Abstract
Background
This analysis was carried out to evaluate the long-term survival of patients with metastatic melanoma who received ipilimumab, a fully human monoclonal antibody that binds to cytotoxic T-lymphocyte antigen-4, in clinical trials.
Patients and methods
Patients received ipilimumab in one of three completed phase II clinical trials (CA184-008, CA184-022, and CA184-007). Previously treated patients were enrolled in all studies, and treatment-naïve patients were also included in study CA184-007. Patients received ipilimumab at a dose of 10 mg/kg in studies CA184-008 and CA184-007, and at doses of 0.3, 3, or 10 mg/kg in study CA184-022. Ipilimumab was given every 3 weeks for four doses, and eligible patients could receive ipilimumab maintenance therapy every 12 weeks. In study CA184-022, patients could cross over to be retreated with ipilimumab at 10 mg/kg upon disease progression. Ongoing survival follow-up is conducted in a companion study, CA184-025.
Results
Four-year survival rates [95% confidence interval (95% CI)] for previously treated patients who received ipilimumab at 0.3, 3, or 10 mg/kg were 13.8% [6.1–22.5], 18.2% [9.5–27.6], and 19.7% [13.4–26.5] to 28.4% [13.9–44.2], respectively. In treatment-naïve patients who received ipilimumab at 10 mg/kg, 4-year survival rates were 37.7% [18.6–57.4] to 49.5% [23.8–75.4].
Conclusions
These results demonstrate durable survival in a significant proportion of patients with metastatic melanoma who received ipilimumab therapy.
Keywords: cytotoxic T-lymphocyte antigen-4, immunotherapy, ipilimumab, long-term survival, metastatic melanoma, survival rate
introduction
Survival times for patients with metastatic melanoma have historically been very poor, with median overall survival (OS) in the range of 6–10 months with chemotherapy [1]. Survival outcomes for patients with advanced disease vary depending on the number of adverse prognostic factors that are present, such as visceral disease or brain metastases, and whether or not serum lactate dehydrogenase (LDH) levels are elevated [2–4]. In general, those with American Joint Committee on Cancer stage IV melanoma (distant metastases present) have 4-year survival rates of ∼15% from the time of initial diagnosis [4]. Two agents, ipilimumab and vemurafenib, have demonstrated an improvement in OS in randomized, controlled, phase III clinical trials involving patients with metastatic melanoma [5]. Ipilimumab can produce long-lasting responses [5], which may translate into a durable or long-term survival benefit for a proportion of patients with metastatic melanoma.
Ipilimumab is a fully human, IgG1 monoclonal antibody that binds to cytotoxic T-lymphocyte antigen-4 to augment antitumor T-cell responses [6, 7]. In two randomized, controlled, phase III trials of patients with metastatic melanoma, a statistically significant improvement in OS was demonstrated with ipilimumab monotherapy at 3 mg/kg (compared with a gp100 vaccine) in previously treated patients [8] and with ipilimumab at 10 mg/kg in combination with dacarbazine (compared with dacarbazine alone) in previously untreated patients [9]. In the first phase III trial, MDX010-20, ipilimumab monotherapy at 3 mg/kg produced 1- and 2-year survival rates of 45.6% and 23.5%, respectively [8]. In the second phase III trial, CA184-024, ipilimumab at 10 mg/kg in combination with dacarbazine produced 1-, 2-, 3-, and 4-year survival rates of 47.5%, 28.8%, 21.2%, and 19.0%, respectively [10]. Here, we conducted survival follow-up analyses (4 years from when the last patient enrolled) for patients who received ipilimumab as monotherapy in one of three completed phase II trials [11–13]. These analyses represent one the longest survival follow-up periods for patients with metastatic melanoma who received ipilimumab in a clinical trial. Safety data for these phase II trials have been previously reported [11–13].
patients and methods
clinical trials
Survival follow-up analyses were conducted on patients who had been randomized to, or treated with, ipilimumab in one of three completed phase II clinical trials [11–13]. In each trial, patients were eligible if they had a histologic diagnosis of unresectable and measurable stage III or stage IV melanoma (patients with mucosal or ocular melanoma were excluded). Upon closure of the trials in 2007, ongoing survival follow-up was conducted through a companion study, CA184-025 [14]. All participating patients (or their legal representatives) provided signed informed consent before enrollment in the parent trials or the companion study.
Table 1 provides an overview of the clinical trials in which patients included in the present survival analyses were originally enrolled. Study CA184-008 was an open-label, single-arm, multinational trial that evaluated the efficacy and safety of ipilimumab monotherapy at 10 mg/kg in a cohort (N = 155) of heavily pretreated patients who progressed on prior therapy [11]. The primary end point was best overall response rate using modified World Health Organization (mWHO) criteria, and secondary end points included median OS and 1-year survival rate. Study CA184-022 was a randomized, double-blind, multicenter, multinational trial which evaluated ipilimumab monotherapy at doses of 0.3, 3, or 10 mg/kg in patients (N = 217 randomized) who progressed on, or were intolerant of, prior therapy [12]. The primary end point was best overall response rate using mWHO criteria. Secondary end points included median OS and 1-year survival rate. Patients with brain metastases (active or stable) were excluded from studies CA184-008 and CA184-022, but there were no exclusion criteria for baseline LDH levels.
Table 1.
Summary of ipilimumab phase II trials included in the survival analyses
| Study | Na | Design | Population | Ipilimumab Treatment | End points |
|---|---|---|---|---|---|
| CA184-008 [10] | 155 | Single-arm, open-label, multicenter study | Heavily pretreated, progressed on prior therapy | 10 mg/kg every 3 weeks × 4 doses (induction); maintenance every 12 weeks from week 24 for eligible patients | Primary: best overall response rate Secondary: median OS; 1-year survival rate |
| CA184-022 [11] | 217 | Randomized, double-blind, parallel-group, multicenter, dose-ranging study | Progressed on or were intolerant of prior therapy | 0.3, 3, or 10 mg/kg every 3 weeks × 4 doses (induction); maintenance every 12 weeks from week 24 for eligible patientsb | Primary: best overall response rate Secondary: median OS; 1-year survival rate |
| CA184-007 [12] | 115 | Randomized, open-label (for ipilimumab), multicenter study | Treatment-naïve and previously treated | 10 mg/kg every 3 weeks × 4 doses (induction), plus oral budesonide or placebo | Primary: rate of grade ≥2 diarrhea Secondary: best overall response rate; median OS; 1-year survival rate |
aNumber of patients treated for study CA184-008 and number of patients randomized for studies CA184-007 and CA184-022.
bAfter disease progression, eligible patients in the 0.3 and 3 mg/kg dose groups could cross over to study CA184-025 to be reinduced with ipilimumab at 10 mg/kg. On-treatment patients without disease progression could receive ipilimumab maintenance therapy at their assigned dose.
Study CA184-007 was a randomized, double-blind, placebo-controlled, multicenter trial in which open-label ipilimumab was given at a dose of 10 mg/kg and patients (N = 115) were randomized 1 : 1 to receive concomitant oral budesonide or placebo [13]. Both patients who had received prior therapy for metastatic disease (previously treated) and those who had not (treatment-naïve) were enrolled. The primary objective of study CA184-007 was to determine whether prophylactic oral budesonide could reduce the rate of grade ≥2 diarrhea in ipilimumab-treated patients. Secondary end points included median OS and 1-year survival rate. Eligible patients included those with stable brain metastases for at least 1 month after prior therapy [15]. There were no exclusion criteria for baseline LDH levels.
treatment
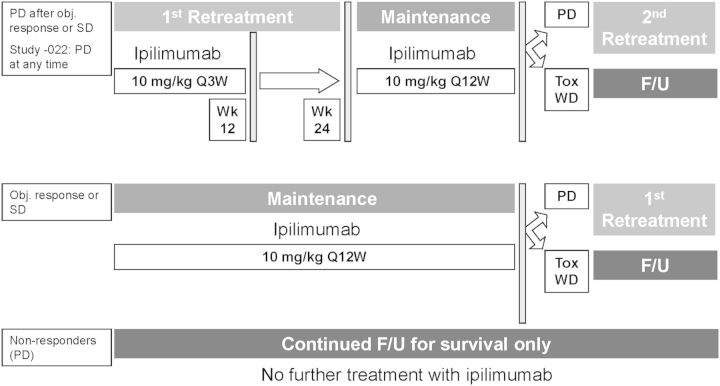
In each study, ipilimumab was given every 3 weeks for up to four doses. Tumor assessments by mWHO criteria were conducted beginning at week 12. Patients with an objective response or stable disease (SD) when the initial phase of each study closed, or patients with an objective response or SD who subsequently progressed, were eligible to receive maintenance therapy at their assigned dose (every 12 weeks) or retreatment with ipilimumab, respectively, in study CA184-025 [14]. In study CA184-022, patients who experienced progressive disease (PD) while receiving ipilimumab could enroll in study CA184-025 at any time and be retreated with ipilimumab at 10 mg/kg (24 of 73 patients who received ipilimumab at 0.3 mg/kg and 30 of 72 patients who received ipilimumab at 3 mg/kg). As per study protocols, patients with PD at week 12 could be followed beyond PD before the use of other anticancer therapies and were eligible (upon study closure) to enroll in study CA184-025 for follow-up only (Figure 1).
Figure 1.
Treatment of patients included in the survival analyses. Patients in studies CA184-007, CA184-008, and CA184-022 could enroll in study CA184-025 for (i) retreatment with ipilimumab [10 mg/kg, every 3 weeks (Q3W) for 4 doses] if they experienced PD after an objective response or SD; (ii) ipilimumab maintenance therapy (Q12W) if they had an objective response or SD but had not progressed; (iii) survival follow-up only without further ipilimumab treatment if PD was their best overall response. In study CA184-022, patients treated with ipilimumab at 0.3 or 3 mg/kg could enroll at any time to receive ipilimumab retreatment. In groups 1 and 2, patients with PD could be reinduced with ipilimumab or followed up for survival if they experienced intolerable toxicity (Tox) or withdrew consent (WD).
survival analyses
Survival data were collected in the parent clinical trials, or upon closure of these studies, in study CA184-025 [14]. In the parent trials, survival was assessed at the end of the observation period (24 weeks after the last patient was treated) and every 6 months thereafter [11–13]. Survival follow-up in study CA184-025 was conducted via routine assessments (per protocol) and/or by periodic data collection on all enrolled patients by the study sponsor (Bristol-Myers Squibb). Each patient was contacted to determine their survival status unless they had withdrawn consent or were lost to follow-up. In some cases, the individual sites contacted the patients by telephone and/or by certified letter to obtain the information. The sponsor (BMS) obtained information from the study sites as of, or beyond, a cutoff date for all surviving patients. The patients with survival information on or after the cutoff date were considered to have current follow-up for the present analysis.
OS was defined as the time from date of randomization in the parent study (or first dose of ipilimumab in study CA184-008) until the date of death, based on the most recent evidence obtained in both the parent trial and study CA184-025. For patients who had not died, OS was censored at the last date the patient was known to be alive. Their survival status and death, or last known alive date, reflected the latest date recorded in either the parent trial or study CA184-025. OS within each randomized treatment group, estimated using the Kaplan–Meier product-limit method, was plotted for each of the three studies. Median OS together with a two-sided 95% confidence interval (95% CI) for the median were calculated using the method of Brookmeyer and Crowley [16]. One-, 2-, 3-, and 4-year survival rates were calculated for each randomized treatment group using the Kaplan–Meier product-limit method. Corresponding two-sided 95% bootstrap CIs were calculated.
results
The evaluation of median OS and 1-year survival rates were secondary end points in each of the phase II trials, CA184-007, CA184-008, and CA184-022 [10–12] (Table 1). The studies were locked for the primary analyses in 2007, but were kept open into 2008 in order to collect survival information for those patients that were not eligible for the rollover study CA184-025. With survival data from both the parent trials and study CA184-025, additional analyses for 2-year survival rates were conducted and were included in the original reports of the studies [11–13]. Follow-up survival information was collected in study CA184-025 and is the source of data for the current analyses. We conducted survival analyses 4 years after the last patient enrolled in the parent trials, and most of the censored patients had survival follow-up of more than 4 years. At the last cutoff date of 9 March 2011, follow-up was considered current (i.e. it was known that the patient had died or the last known alive date was on or after the cutoff date) for 91% of the patients.
Survival analyses were conducted for all treated patients in study CA184-008 (N = 155), and for all randomized patients in studies CA184-022 (N = 217) and CA184-007 (N = 115). Survival data were current for 145 patients (93%) in study CA184-008, 202 patients (93%) in study CA184-022, and 97 patients (84%) in study CA184-007 (40 of 53 treatment-naïve patients, 57 of 62 previously treated patients). The percentages of patients lost to follow-up were 6% in study CA184-008, 7% in study CA184-022, and 8% in study CA184-007 for previously treated patients and 25% in study CA184-007 for treatment-naïve patients. Table 2 provides the median follow-up times for patients who survived >2 years, and who died or were censored at 4 years. Table 3 summarizes the previously reported 1- and 2-year survival rates for each of the trials [11–13], extended here to include data for treatment-naïve and previously treated patients in study CA184-007, along with 3- and 4-year survival rates. Kaplan–Meier survival curves for each of these studies are shown in Figure 2.
Table 2.
Median follow-up times for patients with OS >2 years in phase II clinical trials
| Study | Median follow-up, months (min–max)a |
|
|---|---|---|
| Patients censored | Patients who died | |
| CA184-008 (N = 45) | ||
| 10 mg/kg, previously treated | 51.4 (49.9–56.8), n = 26 | 27.9 (25.2–52.3), n = 19 |
| CA184-022 (N = 46) | ||
| 10 mg/kg, previously treated (n = 18) | 52.1 (47.7–54.5), n = 13 | 31.2 (24.3–41.5) n = 5 |
| 3 mg/kg, previously treated (n = 16)b | 50.9 (49.0–56.0), n = 12 | 30.6 (28.4–40.3), n = 4 |
| 0.3 mg/kg, previously treated (n = 12)b | 49.8 (49.2–58.7), n = 8 | 32.4 (26.6–49.1), n = 4 |
| CA184-007 (N = 35) | ||
| Ipilimumab–placebo (n = 18) | 50.9 (26.1–57.9), n = 14 | 32.5 (28.6–38.3) n = 4 |
| Ipilimumab–budesonide (n = 17) | 54.1 (49.5–61.4), n = 15 | 40.4 (35.7–45.0) n = 2 |
aPatients who died or were censored at 4 years (analyses include all treated patients in study CA184-008 and all randomized patients in studies CA184-022 and CA184-007).
bIn the 0.3 and 3 mg/kg dose groups, 33% and 42% of patients, respectively, crossed over to the 10 mg/kg dose group.
Table 3.
Overall survival with ipilimumab in phase II clinical trials
| Study | Median OS, months [95% CI]a | Overall survival rate, % [95% CI]a |
|||
|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | 4-year | ||
| CA184-008 (N = 155) | |||||
| 10 mg/kg, previously treated | 10.2 [7.6–16.3] | 47.2 [39.5–55.1] | 32.8 [25.4–40.5] | 23.3 [16.7–30.4] | 19.7 [13.4–26.5] |
| CA184-022 (N = 217) | |||||
| 10 mg/kg, previously treated (n = 72) | 11.4 [6.9–16.1] | 48.6 [36.8–60.4] | 29.8 [19.1–41.1] | 24.8 [14.8–35.7] | 21.5 [11.9–32.0] |
| 3 mg/kg, previously treated (n = 72)b | 8.7 [6.9–12.1] | 39.3 [28.0–50.9] | 24.2 [14.4–34.8] | 19.7 [10.7–29.4] | 18.2 [9.5–27.6] |
| 0.3 mg/kg, previously treated (n = 73)b | 8.6 [7.7–12.7] | 39.6 [28.2–51.2] | 18.4 [9.6–28.2] | 13.8 [6.1–22.5] | 13.8 [6.1–22.5] |
| CA184-007 (N = 115) | |||||
| Ipilimumab–placebo (n = 57) | 19.3 [12.0–34.5] | 62.4 [49.4–75.1] | 41.8 [28.3–55.5] | 34.4 [21.1–48.2] | 32.0 [18.9–45.7] |
| 10 mg/kg, treatment-naïve (n = 32) | 30.5 [14.0–NR] | 71.4 [55.2–87.2] | 56.6 [38.4–74.3] | 42.5 [23.0–62.0] | 37.7 [18.6–57.4] |
| 10 mg/kg, previously treated (n = 25) | 14.8 [6.6–20.5] | 50.8 [31.5–71.1] | 24.2 [8.0–42.8] | 24.2 [8.0–42.8] | 24.2 [8.0–42.8] |
| Ipilimumab–budesonide (n = 58) | 17.7 [6.8–45.0] | 55.9 [42.7–68.8] | 41.1 [27.7–54.8] | 38.7 [25.2–52.4] | 36.2 [22.9–49.9] |
| 10 mg/kg, treatment-naive (n = 21) | 45.0 [11.7–NR] | 65.9 [45.0–85.7] | 57.7 [33.3–81.0] | 57.7 [33.3–81.0] | 49.5 [23.8–75.4] |
| 10 mg/kg, previously treated (n = 37) | 8.5 [6.1–22.7] | 49.9 [33.3–66.6] | 31.6 [16.5–47.6] | 28.4 [13.9–44.2] | 28.4 [13.9–44.2] |
aAnalyses include all treated patients in study CA184-008 and all randomized patients in studies CA184-022 and CA184-007.
bIn the 0.3 and 3 mg/kg dose groups, 33% and 42% of patients, respectively, crossed over to the 10 mg/kg dose group.
NR, not reached.
Figure 2.
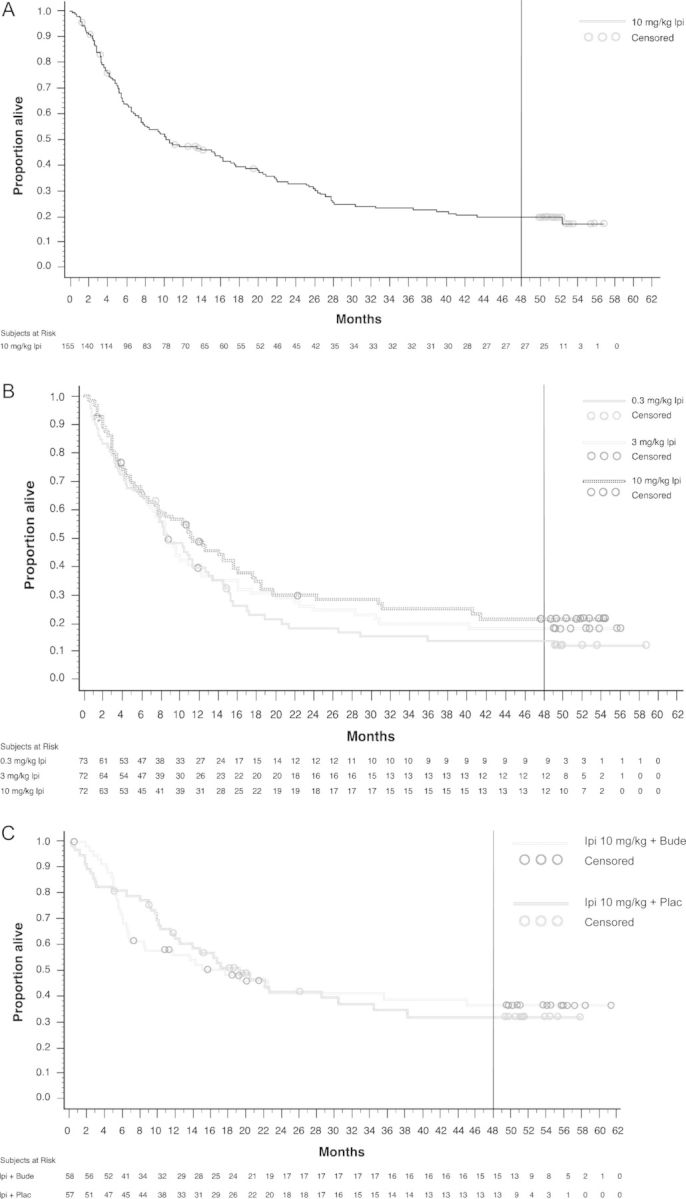
Kaplan–Meier estimates of overall survival. Analyses include all treated patients for study CA184-008 (A) and all randomized patients for studies CA184-022 (B) and CA184-007 (C). For study CA184-022 (B), 33% and 42% of patients in the 0.3 and 3 mg/kg dose groups, respectively, crossed over to the 10 mg/kg dose group. The vertical lines represent the current analyses of 4-year survival rates. Symbols indicate censored observations.
In study CA184-008, which included heavily pretreated patients who received ipilimumab at 10 mg/kg, median OS was 10.2 months [11] with a median follow-up time of 10.1 months (range: 0.23–56.8). The 4-year survival rate was 19.7%. The median OS in study CA184-022 was 11.4, 8.7, and 8.6 months for ipilimumab at 10, 3, and 0.3 mg/kg, respectively [12], with median survival follow-up times of 10.7 months (range: 0.43–54.5), 8.7 months (range: 0.39–56.0), and 8.3 months (range: 0.53–58.7). The corresponding 4-year survival rates were 21.5%, 18.2%, and 13.8%. Importantly, 33% and 42% of patients in the 0.3 and 3 mg/kg dose groups had crossed over to receive ipilimumab at 10 mg/kg in study CA184-025. In study CA184-007 with ipilimumab at 10 mg/kg, median OS was 17.7 and 19.3 months [13] with median follow-up times of 12.7 months (range: 0.56–61.4) and 16.3 months (range: 0.33–57.9) for the ipilimumab plus budesonide and ipilimumab plus placebo groups, respectively. With or without budesonide, respectively, 4-year survival rates were 49.5% and 37.7% for treatment-naïve patients and were 28.4% and 24.2% for previously treated patients. Across studies, most patients alive at 3 years survived to 4 years (84%–100% of the patients alive at 3 years were still alive at 4 years). The longest survival time for a patient was >61 months (treated with 10 mg/kg ipilimumab plus budesonide in study CA184-007).
Finally, we retrospectively evaluated best overall response in patients who survived >2 and in those who survived >4 years, in which the first tumor assessments were conducted in the parent studies at week 12 (after the induction dosing period). We evaluated responses in patients who survived >2 years given that the Kaplan–Meier survival curves began to plateau after this time. The analysis showed that response rates were similar at >2 years OS and >4 years OS. As shown in Table 4, long-term survivors included those with a best overall response of CR, PR, SD, or PD.
Table 4.
Best overall response (BOR) in patients with OS >4 years
| BOR by investigator assessment | >4 years OS n (%) |
||
|---|---|---|---|
| CA184-007 (n = 28) | CA184-008 (n = 27) | CA184-022 (n = 33) | |
| CR | 5 (17.9) | 2 (7.4) | – |
| PR | 11 (39.3) | 8 (29.6) | 9 (27.3) |
| SD | 7 (25) | 13 (48.1) | 9 (27.3) |
| PD | 4 (14.3) | 3 (11.1) | 15 (45.5) |
BOR, best overall response; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
discussion
The present analyses of data from phase II trials represent one the longest survival follow-up periods for patients with metastatic melanoma who received ipilimumab in a clinical study. Recently, long-term survival data were reported for 177 patients with metastatic melanoma who received ipilimumab in one of three clinical trials conducted at the US National Cancer Institute [17]. The patients received ipilimumab (up to 3 mg/kg) with either gp100 peptides (first study) or high-dose interleukin-2 (second study). The third, dose-escalation study evaluated ipilimumab (up to 9 mg/kg) as monotherapy in HLA-A*0201-negative patients (cohort 1) and as monotherapy or with gp100 peptides in HLA-A*0201-positive patients (cohort 2). The 5-year survival rates for the 3 studies were 13%, 25%, and 23%, respectively [17]. These survival outcomes are comparable to the results of the present analyses, where the 4-year survival rate was 18.2% for previously treated patients who received ipilimumab at 3 mg/kg, and ranged from 19.7% to 28.4% for previously treated patients who received ipilimumab at 10 mg/kg. Treatment-naïve patients, who received ipilimumab at 10 mg/kg, had 4-year survival rates of 37.7%–49.5%. Collectively, the results of these studies demonstrate that a significant proportion of patients with metastatic melanoma have long-term survival with ipilimumab therapy.
The 4-year survival rates with ipilimumab therapy in phase II studies are encouraging, given that ∼15% of patients with American Joint Committee on Cancer stage IV melanoma survive 4 years from diagnosis [4]. The data in the present analysis are mature and robust, with most of the patient censoring occurring after the 4-year survival follow-up period (Figure 2). Importantly, patients randomized to, or treated with, ipilimumab in the phase II studies in the present analysis included many with poor prognostic factors, such as elevated serum LDH levels at baseline [11–13]. Only study CA184-007 enrolled patients with brain metastases at baseline, but a recent analysis showed that 3 of these 12 patients survived to 4 years [15]. Limitations of the present analysis were that some patients were lost to follow-up (6–8% for previously treated patients and 25% for treatment-naïve patients). Other limitations were that not all study sites and patients participated in the follow-up companion study (CA184-025), and differential or informative loss to follow-up may have reduced the reliability of the results.
The current analyses extend those previously reported for study CA184-007 [13] by determining the long-term survival benefit for both treatment-naïve and previously treated patients. Based on the observation that no efficacy end point appeared to be affected by budesonide in study CA184-007, a retrospective analysis was carried out on data pooled for the patient subgroups within the placebo and budesonide groups [18]. In this analysis, the median OS for treatment-naïve patients was 30.5 months with 1- and 2-year survival rates of 69.4% and 56.9%, whereas the median OS was 13.6 months for previously treated patients with 1- and 2-year survival rates of 50.0% and 28.5% [18]. Although this analysis and the present data showed that the longest survival times occurred for treatment-naïve patients, further studies are needed to make definitive conclusions regarding outcomes by first versus subsequent lines of ipilimumab therapy. Moreover, given that 42% of patients crossed over from the 3 mg/kg dose group in study CA184-022 to be retreated with ipilimumab at 10 mg/kg in study CA184-025, definitive conclusions regarding survival differences between dose groups cannot be made.
The patients in the current analysis who survived long-term included those with an objective response, SD, or PD as their best overall response according to mWHO criteria. Long-term survivors included those who progressed in the parent studies and were retreated in study CA184-025, those who received maintenance therapy and were subsequently retreated upon disease progression in study CA184-025, and those who did not receive further ipilimumab treatment (followed for survival only). Given the complexity of patient treatment flow in study CA184-025, including patients who had benefit from induction therapy, the relationship between tumor response and long-term survival remains unclear.
In the phase III trial of ipilimumab monotherapy at 3 mg/kg, responses improved over time (beyond week 24) in some patients, including PD to SD [19]. All of these patients were among those that survived more than 2 years [19]. Thus, some patients in the present analysis may have experienced a delayed response to ipilimumab, which could have contributed to the survival outcomes. New patterns of response with ipilimumab have been consistently observed across clinical studies [7, 20, 21]. These include durable SD, which in some patients can be followed by a slow, steady reduction in tumor burden and achievement of an objective response [7, 22]. These results suggest that durable SD may be an important end point for the clinical activity of ipilimumab, possibly including long-term survival. However, OS remains the best end point to capture clinical outcomes for patients treated with ipilimumab.
In summary, the results of the present analysis suggest that a significant proportion of patients with metastatic melanoma survive long-term following ipilimumab monotherapy. This includes both patients who have progressed on prior systemic therapy for metastatic disease and those who have not received prior therapy for metastatic disease. The patients who experienced long-term survival may have included those with good and poor prognostic factors, and future analyses will focus on the characteristics of these patients. Long-term safety data has been reported from the phase III trial, CA184-024, in which eligible patients could receive maintenance therapy with ipilimumab at 10 mg/kg [23], and safety data in retreated patients from study CA184-025 will be the subject of a future report. While a proportion of patients who received ipilimumab at either 3 or 10 mg/kg had long-term survival, further studies are needed to definitively compare the survival results between these two doses. An ongoing phase III trial in patients with advanced melanoma will compare survival outcomes between the 3 and 10 mg/kg doses of ipilimumab [24].
funding
All study sites and institutions received funding from Bristol-Myers Squibb Co. (BMS) to cover expenses of the investigators for undertaking the parent trials.
disclosure
JDW is a paid consultant and receives research funding from BMS. JSW has received honoraria (<$10 000/year) from BMS, and BMS has supported clinical research at his institution (Moffitt Cancer Center). MM has participated in advisory boards for BMS, and BMS has supported preclinical research at his institution (University Hospital of Siena, Istituto Toscano Tumori). CL has participated in advisory boards for BMS. KC, LC, and VdP are currently employed by BMS. RH is a former employee of BMS. All remaining authors have declared no conflicts of interest.
acknowledgements
Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb Co.
references
- 1.Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. doi:10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:5275–5234. doi: 10.1200/JCO.2007.12.7837. doi:10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 3.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624–633. doi: 10.1080/07357900802027073. doi:10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald GE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. doi:10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spagnolo F, Queirolo P. Upcoming strategies for the treatment of metastatic melanoma. Arch Dermatol Res. 2012;304:177–184. doi: 10.1007/s00403-012-1223-7. doi:10.1007/s00403-012-1223-7. [DOI] [PubMed] [Google Scholar]
- 6.Fong L, Small EJ. Anti–cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. doi:10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 7.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. doi:10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. doi:10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. doi:10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Maio M, Bondarenko I, Robert C, et al. Four-year survival update for metastatic melanoma (MM) patients (pts) treated with ipilimumab (IPI) plus dacarbazine (DTIC) in phase III study CA184-024. Ann Oncol. 2012;23(9) abstract 1127P, ix367. [Google Scholar]
- 11.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with previously treated, advanced melanoma: a multicenter, single-arm, phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. doi:10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok J, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with previously treated, advanced melanoma: a randomized, double-blind, multicenter, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. doi:10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo- controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. doi:10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. A companion study for patients enrolled in prior/parent ipilimumab studies; http://clinicaltrials.gov/show/NCT00162123. ( 26 February 2013, date last accessed)
- 15.Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21:530–534. doi: 10.1097/CMR.0b013e32834d3d88. doi:10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]
- 16.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. doi:10.2307/2530286. [Google Scholar]
- 17.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. doi:10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson JA, Hamid O, Minor D, et al. Ipilimumab in treatment-naïve and previously treated patients with metastatic melanoma: retrospective analysis of efficacy and safety data from a phase II trial. J Immunother. 2012;35:73–77. doi: 10.1097/CJI.0b013e31823735d6. doi:10.1097/CJI.0b013e31823735d6. [DOI] [PubMed] [Google Scholar]
- 19.Ottensmeier C, Weber R, Haanen JB, et al. Ipilimumab produces durable objective responses in patients with previously treated, advanced melanoma: results from a phase III trial. Ann Oncol. 2010;21(suppl 8):viii401. Abstract 1326P. [Google Scholar]
- 20.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. doi:10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 21.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. doi:10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmankaya K, Erasim C, Koelblinger C, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol. 2011;28:1140–1144. doi: 10.1007/s12032-010-9606-0. doi:10.1007/s12032-010-9606-0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas L, Wolchok JD, Garbe C, et al. Safety of ipilimumab in patients (pts) with untreated, advanced melanoma alive beyond 2 years: results from a phase III study. J Clin Oncol. 2012;30(suppl 15) abstract 8512. [Google Scholar]
- 24.ClinicalTrials.gov. Phase 3 trial in subjects with metastatic melanoma comparing 3 mg/kg ipilimumab with 10 mg/kg ipilimumab; http://clinicaltrials.gov/ct2/show/NCT01515189. (11 January 2013, date last accessed) [Google Scholar]