mesocorticolimbic dopamine systems in the mediation of FI behavior (Cory-Slechta et al., 1997, 1998, 2002).
Because changes in FI response rate might reflect altered activity rather than impulsive-like behavior, changes in locomotor activity were also examined. As an additional assessment of CAPS behavioral toxicity, a measure of learning/short-term memory, a novel object recognition (NOR) paradigm was utilized. Levels of brain neurotransmitters and markers of neurotoxicity in brain regions known to mediate cognition and attention, as well as neuroinflammation were measured as potential mechanisms of behavioral toxicity. Based on known mechanisms of air pollution and of regions/neurotransmitters mediating these behaviors, we hypothesized that early postnatal CAPS exposure and/or adult CAPS would induce behavioral toxicity mediated by changes in mesocorticolimbic monoamines/glutamate, brain glial activation, and brain histopathology.
MATERIALS AND METHODS
Animals/experimental design/exposure
Young adult male and female C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were bred using a monogamous pairing scheme. Male and female (n = 35 pairs) mice were paired for 3 days, after which males were removed from the home cage. Pregnant dams (n = 24) remained singly housed throughout weaning. On average, litters contained (mean ± SD) 5.91 ± 1.56 pups with (mean ± SD) ∼45 ± 22% males. Litter sizes ranged from four to eight pups. Litters were not culled as allocation of pups into treatment groups was counter-balanced against litter size. Pups were randomly assigned to treatment groups with no more than a single pup per litter per sex being allocated to a given treatment group, with one pup per sex being allocated for each of the four treatment groups when possible. Upon weaning at postnatal day (PND) 25, male and female progeny were housed in same sex pairs under a 12-h light/dark cycle and temperature maintained at 72°F.
The experimental design is shown in Figure 1. Offspring (n = 8–12/treatment group/sex) were exposed to CAPS or HEPA-filtered (99.99% effective) room air in compartmentalized whole body inhalation chambers over PNDs 4–7 and 10–13 for 4 h/day between 0700 h–1300 h. Pups were removed from dams to prevent mothering behavior (e.g., nose-to-fur huddling) from impacting behavior. No gross differences in maternal care by treatment were observed after cessation of exposure. These hours correspond to high levels of vehicular traffic near the ambient air intake valve. Exposures were carried out using the Harvard University Concentrated Ambient Particle System (HUCAPS) as described elsewhere (Allen et al., 2013). Briefly, the HUCAPS system concentrates ambient ultrafine particles from a nearby highly trafficked roadway ∼10 times that of ambient air (see Fig. 2) and delivers them to compartmentalized whole-body inhalation chambers. The variability in these exposures represents the real-world nature of the exposure paradigm and the natural variability in ambient, urban, and outdoor air. Filtered air and CAPS-treated mice experienced similar experimental manipulations. Particle counts were obtained using a condensation particle counter (model 3022A; TSI, Shoreview, MN) and mass concentration was calculated using idealized particle density of 1.5 g/cm3. Both CAPS and control mouse exposure chambers were maintained at 77–79°F and 35–40% relative humidity. At PND56, mice received a secondary challenge with CAPS or filtered air for an additional 4 days to assess cumulative toxicity, generating four treatment groups per sex: postnatal air with (n = 9 males; n = 8 females) and without (n = 9 males; n = 8 females) adult CAPS exposure (designated Air/Air and Air/CAPS, respectively) and postnatal CAPS with (n = 12 males; n = 11 females) and without (n = 12 males and females) adult CAPS exposure (designated CAPS/CAPS and CAPS/Air, respectively).
Fig. 1.
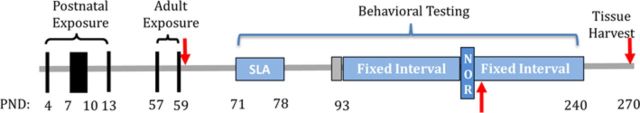
Experimental design. Mice were exposed in the postnatal period from PNDs 4–7 and 10–13, with adult re-exposure occurring at PNDs 57–59. Spontaneous locomotor (SLA) activity was collected every other day for three sessions from PNDs 71–78. Starting on PND93, animals were autoshaped and subsequently placed under the FI schedule of reinforcement until PND240. Novel object recognition (NOR) was carried out at ∼6 months of age. Blood was collected (represented by arrows) on PND60, 6 months of age, and upon sacrifice at approximately PND270 .
Fig. 2.
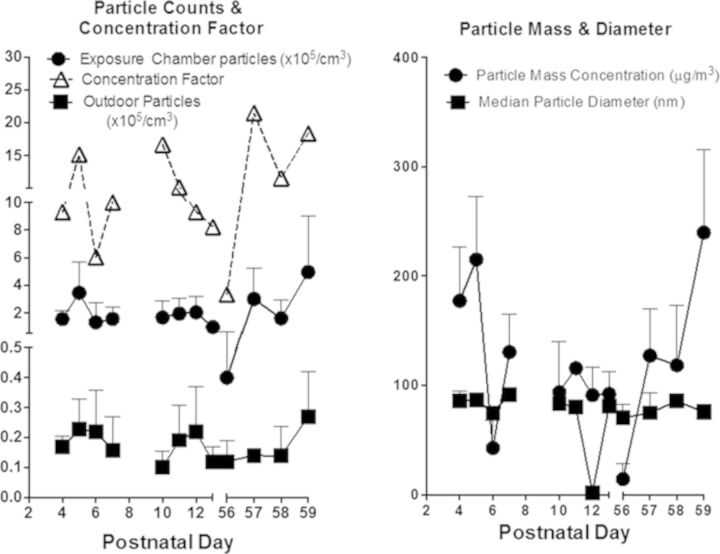
Ambient outdoor UFP and CAPS characterization. Daily outdoor ambient UFP and CAPS counts across all exposure days (left) are reported as number of particles × 105/cm3 ± SDs. Concentration factor (left) was calculated as [CAPS]/[UFP]. Mean particle mass (right) is reported as μg/m3 ± SDs. Daily diameter of CAPS (right) is reported in nm ± SDs.
Ambient outdoor UFP counts ranged from ∼10,000 to 27,000 particles/cm3 that were concentrated 3–21-fold giving rise to CAPS exposure particle count concentrations ranging from ∼40,000 to 496,000 particles/cm3. Reports of UFP particle concentrations in Erfurt, Germany (Wichmann and Peters, 2010) and Atlanta, GA, USA (Woo et al., 2010) ranged from 10,000 to 20,000 particles/cm3, similar to ambient UFP concentrations reported here. However, particle concentrations in Los Angeles, CA, USA and Minneapolis, MN, USA have been reported at 200,000–400,000/cm3, respectively, near highways, with peak episodic counts reaching as high as 2,000,000 particles/cm3 in Minneapolis (Kittelson, 2004; Westerdahl et al., 2005). Diameter of the particles remained <100 nm (i.e., ultrafine) over all exposure days. Thus, particle exposures in this study are relatively low and clearly within human-relevant ranges.
Approximately 24 h after the final CAPS exposure, mice were moved into the behavioral test suite and allowed to acclimate for 10 days prior to commencing behavioral testing on PND71. On PND65, ad libitum weights were obtained, which averaged 25.0 g for males and 19.6 g for females and there were no treatment-related differences in body weight. Four weeks after the conclusion of operant behavior testing (at ∼9 months of age), mice were sacrificed by cervical dislocation without the use of sedatives and fresh brains removed and hemisected. The left side was immersion-fixed in 4% paraformaldehyde for 48 h and cryoprotected in 30% sucrose until it sunk. Fixed frozen brains were sectioned on a sliding microtome at 40 μm for immunohistochemical analysis. Tissue was collected in cryoprotectant (30% sucrose; 30% ethylene glycol in 0.1M PB) into a six-series, with every sixth section being collected into a single well for immunohistochemical analysis. The right hemisphere was dissected into the following brain regions: olfactory bulb, hypothalamus, striatum, midbrain, frontal cortex, and hippocampus, and then fresh-frozen.
Whole blood was collected by submandibular bleed at PND60 (24 h after final adult exposure) and again at ∼6 months of age. Upon sacrifice, trunk blood was collected. Whole blood was collected into prechilled centrifuge tubes and centrifuged for 20 min at 3500 × g for 20 min to obtain serum.
Spontaneous locomotor activity
Automated locomotor activity chambers equipped with infrared photobeams (Opto-Varimex Minor, Columbus Instruments, Columbus, OH) were used to assess spontaneous locomotor activity (SLA). SLA was quantified in three 45-min sessions occurring every other day during PNDs 71–78. Horizontal, vertical, and ambulatory activities were recorded every minute for the duration of the 45-min session. Horizontal activity was defined as any nonambulatory movement in the x–y plane; ambulatory activity required three successive photobeam breaks in the x–y plane and vertical activity was defined as photobeam breaks of the z plane.
Fixed interval schedule-controlled behavior apparatus and procedure
Assessment of FI schedule-controlled behavior was conducted in operant chambers (Med Associates, St Albans, VT) housed in sound-attenuating cabinets equipped with white noise for attenuation of distracting sounds, and fans for ventilation. Three levers were located horizontally across the back wall of the chamber, with a liquid dipper and dual pellet dispenser for reinforcer delivery on the front (opposite wall).
Mice were maintained at 85% of their ad libitum body weights starting at PND88, and starting on PND93, trained to press the left lever via an overnight autoshaping program previously developed in our laboratory (Cory-Slechta et al., 1985) in which they received sucrose pellet reinforcers (20 mg; Bio-serv, Frenchtown, NJ) to a criterion of the delivery of 50 reinforcers on a fixed ratio 1 schedule (each lever press produces a reward delivery). Subsequently, starting on PND107, a FI 60-s schedule (FI60I) was imposed, which was programmed to reward the first response that occurred after an FI of time (60 s). Reward delivery also initiated the next 60-s interval in 20-min sessions. A total of 30 sessions on the FI 60 s schedule were carried out (FI60I). To examine learning of a new time interval, the schedule was shifted to FI 120-s (FI120) for the next five sessions, and to assess memory of the 60 s interval, followed by an additional 10 sessions under FI60 s (FI60II). On the FI schedule, responses occurring prior to the lapse of the interval have no programmed consequences. Behavioral measures of FI performance included overall response rates, run rates, postreinforcement pause time (PRP), and interresponse times (IRT) as defined in previous studies (Rossi-George et al., 2011). Briefly, OR is calculated as the total lever responses/total session time, PRP is measured as the latency between reinforcer delivery and next lever press response, IRT is measured as the time between successive lever press responses, and RR, which provides a more fine-grained measure of response rate, is calculated as total number of lever responses/(session time − PRP time). All FI data session was binned into five session blocks for data presentation. Unbinned data were used to determine statistical effects of session and/or statistical interactions of treatment(s) with session. Binned data were used for subsequent post-hoc analyses.
Novel object recognition
At ∼6 months of age, assessment of learning/short-term memory was carried out using a NOR paradigm. During the training trial, individual mice were placed for 10 min in a 12 in. × 12 in. plastic arena containing two identical objects fixed to the floor. At the end of the session, animals were removed from the arena and placed in the home cage for 1 h, after which the probe trial took place and mice were returned to the arena for 5 min. During the probe trial, one of the novel objects had been replaced with a new and unfamiliar object. Familiar objects were two identical, white, porcelain kitchen cabinet door pulls approximately 3 in. in diameter. The novel object was a gray metal kitchen cabinet door pull slightly smaller in diameter. Placement of the novel object was counterbalanced across treatments to preclude bias. Between animals, the arena and objects were thoroughly cleaned using disinfectant.
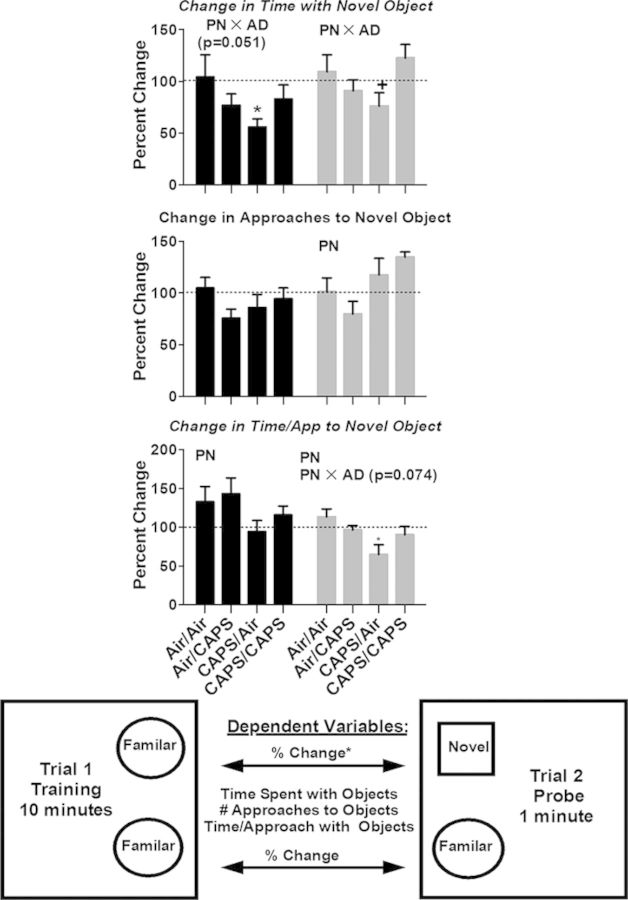
All sessions were videotaped and scored by a blinded reviewer. The entire 10 min of the training trial and the first minute of the probe trial were scored. Measures included time spent with each of the objects and number of approaches and time to approach of each of the objects. Entry of the forward part of the animal (head and front feet) into the object zone (approximately 2 cm around the object), initiated either count. These behavioral measures were used to calculate percent change in time spent with, approaches to, and time per approach with the Familiar-Familiar objects (trial 1) and the Familiar-Novel objects (trial 2). Figure 4 contains a diagram depicting the behavioral measures.
Fig. 4.
Novel object recognition in males and females exposed to postnatal Air or CAPS, adult Air or CAPS, and their combination. Change in time spent with novel object (top) and change in number of approaches to the novel object (middle), and change in time per approach to the novel object (bottom) are reported as percent change ± SEM from trial 1 to trial 2 using the object on the same side as the novel object as baseline. The graphic offers a visual depiction of experimental set up. Position of the novel object was counterbalanced against sex and treatment group to preclude potential side bias. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Neurotransmitter quantification
Level of dopamine (DA), and its metabolites dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), serotonin (5-HT), and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were analyzed in frontal cortex, midbrain (containing substantia nigra and ventral tegmental area), striatum (combined dorsal and ventral), hypothalamus, olfactory bulb, and hippocampus using HPLC with electrochemical detection as previously described (Cory-Slechta et al., 2004, 2009, 2010; Virgolini et al., 2008). Limits of detection (pg/ml) is for each are as follows: NE, 11.77; DOPAC, 7.42; DA, 7.33; HIAA, 5.49; HVA, 11.49; 5HT, 48.68. Levels of glutamate, glutamine, and GABA were determined in hippocampus and frontal cortex using a HPLC coupled with a fluorescence detector in a method described elsewhere (Cory-Slechta et al., 2012) with limits of detection <1 ng/ml for each of the analytes. Concentrations of neurotransmitters were expressed at nanogram or microgram, as appropriate, per milligram protein. DA turnover (DATO) was calculated at the ratio of [DOPAC]/[DA].
Serum corticosterone determination
Serum corticosterone levels were measured using a high sensitivity immunoassay kit (Corticosterone EIA kit AC-15F1; IDS Inc., Scottsdale, AZ) according to manufacturer's instructions (Cory-Slechta et al., 2012). Sensitivity of this assay is 16.9 pg/ml. Serum corticosterone was measured 24 h following cessation of adult CAPS exposure (PND60), at ∼6 months of age, and upon euthanasia.
Immunohistochemistry
Glial fibrillary acidic protein (GFAP) was used to identify brain astrocytes. Briefly, 40 μm free floating sections were washed in 0.1M tris buffered saline (TBS), endogenous peroxidases were deactivated by incubation in 10% methanol/3% hydrogen peroxide in 0.1M TBS for 10 min, blocking with 5% normal goat serum, followed by incubation in primary GFAP antibody (AB5804, Millipore, Billerica, MA) for 24 h at 4°C. Tissue was then incubated in goat antirabbit biotinylated secondary antibody (BA1000, Vector Labs, Burlingame, CA) for 1 h at room temperature followed by incubation in avidin-biotin solution (BA1000, Vector Labs) followed by visualization using 3,3′-diaminobenzidine.
Ionized calcium binding adaptor molecule 1 (IBA-1) was used to identify brain microglia and brain macrophages in order to assess microglial activation. Briefly, 40 μm free floating sections were washed in 0.1M phosphate buffer and blocked in 10% normal goat serum prior to 48 h incubation at 4°C in IBA-1 primary antibody (016-20001, Wako Pure Chemical Industries Ltd., Osaka, Japan). Subsequently, tissue was incubated in Alexa-fluor 594 goat antirabbit IgG secondary antibody (A-11012, Life Technologies Corp, Grand Island, NY) for 24 h at 4°C.
All tissue was mounted on SuperFrost Plus Micro slides (VWR International LLC, Radnor, PA) and coverslipped using Cytoseal-60 for GFAP or Anti-Fade Prolong Gold for IBA-1 mounting medium.
Image analysis
Image Pro Plus 7.0 (MediaCybernetics Inc, Rockville, MD) was used to quantitate relative immunoreactivity levels. GFAP or IBA-1 was analyzed in images of three sections per brain region using brightfield or fluorescent microscopy, as appropriate, at 10× magnification, for each of the following regions: midbrain, hippocampus, striatum, cortex, and corpus callosum at similar bregma across animals. All images underwent identical contrast and brightness enhancement to aid in the image analysis. All analyses were carried out in an identical fashion without regard to treatment-status to preclude any biases. IBA-1 images underwent contrast inversion prior to image analysis. Analysis was performed using count/size tool in analysis software that identifies positively stained cells and provides relative enumeration of the extent of staining. Microscopy and image analysis were carried out by a person blinded to the treatment status of the tissue. Data are reported as percent Air/Air control by sex.
Statistical analysis
Two female mice died prior to completion of behavioral testing due to reasons unrelated to treatment; data from these animals were not included in analysis. Overall analyses that included sex as a between group factor were carried out and for almost all outcomes revealed either main effects or interactions related to sex. Thus, to present a fully accurate representation of effects, all data from male and female mice were analyzed separately.
For assessments involving repeated measures (SLA, FI, and corticosterone), repeated measures analyses of variance (RMANOVA) with postnatal (PN) and adult (AD) treatment as between group factors and session (S) or time point (corticosterone) as a within factor were used. The various outcome measures are analyzed separately as each provides pertinent and different information related to behavioral mechanisms of changes in overall response rate in the case of FI, for example. Results were followed as appropriate depending on main effects or interactions, by Fisher's protected, least-squares differences (PLSDs) tests.
Analyses of variance (ANOVA) with postnatal and adult treatment as the between group factors were used to analyze GFAP and IBA-1 immunoreactivity/fluorescence, neurotransmitter levels and NOR data with Fisher's PLSDs as appropriate. Brain regions were analyzed separately given the potential for differential involvement in behavioral outcomes; further, different neurotransmitters within a brain region were analyzed separately as the different neurotransmitters within a region provide information as to the nature of the specific change in that neurotransmitter's function, e.g., changes in HVA versus DOPAC can signal changes in internal versus external dopamine handling. Statistical analyses were conducted using Statview version 5.0 (SAS Institute Inc., Cary, NC), with p < 0.05 values considered statistically significant and p < 0.1 considered to be marginally significant. Significant outliers were identified by Grubb's test and excluded from ANOVAs, with treatment group mean values substituted to facilitate RMANOVA analysis. To prevent statistical masking, no more than a single outlier per treatment group was removed.
RESULTS
Behavioral, neurochemical, and/or biochemical effects of CAPS by sex are summarized in Table 1.
TABLE 1. Summary of Changes in Behavioral, Neurochemical, and Biochemical Effects of CAPS by Sexa.
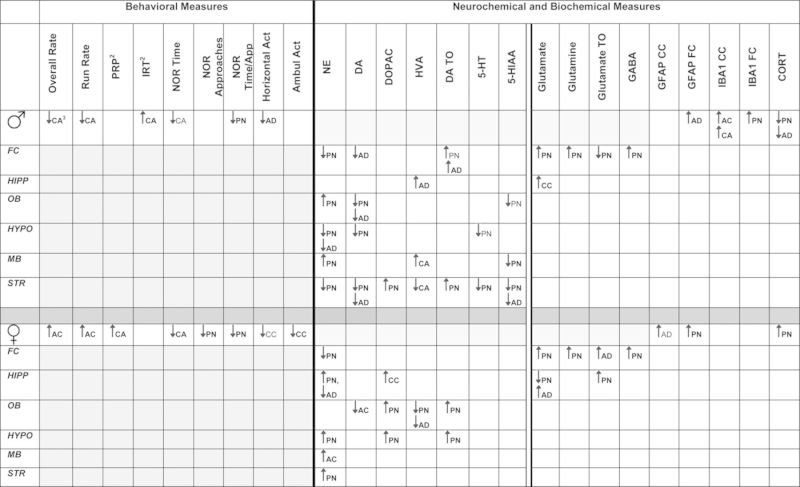 |
aFC, frontal cortex; HIPP, hipocampus; OB, olfactory bulb; HYPO, hypothalamus; MB, midbrain; STR, striatum; CORT, corticosterone . Bolded signifies statistically significant; unbolded signifies marginally significant.
bPRP, postreinforcement pause time; IRT, interresponse time.
cPN, postnatal; AD, adult; AC, Air/CAPS, CA-CAPS/Air, CC-CAPS/CAPS, ∼ marginally significant.
Exposure
Outdoor ultrafine particle counts (mean ± SD) ranged from ∼0.100 ± 0.053 × 105 to 0.270 ± 0.151 × 105 particles/cm3 across all exposure days. Inside the exposure chambers, ultrafine particle counts (mean ± SD) ranged from ∼0.400 ± 0.230 × 105 to 4.960 ± 4.030 × 105 particles/cm3, with the ambient outdoor particles being concentrated from 3.33 to 21.5 times that of ambient outdoor air. Inside the exposure chambers, the mass concentration of particles ranged from 15 to 240 μg/m3 across all exposure days. The diameter of the particles remained <100 nm for all exposure days (Fig. 2). Variability in the particle counts, mass, diameter, and concentration factor reflect the real-world nature of this exposure and the temporal variability in which ambient ultrafine particles are present as described previously (Allen et al., 2013).
Initial FI60 Schedule Controlled Behavior (FI60I)
A statistically significant four-way interaction of FI60I operant session by sex postnatal CAPS by adult CAPS (F(29,2059) = 3.53, p < 0.0001) indicating the sex-dependent nature of the effects of CAPS exposure was observed. As such, all subsequent analyses were performed independently by sex.
Males
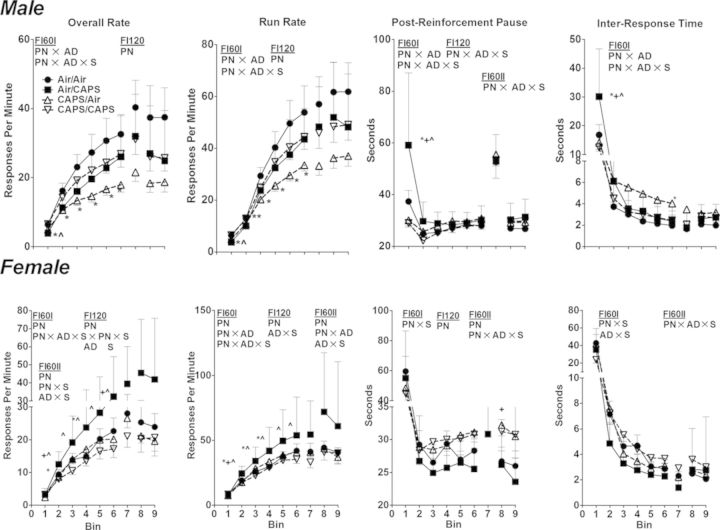
No treatment-related effects on the number of sessions required to meet criterion for lever press training were observed (data not shown). On the initial FI 60 s schedule (Fig. 3; FI60I), CAPS/Air exposed males exhibited significantly reduced overall rates and run rates compared with Air/Air across bins 2–6 (PN × AD × S, F(29,1073) = 1.68, p = 0.014 and F(29,1073) = 1.76, p = 0.008, respectively, all post hocs p < 0.05). Air/CAPS had lower overall rate and run rate for bin 1 only (p < 0.05). Changes in PRP times were restricted to increases in Air/CAPS males compared with all other treatment groups during bin 1 (PN × AD × S, F(29,1073) = 3.15, p < 0.001, post hoc p < 0.05), and decreases in CAPS/CAPs mice compared with Air/CAPS mice during bin 2 (p < 0.05). Similarly, IRTs were increased in Air/CAPS males compared with all other treatment groups during bin 1 (PN × AD × S, F(29,1073) = 3.07, p < 0.0001, post hocs p < 0.05). CAPS/Air males had higher IRT values during bin 6 compared with Air/Air (p < 0.05).
Fig. 3.
Overall rates, run rates, postreinforcement pause times, and interresponse times for male and female mice performing under fixed-interval schedule controlled behavior (mean ± SE). Data binned into five sessions per bin for each of FI60I, FI120, and FI60II. PN, or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Females
No treatment-related effects on the number of sessions required to meet criterion for lever press training were observed (data not shown) in females. Air/CAPS exposure in females (Fig. 3, bottom row) significantly increased overall rates compared with control during bin 4 (PN × AD × S, F(29,986) = 2.05, p = 0.009), whereas overall and run rates were increased compared with CAPS/CAPS during bins 2–6 (post hocs p < 0.05). Overall rates in Air/CAPS females were higher than CAPS/Air females only during bins 2 and 6 (post hoc p < 0.05). Run rates were increased in Air/CAPS exposed females compared with all treatment groups during bin 2 (PN × AD × S, F(29,986) = 2.14, p = 0.0005, post hoc p < 0.05), and as compared with Air/Air and to CAPS/CAPS from bins 2 to 4 (post hocs p < 0.05). PRP times were characterized by a statistical interaction of PN × S (F(29,986) = 2.40, p < 0.0001); females receiving postnatal CAPS (CAPS/Air and CAPS/CAPS) had generally higher PRP values. Similar results were seen for IRT (PN × S, (F(29,986) = 2.23, p = 0.0002 and AD × S, F(29,986) = 1.89, p = 0.003).
FI120 Schedule Controlled Behavior
Males
Reductions in overall rates and run rates under the FI120 schedule were seen in postnatal CAPS exposed males (CAPS/Air and CAPS/CAPS; PN, F(1,37) = 4.33, p = 0.044 and F(1,37) = 4.30, p = 0.045, respectively). No significant treatment-related differences in PRP or IRT were observed in male mice under the FI120 schedule.
Females
Female mice receiving postnatal CAPS exposure (CAPS/Air and CAPS/CAPS) had significantly reduced overall and run rates under the FI120 schedule (PN, F(1,34) = 5.33, p = 0.027 and F(1,34) = 4.40, p = 0.044, respectively). No systematic treatment-related differences on PRP or IRT were observed in female mice under the FI120 schedule.
Return to FI60 Baseline (FI60II)
Males
Despite trends similar to those seen in FI60I, no significant treatment-related differences in overall rates, run rates, or IRT were observed in males on the FI60II schedule. PRP values during the FI60II were similar to those during FI60I (PN × AD × S, F(14,518) = 2.81, p = 0.0005).
Females
Under the FI60II schedule, females that had received postnatal CAPS (CAPS/Air and CAPS/CAPS) had significantly lower overall and run rates (PN, F(1,34) = 5.80, p = 0.022 and F(1,34) = 4.58, p = 0.04, respectively) and significantly longer PRP times (PN, F(1,34) = 6.36, p = 0.017).
Novel Object Recognition
Males
Postnatal CAPS only (CAPS/Air) nearly significantly reduced time in contact with the novel object from the training trial to the probe trial in males (Fig. 4; PN × AD, (F(1,37) = 4.08, p = 0.051, post hoc p < 0.05), whereas both postnatal CAPS alone (CAPS/Air) and postnatal CAPS followed by adult CAPS (CAPS/CAPS) significantly reduced the increase in the percent change in time per approach to the novel object from the training trial to the probe trial (PN, F(1,37) = 5.68, p = 0.02).
Females
As in males, CAPS/Air in females nearly significantly reduced the percent change in time with the novel object from the training trial to the probe trial (Fig. 4; PN × D, F(1,33) = 5.43, p = 0.03), p = 0.051, post hoc p < 0.05). Postnatal CAPS also significantly decreased time per approach to the novel object (PN, F(1,33) = 6.17, p = 0.018), an effect largely attributable to postnatal CAPS only (CAPS/Air) exposure (PN × AD, F(1,33) = 3.70, p = 0.063; post hoc p < 0.05). This occurred in conjunction with a postnatal CAPS-based (CAPS/Air and CAPS/CAPS) increase in the number of approaches to the novel object (PN, F(1,32) = 8.15, p = 0.008) as compared with females receiving postnatal air (Air/Air; Air/CAPS).
Spontaneous Locomotor Behavior
Males
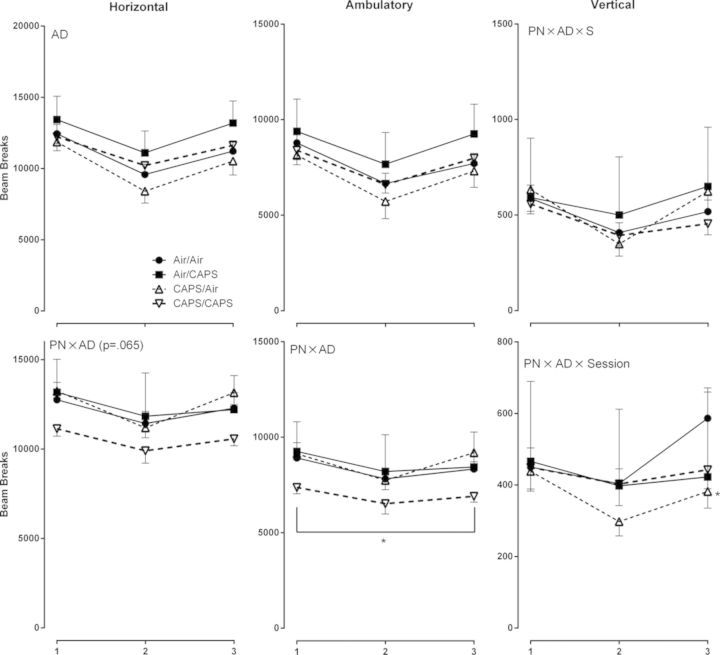
Adult CAPS exposure (Air/CAPS and CAPS/CAPS) significantly reduced horizontal activity levels in comparison to adult air exposure (Fig. 5; AD, F(1,37) = 5.14, p = 0.03). No systematic treatment-related differences in ambulatory or vertical activity were observed in male mice.
Fig. 5.
Ambulatory, horizontal, and vertical activity in male and female mice exposed to postnatal CAPS, adult CAPS, or their combination. Reported as total beam breaks across session ± SE. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Females
A cumulative effect of CAPS/CAPS was observed in females, i.e., activity levels were not significantly affected by postnatal or adult CAPS alone, but only by the combination (Fig. 5, bottom row; PN × AD, F(1,36) = 4.83, p = 0.035) with comparable trends for reduced horizontal activity (PN × AD, F(1,36) = 3.62, p = 0.065) compared with air-treated females. CAPS/Air exposed females had lower vertical activity levels during session 3 compared with Air/Air females (PN × AD × S, F(2,72) = 4.87, p = 0.01, post hoc p < 0.05).
Corticosterone
Males
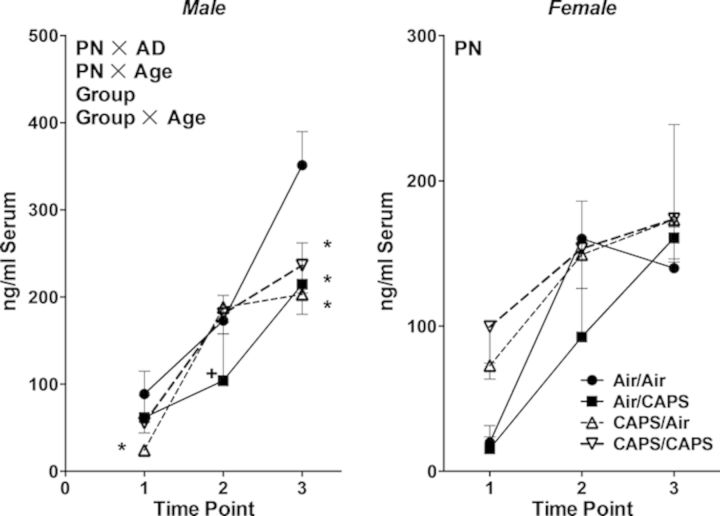
Serum corticosterone increased with age (F(1,37) = 70.03, p < 0.0001) in males (Fig. 6, left). At PND60, CAPS/Air exposed males had lower serum corticosterone levels than did Air/Air males (PN × AD, F(1,37) = 10.28, p = 0.003; Group × Time point, F(6,74) = 2.97, p = 0.01, post hoc p < 0.05). At 6 months of age, Air/CAPS males had lower corticosterone levels than did CAPS/Air males (post hoc p < 0.05). Serum corticosterone levels of all CAPS treated groups were lower than those of Air/Air males at the time of sacrifice (post hocs p < 0.05).
Fig. 6.
Serum corticosterone in male (left) and female (right) animals exposed to Air/Air, Air/CAPS, CAPS/Air, or CAPS/CAPS 24 h following cessation of adult exposure (point 1), at 6 months of age (point 2) or upon sacrifice (point 3). Reported as group mean ± SE. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. Group indicates a main effect of group. Group × Age indicates a statistical interaction of group × age. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Females
Serum corticosterone levels also increased with age in females (Fig. 6, right; F(1,38) = 28.9, p < 0.0001), with those exposed to postnatal CAPS having generally higher levels of serum corticosterone overall (PN, F(1,38) = 6.61, p = 0.01).
Monoamine and Amino Acid Neurochemistry
Plots of changes in monoamines and amino acids depicted in Figures 7 and 8 present only those neurotransmitters within each region that showed significant effects of CAPS.
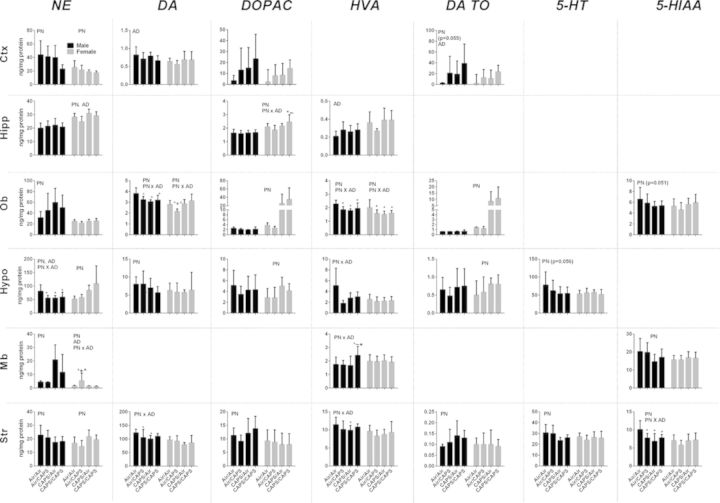
Fig. 7.
Monoamine neurotransmitters and metabolites in male and female Air/Air, Air/CAPS, CAPS/Air, and CAPS/CAPS animals. Data reported as group mean ± SE. DA TO calculated as [DOPAC]/[DA]. Reported in ng neurotransmitter or metabolite/mg protein. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
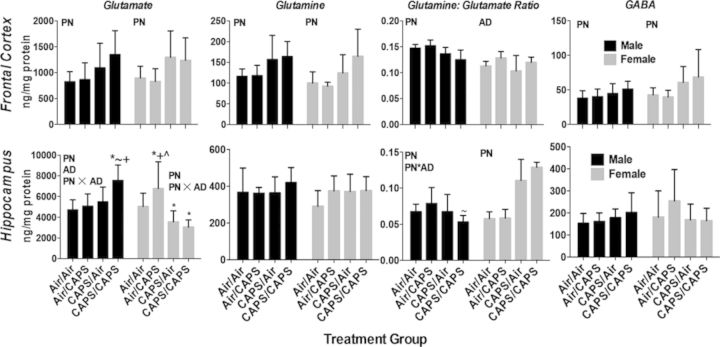
Fig. 8.
Cortical hippocampal glutamate, glutamine, glutamine:glutamate, and GABA in male and female Air/Air, Air/CAPS, CAPS/Air, and CAPS/CAPS mice as indicated. Data reported group mean ± SE. Reported in ng analyte/mg protein. Glutamine:glutamate ratio calculated as [glutamine]/[glutamate]. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Frontal Cortex
Males
Cortical levels of DA turnover (Fig. 7) were nearly increased by both postnatal (PN, F(1,37) = 3.93, p = 0.055) and significantly increased by adult exposures (AD, F(1,37) = 4.75, p = 0.036), with similar but nonsignificant trends for DOPAC. Postnatal CAPS (CAPS/Air and CAPS/CAPS) significantly reduced frontal cortical NE (PN, F(1,37) = 5.56, p = 0.03). Adult only treatments also had impacts, in this case significantly reducing cortical DA levels (AD, F(1, 37) = 5.7, p = 0.02). Levels of cortical glutamate, glutamine, and GABA (Fig. 8) were increased in response to postnatal CAPS relative to Air (PN on glutamate: F(1,37) = 9.03, p = 0.005; on glutamine: F(1,37) = 11.91, p = 0.001, on GABA: F(1,37) = 5.6, p = 0.02), whereas the glutamine:glutamate ratio was reduced (on Gln/Glu: F(1,37) = 19.34, p < 0.0001).
Females
Cortical NE was reduced by postnatal CAPS (PN, F(1,34) = 7.27, p = 0.0108). No other significant monoamine changes were found. Cortical glutamate, glutamine, and GABA (Fig. 8) were increased by postnatal CAPS (PN: glutamate: F(1,34) = 9.7, p = 0.004; glutamine: F(1,34) = 10.4, p = 0.003; GABA: F(1,34) = 7.57, p = 0.009), whereas the glutamine:glutamate ratio was increased in response to adult exposure (AD, F(1,34) = 6.87, p = 0.01).
Hippocampus
Males
No treatment related changes in monoamines were seen in male hippocampus (Fig. 7). However, glutamate was increased (Fig. 8; PN × AD, F(1,34) = 4.20, p < 0.048, post hocs p < 0.05), and the glutamine:glutamate ratio decreased selectively by CAPS/CAPS in males (F(1,34) = 5.11, p = 0.03, post hocs p < 0.05). No treatment-related changes in glutamine, or GABA were observed.
Females
Monoamine changes in hippocampus in females were observed as postnatal and adults CAPS-induced increases in NE (Fig. 7, PN, F(1,34) = 11.74, p = 0.0016; AD, F(1,34) = 6.28, p = 0.02) and to selective increases in CAPS/CAPS females in DOPAC levels relative to Air/Air and Air/CAPS females (PN × AD, F(1,34) = 5.50, p = 0.03, post hocs p < 0.05). Adult only CAPS (Fig. 8; Air/CAPS) selectively increased hippocampal glutamate in females (PN × AD, F(1,34) = 5.71, p = 0.02; post hocs p < 0.05), whereas postnatal CAPS (Air/CAPS, CAPS/CAPS) reduced hippocampal glutamate compared with Air/Air (p < 0.05 for each). The glutamine:glutamate ratio was increased by postnatal CAPS in females (PN, F(1,34) = 112.29, p < 0.0001).
Olfactory Bulb
Males
Olfactory bulb levels of DA and HVA were decreased by any CAPS treatment compared with Air/Air (Fig. 7; DA: PN × AD, F(1,34) = 10.11, p = 0.003, post hocs p < 0.05; HVA: PN × AD, F(1,34) = 9.6, p = 0.004, post hocs p < 0.05), whereas NE increased (PN, F(1,34) = 4.43, p = 0.04), and 5-HIAA was marginally reduced (PN, F(1,34) = 4.08, p = 0.0506) in response to postnatal CAPS.
Females
As with males, any CAPS treatment decreased HVA relative to Air/Air (Fig. 7; PN × AD: F(1,34) = 4.80, p = 0.03, post hocs p < 0.05). DA TO was increased by postnatal CAPS (PN, F(1,34) = 15.59, p = 0.0004), with a similar but nonsignificant trend for DOPAC, whereas adult only exposure (Air/CAPS) selectively reduced DA (PN × AD, F(1,34) = 9.81, p = 0.001, post hocs p < 0.05).
Hypothalamus
Males
Any CAPS treatment was found to reduce hypothalamic NE in males (Fig. 7, PN × AD, F(1,37) = 4.57, p = 0.04, post hocs p < 0.05), whereas HVA showed similar trends (PN × AD, F(1,16) = 5.44, p = 0.03, post hocs p < 0.05), but these were significant only in the Air/CAPS and CAPS/Air groups. DA was reduced by postnatal CAPS (PN: F(1,37) = 4.32, p = 0.045). Postnatal CAPS also marginally reduced hypothalamic 5-HT (PN, F(1,37) = 3.90, p = 0.056).
Females
Postnatal CAPS increased hypothalamic levels of NE, DOPAC, and DA TO in females (PN on NE: F(1,34) = 10.98, p < 0.0001; on DOPAC: F(1,34) = 10.32, p = 0.003; on DA TO: F(1,33) = 6.67, p = 0.01).
Midbrain
Males
Midbrain NE was markedly increased by postnatal CAPS in males (Fig. 7; PN, F(1,37) = 15.66, p = 0.0003) and HVA was selectively increased by CAPS/CAPS (PN × AD, F(1,37) = 4.24, p = 0.047, post hocs p < 0.05). 5-HIAA was decreased in postnatal CAPS exposed males (main effect PN, F(1,37) = 6.36, p = 0.02).
Females
Air/CAPS females had increased midbrain NE compared with all other treatment groups (PN × AD, F(1,34) = 6.67, p = 0.014, post hocs p < 0.05), but no other changes were noted.
Striatum
Males
The most uniform effects of CAPS in males occurred in striatum, where virtually every monoamine was affected (Fig. 7). Postnatal CAPS decreased striatal NE and 5-HT, and increased DOPAC and DA TO (PN on NE: F(1,37) = 6.63, p = 0.014; on 5-HT F(1,37) = 10.59, p = 0.002; on DOPAC: F(1,37) = 4.21, p = 0.047; on DA TO: F(1,37) = 4.81, p = 0.035). Striatal DA was reduced by Air/CAPS and CAPS/Air treatments (PN × AD F(1,37) = 5.96, p = 0.02, post hocs p < 0.05), whereas HVA was decreased only by postnatal CAPS alone (CAPS/Air) (PN × AD, F(1,37) = 4.38, p = 0.04 post hoc p < 0.05). Striatal 5-HIAA was decreased by any CAPS treatment (PN × AD, F(1,37) = 8.43, p = 0.006, post hocs p < 0.05).
Females
The only monoamine change in striatum in females was a postnatal CAPS-induced increase in NE (PN, F(1,34) = 11.66, p = 0.002).
Correlations Between Neurochemical Changes and FI Performance
Males
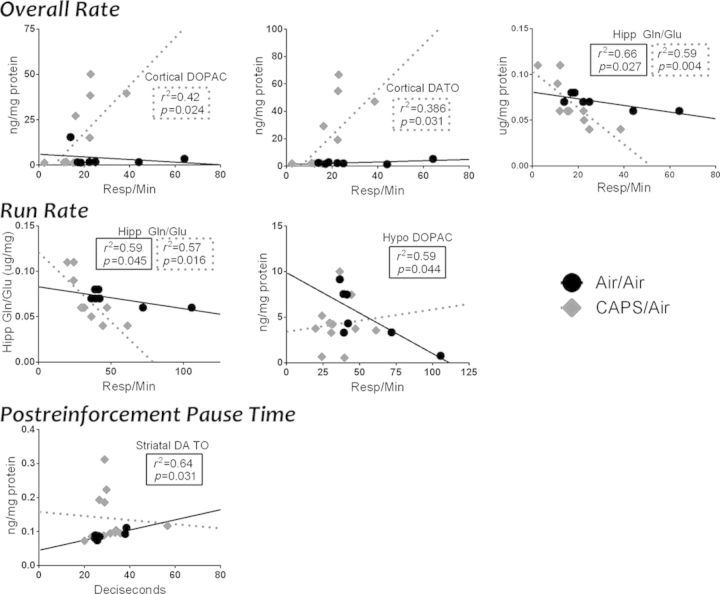
Given the pronounced changes in FI performance with the CAPS/Air group, correlations were examined between performance during the final session of FI60I and brain neurotransmitter levels (Fig. 9). The focus included both correlations in the CAPS/Air group not seen with Air/Air, and correlations in the Air/Air group that were no longer present in the CAPS/Air group. Interestingly, cortical DOPAC and DA TO were positively correlated with overall rate in CAPS/Air-exposed mice, with r2 values of 0.42 and 0.386, respectively, but not in any other treatment groups. Additionally, the hippocampal glutamine/glutamate ratio showed a more negative slope for both overall rate (p = 0.004, r2 = 0.59) and run rate (p = 0.016, r2 = 0.57) in CAPS/Air males than in Air/Air males (p = 0.027, r2 = 0.66 and p = 0.045, r2 = 0.59, respectively). In contrast, the negative correlations between hypothalamic DOPAC and run rate (p = 0.044, r2 = 0.59) and between midbrain DA and IRTS (p = 0.016, r2 = 0.72), as well as the positive correlation between striatal DA TO and postreinforcement pause time (p = 0.031, r2 = 0.64) in Air/Air mice were not evident in other treatment groups.
Fig. 9.
Selected neurochemistry and corticosterone regressed against FI measures in male mice exposed to Air/Air and CAPS/Air. Statistically significant (p < 0.05) regressions and associated r2 and p values are listed in the body of the graph in dashed boxes for the CAPS/Air group and in solid lines for the Air/Air group.
Females
Given the pronounced effects of Air/CAPS in females, correlations were examined between performance during the final session of FI60I and brain neurotransmitter levels (Fig. 10). The focus included both correlations in the Air/CAPS group not seen with Air/Air, and correlations in the Air/Air group that were no longer present in the Air/CAPS group. For Air/CAPS females, significant positive correlations were noted between cortical NE and both overall rate (p = 0.026, r2 = 0.86) and run rate (p = 0.016, r2 = 0.72) that were not found in Air/Air females. As with males, hypothalamic DOPAC was positively correlated with run rate (p = 0.019, r2 = 0.70) in Air/CAPS, but not Air/Air females. IRTs of Air/Air females were positively correlated with hippocampal DOPAC (p = 0.023, r2 = 0.54), an effect no longer evident with Air/CAPS exposure, where instead IRT times were significantly positively associated with striatal DA levels (p = 0.035, r2 = 0.54).
Fig. 10.
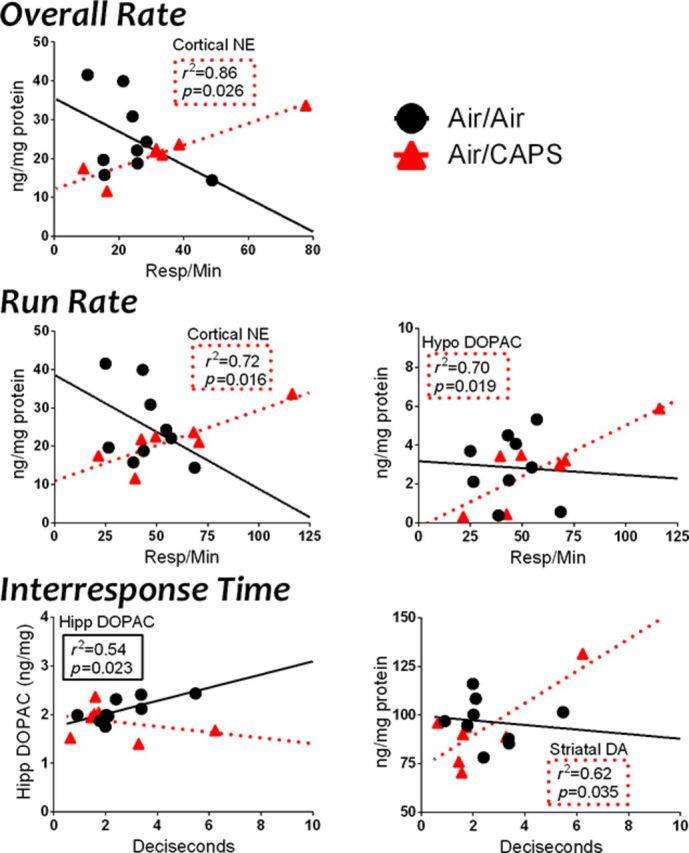
Selected neurochemistry and corticosterone regressed against FI measures in Air/Air and Air/CAPS females. Statistically significant (p < 0.05) regressions and associated r2 and p values are listed in the body of the graph in dashed boxes for the CAPS/Air group and in solid lines for the Air/Air group.
Glial Activation
Males
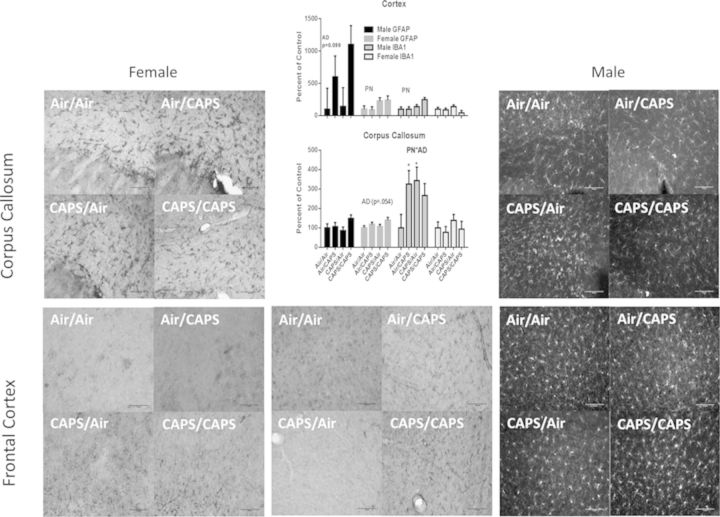
Microglial activation occurred in both the corpus callosum and cortex from CAPS exposure (Fig. 11). Specifically, IBA-1 was prominently increased in corpus callosum in Air/CAPS and CAPS/Air males (PN× AD, F(1,13) = 5.06, p = 0.043; post hocs p < 0.05), with a similar trend in the CAPS/CAPS group, whereas cortical IBA-1 was increased in mice that received postnatal CAPS (PN, F(1,14) = 7.14, p = 0.018). A trend towards adult CAPS-induced increased GFAP in male frontal cortex was observed (F(1,14) = 3.13, p = 0.098), but no effect on GFAP in the corpus callosum were seen. No treatment-related effects on GFAP or IBA-1 immunoreactivity were observed in the other examined brain regions (data not shown).
Fig. 11.
Cortical and corpus callosum astrocyte (GFAP) and microglial/macrophage (IBA-1) activation in CAPS-exposed males and females in indicated treatment groups with representative images of GFAP and IBA1 immunostaining. Relative quantification of GFAP and IBA-1 immunoreactivity in the corpus callosum cortex (left) and corpus callosum (right) of Air/Air, Air/CAPS, CAPS/Air, and CAPS/CAPS exposed males and females. PN or AD indicates statistical main effect of postnatal or adult treatment, respectively. PN × AD indicates statistical interaction of the treatments. * denotes statistical significance from Air/Air, ∼ denotes statistical significance from Air/CAPS, + denotes statistical significance from CAPS/Air, and ∧ denotes statistical significance from CAPS/CAPS.
Females
No IBA-1 changes were seen in either cortex or corpus callosum in females. However, cortical GFAP in females was increased by postnatal CAPS (PN, F(1,17) = 6.4, p = 0.021), as were trends towards adult CAPS-induced increases in GFAP in corpus callosum (AD, F(1,17) = 1.42, p = 0.055). No treatment-related changes were observed in other examined brain regions (data not shown).
DISCUSSION
Collectively, this study demonstrates that low level exposures to ultrafine particles early in development can produce persistent changes in CNS functions that include long-term impairment in measures of learning,/short-term memory, of an impulsivity-linked behavior (FI) and motor function, as well as sustained alterations in brain neurochemistry consistent with a marked glutamate-dopamine imbalance, particularly in frontal cortex, a region critical to mediation of cognitive functions. Moreover, changes in GFAP and IBA-1 indicate a sustained cortical and corpus callosum glial activation, and decrements in corticosterone indicate hypothalamic-pituitary-adrenal (HPA) axis dysfunction, consistent with prior reports (Clougherty et al., 2010; Thomson et al., 2013). The observed changes were highly sex-dependent suggesting a potential interaction with neuroendocrine systems that warrants further investigation.
CAPS altered FI performance in a sex- and time-dependent manner, with greater sensitivity of males to early postnatal exposures, and females to adult exposures. The transition between FI values across sessions, moreover revealed postnatal CAPS-induced changes in females. These alterations could be considered consistent with altered learning and reduced behavioral flexibility. Behavioral inflexibility is a phenotype observed in autism (Memari et al., 2013), bipolar disorder (Weathers et al., 2013), and schizophrenia (Thoma et al., 2007), and air pollution has been associated with both schizophrenia (Pedersen et al., 2004) and autism (Becerra et al., 2013; Volk et al., 2011, 2013). These results support our prior study showing preference of CAPS-exposed mice for immediate reward (Allen et al., 2013), a type of impulsive behavior, given that FI performance has been shown to be a surrogate for impulsivity (Darcheville et al., 1992, 1993), indicating the potential susceptibility of this behavior.
We have previously demonstrated the critical role of the mesocorticolimbic system in mediating FI performance (Cory-Slechta et al., 1997, 1998, 2002; Evans and Cory-Slechta, 2000). Mesocorticolimbic circuit function is dependent upon maintenance of a dopamine/glutamate, or inhibitory/excitatory balance (Lupinsky et al., 2010; Tye et al., 2013; Wang et al., 2012) important to mediation of behavioral flexibility (Chen et al., 2013; Gruber et al., 2010). Prefrontal dopamine-glutamate imbalance is considered to be a key mechanism in schizophrenia (Kehrer et al., 2008; Riederer et al., 1992). Indeed, changes in mesocorticolimbic dopamine and glutamate systems were produced by CAPS in both sexes that would be expected to contribute to the observed behavioral toxicities (Antunes and Biala, 2012; Cory-Slechta et al., 1997, 1998, 2002; Evans and Cory-Slechta, 2000). Indeed, significant correlations between, e.g., cortical dopaminergic neurotransmitter levels and hippocampal glutamine/glutamate ratios with FI overall and run rates in CAPS/Air males, and of cortical NE in females were found. Additionally, marginally increased cortical glutamate/dopamine ratios in PN CAPS males (p = 0.09), and a significant PN and AD (p = 0.004 and 0.0026, respectively) CAPS-related increase in females was seen.
Importantly, these changes in central neurotransmitters also extend to the olfactory bulb and hypothalamus, interactive brain regions (Mucignat-Caretta, 2010). Olfactory bulbectomy has been utilized as a model of depression (Hellweg et al., 2007), and alterations in olfactory bulb monoamines impact aggressive behaviors (Garris, 2003). The olfactory bulb is also influenced by excitatory amino acid receptors (Castro et al., 2007) and its activity influences amygdala, frontal cortex, nucleus accumbens and hippocampus (Hellweg et al., 2007). Future studies should examine animal models of both depression and aggressive behavior.
Notably, both sexes showed increased levels of glutamate in the hippocampus and frontal cortex, consistent with a potential excitotoxic mechanism for the CNS effects of CAPS. Postnatal CAPS increased cortical glutamate in both sexes, whereas increases in hippocampus, were seen only in CAPS/CAPS males. Notably, Air/CAPS females had increased hippocampal glutamate, an outcome that may relate to the increased FI response rates seen after adult CAPS in females (Moore et al., 1999). Postnatal CAPS also increased frontal cortex GABA in both males and females, changes that may relate to alterations seen in both sexes in NOR performance (Damgaard et al., 2011). Clearly future studies will be warranted to determine the extent to which neurochemical changes are directly or indirectly altered by CAPS and their specific contributions to associated behavioral toxicity. Such studies would be aided by inclusion of the current understanding of the known sex differences in both the structure and function of mesolimbic circuitry.
Given the epidemiological associations between exposure to ambient air pollutants and memory impairment in adults (Chen and Schwartz, 2009; Wellenius et al., 2012; Weuve et al., 2012) and children (Suglia et al., 2008; van Kempen et al., 2012), we sought to determine if postnatal CAPS and/or adult CAPS adversely impacted learning/short term memory using an NOR paradigm. Although some differences were evident, generally consistent effects of CAPS were found in males and females, with CAPS/Air reducing time spent with the novel object and less time per approach to the novel object in trial 2. Females also had an increased number of approaches to the novel object. Thus, CAPS/Air females approached the novel object more often, but spent less time with the novel object. Although these altered behavior patterns in both sexes are consistent with short-term memory loss, they may also suggest a fear-like response to the novel object. Interestingly, hippocampal glutamate has been demonstrated to be critical to the mediation of NOR performance (Cohen et al., 2013), and thus these deficits may reflect the alterations in hippocampal glutamate function, particularly in females, where PN CAPS reduced levels of glutamate but increased the glutamine:glutamate ratio, a reflection of turnover. The current findings are consistent with a recent report of nanoparticle rich diesel exhaust exposure-induced deficits in NOR in female mice compared with air-exposed controls (Win-Shwe et al., 2012), although an unexplained finding of that study was that filtered diesel exhaust produced similar impairments.
Patterns of CAPS-related changes in locomotor activity did not correspond to those seen in either FI or NOR performance and were thus not likely behavioral mechanisms of these effects. Specifically, adult CAPS exposures reduced horizontal activity in males, but NOR and FI performance impairments were seen in postnatally exposed CAPS groups. In females, a cumulative reduction in ambulatory and vertical (rearing) counts occurred with combined postnatal and adult exposures (CAPS/CAPS) but not either alone, whereas FI and NOR performance changes occurred in response to adult CAPS, and PN only CAPS was associated with reduced NOR performance.
The hypothalamic-pituitary-adrenal (HPA) gland axis is the mammalian stress-response system; in mice, HPA axis development occurs in two stages during early postnatal life that includes an early stress hypo-responsive period, thought to be centrally mediated, from approximately PNDs 4–14 (Sapolsky and Meaney, 1986), a time period encompassed by our exposure paradigm. The HPA axis shows prominent sex differences (Yoshimura et al., 2003) and can be influenced by excitatory amino acids during this period (Chautard et al., 1993). HPA axis activation produces inflammation with potential behavioral and neurological consequences (Silverman and Sternberg, 2012). Thus, serum corticosterone, the major murine stress-response hormone, was measured at three time points across the lifespan. As with other outcomes, postnatal CAPS influenced serum corticosterone in a sex-dependent manner. CAPS/Air males showed lower corticosterone levels at PND60, and by the time of sacrifice, lower corticosterone levels were evident in all CAPS-treated groups, demonstrating that CAPS at either postnatal or adult life stages alters the age-related trajectory of HPA axis function. Postnatal CAPS-treated females generally sustained higher corticosterone levels across time, consistent with HPA axis hyperreactivity. Interestingly the immune system interacts with the HPA axis across the neuro-immuno-endocrine axis (Costa-Pinto and Palermo-Neto, 2010), which may have implications for neuroinflammation and propagation of a CAPS-induced inflammatory event in the CNS or elsewhere. It will be important to determine whether the HPA axis represents a direct toxicological target of CAPS, or perhaps a response to alterations in excitatory amino acid function in understanding CNS mechanisms of CAPS.
An additional notable finding was the persistent glial activation observed in corpus callosum and cortex of CAPS-exposed mice, observed months posttermination of exposure consistent, with a sustained neuroinflammatory state. IBA-1, a marker of brain microglia and macrophages was increased in the cortex of males receiving postnatal CAPS and in corpus callosum of Air/CAPS and CAPS/Air males, consistent with a CAPS-induced increased macrophage (i.e., microglial) presence in the brain. In females, postnatal CAPS increased frontal cortex GFAP, and adult CAPS increased corpus callosum GFAP levels. Such neuroinflammation, in addition to being present in neurodevelopmental disorders such as autism (Morgan et al., 2010; Rodriguez and Kern, 2011) and schizophrenia (Cropley et al., 2013; Rao et al., 2013), is also a component of age-related neurodegenerative diseases, including Alzheimer's and Parkinson’ disease, multiple sclerosis, Huntington's disease and amyotrophic lateral sclerosis (Frank-Cannon et al., 2009; Hirsch et al., 2012; Li et al., 2011). Thus, the current findings raise the question as to the extent that developmental CAPS exposures may also contribute to the subsequent onset of neurodegenerative diseases. The glial changes in the corpus callosum were discovered incidentally while examining the mesocorticolimbic structures of the brain. The corpus callosum is a central white matter tract that is largely responsible for interhemispheric communication. Neuropathology or dysfunction in the corpus callosum has been implicated in a number of neurological conditions including both schizophrenia and autism (Newbury and Rosen, 2012). Functional disconnectivity, or an aberrant change in connectivity in the central nervous system such that typical neurotransmission is disrupted, is one putative mechanism by which corpus callosum pathology is thought to elict neurological dysfunction. Interestingly, CAPS induces both corpus callosum glial changes and widespread, multi-brain system changes in both monoamine and amino acid neurotransmitters which is consistent with functional disconnection; however, further and more in depth study into this question is required.
Collectively, these data demonstrate persistent neurotoxic changes in response to CAPS during the early postnatal period, young adulthood, or the combination. CAPS produced changes in a sex-specific manner, underscoring the necessity of examining the effects of toxicants in both males and females. Although the molecular mechanism of CAPS-induced neurobehavioral toxicity remains to be fully elucidated, our evidence, coupled with that of others, suggests that exposure to ambient air pollutants in the context of neurodevelopment and/or adulthood may represent a significant and as yet underexplored risk factor for CNS diseases/disorders. If so, air pollution may constitute a significant public health threat even beyond current appreciation.
FUNDING
PI (NIH K12ES019852, NIH R21ES019105 to D.A.C.-S.); PI (NIH P30ES01247 to T.G.); PI (NIH T32ES007026 to B.P.L.).
REFERENCES
- Alessandrini F., Beck-Speier I., Krappmann D., Weichenmeier I., Takenaka S., Karg E., Kloo B., Schulz H., Jakob T., Mempel M., et al. Role of oxidative stress in ultrafine particle-induced exacerbation of allergic lung inflammation. Am. J. Respir. Crit. Care Med. 2009;179:984–991. doi: 10.1164/rccm.200807-1061OC. [DOI] [PubMed] [Google Scholar]
- Allen J. L., Conrad K., Oberdorster G., Johnston C. J., Sleezer B., Cory-Slechta D. A. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ. Health Perspect. 2013;121:32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M., Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W. A., Farr S. A., Morley J. E. Entry of blood-borne cytokines into the central nervous system: Effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Becerra T. A., Wilhelm M., Olsen J., Cockburn M., Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ. Health Perspect. 2013;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Araujo J. A., Li H., Sioutas C., Kleinman M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J. Nanosci. Nanotechnol. 2009;9:5099–5104. doi: 10.1166/jnn.2009.gr07. [DOI] [PubMed] [Google Scholar]
- Castro J. B., Hovis K. R., Urban N. N. Recurrent dendrodendritic inhibition of accessory olfactory bulb mitral cells requires activation of group I metabotropic glutamate receptors. J. Neurosci. 2007;27:5664–5671. doi: 10.1523/JNEUROSCI.0613-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chautard T., Boudouresque F., Guillaume V., Oliver C. Effect of excitatory amino acid on the hypothalamo-pituitary-adrenal axis in the rat during the stress-hyporesponsive period. Neuroendocrinology. 1993;57:70–78. doi: 10.1159/000126344. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Wang E. A., Cepeda C., Levine M. S. Dopamine imbalance in Huntington's disease: A mechanism for the lack of behavioral flexibility. Front. Neurosci. 2013;7:114. doi: 10.3389/fnins.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J. E., Rossi C. A., Lawrence J., Long M. S., Diaz E. A., Lim R. H., McEwen B., Koutrakis P., Godleski J. J. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010;118:769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. J., Munchow A. H., Rios L. M., Zhang G., Asgeirsdottir H. N., Stackman R. W., Jr The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Brockel B. J., O’Mara D. J. Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicology. 2002;23:313–327. doi: 10.1016/s0161-813x(02)00059-1. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Merchant-Borna K., Allen J., Liu S., Weston D., Conrad K. Variations in the nature of behavioral experience can differentially alter the consequences of developmental exposures to lead, prenatal stress and the combination. 2012. doi:10.1093/toxsci/kfs260. [DOI] [PMC free article] [PubMed]
- Cory-Slechta D. A., O’Mara D. J., Brockel B. J. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J. Pharmacol. Exp. Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta D. A., Pazmino R., Bare C. The critical role of the nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Res. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Stern S., Weston D., Allen J. L., Liu S. Enhanced learning deficits in female rats following lifetime pb exposure combined with prenatal stress. Toxicol. Sci. 2010;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Virgolini M. B., Rossi-George A., Weston D., Thiruchelvam M. Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/− stress-related modifications to these trajectories. Behav. Brain Res. 2009;205:76–87. doi: 10.1016/j.bbr.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Virgolini M. B., Thiruchelvam M., Weston D. D., Bauter M. R. Maternal stress modulates the effects of developmental lead exposure. Environ. Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Weiss B., Cox C. Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol. Appl. Pharmacol. 1985;78:291–299. doi: 10.1016/0041-008x(85)90292-3. [DOI] [PubMed] [Google Scholar]
- Costa-Pinto F. A., Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17:196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- Cropley V., Wood S. J., Pantelis C. Brain structural, neurochemical and neuroinflammatory markers of psychosis onset and relapse: Is there evidence for a psychosis relapse signature? Int. Clin. Psychopharmacol. 2013 doi:10.1097/YIC.0b013e32835ab37c. [Google Scholar]
- Damgaard T., Plath N., Neill J. C., Hansen S. L. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology. 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- Darcheville J. C., Riviere V., Wearden J. H. Fixed-interval performance and self-control in children. J. Exp. Anal. Behav. 1992;57:187–199. doi: 10.1901/jeab.1992.57-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcheville J. C., Riviere V., Wearden J. H. Fixed-interval performance and self-control in infants. J. Exp. Anal. Behav. 1993;60:239–254. doi: 10.1901/jeab.1993.60-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino R. J., Staimer N., Tjoa T., Gillen D. L., Polidori A., Arhami M., Kleinman M. T., Vaziri N. D., Longhurst J., Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ. Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden S. F., Tan W. C., Suwa T., Mukae H., Terashima T., Fujii T., Qui D., Vincent R., Hogg J. C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am. J. Respir. Crit. Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Evans S. B., Cory-Slechta D. A. Prefrontal cortical manipulations alter the effects of intra-ventral striatal dopamine antagonists on fixed-interval performance in the rat. Behav. Brain Res. 2000;17:45–58. doi: 10.1016/s0166-4328(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Ferster C. B., Skinner B. F. Schedules of Reinforcement. Englewood Cliffs, NJ: Prentice-Hall; 1957. [Google Scholar]
- Frank-Cannon T. C., Alto L. T., McAlpine F. E., Tansey M. G. Does neuroinflammation fan the flame in neurodegenerative diseases. Mol. Neurodegen. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Hayashi S., Hogg J. C., Vincent R., Van Eeden S. F. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:265–271. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- Garris D. R. Aggression-associated changes in murine olfactory tubercle bioamines. Brain Res. 2003;963:150–155. doi: 10.1016/s0006-8993(02)03963-x. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland M. E., van Berlo D., Cassee F. R., Schins R. P., Wang K., Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A. J., Calhoon G. G., Shusterman I., Schoenbaum G., Roesch M. R., O’Donnell P. More is less: A disinhibited prefrontal cortex impairs cognitive flexibility. J. Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H., Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr. Opin. Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- Hellweg R., Zueger M., Fink K., Hortnagl H., Gass P. Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiol. Dis. 2007;25:1–7. doi: 10.1016/j.nbd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Hirsch E. C., Vyas S., Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Rel. Disord. 2012;18(Suppl. 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Hong Y. C., Lee J. T., Kim H., Kwon H. J. Air pollution: A new risk factor in ischemic stroke mortality. Stroke. 2002;33:2165–2169. doi: 10.1161/01.str.0000026865.52610.5b. [DOI] [PubMed] [Google Scholar]
- Ishii H., Fujii T., Hogg J. C., Hayashi S., Mukae H., Vincent R., van Eeden S. F. Contribution of IL-1 beta and TNF-alpha to the initiation of the peripheral lung response to atmospheric particulates (PM10) Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L176–L183. doi: 10.1152/ajplung.00290.2003. [DOI] [PubMed] [Google Scholar]
- Kehrer C., Maziashvili N., Dugladze T., Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front. Mol. Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher R. T., and Morse, W. H. Determinants of the behavioral effects of drugs. In: Tedeschi D.H. and Tedeschi, T. R.., editor. Importance of Fundamental Principles of Drug Evaluation. New York: Raven Press; 1968. pp. 383–405. [Google Scholar]
- van Kempen E., Fischer P., Janssen N., Houthuijs D., van Kamp I., Stansfeld S., Cassee F. Neurobehavioral effects of exposure to traffic-related air pollution and transportation noise in primary schoolchildren. Environ. Res. 2012;115:18–25. doi: 10.1016/j.envres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Kittelson D. B. Nanoparticle emissions on Minnesota highways. Atmos. Environ. 2004;38:9–19. [Google Scholar]
- Kleinman M. T., Araujo J. A., Nel A., Sioutas C., Campbell A., Cong P. Q., Li H., Bondy S. C. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol. Lett. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman J. P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Levesque S., Taetzsch T., Lull M. E., Kodavanti U., Stadler K., Wagner A., Johnson J. A., Duke L., Kodavanti P., Surace M. J., et al. Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ. Health Perspect. 2011;119:1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisabeth L. D., Escobar J. D., Dvonch J. T., Sanchez B. N., Majersik J. J., Brown D. L., Smith M. A., Morgenstern L. B. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann. Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhao R., Gao K., Wei Z., Yin M. Y., Lau L. T., Chui D., Hoi Yu A. C. Astrocytes: Implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- Lokken R. P., Wellenius G. A., Coull B. A., Burger M. R., Schlaug G., Suh H. H., Mittleman M. A. Air pollution and risk of stroke: Underestimation of effect due to misclassification of time of event onset. Epidemiology. 2009;20:137–142. doi: 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupinsky D., Moquin L., Gratton A. Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J. Neurosci. 2010;30:7624–7633. doi: 10.1523/JNEUROSCI.1187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari A. H., Ziaee V., Shayestehfar M., Ghanouni P., Mansournia M. A., Moshayedi P. Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Res. Dev. Disabil. 2013;34:3218–3225. doi: 10.1016/j.ridd.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Moore N. A., Rees G., Monn J. A. Effects of the group II metabotropic glutamate receptor agonist, LY354740 on schedule-controlled behaviour in rats. Behav. Pharmacol. 1999;10:319–325. doi: 10.1097/00008877-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Morgan J. T., Chana G., Pardo C. A., Achim C., Semendeferi K., Buckwalter J., Courchesne E., Everall I. P. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C. The rodent accessory olfactory system. J. Comp. Physiol. A. 2010;196:767–777. doi: 10.1007/s00359-010-0555-z. [DOI] [PubMed] [Google Scholar]
- Nemmar A., Dhanasekaran S., Yasin J., Ba-Omar H., Fahim M. A., Kazzam E. E., Ali B. H. Evaluation of the direct systemic and cardiopulmonary effects of diesel particles in spontaneously hypertensive rats. Toxicology. 2009;262:50–56. doi: 10.1016/j.tox.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Newbury A. J., Rosen G. D. Genetic, morphometric, and behavioral factors linked to the midsagittal area of the corpus callosum. Front. Genet. 2012;3:91. doi: 10.3389/fgene.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G. Toxicology of ultrafine particles: In vivo studies. Philos. Trans. R. Soc. Lond. A. 2000;358:2719–2740. [Google Scholar]
- Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Lunts A., Kreyling W., Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Pedersen C. B., Raaschou-Nielsen O., Hertel O., Mortensen P. B. Air pollution from traffic and schizophrenia risk. Schizophr. Res. 2004;66:83–85. doi: 10.1016/s0920-9964(03)00062-8. [DOI] [PubMed] [Google Scholar]
- Perry V. H. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Power M.C., Weisskopf M.G., Alexeeff S.E., Coull B.A., Spiro A. 3rd, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environmental Health Perspectives. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J. S., Kim H. W., Harry G. J., Rapoport S. I., Reese E. A. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr. Res. 2013;147:24–31. doi: 10.1016/j.schres.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Lange K. W., Kornhuber J., Danielczyk W. Glutamatergic-dopaminergic balance in the brain. Its importance in motor disorders and schizophrenia. Arzneimittelforschung. 1992;42:265–268. [PubMed] [Google Scholar]
- Rodriguez J. I., Kern J. K. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol. 2011;7:205–213. doi: 10.1017/S1740925X12000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A., Virgolini M. B., Weston D., Thiruchelvam M., Cory-Slechta D. A. Interactions of lifetime lead exposure and stress: Behavioral, neurochemical and HPA axis effects. Neurotoxicology. 2011;32:83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Meaney M. J. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Siddique S., Banerjee M., Ray M. R., Lahiri T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatr. 2011;170:923–929. doi: 10.1007/s00431-010-1379-0. [DOI] [PubMed] [Google Scholar]
- Silverman M. N., Sternberg E. M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S. F., Gryparis A., Wright R. O., Schwartz J., Wright R. J. Association of black carbon with cognition among children in a prospective birth cohort study. Am. J. Epidemiol. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- Thoma P., Wiebel B., Daum I. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr. Res. 2007;92:168–180. doi: 10.1016/j.schres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Vladisavljevic D., Mohottalage S., Kumarathasan P., Vincent R. Mapping acute systemic effects of inhaled particulate matter and ozone: Multiorgan gene expression and glucocorticoid activity. Toxicol. Sci. 2013;135:169–181. doi: 10.1093/toxsci/kft137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye S. J., Miller A. D., Blaha C. D. Ventral tegmental ionotropic glutamate receptor stimulation of nucleus accumbens tonic dopamine efflux blunts hindbrain-evoked phasic neurotransmission: Implications for dopamine dysregulation disorders. Neuroscience. 2013;252C:337–345. doi: 10.1016/j.neuroscience.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Virgolini M. B., Rossi-George A., Lisek R., Weston D. D., Thiruchelvam M., Cory-Slechta D. A. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H. E., Hertz-Picciotto I., Delwiche L., Lurmann F., McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H. E., Lurmann F., Penfold B., Hertz-Picciotto I., McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Dever D., Lowe J., Storey G. P., Bhansali A., Eck E. K., Nitulescu I., Weimer J., Bamford N. S. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J. Physiol. 2012;590(Pt 16):3743–3769. doi: 10.1113/jphysiol.2012.235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers J., Brotman M. A., Deveney C. M., Kim P., Zarate C., Jr, Fromm S., Pine D., Leibenluft E. A developmental study on the neural circuitry mediating response flexibility in bipolar disorder. Psychiatry Res. 2013;214:56–65. doi: 10.1016/j.pscychresns.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius G. A., Boyle L. D., Coull B. A., Milberg W. P., Gryparis A., Schwartz J., Mittleman M. A., Lipsitz L. A. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: Results from the MOBILIZE Boston Study. J. Am. Geriatr. Soc. 2012;60:2075–2080. doi: 10.1111/j.1532-5415.2012.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerdahl D., Fruin S., Sax T., Fine P. M., Sioutas C. Mobile platform measurments of ultrafine particles and associated pollutant concentrations on freeways and residential stresses in Los Angeles. Atomos. Environ. 2005;39:3597–3610. [Google Scholar]
- Weuve J., Puett R. C., Schwartz J., Yanosky J. D., Laden F., Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch. Intern. Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann H. E., Peters A. Epidemiological evidence of the effects of ultrafine particle exposure. Philos. Trans. R. Soc. Lond. A. 2010;358:2751–2769. [Google Scholar]
- Win-Shwe T. T., Fujimaki H., Fujitani Y., Hirano S. Novel object recognition ability in female mice following exposure to nanoparticle-rich diesel exhaust. Toxicol. Appl. Pharmacol. 2012;262:355–362. doi: 10.1016/j.taap.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Woo K. S., Chen D. R., Pui D. Y. H., McMurry P. H. Measurement of Atlanta aerosol size distribution: Observations of ultrafine particle events. Aerosol Sci. Technol. 2010;34:75–87. [Google Scholar]
- Yoshimura S., Sakamoto S., Kudo H., Sassa S., Kumai A., Okamoto R. Sex-differences in adrenocortical responsiveness during development in rats. Steroids. 2003;68:439–445. doi: 10.1016/s0039-128x(03)00045-x. [DOI] [PubMed] [Google Scholar]
- Zanchi A. C., Fagundes L. S., Barbosa F., Jr, Bernardi R., Rhoden C. R., Saldiva P. H., do Valle A. C. Pre and post-natal exposure to ambient level of air pollution impairs memory of rats: The role of oxidative stress. Inhal. Toxicol. 2010;22:910–918. doi: 10.3109/08958378.2010.494313. [DOI] [PubMed] [Google Scholar]
- Zhang Z. F., Yu S. Z., Zhou G. D. Indoor air pollution of coal fumes as a risk factor of stroke, Shanghai. Am. J. Public Health. 1988;78:975–977. doi: 10.2105/ajph.78.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]