Abstract
AIM: To investigate the effect of gastric acid suppressants and prokinetics on peritonitis development in peritoneal dialysis (PD) patients.
METHODS: This was a single-center, retrospective study. The medical records of 398 PD patients were collected from January 2000 to September 2012 and analyzed to compare patients with at least one episode of peritonitis (peritonitis group, group A) to patients who never had peritonitis (no peritonitis group, group B). All peritonitis episodes were analyzed to compare peritonitis caused by enteric organisms and peritonitis caused by non-enteric organisms.
RESULTS: Among the 120 patients who met the inclusion criteria, 61 patients had at least one episode of peritonitis and 59 patients never experienced peritonitis. Twenty-four of 61 patients (39.3%) in group A and 15 of 59 patients (25.4%) in group B used gastric acid suppressants. Only the use of H2-blocker (H2B) was associated with an increased risk of PD-related peritonitis; the use of proton pump inhibitors, other antacids, and prokinetics was not found to be a significant risk factor for PD-related peritonitis. A total of 81 episodes of peritonitis were divided into enteric peritonitis (EP) or non-enteric peritonitis, depending on the causative organism, and gastric acid suppressants and prokinetics did not increase the risk of EP in PD patients.
CONCLUSION: The use of H2B showed a trend for an increased risk of overall PD-related peritonitis, although further studies are required to clarify the effects of drugs on PD-related peritonitis.
Keywords: Proton pump inhibitors, Histamine 2 receptor antagonists, Gastrointestinal motility, Peritoneal dialysis, Peritonitis
Core tip: Bacterial overgrowth/proliferation in an intraluminal environment with a high pH is a well-recognized mechanism for peritonitis development, especially in liver cirrhosis patients. Several studies have investigated whether this mechanism causes peritoneal dialysis (PD)-related peritonitis, although conflicting results have been obtained for the association between acid suppressive therapy and PD-related peritonitis. In addition, no study was conducted to evaluate the association between prokinetics and PD-related peritonitis. Therefore, we sought to assess the effect of gastric acid suppressants and prokinetics on PD-related peritonitis. In the present study, H2B use, but not proton pump inhibitor use, was identified as an independent risk factor for PD-related peritonitis development.
INTRODUCTION
Peritonitis is common in peritoneal dialysis (PD) patients and is associated with significant morbidity and mortality[1,2]. Several studies have evaluated the risk factors for PD-related peritonitis; in particular, ethnicity and body mass index have been identified as independent risk factors for PD-related peritonitis in large cohort studies[3,4]. However, obesity is not a useful predictor of the risk for peritonitis in East Asian PD patients because of the lower prevalence of obesity.
Diverticulosis is also a risk factor for PD-related peritonitis[5]. With mucosal thinning and/or microperforation of the colonic diverticulum, microorganisms in the gastrointestinal tract can easily transmigrate into the peritoneal space. The other potential risk factor for PD-related peritonitis is acid suppressive therapy, as several recent studies have shown that a higher pH in the gastrointestinal tract induced by gastric acid suppressants provides a good environment for microbial proliferation and overgrowth[6-9]. Although these studies showed an association between acid suppression treatment and PD peritonitis, the results remain controversial[9-12]. In addition, few studies have examined the effects of acid suppressive therapy in Asian patients with PD. Furthermore, several drugs such as immunosuppressants and prokinetics can influence the development of PD-related peritonitis and have not been evaluated simultaneously, which may have led to bias in previous studies. Moreover, many patients with end-stage renal disease (ESRD) experience gastroparesis or reduced bowel movements, and several studies have identified an association between gastrointestinal dysmotility and spontaneous bacterial peritonitis in cirrhotic patients and animal models of liver cirrhosis (LC). Indeed, decreased intestinal motility and extended transit time can lead to bacterial overgrowth and bacterial translocation from the gastrointestinal tract[13-16]. However, no study has clarified the effects of prokinetics on PD-related peritonitis.
This study aimed to evaluate whether acid suppressive therapy is associated with an increased risk of PD-related peritonitis, especially peritonitis caused by enteric organisms. In addition, we assessed the effects of prokinetics on PD-related peritonitis.
MATERIALS AND METHODS
Patients
Data from 398 patients with ESRD who underwent PD at our institution between January 2000 and September 2012 were collected. This study was approved by the Institutional Review Board of the Seoul National University Hospital.
This study included only adult patients aged ≥ 20 years; therefore, 54 patients aged < 20 years were excluded. Sixty-three patients were excluded because of incomplete data and 4 patients because of peritonitis related to perforation of the gastrointestinal tract or gallbladder. Twenty-three patients with LC were excluded because there were no standard criteria to distinguish PD-related peritonitis from spontaneous bacterial peritonitis. In addition, 133 patients who were previously administered antibiotics were excluded. One patient who did not receive PD after surgical replacement of the PD catheter was also excluded (Figure 1). Peritonitis episodes were identified by reviewing the medical records of patients. PD-related peritonitis was diagnosed if at least 2 of the following diagnostic criteria were met: (1) abdominal pain or cloudy PD effluent; (2) leukocytosis in the peritoneal fluid effluent (white blood cells > 100/mm3, with at least 50% polymorphonuclear neutrophils); or (3) a positive Gram stain or positive culture from PD effluent[17]. All episodes of peritonitis were initially treated by intraperitoneal administration of first-generation cephalosporin (cefazolin) and third-generation cephalosporin (ceftazidime). Management of peritonitis depended on the clinical course and results of the antibiotic resistance tests for each isolated organism. To minimize potential bias, we excluded individual episodes of recurrent or relapsing peritonitis. According to the International Society for Peritoneal Dialysis recommendations[2], recurrent peritonitis is defined as newly developed peritonitis caused by a different organism within 4 wk of the completion of therapy for a prior episode, whereas relapsing peritonitis is defined as newly developed peritonitis caused by the same organism within 4 wk of the completion of therapy for a prior episode. In addition, we included only up to the third episode for each patient in the analysis to minimize potential bias.
Figure 1.
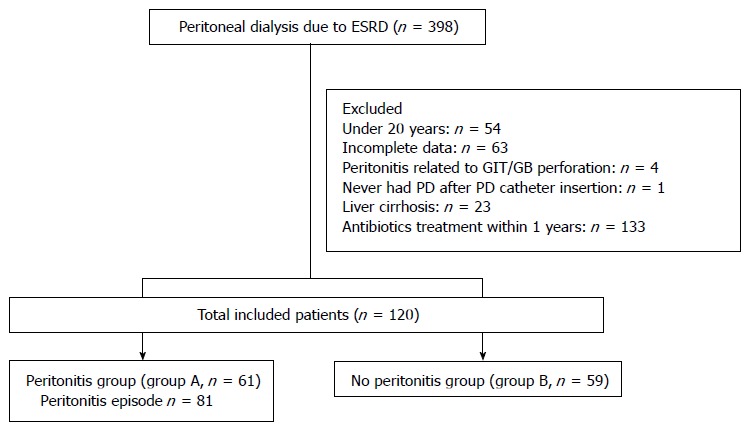
Patient inclusion/exclusion chart. Two hundred seventy-eight patients were excluded, as shown in the flow chart. Among the included 120 patients, 61 patients with at least one episode of peritonitis (group A) and 59 patients without peritonitis (group B) were compared. The data for 81 peritonitis episodes were collected from 61 patients in group A. PD: Peritoneal dialysis; GIT: Gastrointestinal tract; GB: Gallbladder; ESRD: End-stage renal disease.
Data collection and outcome measurement
Medical records were reviewed by a single trained investigator. Baseline characteristics including age, sex, cause of ESRD, modality of PD, initial serum albumin level, and presence of comorbidities such as diabetes mellitus, hypertension, and diverticulosis were recorded. The presence of diverticulosis was determined by reviewing previous findings of abdomen and pelvic contrast-enhanced computed tomography or colonoscopy. Data on treatment with gastric acid suppressants, prokinetics, and immunosuppressants were also collected. Data on acid suppressive therapy with proton pump inhibitors (PPIs), histamine-2 receptor blockers (H2Bs), and other antacids were recorded separately. Ribeiro et al[18] reported that PPI use 48 h after the first dose increases and sustains gastric acid suppression. In addition, a single dose of PPI per day may cause protopathic bias. Therefore, for this study, the use of acid suppressants was defined as the use of any PPIs or H2Bs for at least 2 d. Because the therapeutic doses of PPIs or H2Bs reach a steady state after daily dosing and thus achieve their maximal effective level between 5 to 7 d, the use of gastric acid suppressants was classified into four groups: use within the previous 7 d, use within the previous 30 d but not within the past 7 d, use within the previous 1 year but not within the last 30 d, and no use of acid suppressants. The appropriate indications for treatment with a gastric acid suppressant were defined as follows: gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), Barrett’s esophagus, and concomitant use of steroids or non-steroidal anti-inflammatory drugs. Prokinetic agents were assumed to be administered to patients with clinical symptoms associated with decreased gastrointestinal motility. In most of the cases, the reasons for prescription were not described in the medical records. In group A (the peritonitis group), the use of gastric acid suppressants and other medications was determined by reviewing the electronic medical records from the most recent clinical visits 1 year prior to the first episode of peritonitis. In group B (no peritonitis group), the use of medications was determined by reviewing the records from the most recent clinical visit 1 year prior to the last outpatient visit, renal transplantation, death, or transfer date to hemodialysis or other renal replacement modalities. Each peritonitis episode was classified as enteric peritonitis (EP) or non-enteric peritonitis (NEP) depending on the organism isolated from the PD effluent culture. Microorganisms known to colonize the gastrointestinal tract, such as Gram-negative bacteria and Enterococcus and Candida species, were the causative organisms of EP, whereas all other organisms were pathogens of NEP. For the comparison of mediations used in the EP and NEP groups, the medications used were determined from the records of the most recent clinical visits 1 year prior to the first episode of peritonitis.
Statistical analysis
Baseline characteristics are presented as the mean ± standard deviation or median and percentage for categorical variables. Statistical analysis was performed using Student’s t-test for the comparison of numerical variables and the chi-squared test or Fisher’s exact test for the comparison of categorical variables between groups. All probability values were two-tailed, and the significance levels were set at 0.05. Multivariate logistic regression analysis was performed to assess the association of significant variables in the univariate analysis with the occurrence of peritonitis. All analyses were performed using SPSS version 19.0 for Windows (IBM, New York, United States).
RESULTS
Baseline characteristics of the study patients
A total of 120 patients were included in the study: 61 patients in group A and 59 patients in group B. Table 1 summarizes the clinical characteristics of the two groups. In both groups, the common causes of renal failure were diabetic nephropathy and glomerulonephritis (GN). The GN cases included immunoglobulin A nephropathy (6/19 in group A, 12/20 in group B), focal segment glomerular sclerosis (4/19 in group A, 3/20 in group B), lupus nephritis (1/19 in group A, 1/20 in group B), hepatitis B virus-associated GN (1/19 in group A, none in group B), and other types of GN (7/19 in group A, 4/20 in group B). The baseline concentrations of serum albumin were 3.51 ± 0.47 mg/dL in group A and 3.60 ± 0.48 mg/dL in group B. There was no significant difference in baseline characteristics between the two groups (P = 0.336).
Table 1.
Clinical characteristics of the included patients n (%)
| Variables | Peritonitis group (n = 61) | No peritonitis group (n = 59) | P value |
| Age (yr), [median (range)] | 51 (27-79) | 49 (24-77) | 0.653 |
| Sex (male) | 55 (52.9) | 81 (56.6) | 0.558 |
| Height (cm) | 162.09 ± 6.94 | 163.21 ± 8.45 | 0.435 |
| Bwt (kg) | 57.68 ± 8.41 | 59.49 ± 11.24 | 0.332 |
| BMI [m/(kg)2] | 21.98 ± 2.73 | 22.26 ± 3.48 | 0.628 |
| Cause of ESRD | |||
| Glomerulonephritis | 19 (31.1) | 20 (33.9) | 0.153 |
| DM | 21 (34.4) | 12 (20.3) | |
| HTN | 5 (8.2) | 1 (1.7) | |
| Polycystic kidney disease | 1 (1.6) | 2 (3.4) | |
| Unknown/idiopathic | 11 (18.0) | 17 (28.8) | |
| Other cause | 4 (6.6) | 7 (11.9) | |
| HTN | 50 (82.0) | 53 (89.8) | 0.217 |
| DM | 23 (37.7) | 17 (28.8) | 0.302 |
| Diverticulosis | 1 (2.3) | 3 (6.7) | 0.616 |
| PD modality (CAPD) | 51 (83.6) | 47 (79.7) | 0.577 |
| Initial serum Albumin (mg/dL) | 3.51 ± 0.47 | 3.60 ± 0.48 | 0.336 |
| Peritonitis free time (d), | |||
| [median (range)] | 762 (31-3918) | 1302 (100-4234) | 0.070 |
Data presented as mean ± SD, unless otherwise stated. Bwt: Body weight; BMI: Body mass index; ESRD: End stage renal disease; DM: Diabetes mellitus; HTN: Hypertension; LC: Liver cirrhosis; PD: Peritoneal dialysis; CAPD: Continuous ambulatory peritoneal dialysis.
Drug effects on PD-related peritonitis
Table 2 summarizes the results of the comparison between group A and group B patients in terms of acid suppressive therapy, prokinetics, and immunosuppressants. PPIs or H2Bs were administered to 20 of the 61 patients (32.8%) in group A and 13 of the 59 patients (22.0%) in group B. Pantoprazole was the most frequently prescribed PPI, and famotidine was the most frequently used H2B. The reasons for prescribing PPI or H2B were not recorded in 18 of the 33 patient files (54.5%). Inappropriate indications for drug treatment included gastritis (24.2%) and nausea or vomiting (12.1%). The drugs were administered properly in 3 patients, including 1 patient with GERD (3.0%), 1 patient with PUD (3.0%), and 1 patient who concomitantly received steroids (3.0%).
Table 2.
Acid-suppressive therapy and other medications in peritonitis group and no peritonitis group n (%)
| Peritonitis group | No peritonitis group | P value | |
| (n = 61) | (n = 59) | ||
| Use of PPIs | 6 (9.8) | 10 (16.9) | 0.252 |
| ≤ 7 d | 5 (8.2) | 4 (6.8) | 0.691 |
| 8-30 d | 0 (0.0) | 1 (1.7) | |
| > 30 d-1 yr | 1 (1.6) | 5 (8.5) | |
| No use | 55 (90.2) | 49 (83.1) | |
| Use of H2Bs | 15 (24.6) | 4 (6.8) | 0.011 |
| ≤ 7 d | 7 (11.5) | 3 (5.1) | 0.041 |
| 8-30 d | 1 (1.6) | 0 (0.0) | |
| > 30 d-1 yr | 7 (11.5) | 1 (1.7) | |
| No use | 46 (75.4) | 55 (93.2) | |
| Use of other antacids | 4 (6.6) | 3 (5.1) | 1.000 |
| ≤ 7 d | 3 (1.9) | 1 (1.7) | 0.472 |
| 8-30 d | 1 (0.0) | 1 (1.7) | |
| > 30 d-1 yr | 0 (1.9) | 1 (1.7) | |
| No use | 57 (96.2) | 56 (94.9) | |
| Use of prokinetics | 20 (32.8) | 18 (30.5) | 0.789 |
| ≤ 7 d | 11 (18.0) | 10 (16.9) | 0.712 |
| 8-30 d | 2 (3.3) | 0 (0.0) | |
| > 30 d-1 yr | 7 (11.5) | 8 (13.6) | |
| No use | 41 (67.2) | 41 (69.5) | |
| Use of immunosuppressants | 4 (6.6) | 3 (5.1) | 1.000 |
| ≤ 7 d | 2 (3.3) | 1 (1.7) | 0.610 |
| 8-30 d | 0 (0.0) | 0 (0.7) | |
| > 30 d-1 yr | 2 (3.3) | 2 (3.4) | |
| No use | 57 (93.4) | 56 (94.9) |
PPIs: Proton pump inhibitors; H2B: H2-blockers.
Univariate analysis showed that the use of H2Bs significantly increased the risk of PD-related peritonitis. Using linear-by-linear association analysis, a higher proportion of patients with peritonitis used H2Bs than those without peritonitis for each time interval of treatment (previous 7 d, 8-30 d prior to peritonitis, and within the last 1 year but not in the previous 30 d). No association between PPI, other antacid, or prokinetic use and PD-related peritonitis was found. Furthermore, exposure to immunosuppressants was not associated with PD-related peritonitis. In the multivariate analysis, we found that H2B use within 1 year prior to the development of peritonitis was an independent risk factor for the increased risk of peritonitis development (OR = 6.55; 95%CI: 1.64-26.26; P = 0.008) (Table 3).
Table 3.
Multivariate analysis of potential risk factors for the development of peritoneal dialysis related peritonitis
| Odds ratio | 95%CI | P value | |
| Initial serum albumin level | 0.50 | 0.20-1.25 | 0.139 |
| Use of Immunosuppressants | 1.85 | 0.28-12.40 | 0.527 |
| Use of PPIs | 0.50 | 0.20-1.25 | 0.364 |
| Use of H2Bs | 6.55 | 1.64-26.26 | 0.008 |
| Use of other Antacids | 1.47 | 0.21-10.29 | 0.696 |
| Use of prokinetics | 1.70 | 0.67-4.33 | 0.269 |
PPIs: Proton pump inhibitors; H2Bs: H2-blockers.
Drug effects on enteric peritonitis
During the study period, 54 of the 61 patients in group A experienced a single episode of peritonitis, according to the inclusion criteria, 12 patients had two episodes, and one patient had three episodes. A total of 81 episodes were included in the study, and all episodes were classified as EP or NEP, depending on the causative organism. Baseline characteristics did not show statistically significant differences between the groups (Table 4).
Table 4.
Clinical characteristics of peritonitis patients by the causative pathogen n (%)
| Variables | Included peritonitis episode (n = 81) |
||
| Enteric peritonitis | Non-enteric peritonitis | P value | |
| (n = 26) | (n = 55) | ||
| Age (yr) [median (range)] | 51.5 (29-69) | 51.0 (27-79) | 0.990 |
| Sex (male) | 16 (61.5) | 27 (49.1) | 0.295 |
| Height (cm) | 162.34 ± 7.88 | 162.21 ± 6.82 | 0.943 |
| Bwt (kg) | 56.93 ± 7.88 | 57.95 ± 8.50 | 0.610 |
| BMI [m/(kg)2] | 21.61 ± 2.68 | 22.05 ± 2.81 | 0.504 |
| Cause of ESRD | |||
| Glomerulonephritis | 12 (46.2) | 17 (30.9) | 0.250 |
| DM | 7 (26.9) | 20 (36.4) | |
| HTN | 3 (11.5) | 3 (5.5) | |
| Polycystic kidney disease | 0 (0.0) | 1 (1.8) | |
| Unknown/idiopathic | 3 (11.5) | 11 (20.0) | |
| Other cause | 1 (3.8) | 3 (5.5) | |
| HTN | 21 (80.8) | 43 (78.2) | 0.789 |
| DM | 8 (30.8) | 25 (45.5) | 0.209 |
| Diverticulosis | 0 (0.0) | 0 (0.0) | |
| PD modality (CAPD) | 23 (88.5) | 46 (83.6) | 0.743 |
| Initial serum Albumin (mg/dL) | 3.52 ± 0.45 | 3.47 ± 0.43 | 0.687 |
| Peritonitis free time (d) | |||
| [median (range)] | 692 (31–3696) | 1064 (31-5383) | 0.380 |
Data presented as mean ± SD, unless otherwise stated. Bwt: Body weight; BMI: Body mass index; ESRD: End stage renal disease; DM: Diabetes mellitus; HTN: Hypertension; LC: Liver cirrhosis; PD: Peritoneal dialysis; CAPD: Continuous ambulatory peritoneal dialysis.
The causative microorganisms were identified from peritoneal fluid culture in 61 of the 81 episodes. Escherichia coli (9/26, 34.6%) was the most frequently isolated enteric microorganism, and Streptococcus species (14/55, 24.5%) were the most frequently identified non-enteric microorganisms. The peritoneal fluid cultures from 20 episodes were negative (Table 5). The effects of various medications taken within 1 year prior to peritonitis on the development of EP are summarized in Table 6. When culture-negative peritonitis was included in the NEP group, no statistically significant association was found between exposure to gastric acid suppressants and EP. In addition, the use of prokinetics and immunosuppressants did not influence the development of EP (Table 6). When culture-negative peritonitis was excluded from the NEP group, the overall results were similar to those of the NEP group that included culture-negative peritonitis. The use of PPIs, H2Bs, other antacids, prokinetics, and immunosuppressants did not increase the risk of development of EP (Table 6).
Table 5.
Isolated microorganisms of the peritonitis episodes by the peritoneal dialysis effluent culture
| Causative organism | Peritonitis episodes (n = 81) | Percentage (%) |
| Escherichia coli | 9 | 11.1 |
| Klebsiella species | 4 | 4.9 |
| Acinetobacter species | 3 | 3.7 |
| Enterococcus species | 1 | 1.2 |
| Enterobacter species | 0 | 0 |
| Bacillus species | 4 | 4.9 |
| Pseudomonas species | 0 | 0 |
| Staphylococcus aureus | 0 | 0 |
| Other staphylococcus species (CoNS, etc.) | 9 | 11.1 |
| Streptococcus species | 14 | 17.3 |
| Corynebacterium species | 1 | 1.2 |
| Micrococcus species | 1 | 1.2 |
| Candida species | 2 | 2.5 |
| Other | 3 | 3.7 |
| Polymicrobial | 10 | 12.3 |
| No growth | 20 | 24.7 |
CoNS: Coagulase negative staphylococcus.
Table 6.
Acid-suppressive therapy and other medications in enteric peritonitis group and non-enteric peritonitis group n (%)
| Included peritonitis episode (n = 81) |
||||
| Enteric peritonitis (n = 26) | Non-enteric peritonitis (n = 55) | P value | ||
| Sterile peritonitis included in NEP group | Use of PPIs | 3 (11.5) | 8 (14.5) | 1.000 |
| Use of H2Bs | 6 (23.1) | 15 (27.3) | 0.687 | |
| Use of other antacids | 1 (3.8) | 5 (9.1) | 0.658 | |
| Use of prokinetics | 5 (19.2) | 18 (32.7) | 0.209 | |
| Use of immunosuppressants | 2 (7.7) | 7 (12.7) | 0.501 | |
| Sterile peritonitis excluded in NEP group | Use of PPIs | 3 (11.5) | 5 (14.3) | 1.000 |
| Use of H2Bs | 6 (23.1) | 9 (25.7) | 0.818 | |
| Use of other antacids | 1 (3.8) | 3 (8.6) | 0.629 | |
| Use of prokinetics | 5 (19.2) | 13 (37.1) | 0.129 | |
| Use of immunosuppressants | 2 (7.7) | 5 (14.3) | 0.688 | |
PPIs: Proton pump inhibitors; H2Bs: H2-blockers. NEP: Non-enteric peritonitis.
DISCUSSION
Bacterial overgrowth and proliferation in an intraluminal environment with a high pH is a well-recognized mechanism for the development of peritonitis, especially in LC patients[6,7,10,13]. Several studies have investigated whether this mechanism causes PD-related peritonitis, especially enteric pathogen infections, although conflicting results have been obtained for the association between acid suppressive therapy and PD-related peritonitis. Caravaca et al[12] reported that gastric acid suppressive therapy was an independent risk factor for EP, and Nessim et al[11] reported that H2B use was associated with a higher risk of EP in PD patients. Conversely, del Peso et al[10] did not find any effect of gastric acid suppressants on EP. These studies were valuable but limited in design. Therefore, we carefully excluded patients with liver cirrhosis, prior use of antibiotics, and a history of medication use that might influence peritonitis development to investigate the relationship between acid suppressive therapy and PD-related peritonitis. In the present study, H2B use, but not PPI use, was identified as an independent risk factor for the development of PD-related peritonitis after adjusting for variable factors. Therefore, our results suggest an association between H2Bs and PD-related peritonitis.
Keane et al[19] evaluated the metabolism of PPIs such as rabeprazole and showed that PPI clearance was not affected by renal failure. Conversely, Sica et al[20] analyzed the pharmacokinetics of H2B using ranitidine and reported that H2B clearance was decreased in continuous ambulatory PD patients compared to that observed in individuals with normal kidney function. Therefore, acid suppression with H2B treatment may be more powerful than that obtained with PPIs in PD patients. However, our results showed that neither H2Bs nor PPIs increased the risk of EP development, which suggests that a mechanism independent of acid suppression is involved in the development of PD-related peritonitis. Peritoneal macrophages, mast cells, and migrated leukocytes play crucial roles in the immune response to bacterial invasion. Histamine, which is released by peritoneal mast cells, stimulates vasodilation and encourages leukocyte transmigration. Moreover, histamine induces the aggregation of complement and opsonin, which promotes bacterial phagocytosis[21-23]. H2Bs block these mechanisms and also inhibit inflammation-generated increases in nitric oxide concentrations[24], resulting in reduced phagocytosis and antimicrobial effects. These facts support the hypothesis that H2Bs increase the risk of PD-related peritonitis.
Many ESRD patients experience abdominal discomfort, dyspepsia, and constipation due to decreased dietary fiber content, inadequate liquid intake, electrolyte imbalance, the use of certain medications such as phosphate binders containing aluminum, and calcium- or iron-replacement therapy. Diabetic nephropathy is the most common cause of ESRD[25,26], and most of these patients also show diabetic gastroparesis and decreased intestinal motility. Although the reasons for prescription were not described in detail, these symptoms might be related to the use of gastric acid suppressants and/or prokinetic drugs. Previous studies have shown an association between gastrointestinal dysmotility and spontaneous bacterial peritonitis in patients with LC[13-16]. Therefore, we investigated the effects of prokinetics on PD-related peritonitis, and our data showed that prokinetic use was not associated with a reduced risk of PD-related peritonitis. To our knowledge, this is the first study to investigate the association between prokinetic drugs and PD-related peritonitis, and further studies are therefore needed to confirm the effects of prokinetics on PD-related peritonitis.
The prognosis of EP is worse in patients with PD-related peritonitis, and peritonitis caused by enteric organisms is also associated with increased rates of PD catheter loss, prolonged hospitalization, and mortality[27,28]. In this study, we found no statistically significant variables that increased the risk of EP. However, this result should be interpreted with caution because of the small number of peritonitis episodes that were found with each individual drug use.
This study was superior to previous studies in several aspects. First, we carefully excluded patients with LC or previous antibiotic use. Furthermore, we performed multiple logistic regression analysis using various clinical factors such as the use of immunosuppressive agents and prokinetics, thus eliminating these potentially confounding factors. Second, a complete history of H2B and PPI use was obtained, as both drugs required a prescription in Korea during the study period. In addition, H2B and PPI use was defined as the use of these drugs for at least 2 d to prevent protopathic bias, which increased the causality of the results.
There were also several limitations to this study. First, this was a retrospective study that lacked clinical data on the reasons for gastric acid suppressant prescription. The study design did not permit analysis of individual drug doses and potential confounding factors such as type of PD and exit-site infection. Furthermore, few patients used H2Bs or PPIs, so the sample size was small. When we compared the EP and NEP groups, the number of peritonitis episodes treated with H2B or other medications was considerably smaller than that in groups A and B. To overcome these limitations, a prospective, randomized, placebo-control study is needed, which may put patients at unnecessary risk. As an alternative, a well-designed, prospective cohort study might provide further evidence of the association between acid suppressive therapy and PD-related peritonitis.
In conclusion, H2Bs tended to increase the risk of PD-related peritonitis, whereas PPIs and prokinetics did not. However, further large prospective cohort studies are required to assess the effects of gastric acid suppressants and prokinetics on the development of peritonitis in PD patients.
COMMENTS
Background
Bacterial overgrowth and proliferation in an intraluminal environment with a high pH is a well-recognized mechanism for the development of peritonitis, especially in liver cirrhosis (LC) patients. Several studies have investigated whether this mechanism causes peritoneal dialysis (PD)-related peritonitis, although conflicting results have been obtained for the association between acid suppressive therapy and PD-related peritonitis. In addition, previous studies have shown an association between gastrointestinal dysmotility and spontaneous bacterial peritonitis in patients with LC, although no study was conducted to evaluate the association between prokinetics and PD-related peritonitis.
Research frontiers
The authors sought to assess the effect of not only gastric acid suppressants but also prokinetics on PD-related peritonitis.
Innovations and breakthroughs
In the present study, Histamine 2 receptor blocker (H2B) use, but not proton pump inhibitors (PPIs) or prokinetics use, was identified as an independent risk factor for the development of PD-related peritonitis after adjusting for variable factors. This study was superior to previous studies in the following aspects. First, the authors carefully excluded patients with LC or previous antibiotic use. Furthermore, this study performed multiple logistic regression analysis using various clinical factors such as the use of immunosuppressive agents and prokinetics, thus eliminating confounding factors. Second, a complete history of H2B and PPI use was obtained, as both required a prescription in Korea during the study period.
Applications
The study results suggest that H2Bs tend to increase the risk of PD-related peritonitis, whereas PPIs and prokinetics do not.
Terminology
PPI: a class of drugs whose main action is a pronounced and long-lasting reduction of gastric acid production; H2B: a group of drugs used to block the action of histamine on parietal cells in the stomach, decreasing the production of acid by these cells; Prokinetics: a type of drug that enhances gastrointestinal motility by increasing the frequency of contractions in the small intestine or making them stronger.
Peer review
The authors have studied “The Effect of Gastric Acid Suppressants and Prokinetics on Peritoneal Dialysis-related Peritonitis”. Peritonitis is a very important event in PD patients and may lead to technique failure in many patients. The authors’ study was a retrospective analysis, and a small number of patients were included in the study. The concept is also not new, but may provide useful information for clinicians.
Footnotes
P- Reviewer: Prasad N S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Brown F, Liu WJ, Kotsanas D, Korman TM, Atkins RC. A quarter of a century of adult peritoneal dialysis-related peritonitis at an Australian medical center. Perit Dial Int. 2007;27:565–574. [PubMed] [Google Scholar]
- 2.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393–423. doi: 10.3747/pdi.2010.00049. [DOI] [PubMed] [Google Scholar]
- 3.Farias MG, Soucie JM, McClellan W, Mitch WE. Race and the risk of peritonitis: an analysis of factors associated with the initial episode. Kidney Int. 1994;46:1392–1396. doi: 10.1038/ki.1994.410. [DOI] [PubMed] [Google Scholar]
- 4.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int. 2004;24:340–346. [PubMed] [Google Scholar]
- 5.Tranaeus A, Heimbürger O, Granqvist S. Diverticular disease of the colon: a risk factor for peritonitis in continuous peritoneal dialysis. Nephrol Dial Transplant. 1990;5:141–147. doi: 10.1093/ndt/5.2.141. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SJ, Franco S, Young G, O’Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 7.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried M, Siegrist H, Frei R, Froehlich F, Duroux P, Thorens J, Blum A, Bille J, Gonvers JJ, Gyr K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23–26. doi: 10.1136/gut.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiefel U, Rao A, Pultz MJ, Jump RL, Aron DC, Donskey CJ. Suppression of gastric acid production by proton pump inhibitor treatment facilitates colonization of the large intestine by vancomycin-resistant Enterococcus spp. and Klebsiella pneumoniae in clindamycin-treated mice. Antimicrob Agents Chemother. 2006;50:3905–3907. doi: 10.1128/AAC.00522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Peso G, Bajo MA, Gadola L, Millán I, Codoceo R, Celadilla O, Castro MJ, Aguilera A, Gil F, Selgas R. Diverticular disease and treatment with gastric acid inhibitors do not predispose to peritonitis of enteric origin in peritoneal dialysis patients. Perit Dial Int. 2001;21:360–364. [PubMed] [Google Scholar]
- 11.Nessim SJ, Tomlinson G, Bargman JM, Jassal SV. Gastric acid suppression and the risk of enteric peritonitis in peritoneal dialysis patients. Perit Dial Int. 2008;28:246–251; discussion 236-237. [PubMed] [Google Scholar]
- 12.Caravaca F, Ruiz-Calero R, Dominguez C. Risk factors for developing peritonitis caused by micro-organisms of enteral origin in peritoneal dialysis patients. Perit Dial Int. 1998;18:41–45. [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Albillos A, Barden GE, West AB. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17:1081–1085. [PubMed] [Google Scholar]
- 14.Sandhu BS, Gupta R, Sharma J, Singh J, Murthy NS, Sarin SK. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol. 2005;20:599–605. doi: 10.1111/j.1440-1746.2005.03796.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 16.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154–1163. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 17.Keane WF, Alexander SR, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, Huang CC, Kawaguchi Y, Piraino B, et al. Peritoneal dialysis-related peritonitis treatment recommendations: 1996 update. Perit Dial Int. 1996;16:557–573. [PubMed] [Google Scholar]
- 18.Ribeiro TC, Chebli JM, Kondo M, Gaburri PD, Chebli LA, Feldner AC. Spontaneous bacterial peritonitis: How to deal with this life-threatening cirrhosis complication? Ther Clin Risk Manag. 2008;4:919–925. doi: 10.2147/tcrm.s2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keane WF, Swan SK, Grimes I, Humphries TJ. Rabeprazole: pharmacokinetics and tolerability in patients with stable, end-stage renal failure. J Clin Pharmacol. 1999;39:927–933. doi: 10.1177/00912709922008542. [DOI] [PubMed] [Google Scholar]
- 20.Sica DA, Comstock T, Harford A, Eshelman F. Ranitidine pharmacokinetics in continuous ambulatory peritoneal dialysis. Eur J Clin Pharmacol. 1987;32:587–591. doi: 10.1007/BF02455993. [DOI] [PubMed] [Google Scholar]
- 21.Hall JC, Heel KA, Papadimitriou JM, Platell C. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196. doi: 10.1016/s0016-5085(98)70646-8. [DOI] [PubMed] [Google Scholar]
- 22.Heel KA, Hall JC. Peritoneal defences and peritoneum-associated lymphoid tissue. Br J Surg. 1996;83:1031–1036. doi: 10.1002/bjs.1800830804. [DOI] [PubMed] [Google Scholar]
- 23.Carlos D, Spiller F, Souto FO, Trevelin SC, Borges VF, de Freitas A, Alves-Filho JC, Silva JS, Ryffel B, Cunha FQ. Histamine h2 receptor signaling in the pathogenesis of sepsis: studies in a murine diabetes model. J Immunol. 2013;191:1373–1382. doi: 10.4049/jimmunol.1202907. [DOI] [PubMed] [Google Scholar]
- 24.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 25.Jin DC. Current status of dialysis therapy for ESRD patients in Korea. J Korean Med Assoc. 2013;56:562. [Google Scholar]
- 26.Himmelfarb J, Tuttle KR. New therapies for diabetic kidney disease. N Engl J Med. 2013;369:2549–2550. doi: 10.1056/NEJMe1313104. [DOI] [PubMed] [Google Scholar]
- 27.Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Differing outcomes of gram-positive and gram-negative peritonitis. Am J Kidney Dis. 1998;32:623–628. doi: 10.1016/s0272-6386(98)70026-5. [DOI] [PubMed] [Google Scholar]
- 28.Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int. 1997;52:524–529. doi: 10.1038/ki.1997.363. [DOI] [PubMed] [Google Scholar]


