Abstract
AIM: To assess the effectiveness of endoscopic full-thickness resection (EFR) and laparoscopic surgery in the treatment of gastric stromal tumors arising from the muscularis propria.
METHODS: Out of 62 gastric stromal tumors arising from the muscularis propria, each > 1.5 cm in diameter, 32 were removed by EFR, and 30 were removed by laparoscopic surgery. The tumor expression of CD34, CD117, Dog-1, S-100, and SMA was assessed immunohistochemically. The operative time, complete resection rate, length of hospital stay, incidence of complications, and recurrence rate were compared between the two groups. Continuous data were compared using independent samples t-tests, and categorical data were compared using χ2 tests.
RESULTS: The 32 gastric stromal tumors treated by EFR and the 30 treated by laparoscopic surgery showed similar operative time [20-155 min (mean, 78.5 ± 30.1 min) vs 50-120 min (mean, 80.9 ± 46.7 min), P > 0.05], complete resection rate (100% vs 93.3%, P > 0.05), and length of hospital stay [4-10 d (mean, 5.9 ± 1.4 d) vs 4-19 d (mean, 8.9 ± 3.2 d), P >0.05]. None of the patients treated by EFR experienced complications, whereas two patients treated by laparoscopy required a conversion to laparotomy, and one patient had postoperative gastroparesis. No recurrences were observed in either group. Immunohistochemical staining showed that of the 62 gastric stromal tumors diagnosed by gastroscopy and endoscopic ultrasound, six were leiomyomas (SMA-positive), one was a schwannoglioma (S-100 positive), and the remaining 55 were stromal tumors.
CONCLUSION: Some gastric stromal tumors arising from the muscularis propria can be completely removed by EFR. EFR could likely replace surgical or laparoscopic procedures for the removal of gastric stromal tumors.
Keywords: Gastric stromal tumors, Treatment, Endoscopy, Muscularis propria, Full-thickness resection
Core tip: We used endoscopic full-thickness resection (EFR) to remove gastric stromal tumors arising from the muscularis propria. Out of 62 gastric stromal tumors, each > 1.5 cm in diameter, we found that the 32 gastric stromal tumors treated by EFR and the 30 treated by laparoscopic surgery showed similar operative time and complete resection rate. None of the patients treated by EFR experienced complications, whereas two patients treated by laparoscopy required a conversion to laparotomy and one patient had postoperative gastroparesis. No recurrences were observed in either group. EFR could replace certain surgical or laparoscopic procedures for the removal of gastric stromal tumors.
INTRODUCTION
Gastric stromal tumors typically occur in the fundus, anterior wall of the gastric body, or anterior wall of the gastric antrum. These tumors are the most common type of gastrointestinal tissue-derived mesenchymal tumors. Based on their origin in the stomach wall, gastric stromal tumors can be divided into stromal tumors arising from the muscularis mucosa and those arising from the muscularis propria. Because stromal tumors arising from the muscularis mucosa are located superficially, their endoscopic resection or ligation is not difficult and has therefore been used extensively in clinical practice. However, stromal tumors arising from the muscularis propria are located in deeper layers, especially those that do not grow within cavities. Endoscopic resection may easily lead to perforation, and tumor excision is often incomplete. Hence, stromal tumors arising from the muscularis propria are often considered contraindications to endoscopic resection, and these tumors are usually removed by surgical or laparoscopic procedures[1-5]. In recent years, we have used endoscopic full-thickness resection (EFR) to remove gastric stromal tumors arising from the muscularis propria and have achieved satisfactory results.
MATERIALS AND METHODS
Patients
A total of 62 patients with gastric stromal tumors arising from the muscularis propria were retrospectively analyzed after their diagnosis based on gastroscopy and endoscopic ultrasound. These patients were treated at the Yantai Yuhuangding Hospital Affiliated to Medical College of Qingdao University, China between January 2010 and October 2013. The 62 patients consisted of 34 males and 28 females, ranging in age between 25 and 69 years, with a mean age of 43.4 years. Overall, 21 tumors were present in the gastric antrum; 27 were in the gastric body; and 14 were in the gastric fundus. Tumor sizes ranged from 1.5 to 5.0 cm. All patients had a single tumor, and metastasis was not found in any patient during computed tomography (CT) examination. Before EFR or laparoscopic surgery, all patients underwent routine blood tests for coagulation enzymes and liver and kidney function, in addition to electrocardiography, abdominal CT scan, and other tests. All patients and their families were informed of the benefits and risks of EFR and laparoscopic surgery, chose their treatment, and provided written informed consent. The EFR group consisted of 32 patients, with tumor sizes 1.5-5.0 cm (mean, 3.7 cm); the laparoscopy group consisted of 30 patients, with tumor sizes 2.8-5.0 cm (mean, 3.9 cm).
Instruments
The instruments used included the following: an Olympus GIF-Q260J gastroscope (Olympus, Japan), a D-201-11304 transparent cap (Olympus, Japan), a KD-620LR hook knife (Olympus, Japan), a KD-1L-1 needle knife (Olympus, Japan), an NM-200L-0525 injection needle (Olympus, Japan), an FD-410LR hot biopsy forceps (Olympus, Japan), a KD-611L IT knife (Olympus, Japan), AS-1-S and ASJ-1-S snares (Cook Company, United States), an HX-610-90 (Olympus, Japan), Boston Resolution hemostat (Boston Company, United States), an HX-600-135 (Olympus, Japan), an ERBE VIO 200S high-frequency electrosurgical unit and ERBE APC2 argon plasma coagulator (Erbe Company, Germany), an SBQ 80 HY linear stapler (Johnson and Johnson, United States), and an Olympus OEV191H laparoscope (Olympus, Japan).
EFR method
A transparent cap was mounted on the end of the gastroscope before EFR. Following intravenous anesthesia with propofol, argon plasma coagulation was used to mark the edge of the stromal tumor. The marked submucosal positions were each injected with 2-3 mL of a solution consisting of 2-3 mL indigo carmine, 1 mL epinephrine, and 100 mL saline. A hook knife was used to precut the surrounding mucosa and submucosa along the marked points and to expose the stromal tumor. A hook knife or IT knife was used to isolate the tumor body along the capsule from the muscularis propria down to the serosal layer. The serosa was cut along the edge of the tumor; in most cases, the serosa adhered tightly to the tumor body, making it impossible to remove the tumor directly with an IT knife. Therefore, a needle knife or hook knife was used to penetrate the serosa, resulting in an “artificial perforation.” The liquid in the gastric cavity was fully absorbed, and an IT or hook knife was used to cut the serosa along the edge of the tumor and to remove the tumor completely. Under endoscopic guidance, the incisions on the gastric body from the two ends to the middle were fully closed with titanium clips, and the gastric wound was sealed. For wounds that were too large to seal directly, negative pressure was applied to suck the omentum into the gastric cavity, and titanium clips were used to seal the wound by clipping the omentum to the gastric mucosa (Figure 1). The same endoscopist performed all EFR procedures.
Figure 1.
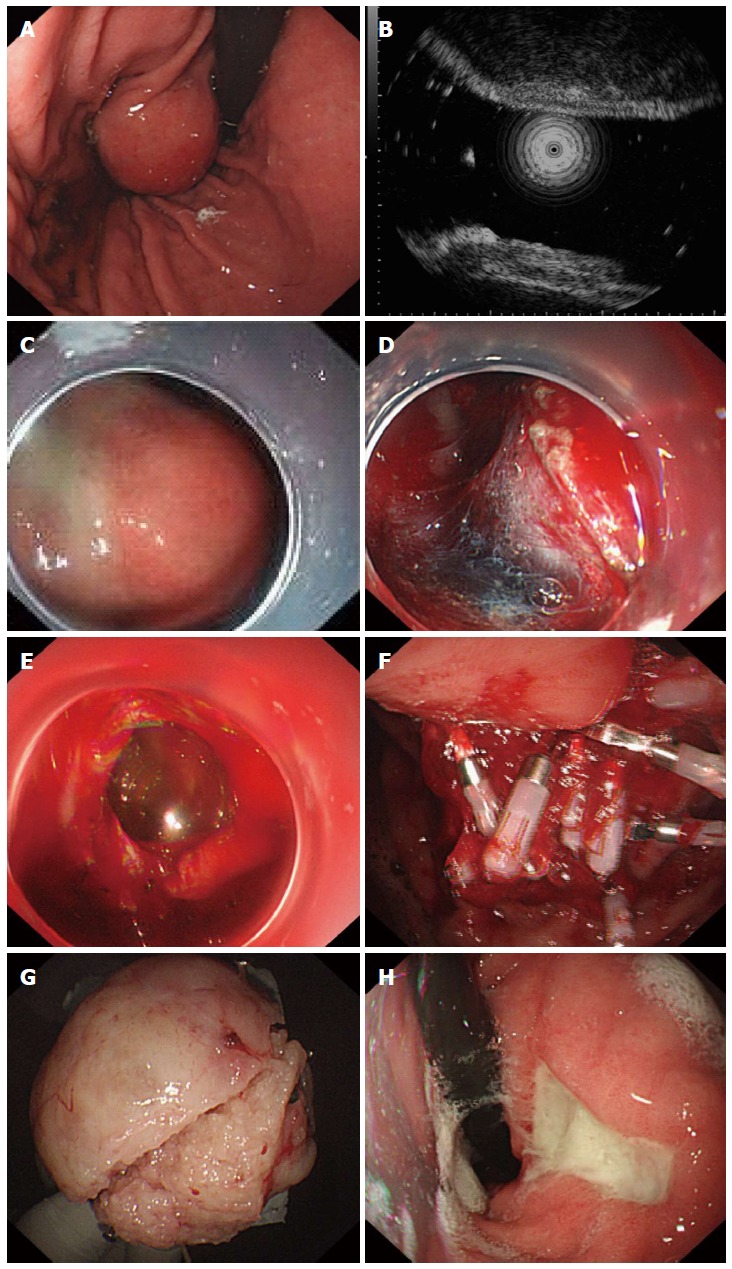
Endoscopic full-thickness resection treatment of gastric stromal tumors arising from the muscularis propria. A: A protruding submucosal lesion in the gastric body; B: Endoscopic ultrasound showing that the lesion arose from the muscularis propria; C: Submucosal injection of saline containing adrenaline and indigo carmine; D: Application of the IT knife to isolate the stromal tumor along its periphery; E: An “artificial perforation” observed after stromal tumor resection, sealed using titanium clips; F: Sealing of the perforation with multiple titanium clips; G: Resected tumor with the mucosa removed (5 cm in diameter); H: View 72 d after the operation, showing that the perforation healed well, with only ulcer residue remaining.
Method for laparoscopic surgery
Laparoscopic resections of gastric stromal tumors were performed under tracheal intubation and general anesthesia. Patients were placed in the supine position with their legs apart. The umbilicus was punctured, and pneumoperitoneum was established while maintaining the intra-abdominal pressure at 12-15 mmHg. A 30° lens was placed into the incision approximately 10 mm above the umbilicus, and a 12-mm trocar was placed horizontally at the main operating site 2 cm above the umbilicus along the left clavicular midline. Finally, 5-mm trocars were placed at auxiliary operating sites located 2 cm above the umbilicus along the right clavicular midline, below the costal margin along the left clavicular midline, and below the costal margin along the right clavicular midline. Based on the location and growth of the gastric stromal tumors, a wedge resection, transgastric tumor-everting resection, sleeve gastrectomy, or partial gastrectomy was performed for patients undergoing laparoscopic resections (Figure 2). The same surgeon performed all laparoscopic operations.
Figure 2.
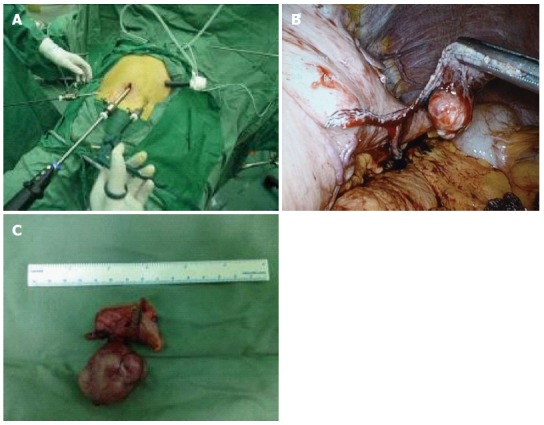
Laparoscopic resection of gastric stromal tumors. A: Layout of instruments for laparoscopic surgery; B: Laparoscopic resection of gastric stromal tumor; C: Removed tumor (4 cm in diameter).
Sample processing
Resected tumors were fixed with neutral formalin and sent for pathological examination; the samples were immunohistochemically stained for CD34, CD117, Dog-1, S-100, and SMA (smooth muscle antibody)[6-10].
Postoperative treatment
Patients with an artificial perforation and significant pneumoperitoneum underwent a puncture of the right upper quadrant of the abdomen with an abdominal puncture needle to reduce bloating, during or after EFR. After EFR, the patients were placed in a semi-supine position and subjected to fasting and gastrointestinal decompression while being closely monitored to determine whether they experienced any abdominal pain, bloating, or peritoneal irritation. Three days after EFR, oral diatrizoate was administered to determine whether contrast agent extravasation and gastric motility had occurred. In addition, an ultrasound examination was performed to determine whether any effusions were present in the abdominal and pelvic cavities. One month after the EFR or laparoscopic resection, an endoscopic examination was performed to observe wound healing and to determine whether there were residual or recurrent tumors.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Continuous data were compared using independent samples t-tests, and categorical data, using χ2 tests. SPSS for Windows Version 17.0 software was used for all statistical analyses, with the level of significance set at P < 0.05.
RESULTS
Hospital stay
The length of hospital stay in the EFR group ranged from 4 to 10 d, with a mean of 5.9 ± 1.4 d. In comparison, the length of hospital stay in the laparoscopy group ranged from 4 to 19 d, with a mean of 8.9 ± 3.2 d. There was no significant difference between the two groups (P > 0.05).
Complete resection rate and operative time
In the EFR group, all 32 of the stromal tumors arising from the muscularis propria were removed successfully in one operation. The complete resection rate was 100%. All perforation wounds were sealed with titanium clips. Operative times ranged from 20 to 155 min, with a mean of 78.5 ± 30.1 min. In the laparoscopic surgery group, 28 of the 30 stromal tumors arising from the muscularis propria were removed laparoscopically; the operative times ranged from 50 to 120 min, with a mean of 80.9 ± 46.7 min. In two patients, the tumors were located near the cardia in the posterior wall of the gastric fundus; because of operational difficulties, the surgeries were converted to open laparotomy half-way through the operation. The complete resection rate was 93.3%. There were no significant between-group differences in the complete resection rate or operative time (P > 0.05).
Complications
In the EFR group, angiography of the upper gastrointestinal tract using diatrizoate 3 d after the operation showed no leakage of contrast agent in any patient. Postoperative reexamination showed good wound healing. There were no complications, such as bleeding, signs of peritonitis, and/or abdominal abscesses, in any patient. In the laparoscopy group, one patient experienced gastroparesis, which was alleviated after conservative treatment. The complication rate was 3.3%, which was not significantly different from that of the EFR group (P > 0.05).
Recurrence rate
Gastroscopy 1 month after EFR or laparoscopic surgery showed good wound healing in both groups, with no residual or recurrent tumors, for a recurrence rate of 0% in both groups.
Immunohistochemical staining
Of the 62 stromal tumors arising from the muscularis propria, 49 (79.0%) were positive for CD34; 50 (80.6%) for CD117; 51 (82.2%) for Dog-1; 1 (1.6%) for S-100; and 6 (9.7%) for SMA. Thus, of these 62 gastric stromal tumors, 6 were leiomyomas, as shown by SMA-positive expression; one was a schwannoglioma, as shown by S-100 positive expression; and 55 were stromal tumors, as shown by CD34 and/or CD117, Dog-1 positive expression.
DISCUSSION
Gastric stromal tumors are commonly found upon gastroscopic examination. Because of their potential malignant tendencies, the resection of gastric stromal tumors is recommended[11-15]. Because stromal tumors arising from the muscularis mucosa are located superficially, their endoscopic resection or ligation is not difficult; thus, these procedures are extensively used in clinical practice. However, stromal tumors arising from the muscularis propria are located within deeper layers, especially those that do not grow within cavities. In this case, endoscopic resection can easily lead to perforation, and the tumor excisions are often incomplete. Hence, stromal tumors arising from the muscularis propria may be considered a contraindication for endoscopic resection and should therefore be removed surgically or laparoscopically, especially when they are larger than 2 cm in diameter[16-19]. In recent years, based on endoscopic submucosal dissection and endoscopic submucosal excavation and due to improvements in the application of titanium clips under endoscopy, EFR treatment of gastrointestinal tumors arising from the muscularis propria has become possible.
Artificial perforations were necessary in all 32 patients with gastric stromal tumors who were treated by EFR. Titanium clips were used to seal the wounds; abdominal punctures were applied to relieve intra-abdominal pressure; and conservative methods were used during the postoperative care. The key to preventing complications was the proper use of titanium clips to seal the wounds under endoscopy. Based on our experience, wounds from large perforations should be sutured from both ends toward the middle; in some cases, some normal mucosa at the periphery may require suturing to reduce the wound size. Successful treatment using EFR required a successful repair of the perforation, thus allowing us to avoid the need for additional surgical repairs or postoperative peritonitis[20,21]. The most common method for repairing perforations was titanium clip repair[22,23]. For small perforations, one or a few titanium clips were sufficient. For larger perforations, the limited span of titanium clips required that the air in the gastric lumen be sucked out to narrow the perforation as much as possible before multiple titanium clips were placed. If the perforation is too large for the direct application of titanium clips, an omental patch technique should be used[24,25]. By this method, negative pressure is continuously applied to suck air from the gastric lumen until the adipose tissue outside the gastric wall covers the perforation; only then should the titanium clips be applied. In addition, a nylon string-purse suture technique can be used to suture overly large perforations. During EFR, perforations cause pneumoperitoneum, which blocks the operative field in the stomach and makes endoscopic operations more difficult. Thus, during EFR, abdominal palpation should be performed repeatedly. If abdominal pressure increases, timely exhaustion should be pursued. The puncture site should be located in the right lower quadrant of the abdomen, and a 20 mL injection needle may be used as the puncture needle. After the puncture, the abdomen should be manipulated to exhaust the air, and the puncture needle should be positioned at the puncture site until the wound has completely sealed and the pneumoperitoneum is significantly improved. The puncture needle should be pulled out after confirming that no air continues to flow through it. Other key elements for successful EFR treatment include avoiding the entrance of excessive gastric acid into the abdominal cavity to prevent postoperative infection; achieving hemostasis to avoid repeated rinsing during the excision; completely removing the gas and fluid from the gastric lumen prior to incision of the serosa; performing continuous effective gastrointestinal decompression postoperatively; and administering proton pump inhibitors and antibiotics postoperatively to prevent abdominal infection[26-27]. None of the patients in our EFR group experienced peritonitis or intra-abdominal abscess.
The complete resection rate in our EFR group was 100%, with no recurrence. The operative time and length of hospital stay were similar to those in the laparoscopic surgery group. Two patients in the latter group required a conversion to an open laparotomy because their stromal tumors were located near the cardia in the posterior wall of the gastric fundus, making a laparoscopic approach difficult. Unlike EFR, which is not affected by the location of the tumor, laparoscopic surgery is difficult and limited to stromal tumors located in the posterior gastric wall or cardia. Thus, tumor size, location, and relationship with the cardia should be clearly determined prior to laparoscopy to avoid conversion to open laparotomy during surgery, causing greater trauma and prolonging the patient’s hospital stay.
One patient in the laparoscopy group experienced a complication of postoperative gastroparesis, compared with none in the EFR group, further suggesting an advantage of the minimally invasive EFR method. Another advantage of EFR is its accurate localization of the tumor. Without the assistance of the gastroscope, it can be difficult during laparoscopic surgery to determine the extent of the excision; in such cases, excess normal gastric tissue may be removed. In patients with giant stromal tumors protruding into the gastric lumen, it is difficult for the laparoscope to distinguish the tumor from the serosal layer and to pull and remove the tumor body. Therefore, gastroscope-assisted laparoscopic EFR without a gastroscope may be favorable for treating patients with gastric stromal tumors arising from the muscularis propria.
In conclusion, EFR can completely remove some stromal tumors arising from the muscularis propria. This technique can replace some surgical and laparoscopic procedures, and its application should be recommended further.
COMMENTS
Background
Gastric stromal tumors typically occur in the fundus, anterior wall of the gastric body, or the anterior wall of the gastric antrum. Based on their origin in the stomach wall, gastric stromal tumors can be divided into stromal tumors arising from the muscularis mucosa and those arising from the muscularis propria. Because stromal tumors arising from the muscularis mucosa are located superficially, their endoscopic resection or ligation is not difficult; thus, this technique has been used extensively in clinical practice. However, stromal tumors arising from the muscularis propria are located in deeper layers, especially those that do not grow within cavities. Because endoscopic resection can easily lead to perforation, tumor excision is often incomplete. Hence, stromal tumors arising from the muscularis propria are often considered contraindications to endoscopic resection, and these tumors are usually removed by surgical or laparoscopic procedures.
Research frontiers
In recent years, endoscopic full-thickness resection (EFR) has been used to remove gastric stromal tumors arising from the muscularis propria and has achieved satisfactory results, avoiding surgical or laparoscopic operations.
Innovations and breakthroughs
The 32 gastric stromal tumors treated by EFR and the 30 treated by laparoscopic surgery showed similar operative time, complete resection rate and length of hospital stay. None of the patients treated by EFR experienced complications, whereas two patients treated by laparoscopy required a conversion to laparotomy, and one patient had postoperative gastroparesis. Therefore, EFR was found to be safe and effective for the removal of gastric stromal tumors arising from the muscularis propria.
Applications
With the successful use of EFR in the treatment of gastric stromal tumors arising from the muscularis propria, this study may present a future strategy for therapeutic intervention in the treatment of patients with these tumors.
Terminology
EFR can remove gastric stromal tumors arising from the muscularis propria completely. Although artificial perforation often occurs, these cases can be sutured with a titanium clip.
Peer review
Gastric stromal tumors are the most common type of gastrointestinal stromal tumors. Surgical procedures, including laparotomy and laparoscopic resection, are extensively used in clinical practice. EFR, a new technique to remove these tumors, generates minimal injury to the patient. The results suggest that gastric stromal tumors originating from the muscularis propria could be treated successfully by EFR, and thus, this study has clinical value.
Footnotes
Supported by Natural Science Foundation of Shandong Province, No. ZR2013HM004
P- Reviewers: Franzen T, Sun XD S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Bamboat ZM, Dematteo RP. Updates on the management of gastrointestinal stromal tumors. Surg Oncol Clin N Am. 2012;21:301–316. doi: 10.1016/j.soc.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orsenigo E, Gazzetta P, Palo SD, Tamburini A, Staudacher C. Experience on surgical treatment of gastrointestinal stromal tumor of the stomach. Updates Surg. 2010;62:101–104. doi: 10.1007/s13304-010-0018-7. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327–330. doi: 10.1007/s00268-011-1387-x. [DOI] [PubMed] [Google Scholar]
- 4.Meza JM, Wong SL. Surgical options for advanced/metastatic gastrointestinal stromal tumors. Curr Probl Cancer. 2011;35:283–293. doi: 10.1016/j.currproblcancer.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Grover S, Ashley SW, Raut CP. Small intestine gastrointestinal stromal tumors. Curr Opin Gastroenterol. 2012;28:113–123. doi: 10.1097/MOG.0b013e32834ec154. [DOI] [PubMed] [Google Scholar]
- 6.Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat. 2010;42:135–143. doi: 10.4143/crt.2010.42.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kara T, Serinsoz E, Arpaci RB, Gubur O, Orekici G, Ata A, Colak T, Arican A. Contribution of DOG1 expression to the diagnosis of gastrointestinal stromal tumors. Pathol Res Pract. 2013;209:413–417. doi: 10.1016/j.prp.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 9.Pelz AF, Agaimy A, Daniels M, Evert M, Schulz HU, Lüders P, Müller G, Lasota J, Röpke A, Wieacker P, et al. Gastrointestinal stromal tumor presenting as a rectovaginal mass. Clinicopathologic and molecular-genetic characterization of a rare tumor with a literature review. Hum Pathol. 2011;42:586–593. doi: 10.1016/j.humpath.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Chugh R. Current directions in systemic therapy for gastrointestinal stromal tumors. Curr Probl Cancer. 2011;35:255–270. doi: 10.1016/j.currproblcancer.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Kubota D, Mukaihara K, Yoshida A, Suehara Y, Saito T, Okubo T, Gotoh M, Orita H, Tsuda H, Kaneko K, et al. The prognostic value of pfetin: a validation study in gastrointestinal stromal tumors using a commercially available antibody. Jpn J Clin Oncol. 2013;43:669–675. doi: 10.1093/jjco/hyt057. [DOI] [PubMed] [Google Scholar]
- 12.Lagarde P, Pérot G, Kauffmann A, Brulard C, Dapremont V, Hostein I, Neuville A, Wozniak A, Sciot R, Schöffski P, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:826–838. doi: 10.1158/1078-0432.CCR-11-1610. [DOI] [PubMed] [Google Scholar]
- 13.Orozakunov E, Akyol C, Tantoglu U, Basceken SI, Kayilioglu IS, Bumin CS, Cakmak A. Surgical glove-port single-incision laparoscopic gastric wedge resection of gastrointestinal stromal tumors: initial experience with 2 cases. Surg Laparosc Endosc Percutan Tech. 2013;23:e160–e161. doi: 10.1097/SLE.0b013e31828b891b. [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Eom BW, Yoon H, Ryu KW, Kim YW, Lee JH. Transumbilical single-incision laparoscopic wedge resection for gastric submucosal tumors: technical challenges encountered in initial experience. J Gastric Cancer. 2012;12:173–178. doi: 10.5230/jgc.2012.12.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888–895. doi: 10.1002/jso.21930. [DOI] [PubMed] [Google Scholar]
- 16.Warsi AA, Peyser PM. Laparoscopic resection of gastric GIST and benign gastric tumours: evolution of a new technique. Surg Endosc. 2010;24:72–78. doi: 10.1007/s00464-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 17.Wan P, Yan C, Li C, Yan M, Zhu ZG. Choices of surgical approaches for gastrointestinal stromal tumors of the stomach: laparoscopic versus open resection. Dig Surg. 2012;29:243–250. doi: 10.1159/000341497. [DOI] [PubMed] [Google Scholar]
- 18.Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol. 2009;6:363–371. doi: 10.1038/nrgastro.2009.43. [DOI] [PubMed] [Google Scholar]
- 19.Grotz TE, Donohue JH. Surveillance strategies for gastrointestinal stromal tumors. J Surg Oncol. 2011;104:921–927. doi: 10.1002/jso.21862. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Sumiyama K, Tajiri H, Yasuda K, Kitano S. Evaluation of a new multitasking platform for endoscopic full-thickness resection. Gastrointest Endosc. 2011;73:117–122. doi: 10.1016/j.gie.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354–4359. doi: 10.1007/s00464-013-3023-3. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal D, Chak A, Champagne BJ, Marks JM, Delaney CP. Endoscopic mucosal resection with full-thickness closure for difficult polyps: a prospective clinical trial. Gastrointest Endosc. 2010;71:1082–1088. doi: 10.1016/j.gie.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Kopelman Y, Siersema PD, Nir Y, Szold A, Bapaye A, Segol O, Willenz EP, Lelcuk S, Geller A, Kopelman D. Endoluminal compression clip: full-thickness resection of the mesenteric bowel wall in a porcine model. Gastrointest Endosc. 2009;70:1146–1157. doi: 10.1016/j.gie.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Fritscher-Ravens A, Cuming T, Jacobsen B, Seehusen F, Ghanbari A, Kahle E, von Herbay A, Koehler P, Milla P. Feasibility and safety of endoscopic full-thickness esophageal wall resection and defect closure: a prospective long-term survival animal study. Gastrointest Endosc. 2009;69:1314–1320. doi: 10.1016/j.gie.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247–258. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–2931. doi: 10.1007/s00464-011-1644-y. [DOI] [PubMed] [Google Scholar]
- 27.Mori H, Rafiq K, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Tani J, et al. Development of pure endoscopic full-thickness resection with mechanical countertraction and double-armed bar suturing systems. Gastrointest Endosc. 2014;79:24–25. doi: 10.1016/j.gie.2013.08.031. [DOI] [PubMed] [Google Scholar]


