Abstract
Alcoholic liver injury represents a progressive process with a range of consequences including hepatic steatosis, steatohepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma. Targeting key molecular regulators involved in the development of alcoholic liver injury may be of great value in the prevention of liver injury. Peroxisome proliferator-activated receptor α (PPARα) plays a pivotal role in modulation of hepatic lipid metabolism, oxidative stress, inflammatory response and fibrogenesis. As such, PPARα may be a potential therapeutic target for the treatment of alcoholic liver disease.
Keywords: Alcoholic liver disease, Oxidative stress, Inflammation, Fibrosis, Peroxisome proliferator-activated receptor α
Core tip: Alcoholic liver disease (ALD) is among the most common chronic liver disease. Modulation of therapeutic genes could potentially provide a novel and more effective treatment option. In this paper, we summarized the potential therapeutic role of PPARα modulation and illustrated the mechanism of PPARα in modulation of hepatic lipid metabolism, oxidative stress, inflammatory response and fibrogenesis in alcoholic liver disease. It is identified that PPARα agonists may serve as an effective therapeutic strategy for ALD.
INTRODUCTION
Long-term and heavy consumption of alcohol is a major risk factor for chronic liver disease. Alcoholic liver disease (ALD) constitutes the major share of alcohol-related morbidity and mortality[1]. More than 90% of individuals who consume excessive amount of alcohol may suffer from hepatic steatosis, 5%-15% of whom may develop fibrosis and cirrhosis, and a small portion of cirrhotic patients may develop hepatocellular carcinoma[2,3]. Accumulation of lipid products such as triglycerides in hepatocytes leads to lipid superoxidation and oxidative stress, further provoking hepatocellular apoptosis, hepatic inflammation and fibrogenesis. During these processes, inflammatory cytokines, mitochondrial perturbation, activation of hepatic stellate cells (HSCs), and activation of transforming growth factor beta (TGF-β) signaling have been reported to play important roles[4]. Targeting the key molecular regulators involved in the above processes may be promising in preventing ALD.
Peroxisome proliferator-activated receptor α (PPARα), a subtype of the PPAR superfamily, interacts with the retinoid X receptor to function as a transcription factor to induce the expression of a series of genes involved in fatty acid transport, mitochondrial fatty acid oxidation, catabolism, inflammatory response and fibrogenesis[5,6]. Many PPARα agonists (such as FA-derived compounds, carbaprostacyclin, nonsteroidal anti-inflammatory drugs, pirinixic acid, phthalate ester plasticizers, and hypolipidemic drug fibrates) have been evaluated for their therapeutic efficacy in animals or patients with ALD[7,8].
In this review, we focus on the protective role of PPARα in preventing hepatic injury induced by alcohol, and highlight the key signaling events mediating lipid metabolism, oxidative stress, hepatic inflammation and fibrosis in ALD (Figure 1).
Figure 1.
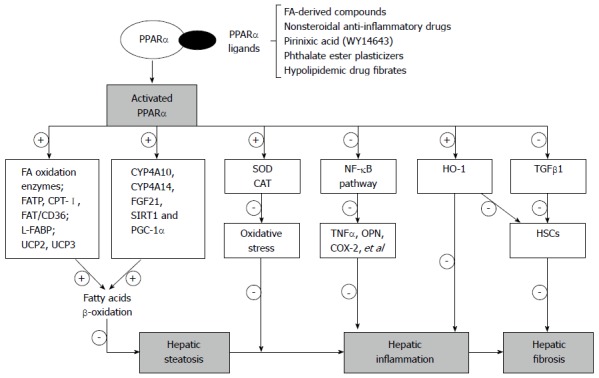
Protective role and partial mechanism of peroxisome proliferator-activated receptor α in alcoholic liver disease. Peroxisome proliferator-activated receptor α (PPARα) plays a critical role in modulation of hepatic lipid metabolism, oxidative stress, inflammatory response and fibrogenesis in alcoholic liver disease. Up-regulation of PPARα by its agonists (e.g., fibrates, WY14643) leads to increased expression of fatty acid oxidation and transport enzymes, alleviated oxidative stress by increasing the expression of genes involved in antioxidant enzymes (e.g., SOD and CAT), inhibited through activating nuclear factor kappa B (NF-κB) signal pathway and decreased fibrogenesis factors (e.g., TGF-β1 and HSC activation). SOD: Superoxide dismutase; CAT: Catalase; TGF-β1: Transforming growth factor beta 1; HSCs: Hepatic stellate cells; COX-2: Cyclooxygenase-2; HO-1: Heme oxygenase-1; TNFα: Tumor necrosis factor α; OPN: Osteopontin.
REGULATORY ROLE OF PPARα IN LIPID METABOLISM IN ALD
Accumulation of fat (mainly triglycerides, phospholipids, and cholesterol esters) in hepatocytes is the first step of alcohol-induced liver injury. Impaired lipid metabolism, mainly increased glycerolipid synthesis and decreased fatty acid oxidation in hepatocytes, is the key mechanism leading to alcoholic liver injury. Alcohol dehydrogenase (ADH) is a key enzyme involved in the conversion of ethanol to acetaldehyde[9], which is then changed to acetate by acetaldehyde dehydrogenase (ALDH), the key mitochondrial enzyme oxidizing aldehyde into acetic acid without producing toxic effect to mitochondria. This process is associated with the reduction of NAD to nicotinamide adenine dinucleotide health (NADH). Increased NADH/NAD ratio inhibits fat acid metabolism through reduced fatty acid β-oxidation[10]. The acetaldehyde not only directly inhibits PPARα activity but also impairs the DNA-binding ability of PPARα[11-13]. Retinoic X receptor α (RXRα) is a heterodimeric partner required for high-affinity DNA binding by many nuclear receptors. An RXRα binding site in the promoter sequence of ALDH has been identified[14]. PPARα can increase or maintain RXRα expression in the liver, resulting in enhanced ALDH expression. This process decreases the level of hepatic aldehyde and prevents the liver injury[15,16].
The imbalance between lipid synthesis and fatty acid oxidation induces excessive fat accumulation in the liver, which plays a pivotal role in the progression of ALD[11]. It has been shown that some of the key enzymes of fatty acid oxidation are regulated by PPARα, including long-chain acyl-CoA dehydrogenase (LCAD), medium-chain acyl-CoA dehydrogenase (MCAD), acyl-CoA oxidase, and very-long-chain acyl-CoA synthetase (VLACS)[17]. PPARα also regulates fatty acid transport protein, carnitine palmitoyltransferase, fatty acid translocase/CD36, liver cytosolic fatty acid-binding protein (LFABP) and uncoupling protein-2 and 3 (UCP2 and UCP3)[18]. Ethanol metabolite acetaldehyde has been shown to suppress PPARα activity, resulting in decreased transcription of PPARα target genes, a subsequent increase in the synthesis of triglycerides and fatty acids, and a decrease in cellular fatty acid uptake and oxidation[19].
There has been much experimental evidence supporting the role of PPARα in regulating fatty acid metabolism. PPARα ligands have been demonstrated to promote fatty acid oxidation and decrease fatty acid accumulation in experimental animals with ALD and such effects have been confirmed in ALD patients[20,21]. Hepatic mRNA expression of cytochrome P450 4A10 (CYP4A10) and CYP4A14, the characteristic PPARα target genes involved in fatty acid oxidation, could be restored by PPARα agonist WY14643[22]. In the experimental C57BL6/J mouse model of ALD (induced by Lieber-DeCarli liquid diet containing 4% ethanol), WY14643 administration could enhance the expression of hepatic fibroblast growth factor 21 and restore the hepatic expression of sirtuin 1 expression[23], which played a positive role in lipid metabolism, gluconeogenesis and fatty acid oxidation in the liver. Fischer et al[21] also found that WY14643 restored the ability of PPAR /RXR receptor complex to bind the specific response element, effectively upregulated the mRNAs expression of LCAD, acetyl-CoA carboxylase, MCAD, VLACS and LFABP, resulting in a higher rate of fatty acid oxidation, normalized serum free fatty acid and triacylglycerol levels, and prevented triacylglycerol accumulation in the liver of ethanol-fed mice.
PPARα SUPPRESSES ALCOHOL-INDUCED LIPID SUPEROXIDATION AND OXIDATIVE STRESS IN ALD
Oxidative stress is one of the key pathogenic factors involved in the development of necroinflammation and ultimately fibrosis and cirrhosis in ALD. Accumulation of fatty acids and generation of excessive amount of lipid peroxidation products can lead to oxidative stress, which in turn leads to mitochondrial dysfunction and enhanced hepatic expression of cytochrome P450 2E1 (CYP2E1)[24]. It is thought that induction of CYP2E1 by fatty acids is a major source of reactive oxygen species (ROS), which can strongly evoke oxidative stress and mitochondrial damage in hepatocytes. This can in turn affect fatty acid β-oxidation, cause hepatic steatosis, and produce necroinflammation[25]. The anti-oxidative effect of PPARα has been demonstrated by many published studies. PPAR-responsive elements (PPREs) have been identified in the promoter regions of several anti-oxidant genes such as catalase (CAT) and Cu2+/Zn2+-superoxide dismutase (SOD). PPARα can bind to PPREs to promote the expression of anti-oxidases, thereby inhibiting oxidative stress in the liver[26-29].
PPARα agonist WY14643 has been shown to prominently up-regulate the mRNA and protein expression of heme oxygenase-1 (HO-1, a stress-responsive protein that is induced by oxidants and plays an anti-oxidative role) in ethanol treated mice, and this was associated with alleviated oxidative stress, reduced lipid superoxidation and CYP2E1 expression[30]. On the other hand, lack of PPARα makes the liver more susceptible to alcohol induced injury. For example, compared with the wild-type mice, PPARα-null mice fed a Lieber-DeCarli diet containing 4% ethanol for 6 mo exhibited hepatomegaly, macrovesicular steatosis, hepatocyte apoptosis, mitochondrial swelling, hepatitis, and hepatic fibrosis[31]. Clearly, PPARα signaling forms a part of the body’s defense mechanism and plays an important role in anti-oxidative stress in the liver.
PPARα AMELIORATES ALCOHOLIC HEPATITIS BY MODULATING INFLAMMATORY FACTORS
Alcoholic steatohepatitis (AH) is an important phase of ALD, characterized by inflammatory cell infiltration and hepatocellular injury. AH develops in patients with steatosis and is usually associated with progressive fibrosis. Lipid peroxidation and oxidative stress are important contributing factors for AH[32]. Ethanol consumption can induce penetration of lipopolysaccharides (LPS) from the gut to the liver where they can act as important cofactors for the progression of ALD[33]. LPS interacts with toll-like receptor 4 on macrophages/Kupffer cells, leading to increased secretion of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), monocyte chemotactic protein 1, vascular cell adhesion molecule-1, and interleukin 6 (IL-6)[34]. In addition, LPS-induced activation of mitogen-activated protein kinases (MAPKs) also contributes to liver injury. Ethanol and its metabolites have also been shown to cause an up-regulation of pro-inflammatory factor osteopontin (OPN) and cyclooxygenase-2 (COX-2) in hepatocytes[35,36]. These factors further promote the synthesis of inflammatory mediators such as TNF-α and CXC chemokines (e.g., IL-8) through activating nuclear factor kappa B (NF-κB), signal transducers and activators of transcription-Janus kinase, and Jun N-terminal kinase pathways in hepatic resident cells. Infiltration of parenchymal neutrophils in the liver is a prominent feature of AH. Many chemokines (e.g., CXCL5, CXCL6 and CXCL4) and cytokines (e.g., TNF-α, IL-1, IL-6 and OPN) are markedly up-regulated in response to alcohol consumption, and they in turn promote infiltration of neutrophils during progression of AH[37].
Studies have shown that PPARα exerts an anti-inflammatory role in ALD by negatively interfering with pro-inflammatory signaling pathway NF-κB and inhibiting the expression of the related inflammatory cytokines[38]. Induction of PPARα by WY14643 attenuated liver inflammatory response by repressing expression of pro-inflammatory cytokines phosphatidylinositol 3-kinase (PI3K), OPN and COX-2, as well as enhancing expression of anti-inflammatory factors adiponectin and HO-1[8]. PPARα could regulate HO-1 transcription directly by binding to a PPRE in the promoter region of HO-1[39]. Thus, up-regulation of PPARα may contribute to the amelioration of ethanol-induced hepatic inflammatory response.
PPARα REVERSES ETHANOL-INDUCED HEPATIC FIBROSIS BY INHIBITING ACTIVATION OF HEPATIC STELLATE CELLS AND EXPRESSION OF PRO-FIBROTIC GENES
Alcoholic liver fibrosis is a severe form of ALD. HSCs, the major source of extracellular matrix (ECM) in hepatic fibrosis, are also stimulated by ethanol metabolites such as acetaldehyde. Activation of HSCs is observed during alcoholic liver injury, resulting in their proliferation and hepatic fibrogenesis[40]. The role of HSC activation in liver fibrosis has been supported by many in vitro and in vivo studies. HSCs, damaged hepatocytes, activated Kupffer cells and infiltrating polymorphonuclear cells can all release fibrogenic mediators such as TGF-β1, visfatin and PI3K, and thereby promote collagen accumulation in ECM[41,42]. Meanwhile, activated TGF-β1 stimulates quiescent HSCs transdifferentiation into myofibroblast-like cells, which forms a positive feedback loop between fibrogenic cells and HSCs[43].
We have investigated the potential role of PPARα in inhibition of ethanol-induced liver fibrosis using a rodent model[23]. Up-regulation of hepatic pro-fibrogenic genes osteopontin, TGF-β1, visfatin, PI3K, matrix metalloproteinase-2 (MMP-2) and MMP-9 was observed in the mice fed a 4% ethanol-containing Lieber-DeCarli diet. Administration of WY14643 could restore the expression of those cytokines altered by ethanol treatment and concomitantly ameliorated the liver injury.
Pro-inflammatory cytokine TNF-α also plays a crucial role in the development of alcoholic liver fibrosis. TNF-α activates Kupffer cells through autocrine and paracrine pathways, and the activated Kupffer cells produce TNF-α, ROS and other inflammatory factors. These processes form a feedback mediation of progression from inflammation to fibrosis in ALD[44]. It has been suggested that WY14643 could prevent the fibrosis progression by dramatically decreasing the expression of TNF-α[23].
Accumulating evidence suggests that the imbalance between pro- and anti-inflammatory cytokines is an important factor in the pathogenesis of fibrosis in ALD. Among the inflammatory cytokines, IL-10 is a potent factor in the regulation of the expression of pro-fibrotic markers such as collagen I, TGF-β1 and MMP-9[45-47]. IL-10 also promotes apoptosis of activated HSCs by modulating the expression of pro-apoptotic genes Fas, Bax and anti-apoptosis gene Bcl-2[48]. In addition, IL-10 was found to up-regulate HO-1 expression through the p38 MAPK pathway[49]. To further support the role of IL-10 and PPARα in the liver fibrosis, PPARα agonist has been shown to prevent the progression of liver fibrosis by modulation of p38 MAPK phosphorylation[50], and hepatic IL-10 and HO-1 expression was up-regulated by PPARα agonist WY14643 in ethanol-fed mice[8].
CONCLUSION
ALD is among the most common chronic liver disease. Modulation of therapeutic genes could potentially provide a novel and more effective treatment option. PPARα might be a crucial target gene in the suppression of hepatic lipid synthesis and oxidative stress, and amelioration of hepatic inflammatory response and fibrosis. PPARα agonists may serve as an effective therapeutic strategy for ALD.
Footnotes
P- Reviewers: Guillou H, Velkov T S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Warren KR, Murray MM. Alcoholic liver disease and pancreatitis: global health problems being addressed by the US National Institute on Alcohol Abuse and Alcoholism. J Gastroenterol Hepatol. 2013;28 Suppl 1:4–6. doi: 10.1111/jgh.12246. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14–32; quiz 33. doi: 10.1038/ajg.2009.593. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, Roerecke M. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 4.Sid B, Verrax J, Calderon PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic Res. 2013;47:894–904. doi: 10.3109/10715762.2013.819428. [DOI] [PubMed] [Google Scholar]
- 5.Bugge A, Mandrup S. Molecular Mechanisms and Genome-Wide Aspects of PPAR Subtype Specific Transactivation. PPAR Res. 2010;2010:pii: 169506. doi: 10.1155/2010/169506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardwick JP, Chiang JY. PPARs, RXRs, and Drug-Metabolizing Enzymes. PPAR Res. 2009;2009:589626. doi: 10.1155/2009/589626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon M. PPARα in Obesity: Sex Difference and Estrogen Involvement. PPAR Res. 2010;2010:pii: 584296. doi: 10.1155/2010/584296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong L, Ren W, Li W, Zhao S, Mi H, Wang R, Zhang Y, Wu W, Nan Y, Yu J. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011;10:246. doi: 10.1186/1476-511X-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haseba T, Ohno Y. A new view of alcohol metabolism and alcoholism--role of the high-Km Class III alcohol dehydrogenase (ADH3) Int J Environ Res Public Health. 2010;7:1076–1092. doi: 10.3390/ijerph7031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23 Suppl 1:S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasineni K, Casey CA. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol. 2012;44:299–303. doi: 10.4103/0253-7613.96297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mello T, Polvani S, Galli A. Peroxisome proliferator-activated receptor and retinoic x receptor in alcoholic liver disease. PPAR Res. 2009;2009:748174. doi: 10.1155/2009/748174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkata NG, Aung CS, Cabot PJ, Monteith GR, Roberts-Thomson SJ. PPARalpha and PPARbeta are differentially affected by ethanol and the ethanol metabolite acetaldehyde in the MCF-7 breast cancer cell line. Toxicol Sci. 2008;102:120–128. doi: 10.1093/toxsci/kfm281. [DOI] [PubMed] [Google Scholar]
- 14.Gyamfi MA, Kocsis MG, He L, Dai G, Mendy AJ, Wan YJ. The role of retinoid X receptor alpha in regulating alcohol metabolism. J Pharmacol Exp Ther. 2006;319:360–368. doi: 10.1124/jpet.106.108175. [DOI] [PubMed] [Google Scholar]
- 15.Alnouti Y, Klaassen CD. Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci. 2008;101:51–64. doi: 10.1093/toxsci/kfm280. [DOI] [PubMed] [Google Scholar]
- 16.Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic Biol Med. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–358. [PubMed] [Google Scholar]
- 18.Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 2012;56:281–290. doi: 10.1002/hep.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 20.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther. 2004;310:417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 21.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 22.Patsouris D, Reddy JK, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- 23.Nan YM, Kong LB, Ren WG, Wang RQ, Du JH, Li WC, Zhao SX, Zhang YG, Wu WJ, Di HL, et al. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol mediated liver fibrosis in mice. Lipids Health Dis. 2013;12:11. doi: 10.1186/1476-511X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsedensodnom O, Vacaru AM, Howarth DL, Yin C, Sadler KC. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis Model Mech. 2013;6:1213–1226. doi: 10.1242/dmm.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T, Yang Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J Cardiol. 2013;5:164–174. doi: 10.4330/wjc.v5.i6.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoo NK, Hebbar S, Zhao W, Moore SA, Domann FE, Robbins ME. Differential activation of catalase expression and activity by PPAR agonists: Implications for astrocyte protection in anti-glioma therapy. Redox Biol. 2013;1:70–79. doi: 10.1016/j.redox.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Jang SS, An Z, Song H, Kim WD, Yu JR, Park WY. Fenofibrate decreases radiation sensitivity via peroxisome proliferator-activated receptor α-mediated superoxide dismutase induction in HeLa cells. Radiat Oncol J. 2012;30:88–95. doi: 10.3857/roj.2012.30.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JL, Wang QY, Luan HY, Kang ZC, Wang CB. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J Biomed Sci. 2012;19:32. doi: 10.1186/1423-0127-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RQ, Nan YM, Wu WJ, Kong LB, Han F, Zhao SX, Kong L, Yu J. Induction of heme oxygenase-1 protects against nutritional fibrosing steatohepatitis in mice. Lipids Health Dis. 2011;10:31. doi: 10.1186/1476-511X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 32.Huang YW, Yang SS, Kao JH. Pathogenesis and management of alcoholic liver cirrhosis: a review. Hepat Med. 2011;3:1–11. doi: 10.2147/HMER.S10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng JH, Cui T, Sun ZL, Huang F, Chen L, Xu L, Feng Q, Hu YY. Effects of Puerariae Radix Extract on Endotoxin Receptors and TNF-α Expression Induced by Gut-Derived Endotoxin in Chronic Alcoholic Liver Injury. Evid Based Complement Alternat Med. 2012;2012:234987. doi: 10.1155/2012/234987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Banerjee A, Ueno Y, Ramaiah SK. Potential relationship between hepatobiliary osteopontin and peroxisome proliferator-activated receptor alpha expression following ethanol-associated hepatic injury in vivo and in vitro. Toxicol Sci. 2008;106:290–299. doi: 10.1093/toxsci/kfn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 38.Moriya T, Naito H, Ito Y, Nakajima T. “Hypothesis of seven balances”: molecular mechanisms behind alcoholic liver diseases and association with PPARalpha. J Occup Health. 2009;51:391–403. doi: 10.1539/joh.k9001. [DOI] [PubMed] [Google Scholar]
- 39.Krönke G, Kadl A, Ikonomu E, Blüml S, Fürnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 40.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 41.Cubero FJ, Nieto N. Ethanol and arachidonic acid synergize to activate Kupffer cells and modulate the fibrogenic response via tumor necrosis factor alpha, reduced glutathione, and transforming growth factor beta-dependent mechanisms. Hepatology. 2008;48:2027–2039. doi: 10.1002/hep.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moschen AR, Gerner R, Schroll A, Fritz T, Kaser A, Tilg H. A key role for Pre-B cell colony-enhancing factor in experimental hepatitis. Hepatology. 2011;54:675–686. doi: 10.1002/hep.24416. [DOI] [PubMed] [Google Scholar]
- 43.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tipoe GL, Liong EC, Casey CA, Donohue TM, Eagon PK, So H, Leung TM, Fogt F, Nanji AA. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin Exp Res. 2008;32:669–682. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 45.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–1429. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou JY, Jiang ZA, Zhao CY, Zhen Z, Wang W, Nanji AA. Long-term binge and escalating ethanol exposure causes necroinflammation and fibrosis in rat liver. Alcohol Clin Exp Res. 2013;37:213–222. doi: 10.1111/j.1530-0277.2012.01936.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LJ, Zheng WD, Chen YX, Huang YH, Chen ZX, Zhang SJ, Shi MN, Wang XZ. Antifibrotic effects of interleukin-10 on experimental hepatic fibrosis. Hepatogastroenterology. 2007;54:2092–2098. [PubMed] [Google Scholar]
- 48.Chen YX, Huang YH, Zheng WD, Chen ZX, Zhang LJ, Wang XZ. Interleukin-10 gene modification attenuates hepatocyte activation of rat hepatic stellate cells in vitro. Mol Med Rep. 2013;7:371–378. doi: 10.3892/mmr.2012.1228. [DOI] [PubMed] [Google Scholar]
- 49.Hsu HY, Chu LC, Hua KF, Chao LK. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J Cell Physiol. 2008;215:603–612. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 50.Huang YC, Liu KC, Chiou YL, Yang CH, Chen TH, Li TT, Liu LL. Fenofibrate suppresses melanogenesis in B16-F10 melanoma cells via activation of the p38 mitogen-activated protein kinase pathway. Chem Biol Interact. 2013;205:157–164. doi: 10.1016/j.cbi.2013.07.008. [DOI] [PubMed] [Google Scholar]


