Abstract
Cytogenetic studies of a male child carrying the 22q11.2 deletion common in patients with velo-cardio-facial/DiGeorge syndrome revealed an unexpected rearrangement of the 22q11.2 region in his normal appearing mother. The mother carries a 3 Mb deletion on one copy and a reciprocal, similar sized duplication on the other copy of chromosome 22q11.2 as revealed by fluorescence in situ hybridization and array comparative genome hybridization analysis. The most parsimonious mechanism for the rearrangement is a mitotic non-allelic homologous recombination event in a cell in the early embryo soon after fertilization. The normal phenotype of the mother can be explained by the theory of genetic dosage compensation. This is the second documented case of such an event for this or any genomic disorder. This finding helps to reinforce this phenomenon in a human model, and has significant implications for genetic counseling of future children.
Introduction
Velo-cardio-facial syndrome (VCFS; MIM#192430)/DiGeorge syndrome (DGS; MIM#188400), has been clinically described since 1968 [DiGeorge, 1968; Shprintzen et al., 1978]. Typical features of patients with VCFS/DGS include mild facial dysmorphism, submucous cleft palate, velo-pharyngeal insufficiency, recurrent infections, and cardiac outflow tract malformations [Ryan et al., 1997; Shprintzen, 2008]. Most have learning disabilities and behavioral disorders including schizophrenia in a subset of adults [Chow et al., 1994; Shprintzen, 2000; Murphy and Owen, 2001; Evers et al., 2009]. Over 90% of affected individuals have a hemizygous 3 million base pair (Mb) deletion on chromosome 22q11.2 [Morrow et al., 1995; Lindsay et al., 1995; Edelmann et al., 1999A, B; Shaikh et al., 2000]. The deletion arises from meiotic non-allelic homologous recombination events between flanking 250 kb (kilobases), low-copy repeats/segmental duplications in the 22q11.2 region termed LCR22 (Edelmann et al., 1999A and B; Shaikh et al., 2000). Although most cases of VCFS/DGS occur as de novo deletions, approximately 5% of cases are inherited in an autosomal dominant pattern [Williams et al., 1985; Digilio et al., 1997; Swillen et al., 1998; Oskarsdóttir et al., 2004]. In this study, we examined a family with an inherited deletion, and found the mother of the proband with 22q11.2 deletion not only carried the same sized deletion, but also carried a duplication on the other chromosome 22. Past reports of patients with a duplication of the 22q11.2 region, for a total of three copies of genes in the interval, report a phenotype with many similar features to those with VCFS/DGS [Edelmann et al., 1999b; Ensenauer et al., 2003; Portnoi et al., 2005; Ou et al., 2008]. This patient’s phenotype was normal. We believe that dosage compensation by the duplicated region on one chromosome 22 occurred in the mother. Last year, the first report of dosage compensation in the syndrome was described [Carelle-Calmels et al., 2009]. The existence of a second family showing the same, suggests that this might not be such a rare event and has implications for similar events in other genomic disorders and for possible genetic counseling for future pregnancies.
Clinical Report
We describe here a mother and a child with an inherited deletion on chromosome 22q11.2. The diagnosis of VCFS/DGS was suspected in a male child who presented with mental retardation and learning disability at the age of 4 (Table 1). The mothers’ antepartum care was complicated by polyhydramnios diagnosed at 28 weeks of gestation. A male child was born via spontaneous vaginal delivery at term. The infant’s birth weight was 3,850 g, length 51 cm, and the head circumference was 35 cm. Apgar scores were 8 and 10 at 1 and 5 minutes respectively. Brain stem audiometry showed conductive deafness likely due to chronic otitis media, commonly occurring in the disorder. Renal ultrasonography was normal. The child also displayed the typical facial features seen in patients with the syndrome (Fig. 1A–C) which include: dolicocephaly, periorbital fullness, narrow upslanting palpebral fissures, epicanthal folds, strabismus, thick lips with everted upper lip, high palate, and small everted ears with an overfolded helix. He had typical hypernasal speech. Over 70% of VCFS/DGS patients have cardiac defects, prevalently conotruncal anomalies [Emanuel et al., 2001; Ryan et al., 2004; Marino et al., 2001]. The child was born with a subaortic ventricular septal defect (VSD; Table 1) identified one day after birth by echocardiography. He had bilateral cryptorchidism, inguinal hernia at right and kyphosis. Bilateral club feet were also noted at birth, and repaired at 6 months of age. Hematologic findings showed T-cell number below the normal range, normal parathyroid function, and thrombocytopenia, common in individuals with the syndrome. The diagnosis of VCFS/DGS was confirmed via fluorescence in situ hybridization (FISH) mapping. The boy is now 14 years old, his weight is 55,400 kg (75%), height 170 cm (90%), head circumference 53.3 cm. At a regional meeting for VCFS/DGS patients, the patients’ mother reported that she had a similar deletion to her son but appeared normal (Fig. 1D–F; Table 1).
Table 1.
| Clinical Features | Mother | Child |
|---|---|---|
| Facial Dysmorphism | ||
| Periorbital Fullness | - | + |
| Upslanding Fullness | - | + |
| Narrow Palpebral Fissures | - | + |
| Epicanthal Folds | - | + |
| Prominent Nose | + | + |
| High Palate | - | + |
| Elevated Upper Lip | - | + |
| Small Dysmorphic Ears | - | + |
| Other Anomalies | ||
| Congenital Heart Defect | - | VSD |
| Developmental Delay | - | + |
| Learning Difficulties | - | + |
| Conductive Deafness | - | + |
| Velopharyngeal Insufficiency | - | + |
| Low T-Cell Number | - | + |
| Hypoparathyroidism | - | + |
| Thrombocytopenia | - | + |
| Club feet | - | + |
Figure 1. Phenotype of the affected child and his normal mother.
Figure 1A–1C shows the phenotype of the VCFS/DGS male child at age 2, 6, and 12 respectively. Note the periorbital fullness, narrow upslanting palpebral fissures, epicanthal folds, strabismus, thick lips with everted upper lip, and small everted ears. Figure 1D–1E shows the appearance of this child's mother at matching time intervals. The mother’s facial phenotype appears normal.
The mother was the fourth child of healthy non-consanguineous parents. Family history was unremarkable. She was born by vaginal delivery at term of an uneventful pregnancy. Birth weight was 3,600 g. Developmental milestones and language were referred in the normal range. She has attended high school without learning difficulties. She is now 44 years old. Weight is 56 kg, height 168 cm, head circumference 54 cm. Facial appearance is normal, with the exception of prominent nose with broad nasal root and a narrow alar base. The voice is normal and velo-pharyngeal insufficiency was excluded by video fluoroscopy. Echocardiography and spiral computed tomography showed a normal heart and aortic arch. Her T-cell number and parathyroid function were within normal range.
This family was enrolled in a research study with their informed consent (Internal Review Board approved program, CCI- 1999-201) to further evaluate the inheritance pattern of the deleted chromosome 22q11.2. A set of samples from the proband (son), his mother, and his grandmother were evaluated for FISH and molecular testing. We obtained a specimen from the boy’s maternal grandmother but, unfortunately, we could not obtain a specimen from the father, who is dead.
Molecular Cytogenetic Analysis
FISH mapping was performed on Epstein Barr virus (EBV) transformed lymphoblastoid cell lines from peripheral blood to evaluate the 22q11.2 region of the proband, his mother, and his grandmother. A commercial probe LSI TUPLE 1 (Abbot Molecular) labeled with rhodamine dye for the chromosome 22q11.2 region with a LSI ARSA (Abbot Molecular) control probe labeled with fluorescein dye was used. A total of 50 nuclei from each specimen was examined by interphase FISH and the experiment was performed twice. The probes covered the minimal deletion region and were used according to manufactures recommendations (Abbott Molecular). Both metaphase and interphase nuclei were evaluated for the mother and son.
Array Comparative Genome Hybridization and SNP Analysis
Genomic DNA was extracted from EBV transformed lymphoblastoid cell lines with the use of the Flexi-Gene DNA kit (Qiagen). Genome-wide Affymetrix human SNP 6.0 microarrays were used for genotyping according to the standard protocol recommended by manufacturer's instructions (www.affymetrix.com).
We determined the genotype of 909,622 SNPs from our three samples, using birdseed 2.0 in Affymetrix Power Tools. The copy number analysis module (CNAM) in Golden Helix was used to process the Affymetrix Genome-Wide SNP 6.0 raw intensity files (.CEL). This information was compared to a reference genome of 96 arrays, which were run and processed in a similar manner at the same facility (AECOM DNA Facility). Log2ratio values were used as a sample-wide genotype quality control measure. The log2ratio was calculated for each probe (both SNP probes and copy number probes) and the results of each microarray was compared with the reference genome. A univariate analysis was implemented in CNAM of Golden Helix to determine the optimal segmentation of the log2ratios for each measured subject. The CNAM optimal segmenting results (red line) overlaid with the original Log2ratios (blue dots) (Fig. 3B). A similar approach was undertaken on Affymetrix Cytogenetics Whole-Genome 2.7 M arrays (run according to manufacturer’s instructions) from the mother and son using the Partek Genomics Suite (http://www.partek.com/partekgs).
Figure 3. Copy number variation analysis of the 22q11.2 region in the VCFS/DGS proband and his normal mother.
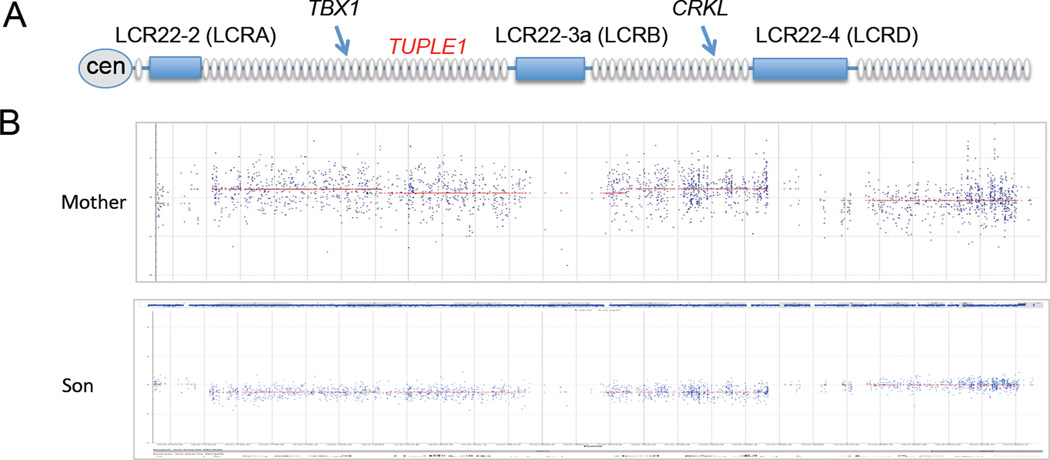
(A) A cartoon of the 22q11.2 region is shown with the genes indicated as ovals. The segmental duplications spanning the interval are indicated as rectangles on the line representing the 22q11.2 region. Two genes, TBX1 and CRKL are shown to aid in orientation. The general position of the TUPLE1 probe used for FISH mapping in Figure 2, is indicated.
(B) The DNA from the mother and son were analyzed by Affymetrix 6.0 arrays (the grandmother had normal dosage and is not shown). The samples were compared to Affymetrix 6.0 data from 100 normal controls run in the same core facility at Albert Einstein College of Medicine. The signal intensity at each CNV is averaged and found to lie near 0 as expected, however, the 22q11.2 region in the mother was slightly above the line, although not great enough to suggest the presence of three alleles. The mothers’ signal intensity of the SNP also revolves around 0, showing compensation for the lost region of one chromosome by the duplicated region in the homologous chromosome. The DNA from the VCFS/DGS son shows presence of one allele flanked by LCR22-2 and LCR22-4. This represents the typical ~3 Mb deleted region in patients with the disorder.
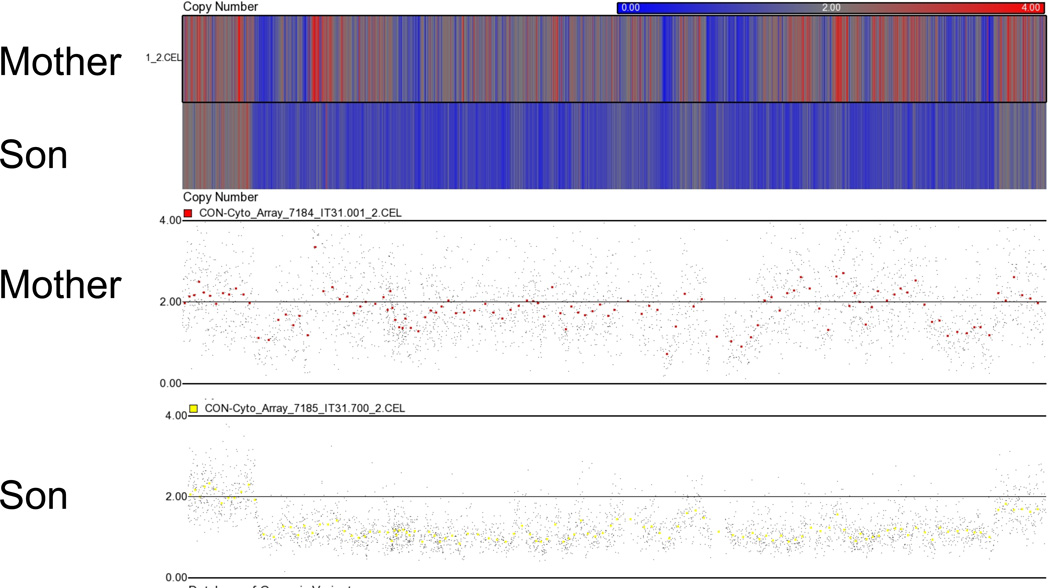
(C) Affymetrix Cytogenetics Whole-Genome 2.7M Arrays were run from the mother and VCFS/DGS son were analyzed using Partek Genomics Suite. The upper track contains a heat map with one row for every sample. Regions appearing at increased in copy number are shown in red, and those at decreased copy number in blue. The lower track shows a detailed view of all the probes in this region.
Results
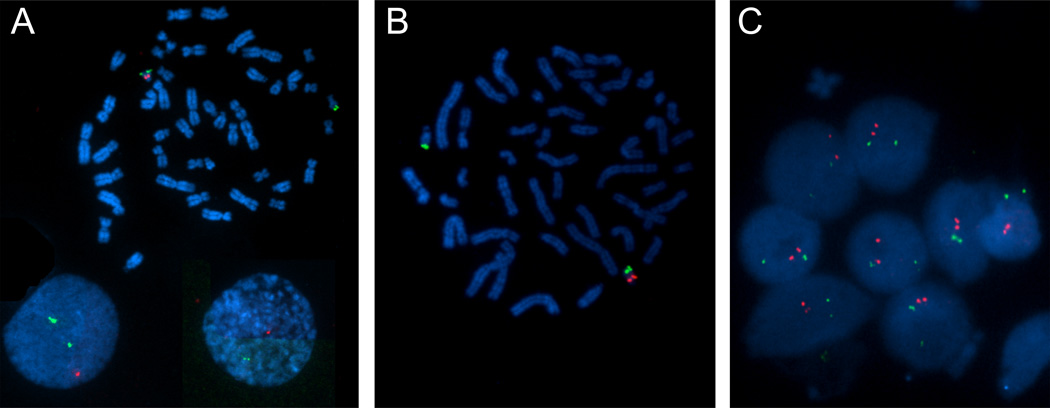
Fluorescence in situ hybridization (FISH) mapping was performed on chromosome spreads using probe LSI TUPLE1 hybridizing near TBX1 in the proximal half of 22q11.2, to confirm the existence of a microdeletion in the proband (Fig. 2A). Metaphase FISH showed the red, q11.2 probe missing on one copy of chromosome 22, while the green LSI ARSA probe hybridizing to the distal 22q13 interval, was present in both. Interphase FISH mapping revealed only one red and two green signals, confirming the deletion. When we carried out the same experiment in the mother, the metaphase FISH analysis revealed an absence of the red signal in one allele of chromosome 22 and a second enlarged red signal on its homologous chromosome (Fig. 2B). On interphase nuclei, two red and two green signals were observed in >50 nuclei (Fig. 2B–C). The two red fluorescent signals appear close together, as compared to the green signals suggesting that they were linked. This implies that there is a deletion on one chromosome and a duplication on the other, consistent with the stronger signal on the metaphase chromosomes (Figure 2C). The cells from the maternal grandmother showed the expected normal pattern in both metaphase and interphase nuclei (data not shown). Therefore this duplication of the q11.2 region was not seen in the son or the grandmother.
Figure 2. Results of fluorescence in situ hybridization analysis of cultured peripheral-blood lymphocytes from the VCFS/DGS son and his mother.
A representative metaphasic spread of chromosomes are shown for the Son (2A) and Mother (2B). The green dots are chromosome 22 control probes (ARSA; 22q13) and the red dots are the 22q11.2 probes (TUPLE1). The son (2A) appears to have one signal for the 22q11.2 probe, showing a deletion of the 22q11.2 region. The mother’s chromosomes during metaphase (2B) have one signal for the 22q11.2 probe (red). However when the mother’s chromosomes were analyzed during interphase (2C), it is evident that two signals for the 22q11.2 probe are seen, likely resulting from a duplication of the 22q11.2 region. This was not seen in the interphase analysis of the son (Figure 2A).
Affymetrix Genome-Wide SNP 6.0 arrays were used to perform array comparative genome hybridization (aCGH) on DNA from the child, mother and grandmother, to determine the size and endpoints of the deletion and duplication. A total of 906,622 SNP and 946,000 CNV probes are present on each array. The genotype calls were plotted against the genes lying on the chromosome 22q11.2 region and the segmental duplications (LCR22-2 or LCRA, LCR22-3a or LCRB and LCR22-4 or LCRD are shown in Fig. 3A). Using the arrays, we found that the proband had the typical 3 Mb microdeletion seen in VCFS/DGS patients (Fig. 3B). The deletion is flanked by LCR22-2 and LCR22-4, intervals that form a gap in SNP and CNV genotypes on aCGH (Fig. 3). The deletion was not seen when examining the SNPs and CNVs in the mother and grandmother, indicating that the deletion on her homologous chromosome 22q11.2 region is being compensated by the duplication of that region in her homologous chromosome. There was a slight increase of signals above the line representing two chromosomes 22 in the 22pter-22q11.2 regions in the mother, but not at the level that would represent a true triplication. We performed a second independent analysis using a different microarray termed the Affymetrix Cytogenetics Whole-Genome 2.7M array and found that the mother had two chromosomes 22 (Fig. 3C), supporting the Affymetrix 6.0 results and confirming the FISH findings.
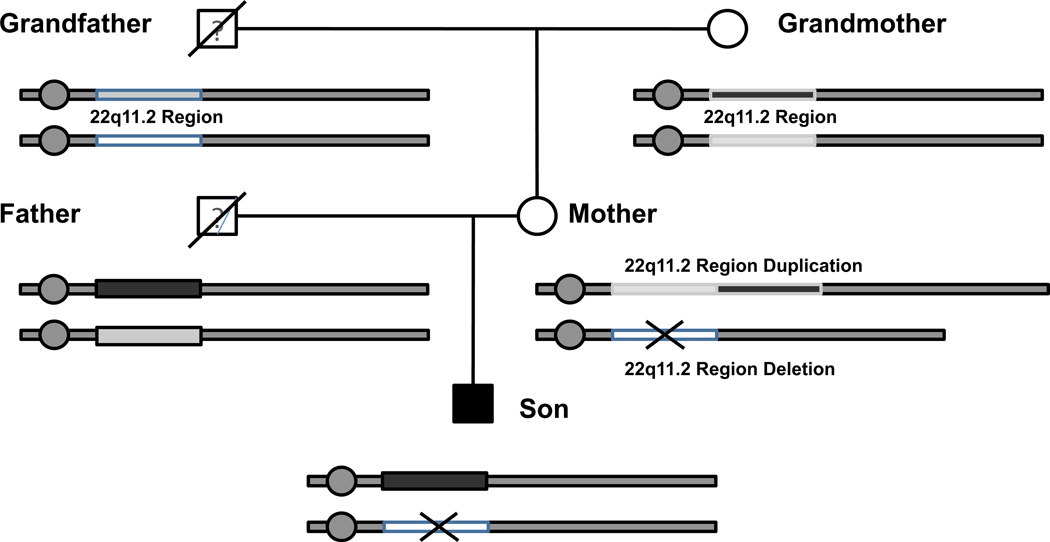
Finally we attempted to determine the origin of the rearrangement observed in the son and the mother using the SNP genotypes among the samples (Table 2). A total of 605 SNPs were successfully determined in the 22q11.2 deletion region (chr 22:17,256,416–19,795,835). The son had 10.9% different SNP genotype calls from that of the mother, within the deleted area and 26.7% different SNP genotype calls outside of the deleted area (Table 2). This indicated that the son received one of his chromosomes from the father as expected. When we analyzed the SNP data between the mother and the grandmother, we saw that the mother and grandmother had a 38.2% SNPs genotype calls difference in the 3 Mb region, and a 32.8% SNP genotype difference outside of the deleted area (Table 2). Overall there is a 33.1% genotype discordance between the mother and grandmother his likely excludes the model of uniparental disomy from the grandmother since the genotypes should be all identical according this model. Therefore, the non-allelic recombination event likely occurred in the germ cell of the grandfather and grandmother (Fig. 4). The data is not consistent with an origin of uniparental disomy from either parent of the mother.
Table 2.
| Mother vs. Son | Grandmother vs. Mother | |
|---|---|---|
| Within 22q11.2 Region | ||
| Same | 539 | 374 |
| 1 Different SNP | 66 | 188 |
| 2 Different SNPs | 0 | 43 |
| Total SNPs | 605 | 605 |
| % Difference | 10.9 | 38.2 |
| Outside 22q11.2 Region | ||
| Same SNP | 7930 | 7266 |
| 1 Different SNP | 2884 | 3310 |
| 2 Different SNPs | 0 | 238 |
| Total SNPs | 10814 | 10814 |
| % Difference | 26.7 | 32.8 |
| Total % Difference | 25.8 | 33.1 |
Figure 4. Pedigree of the proband (son) with the 22q11.2 deletion syndrome and mother with the 22q11.2 deletion and duplication region.
Circles represent female family members and squares represent males. The grandfather is deceased and therefore no genetic information is available. We were unable to obtain DNA samples from the father. It appears that the son received the 22q11.2 deletion region from the mother. However, based on our CNV analysis it appears that the mother did not receive the 22q11.2 duplication region from her mother.
Discussion
We report here a child with VCFS/DGS and the typical 3 Mb 22q11.2 deletion and his mother with a deletion on one and duplication of 3 Mb on the other allele of chromosome 22. This result along with the normal phenotype of the mother, suggest that the duplicated region of the 22q11.2 region in the mother has a compensatory effect to the deleted region on the homologous chromosome. The results are similar to those in a recent report of one family in which a father carried the 3 Mb deletion and duplication and his daughter had the deletion [Carelle-Calmels et al., 2009]. The most parsimonious explanation is that the deletion and reciprocal duplication occurred during the first mitotic divisions after fertilization, as suggested in the previous report [Carelle-Calmels et al., 2009]. Other more complicated events including non-disjunction and trisomy rescue are also possible.
The present study highlights the importance in performing interphase FISH mapping on parents of offspring harboring genomic disorders in addition to metaphase FISH. This is because, for example, the mother was previously only investigated by metaphase FISH for harboring a deletion and was thought of as having an extremely mild spectrum of manifestation of the syndrome [Digilio et al., 2003]. In fact, the present evidence demonstrates that search for deletion 22q11.2 by aCGH in the parents is not sufficient, particularly if their phenotype appears normal. Metaphase and interphase FISH is necessary for future genetic counseling, since a parent carrying 22q11.2 deletion in one chromosome and 22q11.2 duplication on the other chromosome has a reproductive risk of 100% of conceiving a child with chromosomal imbalance (deletion or duplication).
Acknowledgements
We acknowledge Ms. Linda Christenson and Dr. Jidong Shan in the Molecular Cytogenetics Core at Albert Einstein College of Medicine, for performing the FISH mapping in the study. We also thank Dr. Melanie Babcock for running the Cytogenetics Whole-Genome 2.7M Array in the Division of Translational Genetics. We thank the Genomics Core facility at Einstein as well, for running the Affymetrix 6.0 arrays. This work was supported by NIH, HL084410 to B. E. M.
References
- Beckerman JS, Estivill X, Antonarakis SE. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat Rev Genet. 2007;8:639–646. doi: 10.1038/nrg2149. [DOI] [PubMed] [Google Scholar]
- Carelle-Calmels N, Saugier-Veber P, Girard-Lemaire F, Rudolf G, Doray B, Guérin E, Kuhn P, Arrivé M, Gilch C, Schmitt E, Fehrenbach S, Schnebelen A, Frébourg T, Flori E. Genetic Compensation in a Human Genomic Disorder. N Engl J Med. 2009;360:1211–1216. doi: 10.1056/NEJMoa0806544. [DOI] [PubMed] [Google Scholar]
- Chow EW, Bassett AS, Weksberg R. Velo-cardio-facial syndrome and psychotic disorders: implications for psychiatric genetics. Am J Med Genet. 1994;54:107–112. doi: 10.1002/ajmg.1320540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGeorge AM. Congenital absence of the thymus and its immunologic consequences: concurrence with congenital hypoparathyroidism. IV(1) White Plains, NY: March of Dimes-Birth Defects Foundation; 1968. pp. 116–121. [Google Scholar]
- Digilio MC, Marino B, Giannotti A, Dallapiccola B. Familial deletions of chromosome 22q11. Am J Med Genet. 1997;73:95–96. [PubMed] [Google Scholar]
- Digilio MC, Angioni A, De Santis M, Lombardo A, Giannotti A, Dallapiccola B, Marino B. Spectrum of clinical variability in familial deletion 22q11.2: from full manifestation to extremely mild clinical anomalies. Clin Genet. 2003;63:308–313. doi: 10.1034/j.1399-0004.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Marino B, Dallapiccola B. Deletion 22q11 and isolated congenital heart disease. Int J Cardiol. 2008;12:364–365. doi: 10.1016/j.ijcard.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-MB deletion in patients with the velo-cardio-facial syndrome. Am J Hum Genet. 1999;64:1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, Chaganti RS, Magenis E, Shprintzen RJ, Morrow BE. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- Emanuel BS, McDonald-McGinn D, Saitta SC, Zackai EH. The 22q11.2 deletion syndrome. Adv Pediatr. 2001;48:39–73. Review. [PubMed] [Google Scholar]
- Ensenauer RE, Adeyinka A, Flynn HC, Michels VV, Lindor NM, Dawson DB, Thorland EC, Lorentz CP, Goldstein JL, McDonald MT, Smith WE, Simon-Fayard E, Alexander AA, Kulharya AS, Ketterling RP, Clark RD, Jalal SM. Microduplication 22q11.2, an emerging syndrome: clinical, cytogenetic, and molecular analysis of thirteen patients. Am J Hum Genet. 2003;73:1027–1040. doi: 10.1086/378818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers LJ, De Die-Smulders CE, Smeets EE, Clerkx MG, Curfs LM. The velo-cardio-facial syndrome: the spectrum of psychiatric problems and cognitive deterioration at adult age. Genet Couns. 2009;20:307–315. [PubMed] [Google Scholar]
- Lindsay EA, Goldberg R, Jurecic V, Morrow B, Carlson C, Kucherlapati RS, Shprintzen RJ, Baldini A. Velo-cardio-facial syndrome: frequency and extent of 22q11 deletions. Am J Med Genet. 1995;57:514–522. doi: 10.1002/ajmg.1320570339. [DOI] [PubMed] [Google Scholar]
- Marino B, Digilio MC, Toscano A, Anaclerio S, Giannotti A, Feltri C, de Ioris MA, Angioni A, Dallapiccola B. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet Med. 2001;3:45–48. doi: 10.1097/00125817-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Owen MJ. Velo-cardio-facial syndrome: a model for understanding the genetics and pathogenesis of schizophrenia. Br J Psychiatry. 2001;179:397–402. doi: 10.1192/bjp.179.5.397. Review. [DOI] [PubMed] [Google Scholar]
- Oskarsdóttir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch. Dis. Child. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Berg JS, Yonath H, Enciso VB, Miller DT, Picker J, Lenzi T, Keegan CE, Sutton VR, Belmont J, Chinault AC, Lupski JR, Cheung SW, Roeder E, Patel A. Microduplication of 22q11.2 are frequently inherited and are associated with variable phenotypes. Genet Med. 2008;10:267–277. doi: 10.1097/GIM.0b013e31816b64c2. [DOI] [PubMed] [Google Scholar]
- Portnoï MF, Lebas F, Gruchy N, Ardalan A, Biran-Mucignat V, Malan V, Finkel L, Roger G, Ducrocq S, Gold F, Taillemite JL, Marlin S. 22q11.2 duplication syndrome: two new familial cases with some overlapping features with DiGeorge/velocardiofacial syndrome. Am J Med Genet. 2005;137:47–51. doi: 10.1002/ajmg.a.30847. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Beemer FA, Dallapiccola B, Novelli G, Hurst JA, Ignatius J, Green AJ, Winter RM, Brueton L, Brondum-Nielsen K, Stewart F, Van Essen T, Patton M, Paterson J, Scambler PJ. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O'Hare AM, Hu P, Roe BA, Driscoll DA, McDonald-McGinn DM, Zackai EH, Budarf ML, Emanuel BS. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev Disabil Res Rev. 2000;6:142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. Review. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14:3–10. doi: 10.1002/ddrr.2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Vantrappen G, Vogels A, Rommel N, Fryns JP, Eyskens B, Gewillig M, Dumoulin M. Familial deletions of chromosome 22q11: the Leuven experience. Am J Med Genet. 1998;80:531–532. [PubMed] [Google Scholar]
- Williams MA, Shprintzen RJ, Goldberg RB. Male-to-male transmission of the velo-cardio-facial syndrome: a case report and review of 60 cases. J Craniofac Genet Dev Biol. 1985;5:175–180. [PubMed] [Google Scholar]