Abstract
AIM: To determine the extent of colorectal cancer (CRC) mortality and the association between demographic characteristics and CRC mortality in Inner Mongolia.
METHODS: Data were collected from the Death Registry System, maintained by the Inner Mongolia Centers for Disease Control and Prevention, from 2008 to 2012. Deaths were classified according to the International Classification of Disease, 10th Revision. Years of life lost, average years of life lost (AYLL), and mortality were calculated over the five years between 2008 and 2012. A conditional logistic regression model was used to analyze the association between marital status, occupational status, education level, area of residence, and the risk of CRC.
RESULTS: The AYLL of CRC was 17.39 years. The average mortality of CRC was 5.6/100000. People living in urban areas and having a higher education level had a significantly higher risk of CRC (OR = 1.74 and 95%CI: 1.29-2.35, P < 0.001 and OR = 2.39, 95%CI: 1.76-3.25, P < 0.001, respectively). People who were employed had a lower risk of CRC (OR = 0.64, 95%CI: 0.48-0.86, P = 0.003). The mortality of CRC was positively correlated with the education level (P < 0.001). No statistically significant association was observed between marital status and CRC risk (P = 0.259).
CONCLUSION: Living in urban areas, higher education level and unemployment are associated with CRC mortality in Inner Mongolia.
Keywords: Colorectal cancer, Average years of life lost, Mortality, Education level
Core tip: In this article, we used the years of life lost (YLL), average years of life lost (AYLL) and mortality to measure the severity of colorectal cancer (CRC) death. The YLL and AYLL can directly reflect the severity of CRC to life lost. Some demographic characteristics such as marital status, occupation, education level and area of residence, which may be risk factors for CRC, are explored. As a result of this study, living in urban areas, having a higher education level and unemployment are risk factors for CRC mortality.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide and ranks third in men and second in women in terms of mortality, with 608700 deaths estimated to have occurred in 2008, accounting for 8% of all cancer deaths[1]. The highest mortality rates in both sexes are estimated in Central and Eastern Europe (20.3 per 100000 for men, 12.1 per 100000 for women), and the lowest in Middle Africa (3.5 and 2.7, respectively)[2]. The mortality rates of CRC in Africa and Asia are lower as compared to those reported in Europe, North America, New Zealand and Australia[1]. In Asia, higher rates of CRC are observed in Japan and China[3]. Among the Chinese, CRC mortality rates rank fifth among men and fourth among women[4]. In the last few decades, the mortality rates of CRC have increased markedly in China[5].
Early in the last century, the relationship between CRC and rural/urban differences and education level have been extensively studied[6,7], but the results obtained are inconclusive. For example, in the United States, Asians and Pacific Islanders who reside in rural areas are at a greater death risk of CRC than those who reside in urban areas. However, black men who live in urban areas of the United States have a higher risk of CRC than those in rural areas[6]. Similarly, the relation between education level and CRC mortality is controversial[8,9]. A cohort study showed that a lower education level is significantly associated with the risk of CRC mortality[6], but another study[8] found no evidence of this association.
Although the associations between these characteristics and CRC have been investigated by many studies[10], to date, no similar study has been conducted in Inner Mongolia. Therefore, the aim of this study was to examine the extent of CRC mortality, and the associations between demographic characteristics and CRC mortality in Inner Mongolia.
MATERIALS AND METHODS
Data source
This study was conducted from January 2008 to December 2012 in Inner Mongolia. Data were collected through the Death Registry System (DRS). DRS uses a multistage cluster probability sampling strategy with stratification according to east, central and west China, the local gross domestic product and proportion of rural dwellers, the total population of local areas. For the present study, data from eight monitoring points were used. Five of these were from the DRS established by the Chinese Ministry of Health, and another three were from Inner Mongolia established by the Inner Mongolia CDC. The eight monitoring points are divided into two regions for our study: the Eastern region (including Yakeshi City, Kailu County and Bairin Youqi) and the Other region (including Sonid Youqi, Muslims District, Tumd Youqi, Ejin Horo Qi, and Linhe District). The annual average population of eight monitoring points was 2.4 million, accounting for about 10% of the total population of Inner Mongolia.
Population data between 2008 and 2012 were obtained from the Inner Mongolia CDC to calculate CRC mortality rates. Death data were categorized based on the Tenth Revision of the International Classification of Diseases. Death data with CRC as the underlying cause of death were classified as “CRC deaths”, and deaths due to diseases of the circulatory system (DCS) served as controls in multiple analyses. Categories of CRC death included malignant neoplasms of the colon (C18), malignant neoplasms of the rectosigmoid junction (C19), and malignant neoplasms of the rectum (C20). Categories classified as DCS death included ischemic heart diseases (I20-I25) and cerebrovascular diseases (I60-I69).
Diagnostic methods included pathological diagnosis, clinical diagnosis, laboratory examination, surgical diagnosis, and postmortem examination. Clinical diagnosis included imaging diagnosis, the diagnosis based on pathological anatomy, and pathophysiological diagnosis. Laboratory examination included physical, immunological, blood, biochemical, and genetic tests. Surgical diagnosis and postmortem examination were among the pathology methods. Pathological diagnosis can be provided for the CRC deaths. Clinical diagnosis and laboratory examination can be provided for the DCS deaths. Hospitals in Inner Mongolia at which diagnoses were made were divided into four levels: provincial, municipal, county, and township.
Statistical analysis
We calculated the percentages of CRC and DCS deaths diagnosed by the various categories of methods and at different levels of hospitals in each of the monitoring points during the five-year period under analysis. We computed the years of life lost (YLL) and average years of life lost (AYLL) due to CRC in each year and region. The AYLL is the average of the difference between the expected age and the actual age at death due to cancer. CRC mortality (per 100000) and corresponding 95%CI were calculated for the two regions. The χ2 test was used to examine differences between regions and years in CRC mortality. Conditional logistic regression models were applied to analyze the effect of socio-demographic characteristics. Odds ratio (OR) and corresponding 95%CI were calculated. CRC and DCS deaths were matched for age, sex, and region for logistic regression analysis. The variables selected from the DRS included marital status, occupation status, education level, and area of residence. Marital status was divided into married and unmarried, with unmarried as the reference group. Occupation status was divided into employment and unemployment, with unemployment as the reference group. Education level was divided into illiterate and primary school education, and middle school or higher, with illiterate and primary school as the reference group. Area of residence was divided into urban and rural, with rural as the reference group. In the models, OR > 1.0 designated an increased risk and OR < 1.0 indicated a protective factor. Education was further divided into four levels, namely, no school education, primary school, middle school, and university or higher. Average death age was calculated for each level. The χ2 test for trend was used to examine the differences between education levels in terms of CRC death. Finally, we used the χ2 test to examine differences according to occupational and marital status. Statistical significance level was set at P ≤ 0.05 (two-sided). All statistical analyses were performed using SPSS 13.0.
RESULTS
The percentages of CRC and DCS deaths diagnosed by different diagnostic methods and at different levels of hospitals are shown in Table 1. There were 643 CRC deaths recorded by the eight monitoring points of Inner Mongolia between 2008 and 2012. CRC diagnosis was established by pathological diagnosis in 100% of the cases. Laboratory examination and clinical diagnosis were used to diagnose DCS in 98% of the cases. Diagnosis was established in provincial, municipal, county and township level hospitals for 100% of CRC deaths and 100% of DCS deaths.
Table 1.
Percentages of colorectal cancer deaths in Inner Mongolia between 2008 and 2012 n (%)
| CRC | DCS | |
| Diagnostic method | ||
| Clinical and laboratory examinations | 256 (39.8) | 436 (67.8) |
| Pathology | 176 (27.4) | 0 (0.0) |
| Clinical | 131 (20.4) | 194 (30.2) |
| Surgery | 70 (10.9) | 0 (0.0) |
| Postmortem examination | 9 (1.4) | 13 (2.0) |
| Unknown | 1 (0.2) | 0 (0.0) |
| Highest diagnostic institution | ||
| Provincial hospital | 209 (32.5) | 94 (14.6) |
| Municipal hospital | 295 (45.9) | 317 (49.3) |
| County level hospital | 135 (21.0) | 230 (35.8) |
| Township level hospital | 4 (0.6) | 2 (0.3) |
CRC: Colorectal cancer; DCS: Diseases of the circulatory system.
The number of CRC deaths was 391 for men and 252 for women. Age at death ranged between 15 and 91 years with an average age of 65.7 years. DCS deaths were matched to CRC deaths on age and sex (391 men and 252 women; aged 14-91 years, average age 65.7 years).
Table 2 shows AYLL and mortality for CRC in the Eastern region and Other region by year. We observed a total of 3059.5 YLL in the Eastern region and 4691.1 years in the Other region, corresponding to an overall AYLL difference of 2.5 years between the two regions. In the Eastern region, average CRC mortality was 5.2/100000 (95%CI: 4.6-5.9), and there were no significant differences in CRC mortality across the five years included in the study (χ2 = 6.9, P = 0.14). In the Other region, mortality was 5.8/100000 (95%CI: 5.2-6.4) (χ2 = 5.9, P = 0.22). Thus, the data from the five years for each region were merged for further analysis. As no significant differences were found in average mortality between the two regions (χ2 = 1.7, P = 0.19), data from the two regions were merged further.
Table 2.
Years of life lost, average years of life lost and mortality for colorectal cancer in two regions of Inner Mongolia between 2008 and 2012
| Total |
Eastern |
Other |
|||||||
| YLL | AYLL | Mortality (1/105) | YLL | AYLL | Mortality (1/105) | YLL | AYLL | Mortality (1/105) | |
| Annual average | 7737.1 | 17.39 | 5.57 (5.14-6.00) | 3059.5 | 16.63 | 5.23 (4.59-5.88) | 4691.1 | 14.13 | 5.82 (5.24-6.39) |
| 2008 | 1269.3 | 16.27 | 6.15 (5.03-7.26) | 577.7 | 16.05 | 5.24 (3.82-6.67) | 689.0 | 16.41 | 7.13 (5.40-8.86) |
| 2009 | 1393.4 | 16.79 | 5.11 (4.21-6.02) | 519.2 | 16.75 | 4.32 (3.03-5.61) | 896.2 | 13.38 | 5.68 (4.43-6.93) |
| 2010 | 1316.3 | 17.32 | 4.59 (3.74-5.45) | 578.0 | 18.65 | 4.31 (3.02-5.60) | 734.4 | 16.32 | 4.80 (3.65-5.95) |
| 2011 | 1629.0 | 15.51 | 5.98 (5.00-6.95) | 664.3 | 13.84 | 6.49 (4.85-8.14) | 953.2 | 16.72 | 5.66 (4.45-6.87) |
| 2012 | 1813.5 | 17.61 | 6.14 (5.16-7.12) | 703.0 | 18.50 | 5.95 (4.38-7.52) | 1119.1 | 12.86 | 6.25 (4.99-7.51) |
YLL: Years of life lost; AYLL: Average years of life lost.
Table 3 shows the results of multivariate analyses of socio-demographic characteristics and the risk of CRC in the two regions separately. Employment was associated with a decreased likelihood of CRC death, while a higher education level and urban area of residence increased the likelihood of CRC mortality. There was no statistically significant relationship between marital status and CRC death.
Table 3.
Conditional logistic regression analysis of socio-demographic characteristics for colorectal cancer mortality
| Characteristic | P | OR | 95%CI |
| Marital status | 0.259 | ||
| Unmarried | |||
| Married | |||
| Occupation | 0.003 | ||
| Unemployment | 1 | ||
| Employment | 0.64 | 0.48-0.86 | |
| Education level | < 0.001 | ||
| Illiterate and primary school | 1 | ||
| Middle school and higher | 2.39 | 1.76-3.25 | |
| Area of residence | < 0.001 | ||
| Rural | 1 | ||
| Urban | 1.74 | 1.29-2.35 |
To further explore the effects of education, this variable was divided into four levels. The χ2 test for trend showed a significantly increasing trend of CRC mortality according to education level in the two regions (χ2 = 65.6, P < 0.001). Figure 1 shows average CRC mortality and average death age for each of the four education levels in the selected regions. In contrast with CRC mortality, average death age decreased with education level.
Figure 1.
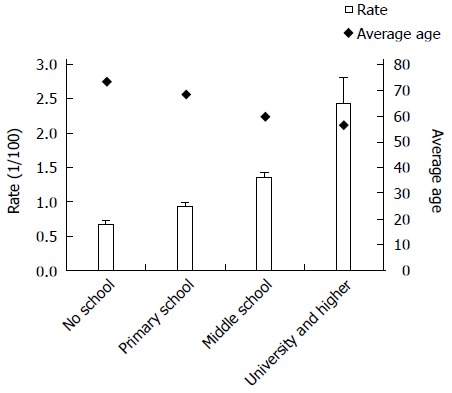
Colorectal cancer rate and average death age among different education levels in Inner Mongolia (2008-2012).
The proportion of married individuals was 76.1% and 85.1% in the unemployment and employment groups, respectively. There was a significant difference (χ2 = 8.2, P < 0.01) between the two groups.
DISCUSSION
Average CRC mortality was 5.6/100000 in Inner Mongolia between 2008 and 2012. This figure is lower than the recently reported CRC mortality in economically developed Asian countries such as Korea[11] and Japan[12], but higher compared to some less developed countries[2]. Corresponding to the mortality, the AYLL of CRC was 17.39 years, higher than that in Japan[12]. We have previously reported the negative contribution of cancer to life expectancy by using potential YLL to estimate the impact of cancers, such as stomach cancer[13] and breast cancer[14], on life expectancy in Japan. In this study, AYLL was used to estimate the effect of CRC on life expectancy. Both YLL and AYLL reflect premature death. Unlike mortality rate, which is usually higher in the elderly, YLL and AYLL give greater weight to diseases that result in younger deaths and less weight to those affecting the elderly[12]. In Inner Mongolia, CRC led to lower mortality than in Japan, but showed higher AYLL. The burden of premature death due to CRC may be more serious in Inner Mongolia.
The results of our study suggest that CRC mortality was higher in urban areas. This finding is consistent with a study of black men[6]. In Western countries, however, most studies revealed a different pattern, and attributed lower CRC mortality in urban areas to higher rates of CRC screening. Some studies showed that CRC screening significantly reduces CRC mortality[15], and the rate of CRC screening is higher in urban than rural areas in Western countries[16]. In Inner Mongolia, both urban and rural areas have few CRC screening programs; therefore, the lack of CRC screening programs may be one of reasons for higher mortality in urban areas. Furthermore, rural areas have lower CRC mortality because people living in rural areas tend to have a better environment, engage in farm work, and eat more fresh fruits and vegetables. Industrial pollution[17], physical inactivity[18] and low consumption of fruits and vegetables[19] were associated with a higher risk of CRC in urban areas.
CRC mortality was found to be the highest among the best educated segment of the population. This finding is related to higher mortality in urban areas, as the urban population was more likely to have received good education. To date, we have found only one study showing an association between a higher education level and an increased risk of CRC for men[8]. Most other existing studies are at odds with our result[9,20], reporting an inverse association between education level and total mortality[21]. In those studies, people with a lower education level suffered a higher burden of cancer[22]. Because the age was matched in multivariate analysis, considering that the age as a confounding factor may affect education level, the average death age is marked out in Figure 1. Clearly, education level shows a different trend compared to mortality of CRC, indicating that the association between education level and CRC mortality is not affected by age factors.
Our results suggest that CRC mortality was higher in the unemployed population. This finding is consistent with some previous studies. Unemployed people have more mental strain and higher standardized mortality rates[23]. In addition, occupational physical activity may reduce the risk of CRC[24]. Furthermore, our results suggest that the proportion of married people was lower in the unemployed group than in the employed group. Married populations are more likely to be diagnosed at earlier stages[25] and receive recommended treatment[26]. Marriage was associated with better outcomes of CRC for both men and women[27].
Several studies have suggested the relationships between urban areas[6], educational levels[10] used as social-economic status indicators, and cancer mortality. Some studies showed that a higher education level can increase opportunities for income security[28]. Living in urban areas and having a higher education level tend to be associated with meat consumption. Meat consumption, especially beef and mutton, was significantly higher in Inner Mongolia (percentages of meat consumption in terms of money value were 15% for beef and 25% for mutton) than other provinces such as Shandong province (beef: 12%, mutton: 7%) and Sichuan province (beef: 11%, mutton: 2%)[29]. The consumption of fish was higher in Western countries. A study from France has shown that the consumption of fish was more frequent among highly educated people[30]. Many studies have found that total fish consumption is not associated with CRC[31], or constitutes a protective factor for CRC[32]. The different types of meat consumption might be one of reasons that CRC mortality was different between Inner Mongolia and Western countries in urban areas and related to a higher education level.
In conclusion, the findings of this study suggest that living in urban areas and having a higher education level are risk factors for CRC mortality in Inner Mongolia, contradicting most previous reports. The effect of occupation status on CRC mortality is consistent with other studies. These results suggest that prevention efforts in Inner Mongolia should focus on populations living in urban areas, having a higher education level and affected by unemployment.
COMMENTS
Background
Colorectal cancer (CRC) is one of the most common cancers worldwide and ranks third in men and second in women in terms of mortality, with 608700 deaths estimated to have occurred in 2008, accounting for 8% of all cancer deaths.
Research frontiers
Early in the last century, the relationship between CRC and rural/urban differences and education level have been extensively studied, but the results obtained are inconclusive.
Innovations and breakthroughs
In this article, authors used the years of life lost (YLL), average years of life lost (AYLL) and mortality to measure the severity of CRC death. The YLL and AYLL can directly reflect the severity of CRC to life lost. Some demographic characteristics such as marital status, occupation, education level and area of residence, which may be risk factors for CRC, are explored.
Peer review
An interesting study, which shows that living in urban areas, higher education level and unemployment are associated with CRC mortality in Inner Mongolia.
Footnotes
Supported by Inner Mongolia Autonomous Region Colleges and Universities of Science and Technology Research Projects, No. NJZY13415; Inner Mongolia Medical University Technology Million Project, No. NY2011BW006; Natural Science Foundation of Inner Mongolia in China, No. 2013MS1124
P- Reviewer: Doll D S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh B, Talole SD, Dikshit R. A case-control study on diet and colorectal cancer from Mumbai, India. Cancer Epidemiol. 2009;33:189–193. doi: 10.1016/j.canep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin SS, Richards TB, Thompson T, Miller BA, VanEenwyk J, Goodman MT, Sherman RL. Rural/nonrural differences in colorectal cancer incidence in the United States, 1998-2001. Cancer. 2006;107:1181–1188. doi: 10.1002/cncr.22015. [DOI] [PubMed] [Google Scholar]
- 7.Faggiano F, Partanen T, Kogevinas M, Boffetta P. Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ. 1997;(138):65–176. [PubMed] [Google Scholar]
- 8.Torres-Cintrón M, Ortiz AP, Ortiz-Ortiz KJ, Figueroa-Vallés NR, Pérez-Irizarry J, Díaz-Medina G, De la Torre-Feliciano T, Suárez-Pérez E. Using a socioeconomic position index to assess disparities in cancer incidence and mortality, Puerto Rico, 1995-2004. Prev Chronic Dis. 2012;9:E15. [PMC free article] [PubMed] [Google Scholar]
- 9.Steenland K, Henley J, Thun M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959-1996. Am J Epidemiol. 2002;156:11–21. doi: 10.1093/aje/kwf001. [DOI] [PubMed] [Google Scholar]
- 10.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 11.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham TM, Fujino Y, Matsuda S, Yoshimura T. Premature mortality due to cancer in Japan, 1995 and 2005. Int J Cancer. 2010;127:190–194. doi: 10.1002/ijc.25021. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Misumi J, Shimaoka A, Aoki K, Kono A. Stomach cancer-related mortality rate is higher in young Japanese women than in men. Public Health. 2002;116:39–44. doi: 10.1038/sj/ph/1900811. [DOI] [PubMed] [Google Scholar]
- 14.Kono A, Misumi J, Misumi J. The time trend of breast cancer mortality in japan. Arch Gynecol Obstet. 2005;272:187–190. doi: 10.1007/s00404-004-0719-6. [DOI] [PubMed] [Google Scholar]
- 15.Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21:182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 17.López-Abente G, García-Pérez J, Fernández-Navarro P, Boldo E, Ramis R. Colorectal cancer mortality and industrial pollution in Spain. BMC Public Health. 2012;12:589. doi: 10.1186/1471-2458-12-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison DS, Parr CL, Lam TH, Ueshima H, Kim HC, Jee SH, Murakami Y, Giles G, Fang X, Barzi F, et al. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: analysis of data from the Asia-Pacific Cohort Studies Collaboration. Asian Pac J Cancer Prev. 2013;14:1083–1087. doi: 10.7314/apjcp.2013.14.2.1083. [DOI] [PubMed] [Google Scholar]
- 19.Safari A, Shariff ZM, Kandiah M, Rashidkhani B, Fereidooni F. Dietary patterns and risk of colorectal cancer in Tehran Province: a case-control study. BMC Public Health. 2013;13:222. doi: 10.1186/1471-2458-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menvielle G, Kunst AE, Stirbu I, Strand BH, Borrell C, Regidor E, Leclerc A, Esnaola S, Bopp M, Lundberg O, et al. Educational differences in cancer mortality among women and men: a gender pattern that differs across Europe. Br J Cancer. 2008;98:1012–1019. doi: 10.1038/sj.bjc.6604274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo V, Mackenbach JP, Ezzati M, Menvielle G, Kunst AE, Rohrmann S, Kaaks R, Teucher B, Boeing H, Bergmann MM, et al. Social inequalities and mortality in Europe--results from a large multi-national cohort. PLoS One. 2012;7:e39013. doi: 10.1371/journal.pone.0039013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puigpinós R, Borrell C, Antunes JL, Azlor E, Pasarín MI, Serral G, Pons-Vigués M, Rodríguez-Sanz M, Fernández E. Trends in socioeconomic inequalities in cancer mortality in Barcelona: 1992-2003. BMC Public Health. 2009;9:35. doi: 10.1186/1471-2458-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin RL, Shah CP, Svoboda TJ. The impact of unemployment on health: a review of the evidence. CMAJ. 1995;153:529–540. [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 25.Chang SM, Barker FG. Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975–1984. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125–3130. [PubMed] [Google Scholar]
- 27.Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417–422. doi: 10.1016/j.canep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Parton KA, Zhou Z-Y, Cox R. At-home meat consumption in china: An empirical study. Aust J Agr Resour Ec. 2009;53:485–501. [Google Scholar]
- 30.Perrin AE, Simon C, Hedelin G, Arveiler D, Schaffer P, Schlienger JL. Ten-year trends of dietary intake in a middle-aged French population: relationship with educational level. Eur J Clin Nutr. 2002;56:393–401. doi: 10.1038/sj.ejcn.1601322. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara Y, Kuriyama S, Kakizaki M, Nagai M, Ohmori-Matsuda K, Sone T, Hozawa A, Nishino Y, Tsuji I. Fish consumption and the risk of colorectal cancer: the Ohsaki Cohort Study. Br J Cancer. 2009;101:849–854. doi: 10.1038/sj.bjc.6605217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]


