Abstract
AIM: To investigate the effect of human leukocyte antigen (HLA) DRB1 and DQB1 alleles on the inactive and advanced stages of chronic hepatitis B.
METHODS: Patient records at a single institution’s hepatology clinic were reviewed. Demographic data, laboratory results, endoscopy results, virological parameters, biopsy scores and treatment statuses were recorded. In total, 355 patients were eligible for the study, of whom 226 (63.7%) were male. Overall, 82 (23.1%) were hepatitis B early antigen (HBeAg) positive, 87 (24.5%) had cirrhosis, and 66 (18.6%) had inactive disease. The presence of DQB1 and DRB1 alleles was determined by polymerase chain reaction with sequence-specific primers. The distribution of the genotyped alleles among patients with cirrhosis and patients with chronic active hepatitis was analyzed.
RESULTS: The most frequent HLA DQB1 allele was DQB1*03:01 (48.2%), and the most frequent HLA DRB1 allele was DRB1*13/14 (51.8%). DQB1*05:01 was more frequent in patients with active disease than in inactive patients (27% vs 9.1%; P = 0.002, Pc = 0.026). DRB1*07 was rare in patients with cirrhosis compared with non-cirrhotics (3.4% vs 16%; P = 0.002, Pc = 0.022). Older age (P < 0.001) and male gender (P = 0.008) were the other factors that affected the presence of cirrhosis. In a multivariate logistic regression analysis, DRB1*07 remained a significant negative predictor of cirrhosis (P = 0.015). A bioinformatics analysis revealed that a polymorphic amino acid sequence in DRB1*07 may alter interaction with the T-cell recognition site.
CONCLUSION: This study demonstrates that HLA alleles may influence cirrhosis development and disease activity in Turkish chronic hepatitis B patients.
Keywords: Chronic active hepatitis, Cirrhosis, Hepatitis B, Human leukocyte antigen DQ, Human leukocyte antigen DR
Core tip: Chronic hepatitis B is a major health problem worldwide. Recent genome-wide association studies revealed a significant association between the human leukocyte antigen (HLA) class II region and chronic hepatitis B. In the present study, we genotyped HLA DQB1 and DRB1 alleles in 355 chronic hepatitis B patients and investigated the effects of the HLA alleles on disease activity and cirrhosis development. We found that DQB1*05:01 was a risk factor for chronic active hepatitis and that DRB1*07 was a protective factor against cirrhosis. A bioinformatics analysis revealed that DRB1*07 might be associated with a hypoimmune response.
INTRODUCTION
Hepatitis B virus (HBV) is observed all around the world, and nearly 350 million people have chronic HBV infections[1]. The estimated number of annual deaths due to the consequences of HBV infection is nearly 600000[2]. The virus itself is non-cytopathic, and liver damage during chronic infection is due to the host immune reaction against the virus. Several host immune mechanisms have been proposed to be involved in viral persistence, beyond viral factors[3]. Human leukocyte antigen (HLA)-restricted T lymphocytes, B lymphocytes in the humoral immunity system, dendritic cells, natural killer cells and numerous cytokines are required to generate an accurate immunologic response against the virus[4,5]. An inaccurate, nonselective cytolytic immune reaction to hepatocytes is believed to cause necroinflammation and further liver fibrosis, rather than eradicating the virus[3].
In the majority of chronic hepatitis B patients, an inactive state, with low DNA levels (HBV DNA levels less than 2000 IU/mL) and normal liver enzyme levels, is observed. Nonetheless, nearly 30% of these inactive carriers (hepatitis B early antigen (HBeAg) negative, normal serum liver enzyme level and low serum HBV DNA level) will develop active disease, and nearly 10% will develop cirrhosis[6,7]. To date, there is no defined test to predict which patient will remain in the inactive state without treatment and which patient will progress to chronic active hepatitis, which may culminate in cirrhosis unless specific antiviral treatment is introduced. It should be noted that when cirrhosis develops, the expected five-year survival decreases to 50% in untreated hepatitis B patients[8]. Additionally, for cirrhotics, the annual risk of developing hepatocellular carcinoma is 5 times higher than for non-cirrhotics[9]. Furthermore, in general, cirrhosis is the most common non-neoplastic cause of death related to the digestive system in the United States[10].
Studies investigating the effect of HLA polymorphism on the disease state of different populations with HBV infection have been performed. Studies in the Chinese population showed that HLA DRB1*03:01, DQB1*03:01, DQB1*03:03, DQB1*05:03, DRB1*06, DRB1*08 and DRB1*16 are associated with viral persistence[11-13]. In Taiwanese patients, DRB1*12:02 was shown to be related to viral persistence, whereas the DRB1*04:01 and DRB1*07:01 alleles were related to viral clearance[14]. In a study from the United States, the DQB1*03:01 allele was related to viral persistence in Afro-Americans[15]. A study from Turkey revealed that HLA DR7, DR13 and DQ3 alleles were related to susceptibility to chronic infection[16].
Recent genome-wide association studies revealed an association between the HLA class II gene region (DR, DQ and DP) and HBV chronicity[17-19]. Moreover, a recent study revealed the effect of DQB1 alleles on the treatment response to nucleoside/nucleotide analogs in chronic HBV infection[20]. In this study, we aimed to investigate the association between HLA DQB1 and DRB1 alleles and the outcome of chronic HBV disease (inactive state, active disease or cirrhosis).
MATERIALS AND METHODS
Patients
Chronic hepatitis B patients who were followed up at a hepatology clinic at a single institution between August 2005 and August 2010 were included in this study. The local ethics committee approved the study. The patients’ case notes were reviewed, and demographic data, laboratory results, endoscopy results, virological parameters, biopsy scores and treatment statuses were noted. There were 628 patients. Patients with delta virus co-infection, hepatitis C virus (HCV) or human immunodeficiency virus infection or liver disease rather than hepatitis B; patients receiving immunosuppressive treatment; and patients under age 18 were excluded. Patients with cirrhosis, patients with inactive disease and patients with active disease were identified according to the following definitions.
Cirrhosis: Cirrhosis was confirmed by biopsy unless portal hypertension (portal ascites or esophageal varices and thrombocytopenia) was present.
Chronic inactive disease: HBeAg-negative patients with persistently normal serum alanine transferase (ALT) levels (lower than 30 IU/mL) and persistently low HBV DNA levels (lower than 2000 IU/mL) for at least three years of follow-up were defined as inactive. A sonographic examination that revealed normal liver function, without any sign of portal hypertension, was mandatory. If the liver was biopsied, the fibrosis score had to be 0, and the hepatic activity index (HAI) had to be less than 4.
Chronic active disease (the patients being treated): The patients who fulfilled the American Association for the Study of Liver Diseases (AASLD) 2009 criteria and were receiving treatment were included[21]. Any patient who received treatment without fulfilling the AASLD criteria was excluded from the study.
In total, 378 patients met the given criteria, among whom 23 did not give consent or did not show up for their appointment (for blood sample collection for HLA analysis). The remaining 355 patients were included and formed the study population. All patients were Caucasian; were born in Turkey; and, when asked, identified themselves to be of Turkish descent. Among the study patients, 63.7% (n = 226) were male, 23.1% (n = 82) were HBeAg positive, 24.5% (n = 87) had cirrhosis, and 18.6% (n = 66) had inactive disease. In all, 81.4% of the patients had active disease and had received at least one course of treatment. The mean age, mean log DNA level and mean ALT level at diagnosis were 37.2 ± 15.1 years, 5.4 ± 2.2 IU/mL and 91.8 ± 35 IU/mL, respectively. The mean follow-up time was 85.5 ± 11 mo. The liver histology scores of 194 patients were available. The mean HAI was 6.8 ± 3.2, and the mean fibrosis score was 2.1 ± 1.8.
HLA analysis
In total, 5 mL of blood was taken from patients and stored in EDTA tubes at -80 °C until the day of DNA extraction. Genomic DNA was extracted from 1 mL of peripheral blood using an Invitrogen PureLink Genomic DNA purification kit (Grand Island, NY). The alleles of HLA DQB1 and DRB1 were detected by a polymerase chain reaction with sequence-specific primers (PCR-SSP)[22]. DQB1 alleles were defined at high resolution, and DRB1 alleles were defined at low resolution. Primer sequences and PCR product sizes are listed in Table 1. Internal positive-control primers were included in the reaction system to eradicate false negatives. The internal control was a fragment of human growth hormone gene 1 (chr 17), consisting of 439 bp. PCR was performed in a 25 μL reaction mixture containing 100 ng genomic DNA, 1 U Crimson Taq DNA Polymerase (New England Biolabs, Ipswich, MA), optimized Crimson Taq polymerase buffer (New England Biolabs, Ipswich, MA) at one-fifth of the total volume, 1 μmol/L MgCl2 (Fermentas, Glen Burnie, MD), 0.2 μmol/L dNTPs (Fermentas, Glen Burnie, MD), 0.2 μmol/L primers and 0.04 μmol/L internal-control primers (C5 and C3). The following cycling conditions were employed: 95 °C for 5 min; 30 cycles at 95 °C for 30 s, 66 °C for 30 s and 68 °C for 1 min; and a single final extension at 68 °C for 7 min. The PCR conditions were optimized to obtain a higher yield and greater fidelity. Following amplification, 10 μL of products stained with SYBR Green were loaded onto a 2% agarose gel in the presence of an O’Range GeneRuler Ultra Low Range marker (Fermentas, Glen Burnie, MD) and then identified under ultraviolet light after electrophoresis. The allelic type was determined according to the presence or absence of PCR products of the desired length.
Table 1.
List of primers used in the study
| DQB1 allele | Primers | PCR product | DRB1 allele | Primers | PCR product |
| DQB1*0501 | 5’ 5’ CGGAGCGCGTGCGGGG 3’ | 128 | DRB1*01 | 5’ TTGTGGCAGCTTAAGTTTGAAT 3’ | 168 |
| 3’ 5’ GCTGTTCCAGTACTCGGCAA 3’ | 5’ GCTGTTCCAGTACTCGGCAT 3’ | ||||
| DQB1*0502 | 5’ 5’ TGCGGGGTGTGACCAGAC 3’ | 117 | DRB1*15/16 | 5’ TCCTGTGGCAGCCTAAGA G 3’ | 310 |
| 3’ 5’ TGTTCCAGTACTCGGCGCT 3’ | 5’ CGCTGCACTGTGAAGCTCTC 3’ | ||||
| DQB1*0503 | 5’ 5’ TGCGGGGTGTGACCAGAC 3’ | 87 | DRB1*03 | 5’ GTTTCTTGGAGTACTCTAGGTC 3’ | 222 |
| 3’ 5’ GCGGCGTCACCGCCCGA 3’ | 5’ TGCAGTAGTTGTCCACCCG 3’ | ||||
| DQB1*0601 | 5’ 5’ GCCATGTGCTACTTCACCAAT 3’ | 198 | DRB1*04 | 5’ GTTTCTTGGAGCAGGTTAAACA 3’ | 262 |
| 3’ 5’ CACCGTGTCCAACTCCGCT 3’ | 5’ CGCTGCACTGTGAAGCTCTC 3’ | ||||
| DQB1*0602 | 5’ 5’ CGTGCGTCTTGTGACCAGAT 3’ | 121 | DRB1*11 | 5’ CACGTTTCTTGGAGTACTCTAC 3’ | 179 |
| 3’ 5’ GCTGTTCCAGTACTCGGCAT 3’ | 5’ CTGGCTGTTCCAGTACTCCT 3’ | ||||
| DQB1*0603 | 5’ 5’ GGAGCGCGTGCGTCTTGTA 3’ | 127 | DRB1*12 | 5’ AGTACTCTACGGGTGAGTGTT 3’ | 198 |
| 3’ 5’ GCTGTTCCAGTACTCGGCAT 3’ | 5’ CTGTTCCAGGACTCGGCGA 3’ | ||||
| DQB1*0604 | 5’ 5’ CGTGTACCAGTTTAAGGGCA 3’ | 254 | DRB1*13/14 | 5’ GTTTCTTGGAGTACTCTACGTC 3’ | 234 |
| 3’ 5’ GCAGGATCCCGCGGTACC 3’ | 5’ CGTAGTTGTGTCTGCA(GA)TAGG 3’ | ||||
| DQB1*0201 | 5’ 5’ GTGCGTATTGTGAGCAGAAG 3’ | 205 | DRB1*07 | 5’ CCTGTGGCAGGG AAGTATA 3’ | 232 |
| 3’ 5’ GCAAGGTCGTGCGGAGCT 3’ | 5’ CCCGTAGTTGTGTCTGCACAC 3’ | ||||
| DQB1*0201/0302 | 5’ 5’ GACGGAGCGCGTGCGTCT 3’ | 129 | DRB1*08 | 5’ AGTACTCTACGGGTGAGTGTT 3’ | 227 |
| 3’ 5’ CTGTTCCAGTACTCGGCGG 3’ | 5’ CCCGTATTGTGTCTGCAG 3’ | ||||
| DQB1*0301 | 5’ 5’ GACGGAGCGCGTGCGTTA 3’ | 122 | DRB1*09 | 5’ GTTTCTTGAAGCAGGATAAGTTT 3’ | 236 |
| 3’ 5’ AGTACTCGGCGTCAGGCG 3’ | 5’ CCCGTAGTTGTGTCTGCACAC 3’ | ||||
| DQB1*0302/0303 | 5’ 5’ GACGGAGCGCGTGCGTCT 3’ | 122 | DRB1*10 | 5’ CGGTTGCTGGAAAGACGCG 3’ | 204 |
| 3’ 5’ AGTACTCGGCGTCAGGCG 3’ | 5’ CTGCACTGTGAAGCT CTCAC 3’ | ||||
| DQB1*0303 | 5’ 5’ GACGGAGCGCGTGCGTCT 3’ | 129 | DRB1-Exon2 | 5’ TTCGTGTCCCCACAGCACGTTTC 3’ | 295 |
| 3’ 5’ CTGTTCCAGTACTCGGCGT 3’ | 5’ GCCGCTGCACTGTGAAGCTCTC 3’ | ||||
| DQB1*0401 | 5’ 5’ CACCAACGGGACCGAGCT 3’ | 200 | Internal control (HGH) | 5′ CAGTGCCTTCCCAACCATTCCCTTA 3′ | 439 |
| 3’ 5’ GGTAGTTGTGTCTGCATACG 3’ | 5’ ATCCACTCACGGATTTCTGTTGTGTTTC 3′ | ||||
| DQB1*0402 | 5’ 5’ CACCAACGGGACCGAGCG 3’ | 200 | |||
| 3’ 5’ GGTAGTTGTGTCTGCATACG 3’ |
Statistical analyses
Statistical analyses were performed to evaluate the difference between cirrhotic and non-cirrhotic patients and between patients with active and inactive disease. During the HLA analysis, 2 × 2 tables and the chi square test were used. When the sample sizes were small or when the expected values in the cells of the chi square table were less than 5, Fisher’s exact test was used. Parametric variables were analyzed using Student’s t test. To analyze the effect of multiple variables on the presence of cirrhosis, multivariate logistic regression was applied.
All P values were double sided, and if the P value was below 0.05, it was considered as statistically significant unless there were multiple comparisons. Bonferroni correction was applied for multiple comparisons, and corrected P values are given. SPSS 20 (Chicago, IL) was used for the statistical analysis. Genotypic analysis was performed using Genepop Population Genetics Software Package Version 4.2[23].
Sequence alignment analysis
The sequence alignment of HLA class II proteins was performed using ClustalW[24].
RESULTS
HLA and active disease
The most frequent HLA DQB1 allele was DQB1*03:01 (48.2%), and the most frequent HLA DRB1 allele was DRB1*13/14 (51.8%). In total, 66 patients had inactive hepatitis B, and 289 patients had chronic active hepatitis. The DQB1*05:01 allele was significantly more frequent in patients with active disease compared with inactive patients (27% vs 9.1%; P = 0.002, Pc = 0.026). The other DQB1 alleles (Table 2) and DRB1 alleles did not have a significant association with inactive disease. In the multivariate logistic regression analysis, the DQB1*05:01 allele had a significant association with chronic active hepatitis (Beta = 1.29, Wald = 7.5, Beta (exp) = 3.63, P = 0.006). This effect was independent of age, HBeAg status and gender.
Table 2.
DQB1 alleles among patients with active disease or inactive disease n (%)
| DQB1 allele | Patients with active disease | Patients with inactive disease | χ2 | P value | OR | 95%CI | |
| n = 289 | n = 66 | ||||||
| 1 | *05:01 | 78 (27) | 6 (9.1) | 9.52 | 0.002a | 3.7 | 1.5-8.9 |
| 2 | *05:02 | 27 (9.3) | 7 (10.6) | 0.1 | 0.75 | 0.9 | 0.4-2.1 |
| 3 | *05:03 | 53 (18.3) | 14 (21.2) | 0.29 | 0.59 | 0.83 | 0.4-1.6 |
| 4 | *06:01 | 40 (13.8) | 12 (18.2) | 0.81 | 0.37 | 0.7 | 0.4-1.5 |
| 5 | *06:02 | 0 | 0 | NA | NA | NA | NA |
| 6 | *06:03 | 0 | 0 | NA | NA | NA | NA |
| 7 | *06:04 | 1 (0.3) | 1 (1.5) | b | 0.34 | 0.2 | 0.01-3.7 |
| 8 | *02:01 | 2 (0.7) | 1 (1.5) | b | 0.46 | 0.5 | 0.04-5.1 |
| 9 | *03:02 | 94 (32.5) | 20 (30.3) | 0.12 | 0.72 | 1.1 | 0.6-2 |
| 10 | *03:01 | 135 (46.7) | 36 (54.5) | 1.32 | 0.25 | 0.7 | 0.4-1.3 |
| 11 | *03:03 | 27 (9.3) | 5 (7.6) | 0.2 | 0.65 | 1.3 | 0.4-3.4 |
| 12 | *04:02 | 7 (2.4) | 0 | b | 0.36 | NA | NA |
| 13 | *04:01 | 0 | 0 | NA | NA | NA | NA |
P < 0.01 (after Bonferroni correction). b: Fisher’s exact test was applied; NA: Not available.
HLA and cirrhosis
In this study, 87 patients had cirrhosis. Patients without cirrhosis carried HLA DRB1*07 more frequently than did the cirrhotic patients (16% vs 3.4%; P = 0.002, Pc = 0.022). The other DRB1 alleles (Table 3) and all of the DQB1 alleles did not exhibit a statistically significant association with the presence of cirrhosis.
Table 3.
DRB1 alleles among patients with cirrhosis or without cirrhosis n (%)
| DRB1 allele | Patients with cirrhosis n = 87 | Patients without cirrhosis n = 268 | χ2 | P value | OR | 95%CI | |
| 1 | *01 | 2 (2.3) | 7 (2.6) | b | 1 | 0.9 | 0.2-4.3 |
| 2 | *15/16 | 17 (19.5) | 56 (20.9) | 0.07 | 0.79 | 0.9 | 0.5-1.7 |
| 3 | *03 | 9 (10.3) | 26 (9.7) | 0.03 | 0.8 | 1.1 | 0.5-2.4 |
| 4 | *04 | 2 (2.3) | 3 (1.1) | b | 0.6 | 2.1 | 0.3-12.7 |
| 5 | *11 | 3(3.4) | 6 (2.2) | b | 0.5 | 1.5 | 0.4-6.4 |
| 6 | *12 | 5 (5.7) | 24 (9) | 0.9 | 0.34 | 0.6 | 0.2-1.7 |
| 7 | *13/14 | 45 (51.7) | 139 (51.9) | 0.01 | 0.98 | 1 | 0.6-1.6 |
| 8 | *07 | 3 (3.4) | 43 (16.0) | 9.2 | 0.002a | 0.2 | 0.06-0.6 |
| 9 | *08 | 4 (4.6) | 5 (1.9) | b | 0.2 | 2.5 | 0.7-9.7 |
| 10 | *09 | 3 (3.4) | 4 (1.5) | b | 0.37 | 2.3 | 0.5-10.7 |
| 11 | *10 | 4 (4.6) | 11 (4.1) | b | 0.77 | 1.1 | 0.4-3.6 |
P < 0.05 (after Bonferroni correction). b: Fisher’s exact test was applied; NA: Not available.
Gender influenced the presence of cirrhosis (28.3% in males vs 17.8% in females; P = 0.03), and cirrhotic patients were older (47.4 vs 33.9; P < 0.001). HBeAg-negative patients were more likely to have cirrhosis compared with HBeAg-positive patients (27.1% vs 15.9%; P = 0.04). Age, HBeAg status, gender and DRB1*07 were analyzed in the multivariate logistic regression analysis. DRB1*07 status remained independently related to the presence of cirrhosis (P = 0.015), in addition to age (P < 0.001) and gender (P = 0.01) (Table 4).
Table 4.
Factors affecting the presence of cirrhosis (multivariate logistic regression analysis)
| Beta | SE | Wald | Exp (Beta) | P value | 95%CI | |
| HBeAg (positive) | -0.08 | 0.38 | 0.05 | 0.92 | 0.83 | 0.44-1.93 |
| DRB1*07 | -1.59 | 0.65 | 5.9 | 0.2 | 0.015 | 0.06-0.73 |
| Age at diagnosis | 0.07 | 0.01 | 39.9 | 1.07 | < 0.001 | 1.05-1.09 |
| Gender (male) | 0.78 | 0.30 | 6.7 | 2.18 | 0.01 | 1.2-3.9 |
HBeAg: Hepatitis B early antigen.
Genotypic analysis
The most frequent DQB1 genotypes were DQB1*03:01 and DQB1*03:02 (n = 35, 9.9%), DQB1*03:01 and DQB1*05:01 (n = 30, 8.5%) and DQB1*0601 and DQB1*0301 (n = 20, 5.6%). The distribution of these DQB1 genotypes did not reveal a significant difference between cirrhotic patients and non-cirrhotics or between inactive patients and patients with chronic active hepatitis (data not shown).
The most frequent DRB1 genotypes were DRB1*13/14 and DRB1*15/16 (n = 28, 7.9%) and DRB1*03 and DRB1*13/14 (n = 14, 3.9%). These genotypes did not significantly correlate with the presence of cirrhosis or active disease (data not shown).
In silico analysis of DRB1*07
When the identified polymorphic sites were mapped to the amino acid sequence of HLA class II molecules, four substitutions were observed at the T-cell interaction site. These substitutions were located at β30, β67, β71 and β86, as obtained by ClustalW analysis (Figure 1).
Figure 1.

Sequence similarity analysis of the β subdomain of three different major histocompatibility complex class II proteins. An asterisks underneath a capital letter denotes a single, fully conserved residue. The semicolons and dots represent amino acids with conservation of strong groups or weak groups, respectively. For a mismatch between amino acids, a space is left underneath. The residues determined to be important for T-cell receptor recognition are shown with yellow shading.
DISCUSSION
In this study, we compared the allele distribution among chronic active hepatitis patients with the distribution among patients in the chronic inactive stage. We defined inactivity according to histology, persistently normal ALT levels and persistently low viremia. Chronic active hepatitis patients more frequently carried the HLA DQB1*05:01 allele than did inactive patients. This allele has been found to be related to a better response to the HBV vaccine[25] and to be a protective factor against chronic HBV infection[17]. Previous data have associated DQB1*05:01 with an appropriate immunologic response, but in the present study, DQB1*05:01 was related to active disease, thus revealing an inappropriate immune response. This finding could be a reflection of genetic divergence between different populations or a consequence of the genotype of the virus; only the D genotype is present in Turkey[26].
Another finding of this study was the more frequent presence of HLA DRB1*07 in the non-cirrhotic group compared with the cirrhotics. Liver histology scores were better in patients with the DRB1*07 allele, and the mean fibrosis score and HAI score of these patients were lower (P = 0.001 and P = 0.008, respectively). Chronic HBV infection is an immunologically driven disease, and HBV is a non-cytopathic virus. Host genetic factors and viral and environmental factors have been proposed to determine disease progression or regression[3]. Immune action against the virus involves HLA-restricted T lymphocytes, antibody-secreting B lymphocytes, natural killer cells and numerous cytokines. The immune recognition of viruses requires the presentation of viral antigens to CD4-positive and CD8-positive lymphocytes by HLA class II and class I molecules, after which viral clearance or persistence occurs[27,28]. When an adequate vigorous polyclonal, multispecific response to HBV antigens is not produced, CD4-positive and CD8-positive T cells that are capable of terminating HBV infection do not accumulate in the liver[4,5]. If HBV-specific CD8-positive and CD4-positive T cell responses are absent, the immune response then becomes nonselective and results in chronic necroinflammation and fibrosis. This long-lasting cytolytic and inappropriate immune response against infected hepatocytes is responsible for liver damage. Previous reports have suggested an association between cirrhosis and HLA DRB1; for instance, two studies from China have demonstrated an association between cirrhosis and HLA DRB1*12[29,30]. Another study, from Brazil, revealed the protective effect of HLA DRB1*11 against severe liver damage in HCV patients[31]. Other studies and a recent meta-analysis implicated DRB1*07 as a risk factor for persistent HBV infection[16,32,33]. Studies from Turkey[34], the United Kingdom[35], Belgium[25], Spain[36], Germany[37] and China[38,39] revealed that the presence of DRB1*07 (or DR7) causes unresponsiveness to the HBV vaccine. Furthermore, in a Turkish study exploring HLA alleles in renal transplant candidates, panel-reactive antibodies were less frequently detected in the patient group with the DRB1*07 allele, despite the fact that this group had a higher predisposition to developing these antibodies[40]. This finding indicates a hypoimmune profile associated with DRB1*07. In the current study, the DRB1*07 allele was less frequent in cirrhotic patients compared with non-cirrhotics. Together with previous reports, the results of the present study may indicate that patients with the DRB1*07 allele are not able to mount a specific immune reaction to HBV and are predisposed to chronic infection. This hypoimmune response also yields less hepatocyte damage and less cirrhosis. The puzzling immune response observed in patients carrying the DRB1*07 allele may be attributable to this allele’s interaction with the T-cell recognition site. The available crystal structures of HLAs reveal that these proteins carry allele-specific motifs[41]. The antigenic peptide specificity of HLA class II proteins comes from the determined polymorphic pockets in the peptide-binding cleft. T-cell receptor recognition is accomplished by both the peptide and HLAs[41]. Certain HLA DRB1 types show polymorphisms specifically at the T-cell recognition site, without significantly altering peptide binding[42,43]. Interestingly, our bioinformatics analysis revealed that DRB1*07 contains four substitutions at the T-cell interaction site, which are located at β30, β67, β71 and β86, according to ClustalW analysis (Figure 1). Two of these residues, β67 and β71, have been determined to be important for peptide binding and T-cell receptor recognition in mutagenesis studies[42,43]. These residues are solvent exposed and are predicted to be critical for direct T-cell receptor binding, so any alteration at these sites would change T-cell receptor recognition. Additionally, the buried residues β30 and β86 have been shown to be important for T-cell recognition in mutagenesis studies[41,42]. Although these residues cannot have a direct effect on T-cell recognition, they might have an indirect conformational influence that can be communicated to the T-cell recognition site (Figure 2). Due to changes in the T-cell recognition residues in the HLA DRB1*07 variant, the immune response can be weakened, resulting in chronic infection without cirrhosis.
Figure 2.
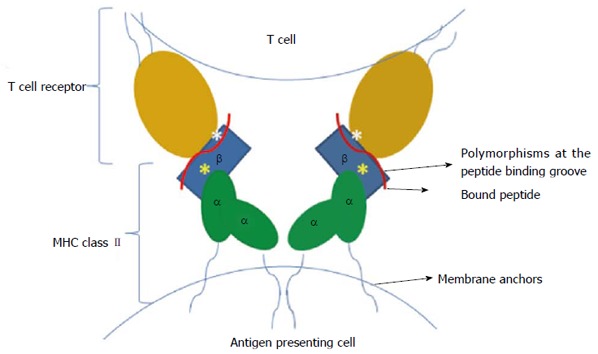
Possible model illustrating the single-nucleotide polymorphic regions of major histocompatibility complex class II that affect T-cell receptor recognition. The yellow asterisks show mutational sites that are buried in the peptide-binding pocket, whereas the white asterisks indicate mutations in the solvent-exposed T-cell recognition regions. This model was built according to the available crystal structures, which were published previously[41].
This study has certain limitations. A larger sample size could be used to identify further associations between HLA subgroups and disease outcomes. This is a cohort study, so it is not possible to conclude that there is a direct causative relationship between our findings and the disease state. Molecular studies are necessary to investigate HBV-specific antigenic peptides and the specific structure of the rearranged T-cell receptor. However, the well-defined patient groups, the long duration of the follow-up period and the medium- to high-resolution genotyping of HLA alleles are the strengths of this study.
In conclusion, the natural course of HBV disease is not easily predictable; viral factors, environmental factors and host factors may affect the disease course. This study suggests that the HLA DQB1*05:01 allele is associated with chronic persistent disease and that the DRB1*07 allele is a protective factor against cirrhosis.
ACKNOWLEDGMENTS
We are grateful to Asli Kumbasar for critically editing the manuscript.
COMMENTS
Background
In certain patients, chronic hepatitis B infection may culminate in cirrhosis, whereas other patients will remain in the inactive state. There is no single test for predicting patient outcomes.
Research frontiers
Recent genome-wide association studies revealed a relationship between human leukocyte antigen (HLA) class II and hepatitis B chronicity.
Innovations and breakthroughs
The authors genotyped HLA DQB1 and DRB1 alleles in their patient cohort. They revealed that the HLA DQB1*05:01 allele is related to chronic persistent disease and that the DRB1*07 allele is a protective factor against cirrhosis.
Applications
Host genetic factors are associated with hepatitis B outcomes. To understand and identify the exact mechanism, future molecular research is needed.
Terminology
The HLA system is the name given to the genes that encode the major histocompatibility complex (MHC) proteins. MHC proteins/antigens are essential for the immune system. MHC class II presents antigens to T-helper cells, which then stimulate B lymphocytes to commence specific antibody production.
Peer review
The authors present the results of a retrospective cohort study in patients with chronic hepatitis B, in which they evaluated the association between HLA DQB1 and DRB1 alleles and the stage of the disease. They identified an association between DQB1*05:01 and active hepatitis B disease. Furthermore, DRB1*07 was associated with the absence of cirrhosis. The topic of the study is very interesting.
Footnotes
Supported by Internal research funds of Istanbul Technical University, No. 36403
P- Reviewers: Gigi E, Gevers TJG S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 3.Vierling JM. The immunology of hepatitis B. Clin Liver Dis. 2007;11:727–759, vii-viii. doi: 10.1016/j.cld.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Das A, Maini MK. Innate and adaptive immune responses in hepatitis B virus infection. Dig Dis. 2010;28:126–132. doi: 10.1159/000282075. [DOI] [PubMed] [Google Scholar]
- 5.Bertoletti A, Kennedy P, Gehring AJ. Role of the immune response in hepatitis B. In: Gershwin ME, Vierling JM, Manns M, edtors , editors. Liver Immunology: Principles and Practice. Totowa, NJ: Humana Press; 2005. pp. 179–191. [Google Scholar]
- 6.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 7.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 8.Weissberg JI, Andres LL, Smith CI, Weick S, Nichols JE, Garcia G, Robinson WS, Merigan TC, Gregory PB. Survival in chronic hepatitis B. An analysis of 379 patients. Ann Intern Med. 1984;101:613–616. doi: 10.7326/0003-4819-101-5-613. [DOI] [PubMed] [Google Scholar]
- 9.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Kim WR, Brown RS, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 11.Jiang YG, Wang YM, Liu TH, Liu J. Association between HLA class II gene and susceptibility or resistance to chronic hepatitis B. World J Gastroenterol. 2003;9:2221–2225. doi: 10.3748/wjg.v9.i10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han YN, Yang JL, Zheng SG, Tang Q, Zhu W. Relationship of human leukocyte antigen class II genes with the susceptibility to hepatitis B virus infection and the response to interferon in HBV-infected patients. World J Gastroenterol. 2005;11:5721–5724. doi: 10.3748/wjg.v11.i36.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi-Lin Z, Te D, Jun-Hong L, Liang-Ping L, Xin-Hui G, Ji-Rong G, Chun-Yan G, Zhuo L, Ying L, Hui L. Analysis of HLA-DQB1 gene polymorphisms in asymptomatic HBV carriers and chronic hepatitis B patients in the Chinese Han population. Int J Immunogenet. 2006;33:249–254. doi: 10.1111/j.1744-313X.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu YF, Wang LY, Lee TD, Lin HH, Hu CT, Cheng ML, Lo SY. HLA phenotypes and outcomes of hepatitis B virus infection in Taiwan. J Med Virol. 2004;72:17–25. doi: 10.1002/jmv.10557. [DOI] [PubMed] [Google Scholar]
- 15.Thio CL, Carrington M, Marti D, O’Brien SJ, Vlahov D, Nelson KE, Astemborski J, Thomas DL. Class II HLA alleles and hepatitis B virus persistence in African Americans. J Infect Dis. 1999;179:1004–1006. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 16.Karan MA, Tascioglu NE, Ozturk AO, Palanduz S, Carin M. The role of HLA antigens in chronic hepatitis B virus infection. J Pak Med Assoc. 2002;52:253–256. [PubMed] [Google Scholar]
- 17.Mbarek H, Ochi H, Urabe Y, Kumar V, Kubo M, Hosono N, Takahashi A, Kamatani Y, Miki D, Abe H, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20:3884–3892. doi: 10.1093/hmg/ddr301. [DOI] [PubMed] [Google Scholar]
- 18.Nishida N, Sawai H, Matsuura K, Sugiyama M, Ahn SH, Park JY, Hige S, Kang JH, Suzuki K, Kurosaki M, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS One. 2012;7:e39175. doi: 10.1371/journal.pone.0039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 20.Doganay L, Tuncer I, Katrinli S, Enc FY, Ozturk O, Colak Y, Ulasoglu C, Dinler G. The effect of HLA-DQB1 alleles on virologic breakthroughs during chronic hepatitis B treatment with genetically low barrier drugs. Clin Res Hepatol Gastroenterol. 2013;37:359–364. doi: 10.1016/j.clinre.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 22.Olerup O, Aldener A, Fogdell A. HLA-DQB1 and -DQA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Tissue Antigens. 1993;41:119–134. doi: 10.1111/j.1399-0039.1993.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 23.Rousset F. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- 24.European Bioinformatics Institute. ClustalW2-Multiple Sequence Alignment. Cambridge UK, 2013. Available from: http://www.ebi.ac.uk/Tools/msa/clustalw2/
- 25.Desombere I, Willems A, Leroux-Roels G. Response to hepatitis B vaccine: multiple HLA genes are involved. Tissue Antigens. 1998;51:593–604. doi: 10.1111/j.1399-0039.1998.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 26.Bozdayi G, Türkyilmaz AR, Idilman R, Karatayli E, Rota S, Yurdaydin C, Bozdayi AM. Complete genome sequence and phylogenetic analysis of hepatitis B virus isolated from Turkish patients with chronic HBV infection. J Med Virol. 2005;76:476–481. doi: 10.1002/jmv.20386. [DOI] [PubMed] [Google Scholar]
- 27.Belz G, Mount A, Masson F. Dendritic cells in viral infections. Handb Exp Pharmacol. 2009;(188):51–77. doi: 10.1007/978-3-540-71029-5_3. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen EM, ten Berge IJ, van Lier RA. Induction and maintenance of CD8+ T cells specific for persistent viruses. Adv Exp Med Biol. 2007;590:121–137. doi: 10.1007/978-0-387-34814-8_9. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YQ, Lin JS, Huang LH, Tian DY, Xiong P. [The association of HLA-DRB1 allele polymorphism with the genetic susceptibility to liver cirrhosis due to hepatitis B virus] Zhonghua Yixue Yichuanxue Zazhi. 2003;20:247–249. [PubMed] [Google Scholar]
- 30.Han Y, Jiang ZY, Jiao LX, Yao C, Lin QF, Ma N, Ju RQ, Yang F, Yu JH, Chen L. Association of human leukocyte antigen-DRB1 alleles with chronic hepatitis B virus infection in the Han Chinese of Northeast China. Mol Med Rep. 2012;5:1347–1351. doi: 10.3892/mmr.2012.800. [DOI] [PubMed] [Google Scholar]
- 31.Marangon AV, Silva GF, de Moraes CF, Grotto RM, Pardini MI, de Pauli DS, Visentainer JE, Sell AM, Moliterno RA. Protective effect of HLA-DRB1 11 and predisposition of HLA-C 04 in the development of severe liver damage in Brazilian patients with chronic hepatitis C virus infection. Scand J Immunol. 2012;76:440–447. doi: 10.1111/j.1365-3083.2012.02755.x. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Liu W, Wang H, Jin X, Fang S, Shi Y, Liu Z, Zhang S, Yang S. The influence of HLA alleles and HBV subgenotyes on the outcomes of HBV infections in Northeast China. Virus Res. 2012;163:328–333. doi: 10.1016/j.virusres.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Yan ZH, Fan Y, Wang XH, Mao Q, Deng GH, Wang YM. Relationship between HLA-DR gene polymorphisms and outcomes of hepatitis B viral infections: a meta-analysis. World J Gastroenterol. 2012;18:3119–3128. doi: 10.3748/wjg.v18.i24.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durupinar B, Okten G. HLA tissue types in nonresponders to hepatitis B vaccine. Indian J Pediatr. 1996;63:369–373. doi: 10.1007/BF02751531. [DOI] [PubMed] [Google Scholar]
- 35.McDermott AB, Zuckerman JN, Sabin CA, Marsh SG, Madrigal JA. Contribution of human leukocyte antigens to the antibody response to hepatitis B vaccination. Tissue Antigens. 1997;50:8–14. doi: 10.1111/j.1399-0039.1997.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 36.Peces R, de la Torre M, Alcázar R, Urra JM. Prospective analysis of the factors influencing the antibody response to hepatitis B vaccine in hemodialysis patients. Am J Kidney Dis. 1997;29:239–245. doi: 10.1016/s0272-6386(97)90036-6. [DOI] [PubMed] [Google Scholar]
- 37.Höhler T, Meyer CU, Notghi A, Stradmann-Bellinghausen B, Schneider PM, Starke R, Zepp F, Sänger R, Clemens R, Meyer zum Büschenfelde KH, et al. The influence of major histocompatibility complex class II genes and T-cell Vbeta repertoire on response to immunization with HBsAg. Hum Immunol. 1998;59:212–218. doi: 10.1016/s0198-8859(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 38.Qian Y, Zhang L, Liang XM, Hou JL, Luo KX. [Association of immune response to hepatitis B vaccine with HLA-DRB1*02, 07, 09 genes in the population of Han nationality in Guangdong Province] Diyi Junyi Daxue Xuebao. 2002;22:67–69. [PubMed] [Google Scholar]
- 39.Liu P, Xu H, Wang X, Li H, Zhuang G, Wu Z, Zhang K. [Field epidemiological and experimental study on relationship between genetic factor and nonresponse or hyporesponse to hepatitis B vaccine] Chin Med J (Engl) 2000;113:547–550. [PubMed] [Google Scholar]
- 40.Karahan GE, Kekik C, Oguz FS, Onal AE, Bakkaloğlu H, Calişkan YK, Yazici H, Turkmen A, Aydin AE, Sever MS, et al. Association of HLA phenotypes of end-stage renal disease patients preparing for first transplantation with anti-HLA antibody status. Ren Fail. 2010;32:380–383. doi: 10.3109/08860221003615803. [DOI] [PubMed] [Google Scholar]
- 41.Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 42.Krieger JI, Karr RW, Grey HM, Yu WY, O’Sullivan D, Batovsky L, Zheng ZL, Colón SM, Gaeta FC, Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991;146:2331–2340. [PubMed] [Google Scholar]
- 43.Coppin HL, Carmichael P, Lombardi G, L’Faqihi FE, Salter R, Parham P, Lechler RI, de Preval C. Position 71 in the alpha helix of the DR beta domain is predicted to influence peptide binding and plays a central role in allorecognition. Eur J Immunol. 1993;23:343–349. doi: 10.1002/eji.1830230207. [DOI] [PubMed] [Google Scholar]


