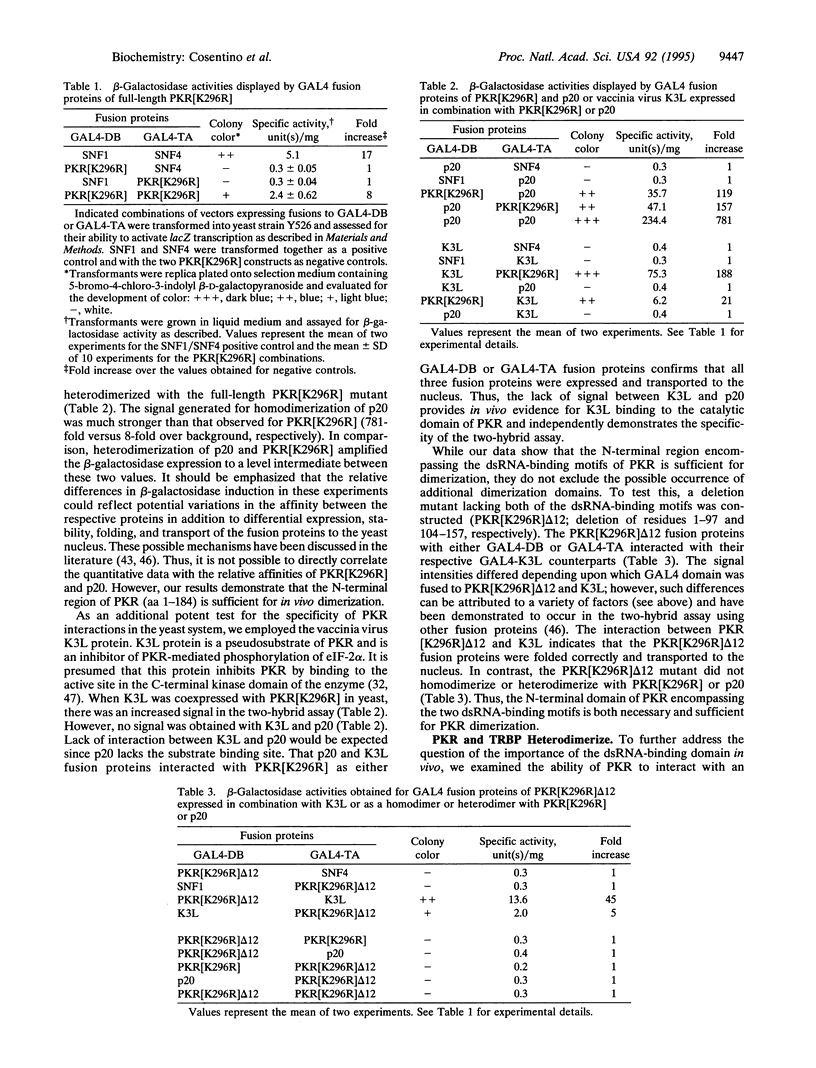
Abstract
The yeast two-hybrid system and far-Western protein blot analysis were used to demonstrate dimerization of human double-stranded RNA (dsRNA)-dependent protein kinase (PKR) in vivo and in vitro. A catalytically inactive mutant of PKR with a single amino acid substitution (K296R) was found to dimerize in vivo, and a mutant with a deletion of the catalytic domain of PKR retained the ability to dimerize. In contrast, deletion of the two dsRNA-binding motifs in the N-terminal regulatory domain of PKR abolished dimerization. In vitro dimerization of the dsRNA-binding domain required the presence of dsRNA. These results suggest that the binding of dsRNA by PKR is necessary for dimerization. The mammalian dsRNA-binding protein TRBP, originally identified on the basis of its ability to bind the transactivation region (TAR) of human immunodeficiency virus RNA, also dimerized with itself and with PKR in the yeast assay. Taken together, these results suggest that complexes consisting of different combinations of dsRNA-binding proteins may exist in vivo. Such complexes could mediate differential effects on gene expression and control of cell growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., De Clercq E. Structural requirements of polynucleotides for the activation of (2' - 5')An polymerase and protein kinase. Nucleic Acids Res. 1981 Oct 10;9(19):4939–4950. doi: 10.1093/nar/9.19.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber G. N., Tomita J., Garfinkel M. S., Meurs E., Hovanessian A., Katze M. G. Detection of protein kinase homologues and viral RNA-binding domains utilizing polyclonal antiserum prepared against a baculovirus-expressed ds RNA-activated 68,000-Da protein kinase. Virology. 1992 Dec;191(2):670–679. doi: 10.1016/0042-6822(92)90242-h. [DOI] [PubMed] [Google Scholar]
- Bartel P., Chien C. T., Sternglanz R., Fields S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques. 1993 Jun;14(6):920–924. [PubMed] [Google Scholar]
- Berry M. J., Knutson G. S., Lasky S. R., Munemitsu S. M., Samuel C. E. Mechanism of interferon action. Purification and substrate specificities of the double-stranded RNA-dependent protein kinase from untreated and interferon-treated mouse fibroblasts. J Biol Chem. 1985 Sep 15;260(20):11240–11247. [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown N. G., Costanzo M. C., Fox T. D. Interactions among three proteins that specifically activate translation of the mitochondrial COX3 mRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1994 Feb;14(2):1045–1053. doi: 10.1128/mcb.14.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Carroll K., Elroy-Stein O., Moss B., Jagus R. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 alpha-specific protein kinase. J Biol Chem. 1993 Jun 15;268(17):12837–12842. [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K. L., Feng L., Schappert K., Meurs E., Donahue T. F., Friesen J. D., Hovanessian A. G., Williams B. R. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992 Apr;11(4):1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. V., Elroy-Stein O., Jagus R., Moss B., Kaufman R. J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992 Apr;66(4):1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever T. E., Chen J. J., Barber G. N., Cigan A. M., Feng L., Donahue T. F., London I. M., Katze M. G., Hinnebusch A. G. Mammalian eukaryotic initiation factor 2 alpha kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4616–4620. doi: 10.1073/pnas.90.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilotter H. E., Hannon G. J., Ruddell C. J., Beach D. Construction of an improved host strain for two hybrid screening. Nucleic Acids Res. 1994 Apr 25;22(8):1502–1503. doi: 10.1093/nar/22.8.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G. S., Chong K., Kumar A., Williams B. R. Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5447–5451. doi: 10.1073/pnas.89.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Sternglanz R. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 1994 Aug;10(8):286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- Galabru J., Katze M. G., Robert N., Hovanessian A. G. The binding of double-stranded RNA and adenovirus VAI RNA to the interferon-induced protein kinase. Eur J Biochem. 1989 Jan 2;178(3):581–589. doi: 10.1111/j.1432-1033.1989.tb14485.x. [DOI] [PubMed] [Google Scholar]
- Gatignol A., Buckler-White A., Berkhout B., Jeang K. T. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991 Mar 29;251(5001):1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- Gatignol A., Buckler C., Jeang K. T. Relatedness of an RNA-binding motif in human immunodeficiency virus type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and Drosophila staufen. Mol Cell Biol. 1993 Apr;13(4):2193–2202. doi: 10.1128/mcb.13.4.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Thompson J. D. Detection of dsRNA-binding domains in RNA helicase A and Drosophila maleless: implications for monomeric RNA helicases. Nucleic Acids Res. 1994 Jul 11;22(13):2552–2556. doi: 10.1093/nar/22.13.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. R., Manche L., Mathews M. B. Two functionally distinct RNA-binding motifs in the regulatory domain of the protein kinase DAI. Mol Cell Biol. 1995 Jan;15(1):358–364. doi: 10.1128/mcb.15.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. R., Mathews M. B. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev. 1992 Dec;6(12B):2478–2490. doi: 10.1101/gad.6.12b.2478. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Demetrick D., Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993 Dec;7(12A):2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Wambach M., Wong M. L., Garfinkel M., Meurs E., Chong K., Williams B. R., Hovanessian A. G., Barber G. N. Functional expression and RNA binding analysis of the interferon-induced, double-stranded RNA-activated, 68,000-Mr protein kinase in a cell-free system. Mol Cell Biol. 1991 Nov;11(11):5497–5505. doi: 10.1128/mcb.11.11.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992 Sep 18;257(5077):1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- Kostura M., Mathews M. B. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989 Apr;9(4):1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland J. O., Jacobs B. L. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr = 66,000 subunits. J Biol Chem. 1992 May 25;267(15):10729–10736. [PubMed] [Google Scholar]
- Lasky S. R., Jacobs B. L., Samuel C. E. Mechanism of interferon action. Characterization of sites of phosphorylation in the interferon-induced phosphoprotein P1 from mouse fibroblasts: evidence for two forms of P1. J Biol Chem. 1982 Sep 25;257(18):11087–11093. [PubMed] [Google Scholar]
- Lee S. B., Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994 Mar;199(2):491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- Lee S. B., Green S. R., Mathews M. B., Esteban M. Activation of the double-stranded RNA (dsRNA)-activated human protein kinase in vivo in the absence of its dsRNA binding domain. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10551–10555. doi: 10.1073/pnas.91.22.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manche L., Green S. R., Schmedt C., Mathews M. B. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol Cell Biol. 1992 Nov;12(11):5238–5248. doi: 10.1128/mcb.12.11.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S. J., Ortega L. G., Doohan J. P., Samuel C. E. Mechanism of interferon action motif I of the interferon-induced, RNA-dependent protein kinase (PKR) is sufficient to mediate RNA-binding activity. Virology. 1994 Jan;198(1):92–99. doi: 10.1006/viro.1994.1011. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E. F., Galabru J., Barber G. N., Katze M. G., Hovanessian A. G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Davies M. V., Langland J. O., Chang H. W., Nam Y. S., Tartaglia J., Paoletti E., Jacobs B. L., Kaufman R. J., Venkatesan S. TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4713–4717. doi: 10.1073/pnas.91.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. C., Stanton P., McMillan N. M., Williams B. R., Sen G. C. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8283–8287. doi: 10.1073/pnas.92.18.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. C., Stanton P., Sen G. C. Role of the amino-terminal residues of the interferon-induced protein kinase in its activation by double-stranded RNA and heparin. J Biol Chem. 1994 Jul 15;269(28):18593–18598. [PubMed] [Google Scholar]
- Romano P. R., Green S. R., Barber G. N., Mathews M. B., Hinnebusch A. G. Structural requirements for double-stranded RNA binding, dimerization, and activation of the human eIF-2 alpha kinase DAI in Saccharomyces cerevisiae. Mol Cell Biol. 1995 Jan;15(1):365–378. doi: 10.1128/mcb.15.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Knutson G. S., Berry M. J., Atwater J. A., Lasky S. R. Purification of double-stranded RNA-dependent protein kinase from mouse fibroblasts. Methods Enzymol. 1986;119:499–516. doi: 10.1016/0076-6879(86)19070-7. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993 Apr 15;268(11):7603–7606. [PubMed] [Google Scholar]
- Schmedt C., Green S. R., Manche L., Taylor D. R., Ma Y., Mathews M. B. Functional characterization of the RNA-binding domain and motif of the double-stranded RNA-dependent protein kinase DAI (PKR). J Mol Biol. 1995 May 26;249(1):29–44. doi: 10.1006/jmbi.1995.0278. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Measures and countermeasures in the modulation of initiation factor activities by viruses. New Biol. 1990 May;2(5):402–409. [PubMed] [Google Scholar]
- St Johnston D., Brown N. H., Gall J. G., Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thomis D. C., Samuel C. E. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J Virol. 1993 Dec;67(12):7695–7700. doi: 10.1128/jvi.67.12.7695-7700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




