Abstract
Intracranial self-stimulation (ICSS) is a behavioral procedure in which operant responding is maintained by pulses of electrical brain stimulation. In research to study abuse-related drug effects, ICSS relies on electrode placements that target the medial forebrain bundle at the level of the lateral hypothalamus, and experimental sessions manipulate frequency or amplitude of stimulation to engender a wide range of baseline response rates or response probabilities. Under these conditions, drug-induced increases in low rates/probabilities of responding maintained by low frequencies/amplitudes of stimulation are interpreted as an abuse-related effect. Conversely, drug-induced decreases in high rates/probabilities of responding maintained by high frequencies/amplitudes of stimulation can be interpreted as an abuse-limiting effect. Overall abuse potential can be inferred from the relative expression of abuse-related and abuse-limiting effects. The sensitivity and selectivity of ICSS to detect abuse potential of many classes of abused drugs is similar to the sensitivity and selectivity of drug self-administration procedures. Moreover, similar to progressive-ratio drug self-administration procedures, ICSS data can be used to rank the relative abuse potential of different drugs. Strengths of ICSS in comparison with drug self-administration include 1) potential for simultaneous evaluation of both abuse-related and abuse-limiting effects, 2) flexibility for use with various routes of drug administration or drug vehicles, 3) utility for studies in drug-naive subjects as well as in subjects with controlled levels of prior drug exposure, and 4) utility for studies of drug time course. Taken together, these considerations suggest that ICSS can make significant contributions to the practice of abuse potential testing.
I. Introduction to Abuse Potential Testing
Drugs may produce therapeutic effects useful in treatment of illness, injury, or disease, but even the most valuable medications produce undesirable effects that limit clinical utility. Abuse potential is one category of undesirable drug effect. Abuse potential refers to the probability that a drug might maintain nonmedical patterns of repeated use leading to adverse consequences in humans. The danger of drug abuse to both the user and the community has stimulated efforts to measure abuse potential of drugs as a guide to government policies for drug regulation, industry strategies for drug development, and consumer decisions for drug use (Ator and Griffiths, 2003; Balster and Bigelow, 2003; Carter and Griffiths, 2009; Horton et al., 2013).
Abuse potential evaluation for any given drug is a multi-tiered process that includes in vitro assessments of receptor binding and functional activity, preclinical behavioral pharmacology studies in animals, and human laboratory studies (Ator and Griffiths, 2003; European Medicines Agency, 2006; Carter and Griffiths, 2009; Food and Drug Administration, 2010). This review article is concerned with procedures for preclinical behavioral pharmacology studies. More specifically, drug use and abuse can be conceptualized as a type of operant behavior. In operant behavior, an “operant” is defined as any active behavior that operates on the environment to generate consequences (Skinner, 1953a), and in the case of drug abuse, the operant is the sequence of behavior that culminates in the consequence of drug administration. Patterns of human drug use can be studied in naturalistic environments as well as in the laboratory (Jones and Comer, 2013). An important advance in the science of drug abuse emerged in the mid-1900s with the discovery that nonhuman animals including chimpanzees (Spragg, 1940), rhesus monkeys (Thompson and Schuster, 1964), and rats (Weeks, 1962) could be trained to behave in ways that produce drug delivery. As one example, James Weeks (1962) reported that rats implanted with intravenous catheters connected to a drug reservoir could be trained to press a lever to self-administer intravenous morphine injections. Subsequent studies determined that laboratory animals would self-administer most drugs abused by humans and would not self-administer many other drugs not abused by humans (Thompson and Schuster, 1964; Deneau et al., 1969; Johanson and Balster, 1978; O’Connor et al., 2011). These findings provided evidence for the sensitivity and selectivity of preclinical drug self-administration procedures to detect drug effects related to abuse potential in humans, and drug self-administration procedures have subsequently emerged as key tools for abuse potential assessment (Ator and Griffiths, 2003; Carter and Griffiths, 2009; Horton et al., 2013).
Although drug self-administration procedures lie at the core of preclinical abuse potential testing, other behavioral procedures can also provide information relevant to abuse potential. Intracranial self-stimulation (ICSS) is one of these procedures. The goal of this review article is to discuss the history of ICSS, its evolution into contemporary methodologies, and its application to abuse potential testing. Major conclusions of this review include the following:
ICSS has made and can continue to make significant contributions to preclinical abuse-potential testing.
ICSS results can be used to rank relative abuse potential of drugs.
ICSS results correlate well with data from drug self-administration procedures.
ICSS has advantages that make it a useful complement to drug self-administration.
There is ample opportunity for future research to refine and enhance ICSS as a tool for abuse potential testing.
II. Intracranial Self-Stimulation Methodology
A. Definition, Discovery, and Neural Substrates
In drug self-administration, experimental subjects are typically implanted with intravenous catheters connected to drug reservoirs and placed into controlled environments where performance of an operant response (e.g., pressing a lever) results in the intravenous delivery of a drug dose. Rates of operant responding can then be quantified and related to independent variables such as the type or dose of drug available for self-administration. ICSS is the generic name for a family of functionally similar behavioral procedures. In ICSS, experimental subjects are implanted with intracranial electrodes that target specific brain regions, and performance of the operant response results in the delivery of electrical stimulation to that target. Rats (Olds and Milner, 1954; Wise, 1996), mice (Cazala et al., 1974; Stoker and Markou, 2011a), nonhuman primates (Rolls et al., 1980), and humans (Bishop et al., 1963; Heath, 1963) will all respond avidly for electrical stimulation of some brain areas.
ICSS was discovered by James Olds and Peter Milner at McGill University in the early 1950s (Olds and Milner, 1954). According to an account of the incident shared later by Milner (1989), the McGill Department of Psychology at that time included a community of scientists interested in the brainstem reticular formation. Milner himself was a graduate student conducting studies on effects of reticular formation stimulation by surgically implanted electrodes, and he had already found in pilot studies that rats would avoid the arm of a maze associated with stimulation. Olds was a new postdoctoral fellow who learned the surgical procedure from Milner and soon implanted his own rats with the goal of targeting the same brain region. Surprisingly though, Olds found that stimulation in one of his rats elicited robust appetitive behaviors, such as forward locomotion and sniffing, rather than avoidance behaviors. Later experiments demonstrated that short bursts of stimulation could be used in this rat to train performance of new behaviors such as pressing a lever. The striking novelty of effects produced by brain stimulation in this rat led Olds and Milner to suspect that the electrode was not in the reticular formation as intended, but was instead in some other brain region. An X-ray confirmed a more rostral placement of the electrode, and they were able later to recapitulate the positive reinforcing effects of brain stimulation with electrodes intentionally implanted into the septal area, a region of forebrain located between the lateral ventricles. Results from subsequent studies testified to the extraordinary reinforcing strength of electrical brain stimulation in rats. It maintained operant response rates in excess of 1 response/second for hours at a time; it maintained not only simple lever-press behaviors but also more complicated maze-running behaviors; and rats would endure high levels of foot shock to gain access to a lever that produced brain stimulation (Olds, 1958b).
ICSS was immediately appreciated to resemble operant behavior maintained by natural reinforcers such as food and sex, and ICSS methodologies were quickly applied to research on the physiology of reward and reinforcement. One noteworthy branch of subsequent research focused on physiologic and emotional responses elicited in humans by stimulation of brain areas that supported self-stimulation. For example, Heath (1963, 1964) implanted electrode arrays that permitted subsequent stimulation of multiple discrete brain areas in patients with neurologic disorders. Their primary goal was to evaluate the therapeutic potential of brain stimulation, but they also found that stimulation of brain sites supporting the behavior of self-stimulation also produced variations on the feeling of pleasure. As an example, one narcoleptic patient was given a portable unit with three response buttons that could be used to stimulate three different brain electrodes implanted in the septal area, hippocampus, or mesencephalic tegmentum (Heath, 1963). The patient initially sampled the effects of responding on all three buttons, but ultimately, he responded exclusively on the button that stimulated the septal area (defined to include not only septum but also adjacent regions including nucleus accumbens). This stimulation promoted alertness and was deemed therapeutic in combatting narcolepsy; however, the authors also noted patient reports that “…the feeling (of stimulation) was ‘good’; it was as if he were building up to a sexual orgasm. He reported that he was unable to achieve the orgastic end point, however, explaining that his frequent, sometimes frantic, pushing of the button was an attempt to reach that end point.” By contrast, stimulation of the hippocampal electrode was reported as only mildly pleasurable, and stimulation of the tegmental electrode led the patient to complain of extreme discomfort, and he devised a method to block the button so it could not be pushed. These early studies inspired by the discovery of ICSS in rats contributed to technologies and research themes that persist today under the rubric of “deep brain stimulation” (Hariz et al., 2010; Schlaepfer et al., 2014).
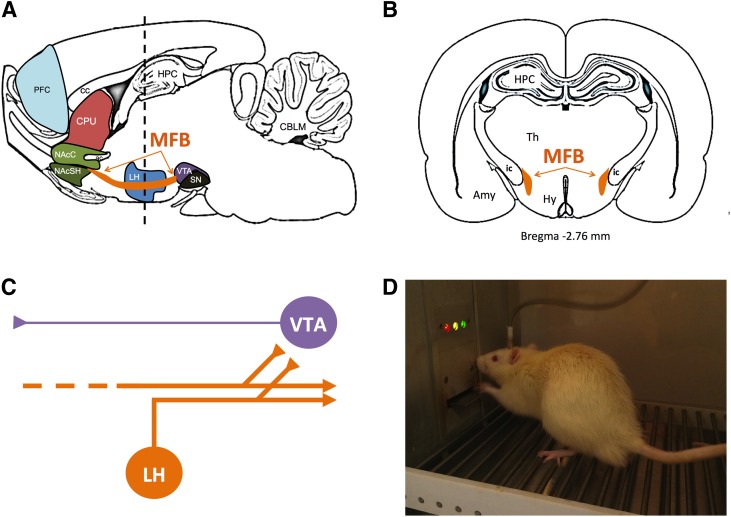
The discovery of ICSS also stimulated preclinical research using three general approaches to examine its neural substrate in rats (Fig. 1). First, in brain mapping studies, the anatomic site of the stimulating electrode was manipulated. For example, the original Olds and Milner study, together with later reports by these and other investigators, found that effective stimulation sites in rats included not only the septal area, but also other regions in forebrain, midbrain, and brainstem, whereas stimulation of other sites was either not effective or functioned to punish behavior (Olds and Milner, 1954; Olds, 1958b; Jacques, 1979; Wise, 1996). The highest rates of self-stimulation were maintained by electrodes in the medial forebrain bundle (MFB) at the level of the lateral hypothalamus (Fig. 1, A and B).
Fig. 1.
Medial forebrain bundle (MFB) as a target for brain stimulation in ICSS studies for abuse potential testing. Sagittal section (A) and coronal section (B) of rat brain showing location of the MFB in orange. (C) Diagram of neurons thought to contribute to ICSS. Electrical stimulation of MFB in ICSS is thought to produce direct activation of “first stage” descending myelinated neurons (orange) that originate in lateral hypothalamus or more rostral regions and project caudally to midbrain and brainstem. Collateral branches of these “first stage” neurons project to and activate “second stage” unmyelinated mesolimbic dopamine neurons (purple) in ventral tegmental area. (D) Photograph of a rat with an MFB electrode in an operant chamber. Amy, amygdala; CBLM, cerebellum; cc, corpus callosum; CPU, caudate/putamen; ic, internal capsule; HPC, hippocampus; Hy, hypothalamus; LH, lateral hypothalamus; NAcC, nucleus accumbens core; NAcSH, nucleus accumbens shell; PFC, prefrontal cortex; SN, substantia nigra; Th, thalamus; VTA, ventral tegmental area.
Second, electrical parameters of stimulation frequency and amplitude were manipulated at single or multiple electrode sites to gain information about the conduction velocity, refractory periods, and connectivity patterns of neurons mediating ICSS (Stellar and Stellar, 1985; Shizgal and Murry, 1989; Yeomans, 1989). These studies implicated low-threshold myelinated neurons as the likely substrate, and these studies also identified multiple parallel networks of neurons capable of supporting ICSS. For example, stimulation at multiple points along the rostrocaudal extent of the MFB appeared to promote activation of a common set of neurons with cell bodies located in forebrain and/or lateral hypothalamus and projecting caudally to brainstem regions including ventral tegmental area (VTA) (Fig. 1C). More recent studies have provided additional insight into this substrate by showing that self-stimulation can be maintained by optogenetic rather than electrical stimulation of glutamate/neurotensin-containing neurons that project from lateral hypothalamus to VTA dopamine (DA) neurons (Kempadoo et al., 2013). Conversely, stimulation of prefrontal cortex maintained self-stimulation by activating a different neural circuit that did not project through MFB.
Lastly, neurochemical and pharmacological studies have implicated mesolimbic DA neurons projecting from VTA to nucleus accumbens as critical contributors to ICSS, particularly when the stimulating electrode is located in the MFB or VTA. For example, ICSS promotes DA release in nucleus accumbens; it is enhanced by drugs that themselves increase extracellular DA levels in nucleus accumbens, and it is blocked by drugs that deplete DA or block DA receptors (Stellar and Stellar, 1985; Phillips et al., 1989; Fiorino et al., 1993; Wise, 1998; You et al., 2001; Cheer et al., 2005) [but see (Miliaressis et al., 1991; Kruk et al., 1998)]. Mesolimbic DA neurons constitute one subset of neurons that project through the MFB (Nieuwenhuys et al., 1982; Veening et al., 1982), and optogenetic studies suggest that direct activation of these neurons is sufficient to maintain ICSS (You et al., 2001; Kim et al., 2012). However, electrical stimulation of the MFB or VTA is not thought to produce direct activation of mesolimbic DA neurons, in part because electrophysiological data summarized above suggest ICSS mediation by descending myelinated axons rather than by ascending unmyelinated axons like those from mesolimbic DA neurons. Consequently, current models of the substrate for ICSS of the MFB and VTA suggest a “first stage” of descending myelinated neurons directly activated by the electrode linked to a “second stage” that includes mesolimbic DA neurons originating in VTA (Fig. 1C). Details of this linkage remain unclear and could involve direct or indirect trans-synaptic connections via cholinergic intermediaries (Rada et al., 2000; Yeomans et al., 2001). Regardless of the precise neurobiology, one implication of this substrate is that MFB stimulation is positioned to activate one source of inputs to mesolimbic DA neurons that can interact with other excitatory or inhibitory inputs, and ICSS maintained by MFB stimulation can be conceptualized as a behavior that integrates these inputs. Figure 1D shows a photograph of a rat engaged in ICSS in our laboratory.
B. Experimental Design
Early studies used drugs as tools to investigate the neural substrates of ICSS, but this work quickly suggested a reciprocal use of ICSS to study abuse-related effects of drugs. The equipment and the experimental designs for ICSS in abuse potential testing have evolved, and technical details regarding the conduct of ICSS in rats and mice can be found in recent excellent reviews (Carlezon and Chartoff, 2007; Stoker and Markou, 2011a). This section will focus on features of experimental design commonly used in contemporary design, conduct, and interpretation of ICSS to examine abuse potential of drugs.
1. Independent Variables.
a. Electrode placement.
Although stimulation at multiple brain sites can maintain ICSS, most studies of abuse-related drug effects use electrodes implanted in the MFB or VTA for two reasons. First, early studies reported that stimulation of MFB/VTA maintained the highest rates of ICSS, so stimulation of these sites is suitable for maintaining high and reliable rates of behavior. Second, stimulation of MFB/VTA activates excitatory inputs to mesolimbic DA neurons known to play a critical role in reinforcing effects of most abused drugs (Koob and Volkow, 2010). As will be discussed in greater detail below, ICSS maintained by MFB/VTA stimulation is sensitive to many abused drugs, and for the remainder of this review, the term “ICSS” will denote behavior maintained by stimulation of the MFB/VTA unless explicitly stated otherwise. However, drug effects on ICSS can vary as a function of electrode placement. For example, amphetamine facilitated ICSS of the MFB more effectively than it facilitated ICSS of prefrontal cortex (Goodall and Carey, 1975; Robertson et al., 1981). As another example, morphine was more efficacious to facilitate ICSS of rostromedial paraventricular nucleus than MFB, whereas cocaine was more efficacious to facilitate ICSS of MFB (Ewan and Martin, 2012). Despite these nuances, prevailing evidence suggests that ICSS of the MFB is either as sensitive or more sensitive than ICSS of other brain regions to abuse-related effects of many drugs.
b. Parameters of electrical stimulation.
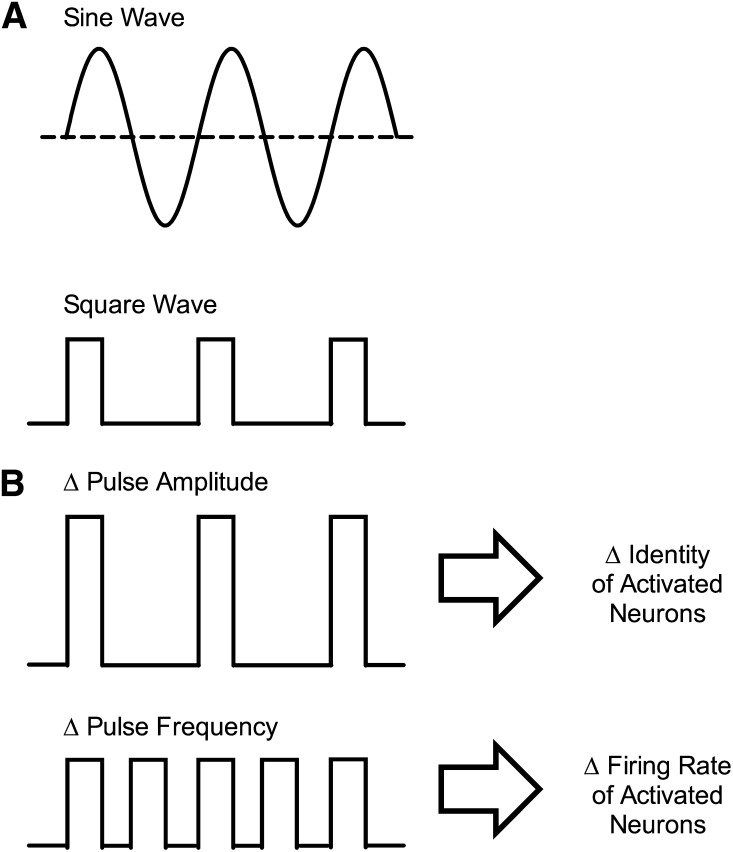
Electric current used in ICSS can vary across multiple parameters that include waveform (sine wave or square wave), amplitude (in units of microamperes, µA), and frequency (in units of cycles per second or Hertz, Hz) (Fig. 2). Early ICSS studies used standard 50- or 60-Hz sine-wave alternating current delivered by conventional wall sockets, and amplitude was manipulated with a variable potentiometer (Olds and Milner, 1954; Olds, 1958b). Later refinements in equipment and procedures permitted delivery of square-wave pulses that allowed more precise control of current onset and offset. In contemporary applications, ICSS is commonly maintained by delivery of short trains of brief square-wave pulses (e.g., 0.5-second trains of 0.1-ms pulses), and the amplitude or frequency of pulses is manipulated as part of experimental design.
Fig. 2.
Example of wave forms and parametric manipulations used for brain stimulation in ICSS. (A) Alternating sine-wave current was often used in early ICSS studies, and monophasic square-wave current is typical of more recent ICSS studies. Square-wave current permits greater control over stimulus onset and offset, and monophasic square waves are typically used when only the cathode is implanted in brain. Biphasic square-wave current is also commonly used with twisted electrodes, in which both the cathode and anode are implanted in brain. (B) Changes in pulse amplitude produce changes in the identity of the activated neurons, such that increased amplitude activates neurons of progressively smaller diameter located progressively farther from the electrode tip. Changes in pulse frequency alter the firing rate of activated neurons.
Changes in pulse amplitude affect the identity of neurons that are activated by electrical stimulation. Activation is most likely for large-diameter axons close to the electrode tip, and increases in pulse amplitude recruit activation both of progressively smaller axons and of axons located progressively farther away from the electrode (BeMent and Ranck, 1969). This impact of pulse amplitude on the anatomic scope of neuronal activation is exploited experimentally to compensate for small differences in electrode placement that result from variability in accuracy of surgical implantation or in anatomy of individual subjects. Thus, when an electrode precisely targets a structure such as the MFB, ICSS can be maintained by relatively low stimulation amplitudes, whereas more distal electrode placements require higher amplitudes to reach and activate MFB. Because there is always some variability in electrode placement, a common first step in ICSS training is adjustment of stimulation amplitude to an optimum level for a given electrode in a given subject. However, the utility of increasing stimulus amplitude has limits because the probability of recruiting off-target pathways also increases. For example, as shown in Fig. 1B, the MFB lies adjacent to the internal capsule, which carries myelinated axons descending from motor cortex to brainstem and spinal cord. A common consequence of excessive stimulation amplitude for MFB electrodes is activation of axons in internal capsule and stimulation of motor responses, such as a head or paw twitch. Overall, pulse amplitude then is typically adjusted early in behavioral training to achieve optimal stimulation of the intended target with minimal stimulation of off-target sites. As will be discussed further below, pulse amplitude may also be manipulated within experimental sessions.
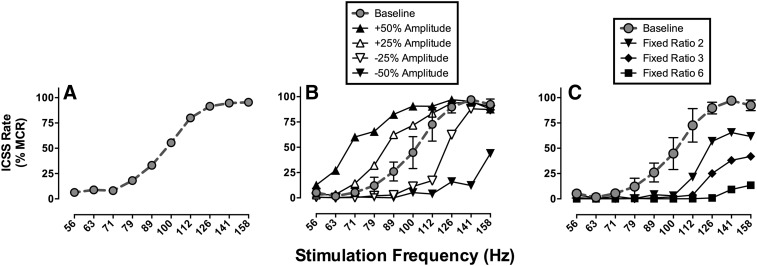
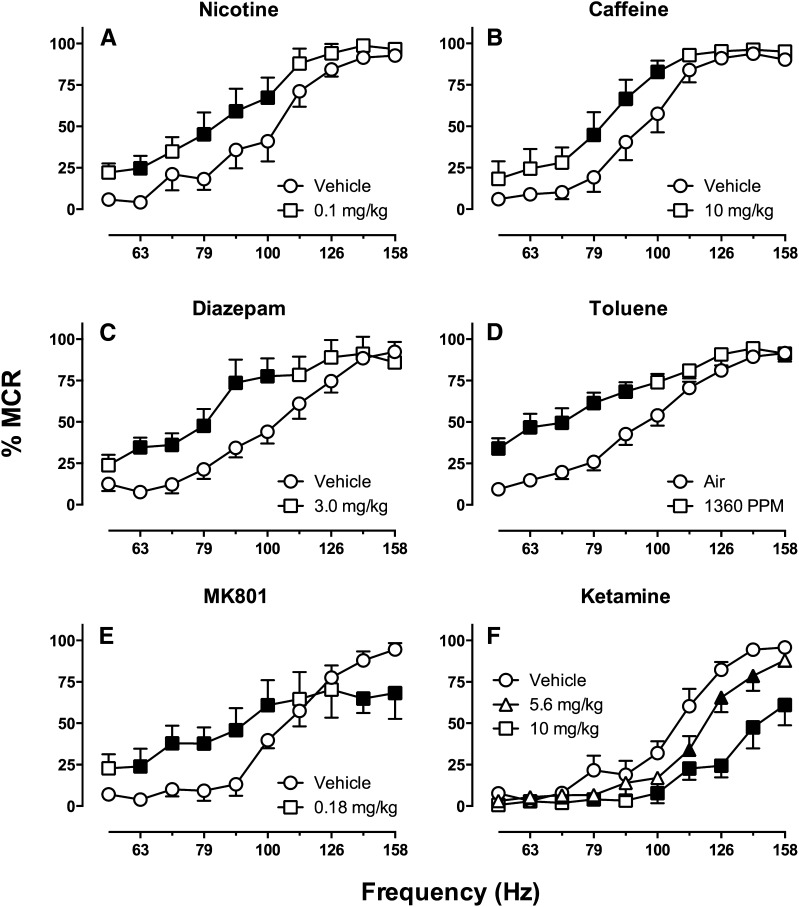
Whereas pulse amplitude governs the identity of activated neurons, pulse frequency governs the firing rate of activated neurons. Neurons affected by a given pulse amplitude have both a basal firing rate and a maximal rate determined by their refractory periods. Manipulation of pulse frequency can synchronize and control neuronal firing rates within this range between basal and maximal rates, and increased rates of neuronal firing are associated with increased rates of operant responding. Contemporary experimental designs often use a broad range of stimulation frequencies to engender a broad range of response rates. Thus, low pulse frequencies maintain little or no responding, whereas higher pulse frequencies maintain higher rates of responding that plateau when pulse frequency meets and exceeds maximal firing rates of ICSS substrate neurons. For example, Fig. 3A shows an ICSS “frequency-rate” curve that relates brain-stimulation frequency to operant response rates in rats. In this example, frequency was manipulated in 0.05-log unit increments from 56 to 158 Hz, and there was a frequency-dependent increase in ICSS rates. This type of frequency-rate curve often serves as the behavioral baseline for studies of drug effects on behavior.
Fig. 3.
ICSS frequency-rate curves obtained with MFB electrodes in Sprague-Dawley rats. Abscissae: frequency of brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as percent maximum control rate (%MCR; see text for details). (A) A baseline frequency-rate curve determined under a fixed-ratio 1 (FR 1) schedule of reinforcement for stimulation amplitude determined individually in each rat. This curve shows mean data from 34 rats used in one study (Negus et al., 2012a). (B) Effects of manipulating stimulation amplitude in a group of 3 rats. Increases/decreases in amplitude produced leftward/rightward shifts in the frequency-rate curve, respectively. (C) Effect of manipulating the FR ratio requirement in the same group of 3 rats. Increasing the ratio requirement produced downward shifts in the frequency-rate curve. Data in (B) and (C) are unpublished but were collected as in studies of drug effects in this frequency-rate procedure.
In addition to producing their distinctive effects on identity and firing rate of ICSS substrate neurons, pulse frequency and amplitude also interact to determine the overall reinforcing strength of brain stimulation. For example, Fig. 3B shows ICSS frequency-rate curves determined in rats at various pulse amplitudes. In this experiment, a baseline pulse amplitude was determined in each rat during initial training as described above, and a baseline frequency-rate curve was determined at this pulse amplitude. Subsequently, the frequency manipulations were held constant, and pulse amplitude was increased or decreased by increments of 25 or 50% from baseline. Increases in pulse amplitude produced parallel leftward shifts in the frequency-rate curve, such that ICSS rates increased at most lower frequencies (e.g., 63–100 Hz). Conversely, decreases in pulse amplitude produced parallel rightward shifts in the frequency-rate curve, such that ICSS rates decreased at higher frequencies (e.g., 89 158 Hz).
Other parameters of stimulation are also occasionally manipulated in ICSS procedures. For example, the duration of the stimulus train can be manipulated, and longer stimulus trains generally maintain higher ICSS rates than shorter trains at given levels of pulse frequency and amplitude (Frank et al., 1987; Hunt and Atrens, 1992). However, stimulus train duration and other parameters are rarely manipulated in studies of abuse potential assessment.
c. The electrode.
Regardless of waveform, amplitude, or frequency, electrical stimulation is delivered by an electrode implanted in brain. ICSS electrodes consist of two wires that permit controlled delivery of stimulation to the anatomic target. At any given moment, one wire serves as the cathode to deliver current (i.e., electrons), and the other wire serves as an anode to extract current. Two types of wire configurations are commonly used. For “twisted” electrodes, both wires are insulated except at the tips. These wires are then twisted together so that their tips are adjacent to each other, and the entire assembly is implanted in brain to position the tips in the target region. Twisted electrodes are optimal for procedures that use alternating sine-wave or square-wave currents (e.g., Fig. 2A), because both wires terminate in or near the target structure, and the identity of the cathode can alternate between the two wires with minimal impact on the anatomic sphere of activation. For “unipolar” electrodes, only one wire, insulated except at its tip, is inserted into brain. This wire always serves as the cathode to deliver unipolar cathodal current (e.g., Fig. 2B). The other wire is typically uninsulated and wrapped around a skull screw outside of brain to serve as the anode. Unipolar electrodes produce less tissue damage than implantation of larger twisted electrodes, and because one wire always serves as cathode, the anatomic sphere of stimulation is constant. Despite these subtle differences, both types of electrode configuration have been used successfully in studies of abuse-related drug effects.
d. The operant manipulandum and schedule of reinforcement.
In ICSS and other forms of operant behavior, subjects engage with an “operant manipulandum” in the experimental environment to generate consequences. The most commonly used type of manipulandum is a lever that can be depressed by the subject to operate a microswitch and register a response (e.g., see Fig. 1D). Another commonly used manipulandum in ICSS resembles a water-wheel that is mounted in a cage wall and can be turned by the subject (Latz et al., 1969; Kornetsky et al., 1979). Typically, a quarter turn of the wheel operates a microswitch and counts as one response.
The “schedule of reinforcement” defines the requirement for responding on the manipulandum to produce brain stimulation. For most ICSS procedures, brain stimulation is delivered under a fixed-ratio 1 (FR 1) schedule of reinforcement, such that each operation of the manipulandum (e.g., each press of the lever) produces brain stimulation. Other more complex schedules of reinforcement are occasionally used to study drug effects on ICSS. For example, FR schedules can use ratio values >1 (e.g., under an FR 10 schedule, brain stimulation is delivered after completion of 10 responses) (West et al., 1983; Neill et al., 2002). Alternatively, under fixed-interval schedules, stimulation is delivered upon the first response after expiration of a designated temporal interval (Elder et al., 1965; Hunt and Atrens, 1992; Schaefer and Michael, 1992), and under progressive-ratio schedules, the number of responses required to produce stimulation increases as successive stimulations are earned (Easterling et al., 2000; Tracy et al., 2014).
Changes in the manipulandum and schedule of reinforcement can produce changes in baseline ICSS. For example, Fig. 3C shows an ICSS frequency-rate curve determined in rats responding under different FR schedules with a lever manipulandum. The baseline frequency-rate curve was determined under an FR 1 schedule, and increases in the FR value produced downward shifts in the frequency-rate curve. This is consistent with other evidence to suggest that maximal ICSS rates can be reduced by manipulations that increase the difficulty of the response (e.g., by weighting the lever so that it is more difficult to press) (Markou and Koob, 1992). However, drug effects on ICSS are generally similar across manipulanda and schedules of reinforcement, and prevailing evidence suggests that ICSS maintained under an FR 1 schedule is either as sensitive or more sensitive to abuse-related drug effects than ICSS maintained under other schedules (Schaefer and Michael, 1992; Tracy et al., 2014).
e. Organization of experimental sessions.
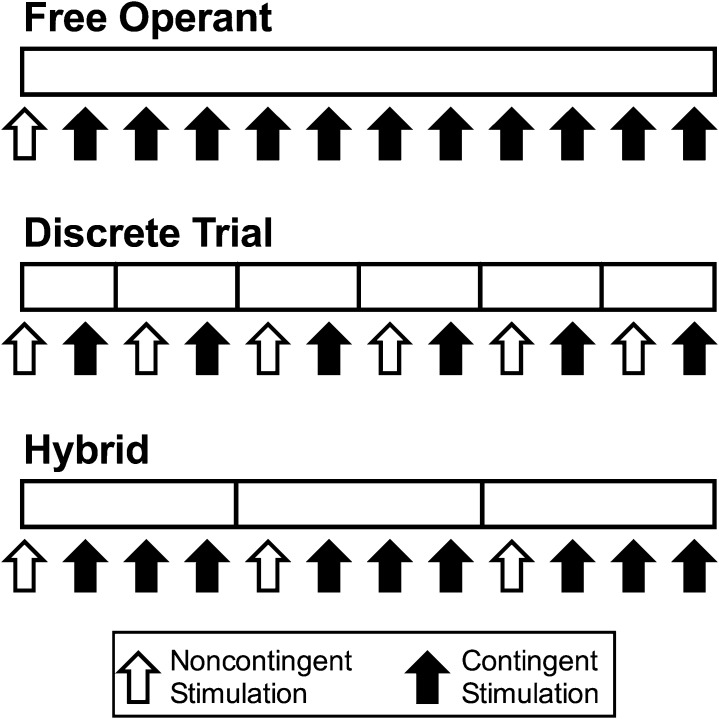
A factor related to schedule of reinforcement is the organization of experimental sessions, and three general approaches will be described here (Fig. 4). First, in free-operant procedures, brain stimulation at fixed parameters of frequency and amplitude is available under some schedule of reinforcement for the duration of the experimental session, and subjects can respond as often and earn as many stimulations as the schedule allows. For example, stimulation might be available under an FR 1 schedule for a 30-minute session, and the subject could respond and be reinforced continuously during that 30-minute period. Sessions often begin with noncontingent delivery of stimulation (i.e., stimulation independent of the subject’s behavior), and this noncontingent stimulation can serve both as a priming stimulus to elicit behavior and as a discriminative stimulus to signal availability of brain stimulation. A common dependent variable in free-operant procedures is the rate of responding or reinforcement during the session. Many early ICSS drug studies used free-operant procedures (Adams et al., 1972; Koob et al., 1975; Holtzman, 1976; Reid, 1987); however, this relatively simple approach was largely abandoned, because interpretation was confounded by the potential for drug effects to vary as a function of baseline response rate (see below).
Fig. 4.
Diagram of different ICSS session types. The open rectangle indicates the time line of an ICSS session, and vertical markers indicate division of the session into “trials” or “components” with varying stimulation parameters. Open/closed arrows indicate delivery of noncontingent/contingent stimulation, respectively. In free-operant sessions, stimulation parameters are constant throughout the session. Sessions can begin with noncontingent stimulation, followed by a period during which the subject can earn contingent stimulation under the prevailing schedule of reinforcement. In discrete-trial sessions, stimulation parameters can be adjusted during each trial within a session. Noncontingent stimulation is typically administered at the beginning of each trial, and subjects can then respond for one contingent stimulation during the trial. In hybrid sessions, stimulation parameters are adjusted across components that usually begin with noncontingent stimulation. Subjects can then earn multiple contingent stimulations by responding under the prevailing schedule of reinforcement.
Discrete trials procedures emerged as one approach to address this issue. In this type of procedure, sessions are partitioned into sequential discrete trials, each lasting a few seconds (Marcus and Kornetsky, 1974; Markou and Koob, 1992). A single noncontingent sample stimulation is delivered at the beginning of each trial, and this is followed by a window of time (a “limited hold”) during which the subject can emit the operant response and receive contingent delivery of one additional stimulation identical to the sample. Trials end after a response is emitted or the limited hold elapses, whichever occurs first, and the primary dependent variable for each trial is the presence or absence of a response. A strength of this approach is that it permits rapid within-session changes in stimulation parameters to produce rapid, within-session changes in response probability. Drug effects on multiple response probabilities maintained by multiple stimulation parameters can then be determined efficiently in a single session. In the most common application of this approach, sometimes denoted as “the discrete-trial current-intensity threshold procedure,” the amplitude of stimulation is manipulated across trials, and the threshold amplitude required to maintain a criterion level of response probability is determined (Marcus and Kornetsky, 1974; Markou and Koob, 1992). Theoretically, the approach could also be used to evaluate effects of stimulation frequency on response probability, but to our knowledge, this has not been done. Discrete-trial procedures are also sometimes described as “rate-free” or “rate-independent” procedures to emphasize their focus on presence or absence of a response rather than rate of response as a dependent measure; however, some minimal response rate is required, and studies using these procedures often report response latency as a reciprocal measure of response rate (i.e., time per response rather than responses per unit time).
Hybrid procedures have characteristics of both free-operant and discrete-trial procedures (Olds, 1958b; Gallistel and Freyd, 1987; Carlezon and Chartoff, 2007). As with discrete-trial procedures, experimental sessions are subdivided into multiple sequential components to permit within-session manipulation of stimulation parameters, and noncontingent stimulation may be delivered at the beginning of each component. However, the components in hybrid procedures last on the order of minutes rather than seconds, and during each component, subjects have free-operant access to multiple stimulations under the prevailing schedule of reinforcement. This approach permits generation and measurement of a wide range of ICSS rates maintained by a wide range of stimulus parameters during each experimental session, and drug effects on this wide range of ICSS rates can then be efficiently examined. Hybrid procedures do not eliminate dependence of drug effects on baseline ICSS rates but rather accommodate this factor by allowing determination of drug effects on multiple ICSS rates within a single session. Hybrid procedures have been used to assess drug effects on ICSS rates maintained by changes in both stimulation frequency and amplitude; however, frequency manipulations are usually preferred for drug studies, perhaps because they do not introduce complications that may be associated with changes in the identity, and potentially the neuropharmacology, of the stimulated neurons. Data reported in Fig. 3 used a hybrid procedure to assess ICSS rates during sequential components of a session in which different stimulation frequencies were available during different components. This type of procedure is commonly referred to as a “frequency-rate” (or “rate-frequency”) procedure. (We prefer the “frequency-rate” nomenclature to state the independent variable first and dependent variable second, as in the term “dose-effect”).
One additional type of procedure, referred to as “autotitration,” has occasionally been used to assess drug effects on ICSS and will be mentioned only briefly here (Stein and Ray, 1960; Nazzaro et al., 1981; Easterling and Holtzman, 1997b). In autotitration procedures, the experimental chamber contains two manipulanda. Responding on one manipulandum produces brain stimulation, and the amplitude or frequency of stimulation declines with successive stimulation deliveries. Responding on the second manipulandum resets the amplitude or frequency of stimulation back to its baseline level, and the primary dependent variable is the amplitude or frequency at which the subject responds on the reset manipulandum.
2. Dependent Variables, Data Analysis, and Interpretation.
The primary dependent variable in any ICSS procedure is a measurement of operant responding. With free-operant procedures, statistical analysis is generally performed directly on raw response rates (e.g., responses per unit time) or on transformations normalized to each subjects baseline rate (e.g., % baseline response rate) (Adams et al., 1972; Koob et al., 1975; Holtzman, 1976). However, with discrete-trial and hybrid procedures, each session yields multiple response measurements at multiple stimulation parameters, and these data can be used to generate derivative metrics that summarize key aspects of drug effects. Common strategies are presented below for analysis of data for hybrid frequency-rate procedures, and similar approaches can be used for hybrid amplitude-rate procedures. This discussion is intended to illustrate basic principles of data analysis, but it should be noted that other variations exist. Strategies for analysis of data from “discrete-trial current-intensity threshold procedures” will also be briefly addressed.
a. Analysis of data from hybrid “frequency-rate” procedures.
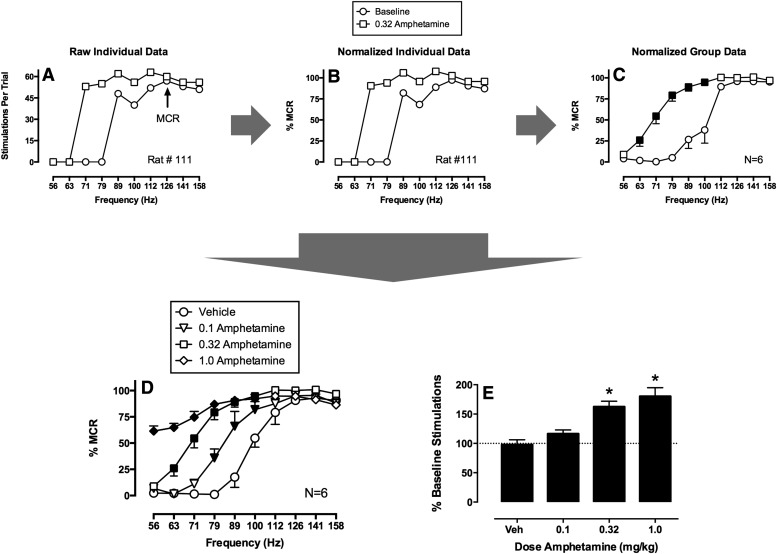
Figure 5 shows the sequence of steps for analysis of effects produced by amphetamine in a frequency-rate ICSS procedure from our laboratory (Bauer et al., 2013b). In this procedure, brain stimulation at 10 different frequencies (56–158 Hz in 0.05-log increments) was available under an FR 1 schedule during sequential 1-minute response periods before and after treatment with amphetamine (vehicle, 0.1–1.0 mg/kg i.p.). The primary dependent variable was the number of active responses emitted and stimulations delivered at each frequency, and Fig. 5A shows ICSS rate as a function of stimulation frequency before treatment (baseline) and after treatment with one amphetamine dose (0.32 mg/kg) in one rat (Rat #111). Before treatment, this rat did not respond at frequencies of 56–79 Hz, and responding increased and plateaued at higher frequencies (89–158 Hz). After amphetamine, the frequency-rate curve shifted to the left, and high ICSS rates were observed at frequencies of 71 Hz and above. Figure 5B shows that these raw data are then normalized to the maximum control rate (MCR), which is defined as the maximum rate observed at any frequency during the baseline frequency-rate determination. In this case, the MCR was 57 stimulations at 126 Hz. Thus, each ICSS rate at each brain-stimulation frequency is converted to %MCR. This normalization step controls for different maximum response rates in different rats and reduces variability when data are averaged across rats as shown in Fig. 5C. At this point, average test data can be compared with average baseline data using repeated-measures two-way analysis of variance with frequency and treatment as the two variables, and significant analyses of variance (ANOVA) can be followed by appropriate post hoc tests. In Fig. 5C, there are significant effects of frequency and treatment and a significant interaction, and filled points show frequencies at which amphetamine significantly increased ICSS rate as determined by a Holm-Sidak post hoc test (P < 0.05 for all analyses). Test data from different experiments with different doses can then be collapsed into a single graph as shown in Fig. 5D, and these data can again be analyzed by two-way ANOVA with frequency and dose as the two factors. In this example, filled points show frequencies at which amphetamine significantly increased ICSS rates relative to vehicle. To provide a summary measure of drug effects on full frequency-rate curves, the total number of stimulations delivered across all frequencies after a given treatment can be summed and expressed as a percent of the baseline number of stimulations, and these data can also be averaged across rats and statistically analyzed as shown in Fig. 5E.
Fig. 5.
Strategy for analysis of data from a frequency-rate procedure for abuse potential testing. Raw baseline and test data (A) for each rat are normalized (B) to that rat’s maximum control rate (MCR) on that day. (C) Normalized data can then be averaged across rats to yield mean baseline and test frequency-rate curves (N = 6). Filled points show test ICSS rates significantly different from baseline as indicated by a significant two-way ANOVA followed by a Holm-Sidak post hoc test (P < 0.05). (D) Alternatively, test data from different test sessions can be compared, and filled points show a data significantly different from vehicle as determined by significant two-way ANOVA followed by a Holm-Sidak post hoc test (P < 0.05). (E) A summary measure of drug effects on ICSS in which the test number of stimulations summed across all frequencies is expressed as a percentage of the baseline number of stimulations across all frequencies. The asterisks indicate significant differences from vehicle as determined by one-way ANOVA followed by Dunnett's post hoc test (P < 0.05). This measure integrates both rate-increasing and rate-decreasing drug effects and has proven useful in correlations with drug self-administration data. Data set has been published (Bauer et al., 2013b).
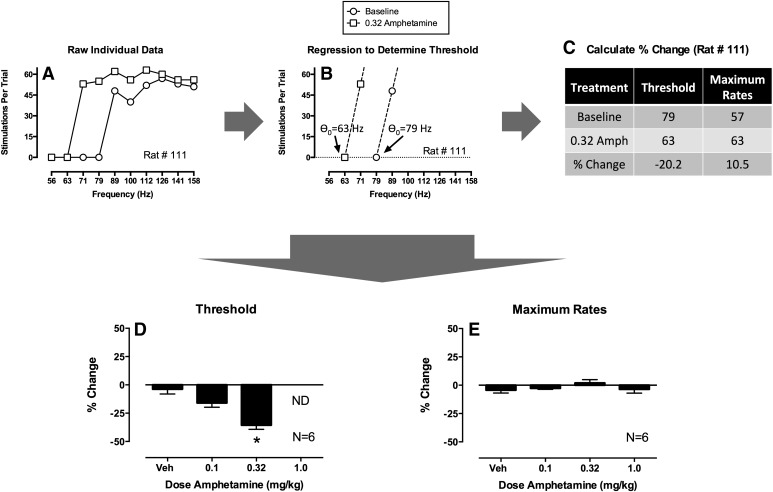
As will be discussed further below, data analyzed and graphed as in Fig. 5, D and E, provide a profile of drug effects that can be useful for evaluating and stratifying abuse potential. However, this approach is relatively uncommon, and a more prevalent approach, sometimes called “curve-shift analysis” (Miliaressis et al., 1986; Carlezon and Chartoff, 2007), is shown in Fig. 6 using the same amphetamine data set. Figure 6A again shows raw data from one rat treated with one dose of amphetamine (0.32 mg/kg). In curve-shift analysis, these data are then submitted to some form of regression analysis. Figure 6B shows linear regression through the ascending portions of the baseline and test frequency-rate curves, but both linear regression and different nonlinear regression equations have been used to fit ICSS data (Miliaressis et al., 1986; Coulombe and Miliaressis, 1987; Carlezon and Chartoff, 2007). Once the regression is established, it is used to calculate a measure of the lateral position of the curve along the X-axis. In Fig. 6B, the X-intercept of each curve (i.e., the point where Y = 0) was determined, and this intercept is often called “θ0” (theta zero), with “theta” being the Greek word for the first letter in the word “threshold,” and “zero” indicating that this threshold is the frequency at which ICSS rate = 0. Other metrics can also be derived to define the lateral position of frequency-rate curves. For example, the frequency that maintains 50% of maximal responding is sometimes derived from linear or nonlinear regression, and this value is often called the “M50” (for 50% of maximal rate) (Miliaressis et al., 1986; Elmer et al., 2010). Alternatively, for curves fit with nonlinear functions, a value called “Locus of Rise” is sometimes calculated to denote the frequency at which the lower inflection point of the sigmoidal frequency-rate curve is observed (O'Neill and Todtenkopf, 2010). In general, metrics anchored to low ICSS rates (e.g., θ0) are preferred over metrics anchored to higher rates (e.g., M50) because they are less sensitive to drug effects that alter maximal rates and the resulting slope of the frequency-rate curve (Miliaressis et al., 1986); however, regardless of the metric and the regression equation used to derive it, these values are taken to represent a measure of threshold frequency for maintenance of responding. Drug effects are then quantified in terms of their effect on this measure of threshold. Figure 6C, for example, shows that 0.32 mg/kg amphetamine produced a 20.2% reduction in θ0 in this rat, and average data for a group of 6 rats are shown in Fig. 6D. The asterisk indicates that 0.32 mg/kg amphetamine significantly reduced ICSS thresholds as determined by a significant one-way ANOVA followed by a Dunnett’s post hoc test (P < 0.05). This analysis of drug effects on thresholds is also routinely complemented by analysis of drug effects on maximum response rates. Again, various approaches can be used to define maximum rates, but to illustrate the approach with this data set, maximum rates were defined simply as the maximum rate observed at any frequency. Thus, the maximum rates for Rat #111 were 57 responses/trial for the baseline frequency-rate curve and 63 responses/trial after treatment with 0.32 mg/kg amphetamine for a 10.5% increase in maximum rates. Average effects are shown in Fig. 6E, and ANOVA indicated that these amphetamine doses did not significantly alter maximum rates.
Fig. 6.
“Curve-shift” strategy for analysis of data from a frequency-rate procedure using the same data set as in Fig. 5. (A) Analysis begins with raw baseline and ICSS data as in Fig. 5. (B) Regression analysis is used to identify metrics of the lateral position of the frequency-rate curve along the X-axis. In this case, linear regression through the ascending portions of the frequency-rate curves were used to determine θ0, the “threshold” frequency at which the regression line crosses the X-axis. (C) Drug-induced changes in threshold and maximum rates can then be determined in each rat, and effects are usually expressed as percent change. (D and E) Values can then be averaged across rats and submitted to statistical analysis. The asterisk in (D) indicates significant differences from vehicle as determined by one-way ANOVA followed by Dunnett’s post hoc test (P < 0.05). Notice that threshold values were not determined (ND) for 1.0 mg/kg amphetamine. See text for details.
Curve-shift analysis has been extremely useful for research on the neurobiology of ICSS because it provides an analytic basis for dissociating drug effects on sensitivity to brain stimulation (reflected in the threshold measure and related to “reward”) from drug effects that alter performance (reflected in the measure of maximum rates). However, our view is that this approach is less useful in the context of abuse potential testing for two reasons. First, curve-shift analysis seeks to dissociate reward-related and performance effects; however, in drug self-administration or human drug abuse, patterns of drug-taking behavior are influenced by both types of effects, and drugs are often self-administered up to doses that produce effects on motor performance. Consequently, metrics that integrate both rewarding and performance effects may be more useful in abuse potential assessment than metrics focused solely on reward. Data to be presented below support this view. Second, drugs often produce effects on ICSS that cannot be accommodated by curve-shift analysis. For example, Fig. 6D shows that thresholds could not be determined after treatment with 1.0 mg/kg amphetamine because ICSS rates were elevated across the entire frequency range for most rats. This limits the range of doses across which drug effects can be analyzed, and such a constraint may not be optimal for comprehensive pharmacological evaluation. We have adopted the analytic approach presented in Fig. 5 because it addresses each of these issues by (1) using an analytic approach (two-way ANOVA) that can accommodate any possible drug effect and (2) generating a summary measure of drug effects (% baseline stimulations) that integrates drug effects on ICSS across the entire frequency range.
b. Analysis of data from “discrete-trial current-intensity” procedures.
Excellent summaries of the strategies for data analysis in discrete-trial procedures have been published previously (Vlachou and Markou, 2011), and a detailed account will not be presented here. In general, though, this approach shares many features with curve-shift analysis described above. In discrete-trial current-intensity procedures, the amplitude of brain stimulation is systematically varied to identify a threshold amplitude required to maintain a criterion probability of responding. Drug effects on this threshold are determined in individual rats, averaged across rats, and analyzed using ANOVA or other appropriate statistics. Thus, threshold values measured with discrete-trial current-intensity procedures are formally similar to threshold values measured with curve-shift analysis in frequency- or amplitude-rate procedures, and drug effects on thresholds are interpreted in the same way as evidence of a reward-related effect. Likewise, measures of response latency are analogous to measures of maximal response rates in frequency- or amplitude-rate procedures, and drug effects on response latency are often interpreted as evidence of performance effects as opposed to rewarding effects. Drug effects in frequency-rate and discrete-trial current-intensity procedures are often similar, and as one example, amphetamine reduces ICSS thresholds in discrete-trial current-intensity procedures (Esposito et al., 1980) as it does in frequency-rate procedures.
c. Data interpretation.
Regardless of the experimental strategy used to collect and analyze drug effects on ICSS, abuse potential is suggested by an amphetamine-like profile to increase low rates or probabilities of behavior maintained by low frequencies or amplitudes of brain stimulation. This profile of drug effects is often referred to as “facilitation” of ICSS, and the relationship of ICSS facilitation to abuse potential is supported by a large literature of studies with drugs from multiple drug classes. Examples will be discussed below, but before proceeding to a review of this literature, we will briefly consider two general mechanisms that may contribute to drug-induced facilitation of ICSS.
First, drugs could increase sensitivity of the ICSS neural substrate to electrical stimulation (Stellar and Rice, 1989; Wise, 1998). Several lines of evidence support this possibility. For example, as noted above, the reinforcing effects of both MFB stimulation in ICSS and of drugs in drug self-administration procedures appear to involve activation of the mesolimbic DA system. Accordingly, one hypothesis regarding drug effects on ICSS is that drugs and brain stimulation produce additive effects on mesolimbic DA activation and on operant behavior maintained by mesolimbic DA activation. Additional support for this hypothesis comes from the similarity in effects on ICSS frequency-rate curves produced both by increasing the amplitude of stimulation (Fig. 3B) and by pretreatment with amphetamine (Fig. 5).
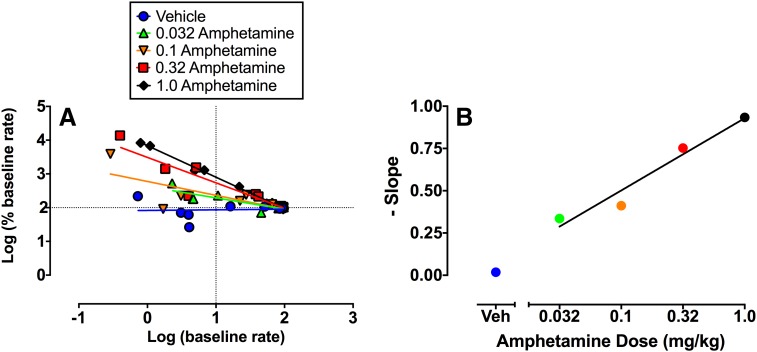
A second possibility, not mutually exclusive with the first, is that drugs could produce nonselective effects on sensory-motor integration or motor capacity that alter performance of the operant response. For example, it is well appreciated that drug-induced decreases in ICSS might reflect motor impairment (e.g., sedation, paralysis). Indeed, drug effects on maximal response rates (in hybrid procedures) or response latencies (in discrete-trial procedures) are often evaluated precisely because of their value in detecting motor impairment (Carlezon and Chartoff, 2007; Vlachou and Markou, 2011). However, it is less well appreciated that drug-induced increases in ICSS might also result from nonselective performance effects (Hernandez et al., 2010; Trujillo-Pisanty et al., 2013), and an even more nuanced principle is that drug effects on operant responding may be rate dependent. “Rate dependency” posits that drug effects on rates of operant responding may be independent of the reinforcing stimulus and may instead be determined by baseline rates of behavior before drug administration (Sanger and Blackman, 1976). A comprehensive discussion of rate dependency is beyond the scope of this review, but recent evidence suggests that drug effects on ICSS often meet criteria for rate dependency (Bauer et al., 2013a). For example, Fig. 7 shows a rate-dependency plot for the same amphetamine data set shown in Figs. 5 and 6. In this log-log plot, the X-axis shows the baseline ICSS rate maintained by each frequency of stimulation before treatment, and the Y-axis shows rates after treatment expressed as a percentage of the baseline. The plot for data from vehicle treatment is horizontal, indicating that ICSS rates after vehicle treatment were similar to baseline ICSS rates at all frequencies of brain stimulation. The negative slope of the plot for each amphetamine dose indicates that amphetamine increased low rates of responding more than it increased high rates of responding, and increasing amphetamine doses produce increasingly negative slopes. It is noteworthy that amphetamine produces strikingly similar evidence for rate-dependent effects under conditions other than ICSS that also maintain variable baseline response rates (e.g., under fixed-interval schedules of reinforcement maintained by food delivery or shock avoidance) (Kelleher and Morse, 1968; Sanger and Blackman, 1976). Taken together, these results suggest the potential for amphetamine and other drugs to alter ICSS in a manner that is at least partially dependent on baseline response rates and independent of sensitivity to the reinforcing electrical stimulus (Bauer et al., 2013a).
Fig. 7.
Rate-dependency analysis of amphetamine effects on ICSS using the same data set as in Fig. 5. (A) Abscissa: Baseline ICSS rate expressed as log of the %MCR value. Ordinate: Treatment-induced change from baseline rate expressed as the log of % baseline rate. The regression line for each treatment is composed of 10 points, one for each of the 10 frequencies of brain stimulation. Amphetamine dose-dependently increased low baseline rates more than it increased high-baseline rates, resulting in plots with progressively steeper negative slopes indicative of rate dependency. (B) Abscissa: amphetamine dose in mg/kg (log scale). Ordinates: Slope of the rate-dependency plots in (A). Amphetamine produced a dose-dependent increase in steepness of slope. For details, see (Bauer et al., 2013a).
Regardless of mechanism, it is useful to appreciate the distinct function that drug stimuli play in ICSS procedures as opposed to the drug self-administration procedures more commonly used in abuse potential testing. Specifically, drug delivery in drug self-administration procedures is contingent upon the behavior of the experimental subject. A drug that maintains self-administration is said to function as a “reinforcer” or “reinforcing stimulus” that produces “reinforcing effects.” ICSS is also an operant behavioral procedure, but in ICSS, it is brain stimulation that functions as the reinforcing stimulus, and drugs function as unconditioned stimuli that may alter the behavior of ICSS. Because drugs do not function as reinforcers in ICSS procedures, it is not appropriate to refer to their effects as “reinforcing” effects. Rather, it is most appropriate to describe drug effects as either increasing or decreasing rates of ICSS behavior, and these changes in behavior may then be interpreted as evidence for changes either in sensitivity to the reinforcing (or “rewarding) effects of brain stimulation or in motor competence to perform the operant behavior.
III. Drug Effects on Intracranial Self-Stimulation by Drug Class
The utility of ICSS as a tool for abuse potential testing has been appreciated for decades, and several previous review articles have addressed the relationship between ICSS effects and abuse potential of drugs (Kornetsky et al., 1979; Reid, 1987; Negus and Dykstra, 1989; Wise, 1996; Vlachou and Markou, 2011). The focus of this section will be to review profiles of ICSS effects produced by systemically administered drugs from different pharmacological classes. Most studies have been conducted using acute drug administration, but drug abuse necessarily involves repeated drug exposure, and abuse potential of drugs can evolve with repeated/chronic treatment. Accordingly, where data are available, effects of repeated drug treatment and drug withdrawal will also be considered.
A. Monoaminergic Drugs
1. Monoamine Releasers.
Amphetamine is one member of a drug class known as monoamine releasers. These drugs function as substrates for dopamine, norepinephrine, and/or serotonin transporters (DAT, NET, SERT, respectively), and they promote neuronal release of dopamine (DA), norepinephrine (NE), and/or serotonin (5HT) independently of neuronal activity (Rothman et al., 2001; Immadisetty and Madura, 2013). Monoamine releasers are used clinically for indications that include attention deficit disorder, obesity, and narcolepsy, but amphetamine and many other drugs in this class also have high abuse liability. Of particular relevance to abuse potential testing, a family of novel monoamine releasers (and uptake inhibitors, see below) with street names such as “bath salts” has recently emerged as a new source of illicit drugs in Europe and North America (Baumann et al., 2013a; De Felice et al., 2014). Table 1 summarizes illustrative data with representative monoamine releasers in free-operant, discrete-trial current-intensity and hybrid frequency-rate ICSS procedures, and text below focuses on results from frequency-rate procedures.
TABLE 1.
Effects of monoamine releasers on intracranial self-stimulation
| Drug Pharmacologya | Doses | Route | Strain/Species | Sex | ICSS Procedureb | Drug Effectc | Dependent Measure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug Name | Selectivity | Structure | Parameter | |||||||
| mg/kg | ||||||||||
| Amphetamine | DA/NE>5HT | 0.1–2.0 | i.p. | Sprague-Dawley rat | Male | Free operant | Facilitation | ↑rate | Carey et al., 1974 | |
| Amphetamine | DA/NE>5HT | 0.25–2.0 | i.p. | Fischer rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Esposito et al., 1980 |
| Amphetamine | DA/NE>5HT | 0.032–1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 ↑rate | Bauer et al., 2013b |
| Amphetamine | DA/NE>5HT | 1.0–4.0 | i.p. | C57Bl6/J mouse | Male | Hybrid | Frequency | Facilitation | ↓M50 | Elmer et al., 2010 |
| Amphetamine | DA/NE>5HT | 1.0–4.0 | i.p. | DBA/2J mouse | Male | Hybrid | Frequency | Facilitation | ↓M50 | Elmer et al., 2010 |
| (+)-Phenmetrazine | DA/NE>5HT | 0.32–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 ↑rate | Bauer et al., 2013b |
| m-Fluroamphetamine | DA/NE>5HT | 1.0–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 ↑rate | Bauer et al., 2013b |
| (+)-Methamphetamine | DA/NE>5HT | 0.032–1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 ↑rate | Bauer et al., 2013b |
| (±)Methcathinone | DA/NE>5HT | 0.1–1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Bonano et al., 2014 |
| PAL-314 | DA/NE>5HT | 0.32–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↓θ0 ↑↓rate | Bauer et al., 2013b |
| PAL-313 | DA/NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↓θ0 ↑↓rate | Bauer et al., 2013b |
| (+)MDMA | DA/NE/5HT | 0.1–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↓θ0 ↑↓rate | Bauer et al., 2013b |
| PAL-287 | DA/NE/5HT | 0.32–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↓θ0 ↑↓rate | Bauer et al., 2013b |
| (−)MDMA | DA<NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Bauer et al., 2013b |
| (±)Methylone | DA/NE/5HT | 1.0–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Bonano et al., 2014 |
| (±)Mephedrone | DA/NE/5HT | 1.0–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Bonano et al., 2014 |
| (±)Mephedrone | DA/NE/5HT | 1.0–10 | i.p. | C57Bl6/J mouse | Male | Hybrid | Frequency | Mixed | ↓θ0 ↓EF50 | Robinson et al., 2012 |
| PAL-544 | DA/NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Banks et al., 2014 |
| PAL-571 | DA/NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Banks et al., 2014 |
| PAL-569 | DA<NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Banks et al., 2014 |
| PAL-542 | DA/5HT>NE | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Banks et al., 2014 |
| Fenfluramine | DA<5HT/NE | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Bauer et al., 2013b |
| Fenfluramine | DA<5HT/NE | 20 | i.p. | Sprague-Dawley rat | Male | Free operant | Depression | ↓rate | Olds, 1995 | |
Rate, response rate; CIT, current-intensity threshold; θ0: theta-0 threshold; M50 or EF50, frequency maintaining 50% maximum rate.
Selectivity for monoamine release.
First colunn indicates structure of experimental session (see text for details). Second column indicates stimulation parameter under manipulation across trials.
Most prominent drug effect on ICSS.
a. Acute administration.
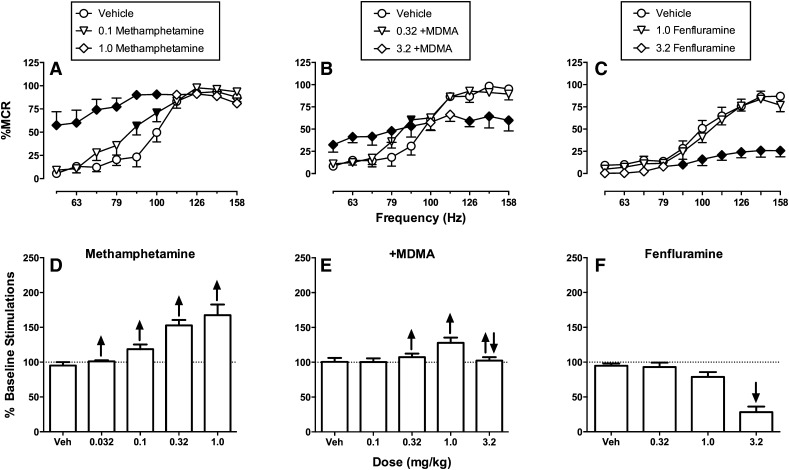
Monoamine releasers can be subclassified along various dimensions, and a key determinant of abuse potential is relative selectivity to promote release of DA, NE, and/or 5HT. Potencies to release DA and NE are usually closely aligned and have been difficult to separate, but selectivity to release DA/NE versus 5HT can vary dramatically (Rothman et al., 2001). For example, based on in vitro functional assays of monoamine release from rat brain synaptosomes, amphetamine and methamphetamine are DA/NE selective (DA/NE > 5HT), whereas methylenedioxymethamphetamine (MDMA) is relatively nonselective (DA/NE ≅ 5HT), and fenfluramine is 5HT selective (5HT > DA/NE). Effects of amphetamine were described above, and Fig. 8 compares effects of methamphetamine, +MDMA, and fenfluramine in the same hybrid frequency-rate ICSS procedure (Bauer et al., 2013b). Like amphetamine, methamphetamine produced a dose-dependent leftward and upward shift in the ICSS frequency-rate curve and a large increase in % baseline stimulations as the summary measure for drug effects (Fig. 8, A and D). Conversely, the 5HT-selective releaser fenfluramine produced a downward shift in the ICSS frequency-rate curve and dose dependently reduced % baseline stimulations (Fig. 8, C and F). Lastly, the mixed-action DA/NE/5HT releaser +MDMA produced mixed effects (Fig. 8, B and E). A low dose of +MDMA produced a modest leftward shift in the frequency-rate curve, but a higher dose produced a biphasic effect consisting of both an increase in low ICSS rates maintained by low brain stimulation frequencies (56–89 Hz) and a decrease in high ICSS rates maintained by high frequencies 126–158 Hz). This recruitment of rate-decreasing effects by higher +MDMA doses functioned to limit the magnitude of increase in % baseline stimulations.
Fig. 8.
Effects of methamphetamine, (+)-methyelenedioxymethamphetamine (+MDMA), and fenfluramine on ICSS in rats. (A–C) Full frequency-rate curves for vehicle and two representative doses of each drug. Abscissae: Frequency of brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as %MCR. Filled points show effects significantly different from vehicle as determined by two-way ANOVA and the Holm-Sidak post hoc test (P < 0.05). (D–F) Summary data for drug effects on ICSS rates across all frequencies of brain stimulation. Abscissae: Drug dose. Ordinates: % Baseline stimulations. Arrows indicate the presence of significant increases (up arrow) or decreases (down arrow) in ICSS rates for at least one frequency of brain stimulation in frequency-rate curve analysis in (A–C). Note that methamphetamine dose dependently increased ICSS rates across a broad dose range, whereas +MDMA produced mixed rate-increasing and rate-decreasing effects, and fenfluramine only decreased ICSS rates. Data set has been published (Bauer et al., 2013b).
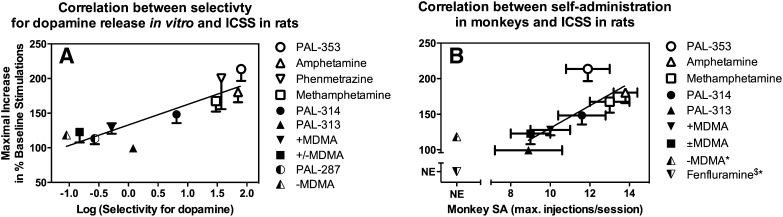
Data with these three compounds suggest a relationship between pharmacological selectivity to release DA/NE versus 5HT and behavioral efficacy to facilitate ICSS. To provide a more quantitative assessment of this relationship, Fig. 9A shows a positive correlation for 10 monoamine releasers between pharmacological selectivity (expressed as selectivity to release DA versus 5HT) and efficacy to facilitate ICSS (expressed as maximal increase in % baseline stimulations) (Bauer et al., 2013b). Moreover, and importantly for consideration of abuse potential testing, previous studies with many of these same monoamine releasers had already shown a similar relationship between pharmacological selectivity to release DA versus 5HT and behavioral efficacy to reinforce drug self-administration under a progressive-ratio procedure in rhesus monkeys (Rothman et al., 2005; Wee et al., 2005; Wang and Woolverton, 2007). Accordingly, we also correlated efficacy of these monoamine releasers to facilitate ICSS with their efficacy to maintain self-administration in monkeys, and the resulting positive correlation is shown in Fig. 9B (Bauer et al., 2013b).
Fig. 9.
Correlation of monoamine releaser effects on ICSS with in vitro selectivity to promote DA versus 5HT release (A) and break points maintained under a progressive-ratio schedule of drug self-administration in rhesus monkeys (B). A, Abscissa: Log selectivity to release DA versus 5HT expressed as EC50 to promote 5HT release ÷ EC50 for DA release in a rat brain synaptosome preparation. Higher values indicate higher selectivity to release DA. Ordinate: Maximum facilitation of ICSS expressed as the maximum increase in the summary measure of % baseline stimulations. Fenfluramine was tested in both procedures but was excluded from this figure because it did not facilitate ICSS at any dose or time and because precise in vitro selectivity could not be quantified because of low potency to release DA. The regression was significant (Pearson r = 0.89, R2 = 0.78, P = 0.0006). B, Abscissa: Maximum break point maintained by any drug dose under a progressive-ratio schedule of drug self-administration in rhesus monkeys (Wee et al., 2005; Wang and Woolverton, 2007). Ordinate. Maximum facilitation of ICSS as in (A). −MDMA and fenfluramine were tested in both procedures but were excluded from the correlation because they did not facilitate ICSS in rats and/or did not reliably maintain self-administration in monkeys (self-administration by <50% of monkeys tested) ( NE, no effect). PAL-287 and phenmetrazine were also excluded, because they have not been tested under the progressive-ratio schedule of drug self-administration in rhesus monkeys. The regression was significant (Pearson r = 0.80, R2 = 0.63, P = 0.0320). For other details, see (Bauer et al., 2013b).
We have interpreted these results to suggest two conclusions relevant to the use of ICSS in abuse potential testing. First, at least for this drug class, “% baseline stimulations” (as determined with methods shown in Fig. 5) provides a useful summary measure of drug effects on ICSS for prediction of drug effects in more established drug self-administration procedures. Drug-induced change in ICSS threshold (as determined with methods shown in Fig. 6) is a different and commonly used dependent measure in frequency-rate ICSS studies, and monoamine releasers that increased % baseline stimulations also reduced ICSS thresholds. However, the magnitude of drug effects on ICSS thresholds did not correlate with either self-administration data (P = 0.67) or with pharmacological selectivity (P = 0.32) (Bauer et al., 2013b). Accordingly, these results provide one source of evidence to suggest that % baseline stimulations may be more useful than ICSS threshold as a dependent variable in ICSS for use in stratifying abuse potential. This approach has also been extended to evaluation of novel cathinone derivatives (a.k.a. “bath salts” such as mephedrone and methylone) that have emerged as drugs of abuse in the United States and Europe (Robinson et al., 2012; Bonano et al., 2014). Second, the summary measure of % baseline stimulations integrates both rate-increasing and rate-decreasing drug effects. It is the rate-increasing effects that are generally interpreted as “abuse-related” effects. By analogy, we suggest that it may be useful to interpret rate-decreasing effects as evidence of effects that might limit abuse potential. ICSS provides an efficient experimental approach to dissect and evaluate these two types of effects. For example, these data with monoamine releasers are consistent with the conclusion that abuse-related rate-increasing effects are mediated by DA, whereas abuse-limiting rate-decreasing effects are mediated by 5HT. This conclusion is consistent with results from other ICSS studies (Lin et al., 1997) and with a large literature of drug self-administration data to suggest that DA mediates reinforcing effects of releasers, whereas 5HT limits expression of reinforcing effects (Wee and Woolverton, 2006; Bradbury et al., 2013). In summary then, ICSS frequency-rate procedures permit simultaneous detection and study of effects that can either promote or limit abuse potential, and “% baseline stimulations” provides a useful summary measure for integration of these effects and prediction of their impact on drug reinforcement in self-administration procedures.
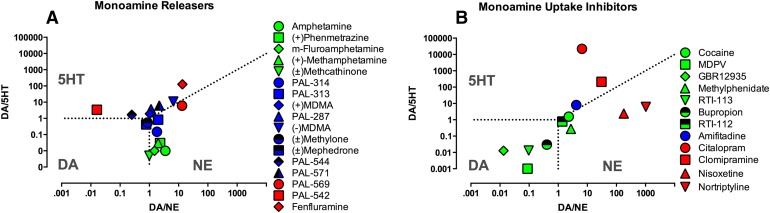
As one final point, Fig. 10A shows a summary of ICSS effects produced in our laboratory by 17 monoamine releasers graphed as a function of pharmacological selectivity to release DA, NE, and 5HT. This plot permits segregation of ICSS effects according to pharmacological selectivity of each drug to release each of the three monoamines, and the nature of drug effect is indicated qualitatively by color (green = exclusive facilitation as with amphetamine; blue = mixed facilitation and depression as with MDMA; red = exclusive depression as with fenfluramine). This type of plot can also be useful in characterizing pharmacological characteristics that contribute to abuse-related effects in ICSS.
Fig. 10.
Relationship between drug effects on ICSS and functional selectivity of drugs to release (A) or inhibit uptake (B) of DA, NE, and 5HT. Abscissae: Selectivity for DA versus NE expressed as potency to release/inhibit uptake of DA ÷ potency to release/inhibit uptake of NE. Ordinate: Selectivity for DA versus 5HT expressed as potency to release/inhibit uptake of DA ÷ potency to release/inhibit uptake of 5HT. Domains of selectivity for each monoamine are indicated by the abbreviation for that monoamine, and dotted lines show borders of equipotency for drugs to release/inhibit uptake of monoamines on either side of the border. Color scheme indicates most prominent drug effect on ICSS: green = facilitation, blue = mixed action, and red = depression. Data for monoamine releaser effects were collected in rat brain synaptosomes (Rothman et al., 2001; Bauer et al., 2013b; Bauer et al., 2014; Bonano et al., 2014). Data for monoamine uptake inhibition were taken from the Psychoactive Drug Screening Program database (pdsp.med.unc.edu/pdsp.php) (Rosenberg et al., 2013; Bonano et al., 2014).
b. Repeated/chronic administration.
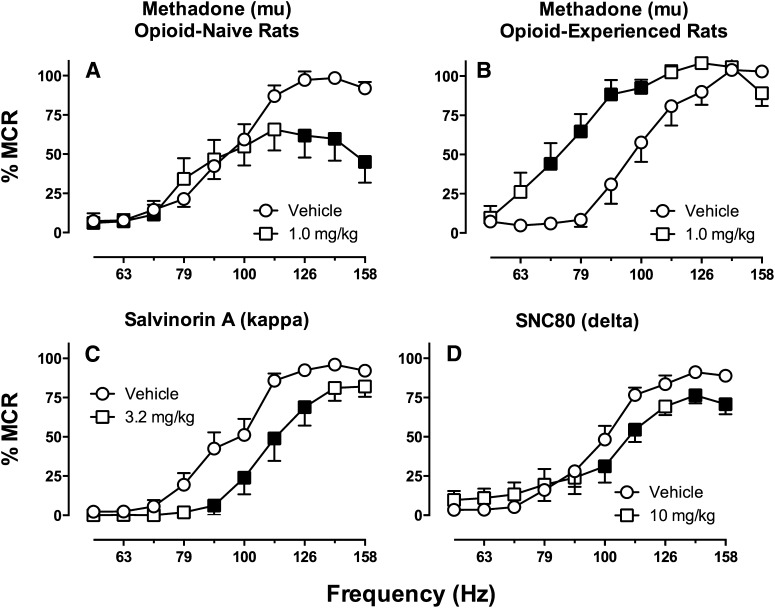
Amphetamine is the monoamine releaser that has been most extensively evaluated with repeated/chronic dosing, and these studies have revealed two general phenomena. First, tolerance to ICSS facilitation fails to develop after amphetamine administered by repeated bolus injections or by continuous infusion (e.g., via osmotic minipump) (Lin et al., 2000; Paterson et al., 2000; Bauer et al., 2014). Rather, amphetamine-induced ICSS facilitation is largely retained over time. In some studies, sensitization to amphetamine-induced facilitation of ICSS has been observed, but sensitization to amphetamine effects in ICSS procedures is less pronounced and less reliable than sensitization to locomotor effects (Lin et al., 2000; Cabeza de Vaca et al., 2004). A similar resistance to both tolerance and sensitization has also been observed with repeated/chronic dosing of cocaine in ICSS procedures (Riday et al., 2012), and this resistance to sensitization suggests a key distinction between stimulant effects on ICSS and locomotor activity. Second, withdrawal from chronic dosing of amphetamine produces transient depression of ICSS that can be reversed by administration with amphetamine or related drugs (e.g., cocaine) (Paterson et al., 2000; Bauer et al., 2014). This ICSS depression has been interpreted as a withdrawal sign that may be related to anhedonic signs of stimulant withdrawal in humans (Cryan et al., 2003).
A similar profile of sustained ICSS facilitation followed by withdrawal-associated ICSS depression has also been observed with methamphetamine (Miyata et al., 2011), but effects of chronic treatment with other releasers have not been explored. Of particular significance for abuse potential testing is whether effects of mixed-action DA/NE/5HT releasers like MDMA or the cathinone derivative mephedrone (Bonano et al., 2014) might change during repeated/chronic treatment. For example, self-administration studies with MDMA suggest that repeated exposure during acquisition of self-administration can produce tolerance to 5HT effects and increased expression of DA-mediated reinforcing effects (Schenk, 2009). Preliminary data from our laboratory suggest that a similar phenomenon occurs during repeated mephedrone treatment, such that repeated treatment produces decreased expression of 5HT-mediated rate-decreasing effects and increased expression of DA-mediated rate-increasing effects (J. Bonano and S.S. Negus, unpublished observations). Although full characterization of this effect will require further studies, these findings illustrate the potential of ICSS not only to assess abuse-related effects produced by acute drug administration, but also to assess changes in abuse-related effects that occur during repeated exposure.
2. Monoamine Uptake Inhibitors.
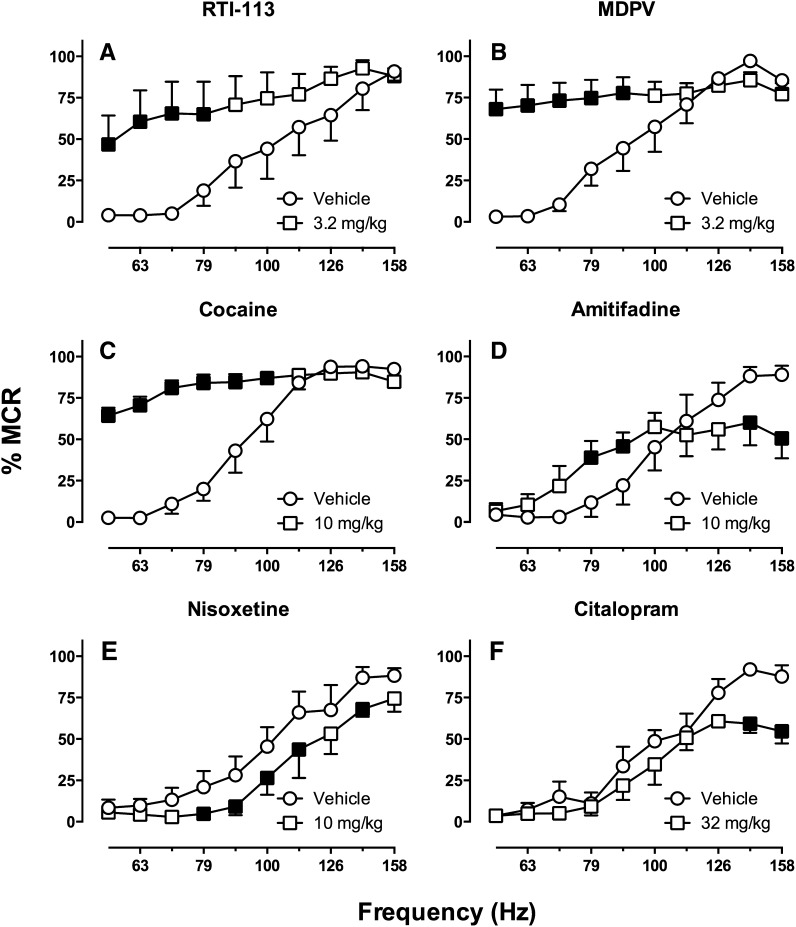
Monoamine uptake inhibitors also act at DA, NE, and/or 5HT transporters, but rather than functioning as substrates that pass through the transporter to promote monoamine release, they occlude the transporter and prevent uptake of synaptically released monoamines (Baldessarini, 2006; O'Brien, 2006). Both releasers and uptake inhibitors increase extracellular monoamine concentrations, but effects of uptake inhibitors are dependent on neuronal activity and are consequently often smaller in magnitude and narrower in anatomic scope than effects of releasers. Similar to the releasers, monoamine uptake inhibitors are used clinically for indications such as attention deficit disorder, obesity, and narcolepsy, and they are also used as front-line antidepressants. However, several members of this drug class, such as cocaine and methylphenidate, also have abuse liability. Table 2 summarizes data with a subset of representative monoamine uptake inhibitors that have been tested in various ICSS procedures.
TABLE 2.
Effects of monoamine uptake inhibitors on intracranial self-stimulation
| Drug Pharmacologya | Doses | Route | Strain/Species | Sex | ICSS Procedureb | Drug Effectc | Dependent Measure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug Name | Selectivity | Structure | Parameter | |||||||
| mg/kg | ||||||||||
| Cocaine | DA/NE/5HT | 0.63–10 | s.c. | Wistar rat | Male | Free operant | Facilitation | ↑rate | Wauquier and Niemergeers, 1974 | |
| Cocaine | DA/NE/5HT | 1–10 | i.p. | Fischer rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Esposito et al., 1978 |
| Cocaine | DA/NE/5HT | 1–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Negus et al., 2012a |
| Cocaine | DA/NE/5HT | 1.25–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Tomasiewicz et al., 2008 |
| Cocaine | DA/NE/5HT | 1–30 | i.p. | C57Bl6/J mouse | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Fish et al., 2010 |
| Cocaine | DA/NE/5HT | 1–30 | i.p. | DBA mouse | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Fish et al., 2010 |
| MDPV | DA/NE>5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Bonano et al., 2014 |
| MDPV | DA/NE>5HT | 0.1–2.0 | i.p. | Sprague-Dawley rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Watterson et al., 2014 |
| GBR 12935 | DA>NE/5HT | 1.0–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | K. Freitas and S. S. Negus, unpublished |
| RTI-113 | DA>NE/5HT | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Rosenberg et al., 2013 |
| Methylphenidate | DA/NE>5HT | 0.1–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | K. Freitas and S. S. Negus, unpublished |
| Buproprion | DA/NE>5HT | 3.2–32 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Rosenberg et al., 2013 |
| RTI-112 | DA/NE/5HT | 0.1–1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Rosenberg et al., 2013 |
| Amitifadine | DA/NE/5HT | 1–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | L. L. Miller and S. S. Negus, unpublished |
| Citalopram | DA/NE<5HT | 3.2–32 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Rosenberg et al., 2013 |
| Clomipramine | DA<NE/5HT | 3.2–32 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Rosenberg et al., 2013 |
| Fluoxetine | DA/NE<5HT | 1.0–10 | i.p. | Sprague-Dawley rat | Male | Free operant | Depression | ↓rate | Katz and Carroll, 1977 | |
| Fluoxetine | DA/NE<5HT | 2.5-20 | i.p. | Wistar rat | Male | Discrete trial | Amplitude | Depression | ↑CIT | Lee and Kornetsky, 1998 |
| Nisoxetine | DA/5HT<NE | 1–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Rosenberg et al., 2013 |
| Nortryptaline | DA<5HT<NE | 1–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Rosenberg et al., 2013 |
Rate, response rate; CIT, current-intensity threshold; θ0, theta-0 threshold.
Selectivity for monoamine uptake inhibition.
First column indicates structure of experimental session (see text for details). Second column indicates stimulation parameter under manipulation across trials.
Most prominent drug effect on ICSS.
a. Acute administration.
Monoamine uptake inhibitors can be subclassified according to their relative selectivities to inhibit DAT, NET, and/or SERT, and in general, effects of uptake inhibitors are similar to effects of releasers with comparable pharmacological selectivities. Figure 11 shows ICSS effects produced by selected doses of representative uptake inhibitors in our laboratory. RTI-113 [phenyl 3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate] is a DAT-selective inhibitor that produces dose-dependent ICSS facilitation across a broad dose range, and maximal facilitation of ICSS is similar to that produced by amphetamine (Rosenberg et al., 2013). Methylenedioxypyrovalerone (MDPV) is a cathinone derivative that has recently emerged as a designer drug of abuse often included in “bath salts,” and it has been designated as a schedule 1 drug by the U.S. Food and Drug Administration (FDA) (Baumann et al., 2013b). MDPV also functions as a relatively selective DAT inhibitor, and it produced dose-dependent and robust ICSS facilitation (Bonano et al., 2014). Cocaine is a nonselective inhibitor at DAT, NET, and SERT (Baumann et al., 2013b), but like the DAT-selective inhibitors, it robustly facilitates ICSS across a broad range of doses and conditions (Bauer et al., 2014) (see Table 2). In accordance with these ICSS data, RTI-113, MDPV, and cocaine are all self-administered by animals (Kimmel et al., 2008; Watterson et al., 2014).
Fig. 11.
Effects of the DA uptake inhibitors RTI-113 and methylenedioxypyrovalerone (MDPV), the mixed-action uptake inhibitors cocaine and amitifadine, the NE uptake inhibitor nisoxetine, and the 5HT uptake inhibitor citalopram on ICSS in rats. Abscissae: frequency of brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as %MCR. Graphs show maximal facilitation produced in dose-effect studies conducted with each drug. Filled points show drug effects significantly different from vehicle as determined by two-way ANOVA and the Holm-Sidak post hoc test (P < 0.05). Data sets are published for RTI-113, nisoxetine and citalopram (Rosenberg et al., 2013), cocaine (Bauer et al., 2014), and MDPV (Bonano et al., 2014). Effects of amitifadine are unpublished but collected with identical procedures.
Amitifadine is a novel monoamine uptake inhibitor with similar potencies to inhibit NET and SERT and slightly weaker potency to inhibit DAT (Skolnick et al., 2003; Golembiowska et al., 2012). The behavioral profile of amitifadine in ICSS resembles the profile of MDMA in that amitifadine facilitates low ICSS rates maintained by low frequencies of brain stimulation but also depresses high rates of ICSS maintained by high frequencies (Prins et al., 2012) (L. L. Miller and S. S. Negus, unpublished observations). Published data on the reinforcing effects of amitifadine are not available, but the related compound bicifadine was reported to function as a less reliable reinforcer in rhesus monkeys than other uptake inhibitors including cocaine (Nicholson et al., 2009). Lastly, nisoxetine and citalopram are NET- and SERT-selective inhibitors, respectively, and both produced only decreases in ICSS consistent with their failure to function as reinforcers in drug self-administration procedures (Hiranita et al., 2009; Rosenberg et al., 2013).
The data summarized above are consistent with many previous ICSS studies that found facilitation of ICSS by cocaine and DAT-selective inhibitors but not by NET- or SERT-selective inhibitors (e.g., see Table 2). Taken together, these results provide additional support for the relationship between ICSS facilitation and maintenance of drug self-administration. In addition, these results are also consistent with the well-established role of DA in mediating abuse-related effects of drugs acting at monoamine transporters. Figure 10B shows the pharmacological selectivity plot for effects of 12 monoamine uptake inhibitors on ICSS in our laboratory, and two observations warrant mention. First, as with monoamine releasers, efficacy to facilitate ICSS increases as selectivity for DAT versus SERT increases. Uptake inhibitors differ from releasers only in appearing to tolerate a greater proportion of 5HT effects than releasers (c.f. MDMA and cocaine are relatively nonselective compounds at DAT versus SERT, but cocaine produces more robust ICSS facilitation). Second, currently available uptake inhibitors include compounds like RTI-113 and nisoxetine that have greater selectivity at DAT versus NET, respectively, than has been obtained with releasers, and data with these compounds implicate DA rather than NE in mediating abuse-related ICSS facilitation.
b. Repeated/chronic administration.
Effects of repeated/chronic treatment with monoamine uptake inhibitors have been evaluated with cocaine and with some SERT- and NET-selective uptake inhibitors that are used clinically as antidepressants. In general, repeated/chronic treatment with cocaine produces effects that are similar to those of amphetamine in that neither tolerance nor sensitization to ICSS facilitation develops during long-term treatment (Frank et al., 1988; Kenny et al., 2003b; Riday et al., 2012; Bauer et al., 2014), and withdrawal produces a transient and cocaine-reversible depression of ICSS (Kokkinidis and McCarter, 1990; Markou and Koob, 1991; Stoker and Markou, 2011b). Conversely, chronic treatment with the SERT-selective inhibitor fluoxetine either did not alter ICSS (Lin et al., 1999) or produced a sustained depression of ICSS (Lee and Kornetsky, 1998), and fluoxetine withdrawal did not alter ICSS. Results have been less consistent with NET-selective inhibitors. For example, as with fluoxetine, several studies have found that chronic treatment with the NET-selective inhibitor desipramine either did not alter ICSS (Markou et al., 1992; Moreau et al., 1992) or produced a sustained depression of ICSS (Hall et al., 1990) without altering ICSS during withdrawal. However, other studies found slight but significant facilitation of ICSS during withdrawal from chronic use of desipramine (Fibiger and Phillips, 1981; McCarter and Kokkinidis, 1988).
3. Monoamine Receptor Agonists and Antagonists.
Drugs acting as monoamine releasers or uptake inhibitors produce indirect effects on DA, NE, and 5HT receptors by increasing extracellular monoamine levels. Drugs that act directly on DA, NE, and 5HT receptors have also been examined extensively in ICSS procedures. DA acts at a receptor family with two main receptor types: D1-like receptors (D1 and D5) and D2-like receptors (D2, D3, and D4) (Baik, 2013). NE acts on a separate family of receptors that also includes two major receptor types with subclassifications within each type (α1–2,β1–3) (Flordellis et al., 2004). 5HT acts on a third and larger family of receptors with seven major receptor types and additional subclassifications (5HT1–7 with several subtypes) (Kranz et al., 2010; Hayes and Greenshaw, 2011). All receptors except the 5HT3 receptor are G-protein-coupled receptors, with different members of each family coupled to excitatory or inhibitory signaling pathways. Monoamine receptor ligands are approved for various clinical applications including Parkinson’s disease (e.g., the nonselective DA agonist apomorphine), hypertension (e.g., the α2 adrenergic agonist clonidine and the nonselective β adrenergic antagonist propranolol), and obesity (e.g., the 5HT2c agonist lorcaserin) (Baldessarini and Tarazi, 2006; Sanders-Bush and Mayer, 2006; Standaert and Young, 2006; Westfall and Westfall, 2006). However, the only drugs in this class currently scheduled by the FDA are hallucinogens [e.g., lysergic acid diethyamide (LSD), psilocybin, and 4-methyl-2,5-dimethoxyamphetamine] that share agonist activity at 5HT2A receptors.
a. Dopamine receptor agonists and antagonists.
Although DA releasers and uptake inhibitors produce robust and reliable ICSS facilitation, direct DA agonists do not (Table 3). For example, the nonselective D1/D2 agonist apomorphine generally produces only depression of ICSS, although in frequency-rate procedures, high doses of apomorphine may produce weak facilitation of low ICSS rates maintained by low brain stimulation frequencies at doses that also depress high ICSS rates maintained by high frequencies (Liebman and Butcher, 1973; Strecker et al., 1982; Depoortere et al., 1996; Singh et al., 1996). Selective D1 agonists such as SKF38393 [(±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide], SKF82958 (3-allyl-6-chloro-1-phenyl-1,2,4,5-tetrahydro-3-benzazepine-7,8-diol), and A77636 [(1R,3S)-3-(1-adamantyl)-1-(aminomethyl)-3,4-dihydro-1H-isochromene-5,6-diol] have been reported to facilitate ICSS in some studies (Nakajima and O'Regan, 1991; Ranaldi and Beninger, 1994; Carr et al., 2001; Gilliss et al., 2002; Malanga et al., 2008), but the magnitude of facilitation is generally weak and may be accompanied by evidence for impaired performance, and other studies have reported only depression by D1 agonists (Hunt et al., 1994; Baldo et al., 1999). Similarly variable effects have been obtained with D2/3 agonists such as bromocriptine, quinpirole, quinelorane, 7-OH-DPAT [7-hydroxy-2-(di-n-propylamino)tetralin], and U99194A (5,6-dimethoxy-N,N-dipropyl-2,3-dihydro-1H-inden-2-amine) (Nakajima and O'Regan, 1991; Hunt et al., 1994; Ranaldi and Beninger, 1994; Kling-Petersen et al., 1995; Depoortere et al., 1996; Hatcher and Hagan, 1998; Carr et al., 2001, 2002; Malanga et al., 2008). For example, quinpirole facilitated ICSS in some studies (Ranaldi and Beninger, 1994; Carr et al., 2001), depressed ICSS in other studies (Rady et al., 1994; Hatcher and Hagan, 1998), and produced variable effects across doses and different ICSS rates in yet other studies (Nakajima and O'Regan, 1991; Depoortere et al., 1996; Malanga et al., 2008). The variable effects of DA agonists on ICSS contrast with the more reliable self-administration of these compounds in rats and nonhuman primates (Woolverton et al., 1984; Caine and Koob, 1993; Weed et al., 1993; Grech et al., 1996; O'Connor et al., 2011; Huskinson et al., 2014). Thus, DA agonists constitute one example of apparent discordance between ICSS and drug self-administration. Given that DA agonists are not scheduled by the FDA and are not typically abused by humans, ICSS may function as a better predictor than drug self-administration for abuse potential of these compounds.
TABLE 3.
Effects of dopamine receptor agonists on intracranial self-stimulation
| Drug Pharmacologya | Doses | Route | Strain/Species | Sex | ICSS Procedureb | Drug Effectc | Dependent Measure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug Name | Selectivity | Structure | Parameter | |||||||
| mg/kg | ||||||||||
| Apomorphine | D1/D2 | 0.03–0.3 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Depoortere et al., 1996 |
| Apomorphine | D1/D2 | 0.1 | s.c. | Wistar rat | Male | Hybrid | Amplitude | Depression | ↓rate | Strecker et al., 1982 |
| SKF81297 | D1 | 0.125–1.5 | s.c. | Wistar rat | Male | Discrete trial | Amplitude | Depression | ↑CIT | Baldo et al., 1999 |
| SKF81297 | D1 | 0.1–10 | i.p. | Swiss-Webster mouse | Male | Hybrid | Frequency | No effect | Malanga et al., 2008 | |
| SKF81297 | D1 | 0.1–10 | i.p. | Swiss-Webster mouse | Female | Hybrid | Frequency | No effect | Malanga et al., 2008 | |
| SKF82958 | D1 | 0.03–0.3 | i.p. | Swiss-Webster mouse | Male | Hybrid | Frequency | Facilitation | ↓M50 | Gilliss et al., 2002 |
| Quinpirole | D2 | 0.03–1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Depoortere et al., 1996 |
| Quinpirole | D2 | 0.1–10 | i.p. | Swiss-Webster mouse | Male | Hybrid | Frequency | Mixed | ↑↓θ0 | Malanga et al., 2008 |
| Quinpirole | D2 | 0.1–10 | i.p. | Swiss-Webster mouse | Female | Hybrid | Frequency | Mixed | ↑↓θ0 | Malanga et al., 2008 |
| Quinpirole | D2 | 0.5–2.0 | i.p. | Long Evans rat | Male | Hybrid | Frequency | Mixed | ↓1/4 threshold | Nakajima and O’Regan, 1991 |
Rate, response rate; CIT, current-intensity threshold; θ0, theta-0 threshold; M50, EF50, or LOR, frequency maintaining 50% maximum rate; 1/4 threshold, frequency maintaining 25% maximum rate.
Dopamine receptor subtype selectivity
First column indicates structure of experimental session (see text for details). Second column indicates stimulation parameter under manipulation across trials.
Most prominent drug effect on ICSS.
In contrast to the variable effects of DA agonists, only decreases in ICSS are observed with nonselective or subtype selective DA antagonists (Olds and Travis, 1960; Fenton and Liebman, 1982; Corbett, 1990; Hunt and Atrens, 1992; Carr et al., 2001; Flagstad et al., 2006; Higley et al., 2011; Negus et al., 2012a; Trujillo-Pisanty et al., 2013). These ICSS results are generally consistent with the lack of self-administration or abuse liability for DA antagonists, although one study did report haloperidol self-administration by rats (Glick and Cox, 1975). In contrast to the ICSS-depressing effects of acute treatment, repeated treatment with the DA antagonists pimozide or haloperidol produced DA receptor supersensitivity in rats that was associated with facilitation of ICSS (Ettenberg and Milner, 1977; Seeger et al., 1981b). This ICSS facilitation after withdrawal from a DA antagonist is opposite to the ICSS depression described above after withdrawal from repeated amphetamine or cocaine.
b. Norepinephrine and serotonin receptor agonists and antagonists.
NE agonists and antagonists have not been extensively evaluated in ICSS procedures, but compounds that have been examined either had no effect on ICSS or only depressed ICSS (Hunt et al., 1976; Liebman et al., 1984; Hunt and Atrens, 1992; Bruijnzeel et al., 2010). One compound of interest in relation to abuse potential testing is the α2 agonist clonidine. Clonidine is not scheduled by the FDA and has not emerged as a significant drug of abuse. Consistent with this profile, it has produced only decreases in ICSS (Hunt et al., 1976; Liebman et al., 1984; Hunt and Atrens, 1992). However, clonidine does maintain drug self-administration in rats and nonhuman primates (Shearman et al., 1981; Woolverton et al., 1982), and consequently, it represents another point of discrepancy between ICSS and drug self-administration.
ICSS is generally not affected or only depressed by systemically administered agonists and/or antagonists for 5HT1 receptors (Harrison et al., 1999; Harrison and Markou, 2001), 5HT2 receptors (Benaliouad et al., 2007; Hayes et al., 2009; Katsidoni et al., 2011), 5HT3 receptors (Greenshaw, 1993; Hatcher et al., 1995), and 5HT4 receptors (Reavill et al., 1998) (see Hayes and Greenshaw, 2011 for review). An exception is that low doses of the 5HT1A agonist 8-OH-DPAT may facilitate ICSS (Harrison and Markou, 2001). Of particular importance for abuse potential testing, the 5HT2A agonist TCB-2 failed to facilitate ICSS (Katsidoni et al., 2011). Although TCB-2 itself is not currently scheduled by FDA, it shares discriminative stimulus and other behavioral effects with schedule 1 hallucinogens like LSD (McLean et al., 2006; Fox et al., 2010). This evidence for poor sensitivity of ICSS to abused hallucinogens with 5HT2A agonist activity is similar to poor sensitivity of drug self-administration to drugs from this same class (Fantegrossi et al., 2008).
B. Opioids
Opioids act at three receptor types: mu, kappa, and delta opioid receptors. These G-protein–coupled receptors are localized throughout the nervous system, including brain areas such as the nucleus accumbens and VTA (Mansour et al., 1987). Moreover, there is evidence for modulation of mesolimbic DA neurons by each opioid receptor subtype (Devine et al., 1993). Mu opioid analgesics, such as the canonical mu agonist morphine, are among the most effective and widely used drugs in the treatment of pain, but unwanted effects such as abuse liability limit their therapeutic potential (Gutstein and Akil, 2006). Kappa and delta opioid agonists have also been considered as candidate therapeutics for pain and other health problems, but their clinical deployment has been prevented by undesirable side effects that include psychotomimetic and convulsant effects, respectively (Chavkin, 2011; Pradhan et al., 2011). Table 4 shows illustrative data with selected opioids in ICSS procedures.
TABLE 4.
Effects of opioids on intracranial self-stimulation
| Drug Pharmacologya | Doses | Route | Strain/Species | Sex | ICSS Procedureb | Drug Effectc | Dependent Measure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug Name | Selectivity | Structure | Parameter | |||||||
| mg/kg | ||||||||||
| Morphine | Mu | 5.25–10.25 | s.c. | Rat/male | Male | Free operant | Depression | ↓rate | Olds and Travis, 1960 | |
| Morphine | Mu | 10 | s.c. | Albino rat | Free operant | Mixed; tolerance | ↑↓rate | Adams et al., 1972 | ||
| Morphine | Mu | 5.0–20 | s.c. | Sprague-Dawley rat | Male | Free operant | Mixed; tolerance | ↑↓rate | Lorens and Mitchell, 1973 | |
| Morphine | Mu | 4.0–12 | s.c. | CDF rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Marcus and Kornetsky, 1974 |
| Morphine | Mu | 1.0–14 | s.c. | CDF rat | Male | Discrete trial | Amplitude | Mixed; tolerance | ↓↑CIT | Esposito and Kornetsky, 1977 |
| Morphine | Mu | 1.0–18 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi and Negus, 2011 |
| Morphine | Mu | 1.0–10 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi et al., 2012 |
| Morphine | Mu | 2.5 | i.p. | Long Evans rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Carlezon and Wise, 1993a |
| Morphine | Mu | 1.0–5.6 | s.c. | C57BL/6J mouse | Male | Hybrid | Frequency | Facilitation | ↓M50 | Elmer et al., 2010 |
| Morphine | Mu | 1.0–5.6 | s.c. | DBA mouse | Male | Hybrid | Frequency | Depression | ↑M50 | Elmer et al., 2010 |
| Heroin | Mu | 5.0 | i.p. | Wistar rat | Male | Free operant | Mixed; tolerance | Koob et al., 1975 | ||
| Methadone | Mu | 0.1–5.6 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi et al., 2012 |
| Methadone | Mu | 0.32–3.2 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi et al., 2013 |
| Fentanyl | Mu | 0.001–0.1 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Altarifi et al., 2012 |
| Fentanyl | Mu | 0.001–0.1 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi et al., 2013 |
| Hydrocodone | Mu | 0.1–10 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Altarifi et al., 2012 |
| Buprenorphine | Mu | 0.001–0.1 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Altarifi et al., 2012 |
| Nalbuphine | Mu | 0.1–10 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↑rate | Altarifi et al., 2012 |
| Nalbuphine | Mu | 0.1–10 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed; tolerance | ↑↓rate | Altarifi et al., 2013 |
| β-Funaltrexamine | Mu | 0.32–3.2 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | No effect | Altarifi et al., 2012 | |
| Naloxone | Mu | 0.1 | s.c. | Sprague-Dawley rat | Male | Hybrid | Frequency | No effect | Altarifi et al., 2012 | |
| Naloxone | Mu | 20 | i.p. | Wistar rat | Male | Hybrid | Amplitude | No effect | θ5 | Borowski and Kokkinidis, 1992 |
| U-69593 | Kappa | 0.0625–0.5 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↑θ0 | Todtenkopf et al., 2004 |
| U-69593 | Kappa | 0.1–0.56 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↑EF50 | Do Carmo et al., 2009 |
| U-69593 | Kappa | 0.056–0.56 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Negus et al., 2010 |
| U-69593 | Kappa | 0.56 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Leitl et al., 2014 |
| Salvinorin A | Kappa | 0.32–3.2 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Negus et al., 2012a |
| Salvinorin A | Kappa | 0.125–2.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↑θ0 | Carlezon et al., 2006 |
| SNC80 | Delta | 10–56 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Do Carmo et al., 2009 |
| SNC80 | Delta | 10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression; tolerance | ↓rate | Negus et al., 2012b |
Rate, response rate; CIT, current-intensity threshold; θ0, theta-0 threshold; θ5, amplitude maintaining five responses per minute; M50 or EF50, frequency maintaining 50% maximum rate.
Opioid receptor subtype selectivity.
First column indicates structure of experimental session (see text for details). Second column indicates stimulation parameter under manipulation across trials.
Most prominent drug effect on ICSS. Tolerance: tolerance to drug-induced depression of ICSS.
1. Mu Opioids.
a. Acute administration.
Among the three classes of opioid receptor ligands, drugs acting at mu receptors have been most widely studied in ICSS, and morphine was among the first drugs studied after the discovery of ICSS (Olds and Travis, 1960). This study used a free-operant procedure that maintained stable high ICSS rates and found only morphine-induced depression of ICSS, with the most robust depression of ICSS seen after administration of the highest doses tested. Results from subsequent studies using similar procedures showed that, early in their time course, morphine and heroin depressed ICSS, but this initial depression was followed by later facilitation (Adams et al., 1972; Lorens and Mitchell, 1973; Koob et al., 1975). These time-dependent effects in free-operant procedures highlighted the difficulty of dissociating abuse-related from motor effects in free-operant procedures and prompted subsequent studies with discrete-trial procedures in an effort to dissociate abuse-related and sedative effects; these studies found facilitation of ICSS even at early time points (Marcus and Kornetsky, 1974; Esposito and Kornetsky, 1977). More recent studies with frequency-rate procedures have confirmed that mu agonists produce complex effects on ICSS determined by factors that include dose, pretreatment time, and efficacy of the agonist at mu receptors (O’Neill and Todtenkopf, 2010; Altarifi and Negus, 2011; Altarifi et al., 2012, 2013). For example, relatively high-efficacy mu agonists such as methadone, fentanyl, and morphine weakly facilitate ICSS at low doses; however, at higher doses, they produce initial ICSS depression followed later by ICSS facilitation. Low-efficacy mu agonists such as buprenorphine and nalbuphine also facilitate ICSS, but even at high doses, these compounds are relatively ineffective at producing ICSS depression. Finally, opioid antagonists like β-funaltrexamine and naloxone do not alter ICSS at doses that block mu agonist effects. Experiments to examine mechanisms of the rate-increasing versus rate-decreasing effects of mu agonists in rats suggest that both types of effect are mediated by pharmacologically similar populations of mu receptors located in distinct regions of brain, with ICSS facilitation mediated by forebrain mu receptors in the vicinity of striatum, whereas ICSS depression is mediated by brainstem receptors in the vicinity of periaqueductal gray (Broekkamp et al., 1976; Altarifi et al., 2012).
b. Repeated/chronic administration.
A major determinant of mu agonist effects on ICSS is opioid exposure history. As discussed above, depression of ICSS is a predominant effect of acute treatment with high-efficacy mu agonists like methadone, fentanyl, and morphine. However, repeated/chronic administration with morphine or other mu agonists produces tolerance to rate-decreasing effects and increased expression of abuse-related rate-increasing effects (Adams et al., 1972; Lorens and Mitchell, 1973; Koob et al., 1975; Carlezon and Wise, 1993a; Easterling and Holtzman, 1997a; Altarifi and Negus, 2011; Altarifi et al., 2012, 2013). It is especially relevant for abuse potential testing to note that, as with monoaminergic drugs discussed above, repeated treatment produces little or no tolerance to abuse-related ICSS facilitation by mu agonists. Rather, it appears that tolerance develops selectively to mu agonist-induced ICSS depression, and this tolerance to rate-decreasing effects unmasks and increases the expression of rate-increasing effects. For example, Fig. 12, A and B, shows effects of 1.0 mg/kg methadone in opioid-naive rats and opioid-experienced rats maintained on 3.2 mg/kg per day morphine (Altarifi et al., 2013). In the naive rats, this methadone dose only depressed high rates of ICSS maintained by high brain stimulation frequencies; however, during morphine treatment, the same methadone dose produced robust facilitation of low ICSS rates maintained by low brain stimulation frequencies. In contrast to these effects with repeated agonist treatment, repeated administration of the mu-selective antagonist naloxone did not alter ICSS (Borowski and Kokkinidis, 1992).
Fig. 12.
Effects of the mu agonist methadone, the kappa agonist salvinorin A, and the delta agonist SNC80, on ICSS in rats. Abscissae: frequency of brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as %MCR. Graphs show illustrative data from dose-effect studies conducted with each drug. Effects of methadone were determined in opioid-naive subjects and again in the same rats during treatment with 3.2 mg/kg per day morphine (opioid-experienced subjects). Filled points show drug effects significantly different from vehicle as determined by two-way ANOVA and the Holm-Sidak post hoc test (P < 0.05). Data sets are published for methadone (Altarifi et al., 2013), salvinorin A (Negus et al., 2012a), and SN80 (Negus et al., 2012b).
Opioid tolerance is often accompanied by dependence as indicated by emergence of abstinence signs during withdrawal, and either spontaneous or antagonist-precipitated withdrawal from mu agonist treatment can produce depression of ICSS (Bermudez-Rattoni et al., 1983; Schulteis et al., 1994; Easterling and Holtzman, 1997a; Easterling et al., 2000; Liu et al., 2008; Altarifi et al., 2013). In one study, for example, rats were treated once per day with an escalating regimen of morphine doses that culminated in a terminal dose of 18 mg/kg per day (Altarifi et al., 2013). Spontaneous withdrawal from this dosing regimen produced ICSS depression manifested as a rightward shift in the frequency-rate curve, with peak depression occurring after 1 day followed by nearly complete recovery after 3 days. In another study, rats responding in a discrete-trial current-intensity procedure were implanted with morphine pellets for continuous morphine delivery, and low naloxone doses that had no effect in control rats depressed ICSS in morphine-treated rats (Schulteis et al., 1994). Spontaneous and antagonist-precipitated withdrawal also produced significant but weaker depression of ICSS after acute treatment with high mu agonist doses, suggesting that dependence can develop rapidly (Easterling et al., 2000; Altarifi et al., 2012). Moreover, this evidence for acute dependence can be obtained with both high- and low-efficacy mu agonists, suggesting that even low-efficacy activation of opioid receptors is sufficient to produce opioid dependence of brain reward systems.
Taken together, these studies with acute and chronic mu agonist treatment have identified a range of conditions under which mu agonists facilitate ICSS. These ICSS findings complement evidence for reinforcing effects by high-, intermediate-, and low-efficacy mu agonists in drug self-administration procedures (O'Connor et al., 2011). In addition, evidence that mu agonist exposure increases expression of abuse-related ICSS facilitation resonates with related findings that mu agonist exposure also increases expression of reinforcing effects in preclinical self-administration procedures (Thompson and Schuster, 1964; Yanagita, 1978; Carrera et al., 1999; Negus and Rice, 2009; O'Connor et al., 2011) and expression of abuse-related subjective and reinforcing effects in humans (Lasagna et al., 1955; Comer et al., 2010; Cooper et al., 2012). Consequently, studies with mu agonists extend the pharmacological range of concordance between ICSS and drug self-administration procedures.
2. Kappa and Delta Opioids.
Selective kappa opioid receptor agonists such as U50,488 (5,6-dimethoxy-N,N-dipropyl-2,3-dihydro-1H-inden-2-amine), U69,593 [N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide], and salvinorin A produce a dose-dependent depression of ICSS (Todtenkopf et al., 2004; Carlezon et al., 2006; Dinieri et al., 2009; Do Carmo et al., 2009; Negus et al., 2010, 2012a; Russell et al., 2013). For example, Fig. 12C shows depression of ICSS by salvinorin A, a psychoactive constituent of the salvia divinorum plant. Moreover, in contrast to effects with mu agonists, repeated salvinorin A treatment failed to produce either tolerance to rate-decreasing effects or enhanced expression of rate-increasing effects (Potter et al., 2011). Taken together, these ICSS effects with kappa agonists are consistent with the lack of evidence for reinforcing effects by kappa agonists in drug self-administration studies (Tang and Collins, 1985; Negus et al., 2008).
The systemically active, nonpeptidic delta opioid agonist SNC80 [4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide] produced only a dose-dependent depression of ICSS; however, the magnitude of this depression was modest compared with kappa agonists, and in contrast to effects of mu agonists, initial rate-decreasing effects were not followed by later ICSS facilitation (Do Carmo et al., 2009; Negus et al., 2012b). Moreover, repeated SNC80 treatment produced complete tolerance to ICSS depression but failed to reveal ICSS facilitation. In agreement with these findings, SNC80 and the related delta agonist BW373U86 [4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide] failed to maintain drug self-administration in rhesus monkeys (Negus, 2004). Central administration of peptidic delta agonists has been reported to both facilitate ICSS and maintain self-administration (Belluzzi and Stein, 1977; Jenck et al., 1987; Negus and Dykstra, 1989; Negus, 2004), suggesting that delta opioid receptors can play a role in reward processes; however, this review focuses on systemically administered drugs most likely to be examined in the course of abuse potential testing.
C. Cholinergic Drugs
Acetylcholine (ACh) acts at two main receptor types, the nicotinic receptors (nAChRs) and muscarinic receptors (mAChRs). nAChRs are ligand-gated ion channels composed of five subunits organized around a cation pore, and diverse subtypes of nAChRs have been identified with different subunit compositions (Taylor, 2006a; Millar and Gotti, 2009). Drugs acting at nAChRs include the prototype agonist nicotine, which is not only a principal psychoactive constituent of tobacco products but is also increasingly available in smoking-cessation products and in formulations that can be used with electronic cigarettes (Palazzolo, 2013). Other clinically available nAChR ligands include the intermediate-efficacy agonist varenicline and the noncompetitive antagonist mecamylamine, both of which are also used in smoking-cessation products. mAChRs are a family of five G-protein–coupled receptors usually coupled to Gi or Gq (Taylor, 2006a). mAChR agonists such as pilocarpine or carbachol are used clinically in ophthalmic applications such as treatment of glaucoma, whereas antagonists such as atropine and scopolamine are used to treat problems associated with excessive activation of the parasympathetic nervous system such as bradycardia or nausea. Acetylcholine is metabolized by the enzyme acetycholinesterase (AChE), and cholinergic drugs also include AChE inhibitors such as physostigmine, which are used for applications that include treatment of Alzheimer’s disease (Taylor, 2006b). Novel drugs that act on cholinergic signaling are also being developed for other applications that include cognitive enhancement (Sarter et al., 2009; Demeter and Sarter, 2013). At present, no cholinergic drugs are scheduled by the FDA.
1. Nicotinic Acetylcholine Receptor Ligands.
By far the most widely studied cholinergic drug in ICSS has been nicotine, which produces its effects primarily by functioning as an agonist at the α4β2 nAChR subtype. Numerous studies have shown that nicotine produces a biphasic effect with modest but significant ICSS facilitation at low doses and depression of ICSS at higher doses (Druhan et al., 1989; Huston-Lyons et al., 1993; Bauco and Wise, 1994; Panagis et al., 2000; Harrison et al., 2002; Tobey et al., 2012) (see Table 5 for illustrative data). For example, Fig. 13A shows facilitation of ICSS by a dose of 0.1 mg/kg i.p. nicotine in rats responding under a frequency-rate procedure, and higher doses recruited rate-decreasing effects and ICSS depression. Nicotine has been shown to facilitate ICSS regardless of whether it was administered noncontingently by the experimenter (as most drugs are in ICSS studies), or if it was self-administered by the experimental subject prior to ICSS testing (Kenny et al., 2009). Moreover, nicotine also facilitated ICSS when it was administered in an aqueous tobacco extract that also included other tobacco alkaloids (Harris et al., 2012). Repeated nicotine treatment produces tolerance to the rate-decreasing effects of high nicotine doses but neither tolerance nor sensitization to ICSS facilitation by lower nicotine doses (Bauco and Wise, 1994; Bozarth et al., 1998). Lastly, as with abused stimulant and opioid drugs described above, termination of chronic nicotine treatment can produce a withdrawal-associated depression of ICSS (Bozarth et al., 1998; Epping-Jordan et al., 1998; Bauzo and Bruijnzeel, 2012). Nicotine is distinguished from stimulant and opioid drugs of abuse primarily by the weak magnitude of maximal ICSS facilitation that can be achieved after either acute or repeated/chronic nicotine treatment. The relatively weak effects of nicotine in ICSS procedures appears to parallel its relatively weak efficacy as a reinforcer in drug self-administration procedures (Caille et al., 2012).
TABLE 5.
Effects of other drugs on intracranial self-stimulation
| Drug Pharmacologya | Doses | Route | Strain/Species | Sex | ICSS Procedureb | Drug Effectc | Dependent Measure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug Name | Mechanism | Structure | Parameter | |||||||
| mg/kg | ||||||||||
| Nicotine | nAChR agonist | 0.025–0.8 | s.c. | Long Evans rat | Male | Hybrid | Frequency | Mixed | ↓θ0 | Bauco and Wise, 1994 |
| Nicotine | nAChR agonist | 0.06–1.0 | s.c. | F-344 rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Huston-Lyons and Kornetsky, 1992 |
| Pentobarbital | GABAA + allosteric modulator | 2.5–10 | i.p. | Long Evans rat | Male | Hybrid | Frequency | Facilitation | ↓M50 | Bossert and Franklin, 2003 |
| Diazepam | GABAA + allosteric modulator | 0.5–4.0 | i.p. | C57Bl6/J mouse | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Straub et al., 2010 |
| Toluene. | GABAA + allosteric modulator/NMDA ant | 480–5000 ppm | C57Bl6/J mouse | Male | Hybrid | Frequency | Mixed | ↓M50 ↑↓rate | Tracy et al., 2014 | |
| Phencyclidine | NMDA antagonist | 0.5–5.0 | i.p. | CDF rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Kornetsky et al., 1979 |
| Phencyclidine | NMDA antagonist | 0.3–5.6 | i.p. | Wistar rat | Male | Discrete trial | Amplitude | No effect | Bespalov et al., 1999 | |
| Phencyclidine | NMDA antagonist | 2.5 and 5.0 | i.p. | Long Evans rat | Male | Hybrid | Frequency | Mixed | ↓θ0 ↑↓rate | Carlezon and Wise, 1993b |
| MK801 | NMDA antagonist | 0.01–0.3 | i.p. | Lister rat | Male | Free operant | Mixed | ↑↓rate | Herberg and Rose, 1989 | |
| MK801 | NMDA antagonist | 0.032–0.32 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓rate | Hillhouse et al., 2014 |
| Ketamine | NMDA antagonist | 0.3–100 | i.p. | Lister rat | Male | Free operant | Mixed | ↑↓rate | Herberg and Rose, 1989 | |
| Ketamine | NMDA antagonist | 3.2–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Hillhouse et al., 2014 |
| THC | CB1/CB2 agonist | 1.0 | i.p. | Fischer 344 rat | Male | Hybrid | Frequency | No effect | Lepore et al., 1996 | |
| THC | CB1/CB2 agonist | 1.0 | i.p. | Lewis rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 ↓M50 | Lepore et al., 1996 |
| THC | CB1/CB2 agonist | 1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Facilitation | ↓θ0 | Lepore et al., 1996 |
| THC | CB1/CB2 agonist | 0.1 and 1.0 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Mixed | ↑↓EF36.7 | Katsidoni et al., 2013 |
| THC | CB1/CB2 agonist | 1.0–10 | i.p. | Sprague-Dawley rat | Male | Hybrid | Frequency | Depression | ↓rate | Kwilasz and Negus, 2012 |
| Caffeine | adenosine antagonist | 1.0–20 | i.p. | Wistar rat | Male | Discrete trial | Amplitude | Mixed | ↑↓CIT | Bespalov et al., 1999 |
| Testosterone | anabolic steroid | 50 μg | Sherman rat | Male | Free operant | Facilitation | ↑rate | Caggiula and Hoebel, 1966 | ||
| Tripelennamine | H1 antagonist | 0.625–20 | i.p. | CDF rat | Male | Discrete trial | Amplitude | Facilitation | ↓CIT | Unterwald et al., 1984 |
Rate, response rate; CIT, current-intensity threshold; θ0, theta-0 threshold; M50 or EF50, frequency maintaining 50% maximum rate; EF36.7, frequency maintaining 36.7% maximum rate.
Mechanism of action or drug class.
First column indicates structure of experimental session (see text for details). Second column indicates stimulation parameter under manipulation across trials.
Most prominent drug effect on ICSS.
Fig. 13.
Effects of the nicotine, caffeine, diazepam, toluene vapor, MK801, and ketamine on ICSS in mice (diazepam, toluene) or rats (all other drugs). Abscissae: frequency of brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as %MCR. Graphs show maximal facilitation produced in dose-effect studies conducted with each drug. Filled points show drug effects significantly different from vehicle as determined by two-way ANOVA and the Holm-Sidak post hoc test (P < 0.05). Data sets are published for diazepam and toluene (Tracy et al., 2014) and for MK801 and ketamine (Hillhouse et al., 2014). Results with nicotine and caffeine are unpublished but collected with identical procedures.
Far fewer studies have been conducted with other nAChR ligands. ICSS was facilitated by the α4-selective agonist SIB-1765F [[6]-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine] and the intermediate-efficacy α4β2 nAChR agonist varenicline, but not by the α7-selective agonist ARR-17779 [(2S)-2′H-spiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxazolidin]-2′-one] (Spiller et al., 2009). Conversely, ICSS was either not affected or depressed by nAChR antagonists, including the nonselective antagonists mecamylamine and dihydro-β-erythroidine, the α4β2-selective antagonist 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine, and the α7 antagonist methyllycaconitine (Panagis et al., 2000; Harrison et al., 2002; Spiller et al., 2009; Tobey et al., 2012).
2. Muscarinic Acetylcholine Receptor Ligands and Enzyme Acetycholinesterase Inhibitors.
Few studies have examined effects of systemically administered mAChR ligands and AChE inhibitors on ICSS. In general, these studies found that ICSS was depressed by mAChR agonists (arecoline and pilocarpine) and the AChE inhibitor physostigmine, but facilitated by the mAChR antagonist scopolamine (Olds and Domino, 1969; Olds, 1972; Druhan et al., 1989). The facilitation of ICSS by scopolamine parallels evidence for reinforcing effects of scopolamine in drug self-administration procedures in rats and mice (Glick and Guido, 1982; Rasmussen and Fink-Jensen, 2000). Other studies have examined effects of centrally administered mAChR ligands, and in contrast to effects of systemic administration, both scopolamine and the other mAChR antagonist atropine depressed ICSS (Kofman and Yeomans, 1988). These latter studies have contributed to the hypothesis that ICSS is mediated in part by activation of cholinergic neurons that, in turn, activate mesolimbic DA neurons by actions at mAChRs. However, the discrepancy in effects of mAChR antagonists after central versus systemic administration has not been investigated. Given interest in development of mAChR ligands and AChE inhibitors for cognitive enhancement and other applications, more extensive studies with these drugs in ICSS procedures are warranted (Conn et al., 2009; Bubser et al., 2012; Jones et al., 2012).
D. GABAergic Drugs
γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the central nervous system, and it binds to two major receptor types, the GABAA and GABAB receptors (Charney et al., 2006; Vlachou and Markou, 2010). GABAA receptors are ligand-gated chloride channels, and because extracellular chloride concentrations are usually greater than intracellular concentrations in neurons, activation of GABAA receptors usually causes an influx of negatively charged chloride anions that hyperpolarizes neurons and reduces neuronal excitability (Farrant and Nusser, 2005; Egawa and Fukuda, 2013). GABAA receptors are composed of five subunits organized around an anion pore, and diverse GABAA receptor subtypes exist with different subunit compositions (including a subtype composed exclusively of rho subunits and known formerly as GABA C receptors). Orthosteric ligands for the GABAA receptor include the agonist muscimol and the antagonist bicuculline, but the more clinically relevant GABAA ligands bind to allosteric sites and function as positive modulators of GABAA function. These positive allosteric modulators include benzodiazepines (e.g., diazepam), barbiturates (e.g., pentobarbital), and neuroactive steroids (e.g., alphaxalone) used for applications that include anxiolysis, sedation, and anesthesia. A variety of other chemicals including ethanol and some volatile inhalants (e.g., toluene) also positively modulate GABAA receptor function. GABAB receptors are Gi/o-protein–coupled receptors that exist as dimers of two GABAB subunits linked to inhibitory signaling pathways, including calcium channel inhibition, potassium channel activation, and inhibition of adenylate cyclase (Vlachou and Markou, 2010; Benarroch, 2012). The only clinically available GABAB ligand is the agonist baclofen, but other drugs have been developed that function as agonists, antagonists and allosteric modulators. Lastly, an array of drugs acting in less clearly specified ways with GABA signaling includes pregabalin, gabapentin, γ-hydroxybutyric acid (GHB), and vigabatrin. Many GABAergic drugs have abuse liability and are scheduled by the FDA in schedule 1 (GHB), schedule 2 (pentobarbital), schedule 3 (hexobarbital), schedule 4 (diazepam, zolpidem, phenobarbital), and schedule 5 (pregabalin). Unscheduled chemicals (e.g., ethanol, toluene) that act at least in part via GABA receptors also have established abuse liability.
1. GABAA Receptor Ligands.
ICSS research with GABAA ligands has focused on studies of positive allosteric modulators, in part because many drugs from this general class have known abuse liability (see Table 5 for illustrative data). In general, ICSS is facilitated by barbiturates including phenobarbital and pentobarbital, although the magnitude of this facilitation is generally small relative to effects of stimulants like amphetamine (Reid et al., 1964; Seeger et al., 1981a; Bossert and Franklin, 2003). ICSS is also typically facilitated by benzodiazepines including midazolam, diazepam, and chlordiazepoxide (Olds, 1966; Ichimaru et al., 1985; Straub et al., 2010; Tracy et al., 2014; Engin et al., 2014). For example, Fig. 13C shows facilitation of ICSS by 3.0 mg/kg i.p. diazepam in mice responding under a hybrid frequency-rate procedure, and higher doses recruited rate-decreasing effects and ICSS depression. To our knowledge, the effects of repeated treatment with these or other barbiturates or benzodiazepines on ICSS have not been examined but would clearly be of interest to assess the potential for tolerance to rate-decreasing effects and enhanced expression of abuse-related rate-increasing effects.
Evidence for abuse-related ICSS facilitation by these barbiturates and benzodiazepines is consistent with evidence both for the reinforcing effects of these drugs in drug self-administration procedures and for their abuse liability in humans (Collins et al., 1984; Griffiths et al., 1991). One possible exception to this concordance may be zolpidem, a nonbenzodiazepine sedative that selectively targets the benzodiazepine binding site of GABAA receptors containing the α1 subunit. In the only study of its effects on ICSS, zolpidem failed to facilitate ICSS in mice (Reynolds et al., 2012); however, numerous drug self-administration studies in nonhuman primates have documented reinforcing effects of zolpidem, and zolpidem is currently classified as a schedule 4 compound by the FDA along with most other benzodiazepines (Griffiths et al., 1992; Licata and Rowlett, 2011). This apparent discrepancy illustrates the opportunity for further studies with established and novel GABAA positive allosteric modulators to assess the predictive validity of ICSS for assessment of abuse potential. This class of drugs includes numerous scheduled and unscheduled drugs that have been extensively evaluated for abuse potential in other procedures, and a rank-ordering of relative abuse liability for 19 hypnotics has been proposed (Griffiths and Johnson, 2005). This previous work provides an empirical foundation that could be used for systematic study of effects produced by the same drugs on ICSS.
2. GABAB Receptor Ligands and Other GABAergic Drugs.
The clinically available and nonscheduled GABAB agonist baclofen only depresses ICSS, and similar effects have been obtained with other GABAB agonists, antagonists, and positive allosteric modulators (Fenton and Liebman, 1982; Macey et al., 2001; Slattery et al., 2005; Paterson et al., 2008; Vlachou et al., 2011). Other drugs acting at GABA receptors have a more varied profile of effects. For example, ethanol typically produces a modest but significant facilitation of ICSS, as do some abused solvents like toluene (Kornetsky et al., 1988; Bespalov et al., 1999; Bespalov et al., 2003; Chan et al., 2012; Fish et al., 2010; Tracy et al., 2014) (Fig. 13D). Conversely, gabapentin and vigabatrin only depressed ICSS under conditions in which pentobarbital facilitated ICSS (Bossert and Franklin, 2003; Paterson et al., 2005). These findings generally agree with the literature on reinforcing effects of these drugs. For example, drug self-administration is maintained by ethanol and toluene, whereas baclofen maintained significant but only low levels of self-administration in baboons, and vigabatrin was not self-administered by rhesus monkeys (Griffiths et al., 1991; Takada and Yanagita, 1997; Blokhina et al., 2004; Green and Grahame, 2008).
E. Glutamatergic Drugs
Glutamate is the primary excitatory neurotransmitter in the mammalian central nervous system, and it binds to an array of ionotropic and metabotropic receptors (Javitt et al., 2011; Osikowicz et al., 2013). Ionotropic glutamate receptors (iGluRs) are each assembled from four subunits structured around a cation pore, and iGluRs are divided into three types named for compounds that bind them selectively: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), kainate, and NMDA (N-methyl-d-aspartate) (Dingledine et al., 1999; Meldrum, 2000). Metabotropic glutamate receptors (mGluRs) are G-protein–coupled receptors divided into three groups (groups I, II, and III), and each group is divided into further subtypes for a total of eight metabotropic glutamate receptor subtypes (Pilc et al., 2008). Group I receptors (mGlu1, mGlu5) are primarily postsynaptic receptors coupled to Gq/G11, and they mediate excitatory postsynaptic effects that include calcium mobilization and diacylglycerlol production. Group II receptors (mGlu2, mGlu3) and group III receptors (mGlu4, mGlu5–8) are primarily presynaptic receptors that couple to Gi/Go to mediate inhibition of adenylate cyclase. Drugs acting at all these receptors have been evaluated as therapeutics, and these drugs include not only orthosteric agonists, partial agonists, and antagonists but also allosteric modulators and, in the case of iGluRs, channel blockers. Glutamate release and extracellular glutamate levels are also regulated by an array of membrane-bound and vesicular glutamate transporters, and drugs targeting these transporters are also under investigation (Divito and Underhill, 2014). The only drugs with current or historical approval as therapeutics are noncompetitive/uncompetitive NMDA receptor antagonists such as memantine, phencyclidine (PCP), and ketamine. PCP and ketamine are scheduled by the FDA, and some other abused compounds including ethanol also appear to have antagonist actions at NMDA receptors (Moykkynen and Korpi, 2012).
1. N-Methyl-d-aspartate Receptor Ligands.
The NMDA receptor has at least four types of binding sites relevant to drug action: a glutamate binding site, a glycine binding site, a binding site in the cation channel, and an array of allosteric binding sites (Monaghan et al., 2012). Ligands that bind directly to the glutamate or glycine sites do not facilitate ICSS and may depress ICSS at high doses. For example, ICSS was not affected or was depressed by the competitive glutamate-site antagonists midafotel and LY23959 [(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid] (Bespalov et al., 2006; Kenny et al., 2009) as well as by an agonist (sarcosine), partial agonist (d-cycloserine), and antagonist [L701324 (7-chloro-4-hydroxy-3-(3-phenoxy)phenyl-2(1H)-quinolinone] of the glycine binding site (Herberg and Rose, 1990; Bespalov et al., 2006; Chan et al., 2012). Studies with systemically administered glutamate site agonists have not been published, but administration of N-methyl-d,l-aspartate directly into the ventral tegmental area also failed to alter ICSS (Willick and Kokkinidis, 1995).
In contrast to these effects of orthosteric ligands, numerous studies have reported facilitation of ICSS by NMDA receptor channel blockers including PCP and the more selective ligand MK801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate] (Kornetsky et al., 1979; Corbett, 1989; Herberg and Rose, 1989; Carlezon and Wise, 1993b; Sundstrom et al., 2002; Bespalov et al., 2006; Hillhouse et al., 2014) (see Table 5 for illustrative data). For example, Fig. 13E shows facilitation of ICSS by 0.18 mg/kg i.p. MK801 in rats responding under a frequency-rate ICSS procedure (Hillhouse et al., 2014). Notably, both MK801 and PCP produced modest facilitation of ICSS at low doses, whereas higher doses recruited abuse-limiting rate-decreasing effects. In general, mixed profiles of ICSS effects that include both facilitation of low ICSS rates maintained by low brain-stimulation frequencies and depression of high ICSS rates maintained by high frequencies are common (Fig. 13E). Repeated PCP administration did not alter its abuse-related rate-increasing effects (Carlezon and Wise, 1993b). Relative to PCP and MK-801, the lower affinity NMDA receptor channel blockers memantine and ketamine produce little or no ICSS facilitation (see Table 5 for illustrative data). For example, relative to MK801, memantine up to doses that recruited rate-decreasing effects produced only weak facilitation of ICSS in rats responding under a frequency-rate procedure (Tzschentke and Schmidt, 1999). Ketamine was also reported to weakly facilitate ICSS in one study that used a free-operant ICSS procedure (Herberg and Rose, 1989); however, Fig. 13F shows that ketamine failed to facilitate ICSS in a hybrid frequency-rate procedure that was sensitive to rate-increasing effects of MK801, and additional studies showed that repeated ketamine produced tolerance to its rate-decreasing effects but still failed to unmask abuse-related rate-increasing effects (Hillhouse et al., 2014) (Table 5). Taken together, these ICSS results suggest a hierarchy of abuse potential for NMDA antagonists with MK801 = PCP > ketamine = memantine. MK801 has not been approved for clinical use and has not been considered for scheduling by the FDA, but for the remaining three compounds, the hierarchy of abuse potential from ICSS studies corresponds roughly to the FDA scheduling hierarchy for these compounds, where PCP is schedule 1, ketamine is schedule 3, and memantine is unscheduled. The relationship of these ICSS data to drug self-administration data is less clear. MK801, PCP, ketamine, and memantine all maintain drug self-administration (Marquis and Moreton, 1987; Koek et al., 1988; Nicholson et al., 1998; Winger et al., 2002). In addition, a behavioral economic analysis of drug self-administration in rhesus monkeys suggested a hierarchy for reinforcing efficacies of ketamine = PCP > MK801, a hierarchy that aligned with their rates of onset (Winger et al., 2002) but not with their relative efficacies to facilitate ICSS. This apparent discrepancy in abuse-related effects for NMDA channel blockers in ICSS versus drug self-administration procedures would benefit from further study. In addition, studies with newer NMDA receptor allosteric modulators, or with antagonists such as ifenprodil that are selective for NMDA receptor subtypes, have yet to be conducted (Mony et al., 2009; Monaghan et al., 2012).
2. Other Glutamate Receptor Ligands.
In studies conducted to date, ICSS has not been facilitated by drugs acting at other iGluRs or at mGluRs. For example, ICSS was not facilitated by either the AMPA antagonist GYKI-53655 [1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride] or the mixed AMPA/kainate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (Kenny et al., 2003a; Bespalov et al., 2006). ICSS was also not altered by an agonist [LY314582, (±)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid] or antagonist [LY341495 ((2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid)] for mGlu2/3 receptors (Kenny et al., 2003a). Lastly, ICSS was not altered or depressed by the mGluR5 antagonist 2-methyl-6-[phenylethynyl]-pyridine (MPEP) (Kenny et al., 2003a; Bespalov et al., 2006), the mGluR5 negative allosteric modulators 3-((2-methyl-4-thiazolyl)ethynyl)pyridine (MTEP) and fenobam, the mGluR5 positive allosteric modulators [CDPPB (3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide) and ADX47273 ((S)-(4-fluorophenyl)-(3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]piperidin-1-yl)methanone)] (Cleva et al., 2012), or by the mGluR7 positive allosteric modulator AMN082 [N,N′-bis(diphenylmethyl)-1,2-ethanediamine dihydrochloride] (Li et al., 2013). The pharmacology of drugs acting at glutamate receptors and transporters is rapidly expanding, and this will clearly be a fruitful area for future research (Javitt et al., 2011).
F. Cannabinoids
Cannabinoids constitute a class of natural and synthetic compounds that bind to cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors, which are widely distributed in the brain and periphery (Cravatt and Lichtman, 2004; Karanian and Bahr, 2006; Vlachou and Panagis, 2014). The prototype cannabinoid receptor agonist is Δ9-tetrahydrocannabinol (THC), a nonselective and intermediate-efficacy agonist at CB1 and CB2 receptors and a primary psychoactive constituent of marijuana products. As of the writing of this review, marijuana retains schedule 1 status under U.S. federal law; however, its legal status is rapidly evolving, and its use is widespread (SAMHSA, 2012). A synthetic isomer of THC (dronabinol; (−)-trans-Δ9-THC) is clinically available as a schedule 3 drug for indications that include treatment of nausea and emesis, and other clinically available cannabinoids include the synthetic THC analog nabilone and the THC/cannabidiol mixture Sativex (GW Pharmaceuticals, Wiltshire, UK). Other synthetic CB receptor agonists have been developed and evaluated for various clinical indications, and some of these compounds have also emerged as designer drugs of abuse with street names such as “spice” and “K2” (Elsohly et al., 2014; Wiley et al., 2014). The class of cannabinoid drugs also includes compounds that modulate synthesis or degradation of the endogenous cannabinoid neurotransmitters anandamide and 2-acylglycerol (Cravatt and Lichtman, 2004; Karanian and Bahr, 2006; Vlachou and Panagis, 2014). In particular, anandamide is degraded by the enzyme fatty acid amide hydrolase (FAAH), whereas 2-acylglycerol is degraded by monoacylglycerol lipase (MAGL). FAAH and MAGL inhibitors can increase endogenous cannabinoid levels and produce CB receptor-mediated effects.
1. Cannabinoid Receptor Ligands.
Cannabinoid receptor ligands with mixed action at both CB1 and CB2 receptors produce little or no facilitation of ICSS. Most of this work has been conducted to evaluate THC, and three studies have reported ICSS facilitation (see Table 5 for illustrative data). An early study reported that 1.5 mg/kg i.p. THC facilitated ICSS in Lewis rats responding under an autotitration procedure (Gardner et al., 1988). A later study from the same group found that a slightly lower dose of 1.0 mg/kg i.p. THC also facilitated ICSS in Lewis and in Sprague-Dawley rats responding under a hybrid frequency-rate ICSS procedure, but this THC dose did not facilitate ICSS in Fischer 344 rats (Lepore et al., 1996). Lastly, a third study using a frequency-rate procedure found that 1.0 mg/kg i.p. THC only depressed ICSS in Sprague-Dawley rats, but ICSS was facilitated by a lower dose of 0.1 mg/kg THC (Katsidoni et al., 2013). In contrast to this evidence for potential strain- and/or dose-dependent facilitation of ICSS, other studies have observed only dose-dependent depression of ICSS by THC doses of 0.32–10 mg/kg i.p. in Sprague-Dawley rats responding under frequency-rate procedures (Vlachou et al., 2007; Kwilasz and Negus, 2012), and we have obtained similar results in C57Bl/6J mice (J. M. Wiebelhaus, S. S. Negus, and A. H. Lichtman, unpublished observations). Moreover, the other CB agonists nabilone, levonantradol, CP55940 [(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol], WIN55212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate], and HU210 [(6aR)-trans-3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol] produced only dose-dependent depression of ICSS in rats of various strains responding under various ICSS procedures (Stark and Dews, 1980; Kucharski et al., 1983; Arnold et al., 2001; Antoniou et al., 2005; Vlachou et al., 2005; Kwilasz and Negus, 2012). Lastly, repeated treatment with THC produced tolerance to its rate-decreasing effects but failed to unmask abuse-related rate-increasing effects (Kwilasz and Negus, 2012), and repeated treatment with the higher efficacy agonist WIN55212-2 produced only sustained ICSS depression (Mavrikaki et al., 2010). Taken together, these findings provide at best only weak evidence for abuse-related facilitation of ICSS by THC and no evidence for facilitation by other CB agonists. This relative insensitivity of ICSS to abuse-related cannabinoid effects is similar to the narrow range of conditions under which cannabinoids maintain self-administration (Panagis et al., 2008; Panlilio et al., 2010; Vlachou and Panagis, 2014). Of potential note for the practice of abuse potential testing, the high-efficacy CB agonist WIN55212-2 was reported to maintain self-administration in rodents (Martellotta et al., 1998; Fattore et al., 2001; Lecca et al., 2006), but it only depressed ICSS (Vlachou et al., 2005; Mavrikaki et al., 2010).
ICSS studies with more selective CB1 or CB2 receptor agonists have not yet been published; however, effects of THC and some other CB agonists have been blocked by doses of the CB1-selective antagonist rimonabant that did not alter ICSS when administered alone (Vlachou et al., 2005, 2007; Kwilasz and Negus, 2012). These findings implicate CB1 receptors as the primary mediator of ICSS effects produced by THC and other mixed-action agonists. Consistent with this conclusion, we found that the CB2-selective agonist GW405833 [1-(2,3-dichlorobenzoyl)-5-methoxy-2-methyl-3-[2-(4-morpholinyl)ethyl]-1H-indole] (Valenzano et al., 2005) at doses up to 32 mg/kg i.p. had little effect on ICSS in rats responding under a frequency-rate procedure (A. Kwilasz and S. S. Negus, unpublished observations).
2. Fatty Acid Amide Hydrolase Inhibitors.
As with direct CB receptor agonists, inhibitors of endocannabinoid degradation have also failed to facilitate ICSS. The only published studies have been conducted with the FAAH inhibitors URB597 [cyclohexylcarbamic acid 3'-(aminocarbonyl)-[1,1′-biphenyl]-3-yl ester] and phenylmethylsulfonyl fluoride, and these compounds depressed ICSS in rats at doses that significantly increased brain anandamide levels (Vlachou et al., 2006; Kwilasz et al., 2014). These findings agree with the failure of URB597 to maintain drug self-administration (Justinova et al., 2008). In unpublished studies conducted in mice, we also found that the other FAAH inhibitor PF3845 [N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide] (Ahn et al., 2009), as well as the MAGL inhibitor JZL184 [4-[bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester] (Long et al., 2009) and the mixed FAAH/MAGL inhibitor SA57 [4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester] (Niphakis et al., 2012), also failed to facilitate ICSS at doses that increased their respective endocannabinoid levels in brain (J. M. Wiebelhaus, S. S. Negus, and A. H. Lichtman, unpublished observations).
G. Other Drugs
In addition to the drug classes reviewed above, ICSS has also been used to evaluate a range of other drugs acting at other targets. Effects of three other drugs will be discussed briefly here to illustrate the breadth of ICSS data that have been collected relevant to abuse potential testing (see Table 5 for illustrative data).
1. Caffeine.
Caffeine is an adenosine receptor antagonist and a primary psychoactive constituent of widely used plant-based beverages, including coffee and some teas (Heckman et al., 2010). Caffeine is also a common additive to soft drinks and energy drinks, and although caffeine is not scheduled, chronic consumption can meet clinical criteria for dependence (Griffiths and Chausmer, 2000; Reissig et al., 2009). Caffeine produced weak but significant facilitation of ICSS in rats responding under a hybrid amplitude-rate procedure (Bespalov et al., 1999), and Fig. 13B shows that caffeine also facilitated ICSS in rats responding under a frequency-rate procedure (M. Lazenka and S. S. Negus, unpublished observations). A recent review of the drug self-administration literature concluded that caffeine does not produce reinforcing effects in preclinical drug self-administration studies (Horton et al., 2013), although other investigators have concluded that caffeine may maintain self-administration under a limited range of conditions (Griffiths and Woodson, 1988). Overall, both ICSS and drug self-administration procedures agree that abuse-related effects of caffeine are weak; however, in the context of the present review, caffeine is the only drug that appears to produce more robust evidence for abuse potential in ICSS than in drug self-administration procedures.
2. Testosterone.
Anabolic steroids constitute a major category of FDA scheduled drugs, and testosterone is the prototype of this class (Kanayama et al., 2010). Steroid use and abuse typically occur in the context of athletic training and competition, but these drugs can also produce other direct effects that may contribute to their abuse potential (Wood, 2008). To our knowledge, only three studies have investigated testosterone effects on ICSS. Two early studies found that daily testosterone treatment facilitated ICSS in rats responding under free-operant procedures (Olds, 1958a; Caggiula and Hoebel, 1966). A significant detail of these studies was the location of the stimulating electrode. Olds (1958a) reported facilitation of ICSS by testosterone at some sites but no effect or depression at other sites. In the study by Caggiula and Hoebel (1966), the electrode was placed in posterior hypothalamus in male rats, and stimulation at this site not only maintained operant self-stimulation but also elicited copulatory behavior if a female rat was present. In both studies, sites where ICSS was responsive to testosterone were distinguished from sites associated with feeding. A more recent study evaluated ICSS in rats responding under a frequency-rate procedure before and during chronic treatment with a steroid mixture that included testosterone (Clark et al., 1996). This treatment produced a small but significant depression of ICSS, but it enhanced the ICSS-facilitating effects of amphetamine. In agreement with these ICSS data, testosterone was also reported to function as a significant but weak reinforcer in drug self-administration procedures (Wood, 2004).
3. Tripelennamine.
Tripelennamine is a histamine H1 receptor antagonist, and during the 1970s, it emerged as a drug of abuse in combination with the opioid agonist pentazocine. This combination acquired the slang name “T’s and Blues” because “T” is the first letter in one trade name for pentazocine (Talwin; Sanofi-Aventis, Bridgewater, NJ), and tripelennamine tablets were usually blue. The emergence of tripelennamine + pentazocine abuse stimulated research on the abuse-related effects of tripelennamine and other antihistamines. In ICSS studies, tripelennamine facilitated ICSS in rats responding under a discrete-trials current-intensity procedure when it was administered alone or in combination with pentazocine (Unterwald and Kornetsky, 1984; Unterwald et al., 1984). These findings agree with evidence for reinforcing effects of tripelennamine in drug self-administration procedures (Beardsley and Balster, 1992).
IV. State and Trait as Determinants of Drug Effects on Intracranial Self-Stimulation
Most studies cited above focused on manipulation of pharmacological variables and parameters of the ICSS procedure. Experimental subjects were usually common strains of male rats and mice housed under normal laboratory conditions, and other biologic or environmental variables were not explicitly manipulated. However, a large literature in ICSS research has addressed the function of “state” and “trait” variables (Chaplin et al., 1988) as determinants of drug effects on ICSS, and this research may be relevant to abuse potential testing insofar as it may identify conditions likely to increase or decrease abuse-related effects of test drugs. For the purposes of this review, “state” variables are defined as manipulations that alter the biology or environment of the test subject for only a portion of the subject’s life span (e.g., transient food deprivation). This temporal attribute of state variables means that drug effects on ICSS can be evaluated before and during state manipulation using a within-subjects experimental designs, and in the case of reversible manipulations, drug effects can also be evaluated after state manipulation. By comparison, “trait” variables are defined here as variables that have a fixed value in a test subject throughout its life span (e.g., male or female sex), and as a result, research on the effect of trait variables requires between-subjects experimental designs. This issue of within- versus between-subjects designs is pertinent in part because of its implications for exerting experimental control over electrode placement. The precise anatomic placement of electrodes varies to some degree across subjects, and the impact of this variable is minimized by within-subject designs, in which electrode placement is consistent within a given subject across time. When between-subject designs are required, then special care must be taken to maximize consistency of electrode placement between groups and minimize the potential that group differences in ICSS are due to differences in electrode placement rather than to differences in the intended target variable. This is typically accomplished by histologic verification of electrode sites to permit comparison of electrode placement between groups. It is noteworthy that the use of identical stereotactic coordinates for electrode implantation is not sufficient to assure consistency of electrode placement, because brain targets such as the MFB may have different spatial relationships to skull coordinates such as bregma in different groups of subjects. The review below is not intended to be exhaustive of the literature on state/trait variable manipulation but rather is intended to illustrate the potential for these variables to influence abuse-related drug effects in ICSS procedures.
A. States
State variables are defined here as manipulations that alter a subject’s biology or environment for only a portion of the subject’s life span, and by this definition, drug treatments are one type of state variable. This section will consider nonpharmacological state variables that may interact with drug effects to modulate ICSS.
1. Food Deprivation.
Operant responding for brain stimulation is similar to operant responding for natural reinforcers such as food, and prior to the discovery of ICSS, it was already established that food-maintained responding could be increased by food deprivation (Skinner, 1950, 1953b). Accordingly, food deprivation was among the first variables examined in early efforts to identify determinants of ICSS. For example, one study examined rats and cats responding for brain stimulation in free-operant procedures (Brady et al., 1957). After stable responding was established, effects of food and water deprivation were examined (1, 4, 24, and 48 hours in cats; 0 and 48 hours in rats). Food and water deprivation resulted in robust facilitation of ICSS. Multiple other studies soon replicated the general phenomenon of food deprivation-induced facilitation of ICSS (Olds, 1958a; Hodos and Valenstein, 1960; Katz et al., 1978), although relatively robust levels of food deprivation leading to more than 10% weight loss may be required to produce this effect (Lin et al., 2002).
Subsequent studies examined the effects of food deprivation on drug-induced changes in ICSS. As one example of such efforts, Cabeza de Vaca and Carr (1998) examined effects of d-amphetamine, nicotine, and the NMDA antagonists phencyclidine and MK801 on ICSS, with and without food deprivation. With a hybrid frequency-rate procedure, they showed that food restriction enhanced facilitation of ICSS after amphetamine, PCP, and MK801 administration, but not nicotine administration. Other studies have extended these findings to include effects on different ICSS procedures (Cabeza de Vaca et al., 2004) and other drugs including D1 and D2 dopamine receptor agonists (Carr et al., 2001). Enhancement of abuse-related drug effects by food deprivation in ICSS procedures is consistent with enhancement of reinforcing drug effects by food deprivation in drug self-administration procedures (O'Connor et al., 2011).
2. Noxious Stimulation and Pain.
In contrast to the enhancement of basal ICSS produced by food deprivation, noxious stimulation and associated pain states can produce a depression of basal ICSS (Negus, 2013). For example, tissue acidification is a common component of inflammation, and intraperitoneal injection of dilute acid is a well-established chemical noxious stimulus that produces a concentration- and time-dependent depression of ICSS in rats responding under a hybrid frequency-rate procedure (Pereira Do Carmo et al., 2009). This acid-induced depression of ICSS is linked to depression of mesolimbic dopamine release (Leitl et al., 2014), and it can be blocked by clinically effective analgesics such as mu opioids and nonsteroidal anti-inflammatory drugs (Pereira Do Carmo et al., 2009; Kwilasz and Negus, 2012; Rosenberg et al., 2013; Leitl et al., 2014). Acid-depressed ICSS has also been used as a behavioral baseline to examine candidate analgesics including kappa and delta opioids (Negus et al., 2010, 2012a,b), cannabinoids (Kwilasz and Negus, 2012; Kwilasz et al., 2014), and monoamine uptake inhibitors (Rosenberg et al., 2013). Of potential relevance to abuse-potential testing, some treatments have been identified (e.g., the delta agonist SNC80, the nonsteroidal anti-inflammatory drug ketoprofen) that block acid-induced depression of ICSS without producing an abuse-related facilitation of ICSS in the absence of acid treatment. These results could be interpreted to suggest heightened potential for abuse of these medications in the presence of some pain states. Conversely, other drugs nonselectively facilitated ICSS (e.g., mu opioid agonists, DA uptake inhibitors) or nonselectively depressed ICSS (e.g., kappa opioid agonists, cannabinoid receptor agonists) in the absence or presence of acid treatment.
Other studies have demonstrated pain-related depression of ICSS in rats by other noxious stimuli, including surgical incision of the hindpaw (Ewan and Martin, 2014), administration of formalin into the hindpaw (M. Leitl and S. S. Negus, unpublished observations), and systemic administration of lipopolysaccharide (Borowski et al., 1998; Barr et al., 2003). However, ICSS was not depressed in rats by spinal nerve ligation, a procedure used to model neuropathic pain (Ewan and Martin, 2011). Moreover, spinal nerve ligation attenuated abuse-related effects of mu opioid agonists in both an ICSS and drug self-administration procedure (Ewan and Martin, 2011, 2013). Thus, these latter data provide added evidence for similar effects of state manipulations on abuse-related drug effects in ICSS and drug self-administration procedures.
3. Stress and Depression.
Anxiety and depressive disorders are significant public health issues (Kessler et al., 2005), and stress is one factor thought to play a significant role in their development (Kendler et al., 1999). A key component of depression is anhedonia, which is defined as diminished interest or pleasure in activities that are usually enjoyable. In one family of preclinical procedures aimed at modeling depressive behaviors, animals are repeatedly exposed to stressors, and anhedonia is inferred from resulting depression of behaviors such as feeding and sexual activity (Der-Avakian and Markou, 2012). Brain reward systems, including the mesolimbic DA system, have been implicated in mood disorders (Nestler and Carlezon, 2006; Russo and Nestler, 2013), and ICSS has been used in preclinical studies aimed at identifying determinants of stress-related depression of positively reinforced behavior. Regimens of repeated stress, including chronic, intermittent exposure to stressors like restraint and soiled bedding (Moreau et al., 1992, 1994) or social defeat (Donahue et al., 2014), have been reported to depress basal ICSS, and ICSS was also depressed by an olfactory bulbectomy model of depression (Slattery et al., 2007). However, other regimens of chronic stress had no effect on ICSS (Fokos and Panagis, 2010) or produced effects that varied across time or across test subjects (Nielsen et al., 2000; Lin et al., 2002). A likely source of these discrepancies is variability in the stress regimens across studies.
In studies of drug effects on ICSS in the context of stress, the monoamine uptake inhibitor antidepressants desipramine and mianserin reversed depression of ICSS by chronic intermittent stress (Moreau et al., 1992, 1994), and these effects were selective for stress states, because neither drug facilitated ICSS in the absence of stress (Rosenberg et al., 2013). Conversely, the NMDA antagonist ketamine, which primarily depresses ICSS in the absence of explicit stress, also failed to reverse depression of ICSS induced by social-defeat stress in mice (Donahue et al., 2014). Other research has shown that repeated stress can enhance amphetamine-induced facilitation of ICSS (Lin et al., 2002), whereas effects of cocaine and THC were not altered by olfactory bulbectomy or repeated stress, respectively (Slattery et al., 2007; Fokos and Panagis, 2010). Together, these results illustrate the potential for differential effects of stress as a modulator of abuse-related drug effects in ICSS procedures, but given the prominent role of stress as a risk factor in drug abuse (Neisewander et al., 2012; Volkow et al., 2012), this is a relatively undeveloped theme of research.
B. Traits
1. Sex.
Rates of drug abuse are higher in males than females (SAMHSA, 2012), and efforts have been made to examine sex differences in drug effects on ICSS. Males and females do not differ with regard to baseline sensitivity to the reinforcing effects of electrical stimulation of the MFB, and sensitivity in females is also stable across stages of the estrous cycle (Stratmann and Craft, 1997; Russell et al., 2013). However, exogenously administered estradiol facilitates ICSS in ovariectomized female rats (Galankin et al., 2010) and exogenous testosterone facilitates ICSS in castrated male rats (Olds, 1958a). These findings suggest that baseline sex differences in ICSS do not exist, but artificial manipulation of sex hormones is sufficient to alter ICSS.
Regarding the role of sex in the effects of drugs of abuse on ICSS, the monoamine releaser amphetamine and the uptake inhibitor cocaine facilitated ICSS to a similar degree in male and female rats responding under a discrete-trial current-intensity threshold procedure (Stratmann and Craft, 1997), but administration of exogenous estradiol enhanced cocaine-induced ICSS facilitation in ovariectomized female rats (Galankin et al., 2010). Sex also does not appear to play a major role in cocaine self-administration in rats (Caine et al., 2004), although estradiol administration to ovariectomized rats increased cocaine self-administration under a progressive ratio procedure (Ramoa et al., 2013).
Acute administration of the mu opioid agonist morphine did not facilitate ICSS in a discrete-trial procedure in either male or female rats (Stratmann and Craft, 1997), but a subsequent study using a hybrid frequency-rate procedure did show sex-dependent effects (Craft et al., 2001). Specifically, this study found that morphine was more potent to depress ICSS in males and more potent to facilitate ICSS in females. Some data also suggest that morphine serves as a stronger reinforcer in females than males in self-administration procedures (Cicero et al., 2003). Similar to these effects with morphine, the kappa opioid agonist U50,488 was also more potent to depress ICSS in male rats than in females responding under a frequency-rate procedure (Russell et al., 2013).
2. Strain.
In contrast to the relatively modest effects of sex, genetic strain has emerged as a more robust modulator of basal and drug-altered ICSS. In an early study of ICSS in DBA/2, C57BL/6, and BALB/c mice responding under a free-operant procedure, consistent differences in ICSS rates were observed with a hierarchy of BALB/c > DBA/2 > C57BL/6 (Cazala et al., 1974). More recent comparisons of C57BL/6J and DBA/2J mice using hybrid frequency-rate procedures found no strain differences in ICSS thresholds, although maximal ICSS rates were greater in C57BL/6 mice (Elmer et al., 2010; Fish et al., 2010). In rats responding under a frequency-rate procedure, ICSS thresholds did not differ between Lewis, Fischer 344, and Sprague-Dawley rats, and in this study, maximal response rates also did not differ across strain (Lepore et al., 1996). Taken together, these data suggest ICSS frequency thresholds do not vary in strains examined to date, although maximum rates may differ across strains.
With regard to strain differences on drug effects, amphetamine and cocaine produce abuse-related reductions in ICSS thresholds to similar degrees in both C57BL/6J and DBA/2J mice (Elmer et al., 2010; Fish et al., 2010). A study examining these strains along with BALB/c mice provided some evidence of potency differences for amphetamine effects on ICSS across these strains (BALB/c > DBA/2 > C57 BL/6), but the effects were qualitatively similar in each strain (Cazala, 1976). Both cocaine and amphetamine maintain self-administration in C57BL/6J and DBA/2J mice (van der Veen et al., 2007; Elmer et al., 2010). Thus, the available data suggest that strain does not play a central role in the effects of amphetamine or cocaine on ICSS, and these findings are consistent with data from self-administration studies.
In contrast to the work with monoaminergic drugs mentioned above, strain can be a primary determinant of morphine effects on ICSS. Morphine administration significantly facilitated ICSS in C57BL/6J mice, whereas morphine only depressed ICSS in DBA/2J mice (Elmer et al., 2010). Similarly, in drug self-administration studies, morphine also served as a more robust reinforcer in C57BL/6J mice compared with DBA/2J mice (Elmer et al., 2010). Thus, strain can determine morphine effects on ICSS in these mouse strains, and it does so in a manner that is consistent with strain differences observed in self-administration studies.
Strain dependency of the effects of ethanol and THC has also been examined. Effects of ethanol were examined in C57BL/6J and DBA/2J mice using a hybrid frequency-rate procedure. Administration of ethanol resulted in ICSS facilitation in each strain and did so across a broader range of doses and pretreatment times in DBA/2J mice compared with C57BL/6J mice (Fish et al., 2010). The ability of ethanol to facilitate ICSS in C57BL/6J mice is consistent with its ability to maintain self-administration in this strain, but the effect in DBA/2J mice is inconsistent with data showing that ethanol does not maintain self-administration in this strain (Risinger et al., 1998). Strain-dependence of the effects of THC on ICSS was examined in Lewis, Fischer 344, and Sprague-Dawley rats using a hybrid frequency-rate procedure. THC facilitated ICSS in Lewis and Sprague-Dawley rats, but not Fischer 344 rats (Lepore et al., 1996), although it should be noted that only one dose of THC was tested in this study. Strain comparisons of THC self-administration have not been conducted, although in general, THC does not serve as a robust reinforcer in rodent drug self-administration procedures (Panlilio et al., 2010; Vlachou and Panagis, 2014).
The studies above used established rodent strains, but novel strains can also be generated by selective breeding for particular phenotypes, and illustrative of this approach are FAST and SLOW mouse lines selectively bread for divergent responses to ethanol. FAST mice exhibit greater motor activation and ethanol consumption relative to SLOW mice (Risinger et al., 1994). These strains were used to study the effects of ethanol and cocaine on ICSS in mice responding under a frequency-rate procedure (Fish et al., 2012). Ethanol facilitated ICSS in FAST mice, but effects of the same ethanol doses in SLOW mice were not significant. Cocaine, on the other hand, facilitated ICSS in both FAST and SLOW mice.
3. Gene-Targeted Manipulations.
Genetic tools offer the opportunity to manipulate expression of specific proteins, and these techniques were recently applied to ICSS studies. One of the first studies to use this approach examined basal and drug-altered ICSS in DA D2 receptor-deficient mice responding under a hybrid frequency-rate procedure (Elmer et al., 2005). Initial experiments in this study demonstrated that D2 receptor knockout (D2Rko) mice required higher intensity stimulation to maintain equivalent ICSS compared with wild-type (WT) mice. Once stimulation parameters were adjusted such that baseline ICSS was similar, effects of amphetamine and morphine were examined. Amphetamine facilitated ICSS regardless of genotype. However, morphine facilitated ICSS in WT mice, produced a mixed, nonsignificant effect in heterozygous mice, and only depressed ICSS in D2Rko mice. This study was interpreted to suggest that expression of D2 receptors is necessary for morphine-induced facilitation of ICSS but not for maintenance of basal ICSS or for amphetamine-induced facilitation of ICSS. Thus, these results resonate with sex and strain manipulations cited above in finding that opioid effects are often more sensitive than monoamine releaser/uptake inhibitor effects to trait manipulations. Moreover, these results agree with the finding that morphine maintained drug self-administration in WT and heterozygous mice but not in D2Rko mice (Elmer et al., 2002).
A related approach was used to study the role of α5 nAChRs in the effects of nicotine on ICSS under a discrete-trial current-intensity procedure (Fowler et al., 2013). WT mice and α5 nAChR subunit knockout mice (α5ko) did not differ with regard to baseline ICSS. Nicotine facilitated ICSS in WT and α5ko mice, but this effect occurred over a broader range of doses in the α5ko mice. In particular, the nicotine dose-effect curve was biphasic in WT mice, and high nicotine doses that failed to facilitate ICSS in WT mice did facilitate ICSS in the α5ko mice. This effect agrees with results from nicotine drug self-administration studies (Fowler et al., 2011) and was interpreted to suggest that α5 nAChRs mediate abuse-limiting effects of nicotine.
Loss-of-function point mutations to α1, α2, and α3 GABAA subunits in mice were used to study the role of these subunits in benzodiazepine effects on ICSS (Reynolds et al., 2012). Baseline ICSS and cocaine-induced facilitation of ICSS were equivalent across genotypes. Point mutations of the α2 or α3 GABAA subunit eliminated diazepam-induced facilitation of ICSS that was observed in WT mice and in mice with a point mutation of the α1 subunit, suggesting a primary role for the α2 and α3 subunits in abuse-related diazepam effects. In contrast, zolpidem administration only depressed ICSS in WT, α2, and α3 mice, but not α1 mice, suggesting that the α1 subunit is necessary for zolpidem-induced depression of ICSS.
Other studies manipulating genotype have done so in an attempt to model a particular pathologic state rather than to examine the role of a certain receptor or receptor subtype in the effects of drugs of abuse. For example, mice lacking a functional FMR1 gene (Fmr1−/Y) have been suggested as a preclinical model of fragile X syndrome and were recently used to examine the effects of drugs of various classes on ICSS (Fish et al., 2013). Cocaine was more potent to facilitate ICSS in Fmr1−/Y mice than WT mice. The dopamine partial agonist aripiprazole depressed ICSS in both genotypes, but was more potent in WT mice. The mGluR5 antagonist MPEP had no effect in WT mice but facilitated ICSS in Fmr1−/Y mice, whereas the M1 cholinergic antagonist trihexyphenidyl had no effect in Fmr1−/Y mice but facilitated ICSS in WT mice. In another study, basal and cocaine-facilitated ICSS were evaluated in mice with a mutation in the CLOCK gene, a central transcriptional activator of molecular rhythms that may contribute to manic and bipolar disorder (Roybal et al., 2007). Relative to WT mice, the CLOCK mutant mice displayed lower ICSS thresholds and greater sensitivity to cocaine-induced ICSS facilitation.
Genotypic manipulations have also been used to study determinants of ICSS outside of the context of studies with abused drugs. For example, 5HT transporter knockout (SERT−/−) rats were used in a discrete-trial procedure to study serotonergic modulation of ICSS depression induced by administration of lipopolysaccharide (LPS) (van Heesch et al., 2013). WT and heterozygous (SERT+/−) rats had reduced baseline ICSS thresholds compared with SERT−/− rats, but only the differences between SERT+/− and SERT−/− achieved statistical significance. SERT −/− rats were also less susceptible to LPS-induced depression of ICSS, suggesting a role for SERT in LPS-induced ICSS depression. In another study, the role of the orexigenic peptide ghrelin on motivated behavior was examined using wild-type and ghrelin receptor knockout rats responding under a hybrid frequency-rate procedure (Wellman et al., 2012). Significantly higher current intensities were required to maintain similar frequency-rate curves in the knockout rats, and these results were interpreted to suggest a role for ghrelin in basal ICSS.
V. Predictive Validity of Intracranial Self-Stimulation in Abuse Potential Testing
A. Prediction of Preclinical Drug Self-Administration
For decades, drug self-administration procedures have been a standard in preclinical abuse potential testing, and results obtained with these procedures have been reviewed extensively (Ator and Griffiths, 2003; Carter and Griffiths, 2009; O'Connor et al., 2011; Horton et al., 2013). Accordingly, one measure of the utility of ICSS in abuse potential testing is the comparison of results obtained with ICSS and drug self-administration procedures. ICSS compares favorably to the drug self-administration standard. A quantitative example of this comparison was presented in Fig. 9B for a series of monoamine releasers, and there was a significant correlation between measures of drug-induced facilitation of ICSS in rats and of reinforcing efficacy in a progressive-ratio procedure in rhesus monkeys. One implication of this correlation is that ICSS might be useful not only for predicting presence or absence of reinforcing effects but also for stratifying those effects along a continuum of reinforcing strength for monoamine releasers (Brady and Griffiths, 1976; Richardson and Roberts, 1996; Horton et al., 2013). Table 6 presents a more qualitative comparison of results in ICSS and drug self-administration procedures for representative drugs discussed in this review article. This type of comparison should be regarded with caution, because it reduces nuanced results from variants of each procedure to simplistic binary assessments of the presence or absence of an abuse potential signal (Horton et al., 2013). Given that caveat, though, it is apparent that ICSS and drug self-administration yield largely concordant results. Some approximate rank ordering of effects is also evident across procedures. For example, many DA releasers (e.g., amphetamine) and uptake inhibitors (e.g., cocaine) reliably facilitate ICSS and maintain self-administration across a wide range of conditions, whereas CB receptor agonists like Δ9-THC facilitate ICSS and maintain self-administration under a far more limited range of conditions. Taken together, these results support the proposition that drug effects in ICSS procedures are often predictive of drug effects in drug self-administration procedures. Moreover, as discussed above in section IV, the concordance between ICSS and drug self-administration results extends not only across multiple drug classes but also to modulation of abuse-related drug effects by many state and trait variables.
TABLE 6.
Comparison of effects produced by representative drugs in intracranial self-stimulation and drug self-administration procedures
Drugs are listed in their order of discussion in the manuscript. A drug was considered to facilitate ICSS or to maintain self-administration if at least one published study supported this characterization. Drugs scheduled by the FDA are shown in boldface italic.
| Facilitate ICSSa | No Change or Depress ICSS | |
|---|---|---|
| Maintain self-administrationb | Amphetamine | Haloperidol |
| Phenmetrazine | Clonidine | |
| MDMA | Zolpidem | |
| Cocaine | Baclofen | |
| Methylphenidate | MPEP | |
| MDPV | WIN55212-2 | |
| Apomorphine | ||
| Methadone | ||
| Morphine | ||
| Nalbuphine | ||
| Nicotine | ||
| Varenicline | ||
| Scopolamine | ||
| Midazolam | ||
| Chlordiazepoxide | ||
| Diazepam | ||
| Pentobarbital | ||
| Phenobarbital | ||
| Toluene | ||
| Ethanol | ||
| MK-801 | ||
| Phencyclidine | ||
| Ketamine | ||
| Δ9-THC | ||
| Testosterone | ||
| Tripelennamine | ||
| Fail to maintain Self-administration | Caffeine | Fenfluramine |
| Citalopram | ||
| Atomoxetine/nisoxetine | ||
| Imipramine/desipramine | ||
| Chlorpromazine | ||
| TFMPP | ||
| SNC80 | ||
| U69593 | ||
| Naloxone | ||
| URB597 |
ICSS references are cited in section III.
Drug self-administration references are as follows: baclofen (Griffiths et al., 1991), MDPV (Watterson et al., 2014), MK-801 (Koek et al., 1988), MPEP (van der Kam et al., 2009), TFMPP (Fantegrossi et al., 2005), SNC80 (Negus et al., 1998), toluene (Blokhina et al., 2004), U69593 (Negus et al., 2008), URB597 (Justinova et al., 2008), WIN55212-2 (Fattore et al., 2001). For all other drugs, results are taken from (O'Connor et al., 2011; Horton et al., 2013).
Table 6 also identifies a few exceptions to this general rule. Several drugs that have failed to facilitate ICSS have been reported to maintain self-administration with varying degrees of reliability in rats and/or nonhuman primates. Conversely, caffeine is the only drug, to our knowledge, that produces stronger evidence for abuse potential in ICSS procedures than in drug self-administration procedures. Determinants of these inconsistencies remain to be discovered, but of note for this review, such inconsistencies are unusual. To the degree that ICSS and drug self-administration procedures yield different results, ICSS appears slightly less likely than drug self-administration to yield evidence of abuse potential.
B. Prediction of Abuse Liability in Humans
The concordance of results from ICSS and drug self-administration procedures provides one source of evidence to support utility of ICSS in preclinical abuse potential testing. However, the value of any preclinical procedure is ultimately determined by its (1) sensitivity for detecting drugs with abuse potential in humans and (2) selectivity for dissociating drugs that have abuse potential in humans from those that do not (Horton et al., 2013). The sensitivity and selectivity of drug self-administration for this purpose has been extensively reviewed (Ator and Griffiths, 2003; Carter and Griffiths, 2009; O'Connor et al., 2011; Horton et al., 2013), and as implied by Table 6, ICSS appears to display similar sensitivity and selectivity. Both procedures are sensitive to effects of abused drugs like DA releasers/uptake inhibitors and mu opioid agonists, and both procedures are also selective for these drugs in comparison with many classes of drugs that are not commonly abused, such as NE or 5HT uptake inhibitors or kappa opioid agonists. Moreover, both procedures share similar vulnerabilities to apparent false negatives and false positives. For example, ICSS and drug self-administration share similarly poor sensitivity to some classes of abused drugs (e.g., CB receptor agonists and LSD-like hallucinogens) and similarly poor selectivity for some other clinically available drugs for which abuse is rare (e.g., scopolamine, bupropion). ICSS and drug self-administration data do not always align, but these exceptions do not strongly favor preference for either procedure to increase predictive validity in abuse potential testing. For example, drug self-administration appears more likely to yield false-positive effects with apomorphine, baclofen, and clonidine, three clinically available drugs that are not scheduled or commonly abused. Conversely, ICSS appears more likely to yield false-negative effects with ketamine and zolpidem, two scheduled drugs that are abused under some circumstances. These examples suggest that ICSS may be more likely than drug self-administration to generate false-negative effects in abuse potential testing, whereas drug self-administration may be more likely to generate false positive effects.
In assessing predictive validity of any preclinical procedure for clinical outcomes, an obvious but often underappreciated issue is the clinical endpoint to which preclinical data are being compared. The discussion above is founded on qualitative judgments of abuse liability for different drugs in humans. More precise validation of preclinical metrics for abuse potential would benefit from more precise clinical metrics. From a regulatory perspective, the most relevant metric of a drug’s abuse liability in the United States is its status under the Controlled Substances Act. The Controlled Substances Act is a federal law that mandates stratification of drugs along an ordinal scale as schedule 1 (high abuse-liability without approved clinical indication), schedule 2 (high abuse liability with approved clinical indications), schedules 3–5 (lower abuse liability with approved clinical indications), or nonscheduled (drugs considered to have little or no abuse liability) (Rocha, 2013). This scale explicitly acknowledges a regulatory perspective that drugs have graded levels of abuse liability, but the relationship of this scale to graded metrics of abuse potential from preclinical procedures has been only superficially explored. For example, one recent study compiled data from a set of 100 drugs and reported that the binary identity of a drug as scheduled or not scheduled correlated with binary preclinical metrics such as whether the drug maintained self-administration or not (Horton et al., 2013). However, this type of correlation is largely tautological insofar as preclinical data often guide scheduling decisions, and no attempt has been made to correlate preclinical metrics with different levels of scheduling. Moreover, there are prominent discrepancies between preclinical metrics and drug scheduling (e.g., the evolving scheduling status of marijuana, the exemptions of ethanol and tobacco products, the schedule 4 status of fenfluramine).
These limitations to scheduling status as an appropriate clinical metric suggest the need for other objective and quantitative endpoints to validate preclinical procedures. It is noteworthy that preclinical metrics from ICSS and drug self-administration do not correspond to prevalence of use (SAMHSA, 2012), which is governed by social and economic factors such as cost, availability, and legal status as well as by pharmacological effects (Katz and Goldberg, 1988). The most widely used drugs are legal compounds (e.g., ethanol and nicotine) that are marketed primarily as constituents of plant-based products (e.g., wine and tobacco) and that produce relatively weak effects in ICSS or drug self-administration procedures. Alternative, data-driven and clinically relevant metrics of abuse liability in humans might consider the risk or prevalence of harm among recreational users of a given drug. As one example of this approach, Nutt and colleagues (2007) recently devised a questionnaire to score harm associated with drug use, and scores for different drugs and drug classes were processed by a panel of experts using a “delphic” approach. Thus, harm scores assigned by individual experts were averaged to yield a rank order of perceived harm, and by this approach, heroin and cocaine emerged as the most harmful of the drugs considered, whereas other illicit drugs including cannabis, LSD, and GHB had much lower harm scores. Tobacco and alcohol had intermediate harm scores by this approach. As another example that might rely on existing quantitative databases rather than on expert opinions, Fig. 14 plots data for a series of drugs and drug classes from two surveys conducted by the Substance Abuse and Mental Health Services Administration. Shown for each drug or drug class is a measure of negative consequences (emergency room mentions as reported by the Drug Abuse Warning Network) expressed as a proportion of use prevalence (past year usage from the National Survey on Drug Use and Health) (SAMHSA, 2012, 2013). Users of opioid agonists (e.g., heroin) and DA releasers/uptake inhibitors (e.g., cocaine) were more likely than users of other drugs/drug classes to experience negative consequences leading to emergency room visits. Phencyclidine (PCP), which facilitates ICSS and maintains drug self-administration, scored especially highly on this measure despite relatively low overall prevalence. Conversely, alcohol scored low on this measure despite high overall prevalence.
Fig. 14.
Rate of emergency room mentions per 1000 users for a series of drugs and drug classes as calculated from surveys conducted by the Substance Abuse and Mental Health Services Administration in 2011. Abscissa shows drug or drug class. Ordinate shows the ratio of Emergency Room Mentions (from Drug Abuse Warning Network) per 1000 Past Year Users (from the National Survey on Drug Use and Health) (log scale). Drugs associated with high rates of emergency room mentions per 1000 users also tend to produce robust facilitation of ICSS and maintain self-administration across a broad range of conditions. This analysis illustrates one possible approach to quantifying negative consequences of drug use as a metric for abuse liability in humans.
These two approaches based on expert opinions (Nutt et al., 2007) or on government databases (Fig. 14) yield largely similar rank orders, with the notable exception of alcohol, which received high harm scores but yielded a relatively low ratio of emergency room mentions to use prevalence. Nonetheless, these approaches illustrate the types of strategies that might provide useful clinical comparators for interpretation of outcomes from preclinical procedures for abuse potential testing. A rough implication of these findings is that preclinical ICSS and drug self-administration procedures might function best to predict potential for harm associated with relatively acute recreational use. For example, robust facilitation of ICSS, or reliable maintenance of drug self-administration, may be predictive of drug effects in humans that promote sufficiently high and frequent rates of drug consumption that users are likely to achieve toxic doses that prompt clinical intervention.
C. Intracranial Self-Stimulation as a Complement to Drug Self-Administration in Abuse Potential Testing
Given the largely similar outcomes produced by ICSS and drug self-administration procedures, the selection of procedure for any particular task must be guided by other considerations. Drug self-administration has two particular advantages as a tool for abuse potential testing. First, it has high face validity with drug abuse by humans and provides a direct measure of drug-taking behavior (Brady and Griffiths, 1976; Katz and Goldberg, 1988; Ator and Griffiths, 2003; Carter and Griffiths, 2009). Second, it is deeply embedded in the regulatory infrastructure of abuse potential testing, and its long use has contributed to standardization of procedures, construction of large databases, and education of policy makers in common strategies for data analysis and interpretation (Balster and Bigelow, 2003; European Medicines Agency, 2006; Food and Drug Administration, 2010).
ICSS by comparison provides an indirect measure of abuse-related effects and historically has had a limited role in generating data for regulatory guidance. However, ICSS is also distinguished by several procedural attributes that can make it valuable as a complement to drug self-administration. Four of those attributes will be mentioned here. First, ICSS procedures can generate baseline patterns of operant behavior that include both low ICSS rates sensitive to abuse-related rate-increasing drug effects and high ICSS rates sensitive to abuse-limiting rate-decreasing drug effects. Both types of effect likely contribute to patterns of self-administration and abuse, and ICSS procedures permit efficient detection and dissociation of these effects. For example, we have argued in this review that coordinated assessment of abuse-related and abuse-limiting drug effects with hybrid frequency-rate ICSS procedures permits a rank ordering of abuse potential similar to that provided by progressive-ratio self-administration procedures (Bauer et al., 2013b). Second, ICSS is compatible with a wide range of drug vehicles and routes of administration. For example, ICSS can be readily adapted to studies of inhaled solvents, orally administered drugs, or drugs dissolved or suspended in vehicles that would be difficult to deliver by the intravenous route preferred for drug self-administration procedures. Third, ICSS evaluates unconditioned drug effects that can be detected immediately in otherwise drug-naive subjects, and changes in ICSS effects can be tracked during regimens of repeated drug exposure and withdrawal. By contrast, reinforcing drug effects in drug self-administration procedures are conditioned behaviors that develop over time during drug availability and that adjust over time when drug dose is changed or drug is removed. Lastly, ICSS permits detailed assessment of the time course of abuse-related drug effects. By contrast, drug self-administration procedures are sensitive to drug time course, but they are not ideally suited to characterization of that time course. Taken together, these considerations suggest that ICSS is unlikely to replace drug self-administration as a core procedure for preclinical abuse potential testing. However, ICSS may be especially useful either to evaluate drugs that are difficult to study in standard drug self-administration procedures or to collect data that can guide the design and interpretation of drug self-administration experiments.
VI. Conclusions and Future Directions
A. Conclusions from Existing Data
Evidence reviewed here suggests three major conclusions regarding the use of ICSS as a tool for abuse potential testing. First, the most effective and widely used procedures involve (1) electrode placements that target the MFB and (2) schedule parameters that permit within-session manipulation of the frequency or amplitude of electrical stimulation to maintain a wide range of baseline response rates or response probabilities during each experimental session. Under these conditions, drug-induced increases in low ICSS rates maintained by low frequencies or amplitudes of stimulation can be interpreted as an abuse-related effect, whereas drug-induced decreases in high ICSS rates maintained by high frequencies or amplitudes of stimulation can be interpreted as an abuse-limiting effect. Drugs can vary in their efficacies to produce these rate-increasing and rate-decreasing effects, and importantly, both types of effects can be detected simultaneously. Net abuse potential appears to reflect an integration of these abuse-related and abuse-limiting effects as summarized in Fig. 15. Moreover, within these profiles, abuse potential would be greatest for drugs with rapid onsets and short durations of action and for drugs that display increased expression of abuse-related effects with repeated treatment. Consequently, thorough evaluation of the potency and time course of acute and chronic drug effects on ICSS provides a strategy not only for detecting presence or absence of abuse-related effects but also for ranking the expression of those effects relative to expression of abuse-limiting effects. It is our view that hybrid frequency-rate ICSS procedures are especially well-suited for this task.
Fig. 15.
Summary diagram for relating profiles of drug effects on ICSS to net abuse potential. Drugs can vary in their efficacies to produce abuse-related rate-increasing effects and abuse-limiting rate-decreasing effects, and both effects can be produced simultaneously. See Fig. 8 for examples. Net abuse potential can be conceptualized as an integration of these rate-increasing and rate-decreasing effects. In addition, the profile of rate-increasing and rate-decreasing effects varies over time after administration of a single drug dose, and may also change with repeated dosing.
Second, evidence of abuse potential from ICSS procedures closely matches evidence from drug self-administration procedures for a wide range of drugs and drug classes. Drug self-administration is a core procedure in preclinical abuse potential testing, and the close alignment of results from ICSS and drug self-administration procedures suggests that ICSS could be comparably informative in providing preclinical data on abuse potential of novel drugs. To the degree that ICSS and drug self-administration yield different results, ICSS appears to be slightly less likely than drug self-administration to yield evidence of abuse potential. In terms of predictive validity, this suggests that ICSS may be slightly less likely than drug self-administration to yield false-positive results but slightly more likely to yield false negatives. However, discrepancies between ICSS and drug self-administration are unusual with compounds studied to date, and these discrepancies are generally restricted to drugs that generate weak signals in one or the other procedure. Moreover, of particular importance to abuse potential testing, ICSS permits a ranking of abuse-related effects similar to the ranking of relative reinforcing efficacy that can be achieved with progressive-ratio drug self-administration procedures. This point warrants special emphasis. Current regulatory practice seeks to rank drugs by their perceived abuse potential (e.g., schedule 1–5 in the United States), and there is general agreement that progressive-ratio schedules of drug self-administration provide one strategy for assigning rank (Brady and Griffiths, 1976; Richardson et al., 1994; European Medicines Agency, 2006; Horton et al., 2013). Evidence presented in this review article suggests that ICSS may provide an alternative to progressive-ratio drug self-administration procedures for generating data that can be used to rank relative abuse potential of different drugs. Evidence for this claim is strongest for monoaminergic stimulants (Bauer et al., 2013b), weakest for uncompetitive NMDA channel blockers (Marquis and Moreton, 1987; Hillhouse et al., 2014), and further study with other drug classes is warranted.
Third and finally, ICSS has procedural advantages that contribute to its value as a complement to drug self-administration procedures. These include its usefulness for (1) studies of both abuse-related and abuse-limiting effects, (2) studies using various routes of drug administration or drug vehicles, (3) studies in drug-naive subjects as well as in subjects with controlled levels of prior drug exposure, and (4) studies of drug time course.
B. Opportunities for Future Research
Although ICSS procedures have long been used to examine abuse-related drug effects (Kornetsky et al., 1979; Wise, 1996; Carlezon and Chartoff, 2007; Vlachou and Markou, 2011), these procedures do not have a formal association with abuse potential testing for regulatory purposes. For example, ICSS is not mentioned in guidance for abuse potential testing provided by either the FDA or the European Medicines Agency (European Medicines Agency, 2006; Food and Drug Administration, 2010), nor is it considered in recent academic reviews of strategies for abuse potential testing (Ator and Griffiths, 2003; Carter and Griffiths, 2009; Horton et al., 2013). As a consequence, a clear first step in advancing ICSS as a viable tool in abuse potential testing is to raise awareness in the testing community about its existence, its predictive validity, and its strengths and weaknesses relative to established procedures. Moreover, the integration of ICSS into abuse potential testing would benefit from standardization of procedures. This review has presented evidence to suggest that optimal test strategies will include evaluation of drug potency and time course in hybrid frequency-rate procedures, followed by analysis using procedures described in Fig. 5 to integrate both rate-increasing and rate-decreasing drug effects. Also, because repeated drug exposure and withdrawal may modify both drug effects on ICSS and abuse liability in humans (e.g., with mu opioid receptor agonists), we also feel that evaluation of drug effects before, during, and after repeated dosing is often warranted. Overall, it is our view that ICSS research has already made significant contributions to the general practice of abuse potential testing and that future research could refine and enhance those contributions. Five possible topics of future research are mentioned briefly below.
First, a substantial database already exists on abuse potential of drugs and drug classes that have been evaluated in other procedures such as drug self-administration more thoroughly than they have been examined in ICSS. Systematic evaluation of these compounds would help to clarify the range of conditions across which ICSS is predictive of reinforcing effects in drug self-administration procedures and abuse liability in humans. As one example, benzodiazepines and related positive allosteric modulators of GABAA receptors constitute a major set of abused and scheduled drugs, but relatively little work has been conducted with these compounds in ICSS procedures.
Second, we live in an age of proliferating drug development that requires an expanding capability for abuse potential testing. Much of this drug development is focused on discovery of new medications in drug classes populated with known drugs of abuse. Examples include development of anorectics with novel mechanisms of action, biased mu opioid agonists to target selected signaling pathways coupled to mu receptors, selective ligands for GABAA receptor subtypes, and allosteric modulators of NMDA receptors (Mohler, 2012; Burgdorf et al., 2013; DeWire et al., 2013; Fleming et al., 2013). Development of illicit “designer” drugs is also proliferating, and most recently, this has included emergence of novel cathinone derivatives and synthetic cannabinoids (Baumann et al., 2013a; Cottencin et al., 2013; Wiley et al., 2014). All of these novel compounds will require abuse potential testing, and ICSS is poised to contribute to this effort. For example, ICSS has already contributed to abuse potential evaluation of novel cathinone derivatives (Robinson et al., 2012; Watterson et al., 2014; Bonano et al., 2014), and further studies with these and other novel compounds will aid in building the ICSS database and informing the validation of ICSS as viable tool for abuse potential testing.
Third, abuse potential testing with ICSS typically uses electrodes that target the MFB, but ICSS at this site is relatively insensitive to facilitation by some known classes of abused drugs including cannabinoids and hallucinogens. As discussed above, drug effects on ICSS can vary as a function of electrode placement (Goodall and Carey, 1975; Robertson et al., 1981; Ewan and Martin, 2012). It would be of interest to explore the degree to which ICSS of other brain targets might alter, and potentially improve, sensitivity to cannabinoids or other drugs that yield weak signals in procedures that use MFB electrodes.
Fourth, sensitivity might also be improved by manipulating state and/or trait variables in ICSS procedures. For example, nearly all ICSS studies have been conducted in male subjects, but the few studies that have been conducted with females have identified intriguing sex differences in effects of some drugs such as opioids (Craft et al., 2001; Russell et al., 2013). Systematic examination of state/trait variables on drug effects in ICSS procedures may help to identify conditions under which selected drugs are most likely to produce an abuse-related effect.
Finally, brain stimulation in standard ICSS procedures is accomplished by electrical stimulation delivered via electrodes. However, electrical stimulation preferentially activates large myelinated neurons proximal to the electrode, and it provides limited flexibility for targeting neurons with selected functional or chemical phenotypes. Emerging optogenetic technologies permit targeted expression of channel rhodopsins in neurons of interest, and these neurons can then be selectively activated or inhibited by light stimuli delivered via fiber optic probes. ICSS maintained by light rather than electrical stimulation has been demonstrated (You et al., 2001; Kim et al., 2012; Kempadoo et al., 2013), and the potential of this technology for abuse potential testing remains to be determined.
Acknowledgments
The authors thank Robert L. Balster, Ahmad Altarifi, and Travis Grim for comments on earlier versions of the manuscript. In addition, the authors thank Matthew Lazenka for assistance in preparing anatomical diagrams in Fig. 1, as well as the following graduate students who contributed to literature review and analysis as part of a graduate class on the use of ICSS in behavioral pharmacology: Ahmad Altarifi, Clayton Bauer, Julie Bonano, Kelen Freitas, Travis Grim, Todd Hillhouse, Andrew Kwilasz, Michael Leitl, and Matthew Tracy.
Abbreviations
- α5ko
α5 nAChR subunit knockout mice
- 5HT
serotonin
- 7-OH-DPAT
7-hydroxy-2-(di-n-propylamino)tetralin
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- A77636
(1R,3S)-3-(1-adamantyl)-1-(aminomethyl)-3,4-dihydro-1H-isochromene-5,6-diol
- ACh
acetylcholine
- AChE
enzyme acetycholinesterase
- ADX47273
(S)-(4-fluorophenyl)-(3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]piperidin-1-yl)methanone
- AMN082
N,N′-bis(diphenylmethyl)-1,2-ethanediamine dihydrochloride
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- ARR-17779
(2S)-2′H-spiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxazolidin]-2′-one
- BW373U86
4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide
- CB
cannabinoid
- CDPPB
3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide
- CP55940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DA
dopamine
- DAT
dopamine transporter
- D2Rko
D2 receptor knockout
- FAAH
fatty acid amide hydrolase
- FDA
U.S. Food and Drug Administration
- FR
fixed ratio
- GABA
γ-aminobutyric acid
- GHB
γ-hydroxybutyric acid
- GW405833
1-(2,3-dichlorobenzoyl)-5-methoxy-2-methyl-3-[2-(4-morpholinyl)ethyl]-1H-indole
- GYKI-53655
1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride
- HU210
(6aR)-trans-3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol
- ICSS
intracranial self-stimulation
- L701324
7-chloro-4-hydroxy-3-(3-phenoxy)phenyl-2(1H)-quinolinone
- LPS
lipopolysaccharide
- LSD
lysergic acid diethyamide
- LY314582
(±)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid
- LY341495
(2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid
- LY93959
(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid
- mAChR
muscarinic acetylcholine receptor
- MAGL
monoacylglycerol lipase
- MCR
maximum control rate
- MDMA
methylenedioxymethamphetamine
- MDPV
methylenedioxypyrovalerone
- MFB
medial forebrain bundle
- mGluR
metabotropic glutamate receptor
- MK801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- MPEP
2-methyl-6-[phenylethynyl]-pyridine
- nAChR
nicotinic acetylcholine receptor
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline
- NE
norepinephrine
- NET
norepinephrine transporter
- NMDA
N-methyl-d-aspartate
- PCP
phencyclidine
- SERT
serotonin transporter
- RTI-113
phenyl 3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- SIB-1765F
[6]-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine
- SKF38393
(±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide
- SKF82958
3-allyl-6-chloro-1-phenyl-1,2,4,5-tetrahydro-3-benzazepine-7,8-diol
- SNC80
4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide
- TCB-2
1-[(7R)-3-bromo-2,5-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methanamine
- THC
Δ9-tetrahydrocannabinol
- U50,488
5,6-dimethoxy-N,N-dipropyl-2,3-dihydro-1H-inden-2-amine
- U69,593
N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide
- U99194A
5,6-dimethoxy-N,N-dipropyl-2,3-dihydro-1H-inden-2-amine
- URB597
cyclohexylcarbamic acid 3′-(aminocarbonyl)-[1,1′-biphenyl]-3-yl ester
- VTA
ventral tegmental area
- WIN55212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
- WT
wild type
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Negus, Miller.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA026946, R01-DA033930, R01-DA030404]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS070715].
References
- Adams WJ, Lorens SA, Mitchell CL. (1972) Morphine enhances lateral hypothalamic self-stimulation in the rat. Proc Soc Exp Biol Med 140:770–771 [DOI] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, et al. (2009) Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol 16:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Miller LL, Negus SS. (2012) Role of µ-opioid receptor reserve and µ-agonist efficacy as determinants of the effects of µ-agonists on intracranial self-stimulation in rats. Behav Pharmacol 23:678–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. (2011) Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22:663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. (2013) Abuse-related effects of µ-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24:459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Galanopoulos A, Vlachou S, Kourouli T, Nahmias V, Thermos K, Panagis G, Daifoti Z, Marselos M, Papahatjis D, et al. (2005) Behavioral pharmacological properties of a novel cannabinoid 1′,1′-dithiolane delta8-THC analog, AMG-3. Behav Pharmacol 16:499–510 [DOI] [PubMed] [Google Scholar]
- Arnold JC, Hunt GE, McGregor IS. (2001) Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life Sci 70:97–108 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70(3, Suppl)S55–S72 [DOI] [PubMed] [Google Scholar]
- Baik JH. (2013) Dopamine signaling in reward-related behaviors. Front Neural Circuits 7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ. (2006) Drug therapy of depression and anxiety disorders, in Goodman and Gilman's The Pharmacological Basis of Therapeutics,11th ed (Brunton L, Lazo J, Parker K, eds) pp 429–459, McGraw-Hill, New York [Google Scholar]
- Baldessarini RJ, Tarazi FI. (2006) Pharmacotherapy of psychosis and mania, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 461–500, McGraw-Hill, New York [Google Scholar]
- Baldo BA, Jain K, Veraldi L, Koob GF, Markou A. (1999) A dopamine D1 agonist elevates self-stimulation thresholds: comparison to other dopamine-selective drugs. Pharmacol Biochem Behav 62:659–672 [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. (2003) Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend 70(3, Suppl)S13–S40 [DOI] [PubMed] [Google Scholar]
- Banks ML, Bauer CT, Blough BE, Rothman RB, Partilla JS, Baumann MH, Negus SS. (2014) Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys. Exp Clin Psychopharmacol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Song C, Sawada K, Young CE, Honer WG, Phillips AG. (2003) Tolerance to the anhedonic effects of lipopolysaccharide is associated with changes in syntaxin immunoreactivity in the nucleus accumbens. Int J Neuropsychopharmacol 6:23–34 [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. (1994) Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther 271:294–301 [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. (2013a) Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behav Pharmacol 24:448–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. (2013b) Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168:850–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS. (2014) The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl) 10.1007/s00213-013-3405-1. [DOI] [PMC free article] [PubMed]
- Baumann MH, Partilla JS, Lehner KR. (2013a) Psychoactive “bath salts”: not so soothing. Eur J Pharmacol 698:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, et al. (2013b) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Bruijnzeel AW. (2012) Animal models of nicotine withdrawal: intracranial self-stimulation and somatic signs of withdrawal. Methods Mol Biol 829:257–268 [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL. (1992) The intravenous self-administration of antihistamines by rhesus monkeys. Drug Alcohol Depend 30:117–126 [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Stein L. (1977) Enkephalin may mediate euphoria and drive-reduction reward. Nature 266:556–558 [DOI] [PubMed] [Google Scholar]
- BeMent SL, Ranck JB., Jr (1969) A quantitative study of electrical stimulation of central myelinated fibers. Exp Neurol 24:147–170 [DOI] [PubMed] [Google Scholar]
- Benaliouad F, Kapur S, Rompré PP. (2007) Blockade of 5-HT2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology 32:551–561 [DOI] [PubMed] [Google Scholar]
- Benarroch EE. (2012) GABAB receptors: structure, functions, and clinical implications. Neurology 78:578–584 [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Cruz-Morales S, Reid LD. (1983) Addictive agents and intracranial stimulation (ICS): novel antagonists and agonists of morphine and pressing for ICS. Pharmacol Biochem Behav 18:777–784 [DOI] [PubMed] [Google Scholar]
- Bespalov A, Dravolina O, Belozertseva I, Adamcio B, Zvartau E. (2006) Lowered brain stimulation reward thresholds in rats treated with a combination of caffeine and N-methyl-d-aspartate but not alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate or metabotropic glutamate receptor-5 receptor antagonists. Behav Pharmacol 17:295–302 [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. (1999) Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharmacol 9:377–383 [DOI] [PubMed] [Google Scholar]
- Bespalov A, Sukhotina I, Medvedev I, Malyshkin A, Belozertseva I, Balster R, Zvartau E. (2003) Facilitation of electrical brain self-stimulation behavior by abused solvents. Pharmacol Biochem Behav 75:199–208 [DOI] [PubMed] [Google Scholar]
- Bishop MP, Elder ST, Heath RG. (1963) Intracranial self-stimulation in man. Science 140:394–396 [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Dravolina OA, Bespalov AY, Balster RL, Zvartau EE. (2004) Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol 485:211–218 [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. (1998) Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport 9:3797–3802 [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. (1992) Long-term influence of d-amphetamine on mesolimbic brain-stimulation reward: comparison to chronic haloperidol and naloxone effects. Pharmacol Biochem Behav 43:1–15 [DOI] [PubMed] [Google Scholar]
- Bossert JM, Franklin KB. (2003) Reinforcing versus anticonvulsant drugs: effects on intracranial self-stimulation rate-frequency M50 indices. Behav Brain Res 144:243–247 [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Pudiak CM, KuoLee R. (1998) Effect of chronic nicotine on brain stimulation reward. II. An escalating dose regimen. Behav Brain Res 96:189–194 [DOI] [PubMed] [Google Scholar]
- Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte G, Schenk S. (2013) Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addict Biol 10.1111/adb.12069. [DOI] [PubMed] [Google Scholar]
- Brady JV, Boren JJ, Conrad D, Sidman M. (1957) The effect of food and water deprivation upon intracranial self-stimulation. J Comp Physiol Psychol 50:134–137 [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR. (1976) Behavioral procedures for evaluating the relative abuse potential of CNS drugs in primates. Fed Proc 35:2245–2253 [PubMed] [Google Scholar]
- Broekkamp CL, Van den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM. (1976) Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol 36:443–446 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. (2010) Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 212:485–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Byun N, Wood MR, Jones CK. (2012) Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb Exp Pharmacol 208:121–166 [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. (2013) GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38:729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. (1998) Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18:7502–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Krahne LL, Carr KD. (2004) A progressive ratio schedule of self-stimulation testing in rats reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacology (Berl) 175:106–113 [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Hoebel BG. (1966) “Copulation-reward site” in the posterior hypothalamus. Science 153:1284–1285 [DOI] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. (2012) Modeling nicotine addiction in rats. Methods Mol Biol 829:243–256 [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. (2004) Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology 29:929–942 [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816 [DOI] [PubMed] [Google Scholar]
- Carey RJ, Goodall EB, Procopio GF. (1974) Differential effects of d-amphetamine on fixed ratio 30 performance maintained by food versus brain stimulation reinforcement. Pharmacol Biochem Behav 2:193–198 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316:440–447 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. (1993a) Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Res 620:339–342 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. (1993b) Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacology (Berl) 111:402–408 [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. (2001) Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology (Berl) 154:420–428 [DOI] [PubMed] [Google Scholar]
- Carr KD, Yamamoto N, Omura M, Cabeza de Vaca S, Krahne L. (2002) Effects of the D(3) dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology (Berl) 163:76–84 [DOI] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF. (1999) Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology (Berl) 144:111–120 [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105 (Suppl 1):S14–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazala P. (1976) Effects of d- and l-amphetamine on dorsal and ventral hypothalamic self-stimulation in three inbred strains of mice. Pharmacol Biochem Behav 5:505–510 [DOI] [PubMed] [Google Scholar]
- Cazala P, Cazals Y, Cardo B. (1974) Hypothalamic self-stimulation in three inbred strains of mice. Brain Res 81:159–167 [DOI] [PubMed] [Google Scholar]
- Chan MH, Chung SS, Stoker AK, Markou A, Chen HH. (2012) Sarcosine attenuates toluene-induced motor incoordination, memory impairment, and hypothermia but not brain stimulation reward enhancement in mice. Toxicol Appl Pharmacol 265:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin WF, John OP, Goldberg LR. (1988) Conceptions of states and traits: dimensional attributes with ideals as prototypes. J Pers Soc Psychol 54:541–557 [DOI] [PubMed] [Google Scholar]
- Charney DS, Mihic SJ, Adron Harris R. (2006) Hypnotics and sedatives, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 401–427, McGraw-Hill, New York [Google Scholar]
- Chavkin C. (2011) The therapeutic potential of κ-opioids for treatment of pain and addiction. Neuropsychopharmacology 36:369–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM. (2005) Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: implications for intracranial self-stimulation. Proc Natl Acad Sci USA 102:19150–19155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. (2003) Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74:541–549 [DOI] [PubMed] [Google Scholar]
- Clark AS, Lindenfeld RC, Gibbons CH. (1996) Anabolic-androgenic steroids and brain reward. Pharmacol Biochem Behav 53:741–745 [DOI] [PubMed] [Google Scholar]
- Cleva RM, Watterson LR, Johnson MA, Olive MF. (2012) differential modulation of thresholds for intracranial self-stimulation by mGlu5 positive and negative allosteric modulators: implications for effects on drug self-administration. Front Pharmacol 2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. (1984) Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 82:6–13 [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. (2010) Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend 109:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci 30:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Evans SM, Kowalczyk WJ, Saccone PA, Comer SD. (2012) Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol 23:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D. (1989) Possible abuse potential of the NMDA antagonist MK-801. Behav Brain Res 34:239–246 [DOI] [PubMed] [Google Scholar]
- Corbett D. (1990) Differences in sensitivity to neuroleptic blockade: medial forebrain bundle versus frontal cortex self-stimulation. Behav Brain Res 36:91–96 [DOI] [PubMed] [Google Scholar]
- Cottencin O, Rolland B, Karila L. (2013) New designer drugs (synthetic cannabinoids and synthetic cathinones): review of literature. Curr Pharm Des, in press [DOI] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. (1987) Fitting intracranial self-stimulation data with growth models. Behav Neurosci 101:209–214 [DOI] [PubMed] [Google Scholar]
- Craft RM, Stoffel EC, Stratmann JA. (2001) Effects of chronic morphine treatment on responding for intracranial stimulation in female versus male rats. Exp Clin Psychopharmacol 9:198–208 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. (2004) The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol 61:149–160 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. (2003) Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54:49–58 [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. (2014) Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci 97:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M. (2013) Leveraging the cortical cholinergic system to enhance attention. Neuropharmacology 64:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. (1969) Self-administration of psychoactive substances by the monkey. Psychopharmacology (Berl) 16:30–48 [DOI] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. (1996) Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacology (Berl) 124:231–240 [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266:1236–1246 [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, et al. (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61 [PubMed] [Google Scholar]
- Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, Neve RL, Nestler EJ, Carlezon WA., Jr (2009) Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci 29:1855–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divito CB, Underhill SM. (2014) Excitatory amino acid transporters: Roles in glutamatergic neurotransmission. Neurochem Int 10.1016/j.neuint.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. (2009) The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol 604:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr (2014) Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druhan JP, Fibiger HC, Phillips AG. (1989) Differential effects of cholinergic drugs on discriminative cues and self-stimulation produced by electrical stimulation of the ventral tegmental area. Psychopharmacology (Berl) 97:331–338 [DOI] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. (1997a) Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther 281:188–199 [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. (1997b) Parametric changes in response equilibrium during an intra-cranial self stimulation (ICSS) task: can reward value be assessed independently of absolute threshold? Neurosci Biobehav Rev 21:55–65 [DOI] [PubMed] [Google Scholar]
- Easterling KW, Plovnick RM, Holtzman SG. (2000) Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology (Berl) 148:263–271 [DOI] [PubMed] [Google Scholar]
- Egawa K, Fukuda A. (2013) Pathophysiological power of improper tonic GABA(A) conductances in mature and immature models. Front Neural Circuits 7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder ST, May JG, Rye MM. (1965) Establishment and control of a bar-pressing habit by means of fixed interval ICSS reinforcement. Psychol Rep 17:607–618 [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Hamilton LR, Wise RA. (2010) Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology (Berl) 208:309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. (2005) Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl) 182:33–44 [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. (2002) Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci 22:RC224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly MA, Gul W, Wanas AS, Radwan MM. (2014) Synthetic cannabinoids: analysis and metabolites. Life Sci 97:78–90 [DOI] [PubMed] [Google Scholar]
- Engin E, Bakhurin KI, Smith KS, Hines RM, Reynolds LM, Tang W, Sprengel R, Moss SJ, Rudolph U. (2014) Neural basis of benzodiazepine reward: requirement for α2 containing GABA receptors in the nucleus accumbens. Neuropsychopharmacology 10.1038/npp.2014.41. [DOI] [PMC free article] [PubMed]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79 [DOI] [PubMed] [Google Scholar]
- Esposito R, Kornetsky C. (1977) Morphine lowering of self-stimulation thresholds: lack of tolerance with long-term administration. Science 195:189–191 [DOI] [PubMed] [Google Scholar]
- Esposito RU, Motola AH, Kornetsky C. (1978) Cocaine: acute effects on reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacol Biochem Behav 8:437–439 [DOI] [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. (1980) Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology (Berl) 69:187–191 [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Milner PM. (1977) Effects of dopamine supersensitivity on lateral hypothalamic self-stimulation in rats. Pharmacol Biochem Behav 7:507–514 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2006) Guideline on the non-clinical investigation of the dependence potential of medicinal products, in (http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000397.jsp&mid=WC0b01ac058002956f ed).
- Ewan EE, Martin TJ. (2012) Intracranial self-stimulation of the paraventricular nucleus of the hypothalamus: increased faciliation by morphine compared to cocaine. Anesthesiology 116:1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. (2011) Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology 114:624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. (2013) Analgesics as reinforcers with chronic pain: Evidence from operant studies. Neurosci Lett 557:60–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. (2014) Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activitty by paw incision and spinal nerve ligation in rats. Anaesthesiology 10.1213/ANE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. (2008) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75:17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Winger G, Woods JH, Woolverton WL, Coop A. (2005) Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend 77:161–168 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229 [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. (2001) Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 156:410–416 [DOI] [PubMed] [Google Scholar]
- Fenton HM, Liebman JM. (1982) Self-stimulation response decrement patterns differentiate clonidine, baclofen and dopamine antagonists from drugs causing performance deficit. Pharmacol Biochem Behav 17:1207–1212 [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. (1981) Increased intracranial self-stimulation in rats after long-term administration of desipramine. Science 214:683–685 [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Coury A, Fibiger HC, Phillips AG. (1993) Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res 55:131–141 [DOI] [PubMed] [Google Scholar]
- Fish EW, Krouse MC, Stringfield SJ, Diberto JF, Robinson JE, Malanga CJ. (2013) Changes in sensitivity of reward and motor behavior to dopaminergic, glutamatergic, and cholinergic drugs in a mouse model of fragile X syndrome. PLoS ONE 8:e77896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, Malanga CJ. (2010) Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol Clin Exp Res 34:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Robinson JE, Krouse MC, Hodge CW, Reed C, Phillips TJ, Malanga CJ. (2012) Intracranial self-stimulation in FAST and SLOW mice: effects of alcohol and cocaine. Psychopharmacology (Berl) 220:719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, Arnt J, Olsen CK. (2006) Classical as well as novel antipsychotic drugs increase self-stimulation threshold in the rat—similar mechanism of action? Eur J Pharmacol 544:69–76 [DOI] [PubMed] [Google Scholar]
- Fleming JW, McClendon KS, Riche DM. (2013) New obesity agents: lorcaserin and phentermine/topiramate. Ann Pharmacother 47:1007–1016 [DOI] [PubMed] [Google Scholar]
- Flordellis C, Paris H, Karabinis A, Lymperopoulos A. (2004) Pharmacogenomics of adrenoceptors. Pharmacogenomics 5:803–817 [DOI] [PubMed] [Google Scholar]
- Fokos S, Panagis G. (2010) Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol 24:767–777 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2010) Guidance for Industry: assessment of abuse potential of drugs (draft guidance), in http://www.fda.gov/downloads/Drugs/ and GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf.eds
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. (2011) Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Tuesta L, Kenny PJ. (2013) Role of alpha5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl) 10.1007/s00213-013-3235-1. [DOI] [PMC free article] [PubMed]
- Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. (2010) The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 212:13–23 [DOI] [PubMed] [Google Scholar]
- Frank RA, Markou A, Wiggins LL. (1987) A systematic evaluation of the properties of self-stimulation train-duration response functions. Behav Neurosci 101:546–559 [DOI] [PubMed] [Google Scholar]
- Frank RA, Martz S, Pommering T. (1988) The effect of chronic cocaine on self-stimulation train-duration thresholds. Pharmacol Biochem Behav 29:755–758 [DOI] [PubMed] [Google Scholar]
- Galankin T, Shekunova E, Zvartau E. (2010) Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav 58:827–834 [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Freyd G. (1987) Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav 26:731–741 [DOI] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. (1988) Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 96:142–144 [DOI] [PubMed] [Google Scholar]
- Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr (2002) Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl) 163:238–248 [DOI] [PubMed] [Google Scholar]
- Glick SD, Cox RS. (1975) Self-admimistration of haloperidol in rats. Life Sci 16:1041–1045 [DOI] [PubMed] [Google Scholar]
- Glick SD, Guido RA. (1982) Scopolamine self-administration: cholinergic involvement in reward mechanisms. Life Sci 31:909–913 [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Kowalska M, Bymaster FP. (2012) Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity. Synapse 66:435–444 [DOI] [PubMed] [Google Scholar]
- Goodall EB, Carey RJ. (1975) Effects of d- versus l-amphetamine, food deprivation, and current intensity on self-stimulation of the lateral hypothalamus, substantia nigra, and medial frontal cortex of the rat. J Comp Physiol Psychol 89:1029–1045 [DOI] [PubMed] [Google Scholar]
- Grech DM, Spealman RD, Bergman J. (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 125:97–104 [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. (2008) Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol 42:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshaw AJ. (1993) Differential effects of ondansetron, haloperidol and clozapine on electrical self-stimulation of the ventral tegmental area. Behav Pharmacol 4:479–485 [PubMed] [Google Scholar]
- Griffiths RR, Chausmer AL. (2000) Caffeine as a model drug of dependence: recent developments in understanding caffeine withdrawal, the caffeine dependence syndrome, and caffeine negative reinforcement. Nihon Shinkei Seishin Yakurigaku Zasshi 20:223–231 [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW. (2005) Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 66 (Suppl 9):31–41 [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. (1991) Self-injection of barbiturates, benzodiazepines and other sedative-anxiolytics in baboons. Psychopharmacology (Berl) 103:154–161 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. (1992) Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther 260:1199–1208 [PubMed] [Google Scholar]
- Griffiths RR, Woodson PP. (1988) Reinforcing properties of caffeine: studies in humans and laboratory animals. Pharmacol Biochem Behav 29:419–427 [DOI] [PubMed] [Google Scholar]
- Gutstein H, Akil H. (2006) Opioid analgesics, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 547–590, McGraw-Hill, New York [Google Scholar]
- Hall FS, Stellar JR, Kelley AE. (1990) Acute and chronic desipramine treatment effects on rewarding electrical stimulation of the lateral hypothalamus. Pharmacol Biochem Behav 37:277–281 [DOI] [PubMed] [Google Scholar]
- Hariz MI, Blomstedt P, Zrinzo L. (2010) Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus 29:E1. [DOI] [PubMed] [Google Scholar]
- Harris AC, Stepanov I, Pentel PR, Lesage MG. (2012) Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl) 220:565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. (2002) Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160:56–66 [DOI] [PubMed] [Google Scholar]
- Harrison AA, Markou A. (2001) Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther 297:316–325 [PubMed] [Google Scholar]
- Harrison AA, Parsons LH, Koob GF, Markou A. (1999) RU 24969, a 5-HT1A/1B agonist, elevates brain stimulation reward thresholds: an effect reversed by GR 127935, a 5-HT1B/1D antagonist. Psychopharmacology (Berl) 141:242–250 [DOI] [PubMed] [Google Scholar]
- Hatcher JP, Boyland P, Hagan JJ. (1995) The 5 -HT3 receptor antagonists, granisetron and ondansetron, do not affect cocaine-induced shifts in intra-cranial self-stimulation thresholds. J Psychopharmacol 9:342–347 [DOI] [PubMed] [Google Scholar]
- Hatcher JP, Hagan JJ. (1998) The effects of dopamine D3/D2 receptor agonists on intracranial self stimulation in the rat. Psychopharmacology (Berl) 140:405–410 [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Clements R, Greenshaw AJ. (2009) Effects of systemic and intra-nucleus accumbens 5-HT2C receptor compounds on ventral tegmental area self-stimulation thresholds in rats. Psychopharmacology (Berl) 203:579–588 [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ. (2011) 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev 35:1419–1449 [DOI] [PubMed] [Google Scholar]
- Heath RG. (1963) Electrical Self-Stimulation of the Brain in Man. Am J Psychiatry 120:571–577 [DOI] [PubMed] [Google Scholar]
- Heath RG.(1964) Pleasure response of human subjects to direct stimulation of the brain: Physiologic and psychodynamic considerations, in The Role of Pleasure in Behavior (Heath RG. ed) pp 219–243, Hoeber, Harper and Row, New York [Google Scholar]
- Heckman MA, Weil J, Gonzalez de Mejia E. (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75:R77–R87 [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. (1989) The effect of MK-801 and other antagonists of NMDA-type glutamate receptors on brain-stimulation reward. Psychopharmacology (Berl) 99:87–90 [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. (1990) Excitatory amino acid pathways in brain-stimulation reward. Behav Brain Res 39:230–239 [DOI] [PubMed] [Google Scholar]
- Hernandez G, Breton YA, Conover K, Shizgal P. (2010) At what stage of neural processing does cocaine act to boost pursuit of rewards? PLoS ONE 5:e15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. (2011) PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol 25:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, Negus SS. (2014) Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacology (Berl) 10.1007/s00213-014-3451-3. [DOI] [PMC free article] [PubMed]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Valenstein ES. (1960) Motivational variables affecting the rate of behavior maintained by intracranial stimulation. J Comp Physiol Psychol 53:502–508 [DOI] [PubMed] [Google Scholar]
- Holtzman SG. (1976) Comparison of the effects of morphine, pentazocine, cyclazocine and amphetamine on intracranial self-stimulation in the rat. Psychopharmacology (Berl) 46:223–227 [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. (2013) A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol 24:410–436 [DOI] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM. (1992) Reward summation and the effects of pimozide, clonidine, and amphetamine on fixed-interval responding for brain stimulation. Pharmacol Biochem Behav 42:563–577 [DOI] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM, Chesher GB, Becker FT. (1976) Alpha-noradrenergic modulation of hypothalamic self-stimulation: studies employing clonidine, 1-phenylephrine and alpha-methyl-p-tyrosine. Eur J Pharmacol 37:105–111 [DOI] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM, Jackson DM. (1994) Reward summation and the effects of dopamine D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacol Biochem Behav 48:853–862 [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Rowlett JK, Freeman KB. (2014) Predicting abuse potential of stimulants and other dopaminergic drugs: Overview and recommendations. Neuropharmacology 10.1016/j.neuropharm.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. (1992) Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav 41:755–759 [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Sarkar M, Kornetsky C. (1993) Nicotine and brain-stimulation reward: interactions with morphine, amphetamine and pimozide. Pharmacol Biochem Behav 46:453–457 [DOI] [PubMed] [Google Scholar]
- Ichimaru Y, Moriyama M, Gomita Y. (1985) Auto-titration technique using intracranial self-stimulation and effects of antianxiety drugs. Jpn J Pharmacol 39:331–338 [DOI] [PubMed] [Google Scholar]
- Immadisetty K, Madura JD. (2013) A review of monoamine transporter-ligand interactions. Curr Comput Aided Drug Des 9:556–568 [DOI] [PubMed] [Google Scholar]
- Jacques S. (1979) Brain stimulation and reward: “pleasure centers” after twenty-five years. Neurosurgery 5:277–283 [DOI] [PubMed] [Google Scholar]
- Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, Bear MF, Umbricht D, Hajos M, Potter WZ, et al. (2011) Translating glutamate: from pathophysiology to treatment. Sci Transl Med 3:102mr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Gratton A, Wise RA. (1987) Opioid receptor subtypes associated with ventral tegmental facilitation of lateral hypothalamic brain stimulation reward. Brain Res 423:34–38 [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. (1978) A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc 30:43–54 [PubMed] [Google Scholar]
- Jones CK, Byun N, Bubser M. (2012) Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 37:16–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD. (2013) A review of human drug self-administration procedures. Behav Pharmacol 24:384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, et al. (2008) Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry 64:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr (2010) Illicit anabolic-androgenic steroid use. Horm Behav 58:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian DA, Bahr BA. (2006) Cannabinoid drugs and enhancement of endocannabinoid responses: strategies for a wide array of disease states. Curr Mol Med 6:677–684 [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Apazoglou K, Panagis G. (2011) Role of serotonin 5-HT2A and 5-HT2C receptors on brain stimulation reward and the reward-facilitating effect of cocaine. Psychopharmacology (Berl) 213:337–354 [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G. (2013) Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol 16:2273–2284 [DOI] [PubMed] [Google Scholar]
- Katz JL, Goldberg SR. (1988) Preclinical assessment of abuse liability of drugs. Agents Actions 23:18–26 [DOI] [PubMed] [Google Scholar]
- Katz RJ, Baldrighi G, Roth K. (1978) Appetitive determinants of self-stimulation. Behav Biol 23:500–508 [DOI] [PubMed] [Google Scholar]
- Katz RJ, Carroll BJ. (1977) Intracranial reward after Lilly 110140 (fluoxetine HCl): evidence for an inhibitory role for serotonin. Psychopharmacology (Berl) 51:189–193 [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. (1968) Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol 60:1–56 [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, et al. (2013) Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci 33:7618–7626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. (2009) NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology 34:266–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. (2003a) Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther 306:1068–1076 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Koob GF, Markou A. (2003b) Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci 117:1103–1107 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602 [DOI] [PubMed] [Google Scholar]
- Kim KM, Baratta MV, Yang A, Lee D, Boyden ES, Fiorillo CD. (2012) Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE 7:e33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. (2008) Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav 90:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling-Petersen T, Ljung E, Wollter L, Svensson K. (1995) Effects of dopamine D3 preferring compounds on conditioned place preference and intracranial self-stimulation in the rat. J Neural Transm 101:27–39 [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Winger GD. (1988) MK-801, a proposed noncompetitive antagonist of excitatory amino acid neurotransmission, produces phencyclidine-like behavioral effects in pigeons, rats and rhesus monkeys. J Pharmacol Exp Ther 245:969–974 [PubMed] [Google Scholar]
- Kofman O, Yeomans JS. (1988) Cholinergic antagonists in ventral tegmentum elevate thresholds for lateral hypothalamic and brainstem self-stimulation. Pharmacol Biochem Behav 31:547–559 [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, McCarter BD. (1990) Postcocaine depression and sensitization of brain-stimulation reward: analysis of reinforcement and performance effects. Pharmacol Biochem Behav 36:463–471 [DOI] [PubMed] [Google Scholar]
- Koob GF, Spector NH, Meyerhoff JL. (1975) Effects of heroin on lever pressing for intracranial self-stimulation, food and water in the rat. Psychopharmacology (Berl) 42:231–234 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Bain GT, Unterwald EM, Lewis MJ. (1988) Brain stimulation reward: effects of ethanol. Alcohol Clin Exp Res 12:609–616 [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. (1979) Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry 36:289–292 [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. (2010) Reward and the serotonergic system. Neuroscience 166:1023–1035 [DOI] [PubMed] [Google Scholar]
- Kruk ZL, Cheeta S, Milla J, Muscat R, Williams JE, Willner P. (1998) Real time measurement of stimulated dopamine release in the conscious rat using fast cyclic voltammetry: dopamine release is not observed during intracranial self stimulation. J Neurosci Methods 79:9–19 [DOI] [PubMed] [Google Scholar]
- Kucharski LT, Williams JE, Kornetsky C. (1983) The effects of levonantradol on rewarding brain stimulation thresholds in the rat. Pharmacol Biochem Behav 19:149–151 [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Abdullah RA, Poklis JL, Lichtman AH, Negus SS. (2014) Effects of the fatty acid amide hydrolase inhibitor URB597 on pain-stimulated and pain-depressed behavior in rats. Behav Pharmacol 25:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. (2012) Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna L, Von Felsinger JM, Beecher HK. (1955) Drug-induced mood changes in man. I. Observations on healthy subjects, chronically ill patients, and postaddicts. J Am Med Assoc 157:1006–1020 [DOI] [PubMed] [Google Scholar]
- Latz A, Bain GT, Kornetsky C. (1969) Attenuated effect of chlorpromazine on conditioned avoidance as a function of rapid acquisition. Psychopharmacology (Berl) 14:23–32 [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Di Chiara G. (2006) Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology (Berl) 188:63–74 [DOI] [PubMed] [Google Scholar]
- Lee K, Kornetsky C. (1998) Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav 60:539–544 [DOI] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. (2014) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology 39:614–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. (1996) Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci 58:PL365–PL372 [DOI] [PubMed] [Google Scholar]
- Li X, Xi ZX, Markou A. (2013) Metabotropic glutamate 7 (mGlu7) receptor: a target for medication development for the treatment of cocaine dependence. Neuropharmacology 66:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. (2011) Self-administration of bretazenil under progressive-ratio schedules: behavioral economic analysis of the role intrinsic efficacy plays in the reinforcing effects of benzodiazepines. Drug Alcohol Depend 113:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman JM, Butcher LL. (1973) Effects on self-stimulation behavior of drugs influencing dopaminergic neurotransmission mechanisms. Naunyn Schmiedebergs Arch Pharmacol 277:305–318 [DOI] [PubMed] [Google Scholar]
- Liebman JM, Hall NR, Prowse J, Gerhardt S, Noreika L, Fenton HM. (1984) Comparative effects of beta 2-adrenoceptor agonists on intracranial self-stimulation, Sidman avoidance, and motor activity in rats. Psychopharmacology (Berl) 84:336–341 [DOI] [PubMed] [Google Scholar]
- Lin D, Bruijnzeel AW, Schmidt P, Markou A. (2002) Exposure to chronic mild stress alters thresholds for lateral hypothalamic stimulation reward and subsequent responsiveness to amphetamine. Neuroscience 114:925–933 [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. (1999) Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat—interactions between the two drugs. Psychopharmacology (Berl) 145:283–294 [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. (2000) Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav 65:407–417 [DOI] [PubMed] [Google Scholar]
- Lin HQ, Jackson DM, Atrens DM, Christie MJ, McGregor IS. (1997) Serotonergic modulation of 3,4-methylenedioxymethamphetamine (MDMA)-elicited reduction of response rate but not rewarding threshold in accumbal self-stimulation. Brain Res 744:351–357 [DOI] [PubMed] [Google Scholar]
- Liu J, Pan H, Gold MS, Derendorf H, Bruijnzeel AW. (2008) Effects of fentanyl dose and exposure duration on the affective and somatic signs of fentanyl withdrawal in rats. Neuropharmacology 55:812–818 [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. (2009) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorens SA, Mitchell CL. (1973) Influence of morphine on lateral hypothalamic self-stimulation in the rat. Psychopharmacology (Berl) 32:271–277 [DOI] [PubMed] [Google Scholar]
- Macey DJ, Froestl W, Koob GF, Markou A. (2001) Both GABA(B) receptor agonist and antagonists decreased brain stimulation reward in the rat. Neuropharmacology 40:676–685 [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. (2008) Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry 63:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. (1987) Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7:2445–2464 [PMC free article] [PubMed] [Google Scholar]
- Marcus R, Kornetsky C. (1974) Negative and positive intracranial reinforcement thresholds: Effects of morphine. Psychopharmacologia (Berl) 38:1–13 [Google Scholar]
- Markou A, Hauger RL, Koob GF. (1992) Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacologia (Berl) 109:305–314 [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. (1991) Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26 [PubMed] [Google Scholar]
- Markou A, Koob GF. (1992) Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51:111–119 [DOI] [PubMed] [Google Scholar]
- Marquis KL, Moreton JE. (1987) Animal models of intravenous phencyclinoid self-administration. Pharmacol Biochem Behav 27:385–389 [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. (1998) Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85:327–330 [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Markaki E, Nomikos GG, Panagis G. (2010) Chronic WIN55,212-2 elicits sustained and conditioned increases in intracranial self-stimulation thresholds in the rat. Behav Brain Res 209:114–118 [DOI] [PubMed] [Google Scholar]
- McCarter BD, Kokkinidis L. (1988) The effects of long-term administration of antidepressant drugs on intracranial self-stimulation responding in rats. Pharmacol Biochem Behav 31:243–247 [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. (2006) 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem 49:5794–5803 [DOI] [PubMed] [Google Scholar]
- Meldrum BS. (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130(4S, Suppl)1007S–1015S [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Emond C, Merali Z. (1991) Re-evaluation of the role of dopamine in intracranial self-stimulation using in vivo microdialysis. Behav Brain Res 46:43–48 [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. (1986) The curve-shift paradigm in self-stimulation. Physiol Behav 37:85–91 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Milner PM. (1989) The discovery of self-stimulation and other stories. Neurosci Biobehav Rev 13:61–67 [DOI] [PubMed] [Google Scholar]
- Miyata H, Itasaka M, Kimura N, Nakayama K. (2011) Decreases in brain reward function reflect nicotine- and methamphetamine-withdrawal aversion in rats. Curr Neuropharmacol 9:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H. (2012) The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62:42–53 [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. (2012) Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem Int 61:581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony L, Kew JN, Gunthorpe MJ, Paoletti P. (2009) Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol 157:1301–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Bourson A, Jenck F, Martin JR, Mortas P. (1994) Curative effects of the atypical antidepressant mianserin in the chronic mild stress-induced anhedonia model of depression. J Psychiatry Neurosci 19:51–56 [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. (1992) Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol 2:43–49 [DOI] [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER. (2012) Acute effects of ethanol on glutamate receptors. Basic Clin Pharmacol Toxicol 111:4–13 [DOI] [PubMed] [Google Scholar]
- Nakajima S, O’Regan NB. (1991) The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacol Biochem Behav 39:465–468 [DOI] [PubMed] [Google Scholar]
- Nazzaro JM, Seeger TF, Gardner EL. (1981) Morphine differentially affects ventral tegmental and substantia nigra brain reward thresholds. Pharmacol Biochem Behav 14:325–331 [DOI] [PubMed] [Google Scholar]
- Negus SS. (2004) Delta Opioids and Substance Abuse, in The Delta Receptor (Chang K-J, Porreca F, Woods JH, eds) pp 401–430, Marcel Dekker, Inc., New York [Google Scholar]
- Negus SS. (2013) Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Dykstra LA. (1989) Neural substrates mediating the reinforcing properties of opioid analgesics, in Biochemistry and Physiology of Substance Abuse, Vol. 1 (Watson RW, ed) pp 211–242, CRC Press, Boca Raton [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. (1998) Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther 286:362–375 [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. (2010) Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. (2012a) Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rice KC. (2009) Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology 34:899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. (2012b) Effects of the δ opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain 13:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW. (2008) Micro/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 16:386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DB, Fenton H, Justice JB., Jr (2002) Increase in accumbal dopaminergic transmission correlates with response cost not reward of hypothalamic stimulation. Behav Brain Res 137:129–138 [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. (2012) Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 224:33–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159 [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Balster RL, Golembiowska K, Kowalska M, Tizzano JP, Skolnick P, Basile AS. (2009) Preclinical evaluation of the abuse potential of the analgesic bicifadine. J Pharmacol Exp Ther 330:236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Jones HE, Balster RL. (1998) Evaluation of the reinforcing and discriminative stimulus properties of the low-affinity N-methyl-d-aspartate channel blocker memantine. Behav Pharmacol 9:231–243 [PubMed] [Google Scholar]
- Nielsen CK, Arnt J, Sánchez C. (2000) Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindividual differences. Behav Brain Res 107:21–33 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LM, Veening JG. (1982) The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol 206:49–81 [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Johnson DS, Ballard TE, Stiff C, Cravatt BF. (2012) O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci 3:418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C. (2007) Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369:1047–1053 [DOI] [PubMed] [Google Scholar]
- O’Brien CP. (2006) Drug addiction and drug abuse, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 607–628, McGraw-Hill, New York [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938 [DOI] [PubMed] [Google Scholar]
- O’Neill KS, Todtenkopf MS. (2010) Using a rate-frequency curve method to assess the rewarding properties of morphine in the intracranial self-stimulation paradigm in rats. J Neurosci Methods 189:75–79 [DOI] [PubMed] [Google Scholar]
- Olds J. (1958a) Effects of hunger and male sex hormone on self-stimulation of the brain. J Comp Physiol Psychol 51:320–324 [DOI] [PubMed] [Google Scholar]
- Olds J. (1958b) Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science 127:315–324 [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–427 [DOI] [PubMed] [Google Scholar]
- Olds J, Travis RP. (1960) Effects of chlorpromazine, meprobamate, pentobarbital and morphine on self-stimulation. J Pharmacol Exp Ther 128:397–404 [PubMed] [Google Scholar]
- Olds ME. (1966) Facilitatory action of diazepam and chloridiazepoxide on hypothalamic reward behavior. J Comp Physiol Psychol 62:136–140 [DOI] [PubMed] [Google Scholar]
- Olds ME. (1972) Comparative effects of amphetamine, scopolamine and chlordiazepoxide on self-stimulation behavior. Rev Can Biol 31 (Suppl):25–47 [PubMed] [Google Scholar]
- Olds ME. (1995) Dopamine agonists prevent or counteract the suppression of brain stimulation reward by fenfluramine. Pharmacol Biochem Behav 50:41–48 [DOI] [PubMed] [Google Scholar]
- Olds ME, Domino EF. (1969) Comparison of muscarinic and nicotinic cholinergic agonists on self-stimulation behavior. J Pharmacol Exp Ther 166:189–204 [PubMed] [Google Scholar]
- Osikowicz M, Mika J, Przewlocka B. (2013) The glutamatergic system as a target for neuropathic pain relief. Exp Physiol 98:372–384 [DOI] [PubMed] [Google Scholar]
- Palazzolo DL. (2013) Electronic cigarettes and vaping: A new challenge in clinical medicine and public health. A literature review. Front Public Health 1:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A, Spyraki C, Nomikos G. (2000) Effects of methyllycaconitine (MLA), an alpha 7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berl) 149:388–396 [DOI] [PubMed] [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG. (2008) Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1:350–374 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. (2010) Animal models of cannabinoid reward. Br J Pharmacol 160:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Bruijnzeel AW, Kenny PJ, Wright CD, Froestl W, Markou A. (2005) Prolonged nicotine exposure does not alter GABA(B) receptor-mediated regulation of brain reward function. Neuropharmacology 49:953–962 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. (2000) Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 152:440–446 [DOI] [PubMed] [Google Scholar]
- Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A. (2008) Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther 326:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Blaha CD, Fibiger HC. (1989) Neurochemical correlates of brain-stimulation reward measured by ex vivo and in vivo analyses. Neurosci Biobehav Rev 13:99–104 [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. (2008) Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol 75:997–1006 [DOI] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. (2011) Repeated exposure to the κ-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry 70:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL. (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins J, Kenny PJ, Doomernik I, Schreiber R, Olivier B, Mechiel Korte S. (2012) The triple reuptake inhibitor DOV 216,303 induces long-lasting enhancement of brain reward activity as measured by intracranial self-stimulation in rats. Eur J Pharmacol 693:51–56 [DOI] [PubMed] [Google Scholar]
- Rada PV, Mark GP, Yeomans JJ, Hoebel BG. (2000) Acetylcholine release in ventral tegmental area by hypothalamic self-stimulation, eating, and drinking. Pharmacol Biochem Behav 65:375–379 [DOI] [PubMed] [Google Scholar]
- Rady JJ, Takemori AE, Portoghese PS, Fujimoto JM. (1994) Supraspinal delta receptor subtype activity of heroin and 6-monoacetylmorphine in Swiss Webster mice. Life Sci 55:603–609 [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Naim DW, Lynch WJ. (2013) Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology 38:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. (1994) The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Res 651:283–292 [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Fink-Jensen A. (2000) Intravenous scopolamine is potently self-administered in drug-naive mice. Neuropsychopharmacology 22:97–99 [DOI] [PubMed] [Google Scholar]
- Reavill C, Hatcher JP, Lewis VA, Sanger GJ, Hagan J. (1998) 5-HT4 receptor antagonism does not affect motor and reward mechanisms in the rat. Eur J Pharmacol 357:115–120 [DOI] [PubMed] [Google Scholar]
- Reid LD. (1987) Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs, in Methods of Assessing the Reinforcing Properties of Abused Drugs (Bozarth MJ, ed) pp 391–420, Springer, Berlin [Google Scholar]
- Reid LD, Gibson WE, Gledhill SM, Porter PB. (1964) Anticonvulsant drugs and self-stimulating behavior. J Comp Physiol Psychol 57:353–356 [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. (2009) Caffeinated energy drinks—a growing problem. Drug Alcohol Depend 99:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Engin E, Tantillo G, Lau HM, Muschamp JW, Carlezon WA, Jr, Rudolph U. (2012) Differential roles of GABA(A) receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology 37:2531–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11 [DOI] [PubMed] [Google Scholar]
- Richardson NR, Smith AM, Roberts DCS. (1994) A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug Alcohol Depend 36:23–25 [DOI] [PubMed] [Google Scholar]
- Riday TT, Kosofsky BE, Malanga CJ. (2012) The rewarding and locomotor-sensitizing effects of repeated cocaine administration are distinct and separable in mice. Neuropharmacology 62:1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. (1998) Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res 22:677–684 [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. (1994) Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 116:207–216 [DOI] [PubMed] [Google Scholar]
- Robertson A, Laferrière A, Franklin KB. (1981) Amphetamine and increases in current intensity modulate reward in the hypothalamus and substantia nigra but not in the prefrontal cortex. Physiol Behav 26:809–813 [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. (2012) Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res 234:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA. (2013) Principles of assessment of abuse liability: US legal framework and regulatory environment. Behav Pharmacol 24:403–409 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Burton MJ, Mora F. (1980) Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res 194:339–357 [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. (2013) Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain 14:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. (2005) Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther 313:1361–1369 [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, et al. (2007) Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 104:6406–6411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH. (2013) Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry DOI: 10.1016/j.biopsych.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2012) Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, in http://www.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm#2017.2011, Substance Abuse and Mental Health Services Administration, Rockville, MD.
- SAMHSA (2013) Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits, in http://www.samhsa.gov/data/2k13/DAWN12k11ED/DAWN12k11ED.htm, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed]
- Sanders-Bush E, Mayer SE. (2006) 5Hydroxytryptamine (serotonin): receptor agonists and antagonists, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 297–315, McGraw-Hill, New York [Google Scholar]
- Sanger DJ, Blackman DE. (1976) Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav 4:73–83 [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. (2009) nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol 78:658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. (1992) Interactions between alcohol and nicotine on intracranial self-stimulation and locomotor activity in rats. Drug Alcohol Depend 30:37–47 [DOI] [PubMed] [Google Scholar]
- Schenk S. (2009) MDMA self-administration in laboratory animals: a summary of the literature and proposal for future research. Neuropsychobiology 60:130–136 [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH, Kayser S, Hurlemann R, Coenen VA. (2014) Deep brain stimulation of the human reward system for major depression-rationale, outcomes and outlook. Neuropsychopharmacology 39:1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. (1994) Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther 271:1391–1398 [PubMed] [Google Scholar]
- Seeger TF, Carlson KR, Nazzaro JM. (1981a) Pentobarbital induces a naloxone-reversible decrease in mesolimbic self-stimulation threshold. Pharmacol Biochem Behav 15:583–586 [DOI] [PubMed] [Google Scholar]
- Seeger TF, Gardner EL, Bridger WF. (1981b) Increase in mesolimbic electrical self-stimulation after chronic haloperidol: reversal by L-DOPA or lithium. Brain Res 215:404–409 [DOI] [PubMed] [Google Scholar]
- Shearman GT, Hynes M, Lal H. (1981) Self-administration of clonidine by the rat. Prog Clin Biol Res 71:259–276 [PubMed] [Google Scholar]
- Shizgal P, Murry B. (1989) Neuronal basis of intracranial self-stimulation, in The Neuropharmacological Basis of Reward (Liebman JM, Cooper SJ, eds) Oxford University Press, New York [Google Scholar]
- Singh J, Desiraju T, Raju TR. (1996) Dose-response functions of apomorphine, SKF 38393, LY 171555, haloperidol and clonidine on the self-stimulation evoked from lateral hypothalamus and ventral tegmentum. Indian J Physiol Pharmacol 40:15–22 [PubMed] [Google Scholar]
- Skinner BF. (1950) Are theories of learning necessary? Psychol Rev 57:193–216 [DOI] [PubMed] [Google Scholar]
- Skinner BF. (1953a) Science and Human Behavior, Macmillan, New York [Google Scholar]
- Skinner BF. (1953b) Some contributions of an experimental analysis of behavior to psychology as a whole. Am Psychol 8:69–78 [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. (2003) Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 461:99–104 [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Cryan JF. (2007) Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 190:555–568 [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Froestl W, Cryan JF. (2005) The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology 30:2065–2072 [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. (2009) Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology 57:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg SDS. (1940) Morphine addiction in chimpanzees. Comp Psychol Mono 15:5–132 [Google Scholar]
- Standaert DG, Young AB. (2006) Treatment of central nervous system degenerative disorders, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 527–545, McGraw-Hill, New York [Google Scholar]
- Stark P, Dews PB. (1980) Cannabinoids. I. Behavioral effects. J Pharmacol Exp Ther 214:124–130 [PubMed] [Google Scholar]
- Stein L, Ray OS. (1960) Brain stimulation reward “thresholds” self-determined in rat. Psychopharmacology (Berl) 1:251–256 [DOI] [PubMed] [Google Scholar]
- Stellar JR, Rice MB. (1989) Pharmacological basis of intracranial self-stimulation reward, in The Neuropharmacological Basis of Reward (Liebman JM, Cooper SJ, eds) pp 14–65, Oxford University Press, New York [Google Scholar]
- Stellar JR, Stellar E. (1985) The Neurobiology of Motivation and Reward, Springer-Verlag, New York [Google Scholar]
- Stoker AK, Markou A.(2011a) The intracranial self-stimulation procedure provides quantitative measures of brain reward function, in Mood and Anxiety Related Phenotypes in MIce: Characterization Using Behavioral Tests, Volume II. Neuromethods Volume 63 (Gould TD, ed) pp 307–331, Humana Press, Totowa, NJ [Google Scholar]
- Stoker AK, Markou A. (2011b) Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav Brain Res 223:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JA, Craft RM. (1997) Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend 46:31–40 [DOI] [PubMed] [Google Scholar]
- Straub CJ, Carlezon WA, Jr, Rudolph U. (2010) Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav Brain Res 206:17–20 [DOI] [PubMed] [Google Scholar]
- Strecker RE, Roberts DC, Koob GF. (1982) Apomorphine-induced facilitation of intracranial self-stimulation following dopamine denervation of the nucleus accumbens. Pharmacol Biochem Behav 17:1015–1018 [DOI] [PubMed] [Google Scholar]
- Sundstrom JM, Hall FS, Stellar JR, Waugh EJ. (2002) Effects of isolation-rearing on intracranial self-stimulation reward of the lateral hypothalamus: baseline assessment and drug challenges. Life Sci 70:2799–2810 [DOI] [PubMed] [Google Scholar]
- Takada K, Yanagita T. (1997) Drug dependence study on vigabatrin in rhesus monkeys and rats. Arzneimittelforschung 47:1087–1092 [PubMed] [Google Scholar]
- Tang AH, Collins RJ. (1985) Behavioral effects of a novel kappa opioid analgesic, U-50488, in rats and rhesus monkeys. Psychopharmacology (Berl) 85:309–314 [DOI] [PubMed] [Google Scholar]
- Taylor P. (2006a) Agents acting at the neuromuscular junction and autonomic ganglia, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 217–236, McGraw-Hill, New York [Google Scholar]
- Taylor P. (2006b) Anticholinesterase agents, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 201–216, McGraw-Hill, New York [Google Scholar]
- Thompson T, Schuster CR. (1964) Morphine self-administration, food-reinforced, and avoidance behaviors in rhesus monkeys. Psychopharmacology (Berl) 5:87–94 [DOI] [PubMed] [Google Scholar]
- Tobey KM, Walentiny DM, Wiley JL, Carroll FI, Damaj MI, Azar MR, Koob GF, George O, Harris LS, Vann RE. (2012) Effects of the specific α4β2 nAChR antagonist, 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine, on nicotine reward-related behaviors in rats and mice. Psychopharmacology (Berl) 223:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470 [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr (2008) The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry 64:982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy ME, Slavova-Hernandez GG, Shelton KL. (2014) Assessment of reinforcement enhancing effects of toluene vapor and nitrous oxide in intracranial self-stimulation. Psychopharmacology (Berl) 7: 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Pisanty I, Conover K, Shizgal P. (2013) A new view of the effect of dopamine receptor antagonism on operant performance for rewarding brain stimulation in the rat. Psychopharmacology (Berl) 10.1007/s00213-013-3328-x. [DOI] [PubMed]
- Tzschentke TM, Schmidt WJ. (1999) Memantine does not substantially affect brain stimulation reward: comparison with MK-801. Brain Res 845:192–198 [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Kornetsky C. (1984) Effects of concomitant pentazocine and tripelennamine on brain-stimulation reward. Pharmacol Biochem Behav 21:961–964 [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Kucharski LT, Williams JE, Kornetsky C. (1984) Tripelennamine: enhancement of brain-stimulation reward. Life Sci 34:149–153 [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, et al. (2005) Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 48:658–672 [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. (2009) The mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) supports intravenous self-administration and induces conditioned place preference in the rat. Eur J Pharmacol 607:114–120 [DOI] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. (2007) Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 193:179–186 [DOI] [PubMed] [Google Scholar]
- van Heesch F, Prins J, Konsman JP, Westphal KG, Olivier B, Kraneveld AD, Korte SM. (2013) Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: an intracranial self-stimulation study. Brain Behav Immun 29:98–103 [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Cowan WM, Nieuwenhuys R, Geeraedts LM. (1982) The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J Comp Neurol 206:82–108 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Markou A. (2011) Intracranial self-stimulation, in Animal Models of Drug Addiction (Olmstead MC, ed) pp 3–56, Humana Press, New York [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. (2005) CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology (Berl) 179:498–508 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. (2006) Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology (Berl) 188:293–305 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. (2007) Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Panagis G. (2014) Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des 20:2072–2088 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Paterson NE, Guery S, Kaupmann K, Froestl W, Banerjee D, Finn MG, Markou A. (2011) Both GABA(B) receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats. Eur J Pharmacol 655:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. (2007) Estimating the relative reinforcing strength of (+/-)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology (Berl) 189:483–488 [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauquier A, Niemergeers CJ. (1974) Intracranial self-stimulation in rats as a function of various stimulus parameters. V. Influence of cocaine on medial forebrain bundle stimulation with monopolar electrodes. Psychopharmacology (Berl) 38:201–210 [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854 [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. (2006) Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav 84:337–343 [DOI] [PubMed] [Google Scholar]
- Weed MR, Vanover KE, Woolverton WL. (1993) Reinforcing effect of the D1 dopamine agonist SKF 81297 in rhesus monkeys. Psychopharmacology (Berl) 113:51–52 [DOI] [PubMed] [Google Scholar]
- Weeks JR. (1962) Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science 138:143–144 [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Clifford PS, Rodriguez JA, Hughes S, Di Francesco C, Melotto S, Tessari M, Corsi M, Bifone A, Gozzi A. (2012) Brain reinforcement system function is ghrelin dependent: studies in the rat using pharmacological fMRI and intracranial self-stimulation. Addict Biol 17:908–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Schaefer GJ, Michael RP. (1983) Increasing the work requirements lowers the threshold of naloxone for reducing self-stimulation in the midbrain of rats. Pharmacol Biochem Behav 18:705–710 [DOI] [PubMed] [Google Scholar]
- Westfall TC, Westfall DP. (2006) Adrenergic agonists and antagonists, in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th ed (Brunton L, Lazo J, Parker K, eds) pp 237–295, McGraw-Hill, New York [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW. (2014) Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci 97:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willick ML, Kokkinidis L. (1995) The effects of ventral tegmental administration of GABAA, GABAB and NMDA receptor agonists on medial forebrain bundle self-stimulation. Behav Brain Res 70:31–36 [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. (2002) Relative reinforcing strength of three N-methyl-d-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther 301:690–697 [DOI] [PubMed] [Google Scholar]
- Wise RA. (1996) Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19:319–340 [DOI] [PubMed] [Google Scholar]
- Wise RA. (1998) Drug-activation of brain reward pathways. Drug Alcohol Depend 51:13–22 [DOI] [PubMed] [Google Scholar]
- Wood RI. (2004) Reinforcing aspects of androgens. Physiol Behav 83:279–289 [DOI] [PubMed] [Google Scholar]
- Wood RI. (2008) Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol 29:490–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683 [PubMed] [Google Scholar]
- Woolverton WL, Wessinger WD, Balster RL. (1982) Reinforcing properties of clonidine in rhesus monkeys. Psychopharmacology (Berl) 77:17–23 [DOI] [PubMed] [Google Scholar]
- Yanagita T. (1978) Drug dependence studies in laboratory animals. NIDA Res Monogr 19:179–190 [PubMed] [Google Scholar]
- Yeomans J, Forster G, Blaha C. (2001) M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sci 68:2449–2456 [DOI] [PubMed] [Google Scholar]
- Yeomans JS. (1989) Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci Biobehav Rev 13:91–98 [DOI] [PubMed] [Google Scholar]
- You ZB, Chen YQ, Wise RA. (2001) Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience 107:629–639 [DOI] [PubMed] [Google Scholar]