Summary
Background
Medical treatment in patients suffering from Parkinson’s disease is very difficult as dose-finding is mainly based on selective and subjective impressions by the physician.
Objectives
To allow for the objective evaluation of patients’ symptoms required for optimal dosefinding, a telemonitoring system tracks the motion of patients in their surroundings. The system focuses on providing interoperability and usability in order to ensure high acceptance.
Methods
Patients wear inertia sensors and perform standardized motor tasks. Data are recorded, processed and then presented to the physician in a 3D animated form. In addition, the same data is rated based on the UPDRS score. Interoperability is realized by developing the system in compliance with the recommendations of the Continua Health Alliance. Detailed requirements analysis and continuous collaboration with respective user groups help to achieve high usability.
Results
A sensor platform was developed that is capable of measuring acceleration and angular rate of motions as well as the absolute orientation of the device itself through an included compass sensor. The system architecture was designed and required infrastructure, and essential parts of the communication between the system components were implemented following Continua guidelines. Moreover, preliminary data analysis based on three-dimensional acceleration and angular rate data could be established.
Conclusion
A prototype system for the telemonitoring of Parkinson’s disease patients was successfully developed. The developed sensor platform fully satisfies the needs of monitoring patients of Parkinson’s disease and is comparable to other sensor platforms, although these sensor platforms have yet to be tested rigorously against each other. Suitable approaches to provide interoperability and usability were identified and realized and remain to be tested in the field.
Keywords: Parkinson’s disease, telehealth, medical informatics, acceleration, rotation
Introduction
Parkinson’s disease (PD) is one of the most common chronic neurological disorders with prevalence in 0.2% of the global population [1]. This degenerative disease particularly impairs the motor abilities of the affected patients. Although these symptoms can be well medicated, the respective drugs implicate inevitable side effects through long-term usage. They must be dosed in precisely required amounts while aiming to maintain minimal dosage. Until now, dose-finding has been based merely on the anamnestic and visual impressions of the physician during the patient’s visit. An objective evaluation of the patient’s symptoms in daily life that would be required for optimal dose-finding is not possible through this method. This often results in dissatisfaction in both patients and physicians. There exist various telemonitoring systems for PD patients using inertia sensors. However, they still do not obtain wide acceptance by physicians [2].
Objectives
The research project described in this paper aims at providing the physician with a tool for an objective evaluation of Parkinson disease specific symptoms of the patient. For this purpose, a telemonitoring system was developed that records motions of patients in their domestic environment by means of inertia sensors. The system is intended to integrate seamlessly into the daily life of the patient and neurologists alike. The goal is to achieve acceptance by all user groups and the establishment within the healthcare system. Accordingly, the telemonitoring system described here is focused on 1) usability and 2) interoperability. These objectives have been identified as key technology related factors with regard to acceptance in [2].
Related Work
There are several telemonitoring systems that focus on chronic diseases and PD in particular. The focus of the system for chronic disease management presented in [2] is set on usability and interoperability. The relevance of these two points has also been identified for the telemonitoring system described in the paper at hand. Patel et al. present a system that aims “to identify movement characteristics associated with motor fluctuations in patients with PD relying on wearable sensors” [3]. For this purpose, patients wear 8 accelerometers, based on the Shimmer sensor platform, continuously for 12 to 18 hours a day on their upper and lower limbs. The collected data are processed to derive estimates of the Unified Parkinson’s Disease Rating Scale (UPDRS). Based on the preliminary findings of Patel et al., a similar data analysis has been established for the intended telemonitoring system. However, in contrast to Patel et al., only up to 5 sensor units have been used to derive estimated UPDRS scores. Herrlich et al. [4] implemented a telemonitoring system for ambulant treatment of PD patients by means of a set of different sensors, amongst others inertia sensors. A smartphone is utilized as a mobile gateway and a web-based user interface presents data at the point of care. Additionally, they equipped patients with actuators for automatic drug delivery. In its composition and architecture, this system resembles the design of the system described in the paper at hand.
There exist a large number of publications on motion analysis algorithms using data delivered by inertia sensors. They often focus on the analysis of single Parkinson specific motor symptoms like tremor [5], gait abnormalities including freeze of gait [6] and lower extremity bradykinesia [7]. The intended data analysis will be based on these symptom-specific algorithms.
Methods
In the following section, the project vision and two key aspects will be explained.
Project Vision
The vision of the project comprises of the idea of monitoring patients during their day-to-day activities by means of a sensor system. This system records information about their current motor symptoms. Collected data enable the physician to objectively evaluate the health situation of patients.
Patients wear sensor units that are fixed with elastic bands to their limbs and the center of their body (►Figure 1). A smartphone application that is able to communicate with the sensor units monitors the patient during the course of the day and requests the patient to execute standardized motor tasks on a regular basis. Depending on the manifestation of symptoms, the position of sensor units can vary and must therefore be documented by the patient via the smartphone application. The system verifies the patient’s indication of sensor positioning with automatically performed plausibility checks. The smartphone application is also used to document times of drug and food intake, and accordingly prompts the patient to perform the motor tasks. In addition to a pre-evaluation function informing the patient about his or her medical condition, the mobile application can upload measured and documented data to a central server. There, motion data recorded during the motor tasks are preprocessed, analyzed and then presented to the physician in a web-based user interface. A 3D representation of the patient’s body, a so called avatar, as well as standardized medical scores (e.g. UPDRS), provide the neurologist with a data representation that corresponds to his clinical experience. Based on these results the physician can evaluate the correlation between medication intake and drug-induced motor features and thereby regulate the patient’s medication.
Fig. 1.
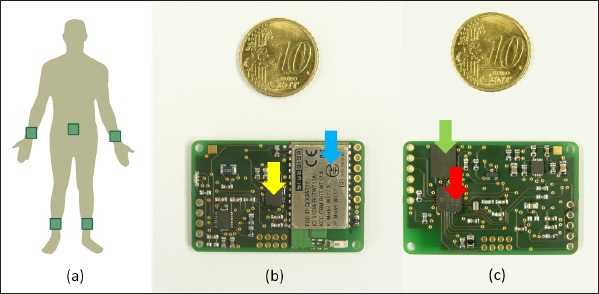
(a) Positioning of sensor units at the limbs and the center of body, (b) upper side of the sensor platform with Bluetooth Module (blue arrow) and inertia sensor (yellow arrow), (c) bottom side of the sensor platform with microcontroller (red arrow) and flash memory (green arrow)
As an additional feature, the patient may be able to share results with relatives or other people involved in therapy.
Usability
The research project aims to develop a system that integrates seamlessly into both the patients’ everyday lives, and the clinical routines of the physicians. Therefore, a close cooperation with all user groups is an essential part of the development process. The two main user groups can be identified as patients with Parkinson’s disease, and neurologists. In order to achieve the intended usability and the accompanying high acceptance, these user groups have been involved for the entirety of the development process.
In line with requirements analysis, interviews and questionnaires have been determined to be the most suitable method to identify the needs of the respective user groups. For this purpose, expert interviews were conducted with neurologists and collaborations with PD support groups were established. All functional and non-functional requirements were documented and considered during the development of the telemonitoring system.
In addition to the normal requirements analysis performed with potential users, the special characteristics of the patient group had to be considered. Since PD predominantly occurs at an older age, it was essential to ensure that the technology used by the patients (i.e. the smartphone application and the sensor units) is comprehensible and intuitive in handling. In a master’s thesis connected to this project, particular requirements of older people have been examined and added to the requirements list for smartphone application and sensor units.
In order to receive immediate and continuous feedback during the subsequent parts of the development process, results are presented to the respective user groups on a regular basis.
Interoperability
Interoperability is defined as the ability of a system or a product to work together with other systems or products without special effort by the user or customer. In the particular case of healthcare, interoperability enables information systems, medical devices and software applications to collaborate across organizational boundaries, in order to improve the health status of individuals and communities. Compliance with well-established standards is the essential precondition for achieving interoperability [8, 9].
The Continua Health Alliance is a non-profit and open-industry organization that aims at improving interoperability for personal health devices. Its special focus lies on fitness, wellness, independent aging and the management of chronic diseases. To achieve this goal, Continua has published design guidelines in which appropriate standards for the communication between typical components of a telemonitoring system were recommended. For the interface between the respective medical devices and a gateway device (e.g. a smartphone), they promote the application of the ISO/IEEE 11073 Personal Health Device (PHD). This standard family specifies an exchange format for data of medical devices and has been introduced to replace proprietary protocols. It comprises of a core standard that applies to all medical devices and a set of specialized standards for selected devices like pulse oximeters or weighing scales. The central concept of the ISO/IEEE 11073 standard family is about agents and managers. The software component within the medical device is referred to as agent, and the component that receives the data from the agent is called manager. Managers are typically smart phones or personal computers, and can also forward data to remote analysis services.
Since data are typically forwarded and collected by a central database, the interface between a gateway device and a remote medical service is also specified by Continua. Here, HL7 Version 2, one of the most widely implemented standards for healthcare information, is used [10].
Results
Hardware
The hardware used in this project was developed and built by students from the University of Applied Science at Ulm during the course of several theses. The developed sensor platform (as shown in ►Figure 1) comprises of a PIC18F26K22 8-Bit microcontroller, a MPU9150 inertia sensor-chip including an accelerometer, a gyroscope and a magnetic field sensor, a WT12 Bluetooth module manufactured by Bluegiga and an on-board 64MB serial flash memory. A lithium polymer battery provides a maximum runtime of about 24 hours. This battery can be charged inductively by placing it on a Qi wireless charging plate. Once packaged, the sensor unit measures 50 mm x 35 mm x 20 mm and weighs 25 g. The casing features eyelets to attach an elastic band for comfortable wearing.
With this configuration, the sensor unit is able to measure acceleration and angular rate, as well as the absolute orientation through an included compass sensor. The sensor data are sampled with 200 Hz. The measured data can be transmitted directly to an Android based smartphone and can also be stored on the flash memory in the absence of an active Bluetooth link. Additionally, the serial-flash memory prevents data loss in cases where the Bluetooth connection between the smartphone and the sensor unit breaks down. Such a break-down could happen due to a critical battery level of the smartphone or if the distance between sensor unit and smartphone exceeds the Bluetooth transmission range. The Bluetooth connection is not only used for the transmission of data from sensor unit to smartphone application, but also to handle the sensor unit via smartphone. To provide minimal information about the sensor unit’s state, an LED is positioned on the sensor unit and can display different colors. With this method, the sensor unit does not require a more complex user interface.
Further features of the sensor unit are four external analog ports, which could be utilized for the connection of analog sensors, e.g. force-sensing resistors (FSR). These ports were integrated into a prototypic sensor insole to allow for the analysis of gait patterns. Additionally, an integrated phototransistor is used to detect whether a sensor unit has been removed from the patient’s body.
System Architecture
Following the guidelines of the Continua Health Alliance, the architecture of the intended telemonitoring system, including all system components and communication paths, was realized as illustrated in ►Figure 2.
Fig. 2.
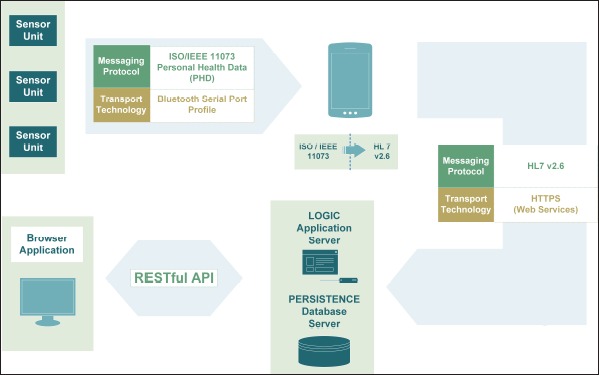
Overview of the system architecture and respective interfaces between sensor units, smartphone, application server, database server and browser application
As specified by the recommendations of Continua, the messaging protocol IEEE 11073 PHD was deployed for the communication between sensor units and smartphone based on Bluetooth SPP as transport technology. Although there is currently no device specialization that exactly fits the data delivered by the purpose-built sensor unit, a similar device specialization could be adapted for the particular requirements of motion data. Originally, the standard allows for transmitting acceleration and gyroscope data. Extending the standard enabled the additional transmission of magnetic field, phototransistor and quality assurance data. At first connection, establishment information about the agent and its capabilities is transmitted from agent to manager in the form of a configuration. On the basis of this configuration, the manager decides if it can communicate with the respective agent. Due to the extension of the standard that was required to satisfy the Parkinson monitoring specific needs, it cannot be assumed that other managers can understand the configuration. However, to ensure interoperability, the agent offers a second configuration that fully conforms to the standard. In case of managers who do not understand the extended configuration, the agent may fall back on this default configuration.
In order to enable communication between smartphone and central server according to the guidelines of the Continua Health Alliance, the mobile application on the smartphone has to map the received IEEE 11073 messages to the HL7 Version 2 data exchange format. These HL7 messages are then sent to the server via web services.
The server component includes an application server offering an interface for the upload and download of data to its clients (i.e. smartphone and the browser application), for the pre-processing of data and for the execution of analytic algorithms. A database server stores all received data.
Finally, a browser application can retrieve data via the restful application programming interface offered by the server component and enables the display of preprocessed data in form of 3D animations and medical scores. The browser application also provides the possibility of making annotations, tagging data and uploading this information to the server again.
Software Components
A prototype of the described system architecture was implemented. In order to ensure communication over the specified standard, an IEEE 11073 capable software component was implemented on the sensor unit. The mobile application prototype was developed for Android Version 2.2 (API 8) and higher, and was tested on various android devices. In addition to the respective counterpart for IEEE 11073 compliant communication, the mobile application includes software modules for the generation of HL7 messages and for the consumption of web services offered by the server.
Furthermore, the required server infrastructure was set up and respective web services, capable of receiving data from the mobile application, were implemented and deployed. It is now possible to record motion data, communicate them in a standards compliant way from the sensor system to the central server where data are extracted and stored in a database.
Data Analysis and algorithms
The existing data analysis was based on three-dimensional acceleration and angular rate data from a previous version of the sensor platform. The new sensor platform includes a compass sensor providing additional data which is not yet used by the existing algorithms presented below. They should hence be regarded as preparatory work for future data analysis and visual presentation.
Using the gradient descent algorithm presented by [11], the orientation of the sensor unit was calculated. Based on this calculation, initial experiments on tracking limb movements for later presentation of the 3D human avatar [12] were performed. The data of two sensor units on the upper and lower arm were fused with the information obtained from an optical system (Microsoft Kinect sensor) to move the arm of the avatar. However, the avatar could be animated with acceptable accuracy without the use of the optical system.
For evaluation of Parkinson specific gait patterns, a gait analysis and characterization were performed [13]. With a three layer neural network, individual footsteps were extracted from walking datasets. Gait features like step length, step height and direction of movement were calculated to detect the walking path and characterize the gait of individuals.
Discussion
A telemonitoring system was developed that records the movement of patients, transmits the data in compliance with the recommendations of the Continua Health Alliance and implements first algorithms for data analysis. The development process aimed at achieving a user-friendly and interoperable system that allowed realistic representation and analysis of motion data. The developed sensor unit seems to be comparable to other sensor platforms (e.g. Shimmer applied by [3]), but respective comparative tests are yet to be performed. The sensor unit is user-friendly and efficient because of its waterproof casing and inductive charging capability, in addition to an intelligent energy management system that maximizes battery run-time.
Interoperability of the system was achieved by following the recommendations of the Continua Health Alliance during the system design process. Herrlich et al. [4] renounced using the Continua guidelines due to the incompatibility of their developed inertia sensor. In contrast, this project extended the relevant Continua standards, so that they could be applied for the developed sensor system. It is noteworthy that the application of an individually extended version of the standard interferes with the aim of having an interoperable system. Consequently, interoperability had to be traded off against the accomplishment of individual needs. For the communication of motion data, a concept could be elaborated and realized that uses extended standards for individual requirements and ensures interoperability with third-party systems. The integration of third-party medical devices into the described telemonitoring system requires the ability to interpret all messages compliant with the ISO/IEEE 11073 communication standard, and is under development. Interoperability with third-party devices and with third-party systems is to be tested in forthcoming studies.
By definition, telemonitoring does not imply real-time evaluation of data by healthcare professionals. Indeed, there are diseases where real-time evaluation of data is a useful approach. For the Parkinson’s disease telemonitoring scenario it is assumed that the physician uses the system immediately before or during a patient’s visit to view prepared statistics or selected animations revealing the development of symptoms since the last visit. Neurologists who were consulted in line with this project confirmed that no real-time evaluation of data is required. The authors of [14] also state that real time monitoring is not needed for the use of Parkinson’s disease use cases.
To rate the symptoms, the patient will perform standardized motor tasks which are derived from the UPDRS catalog. This approach is also described by [3]. In addition to the UPDRS rating, an animated avatar will show the patient’s movements, so that the evaluation by the physician is as close as possible to a face-to-face examination. These results should also be accessible to the patients and persons authorized by them. A simple pre-evaluation will also be implemented on the smartphone to give patients a quick feedback about their current state of health. This feedback will enable them better to assess their symptoms. For this purpose, appropriate analysis routines for the smartphone application have to be identified.
In order to minimize the impairment of patients, only as few sensor units as necessary are to be worn. Since this limits the available knowledge of the patient’s movements, the animation of the avatar is an under-determined problem. The animation is made possible by constructing the avatar’s movement, which could be based on biomechanical, mathematical or activity based models. More research on this topic will be done in the near future. In the previous algorithm for tracking the motion of an arm described in [12], the optical system was used to correct the drift. Because the sensor platform has been expanded with a compass sensor, the animation can be optimized to work without optical support. In the long term, the analysis of motor tasks within the intended telemonitoring system should not require an optical system. However, optical support may possibly be used in clinics to measure the patient’s constitution in order to provide a more realistic animation of the human avatar.
To continuously improve the algorithms in use, a machine learning algorithm is planned. The physician will supply his or her own UPDRS rating based on the movement of the avatar, which will be used to correct the algorithmically determined rating and allow the algorithm to “learn”. As long as the avatar is in a testing phase as well, video recording of the standardized motor tasks will be displayed for additional examination.
In summary, it can be said, that the initially determined goal of providing high usability could be realized by establishing respective requirements and by considering them during the design process of the system. In the near future, usability and applicability have to be checked in studies that will be conducted with a small group of patients. Interoperability, as the second goal contributing to a widely accepted system, has been almost fully realized. A prototype system has been implemented that complies in its design with Continua guidelines. As a final step before the full realization of an interoperable system, this prototype will be tested against other systems and devices compliant with Continua guidelines.
Moreover, further research and detailed planning is required in order to provide an adequate data representation to the physician. It is to be avoided that the physician must become acquainted with the interpretation of new medical key figures. Therefore familiar medical scores and visual impressions of the patient’s symptoms should be available. This is considered as one major key to establish the system in clinical routine.
Conclusions
A complete concept for the remote monitoring of Parkinson’s disease patients was developed and partially implemented. A suitable approach for providing interoperability was extrapolated from the recommendations of the Continua Health Alliance, adapted and introduced into the system design. Both interoperability and usability are to be tested further in upcoming studies, in continuous collaboration with concerned user groups.
A sensor platform serving the needs of motion tracking in Parkinson’s disease patients has successfully been developed and is ready to use. Although the sensor platform seems to be comparable to other platforms, respective comparative tests are still outstanding. The elaborated system architecture including required services has been set up; required software components for communication between sensor unit and smart phone as well as smartphone and server has also been implemented. Desired data analysis is in a preliminary state and requires further development.
Acknowledgments
The authors would like to thank Professor Karla Pollmann, Abhiram Mamandur Kidambi and Andreas Pflugrad for proofreading this paper. Their detailed advices on grammar, syntax and their valuable comments were very helpful.
This work was supported by a grant from the Ministry of Science, Research and the Arts of Baden-Wuerttemberg (Az: 33–7533–7–11.6–10/2).
Footnotes
Clinical Relevance Statement
The daily recording of the patient’s movement at home enables the practitioner to optimize the medication with as low dosages as possible. Hence, negative long-term effects of the therapy can be deferred, leading to a better quality of living. Additionally the patient gets feedback on his or her improvements of performing physical exercises, which leads to a higher compliance of the physiotherapy.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest associated with the work presented in this manuscript.
Protection of Human Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. Upcoming studies will be reviewed by the Institutional Review Board of the University of Ulm.
References
- 1.Hacke W.Neurologie. 13th ed Heidelberg: Springer; 2010 [Google Scholar]
- 2.Jimenez-Fernandez S, de Toledo P, del Pozo F.Usability and Interoperability in Wireless Sensor Networks for Patient Telemonitoring in Chronic Disease Management. IEEE Trans Biomed Eng 2013September5; 60(12): 3331-3339 [DOI] [PubMed] [Google Scholar]
- 3.Patel S, Chen BR, Buckley T, Rednic R, McClure D, Tarsy D, Shih L, Dy J, Welsh M, Bonato P.Home monitoring of patients with Parkinson’s disease via wearable technology and a web-based application. Conf Proc IEEE Eng Med Biol Soc 2010; 2010: 4411-4414 [DOI] [PubMed] [Google Scholar]
- 4.Herrlich S, Spieth S, Nouna R, Zengerle R, Giannola LI, Pardo-Ayala DE, Federico E, Garino P.Ambulatory Treatment and Telemonitoring of Patients with Parkinson’s Disease. : Wichert R, Eberhardt B, Ambient Assisted Living: Proceedings of the 4th AAL-Congress; 2011 January 25–26; Berlin, Germany. Berlin: Springer; 2011. P 295–305 [Google Scholar]
- 5.Pulliam CL, Eichenseer SR, Goetz CG, Waln O, Hunter CB, Jankovic J, et al. Continuous in-home monitoring of essential tremor. Parkinsonism Relat Disord 2014January; 20(1): 37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore ST, Yungher DA, Morris TR, Dilda V, MacDougall HG, Shine JM, Naismith SL, Lewis SJ.Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental accelerometry. J Neuroeng Rehabil 2013; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heldman DA, Filipkowski DE, Riley DE, Whitney CM, Walter BL, Gunzler SA, Giuffrida JP, Mera TO.Automated motion sensor quantification of gait and lower extremity bradykinesia, Conf Proc IEEE Eng Med Biol Soc 2012; 2012: 1956-1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IEEE. IEEE Standards Glossary. New York: IEEE; c2014 [cited 2014 Mar 28]. Available from: http://www.ieee.org/education_careers/education/standards/standards_glossary.html [Google Scholar]
- 9.HIMSS. Definition of Interoperability. Chicago: HIMSS; c2012–14 [cited 2014 Mar 28]. Available from: http://www.himss.org/library/interoperability-standards/what-is?navItemNumber=17333 [Google Scholar]
- 10.Continua Health Alliance. Continua Design Guidelines Version 2012. Beaverton (OR): Continua Health Alliance; c2013 [cited 2014 Mar 28] Available from: http://www.continuaalliance.org [Google Scholar]
- 11.Madgwick SOH, Harrison AJL, Vaidyanathan R.Estimation of IMU and MARG orientation using a gradient descent algorithm. IEEE Int Conf Rehabil Robot 2011; 2011: 5975346. [DOI] [PubMed] [Google Scholar]
- 12.Kalkbrenner C, Hacker S, Algorri ME, Blechschmidt-Trapp R.Motion capturing with Inertial Measurement Units and Kinect Tracking of limb movement using optical and orientation information. Proceedings of the 7th International Conference on Biomedical Electronics and Devices: Biodevices 2014; 2014Mar 03–06; Angers, France; p 120-126 [Google Scholar]
- 13.Hacker S, Kalkbrenner C, Algorri ME, Blechschmidt-Trapp R.Gait Analysis with IMU Gaining new Orientation Information of the Lower Leg. Proceedings of the 7th International Conference on Biomedical Electronics and Devices: Biodevices 2014; 2014Mar 03–06; Angers, France; p 127-133 [Google Scholar]
- 14.Chen BR, Patel S, Buckley T, Rednic R, McClure DJ, Shih L, Tarsy D, Welsh M, Bonato P.A Web-Based System for Home Monitoring of Patients With Parkinson Disease Using Wearable Sensors, IEEE Trans Biomed Eng 2011; 58(3): 831-836 [DOI] [PubMed] [Google Scholar]


