Abstract
Background
The purpose of the study was to assess the outcome of patients with advanced melanoma treated with matched molecularly targeted therapy.
Patients and methods
We reviewed 160 consecutive patients with metastatic melanoma treated in the phase I program (N = 35 protocols). Treatment was considered to be ‘matched’ (N = 84) if at least one drug in the regimen was known to inhibit the functional activity of at least one of the patient's mutations.
Results
Of 160 patients, 134 (83.7%) had adequate tissue for molecular analysis; 69% (110 of 160) had ≥1 mutation: 61.2% (82 of 134), BRAF; 20.7% (23 of 111), NRAS; 2.6% (2 of 77), KIT; 2.3% (1 of 44), KRAS; 20% (1 of 5), GNAQ; 11.1% (1 of 9), P53 and 2.6% (1 of 39), coexisting mutations in BRAF and PIK3CA. Eighty-four patients (52.4%) were treated with matched-targeted agents, most of whom had BRAF mutations (N = 74). Twenty-six percent of patients (41 of 160) achieved a complete or partial remission (CR/PR) [40% (34 of 84)) on a matched phase I protocol versus 9.2% (7 of 76) for those on a non-matched study (P ≤ 0.0001)]. The median progression-free survival (PFS) (95% CI) was longer for patients treated on a matched phase I trial than on their prior first standard treatment [5.27 (4.10, 6.44) versus 3.10 (1.92, 4.28) months, P = 0.023], but not on non-matched phase I treatment. Multivariable analysis showed that matched therapy was an independent predictor of higher CR/PR rates, prolonged PFS and survival.
Conclusions
For melanoma patients, especially those with BRAF mutations, administering molecularly matched agents can be associated with better outcomes, including longer PFS compared with their first-line systemic therapy.
Keywords: melanoma, targeted therapy, metastatic melanoma, matched therapy, phase I
introduction
Patients with advanced melanoma are treated with palliative surgery, immunotherapy and/or chemotherapy and sometimes radiation therapy [1–4]. Metastatic melanoma is rarely curable with standard therapeutic modalities. Current chemotherapy and cytokine-based immunotherapy [1–4] approaches benefit only a small percentage of patients with advanced disease. High-dose interleukin-2 (IL-2) [5, 6] has been reported to produce durable responses in only a small number of patients (<10%). Single-agent dacarbazine [7] has historically been the chemotherapy of choice for patients with advanced melanoma, with a response rate of 7%–15% and no overall survival (OS) benefit [7]. Other standard therapies according to National Comprehensive Cancer Network guidelines include temozolomide-based combination chemotherapy [5, 6], including cisplatin [5, 6] and vinblastine [2, 3] with or without IL-2/interferon alpha.
Newer agents have also been adopted. For instance, breakthroughs in understanding T-cell activation and anergy [8, 9] led to the development of ipilimumab, [9, 10] a CTLA4-blocking antibody. The drug improved survival and measurable responses in ∼10% of patients with OS benefits [9, 10].
The discovery of BRAF, NRAS and KIT mutations in melanoma [11–16] led to various rational therapeutic approaches. Promising treatment results [17–21] highlighted a new paradigm in melanoma treatment based on molecular analysis translated into personalized therapeutic approaches and increasing clinical benefit. For instance, the BRAF inhibitor vermurafenib [22, 23] is effective only in patients with a BRAF mutation and results in responses in ∼48% of such patients [22, 23] versus 5% for those treated with dacarbazine, the previous standard therapy. Vemurafenib [22, 23] is now approved in both the United States and Europe for the treatment of metastatic melanoma. Additionally, a plethora of other promising agents targeting the RAS/RAF/MEK [17–21] pathway have entered clinical trials, with early evidence of activity [17–21]. The primary goals of phase I trials [17–19] are to determine the maximum-tolerated dose of a drug or a combination of drugs, define safety profiles and observe early response signals. Thus far, the overall objective response rate for unselected patients treated on phase I trials [17–19] has ranged from 4% to 11% [20], which is likely to increase for selected patients with specific biomarkers fitted to trials with therapies aimed at those targets [21, 24]. This study analyzed patients with advanced melanoma for diverse aberrations, including BRAF, NRAS, KRAS, KIT, PIK3CA, P53 and GNAQ mutations. We hypothesized that melanoma patients whose therapy was matched to their oncogenic mutations would have improved progression-free survival (PFS) compared with treatment with their prior systemic therapies.
patients and methods
We retrospectively reviewed the clinical outcome of 160 consecutive patients with metastatic melanoma referred to the phase I clinic (Clinical Center for Targeted Therapy) at The University of Texas MD Anderson Cancer Center starting in June 2008, who had participated in treatment as per phase I protocols. Patient records were reviewed for medical history, laboratory results, mutation status and outcome of therapy. The Royal Marsden Hospital score (RMH score) [25, 26] and the MD Anderson prognostic score (MDACC score) [1] were used to evaluate the prognostic status of the patients. The RMH score [27, 28] classified patients according to three variables: lactate dehydrogenase (LDH) normal (0) versus LDH >upper limit of normal (ULN) (+1); albumin >3.5 g/dl (0) versus albumin <3.5 g/dl (+1) and number of metastatic sites of disease ≤2 (0) versus metastatic sites of disease ≥3 (+1).The MDACC score [1] includes, in addition to those in the RMH score [27, 28], two other variables: gastrointestinal tumor type (+1) versus non-gastrointestinal tumor type (0) and Eastern Cooperative Oncology Group performance status [29] (ECOG) ≥1 (+1) versus (0) for ECOG of 0. All patients provided written informed consent before enrollment on a clinical trial, and all trials as well as this analysis were approved by the MD Anderson Institutional Review Board.
We collected baseline characteristics that included age, gender, tumor histology, ECOG performance status [29], number of prior systemic therapies for metastatic disease, number of metastatic sites, location of metastatic disease, LDH level, disease staging, prior systemic therapies, PFS on first-line systemic therapy in the metastatic setting, best response to matched-targeted investigational therapy based on RECIST response criteria [30, 31] and date of death or date lost to follow-up. For patients who had been treated on more than one phase I clinical trial, we considered in our analysis only the phase I clinical trial on which the patient had the best response.
Patients were allocated to investigational treatments, which varied according to the protocol availability. Treatment on a phase I clinical trial was considered to be ‘matched’ to a patient if at least one drug in the regimen was known to inhibit the functional activity of at least one of the patient's mutations at nanomolar concentrations. For patients with GNAQ, RAS or BRAF mutations, treatment was considered matched if they were treated with MEK or RAF inhibitors [17, 19, 32]. Treatment with AKT, mTOR or PI3K inhibitors was considered matched therapy for patients with PIK3CA mutations. For patients with KIT mutations, treatment was considered matched if the patients were treated with KIT inhibitors.
molecular analysis
Patients who had adequate tissue available had analysis of molecular aberrations carried out in the Clinical Laboratory Improvement Amendments-certified Molecular Diagnostics Laboratory at MD Anderson using standard operating procedures and a polymerase chain reaction-based sequencing technology was used for all tests [33, 34]. DNA was extracted from microdissected paraffin-embedded tumor samples, and analysis was carried out on specific exons, depending on the tests ordered, for the following genes: BRAF (exon 15: codons 595–600); KRAS and NRAS (exon 2: codons 12, 13 and 61); PIK3CA (exon 9: codons 532–554; exon 20: codons 1011–1062); KIT (exons 9, 11, 13 and 17); and GNAQ (exon 5); TP53 (exons 4–9).
statistical evaluation
All statistical evaluations were carried out by our statisticians (SW and GG). The response was assessed approximately every two cycles (one cycle 3 to 4 weeks, depending on the protocol) by an MD Anderson radiologist and verified by a tumor measurement team within the Department of Investigational Cancer Therapeutics using RECIST [30, 31] guidelines. PFS was defined as the time from the start of best protocol treatment to the time of initial disease progression or death, whichever came first. For patients enrolled on more than one clinical trial, the patient's best phase I treatment was defined as the study on which the patient had the longest PFS. First-line treatment PFS was defined as the time from the start of the patient's first conventional systemic treatment in the metastatic setting to the time of initial disease progression on that treatment. For PFS, patients were censored at the time of their last follow-up if they were progression-free. Survival was measured from the date of treatment on the best phase I therapy (either matched or non-matched) until death from any cause or last follow-up. Patients were censored at the time of their last follow-up if they were alive.
Patients' characteristics were analyzed using descriptive statistics. Categorical data were described using frequencies and contingency tables, and continuously scaled measures were summarized with median and range. Waterfall plot analysis was used to graph individual patients' best response on protocol treatments (Figure 1). PFS and OS hazard functions were estimated using the Kaplan–Meier method (Figure 2), and the PFS or OS curves among groups were compared using a two-sided log-rank test. PFS on the best protocol treatment versus first-line treatment was assessed using a regression analysis modeling technique for repeat failure time observations. The multivariable Cox proportional hazards regression model was used to examine risk factors related to PFS and OS, after adjusting for other factors. A chi-square test was used to determine associations between individual risk factors and best response on phase I trials, and a multivariable logistic regression model was used to identify predictors of complete response (CR) or partial response (PR) as defined by RECIST criteria [30, 31]. Covariates included in the multivariable models (the Cox model and the multiple logistic regression model) were gender, age, race, number of prior therapies, MDACC score [1], and whether or not the patient was being treated with matched therapy. Statistical analyses were carried out using SPSS software, version 17.0. P values were reported for two-sided tests, and P < 0.05 was considered statistically significant.
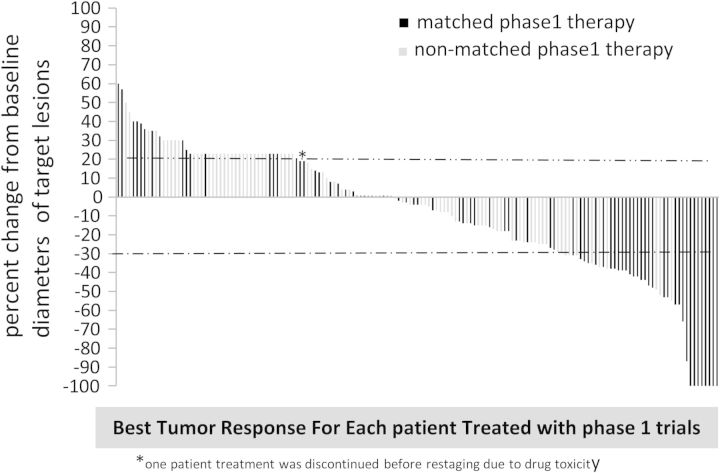
Figure 1.
Waterfall graph of all melanoma patients. Best response by RECIST. RECIST [40, 41] criteria were used to evaluate the response to treatment and state that a >20% increase in tumor measurement indicates progression. Patients who had progression due to new lesions were assigned a value of 21% to indicate progression as no absolute value can be calculated.
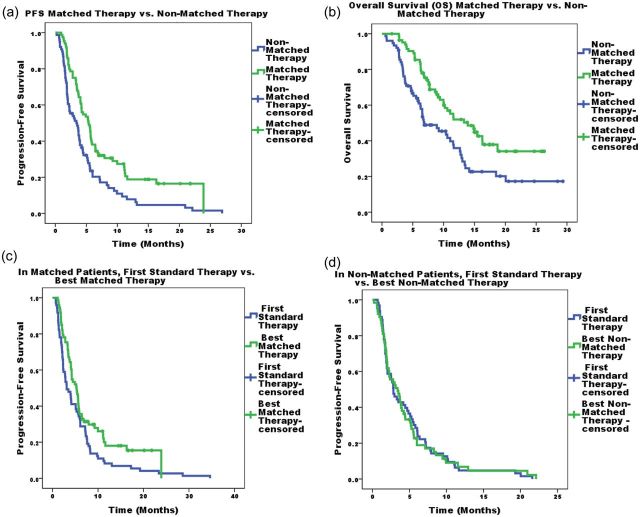
Figure 2.
(a) Kaplan–Meier log-rank estimates of PFS best phase I matched therapy versus best phase I non-matched therapy. Patients without progression at their last follow-up appointment were censored. (b) Kaplan–Meier log-rank estimates of overall survival (OS) of best phase I matched therapy versus best phase I non-matched therapy. Patients who were still alive at their last follow-up appointment were censored. (c) Kaplan–Meier log-rank estimates of PFS of best phase I matched therapy versus their first-line systemic therapy. Patients without progression at their last follow-up appointment were censored. (d) Kaplan–Meier log-rank estimates of PFS of best phase I non-matched therapy versus their first-line systemic therapy. Patients without progression at their last follow-up appointment were censored.
results
patient characteristics
One-hundred and sixty consecutive patients with metastatic melanoma who participated in a protocol were included in this analysis; 93 patients were men and their median age was 59 years (range 23–90 years); 130 (81.2%) had cutaneous melanoma, 18 (11.3%) had ocular melanoma and 12 (7.5%) had mucosal melanoma. The majority of the patients who presented to the phase I clinic had stage IV M1C disease (83%; N = 132) (Table 1).
Table 1.
Baseline characteristics of patients
| Characteristic | (N = 160) |
|---|---|
| Age, years | |
| Median | 59 |
| Range | 23–90 |
| Sex, n (%) | |
| Men | 93 (58%) |
| Women | 67 (42%) |
| Melanoma types | |
| Cutaneous | 130 (81.2%) |
| Ocular | 18 (11.3%) |
| Mucosal | 12 (7.5%) |
| Extent of metastatic disease, n (%) | |
| Stage IV M1a | 11 (7%) |
| Stage IV M1b | 14 (9%) |
| Stage IV M1c | 132 (83%) |
| Unresectable IIIc | 3 (1%) |
| Lactate dehydrogenase (LDH), n (%) | |
| ≤upper limit of normal (ULN) | 72 (45%) |
| >ULN | 88 (55%) |
| ECOG [42] performance status score, n (%) | |
| 0 | 33 (21%) |
| 1 | 125 (78%) |
| 2 | 2 (1%) |
| No. of prior therapies for metastatic disease before referral to phase I– | (n = 160) (%) |
| Median | 2 |
| Range | 0–4 |
| 0 | 24 (15%) |
| 1 | 54 (33.8%) |
| 2 | 44 (27.5%) |
| ≥3 | 38 (23.7%) |
| aRMH prognostic score [25, 26] | |
| Good (0–1) | 79 (49%) |
| Poor (2–3) | 81 (51%) |
| bMD Anderson prognostic score [1] | |
| 0 (low risk) | 9 (5.2%) |
| 1 (low-intermediate risk) | 33 (21%) |
| 2 (intermediate risk) | 46 (29%) |
| 3 (high-intermediate) | 69 (43%) |
| 4 (high risk) | 2 (1.2%) |
| 5 (high risk) | 1 (0.6%) |
analysis of molecular aberrations
Of 160 patients, 134 (83.7%) had adequate tissue for molecular analysis (Table 2).One hundred and ten patients (68.8%) had more than one molecular aberration and 24 (15%) had no molecular aberrations. Of the 134 patients with adequate tissue for molecular analysis, 134 were tested for BRAF mutation, 111 were tested for NRAS mutation, 77 for KIT mutation, 44 for KRAS mutation, 5 for PIK3CA mutation, 5 for GNAQ mutation and 9 for P53 mutation. Not all patients were tested for all mutations because of the limited tissue availability. Molecular analysis of patients who had adequate tissue for molecular analysis showed that 61.2% (82 of 134) had a BRAF mutation, 20.7% (23 of 111) had a NRAS mutation, 2.6% (2 of 77) had a KIT mutation, 2.3% (1 of 44) had a KRAS mutation, 11.1% (1 of 9) had a P53 mutation and 2.6% (1 of 39) had coexisting mutations in both BRAF and PIK3CA. Out of the 18 patients with uveal melanoma, 5 patients had adequate tissue for molecular analysis, 20% (1 of 5) had a GNAQ mutation.
Table 2.
Proportion of molecular aberrations and protocol therapy
| Mutation | No. of patients tested | No. of patients with aberrations |
|---|---|---|
| No. of patients had one or more molecular aberrations | 134 | 110 (68.8%) |
| BRAF | 134 | 82 (61.2%) |
| NRAS | 111 | 23 (20.7%) |
| KIT | 77 | 2 (2.6%) |
| GNAQ | 5 | 1 (20%) |
| P53 | 9 | 1 (11.1%) |
| KRAS | 44 | 1 (2.3%) |
| BRAF + PIK3CA | 39 | 1 (2.6%) |
| No mutation | 134 | 24 (15%) |
| Agents used in matched therapy clinical trials | No. of patients treated with matched phase I therapy, n = 84 | |
| BRAF inhibitor as a single agent | 44 (53.4%) | |
| MEK Inhibitor as a single agent | 19 (21.6%) | |
| BRAF and MEK inhibitors in combination | 14 (16.7%) | |
| MEK inhibitor combinationsa | 6 (7.1%) | |
| KIT inhibitor as a single agent | 1 (1.2%) | |
aMEK inhibitor in combination with EGFR inhibitor (2), AKT inhibitor (2), PI3K inhibitor (1) and decarbazine (1).
prior therapies before referral to phase I clinic
Of 160 patients, 136 (85%) received at least one systemic therapy before referral to the phase I clinic; 82 patients (51.3%) received two or more systemic therapies. The median number of prior systemic therapies was 2 (range 0–4).The most commonly received first-line therapies were immunotherapy-based regimens (49.2%; n = 64), cytotoxic-based chemotherapy regimens (28.7%; n = 40), biochemotherapy (15.8%; n = 22) and other biological agents (7.3%; n = 10).
protocol therapy
One-hundred and sixty patients were treated on 35 different protocols. Of 160 patients, 84 (52.5%) were treated on phase I clinical trials with matched therapies. The most common types of targeted drugs used were BRAF inhibitors as a single agent (53.4%; n = 44) and MEK inhibitors as a single agent (21.6%; n = 19). Targeted agents used in various clinical protocols are outlined in Table 2.
clinical and tumor response to protocol therapy
One hundred and sixty patients were assessable for response according to RECIST criteria [30, 31]. Of 160 patients, 41 (26%; 41 of 160) achieved a CR/PR on their best phase I protocol. Forty percent (34 of 84) versus 9.2% (7 of 76) of patients treated with matched versus non-matched therapy achieved a CR/PR (P ≤ 0.0001) from their best phase I matched protocol versus best phase I protocol for patients who were never treated with matched therapy.
tumor response to protocol therapy in different molecular subgroups
BRAF-mutant patients
Of the 134 patients who had adequate tissue for molecular analysis, 82 had a BRAF mutation and 74of 82 (90.2%) were treated on matched phase I clinical trials. Forty-four (59.4%) were treated with a BRAF inhibitor as a single agent, 14 (81.9%) were treated with a combination of BRAF inhibitor and MEK inhibitor, 13 (17.6%) were treated with an MEK inhibitor as a single agent, and 3 (4.1%) were treated with an MEK inhibitor in combination with other targeted agents. Of the 74 patients who had a BRAF mutation and were treated on a matched phase I trial, 34 (45.9%) achieved a CR/PR.
BRAF wild-type patients
Of the 134 patients who had adequate tissue for molecular analysis, 52 (38.8%) did not have a BRAF mutation. Of the 23 patients who had an NRAS mutation, 9 (39.1%) were treated on a matched phase I clinical trial with an MEK inhibitor. None achieved CR/PR.
Of the two patients who had a KIT mutation, only one was treated on a matched phase I clinical trial with a KIT inhibitor. This patient achieved 25% regression according to RECIST criteria with a PFS of 12 months.
None of the patients who had mutations of GNAQ (n = 1), PIK3CA (n = 1) or KRAS (n = 1) were treated on a matched phase I clinical trial.
progression-free survival versus first-line treatment
The overall median PFS following best phase I treatment was 4.05 months. PFS was longer for patients on matched (n = 84) versus non-matched (n = 76) therapy (5.33 versus 3.40 months, P = 0.001).
All 136 patients who had systemic therapies before being enrolled on phase I protocols had disease progression after their first-line treatment. For the 136 patients who had at least one systemic therapy before phase I therapy, 73 (53.6%) were treated on a matched phase I clinical trial versus 63 (46.4%) who were treated on a non-matched phase I clinical trial. Median PFS (95% CI) in months was longer for patients treated on a matched phase I trial than on the first standard treatment [5.27 (4.10, 6.44) versus 3.10 (1.92, 4.28) months, P = 0.023], but not on non-matched phase I treatment versus the first standard treatment (3.40 (1.92, 4.88) versus 2.83 (1.98, 3.68) months; P = 0.303).
univariate and multivariate analyses of factors predicting response, prolonged PFS and survival
PFS and response (CR/PR)
Univariate analyses (supplementary Table S1 and S2, available at Annals of Oncology online) showed that treatment with a matched phase I therapy (P ≤ 0.001) was positively associated with prolonged PFS, whereas ECOG [29] ≥1 (P ≤ 0.001), elevated LDH levels (P ≤ 0.0001), three or more metastatic sites (P = 0.001), the overall RMH score [27, 28] ≥2 (<0.001) and overall MDACC [1] score ≥3 (P ≤ 0.001) were inversely associated with PFS in patients treated on phase I clinical trials.
Univariate correlates of CR/PR, as defined by RECIST criteria [30, 31], were treatment with matched therapy (P ≤ 0.0001), normal LDH (P = 0.001), less than three metastatic sites (P = 0.001), RMH score [25, 26] <2 (P = 0.005), overall MDACC score [35] < 3 (P = 0.007), ECOG [29] < 1 (P = 0.013) and age <60 years (P = 0.047).
A multivariable Cox proportional hazards model (Table 3) showed that matched therapy (P ≤ 0.001) was an independent factor for response (CR/PR), whereas matched therapy (P = 0.004), overall MDACC score [35] <3 (P = 0.005) and female gender (P = 0.046) were independent predictors of prolonged PFS.
Table 3.
Multivariate analyses for progression-free survival (PFS), responseaCR/PR and overall survival (OS)
| Variable | PFSb |
Responsec |
OSb |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | OR (95% CI) | P value | HR (95% CI) | P value | |
| Matched therapy, yes (versus no) | 0.58 (0.40, 0.84) | 0.004 | 5.46 (2.14, 13.94) | <0.001 | 0.55 (0.36, 0.86) | 0.009 |
| MD Anderson scored ≥ 3 (versus <3) | 1.69 (1.18, 2.43) | 0.005 | 0.48 (0.20, 1.12) | 0.094 | 1.70 (1.10, 2.61) | 0.017 |
| Male gender (versus female gender) | 1.46 (1.00, 2.10) | 0.046 | 0.87 (0.39, 1.97) | 0.720 | 1.55 (1.02, 2.36) | 0.043 |
| Number of prior therapies ≥3 (versus <3) | 1.12 (0.74, 1.69) | 0.590 | 1.25 (0.49, 3.18) | 0.661 | 1.43 (0.90, 2.27) | 0.133 |
| Age ≥ 60 years (versus <60 years) | 0.75 (0.53, 1.08) | 0.124 | 0.74 (0.33, 1.68) | 0.484 | 0.78 (0.51, 1.19) | 0.247 |
| Caucasian race (versus non-Caucasian) | 1.25 (0.72, 2.18) | 0.428 | 0.92 (0.24, 3.51) | 0.920 | 1.36 (0.65, 2.84) | 0.417 |
aResponse defined as CR or PR by RECIST criteria [40, 41], HR = hazard ratio (<1 is associated with longer PFS and OS); OR = odds ratio (>1 is associated with response CR/PR).
bMultivariate Cox proportional hazards model was used to identify predictors of PFS and OS.
cA multivariate logistic regression model was used to identify predictors of response (defined as CR or PR by RECIST criteria [40, 41]) on best-matched or non-matched treatment.
dMD Anderson score[1] includes the RMH score [25, 26] (with component scores for LDH, albumin and two or more metastatic sites), and scores for GI tumor type and performance status; MD Anderson scores ≥ 3 are associated with poorer prognosis, and scores <3 with better prognosis.
CI = confidence intervals.
overall survival
Univariate analysis of survival (supplementary Table S3, available at Annals of Oncology online) from the start of best phase I therapy showed that treatment with a matched therapy (P = 0.002) was positively associated with longer survival, whereas ECOG [27, 28] ≥1 (P = 0.026), elevated LDH levels (P = 0.001), three or more metastatic sites (P = 0.008), overall RMH score [25, 26] ≥2 (P = 0.0002) and overall MDACC score [1] ≥3 (P = 0.001) were inversely associated with longer survival in patients treated on phase I clinical trials.
A multivariable Cox proportional hazards model (Table 3) showed that matched therapy (P = 0.009), overall MDACC score [1] <3 (P = 0.017) and female gender (P = 0.043) were independent predictors of longer survival.
discussion
Historically, the overall objective response rate for unselected patients treated on phase I trials ranges from 4% to 11% [24, 27–29, 35]. In this study, we demonstrated an association between better outcomes, including higher response rates and prolonged PFS and survival, and treatment with molecularly matched-targeted therapy. Indeed, 40% (34 of 84) versus 9.2% (7 of 76) of patients treated with a matched versus a non-matched therapy achieved a CR/PR (P ≤ 0.0001), and the median PFS was 5.33 versus 3.40 months (P = 0.001).
We also demonstrated that patients treated with matched therapy had a longer PFS compared with first standard systemic therapy in the metastatic setting [median (95% CI)] [5.27 (4.1–6.44) versus 3.10 (1.92–4.28) months, P = 0.023] but not on non-matched phase I treatment versus the first standard systemic treatment [3.40 (1.92, 4.88) versus 2.83 (1.98, 3.68) months; P = 0.303]. Most of the high response rates as well as the longer PFS were dependent on the BRAF-mutant population treated on BRAF, MEK or BRAF and MEK inhibitor combinations. Indeed, only 10 patients who were matched had aberrations other than a BRAF mutation.
Of the 23 patients who had an NRAS mutation, nine (39.1%) were treated on a phase I clinical trial with an MEK inhibitor; none achieved a CR/PR. The lower response rate to MEK inhibitors among the NRAS-mutant melanoma patients is consistent with previous reports [36, 37] that demonstrated that none of the seven patients with NRAS-mutant melanoma treated with the MEK inhibitor trametinib achieved a CR/PR [36, 37].
Somatic mutations in GNAQ have been found in ∼32% of primary uveal melanomas; however, in uveal melanoma metastases, it is 57% [38, 39]. We have identified GNAQ mutations in 20% of patients (1 of 5) tested; the relative lower rate of GNAQ mutations in our report could be attributed to the fact that the majority of our patients (87.7%) had extra-ocular melanoma in which GNAQ mutations are rare [39]. Alternatively, the small number of patients tested could account for the differences in percent positivity.
To date, most evidence suggests high initial rates of tumor responses when RAF/MEK pathway inhibitors are given to patients with cognate aberrations; few data are available to demonstrate the outcome of inhibiting the activity of other pathways such as PIK3CA or AKT. Several clinical trials are under way combining MEK inhibitors with PI3K or AKT inhibitors, which also test the potential benefit of blocking both signaling pathways.
Our study had several limitations. First, lack of randomization and the retrospective nature of the current study could lead to overestimation of the benefits of matching therapy, as we cannot rule out the possibility that the significantly higher response rate and PFS of patients with mutations treated with matched-targeted therapy compared with non-matched could have been due to differences in the proportion of patients on specific protocols or to unknown confounding factors in the two groups. Furthermore, it is possible that BRAF mutations themselves may act as a good prognostic factor [30]. However, these potential biases would not account for the finding from our paired analysis showing that only patients treated with molecular matching had a higher PFS on their matched-targeted treatment compared with their first-line systemic therapy. This contention is further supported by the multivariable Cox proportional hazards model, which showed that matched therapy was an independent predictor of CR/PR, prolonged PFS and longer survival. Various factors might also have attenuated the benefit of matched therapy in patients who achieved less than a CR/PR. For instance, because patients were enrolled in phase I trials and because the dose levels varied, as is standard on phase I trials, some individuals may have received lower than optimal drug doses and/or doses of suboptimal targeted agents. It should, however, be noted that responses have not necessarily been worse in phase I trials with agents given at lower doses [27]. Another limitation of the study was the high proportion of patients with BRAF mutations. Whether or not matching is associated with the same level of improved outcomes in other groups will require additional studies. Finally, these patients were heterogeneous in their molecular profiles and were also treated with several different BRAF, MEK and other targeted agents. Therefore, the relationship between any one mutation and/or agent and outcome cannot be clearly delineated. On the other hand, these observations suggest that the implications of matching may not be restricted to a single-targeted agent.
In conclusion, we demonstrated that targeting advanced melanoma with molecularly matched agents, especially individuals with BRAF-mutant disease given BRAF and/or MEK inhibitors, is associated with longer PFS compared with their first-line systemic therapy. Further, in multivariable analysis, molecular matching between a tumor's aberrations and the targeted therapy administered is an independent prognostic factor predicting response, PFS and survival. Because outcomes across a spectrum of agents were analyzed, this effect is likely related to matching patients based on a known target rather than matching them to individual agents. Further investigation of administering a matched-targeted therapy earlier in the course of melanoma may be warranted.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors acknowledge Joann Aaron, in the MD Anderson Department of Investigational Cancer Therapeutics for scientific editing and review of this manuscript.
references
- 1.Wheler J, Tsimberidou AM, Hong D, et al. Survival of 1181 patients in a phase I clinic: the MD Anderson clinical center for targeted therapy experience. Clin Cancer Res. 2012;18:2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MH, Johnson JR, Middleton MR. Temozolomide for advanced, metastatic melanoma. J Clin Oncol. 2000;18:2185. doi: 10.1200/JCO.2000.18.10.2185. [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 6.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 8.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lens M, Ferrucci PF, Testori A. Anti-CTLA4 monoclonal antibody Ipilimumab in the treatment of metastatic melanoma: recent findings. Recent Pat Anticancer Drug Discov. 2008;3:105–113. doi: 10.2174/157489208784638767. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 13.Wallander ML, Layfield LJ, Emerson LL, et al. KIT mutations in ocular melanoma: frequency and anatomic distribution. Mod Pathol. 2011;24:1031–1035. doi: 10.1038/modpathol.2011.57. [DOI] [PubMed] [Google Scholar]
- 14.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 15.Akslen LA, Puntervoll H, Bachmann IM, et al. Mutation analysis of the EGFR-NRAS-BRAF pathway in melanomas from black Africans and other subgroups of cutaneous melanoma. Melanoma Res. 2008;18:29–35. doi: 10.1097/CMR.0b013e3282f32517. [DOI] [PubMed] [Google Scholar]
- 16.Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–824. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smalley KS, Flaherty KT. Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br J Cancer. 2009;100:431–435. doi: 10.1038/sj.bjc.6604891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Euw E, Atefi M, Attar N, et al. Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol Cancer. 2012;11:22. doi: 10.1186/1476-4598-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 24.Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352:930–932. doi: 10.1056/NEJMe058007. [DOI] [PubMed] [Google Scholar]
- 25.Arkenau HT, Olmos D, Ang JE, et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrido-Laguna I, Janku F, Vaklavas C, et al. Validation of the Royal MarsdenHospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, Lee JJ, Hong D, et al. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res. 2010;16:1289–1297. doi: 10.1158/1078-0432.CCR-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 29.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 30.El-Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS One. 2011;6:e25806. doi: 10.1371/journal.pone.0025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosini G, Pratilas CA, Qin L-X, et al. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012;18:1–10. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo Z, Chen SS, Chandra PK, et al. Application of COLD-PCR for improved detection of KRAS mutations in clinical samples. Mod Pathol. 2009;22:1023–1031. doi: 10.1038/modpathol.2009.59. [DOI] [PubMed] [Google Scholar]
- 34.Margraf RL, Durtschi JD, Dames S, et al. Multi-sample pooling and illumina genome analyzer sequencing methods to determine gene sequence variation for database development. J Biomol Tech. 2010;21:126–140. [PMC free article] [PubMed] [Google Scholar]
- 35.Joffe S, Miller FG. Rethinking risk–benefit assessment for phase I cancer trials. J Clin Oncol. 2006;24:2987–2990. doi: 10.1200/JCO.2005.04.9296. [DOI] [PubMed] [Google Scholar]
- 36.Infante JR, Fecher LA, Nallapareddy S, et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 37.Falchook GS, Lewis KD, Infante R, Jr, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:5230–5234. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishino M, Jagannathan JP, Ramaiya NH, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 2010;195:W221–W228. doi: 10.2214/AJR.09.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishino M, Jackman DM, Hatabu H, et al. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 42.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.