Abstract
Objective
To test transethnic replication of a genetic risk score for obesity in white and black young adults using a national sample with longitudinal data.
Design and Methods
A prospective longitudinal study using the National Longitudinal Study of Adolescent Health Sibling Pairs (n = 1,303). Obesity phenotypes were measured from anthropometric assessments when study members were aged 18–26 and again when they were 24–32. Genetic risk scores were computed based on published genome-wide association study discoveries for obesity. Analyses tested genetic associations with body-mass index (BMI), waist-height ratio, obesity, and change in BMI over time.
Results
White and black young adults with higher genetic risk scores had higher BMI and waist-height ratio and were more likely to be obese compared to lower genetic risk age-peers. Sibling analyses revealed that the genetic risk score was predictive of BMI net of risk factors shared by siblings. In white young adults only, higher genetic risk predicted increased risk of becoming obese during the study period. In black young adults, genetic risk scores constructed using loci identified in European and African American samples had similar predictive power.
Conclusion
Cumulative information across the human genome can be used to characterize individual level risk for obesity. Measured genetic risk accounts for only a small amount of total variation in BMI among white and black young adults. Future research is needed to identify modifiable environmental exposures that amplify or mitigate genetic risk for elevated BMI.
Introduction
Genome-wide association studies (GWAS) have discovered genetic loci that associate with obesity risk [1]. Genetic risks manifest early in life; children at higher genetic risk gain weight more rapidly during infancy and early childhood and reach adiposity rebound earlier in life and at higher body-mass-index (BMI) [2]–[4]. In turn, this rapid growth early in life functions as a mediator of genetic risk for adult obesity [4]. These observations suggest the possibility that genetic information can inform research to understand pathogenesis of obesity in childhood, with the goal of improving prevention and treatment [5], [6].
Single-nucleotide polymorphisms (SNPs) discovered in GWAS of obesity phenotypes have small effects; the most penetrant SNP predicts an increase of less than one half of one BMI point in adult samples [7]. As a result, many samples designed to investigate obesity etiology are underpowered to study individual GWAS discoveries. Combining information on multiple GWAS discovered SNPs to compute a “genetic risk score” can provide a tool for investigating genetic contributions to obesity etiology in samples far smaller than are needed for GWAS [8], [9].
To date, most genetic risk score research on obesity has focused on European-descent samples [2]–[4], [10], [11]. Expanding the scope of genetic research to consider other populations is a public health priority [12]. A challenge is that GWAS-discovered SNPs may not cause disease themselves, but may instead serve as proxy measures of causal variants elsewhere in the DNA sequence. Allele frequencies and patterns of linkage disequilibrium vary across racial and ethnic groups [13]. One implication of these differences is that a SNP measured in a GWAS may serve as a proxy for a given causal variant in the GWAS discovery population, but not in a new sample drawn from a different ethnic group. This problem is compounded when GWAS discoveries are followed up in existing databases that may not contain the original GWAS-discovered SNP and proxies must be selected [14].
An increasing number of large and representative samples of adults from diverse populations are available with genome wide data from respondents. Genetic risk scores are a promising tool for population health research using such datasets but relatively small differences in allele frequency and LD patterns across groups may complicate the interpretation of genetic risk associations [15], [16]. A necessary first step is to test scores in different ethnic populations and establish whether a genetic risk score based on discoveries made in one population will translate to another.
This study tests transethnic replication of a genetic risk score for obesity in white and black young adults in the National Longitudinal Study of Adolescent Health (Add Health) Sibling Pairs Study. We tested genetic associations with obesity at two waves (when respondents were roughly 18–26 and 24–32 years old, respectively). We next tested genetic associations with change in obesity over the 7-year interval between waves. We conducted tests separately in the white and black samples and then tested for differences in genetic associations between the two groups. We also compared the performance of the GRSs computed from SNPs identified in samples of European ancestry to additional GRSs that incorporate SNPs discovered in a recent GWAS of African Americans.
Materials and Methods
Sample
Add Health is a nationally representative cohort (n = 20,745, aged 12–20 years at Wave 1 in 1994–95) drawn from a probability sample of 80 US high schools and 52 US middle schools, representative of US schools in 1994–95 with respect to region, urban setting, school size, school type and race or ethnic background. The Wave 3 (2001–2002) and 4 (2008–2009) data collection originally contained n = 15,197 individuals (then aged 18–26 years, mean age 22.3 years) and n = 15,701 individuals (then aged 24–32 years, mean age 28.9 years) respectively. The Add Health Sibling Pairs [17] data used here consists of 1,595 individuals (58% white, 42% black) from 965 families (564 sibling pairs, 30 sibling trios, 2 sibling quads, and 369 singletons) who were genotyped from samples collected during Wave 4 of the Add Health study (this phase of the Add Health study genotyped only the Sibling Pairs). The Sibling Pairs cohort oversampled black respondents (42.1% of Sibling Pairs as compared to 28.4% of all Add Health are black). The Sibling Pairs cohort did not differ from the full Add Health sample in terms of gender, age, maternal education, or health of the respondents (detailed results available upon request). The Siblings Pairs cohort was roughly 0.25 BMI units above the full Add Health sample at both Waves 3 and 4 but the waist to height ratios were identical in both groups. Missing data for phenotypic information reduced the number of respondents that could be used in the below analyses. The exact reduction in sample varied by phenotype, but the minimum white sample used in analysis was 773 respondents and the minimum black sample was 530 respondents (n = 1,303).
Genetic Risk Score
Genotyping was conducted with the Illumina HumanOmni1-Quad v1 platform using DNA extracted (via Oragene saliva collection) from 1,946 individuals at Wave 4. After quality controls (see http://ibs.colorado.edu/jb/pairsgwasqc.pdf), the genetic database included 1,886 individuals with valid data on 940,862 single nucleotide polymorphisms. Our analysis focused on non-Hispanic white and black individuals as indicated by self-report (n = 1,303) and SNPs with missing call rates below 5% (this criteria resulted in the removal of 18,665 SNPs from the original set of 959,862 SNPs). Principal components, which are commonly used to adjust for population stratification in GWAS [13], were computed with 231, 649 SNPs (selected from the full set of SNPs to be in linkage equilibria) from chromosomes 1–22.
We constructed three multi-locus indicators of genetic risk for obesity. The genetic risk score for European-descent populations (GRS-E) included 31 SNPs discovered in GWAS of adult BMI in European-descent individuals [7] (this risk score is available through the restricted use mechanism of Add Health). The genetic risk score for African-Americans (GRS-A) included 8 SNPs discovered in GWAS of adult BMI in African American and African individuals [18]. Genetic risk scores were created from sets of SNPs identified as genome-wide significant in their respective studies. We constructed a third genetic risk score (GRS-Omni) from the complete set of loci discovered in either GWAS. For loci in or near the genes FTO, SEC16B, and GNPDA2, the two GWAS identified loci in high linkage disequilibrium (r>0.9) and a single tag SNP was selected. The method used to compute the risk scores was the same for each set of SNPs. We summed the BMI-increasing alleles (as identified in each GWAS) for each SNP and then summed these counts of BMI-increasing alleles across the SNPs. Due to the lack of a comparable effect size metric between the Speliotes et al. [7] and Monda et al. [18], we use unweighted risk scores in most analyses although we also report weighted risk scores for GRS-E (using weights from [7]) to examine sensitivity to the weighting. For individuals with missing information on SNPs to be included in a risk score (8% of individuals were missing information on at least one SNP in GRS-Omni but no individual was missing information on more than four SNPs), we calculated pro-rated genetic risk scores by dividing the calculated genetic risk score by the number of SNPs with available calls and multiplying by the total number of SNPs in the score.
The SNPs included in the genetic risk scores are reported in Tables 1 and 2 . Since base rates of the risk alleles varied between the white and black samples, we standardized the weighted sums of risk alleles to have a mean of 0 and a SD of 1 separately in each of the white and black samples for each genetic risk score. (For analyses conducted using both white and black samples, results were consistent if risk scores are instead standardized across race). In the black sample, GRS-E and GRS-Omni were highly correlated (r = 0.91); GRS-A was less correlated with both (with GRS-E, r = 0.23; with GRS-Omni, r = 0.54). Effect-sizes reported from genetic risk score analyses reflect the effect of a one standard-deviation increase in genetic risk on obesity outcomes.
Table 1. Single-nucleotide polymorphisms included in the genetic risk score for Europeans (GRS-E).
| Chr | Nearest Gene | Speliotes et al. [7] SNP | Add Health SNP | R2 with GWAS SNP* | Risk Allele | Other | White MAF | Black MAF | Weight [7] |
| 1 | PTBP2 | rs1555543 | rs10489741 | 1 | a | g | 0.46 | 0.41 | 0.06 |
| TNNI3K | rs1514175 | rs1514175 | 1 | a | g | 0.43 | 0.35 | 0.07 | |
| NEGR1 | rs2815752 | rs2815752 | 1 | a | g | 0.37 | 0.46 | 0.13 | |
| SEC16B | rs543874 | rs543874 | 1 | g | a | 0.20 | 0.26 | 0.22 | |
| 2 | LRP1B | rs2890652 | rs1523702 | 0.702 | c | t | 0.13 | 0.06 | 0.09 |
| FANCL | rs887912 | rs2192497 | 1 | c | t | 0.26 | 0.09 | 0.10 | |
| TMEM18 | rs2867125 | rs2867125 | 1 | c | t | 0.16 | 0.11 | 0.31 | |
| RBJ | rs713586 | rs713587 | 0.967 | t | c | 0.47 | 0.16 | 0.14 | |
| 3 | ETV5 | rs9816226 | rs7635103 | 0.618 | a | c | 0.27 | 0.47 | 0.14 |
| CADM2 | rs13078807 | rs9852127 | 1 | a | g | 0.25 | 0.06 | 0.10 | |
| 4 | GNPDA2 | rs10938397 | rs10938397 | 1 | g | a | 0.43 | 0.20 | 0.18 |
| SLC39A8 | rs13107325 | rs13107325 | 1 | t | c | 0.13 | 0.02 | 0.19 | |
| 5 | FLJ35779 | rs2112347 | rs10057967 | 1 | t | c | 0.39 | 0.48 | 0.10 |
| ZNF608 | rs4836133 | rs4836133 | 1 | a | c | 0.48 | 0.22 | 0.07 | |
| 6 | NUDT3 | rs206936 | rs206936 | 1 | g | a | 0.21 | 0.48 | 0.06 |
| TFAP2B | rs987237 | rs987237 | 1 | g | a | 0.16 | 0.09 | 0.13 | |
| 9 | LRRN6C | rs10968576 | rs10968576 | 1 | g | a | 0.30 | 0.17 | 0.11 |
| 11 | RPL27A | rs4929949 | rs11041994 | 0.966 | c | a | 0.46 | 0.47 | 0.06 |
| BDNF | rs10767664 | rs7103411 | 1 | t | c | 0.15 | 0.07 | 0.19 | |
| MTCH2 | rs3817334 | rs7124681 | 1 | a | c | 0.39 | 0.26 | 0.06 | |
| 12 | FAIM2 | rs7138803 | rs7138803 | 1 | a | g | 0.36 | 0.16 | 0.12 |
| 13 | MTIF3 | rs4771122 | rs9512699 | 0.874 | g | a | 0.19 | 0.13 | 0.09 |
| 14 | NRXN3 | rs10150332 | rs17109256 | 1 | a | g | 0.19 | 0.23 | 0.13 |
| 15 | MAP2K5 | rs2241423 | rs2241423 | 1 | g | a | 0.27 | 0.38 | 0.13 |
| 16 | GPRC5B | rs12444979 | rs12444979 | 1 | c | t | 0.14 | 0.08 | 0.17 |
| FTO | rs1558902 | rs1421085 | 1 | c | t | 0.42 | 0.08 | 0.39 | |
| SH2B1 | rs7359397 | rs3888190 | 0.965 | a | c | 0.44 | 0.27 | 0.15 | |
| 18 | MC4R | rs571312 | rs571312 | 1 | a | c | 0.16 | 0.37 | 0.23 |
| 19 | QPCTL | rs2287019 | rs2287019 | 1 | c | t | 0.21 | 0.10 | 0.15 |
| KCTD15 | rs29941 | rs29942 | 1 | g | a | 0.29 | 0.15 | 0.06 | |
| TMEM160 | rs3810291 | rs3810291 | 1 | a | g | 0.35 | 0.18 | 0.09 |
Note: SNPs are the set of genome-wide significant SNPs discovered in the GWAS meta-analysis by the GIANT Consortium [7]. Weights are the effect-sizes estimated in that analysis. In cases where the original GWAS SNP was not available in the Add Health genotype database, linkage proxies were identified using the Broad Institute’s SNAP tool [35] (1000 Genomes (Pilot 1) CEU reference sample). No proxy was available for rs11847697 near PRKD1. Alleles are reported according to dbSNP. Frequencies of BMI-increasing alleles are reported separately for white and black Add Health study members meeting genotype quality control criteria.
Table 2. Single nucleotide polymorphisms (SNPs) included in the African American genetic risk score (GRS-A).
| Chr | Nearest Gene | Monda et al. [18] SNP | Add Health SNP | R2 with GWAS SNP* | Risk Allele | Other | White MAF | Black MAF |
| 1 | SEC16B | rs543874 | rs543874 | 1 | G | A | 0.2019 | 0.0571 |
| 2 | ADCY3 | rs7586879 | rs6752483 | 0.849 | T | C | 0.4903 | 0.042 |
| 4 | GNPDA2 | rs348495 | rs10938397 | 0.698 | G | A | 0.4307 | 0.048 |
| 5 | GALNT10 | rs7708584 | rs7719067 | 0.836 | A | G | 0.4471 | 0.05 |
| 6 | KLHL32 | rs974417 | rs974417 | 1 | G | T | 0.1154 | 0.04 |
| 7 | MIR148A-NFE2L3 | rs10261878 | rs1966841 | 0.961 | G | A | 0.399 | 0.03 |
| 16 | FTO | rs17817964 | rs3751812 | 0.707 | T | C | 0.399 | 0.074 |
| 18 | MC4R | rs6567160 | rs6567160 | 1 | C | T | 0.1635 | 0.062 |
Note: SNPs are the set of genome-wide significant SNPs discovered in the GWAS meta-analysis by Monda and colleagues [17]. In cases where the original GWAS SNP was not available in the Add Health genotype database, linkage proxies were identified using the Broad Institute’s SNAP tool [35] (1000 Genomes (Pilot 1) YRI reference sample). Frequencies of BMI-increasing alleles are reported separately for white and black Add Health study members meeting genotype quality control criteria.
Anthropometry
Anthropometric assessments of the Sibling Pairs were conducted at Add Health waves 3 and 4. Weight and height were measured during in-person interviews [19]. Participants were weighed without shoes on a digital bathroom scale (to the nearest half-pound in Wave 3 and tenth of a kilogram in Wave 4). The scales had a maximum of 330 pounds (200 kg); individuals above these thresholds were coded as being at the maximum scale weights (9 and 19 individuals were coded at this maximum weight for Waves 3 and 4 respectively). Heights were measured to the nearest 1/8th of an inch. BMI was computed as kilograms per height in meters squared. Obesity was defined as BMI≥30. Anthropometric characteristics of the white and black samples are described in Table 3 .
Table 3. Characteristics of white and black young adults in the Add Health Sibling Pairs sample.
| Whites (N = 918) | Blacks (N = 677) | p-value for difference | |||
| Mean | SD | Mean | SD | ||
| % Male | 0.48 | 0.50 | 0.46 | 0.50 | 0.44 |
| BMI-Wave 3 | 25.78 | 5.80 | 26.39 | 6.32 | 0.07 |
| BMI-Wave 4 | 27.86 | 6.60 | 29.34 | 7.44 | 0.00 |
| BMI Change | 2.10 | 3.93 | 2.69 | 3.99 | 0.01 |
| Waist/Height-Wave 4 | 0.57 | 0.10 | 0.58 | 0.11 | 0.15 |
| % Obese-Wave 3 | 0.22 | 0.42 | 0.26 | 0.44 | 0.13 |
| % Obese-Wave 4 | 0.33 | 0.47 | 0.40 | 0.49 | 0.01 |
Note: Data are for the Sibling Pairs of the National Longitudinal Study of Adolescent Health [17].
Statistical Analysis
We tested genetic associations with BMI and obesity using linear and logistic regression models, respectively. Analyses were conducted separately in whites and blacks. We analyzed change in BMI and obesity from Wave 3 to Wave 4 by including the level of Wave 3 BMI (or obesity) as a predictor in multivariate regression models predicting Wave 4 BMI (or obesity). All regressions were estimated using multilevel models (random intercept) to account for the non-independence of observations within families [20] and were adjusted for age and sex. All continuous outcomes were standardized within race. There was greater variability in the BMI of black respondents, see Table 3 . Effect sizes reflect the effect in SDs of a 1 SD increase in genetic risk score on BMI or BMI change or on the log odds of obesity or in log odds of change in obesity (reported as odds ratios).
We conducted two additional sets of analyses. First, because a previous study reported that the predictive performance of an obesity genetic risk score differed in black and white populations [4], we tested for differences in genetic associations with obesity phenotypes between blacks and whites. These analyses combined black and white respondents were into a single dataset. The models included a main effect term for race, a main effect term for genetic risk, and an interaction term testing race differences in the magnitude of the genetic effect.
Second, to rule out confounding by unmeasured population stratification [21], we conducted a sibling difference analysis using family fixed effects. The sibling difference analysis tested whether, within a pair of siblings who grew up in the same household, the sibling with the higher genetic risk score had the higher BMI. Sibling difference analyses provide a control for any unmeasured population stratification [22]. To maximize statistical power for these models, we analyzed all available data from waves 3 and 4 and introduced an individual-level random intercept to account for the non-independence of observations within individuals.
Results
White and black young adults at higher genetic risk as measured by GRS-E had higher BMIs compared to their lower genetic risk age-peers ( Table 4 ). For whites, genetic associations with BMI were 0.16 at Wave 3 and 0.17 at Wave 4 (p<0.001 for both; results with unweighted risk scores are reported unless indicated otherwise). For blacks, genetic associations with BMI were r = 0.14 at Wave 3 and r = 0.13 at Wave 4 (p<0.01 for both). Genetic associations with BMI did not differ between the white and black samples at either Wave (p>0.75 for both tests).
Table 4. Genetic associations with body-mass index and obesity in white and black young adults in the Add Health Sibling Pairs sample estimated using the genetic risk score for Europeans (GRS-E).
| Obesity Phenotype | White Sample | Black Sample | p-value for difference | |||
| Unweighted | B [95% CI] | |||||
| BMI-Wave 3 | 0.16*** | [0.09, 0.23] | 0.14** | [0.06, 0.23] | 0.96 | |
| BMI-Wave 4 | 0.17*** | [0.10, 0.24] | 0.13** | [0.04, 0.21] | 0.76 | |
| Change | 0.06* | [0.01, 0.10] | 0.01 | [−0.04, 0.05] | 0.23 | |
| OR [95% CI] | ||||||
| Obesity-Wave 3 | 1.42** | [1.14, 1.78] | 1.19 | [0.96, 1.48] | 0.38 | |
| Obesity-Wave 4 | 1.54*** | [1.30, 1.83] | 1.19 | [0.98, 1.45] | 0.06 | |
| Change | 1.43** | [1.14, 1.79] | 1.09 | [0.83, 1.45] | 0.12 | |
| Weighted | B [95% CI] | |||||
| BMI-Wave 3 | 0.16*** | [0.09, 0.23] | 0.16*** | [0.07, 0.24] | 0.83 | |
| BMI-Wave 4 | 0.18*** | [0.10, 0.25] | 0.14*** | [0.06, 0.22] | 0.85 | |
| Change | 0.06** | [0.02, 0.11] | 0.01 | [−0.03, 0.06] | 0.21 | |
| OR [95% CI] | ||||||
| Obesity-Wave 3 | 1.37** | [1.10, 1.71] | 1.25* | [1.01, 1.56] | 0.68 | |
| Obesity-Wave 4 | 1.56*** | [1.31, 1.85] | 1.22* | [1.00, 1.48] | 0.05 | |
| Change | 1.48*** | [1.18, 1.86] | 1.10 | [0.83, 1.46] | 0.07 | |
* p<.05; ** p<.01; *** p<.001.
Note: All data come from the National Longitudinal Study of Adolescent Health Sibling Pairs [17]. Genetic risk was measured using the genetic risk score for Europeans (GRS-E). Regressions were estimated using multi-level models [20] to account for the clustering of observations within families and adjusted for age and sex. Change models were estimated by including Wave 3 outcomes as covariates in regression models predicting Wave 4 outcomes.
White and black young adults at higher genetic risk as measured by GRS-E were also more likely to be obese compared to those at lower genetic risk. For whites, genetic associations with obesity were OR = 1.42 [1.14–1.78] at Wave 3 and OR = 1.54 [1.30–1.83] at Wave 4. For blacks, genetic associations with obesity were OR = 1.19 [0.96–1.48] at Wave 3 and OR = 1.19 [0.98–1.45] at Wave 4. Genetic associations with obesity were similar in blacks and whites at Wave 3 (p = 0.38). At Wave 4, the effect magnitude was larger among whites as compared to blacks (p = 0.06). Over the 7-year interval between waves 3 and 4, white young adults at higher genetic risk gained more weight and were more likely to become obese as compared to their lower genetic risk age-peers (for BMI, r = 0.06, p<0.05; for obesity status, OR = 1.43 [1.14–1.79]). Genetic risk was not associated with change in BMI and obesity among blacks. Insufficient statistical power is a possible, but unlikely, explanation for the failure to detect an association between the GRS and BMI change in the black Sibling Pairs; based on the effect observed in the white Sibling Pairs, we have 73% power in the black Sibling Pairs sample. However, the Add Health Sibling Pairs is slightly underpowered as a dataset to test black-white differences in genetic risk score associations with BMI change; power for these analyses was below 50%.
Weighting GRS SNPs by the effect-sizes estimated in GWAS had a modest effect on genetic risk score performance. Because weights customize the contribution of each SNP to the GRS according to its effect on BMI, the expectation is that a weighted GRS will provide superior prediction as compared to a GRS in which all SNPs are weighted equally. For most of the phenotypes analyzed, test-statistics and effect sizes were similar for weighted and un-weighted scores ( Table 4 ).
We also analyzed genetic associations with a more direct measure of adiposity: the ratio of waist-circumference to height [23]–[25]. Similar to results for BMI, white and black young adults at higher genetic risk as measured by GRS-E had higher waist-height ratios as compared to their lower genetic risk peers (for whites r = 0.16, for blacks r = 0.13; p<0.001 for whites and p<0.01 for blacks; as with BMI, the waist-height ratio was standardized in each racial group). Genetic associations did not differ between white and black samples (p = 0.86).
As a final test of the performance of GRS-E, we examined sibling differences in BMI using fixed effects regression techniques. Because previous race-stratified analyses yielded parallel results for whites and blacks with BMI, we pooled samples for the sibling differences analysis (for added stringency, we also included the first four principal components). Results were little changed from our original analyses. Across the Wave 3 and 4 assessments, the sibling with the higher genetic risk score had higher BMI (b = 0.62, p = 0.06; BMI was unstandardized in this analysis).
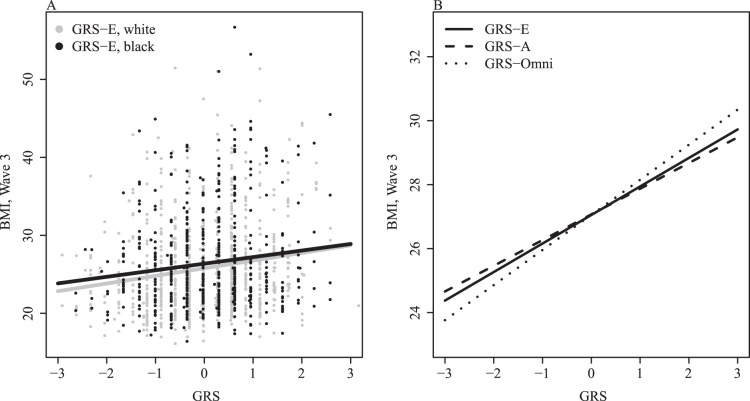
To test whether incorporating SNPs discovered in GWAS of African-descent individuals would improve GRS performance in African Americans, we compared the performance of GRS-E to that of GRS-A and GRS-Omni in the black Add Health Sibling Pairs (n = 667). Table 5 reports results for the analyses described above using the alternate genetic risk scores. For BMI, results were similar for all genetic risk scores (see also Figure 1 ). Inclusion of SNPs discovered in the Monda et al. [18] GWAS of BMI in African Americans and Africans modestly improved the performance of the genetic risk score (GRS-A and GRS-Omni scores performed better than GRS-E in the black Sibling Pairs).
Table 5. Genetic associations with body-mass index and obesity among black young adults in the Add Health Sibling Pairs Sample estimated using the genetic risk scores for Europeans (GRS-E), African Americans (GRS-A), and the composite genetic risk score (GRS-Omni).
| Obesity Phenotype | GRS-E | GRS-A | GRS-Omni | |||
| B [95% CI] | ||||||
| BMI-Wave 3 | 0.14** | [0.06, 0.23] | 0.12** | [0.04, 0.20] | 0.17*** | [0.09, 0.26] |
| BMI-Wave 4 | 0.13** | [0.04, 0.21] | 0.12** | [0.04, 0.20] | 0.15*** | [0.07, 0.24] |
| Change | 0.01 | [−0.04, 0.05] | 0.03 | [−0.02, 0.07] | 0.02 | [−0.03, 0.06] |
| OR [95% CI] | ||||||
| Obesity-Wave 3 | 1.19 | [0.96, 1.48] | 1.29* | [1.05, 1.59] | 1.27* | [1.03, 1.58] |
| Obesity-Wave 4 | 1.19 | [0.98, 1.45] | 1.26* | [1.04, 1.52] | 1.28* | [1.06, 1.55] |
| Change | 1.09 | [0.83, 1.45] | 1.19 | [0.90, 1.56] | 1.18 | [0.89, 1.55] |
* p<.05; ** p<.01; *** p<.001.
Note: All data come from the National Longitudinal Study of Adolescent Health Sibling Pairs [17]. Regressions were estimated using multi-level models [20] to account for the clustering of observations within families and adjusted for age and sex. Change models were estimated by including Wave 3 outcomes as covariates in regression models predicting Wave 4 outcomes.
Figure 1. Comparison of GRS predictions.
Panel A compares the predictive performance of GRS-E in both white and black samples of Add Health respondents based on a model where Wave 3 BMI is predicted by only GRS (separately in each racial group). Panel B focuses on predictions based on the three risk scores for only the black sample of respondents. The fitted lines are based on linear models controlling for age, sex, and one of the risk scores. The predictions assume an age of 21 and female.
Discussion
We used data from a prospective longitudinal study to examine the effects of cumulative genetic risk on body-mass phenotypes in white and black young adults. Consistent with findings from previous studies using samples of white adults [4], [14], [26], individuals at higher genetic risk had higher BMI, were more likely to be obese, and had higher levels of body fat (as measured by waist-height ratio) compared to their lower genetic risk peers. A novel finding of our study is that magnitudes of genetic associations, especially with BMI, were similar in white and black samples. Results for white and black samples differed in analyses of change over time. In the white sample, young adults at higher genetic risk gained more weight and were more likely to become obese as compared to those at lower genetic risk. In the black sample, these associations were in the same direction, but were smaller in magnitude and not statistically significant. We further showed that alternate genetic risk scores derived from GWAS of Europeans and from GWAS of African Americans and Africans performed similarly in predicting BMI and obesity in African American young adults. We also note that although the risk score created from the GWAS on African Americans utilized a small number of SNPs, the association is unlikely to be driven by a single SNP given the fact that the weights cited in the GWAS [18] are relatively consistent with the least predictive SNP being only half as predictive as the most predictive SNP. In contrast, the most predictive SNP in the Speliotes et al. [7] GWAS is ten times predictive as many of the other SNPs.
The magnitudes of genetic associations with obesity phenotypes were small; e.g. a one SD increase in GRS-E predicted a 0.14 SD increase in BMI at Wave 3 for those in the black sample. These translate to roughly a 0.9 point increase in BMI. Using the genetic risk score as an individual-level risk assessment would produce too many false positive and false negative results to recommend immediate clinical translation [27]. Nevertheless, the small effects we report are consistent with effect sizes for many other biomarkers routinely assessed in clinical settings [28]. Moreover, sibling comparison analyses showed that genetic associations with BMI were detectable within sibling pairs, indicating that genetic effects, although small, are apparent even in individuals who share those risk factors for obesity defined by the family environment. More research is needed to understand how GWAS-discovered genetic risks combine with other risk factors in order to understand complex traits [16].
Our findings have important implications for the use of genetic risk scores in obesity research. Our study provides evidence of transethnic replication of a genetic risk score for obesity based on GWAS discoveries in European-descent samples in a population-based black cohort. Some of the SNPs discovered in GWAS of obesity in European-descent samples have been replicated in black samples [18], [29], but transethnic replication a GWAS-based genetic risk score for obesity was uncertain. A recent analysis of data from the Atherosclerosis Risk in Communities (ARIC) study found that associations between the genetic risk score and obesity were weaker in blacks as compared to whites [14]. In that study, the genetic association with body-mass-index was r = 0.13 in whites, a little less than what we report from Add Health, but among blacks the magnitude of this association was reduced by two thirds. Research is needed to determine the cause of the discrepancy between results from the ARIC and Add Health cohorts. Three obvious differences in the cohorts are their age–ARIC participants are in their 50s and 60s whereas Add Health participants are in their 20s; the timing of assessments–the ARIC cohort was assessed in the late 1980s and 1990s whereas the Add Health cohort was assessed in the 2000s; and the geographic locations where individuals in the samples lived–black ARIC participants lived in North Carolina and Mississippi whereas Add Health participants were representatively drawn from across the United States. Thus, age, period, and cohort factors as well as factors related to place all represent candidate explanations [4], [26], [30].
Developmental processes, gene-environment correlations, and gene-environment interactions are promising targets for future inquiry into variation in the effects of obesity genetic risk scores [31]–[33]. Research in European-descent samples has identified rapid childhood growth, partly arising from decreased satiety response, as a mediator of genetic risk for obesity and points to sedentary lifestyle and poor diet as important moderators of genetic risk for obesity [10], [11], [14], [34]. These factors and others may differ between Add Health black respondents and the older African Americans examined in other studies, contributing to the small differences in genetic associations with obesity that we observe at Wave 4. Now that this study has provided evidence for transethnic replication of the genetic risk score in black young adults, future research can investigate the role of gene-environment interactions in determining genetic associations with obesity in blacks.
We acknowledge limitations. First, we provide evidence for transethic replication of genetic risk score associations with obesity phenotypes in white and black young adults, but results may not generalize to other ethnic groups. Additional studies focusing on other populations are needed. Second, the obesity phenotypes we examined were derived from anthropometric assessments that may capture body-size variation due to muscle mass as well as adiposity. We did replicate genetic associations with body-mass index using waist-circumference-to-height ratio, a superior measure of adiposity (that was available only for Wave 4). Finally, Add Health is a nationally representative sample, but the Sibling Pairs Study subsample that we analyzed is smaller and may not represent children who do not have siblings. As with all genetic research, replication of findings in additional samples is a priority.
Funding Statement
Add Health is funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Genotyping of the Sibling Pairs data was funded by the NIH/NICHD (R01HD060726). Additional research funds were provided by the NIH/NICHD funded CU Population Center (R24HD066613). DWB is supported in part by T32-AG000029. JDB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. El-Sayed Moustafa JS, Froguel P (2013) From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol 9: 402–413. [DOI] [PubMed] [Google Scholar]
- 2. Elks CE, Loos RJF, Sharp SJ, Langenberg C, Ring SM, et al. (2010) Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med 7/5: e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elks C, Loos R, Hardy R (2012) Adult obesity susceptibility variants are associated with greater childhood weight gain and a faster tempo of growth: the 1946 British Birth Cohort Study. Am J Clin Nutr 95/5: 1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, et al. (2012) Polygenic Risk, Rapid Childhood Growth, and the Development of Obesity: Evidence from a 4-Decade Longitudinal Study. Arch Pediatr Adolesc Med 166/6: 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramachandrappa S, Farooqi I (2011) Genetic approaches to understanding human obesity. J Clin Invest121/6: 2080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy MI (2010) Genomics, type 2 diabetes, and obesity. N Engl J Med 363/24: 2339–50. [DOI] [PubMed] [Google Scholar]
- 7. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet42/11: 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plomin R, Haworth CM, Davis OSP (2009) Common disorders are quantitative traits. Nat Rev Genet10/12: 872–8. [DOI] [PubMed] [Google Scholar]
- 9. Dudbridge F (2013) Power and predictive accuracy of polygenic risk scores. PLoS Genet 9/3: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li S, Zhao JH, Luan J, Ekelund U, Luben RN, et al. (2010) Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med 7/8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, et al. (2012) Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med 367/15: 1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khoury MJ, Gwinn M, Bowen MS, Dotson WD (2012) Beyond base pairs to bedside: a population perspective on how genomics can improve health. Am J Public Health 102/1: 34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Gen 38: 904–909. [DOI] [PubMed] [Google Scholar]
- 14. Belsky DW, Moffitt TE, Sugden K, Williams B, Houts R, et al. (2013) Development and evaluation of a genetic risk score for obesity. Biodemography Soc Biol 59/1: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xing J, Watkins WS, Witherspoon DJ, Zhang Y, Guthery SL, et al. (2009) Fine-scaled human genetic structure revealed by SNP microarrays. Genome Res 19: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belsky DW, Moffitt TE, Caspi A (2013). Genetics in Population Health Science: Strategies and Opportunities. Am J Public Health doi: 10.2105/AJPH.2012.301139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris KH, Halpern CT, Haberstick BC Smolen A (2013) The National Longitudinal Study of Adolescent Health (Add Health) Sibling Pairs Data. Twin Res Hum Genet 16: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, et al. (2013) A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Gen 45/6: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Entzel P, Whitsel EA, Richardson A, Tabor J, Hallquist S, et al. (2009). Add Health Wave IV Documentation Report: Cardiovascular and Anthropometric Measures. [WWW Document]. Available: http://www.cpc.unc.edu/projects/addhealth/data/guides/ Wave%20IV%20cardiovascular%20and%20anthropometric%20documentation%20110209.pdf.
- 20.Raudenbush SW, Bryk AS. (2002). Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Sage Publications: Thousand Oaks, CA, 2002. [Google Scholar]
- 21.Cardon LR, Palmer LJ (2003). Population stratification and spurious allelic association. The Lancet, 361(9357), 598–604. Chicago. [DOI] [PubMed]
- 22. Price AL, Zaitlen NA, Reich D, Patterson N (2010) New approaches to population stratification in genome-wide association studies. Nat Rev Gen 11: 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garnett SP, Baur LA, Cowell CT (2008) Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int J Obes 32/6: 1028–30. [DOI] [PubMed] [Google Scholar]
- 24. Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, et al. (2010) The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab 95(4): 1777–1785. [DOI] [PubMed] [Google Scholar]
- 25. Ashwell M, Gunn P, Gibson S (2012) Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes Rev 13(3): 275–286. [DOI] [PubMed] [Google Scholar]
- 26. Demerath EW, Choh AC, Johnson W, Curran JE, Lee M, et al. (2013) The Positive Association of Obesity Variants with Adulthood Adiposity Strengthens over an 80-Year Period: A Gene-by-Birth Year Interaction. Hum Hered 75: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loos RJF (2012) Genetic determinants of common obesity and their value in prediction. Best Pract Res Clin Endocrinol Metab 26/2: 211–26. [DOI] [PubMed] [Google Scholar]
- 28. Ioannidis J, Panagiotou O (2011) Comparison of Effect Sizes Associated With Biomarkers Reported in Highly Cited Individual Articles and in Subsequent Meta-analyses. JAMA. 305/21: 2200–10. [DOI] [PubMed] [Google Scholar]
- 29. Hester JM, Wing MR, Li J, Palmer ND, Xu J, et al. (2012) Implication of European-derived adiposity loci in African Americans. Int J Obes 36/3: 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boardman JD, Blalock CL, Pampel FC, Hatemi PK, Heath AC, et al. (2011) Population composition, public policy, and the genetics of smoking. Demography 48(4): 1517–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez JR, Klimentidis YC, Dulin-Keita A, Casazza K (2012) Genetic influences in childhood obesity: Recent progress and recommendations for experimental designs. Int J Obes 36/4: 479–84. [DOI] [PubMed] [Google Scholar]
- 32. Boardman JD, Daw J, Freese J (2013) Defining the Environment in Gene–Environment Research: Lessons From Social Epidemiology. Am J Public Health 103/S1: S64–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boardman JD, Domingue BW, Blalock CL, Haberstick BC, Harris KM, et al. (2013). Is the gene-environment interaction paradigm relevant to genome-wide studies? The case of education and body mass index. Demography, doi 10.1007/s13524-013-0259-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Llewellyn CH, Trzaskowski M, Hendrika C, van Jaarsveld M, Plomin R, et al. (2013). Satiety mechanisms in genetic risk of obesity. JAMA Pediatrics (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson AD, Handsaker RE, Pulit S, Nizzari MM, O’Donnell CJ, et al. (2008) SNAP: A web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24: 2938–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]