Background: Bispecific antibodies are currently emerging as a promising new class of cancer therapeutics.
Results: The novel one-arm single chain Fab IgG bispecific antibody (XGFR) targeting IGF-1R and EGFR demonstrated potent signaling inhibition and enhanced ADCC induction.
Conclusion: XGFR has shown in vitro and in vivo anti-tumor activity in pancreatic, lung, and colorectal mouse xenograft tumor models.
Significance: Rational design can help to overcome low expression yields and impaired effector functions of bispecific antibodies.
Keywords: Antibody Engineering, Epidermal Growth Factor Receptor (EGFR), Glycosylation, Insulin-like Growth Factor (IGF), Tumor Therapy
Abstract
In the present study, we have developed a novel one-arm single chain Fab heterodimeric bispecific IgG (OAscFab-IgG) antibody format targeting the insulin-like growth factor receptor type I (IGF-1R) and the epidermal growth factor receptor (EGFR) with one binding site for each target antigen. The bispecific antibody XGFR is based on the “knob-into-hole” technology for heavy chain heterodimerization with one heavy chain consisting of a single chain Fab to prevent wrong pairing of light chains. XGFR was produced with high expression yields and showed simultaneous binding to IGF-1R and EGFR with high affinity. Due to monovalent binding of XGFR to IGF-1R, IGF-1R internalization was strongly reduced compared with the bivalent parental antibody, leading to enhanced Fc-mediated cellular cytotoxicity. To further increase immune effector functions triggered by XGFR, the Fc portion of the bispecific antibody was glycoengineered, which resulted in strong antibody-dependent cell-mediated cytotoxicity activity. XGFR-mediated inhibition of IGF-1R and EGFR phosphorylation as well as A549 tumor cell proliferation was highly effective and was comparable with a combined treatment with EGFR (GA201) and IGF-1R (R1507) antibodies. XGFR also demonstrated potent anti-tumor efficacy in multiple mouse xenograft tumor models with a complete growth inhibition of AsPC1 human pancreatic tumors and improved survival of SCID beige mice carrying A549 human lung tumors compared with treatment with antibodies targeting either IGF-1R or EGFR. In summary, we have applied rational antibody engineering technology to develop a heterodimeric OAscFab-IgG bispecific antibody, which combines potent signaling inhibition with antibody-dependent cell-mediated cytotoxicity induction and results in superior molecular properties over two established tetravalent bispecific formats.
Introduction
Over the last years, numerous bispecific antibody formats have been reported, and the approach of simultaneously modulating two molecular targets on a tumor cell or redirecting immune effector cells to kill tumor cells may develop into an important therapeutic alternative to monoclonal antibodies. Notably, blinatumomab, a bispecific antibody targeting CD3 on T cells and CD19 on B cells in patients with acute lymphoblastic leukemia (1) has demonstrated impressive clinical anti-tumor activity. Various other bispecific formats are currently in preclinical and clinical evaluation. One of the first published formats, the dual variable domain immunoglobulin (DVD-Ig)3 format is a tetravalent, dual targeting single agent generated by fusion of VH and VL domains to the N terminus of a second antibody by a short amino acid linker (2). However, for this format, depending on the epitopes being targeted by the applied VH and VL domains, a loss of affinity for the inner variable domains was observed in one example caused by steric hindrance of ligand binding by the proximity of the outer variable domain (3). Another tetravalent bispecific format that has been extensively characterized is a monoclonal antibody carrying fusions of disulfide-stabilized scFvs at the C terminus of the heavy chain (4, 5). Recently, we have described a loss of ADCC activity with this bispecific format most likely due to steric hindrance caused by the attached scFv moieties preventing binding to the Fc γ receptor IIIA on immune effector cells (6). These findings suggest that a thorough analysis of the effects of antibody modification on the in vitro and in vivo properties of novel bispecific antibody formats is essential. Here, we rationally designed a heterodimeric one-arm scFab-IgG antibody format targeting EGFR and IGF-1R, which combines potent signaling inhibition with effective ADCC induction through glycoengineering of the Fc region. Glycoengineered antibodies were produced using a method first described by Umaña et al. (7). Glycosylation of human IgGs occurs in the Fc region at a conserved N-glycosylation site within the CH2 domain, where Asn-297 is linked to carbohydrates. The carbohydrate chain at this site contains a core of N-acetylglucosamine, mannose, galactose, sialic acid, and fucose residues. Afucosylation or glycoengineering leads to an up to 100-fold increase in the affinity to FcγRIIIa receptors and subsequently to an increase in ADCC-mediated cell death (8). Therefore, the antibodies in this study are absent of the core fucose at the Asn-297 of the Fc region.
The receptor tyrosine kinases EGFR and IGF-1R are frequently overexpressed or show enhanced activation in human tumors (9–11). EGFR and IGF-1R contribute to tumor development and progression through their effects on tumor cell proliferation, inhibition of apoptosis, and induction of angiogenesis (12–14). Several small molecules, such as erlotinib or gefitinib, as well as monoclonal antibodies, such as cetuximab and panitumumab, inhibit EGFR downstream signaling pathways and are approved for tumor treatment in the clinic (15, 16). We have reported the development of a glycoengineered and ADCC enhanced EGFR antibody termed imgatuzumab (GA201), which is currently in PhII clinical trials (17). In a phase I study, imgatuzumab (GA201) was well tolerated and showed early signs of clinical efficacy in patients suffering from colorectal cancer (18). In addition, several monoclonal antibodies targeting IGF-1R, such as AMG-479 or IMC-A12, are currently in clinical development either as monotherapy or in combination with chemotherapeutics or EGFR inhibitors (19–22). We have developed an IGF-1R antibody termed R1507 that has been evaluated in combination with erlotinib in phase II clinical trials for advanced stage non-small cell lung cancer (19). Both receptor tyrosine kinases contribute to tumor growth via activation of the PI3K-AKT and RAS-RAF-MAPK signaling pathways, and cross-talk between EGFR and IGF-1R signaling has been reported. Preclinical and clinical studies have shown that signaling through the IGF-1 receptor can overcome resistance to EGFR inhibitors (23, 24), and EGFR-dependent signaling can confer resistance to IGF-1R inhibitors (25–29).
Targeting EGFR and IGF-1R using bispecific antibodies to induce inhibition of tumor growth has been described in several publications; however, the applied bispecific antibody formats suffered from low production yields, inherent stability problems, or lack of important anti-tumor effector mechanisms, such as ADCC or inferior tumor cell proliferation inhibition, compared with the parental antibodies. One example is a di-diabody with fusion of IFG-1R and EGFR targeting crossover scFvs directly to the Fc domain, which showed a significantly reduced antiproliferative activity compared with the respective monospecific EGFR antibody (26). In addition, a tetravalent bispecific antibody has been described devoid of ADCC effector functions, containing a C-terminal attachment of an IGF-1R scFv to the Fc part of an EGFR antibody (4). Another molecule lacking ADCC activity is a bispecific EGFR-IGF-1R inhibitor based on a human fibronectin scaffold, which has been PEGylated to increase serum half-life (30).
In the past, monospecific scFab antibodies have been mostly expressed in bacteria and yeasts and were found to be compatible for use in phage display (31). However, upon expression in HEK293 cells, the formation of large amounts of oligomers was observed (32, 33), which renders this approach not viable for development of therapeutic proteins. In the present study, we rationally designed a bispecific heterodimeric and bivalent one-arm scFab IgG (OAscFab-IgG) antibody format based on the “knob-into-hole” technology (34) targeting EGFR and IGF-1R with distinct binding arms derived from the parental antibodies GA201 (EGFR) and R1507 (IGF-1R) (18, 20). This novel antibody format combines robust expression and overcomes the light chain association issue in bispecific heterodimeric IgG antibodies (35). Furthermore, it combines potent signaling inhibition as well as reduced IGF-1R internalization with effective ADCC induction through glycoengineering of the Fc region. We also compared the OAscFab-IgG XGFR with two other tetravalent bispecific antibodies in vitro and were able to improve impaired ligand binding and reduced Fc receptor activation of these formats. In several ADCC-competent mouse xenograft models, antibody XGFR demonstrated highly effective antitumoral activity.
EXPERIMENTAL PROCEDURES
Generation of Bispecific Antibodies
All antibody gene segments were generated by gene synthesis and cloned by unique restriction sites into pUC expression vectors. Bispecific and control antibodies were expressed by transient transfection of human embryonic kidney (HEK) cells growing in suspension. HEK cell culture supernatants were harvested 7 days after transfection and purified in two steps by affinity chromatography using protein A-SepharoseTM (GE Healthcare) and Superdex 200 size exclusion chromatography. Glycoengineered antibodies were produced by co-transfection of the cells with two plasmids coding for the carbohydrate-modifying enzymes β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II. XGFR was also expressed from stable Chinese hamster ovary (CHO) K1 cell lines engineered to constitutively overexpress the GA201 “knob” heavy and light chain and the R1507 OAscFAb “hole” heavy chain as well as recombinant β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II using the glutamine synthetase expression system (Lonza Biologics). The XGFR bispecific antibody was produced in a fed batch fermentation process with the engineered CHO cells in a chemically defined animal component-free medium and was subsequently purified by protein A and ion-exchange chromatographic techniques. Fractions containing purified antibodies with less than 5% high molecular weight aggregates were pooled and stored in 6.0 mg/ml aliquots at −80 °C.
Biochemical and Biophysical Analysis of Purified Recombinant Proteins
The protein concentration of purified protein samples was determined by measuring the optical density (OD) at 280 nm, using the molar extinction coefficient calculated on the basis of the amino acid sequence. Purity, antibody integrity, and molecular weight of bispecific and control antibodies were analyzed by SDS-PAGE and CE-SDS using microfluidic Labchip technology (Caliper Life Sciences). Aggregation of bispecific antibody samples was analyzed by high-performance size exclusion chromatography using a Superdex 200 analytical size exclusion column (GE Healthcare) in 200 mm KH2PO4, 250 mm KCl, pH 7.0, running buffer at 25 °C. The integrity of the amino acid backbone and the molecular weight of reduced bispecific antibody light and heavy chains was verified by electrospray Q-TOF mass spectrometry after removal of N-glycans by enzymatic treatment with peptide-N-glycosidase F (Roche Applied Science).
Oligosaccharide Analysis
Oligosaccharides were enzymatically released from the antibodies by peptide-N-glycosidase F (Roche Applied Science). A fraction of the peptide-N-glycosidase F-treated sample was subsequently digested with endoglycosidase H (Roche Applied Science). The released oligosaccharides were incubated in 150 mm acetic acid prior to purification through a cation exchange resin and analyzed using an Autoflex MALDI-TOF (Bruker Daltonics) in positive ion mode.
Surface Plasmon Resonance (SPR) Analysis
All SPR experiments were performed using a Biacore T200 instrument with PBS, 0.05% Tween 20 (v/v). Standard amine coupling to N-ethyl-N(dimethylaminopropyl)-carbodiimide hydrochloride/N-hydroxysuccinimide-activated chip surfaces was performed as recommended by the provider (GE Healthcare). Antibodies were captured via anti-human IgG-Fc antibody. A CM5 chip was used for detection of EGFR binding with a ligand density of capture molecule of ∼1000 response units and capture levels of ∼40–46 response units of the tested antibodies. A C1-Chip with low ligand density (∼200 response units, capture levels of antibody ∼6–10 response units) was used to achieve monovalent binding of the IGF-1R dimer. Five increasing concentrations of the receptors were injected at a flow rate of 50 μl/min for a 180-s association time and a dissociation time of 1200 s for EGFR and 1600 s for IGF-1R at 37 °C. Final regeneration was performed after each cycle using 10 mm glycine, pH 1.75, contact time 60 s, and a flow rate of 50 μl/min. Kinetic constants were evaluated by fitting the association and dissociation phase of the analyzed interaction with a Langmuir 1:1 binding model (refractive index = 0) using usual double referencing (flow cell 1 reference surface with capture molecule and concentration = 0 nm) by Biacore Evaluation software.
Cell Culture
A549 (ATCC), AsPC1 (ATCC), LS174T (ATCC), and NCI-H322M cells (NCI, National Institutes of Health) were cultivated in RPMI 1640 medium (PAA, Pasching, Austria) supplemented with 10% fetal bovine serum (PAA) and 2 mm l-glutamine (Sigma) at 37 °C and 5% CO2.
IGF-1R/EGFR Phosphorylation Assay
A549 NCSLC cells (3 × 104) were seeded in 96-well plates and starved in serum-free medium (1 mg/ml bovine serum albumin, 10 mm HEPES, 1% penicillin/streptomycin) for 2 h. To assess phosphorylation, cells were first incubated with either bispecific or control antibodies for 30 min, followed by the addition of 5 nm IGF-1 (PreProtech) or 10 nm EGF (PreProtech) for 10 min and final lysis in 100 μl/well lysis buffer (Cell Lysis Kit, Bio-Rad). Samples were analyzed for EGFR or IGF-1R phosphorylation using the P-EGFR (Tyr) bead kit (Millipore) or P-IGF-1R (Tyr-1131) bead kit (Bio-Rad) combined with a phosphoprotein detection reagent (Bio-Rad) by the Luminex detection system.
Signaling Pathway Analysis
LS174T cells (1.5 × 105) were seeded in 6-well plates overnight and incubated with 1000 nm bispecific or control antibodies for 0.5, 6, 24, and 48 h. Cells were lysed by the addition of 10 ml of lysis buffer (Cell Lysis Kit, Bio-Rad). Samples were analyzed for PI3K (Bio-Plex Assay, Bio-Rad) and MAPK pathway (WideScreen EpiTag assay, Novagen) activation markers using the Luminex detection system.
Three-dimensional Cell Viability Assay
Tumor cells were seeded in 96-well poly-HEMA-coated plates with increasing concentrations of bispecific or control antibodies and incubated for 7 days. Cell viability was determined using a CellTiterGlo® (Promega) assay according to the manufacturer's instructions.
IGF-1R Levels in Cell Lysates
Tumor cells were cultivated in the presence of 50 nm concentrations of bispecific or control antibodies in standard culture medium for 24 h at 37 °C and 5% CO2. Cells were then lysed with ice-cold cell signaling lysis buffer (Millipore) supplemented with protease inhibitors (Roche Applied Science). Plates were stored at −20 °C until further analysis. IGF-1R levels were detected using an IGF-1R sandwich ELISA using a biotinylated anti-IGF-1Rhu-1a-IgG (Roche Applied Science) capture antibody and an anti-IGF-1Rβ rabbit (Santa Cruz Biotechnology, Inc.) detection antibody.
Quantification of Antibodies on the Surface of Tumor Cells
A549 cells were seeded into poly-HEMA-coated 6-well tissue culture plates to prevent adhesion of cells to the plastic surface for three-dimensional cultures. Coating was performed using 4% poly-HEMA (Polysciences) in EtOH and subsequently drying for at least 5 days at 37 °C. After 4 h of cell seeding, Matrigel (BD Biosciences) was added, and spheroids were collected 24 h later, washed, and trypsinized to generate single cell suspensions. Subsequently, cells were washed twice with ice-cold medium and stained with 10 μg/ml phycoerythrin-conjugated EGFR or IGF-1R antibodies (BD Biosciences) or matching isotype controls (BD Biosciences). Acquisition of data was performed using FACSCanto II, and data were analyzed by FlowJo software. The number of antibodies bound per cell was determined using a phycoerythrin fluorescence quantification kit (BD Biosciences), and receptor density per cell was calculated.
In Vitro ADCC Assay
The in vitro ADCC assay was performed as described previously (6).
In Vivo Studies
Human pancreatic cancer AsPC-1-LUC cells were orthotopically inoculated into the pancreas of female SCID beige mice (1 × 106 cells/mouse). Groups of n = 5 animals were treated with intraperitoneal injection of control antibody (Xolair, Roche Applied Science) or the bispecific antibody XGFR on days 7, 14, and 21 after tumor inoculation at a 20 mg/kg dose. Orthotopic tumor growth of luciferase-positive AsPC-1 xenografts was assessed by Bioluminescence Imaging using the IVIS Xenogen system on study day 7, 14, 21, and 27 after tumor inoculation. In the A549 lung tumor model, A549 cells were injected intravenously in the tail vein of female SCID beige mice. Groups of n = 10–15 mice were treated once weekly by intraperitoneal injections of compounds or vehicle control starting on day 7 after tumor cell inoculation, when evidence of tumor growth was visible in the lungs of sacrificed scout animals. GA201 and R7072 (glycoengineered R1507) were applied at 10 mg/kg, whereas XGFR was applied at 20 mg/kg to ensure equimolar concentrations of dosing between XGFR and GA201/R7072 combination therapy. In the LS174T colon carcinoma metastasis model, 3 × 106 human colorectal adenocarcinoma LS174T cells were inoculated into the splenic tissue of female SCID beige mice. Weekly intraperitoneal administration of vehicle or test compounds (20 mg/kg XGFR, 20 mg/kg XGFR-wt, and 10 mg/kg AMG-479 (resynthesized according to patent information) plus 10 mg/kg cetuximab (Merck)) was started on day 7 after tumor cell inoculation (n = 10/group). Animals with severe clinical symptoms (e.g. body weight loss >20%) were excluded according to animal welfare guidelines and classified as non-survivors. Survival data were analyzed using Kaplan-Meier curves, and statistical analysis between treatment groups was assessed by a pairwise log-rank test.
RESULTS
Design of a Heterodimeric One-arm scFab Bispecific Antibody Targeting EGFR and IGF-1R (XGFR)
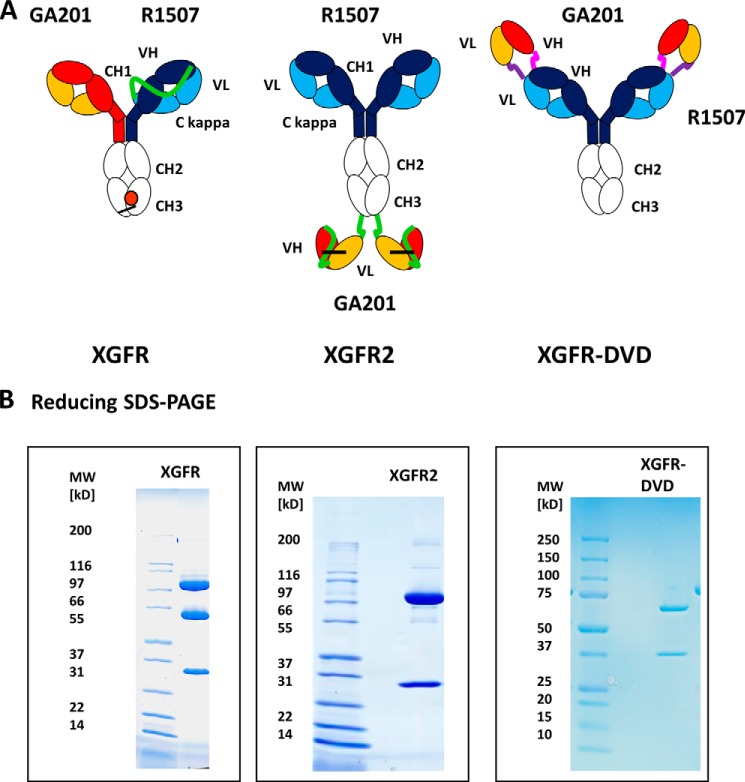
The bispecific antibodies generated in this study are based on a human IgG1 isotype with heavy chains composed of a variable VH domain and three constant domains CH1, CH2, and CH3. The corresponding light chains are composed of a variable VL domain and a constant Cκ domain. XGFR bispecific antibodies were assembled with an EGFR binding arm composed of the GA201 light and heavy chain. The IGF-1R binding arm is composed of a single-chain Fab fragment (scFab) of R1507 with the light chain attached to the N terminus of the VH domain by a 32-amino acid glycine serine linker to form the second heavy chain (Fig. 1A). Heterodimerization of the two heavy chains in this novel bispecific antibody format was achieved by application of the knobs-into-hole technology (34). The knob mutation (T366W) was introduced into the CH3 domain of the GA201 heavy chain, and three mutations to form a hole (T366S, L368A, and Y407V) were introduced into the CH3 domain of the scFab heavy chain of R1507. In addition, two cysteine residues were introduced (S354C on the knob and Y349C on the hole side) to form a stabilizing disulfide bond between the heterodimeric heavy chains. To compare the in vitro properties of the bivalent bispecific antibody XGFR with other bispecific formats, we generated two established tetravalent bispecific formats XGFR-DVD (dual variable domain-IgG) and XGFR2 (single chain Fv-IgG) (2, 6). In the XGFR-DVD (Fig. 1A), additional GA201 VH and VL variable domains were fused by a short peptide linker on the N terminus of heavy and light chain of the R1507 antibody. XGFR2 (Fig. 1A) was constructed with disulfide-stabilized GA201 scFvs fused to the C terminus of the R1507 heavy chains by a 10-amino acid (G4S)2 linker (6). The VH and VL region of the GA201 scFvs were tethered by a glycine serine peptide (G4S)4 linker and stabilized to eliminate aggregation by the previously described VH44 and VL100 disulfide bond (36, 37).
FIGURE 1.
Design of XGFR (EGFR/IGF-1R) bispecific antibodies. A, schematic diagram of the one-arm single chain Fab bispecific antibody XGFR, the XGFR2 antibody with C-terminal attachment of disulfide-stabilized scFvs, and the dual V domain (DVD) antibody XGFR-DVD. All VH and VL domains in the bispecific antibodies are derived from the parental antibodies GA201 (EGFR; red and yellow) and R1507 (IGF-1R; blue and light blue). The R1507 light chain in the XGFR molecule was fused by a 32-amino acid (G4S)6GG linker (green) to the N terminus of the VH domain. Dimerization of the two different heavy chains in the XGFR antibody was facilitated by the knob-into-hole mutations (orange) in the CH3 domain and an additional disulfide bond (black bar). The XGFR molecule design is based on the R1507 master antibody with a GA201 scFv fused by a (G4S)2 connector (green) to the C terminus of the CH3 domain. VH and VL domains are joined by a (G4S)4 linker (green). In the XGFR-DVD molecule, the GA201 VH and VL domains were fused to the R1507 antibody by an ASTKGP (pink) heavy chain and a TVAAP (purple) light chain linker. B, SDS-PAGE of bispecific XGFR antibody variants under reducing conditions after purification by protein A and SEC.
Expression and Purification of XGFR Bispecific Antibodies
Monoclonal antibodies GA201 and R1507 as well as XGFR bispecific antibodies were produced by transient expression in HEK293 cells. The parental antibodies as well as XGFR, XGFR-DVD, and XGFR2 were purified to homogeneity by protein A and size exclusion chromatography from cell culture supernatants, as demonstrated by SDS-PAGE analysis under reducing conditions (Fig. 1B). Reduced SDS-PAGE analysis of XGFR shows a 74.7-kDa R1507 scFab hole heavy chain band, a 49.3-kDa GA201 knob heavy chain, and a 23.4-kDa light chain (Fig. 1B). Upon transient expression, purification yields of the novel one-arm scFab bispecific antibody XGFR (20.6 mg/liter) were similar to the GA201 and R1507 parental antibodies, with yields of 24.0 and 23.6 mg/liter, respectively (Table 1). XGFR2 with C-terminal fusion of scFvs showed ∼2-fold reduced expression, whereas attachment of N-terminal VDs led to an 18-fold reduction in expression of XGFR-DVD compared with the parental antibodies (Table 1).
TABLE 1.
Protein purification yields of XGFR molecules compared with parental antibodies GA201 and R1507
| Molecule (transient HEK293 expression) | Purification yield |
|
|---|---|---|
| Protein A | SEC | |
| mg/liter | ||
| XGFR | 24.0 | 20.6 |
| XGFR-DVD | 1.5 | 1.3 |
| XGFR2 | 13.3 | 11.8 |
| GA201 | 26.4 | 24.0 |
| R1507 | 32.5 | 23.6 |
Generation of Stable CHO Cell Lines
Most therapeutic antibodies in clinical development are currently produced in stable CHO cell lines. Therefore, the manufacturing scalability of the novel bispecific antibody format XGFR was evaluated in CHO cells. GA201 knob heavy and light chain and the R1507 OAscFAb hole heavy chain as well as recombinant β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II were overexpressed in CHO K1 cells using a glutamine synthetase expression system. A total number of 415 clones were analyzed for IgG titer and afucosylation, and 26 clones were selected for generic fed batch production. Several stable production CHO clones with yields of 2–3 g/liter and afucosylation levels above 70% were identified and demonstrate the excellent manufacturing feasibility of the XGFR bispecific antibody.
Characterization of the By-product Profile of XGFR
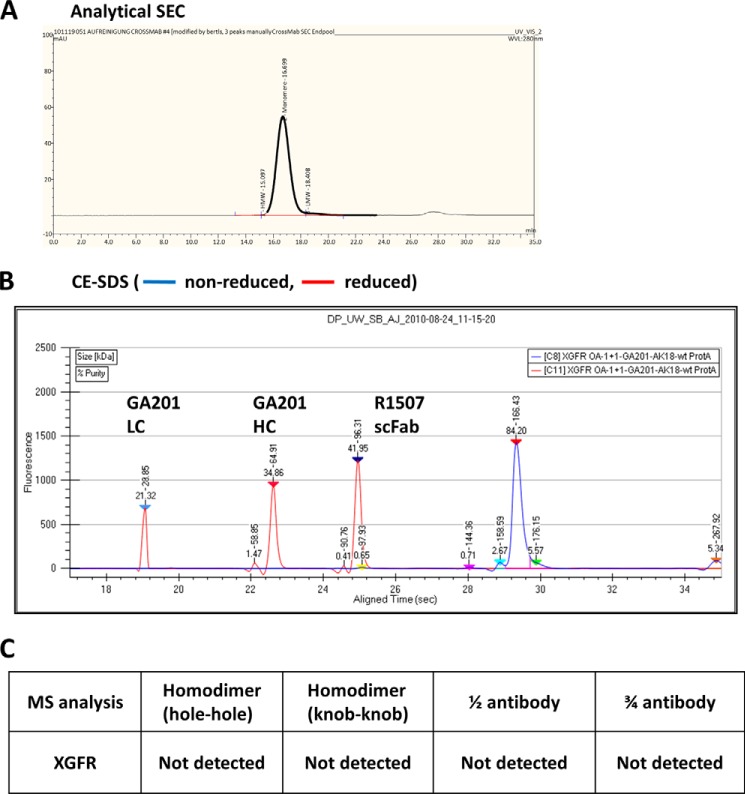
The product quality of purified XGFR was further analyzed by analytical size exclusion chromatography, CE-SDS, and mass spectroscopy (MS) analysis. Initially, analytical size exclusion chromatography was used to evaluate the levels of high molecular weight aggregates, and XGFR was found to be 99% pure after protein A and size exclusion chromatography (SEC) purification (Fig. 2A). Product homogeneity of the GA201 light and heavy chain and the OAscFAb R1507 heavy chain was confirmed by CE-SDS under reducing conditions, and a single monomer peak of XGFR was found under non-reducing conditions (Fig. 2B). The expected molecular mass of 147.4 kDa of XGFR was confirmed by mass spectroscopy. In addition, the potential formation of knob-into-hole antibody by-products, such as hole-hole and knob-knob heavy chain homodimers, half-antibodies lacking a knob, or hole heavy chain and three-quarter antibodies lacking the GA201 light chain, was analyzed, and none of the mentioned by-products was detectable by MS analysis (Fig. 2C).
FIGURE 2.
Biochemical and biophysical analysis of purified XGFR. A, analytical SEC was used to estimate the presence of aggregates in the one-arm scFab XGFR molecule after protein A and SEC purification. The chromatogram represents a 20-μg injection. B, purity, antibody integrity, and molecular weight of XGFR was further characterized by CE-SDS under reducing (red) and non-reducing (blue) conditions. C, MS analysis confirmed sequence integrity of XGFR, and no by-products, such as hole-hole dimers, knob-knob dimers, half-antibodies, or three-quarter antibodies lacking the GA201 light chain, were detectable.
SPR Analysis of the XGFR Bispecific Molecule Binding Affinities
The bispecific XGFR antibodies were analyzed for association rate constants (ka), dissociation rate constants (kd), and equilibrium constants (KD) using surface plasmon resonance analysis. First, the EGFR extracellular domain was coupled to the chip surface, and XGFR antibodies were injected, followed by the IGF-1R extracellular domain, which confirmed that all of the bispecific antibodies could simultaneously bind to both receptors (data not shown). All XGFR bispecific molecules could bind to EGFR within the same low nanomolar range as the parental antibody GA201 (Table 2). Binding to the IGF-1R extracellular domain was strongly dependent on the design of the bispecific antibodies. XGFR and XGFR2 showed KD values of 4 and 6 nm, which are similar to the parental R1507 KD of 5 nm. In contrast, XGFR-DVD with a rigid 5-amino acid attachment of GA201 variable domains to R1507 completely lost the ability to bind IGF-1R and therefore was not further evaluated in vitro and in vivo (Table 3).
TABLE 2.
SPR analysis of the binding affinities of XGFR molecules to EGFR
| Binding to EGFR |
ka | kd | |
|---|---|---|---|
| Molecule | KD | ||
| nm | m−1 s−1 | s−1 | |
| XGFR | 4 | 8.20E + 04 | 5.80E − 04 |
| XGFR-DVD | 3 | 6.55E + 04 | 1.99E − 04 |
| XGFR2 | 6 | 4.98E + 04 | 3.11E − 04 |
| GA201 | 3 | 5.92E + 04 | 1.91E − 04 |
TABLE 3.
SPR analysis of the binding affinities of XGFR molecules to IGF-1R
| Binding to IGF-1R |
ka | kd | |
|---|---|---|---|
| Molecule | KD | ||
| nm | m−1 s−1 | s−1 | |
| XGFR | 4 | 7.90E + 05 | 3.30E − 03 |
| XGFR-DVD | No binding | ||
| XGFR2 | 6 | 6.79E + 04 | 3.78E − 04 |
| R1507 | 5 | 6.47E + 04 | 3.47E − 04 |
XGFR Bispecific Antibodies Maintain High Levels of IGF-1R Cell Surface Expression
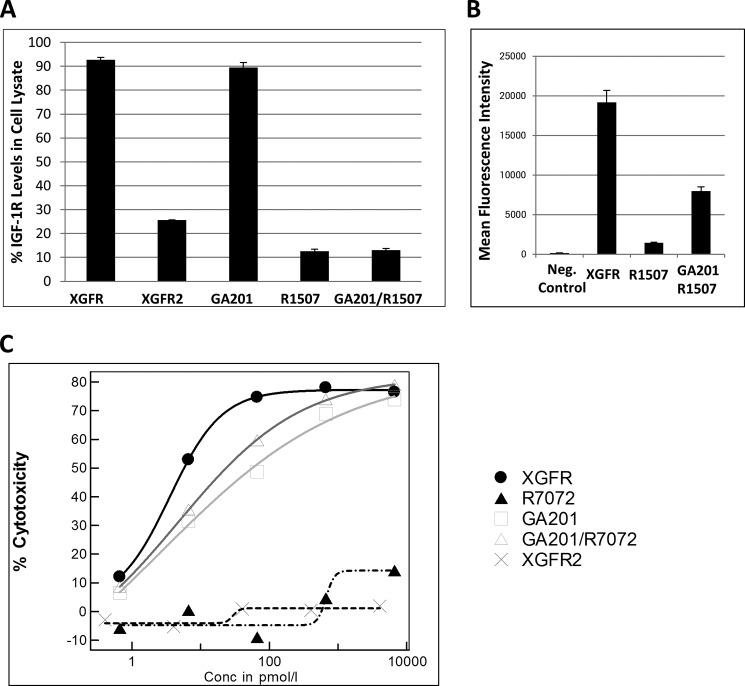
Previous studies have demonstrated that IGF-1R- and EGFR-targeting antibodies can reduce receptor cell surface expression by inducing internalization and subsequent degradation in the endosomal-lysosomal cell compartment. Whereas GA201 only induces minimal EGFR degradation in vitro (data not shown), the IGF-1R antibody R1507 induces strong receptor degradation of 87% in A549 non-small cell lung cancer cell lysates after 24 h (Fig. 3A). Because bispecific XGFR antibodies should simultaneously bind both receptors on the cell surface and may cross-link the two receptor tyrosine kinases, we next investigated whether binding by a bivalent or tetravalent bispecific antibody format affects total IGF-1R and EGFR levels in cell lysates. EGFR degradation following XGFR incubation was similar compared with the parental antibody GA201 (data not shown). IGF-1R levels in lysates of cells treated with XGFR were 92.7% of untreated controls, whereas treatment with the parental IGF-1R antibody R1507 or the combination of R1507 + GA201 induced receptor degradation to levels of 13.0% after 24 h (Fig. 3A). The tetravalent bispecific antibody XGFR2 induced IGF-1R internalization and degradation similar to R1507 (Fig. 3A). In agreement with these findings, high levels of XGFR on the cell surface were still detectable after 24-h incubation by FACS analysis (Fig. 3B). Incubation of H322M cells with the parental IGF-1R antibody in the same time period showed only small residual levels of antibody on the cell surface (Fig. 3B). In summary, a 13-fold increased antibody density of XGFR compared with R1507 and a 2.4-fold increased density toward the R1507/GA201 combination was observed on the cell surface of tumor cells after 24-h incubation. Because reduced receptor down-regulation and remaining antibody density on the cell surface may affect induction of effector cell-mediated functions, we next compared ADCC induction of the different bispecific antibody formats.
FIGURE 3.
Reduction in IGF-1R protein levels after antibody treatment on non-small cell lung cancer cells and induction of ADCC. A, reduction in total IGF-1R protein levels was analyzed by IGF-1R ELISA 24 h after incubation of A549 cells with 50 nm XGFR, XGFR2, GA201, R1507, and the R1507/GA201 combination and subsequent cell lysis. The means of triplicate experiments are shown. B, FACS analysis of XGFR, R1507, and R1507/GA201 on the surface of A549 cells after a 24-h incubation at 37 °C using an anti-human IgG1-phycoerythrin-labeled detection antibody. Acquisition of data was performed on a FACS Canto II, and analysis was performed by FlowJo software. C, ADCC induction of XGFR was determined with H322M tumor cells and NK92 effector cells. Cells were incubated at an effector/tumor cell ratio of 3:1 for 5 h at the indicated concentrations of XGFR, XGFR2, and the parental control antibodies R7072 and GA201 in triplicate in two or more independent experiments. The xCELLigence technology and software was used for data analysis. Error bars, S.D.
Antibody-dependent Cellular Cytotoxicity of the XGFR Bispecific Antibodies
ADCC activity relative to the afucosylated parental antibody R7072 (glycoengineered version of R1507) and GA201 combination was measured with H322M target cells and the NK92 effector cell line. XGFR showed a maximal killing efficiency of 75% with a highly potent IC50 of 7 pm and was slightly superior to the combination of the parental antibodies (Fig. 3C). The XGFR2 bispecific molecule exhibited significantly reduced ADCC activity. Here, the scFvs were fused to the C terminus of the heavy chain, which results in a loss of ADCC activity (Fig. 3C). Interestingly, ADCC activity correlated with receptor density on the cell surface; GA201, which induces modest receptor internalization, showed more potent ADCC activity than R7072 (Fig. 3C) with strong receptor down-modulation (Fig. 3A) despite a similar receptor density of IGF-1R and EGFR on H322M cells (Table 4). Increased density of XGFR on the cell surface (Fig. 3B) translated into slightly enhanced ADCC induction compared with the parental antibody combination GA201 and R7202. In conclusion, the XGFR OAscFab bispecific bivalent antibody format was optimized for ADCC induction and clearly superior to the tetravalent XGFR2 scFv antibody.
TABLE 4.
FACS quantification of EGF and IGF-1 receptor density on tumor cell lines
| Cell line | EGF receptors/cell | IGF-1 receptors/cell |
|---|---|---|
| AsPC-1 | 96,645 | 15,019 |
| HT-29 | 18,165 | 7287 |
| H322M | 62,480 | 41,188 |
| A549 | 39,695 | 43,205 |
Inhibition of EGFR and IGF-1R Phosphorylation by XGFR Bispecific Antibodies in Vitro
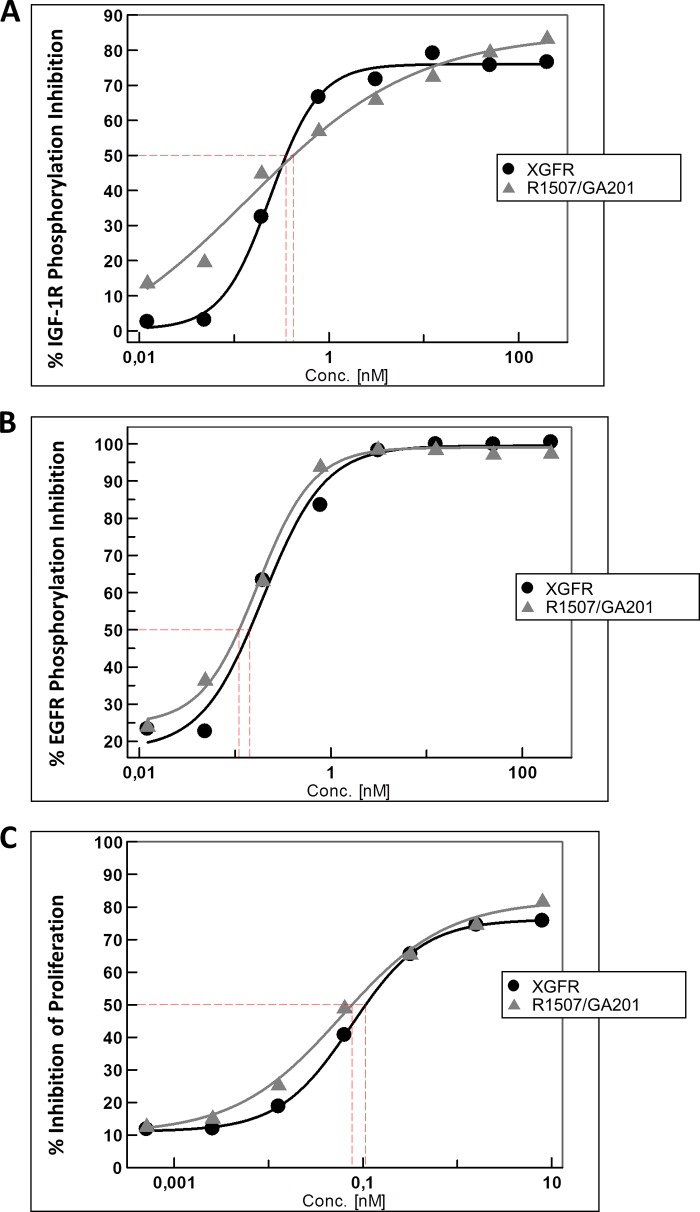
In order to evaluate the capacity of XGFR to inhibit IGF-1R and EGFR phosphorylation in comparison with the bivalent parental antibodies GA201 and R1507, we investigated the inhibition of EGFR and IGF-1R phosphorylation on A549 human NSCLC cells. The A549 cell line expresses high levels of both receptors on the cell surface (see Table 4). XGFR potently inhibited IGF-R phosphorylation with a maximum inhibition level of ∼80% and an IC50 value of 0.35 nm (Fig. 4A). The combination of the parental antibodies with an active R1507 component resulted in a comparable IC50 of 0.42 nm, indicating that IGF-1R phosphorylation inhibition by XGFR was similarly potent as the bivalent R1507 antibody despite being monovalent for the respective receptor. The bispecific tetravalent antibody XGFR2 also showed IGF-1R phosphorylation inhibition in a similar range compared with the parental antibody R1507, as we have published previously (6). Due to the lack of binding to IGF-1R, the XGFR-DVD bispecific antibody format was not tested for phosphorylation inhibition. EGFR phosphorylation was inhibited even more strongly by XGFR, with a maximal inhibition of 100% and an IC50 value of 0.23 nm (Fig. 4B). Here, the combination of the bivalent antibodies with an active GA201 antibody resulted in IC50 of 0.18 nm and was comparable with XGFR (Fig. 4B). As expected, XGFR2 induced phosphorylation inhibition in a similar manner as the parental GA201 in combination with R1507 (6). In summary, XGFR efficiently inhibits IGF-1R- and EGFR-dependent signaling in vitro and retains the potent signaling inhibition properties of the parental antibodies.
FIGURE 4.
Inhibition of EGFR and IGF-1R receptor phosphorylation and three-dimensional tumor cell proliferation in A549 cells. A549 NCSLC cells were incubated with XGFR or GA201/R1507 parental antibodies at concentrations between 200 and 0.0122 nm for 30 min in the presence of 5 nm IGF-1 or 10 nm EGF. Phosphorylation of IGF-1R (A) and EGFR (B) was analyzed after cell lysis by P-EGFR (Tyr) or P-IGF-1R (Tyr) beads combined with a phosphoprotein detection reagent. The data were recorded in triplicate by the Luminex detection system. C, inhibition of cell viability by XGFR at concentrations between 8 and 0.0005 nm in comparison with GA201/R1507 was determined in a three-dimensional cellular proliferation assay after 7 days of incubation.
Inhibition of Downstream Signaling Pathways by XGFR Bispecific Antibodies in Vitro
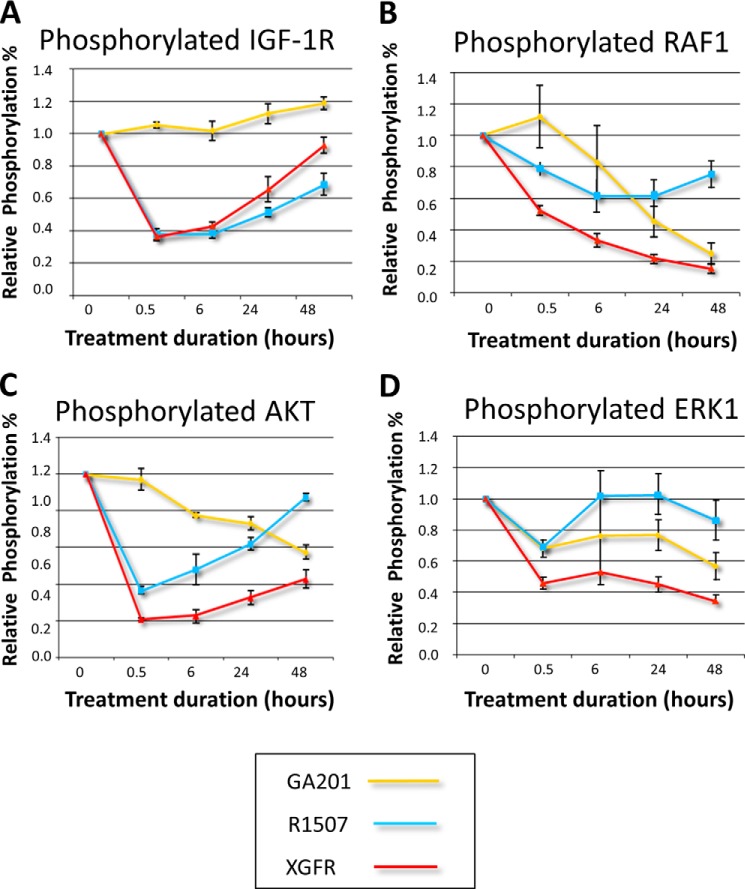
In order to evaluate the capacity of XGFR to inhibit IGF-1R- and EGFR-dependent signaling pathways, LS174T colorectal carcinoma cells were incubated with XGFR, R1507, or GA201, and levels of phosphorylated IGF-1R, phosphorylated RAF1, phosphorylated AKT, and phosphorylated ERK1 were analyzed. Whereas XGFR inhibited IGF-1R phosphorylation comparable with R1507 (Fig. 5A), inhibition of downstream signaling pathway components RAF1, AKT, and ERK1 by XGFR was superior to the individual parental antibody GA201 or R1507 (Fig. 5, B–D).
FIGURE 5.
Inhibition of EGFR and IGF-1R downstream signaling pathways by XGFR. LS174T colon carcinoma cells were incubated with XGFR, GA201, or R1507 parental antibodies at a concentration of 1000 nm in triplicates for 0.5, 6, 24, and 48 h. Levels of phosphorylated IGF-1R (A), RAF1 (B), AKT (C), and ERK1 (D) in total cell lysates were determined by the Luminex assay technology. Mean levels of relative phosphorylation in comparison with IgG-treated controls are depicted. Error bars, S.E.
Inhibition of Three-dimensional Tumor Cell Proliferation Mediated by XGFR
Inhibition of IGF-1R- and EGFR-dependent signaling by XGFR also translated into potent inhibition of tumor cell viability in a three-dimensional cell proliferation assay with NSCLC A459 tumor cells (Fig. 4C). XGFR inhibited A549 tumor cell growth by ∼80% with an IC50 value of 0.11 nm after 7 days of incubation. Tumor cell proliferation inhibition mediated by XGFR was comparable with the combination of the parental antibodies R1507 and GA201, which inhibited A549 spheroid proliferation with an IC50 value of 0.08 nm (Fig. 4C). The tumor cell proliferation inhibition of XGFR2 was evaluated with NSCLC H322M cells and resulted in potent tumor growth inhibition within a similar range as the parental antibody R1507 and GA201 combination. XGFR-DVD was not tested for tumor cell proliferation inhibition because we have shown previously that single agent GA201 tumor cell proliferation inhibition was less efficient than the combined inhibition of EGFR and IGF-1R signaling (6).
XGFR Inhibits Tumor Growth in Preclinical in Vivo Models
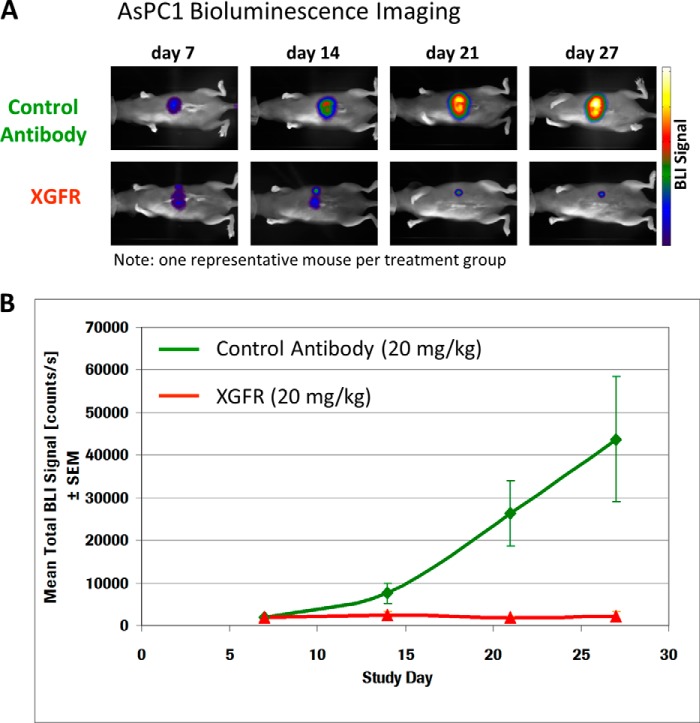
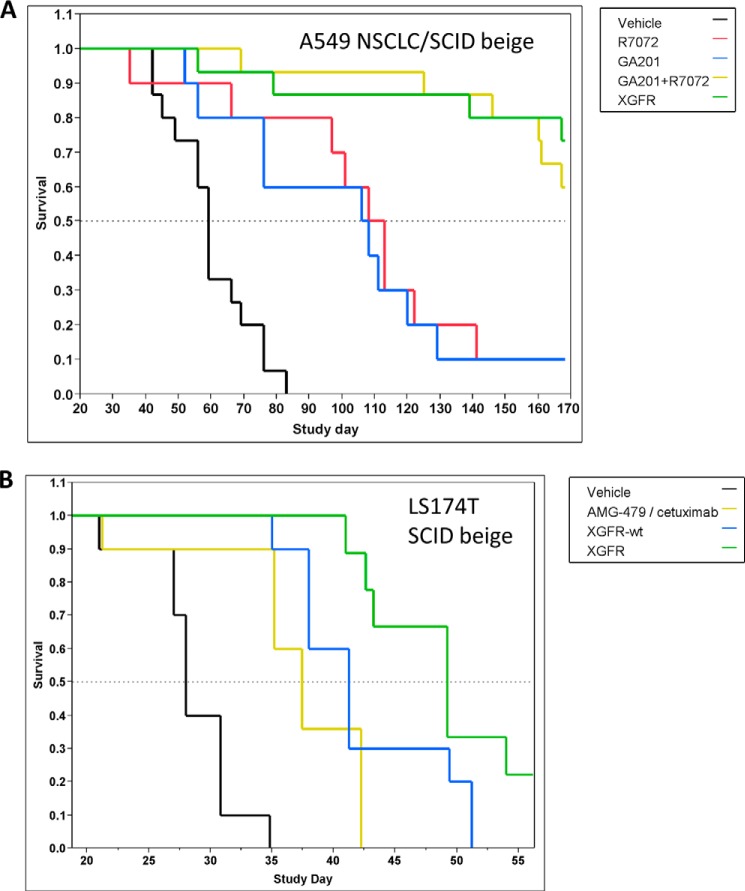
To confirm the efficacy of XGFR in vivo, its anti-tumor activity was evaluated in several tumor models in SCID beige mice. First, human pancreatic cancer AsPC-1 cells, which express high levels of EGFR and 6.4-fold lower levels of IGF-1R on the cell surface (Table 4), were transfected with the luciferase gene and orthotopically inoculated into the pancreas of female SCID beige mice. Mice were treated with intraperitoneal XGFR or control antibody (omalizumab) injections on days 7, 14, and 21 after tumor inoculation at a dose of 20 mg/kg. Orthotopic tumor growth of luciferase-positive AsPC-1 xenografts was assessed by bioluminescence imaging on study days 7, 14, 21, and 27. Whereas signal intensity strongly increased over time in control animals, luminescence signal in XGFR-treated animals remained low over the entire observation period, demonstrating potent anti-tumor activity of XGFR (Fig. 6, A and B). Moreover, human A549 NSCLC cells were injected in the tail vein of SCID beige mice, resulting in formation of orthotopic lung metastases. Treatment with weekly injections of the bispecific antibody XGFR at 20 mg/kg resulted in statistically significant increased survival compared with treatment with the single agent GA201 or glycoengineered R1507 (R7072) (p < 0.0001). XGFR treatment was as efficacious as the combination of GA201 and R7072, confirming the strong anti-tumor activity of XGFR (Fig. 7A). XGFR was also evaluated in the LS174T colon carcinoma metastasis model, where LS174T cells were inoculated in the spleen of SCID beige mice as described previously (17). Here, the in vivo effects of afucosylation of the Fc region were assessed by comparing XGFR with XGFR-wt (non-glycoengineered version of XGFR). In addition, a combination of the therapeutic EGFR antibody cetuximab with the IGF-1R antibody AMG-479 (resynthesized based on patent information) was evaluated. Treatment with each bispecific antibody at a weekly dose of 20 mg/kg resulted in significant improvement of survival compared with vehicle control (p = 0.000005 or 0.00001, respectively). Survival of animals treated with XGFR was significantly improved compared with XGFR-wt (p = 0.033) as well as the cetuximab/AMG-479 combination (p = 0.0005) in this model, indicating the benefit of a glycoengineered Fc region for the potent anti-tumor activity of the bispecific antibody (Fig. 7B).
FIGURE 6.
XGFR in vivo efficacy in human AsPC-1 luciferase pancreatic carcinoma mouse model. A, bioluminescence signal of a representative example derived from AsPC-1 luc cells implanted orthotopically into the pancreas of SCID mice on days 7, 14, 21, and 27 after tumor inoculation. Top, treatment with Omalizumab (control antibody); bottom, treatment with XGFR at 20 mg/kg, intraperitoneally once weekly. B, quantification of mean bioluminescence intensity (BLI) in n = 5 animals treated with Omalizumab (control antibody) or XGFR (20 mg/kg, intraperitoneally once weekly) at the indicated time points after orthotopic tumor cell inoculation. Error bars, S.E.
FIGURE 7.
XGFR in vivo efficacy in lung and colon carcinoma in vivo models. A, Kaplan-Meier plot of the indicated treatment groups (n = 10–15) in an orthotopic A549 NSCLC survival model in SCID beige mice. B, Kaplan-Meier plot of indicated treatment groups (n = 10) in an intrasplenic LS174T colon carcinoma survival model in SCID beige mice. Treatment start was at day 7 after tumor inoculation in both models with weekly intraperitoneal administration of vehicle or test compounds.
DISCUSSION
We have constructed a novel afucosylated heterodimeric one-arm scFab bispecific IgG1 antibody with enhanced ADCC activity that is capable of simultaneously binding IGF-1R and EGFR and inducing potent inhibition of receptor signaling as well as tumor cell proliferation. In the present study, we have performed a detailed analysis of protein expression, receptor binding and down-modulation, phosphorylation inhibition, ADCC activity, and in vivo efficacy in ADCC competent mouse tumor models.
The bispecific antibody XGFR uses a single chain Fab with a glycine serine linker on one arm to overcome the light chain mispairing issue in bispecific heterodimeric IgG antibodies and achieves heterodimerization of two antibody heavy chains with different binding specificities applying the knob-into-hole technology. The parental antibody GA201 (EGFR) was introduced into the bispecific antibody as knob heavy and light chain and the R1507 (IGF-1R) antibody as OAscFAb hole heavy chain. The introduction of an scFab arm allowed us to rapidly generate a bispecific antibody without the need for variable region engineering, such as disulfide stabilization, usually applied in tetravalent scFv (6, 37) antibody formats or time- and labor-intensive optimization by phage display in the “two-in-one” antibody technology (38, 39). The only modifications to the native structure of an IgG antibody were the introduction of the knob-into-hole mutations into the CH3 domain of each heavy chain (34) and the addition of a 32-amino acid glycine serine linker between the N terminus of the VH region and the C terminus of the Cκ domain in the IGF-1R binding arm. The knob-into-hole technology is currently being evaluated in phase II clinical trials in a one-arm 5D5 anti-cMet antibody (40) as well as in an Ang-2-VEGF CrossMAb in phase I clinical trials, and no negative effect on the safety profile of the engineered antibodies was reported (41). Moreover, the glycine serine recognition motif has not been identified for any of the known MHC alleles (42) and immunogenicity of a 15-amino acid glycine serine linker has been examined in the clinic for a bispecific T cell-engaging molecule with no adverse events being described (43), indicating that the glycine serine linker is currently the best available peptide linker to tether scFv or scFab molecules in the engineering of antibodies. The described OAscFab XGFR bispecific antibody showed no aggregation when analyzed by analytical SEC, in agreement with the results of Lee et al. (42), who published a monospecific scFab antibody targeting the tumor-associated glycoprotein (TAG)-72 and expressed this molecule in mammalian CHO cells in 1999. Interestingly, when Dübel and colleagues (31) characterized scFab fragments expressed in Escherichia coli, high amounts of multimers and dimers were found as final product, leading to the expression of monospecific single chain IgG molecules in HEK293T cells with the formation of large amounts of oligomers (32). Here, we have combined the scFab technology with knob-into-hole heterodimerization of two distinct heavy chains to generate a novel bispecific antibody with excellent production yields of 2–3 g/liter in CHO cells and low formation of multimers.
For comparison, we also generated XGFR2, a full-length IgG1 antibody composed of an IGF-1R master molecule with EGFR disulfide-stabilized scFv moieties attached to the CH3 domain by a (G4S)4 linker (6) and a DVD-Ig molecule based on an R1507 IGF-1R master antibody with GA201 VH and VL domains attached to the N terminus by a 5-amino acid linker as described previously (2). In this study, the OAscFab XGFR and the scFv-containing derivative XGFR2 showed transient expression levels that correlated with the parental antibodies; however, the DVD-Ig molecule showed significantly reduced expression levels in the HEK293 transient expression system. In addition, the XGFR-DVD molecule with its short 5-amino acid linker between the IGF-1R and EGFR variable domains completely lost the ability to bind IGF-1R, as demonstrated by SPR analysis. A similar observation was made for an EGFR/IGF-1R-targeting di-diabody with crossover scFvs joined by a short 5-amino acid (RTVAA) linker, where the molecule displayed lower binding efficiency to the target antigens compared with the monospecific counterparts (26). The OAscFab format clearly showed the advantage of converting an existing antibody in a bispecific molecule without loss of target antigen binding and evaluation of a linker optimization strategy. Notably, monovalent binding of target antigens EGFR and IGF-1R by XGFR resulted in highly potent inhibition of receptor phosphorylation and tumor cell proliferation comparable with the bivalent parental antibodies GA201 and R1507. Other IGFR/EGFR bispecific antibodies, such as the EGFR/IGF-1R di-diabody, showed a 25-fold reduced tumor cell proliferation inhibition in comparison with their parental antibody combination despite tetravalent binding of the target antigens, presumably due to a reduced binding affinity for EGFR (26). The most striking property of the OAscFab bispecific format was the significantly reduced IGF-1R internalization and subsequent degradation in tumor cells compared with the parental antibody R1507 or the tetravalent bispecific antibody XGFR2. Here, we have shown by FACS analysis that reduced IGF-1R down-modulation on tumor cells leads to an increased antibody density of XGFR on the tumor cell surface. Interestingly, the increased amounts of XGFR on the tumor cell surface translated into slightly enhanced ADCC induction compared with the EGFR-targeting antibody GA201 with low receptor internalization and strongly increased activity over the IGF-1R-targeting antibody R7202 with high receptor down-modulation. In conclusion, we report for the first time a glycoengineered bispecific antibody format optimized for ADCC induction with unique effects on IGF-1R down modulation. In addition, we demonstrate clear superiority of the OAscFab format compared with the tetravalent XGFR2 scFv antibody, which shows a loss of ADCC activity in vitro most likely due to steric hindrance of FcγIII receptor binding on effector cells by C-terminal attachment of scFvs on the C terminus of the CH3 domain of the Fc region (6). Despite the lack of internalization, the OAscFab XGFR showed potent IGF-1R and EGFR phosphorylation inhibition in vitro attributed to the inhibition of receptor dimerization and subsequent activation of the kinase domain. Inhibition of AKT, ERK1, and RAF1 as representative signaling components of IGF-1R/EGFR downstream signaling pathways was superior with XGFR compared with individual EGFR (GA201) or IGF-1R antibodies. Furthermore, XGFR retained the ability to inhibit the growth of tumor cells comparable with the parental antibodies, indicating that receptor internalization, at least in vitro, was not required or essential for tumor growth inhibition.
Potent in vivo efficacy of the bispecific OAscFab antibody XGFR was shown in several mouse xenograft models. XGFR treatment led to complete tumor growth arrest in the orthotopic human pancreatic carcinoma model AsPC-1-luc and an improvement of survival in the orthotopic lung carcinoma model A549 and the colon carcinoma metastasis model LS174T. In vivo efficacy of OAscFab XGFR was shown to be comparable with the parental antibody combination, confirming the full retention of anti-tumor activity of GA201 and R7072 in the A549 mouse xenograft model. The mouse model reflects the in vitro experimental data of XGFR, showing similar phosphorylation and tumor cell proliferation inhibition and slightly improved ADCC activity compared with the parental antibody combination. However, the combination of targeting both receptors in a single molecule is clearly more potent than dosing of an individual monospecific antibody in vivo. The contribution of glycoengineering to anti-tumor efficacy of XGFR in vivo was investigated in the LS174T colon carcinoma model in SCID beige mice. Anti-tumor activity of XGFR was improved over the non-glycoengineered antibody XGFR-wt or a combination of the therapeutic EGFR antibody cetuximab with the IGF-1R antibody AMG-479, showing the therapeutic potential of combining IGF-1R/EGFR signaling inhibition with potent ADCC induction in a bispecific antibody format. This unique feature provides XGFR an important advantage over other bispecific antibodies targeting EGFR and IGF-1R, such as a tetravalent bispecific antibody with IGF-1R scFvs fused to the Fc part of an EGFR antibody devoid of ADCC effector functions (4) or a fibronectin-based bispecific scaffold (30), which lack direct recruitment of immune cells through the Fcγ receptor. Furthermore, bispecific antibody design with enhanced ADCC induction may lead to an important improvement of cytotoxic activity for targeting tumors with mutated signaling pathways, such as K-Ras, where ADCC can aid in the killing of resistant tumor cells. Recently, we have shown that the glycoengineered EGFR antibody GA201 significantly enhanced in vitro induction of ADCC compared with cetuximab and in vivo efficacy in a series of mouse xenograft models (17). Generation of the bispecific antibody XGFR could even further increase the potent activity of GA201 against tumors that express EGFR and IGF-1R on the cell surface.
In summary, we have demonstrated that rational antibody design can help to overcome technical hurdles related to bispecific antibody design and generated a bispecific anti-IGF-1R/EGFR antibody XGFR, which induces more potent phosphorylation inhibition of downstream signaling mediators RAF-1, AKT, and ERK1 than the parental antibodies. In addition, IGF-1R internalization was reduced after XGFR treatment, leading to higher receptor levels at the cell surface and enhanced ADCC activity compared with the combination of GA201 and R1507. Antitumor activity of afucosylated XGFR was superior to individual anti-EGFR and anti-IGF-1R antibodies and combined therapeutic EGFR/IGF-1R antibody cetuximab/AMG-479 (resynthesized) treatment in various mouse tumor models. Therefore, XGFR may be a promising new therapeutic option for the treatment of pancreatic, pulmonary, or colorectal cancer.
Acknowledgments
We thank Ute Jucknischke, Sabine Bertl, Carsten Wolter, Petra Ulrich, Theresia Manger-Harasim, Michael Antony, Andreas Hinz, Silke Kirchner, Marion Lichtenauer, Ulrike Thomas, Alexandra Baumgartner, Barbara Threm, Sandra Bunte, Andrea Kleine, Esther Abraham, and Stefanie Lechner for excellent technical assistance.
This work was supported in part by a grant from the Bavarian Ministry for Science and Education.
- DVD
- dual variable domain
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- CE
- capillary electrophoresis
- poly-HEMA
- poly(2-hydroxyethyl methacrylate)
- EGFR
- epidermal growth factor receptor
- IGF-1R
- insulin-like growth factor receptor type I
- OAscFab
- one-arm single chain Fab
- scFab and scFv
- single chain Fab and Fv, respectively
- HEK
- human embryonic kidney
- SPR
- surface plasmon resonance
- SEC
- size exclusion chromatography.
REFERENCES
- 1. Topp M. S., Kufer P., Gökbuget N., Goebeler M., Klinger M., Neumann S., Horst H. A., Raff T., Viardot A., Schmid M., Stelljes M., Schaich M., Degenhard E., Köhne-Volland R., Brüggemann M., Ottmann O., Pfeifer H., Burmeister T., Nagorsen D., Schmidt M., Lutterbuese R., Reinhardt C., Baeuerle P. A., Kneba M., Einsele H., Riethmüller G., Hoelzer D., Zugmaier G., Bargou R. C. (2011) Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 [DOI] [PubMed] [Google Scholar]
- 2. Wu C., Ying H., Grinnell C., Bryant S., Miller R., Clabbers A., Bose S., McCarthy D., Zhu R. R., Santora L., Davis-Taber R., Kunes Y., Fung E., Schwartz A., Sakorafas P., Gu J., Tarcsa E., Murtaza A., Ghayur T. (2007) Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 25, 1290–1297 [DOI] [PubMed] [Google Scholar]
- 3. Digiammarino E. L., Harlan J. E., Walter K. A., Ladror U. S., Edalji R. P., Hutchins C. W., Lake M. R., Greischar A. J., Liu J., Ghayur T., Jakob C. G. (2011) Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs 3, 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong J., Sereno A., Snyder W. B., Miller B. R., Tamraz S., Doern A., Favis M., Wu X., Tran H., Langley E., Joseph I., Boccia A., Kelly R., Wortham K., Wang Q., Berquist L., Huang F., Gao S. X., Zhang Y., Lugovskoy A., Martin S., Gouvis H., Berkowitz S., Chiang G., Reff M., Glaser S. M., Hariharan K., Demarest S. J. (2011) Stable IgG-like bispecific antibodies directed toward the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J. Biol. Chem. 286, 4703–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michaelson J. S., Demarest S. J., Miller B., Amatucci A., Snyder W. B., Wu X., Huang F., Phan S., Gao S., Doern A., Farrington G. K., Lugovskoy A., Joseph I., Bailly V., Wang X., Garber E., Browning J., Glaser S. M. (2009) Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTβR. MAbs 1, 128–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Croasdale R., Wartha K., Schanzer J. M., Kuenkele K. P., Ries C., Mayer K., Gassner C., Wagner M., Dimoudis N., Herter S., Jaeger C., Ferrara C., Hoffmann E., Kling L., Lau W., Staack R. F., Heinrich J., Scheuer W., Stracke J., Gerdes C., Brinkmann U., Umana P., Klein C. (2012) Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Arch. Biochem. Biophys. 526, 206–218 [DOI] [PubMed] [Google Scholar]
- 7. Umaña P., Jean-Mairet J., Moudry R., Amstutz H., Bailey J. E. (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 [DOI] [PubMed] [Google Scholar]
- 8. Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., Umaña P., Benz J. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U.S.A. 108, 12669–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollak M. N., Perdue J. F., Margolese R. G., Baer K., Richard M. (1987) Presence of somatomedin receptors on primary human breast and colon carcinomas. Cancer Lett. 38, 223–230 [DOI] [PubMed] [Google Scholar]
- 10. Baselga J., Mendelsohn J. (1994) The epidermal growth factor receptor as a target for therapy in breast carcinoma. Breast Cancer Res. Treat. 29, 127–138 [DOI] [PubMed] [Google Scholar]
- 11. Ludovini V., Bellezza G., Pistola L., Bianconi F., Di Carlo L., Sidoni A., Semeraro A., Del Sordo R., Tofanetti F. R., Mameli M. G., Daddi G., Cavaliere A., Tonato M., Crinò L. (2009) High coexpression of both insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth factor receptor (EGFR) is associated with shorter disease-free survival in resected non-small-cell lung cancer patients. Ann. Oncol. 20, 842–849 [DOI] [PubMed] [Google Scholar]
- 12. Bowers G., Reardon D., Hewitt T., Dent P., Mikkelsen R. B., Valerie K., Lammering G., Amir C., Schmidt-Ullrich R. K. (2001) The relative role of ErbB1–4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cells. Oncogene 20, 1388–1397 [DOI] [PubMed] [Google Scholar]
- 13. Ueda S., Tsuda H., Sato K., Takeuchi H., Shigekawa T., Matsubara O., Hiraide H., Mochizuki H. (2006) Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1. Cancer Sci. 97, 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pollak M. (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8, 915–928 [DOI] [PubMed] [Google Scholar]
- 15. Ciardiello F., Tortora G. (2002) Anti-epidermal growth factor receptor drugs in cancer therapy. Expert Opin. Investig. Drugs 11, 755–768 [DOI] [PubMed] [Google Scholar]
- 16. Vecchione L., Vecchione L., Jacobs B., Jacobs B., Normanno N., Normanno N., Ciardiello F., Ciardiello F., Tejpar S., Tejpar S. (2011) EGFR-targeted therapy. Exp. Cell Res. 317, 2765–2771 [DOI] [PubMed] [Google Scholar]
- 17. Gerdes C. A., Nicolini V. G., Herter S., van Puijenbroek E., Lang S., Roemmele M., Moessner E., Freytag O., Friess T., Ries C. H., Bossenmaier B., Mueller H. J., Umaña P. (2013) GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin. Cancer Res. 19, 1126–1138 [DOI] [PubMed] [Google Scholar]
- 18. Paz-Ares L. G., Gomez-Roca C., Delord J. P., Cervantes A., Markman B., Corral J., Soria J. C., Bergé Y., Roda D., Russell-Yarde F., Hollingsworth S., Baselga J., Umana P., Manenti L., Tabernero J. (2011) Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J. Clin. Oncol. 29, 3783–3790 [DOI] [PubMed] [Google Scholar]
- 19. Pappo A. S., Patel S. R., Crowley J., Reinke D. K., Kuenkele K. P., Chawla S. P., Toner G. C., Maki R. G., Meyers P. A., Chugh R., Ganjoo K. N., Schuetze S. M., Juergens H., Leahy M. G., Geoerger B., Benjamin R. S., Helman L. J., Baker L. H. (2011) R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J. Clin. Oncol. 29, 4541–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramalingam S. S., Spigel D. R., Chen D., Steins M. B., Engelman J. A., Schneider C. P., Novello S., Eberhardt W. E., Crino L., Habben K., Liu L., Jänne P. A., Brownstein C. M., Reck M. (2011) Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 29, 4574–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosen L. S., Puzanov I., Friberg G., Chan E., Hwang Y. C., Deng H., Gilbert J., Mahalingam D., McCaffery I., Michael S. A., Mita A. C., Mita M. M., Mulay M., Shubhakar P., Zhu M., Sarantopoulos J. (2012) Safety and pharmacokinetics of ganitumab (AMG 479) combined with sorafenib, panitumumab, erlotinib, or gemcitabine in patients with advanced solid tumors. Clin. Cancer Res. 18, 3414–3427 [DOI] [PubMed] [Google Scholar]
- 22. Weickhardt A., Doebele R., Oton A., Lettieri J., Maxson D., Reynolds M., Brown A., Jackson M. K., Dy G., Adjei A., Fetterly G., Lu X., Franklin W., Varella-Garcia M., Hirsch F. R., Wynes M. W., Youssoufian H., Adjei A., Camidge D. R. (2012) A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 7, 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chakravarti A., Loeffler J. S., Dyson N. J. (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 62, 200–207 [PubMed] [Google Scholar]
- 24. Jones H. E., Goddard L., Gee J. M., Hiscox S., Rubini M., Barrow D., Knowlden J. M., Williams S., Wakeling A. E., Nicholson R. I. (2004) Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839, Iressa) in human breast and prostate cancer cells. Endocr. Relat. Cancer 11, 793–814 [DOI] [PubMed] [Google Scholar]
- 25. Goetsch L., Gonzalez A., Leger O., Beck A., Pauwels P. J., Haeuw J. F., Corvaia N. (2005) A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int. J. Cancer 113, 316–328 [DOI] [PubMed] [Google Scholar]
- 26. Lu D., Zhang H., Koo H., Tonra J., Balderes P., Prewett M., Corcoran E., Mangalampalli V., Bassi R., Anselma D., Patel D., Kang X., Ludwig D. L., Hicklin D. J., Bohlen P., Witte L., Zhu Z. (2005) A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J. Biol. Chem. 280, 19665–19672 [DOI] [PubMed] [Google Scholar]
- 27. Lu D., Zhang H., Ludwig D., Persaud A., Jimenez X., Burtrum D., Balderes P., Liu M., Bohlen P., Witte L., Zhu Z. (2004) Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J. Biol. Chem. 279, 2856–2865 [DOI] [PubMed] [Google Scholar]
- 28. Haluska P., Carboni J. M., TenEyck C., Attar R. M., Hou X., Yu C., Sagar M., Wong T. W., Gottardis M. M., Erlichman C. (2008) HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol. Cancer Ther. 7, 2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scartozzi M., Mandolesi A., Giampieri R., Pierantoni C., Loupakis F., Zaniboni A., Galizia E., Giustini L., Silva R. R., Bisonni R., Berardi R., Biagetti S., Menzo S., Falcone A., Bearzi I., Cascinu S. (2010) Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. Int. J. Cancer 127, 1941–1947 [DOI] [PubMed] [Google Scholar]
- 30. Emanuel S. L., Engle L. J., Chao G., Zhu R. R., Cao C., Lin Z., Yamniuk A. P., Hosbach J., Brown J., Fitzpatrick E., Gokemeijer J., Morin P., Morse B. A., Carvajal I. M., Fabrizio D., Wright M. C., Das Gupta R., Gosselin M., Cataldo D., Ryseck R. P., Doyle M. L., Wong T. W., Camphausen R. T., Cload S. T., Marsh H. N., Gottardis M. M., Furfine E. S. (2011) A fibronectin scaffold approach to bispecific inhibitors of epidermal growth factor receptor and insulin-like growth factor-I receptor. MAbs 3, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hust M., Jostock T., Menzel C., Voedisch B., Mohr A., Brenneis M., Kirsch M. I., Meier D., Dübel S. (2007) Single chain Fab (scFab) fragment. BMC. Biotechnol. 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schirrmann T., Menzel C., Hust M., Prilop J., Jostock T., Dübel S. (2010) Oligomeric forms of single chain immunoglobulin (scIgG). MAbs 2, 73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kontermann R. E., Wing M. G., Winter G. (1997) Complement recruitment using bispecific diabodies. Nat. Biotechnol. 15, 629–631 [DOI] [PubMed] [Google Scholar]
- 34. Ridgway J. B., Presta L. G., Carter P. (1996) “Knobs-into-holes” engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 9, 617–621 [DOI] [PubMed] [Google Scholar]
- 35. Klein C., Sustmann C., Thomas M., Stubenrauch K., Croasdale R., Schanzer J., Brinkmann U., Kettenberger H., Regula J. T., Schaefer W. (2012) Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 4, 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reiter Y., Brinkmann U., Kreitman R. J., Jung S. H., Lee B., Pastan I. (1994) Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry 33, 5451–5459 [DOI] [PubMed] [Google Scholar]
- 37. Reiter Y., Brinkmann U., Webber K. O., Jung S. H., Lee B., Pastan I. (1994) Engineering interchain disulfide bonds into conserved framework regions of Fv fragments: improved biochemical characteristics of recombinant immunotoxins containing disulfide-stabilized Fv. Protein Eng. 7, 697–704 [DOI] [PubMed] [Google Scholar]
- 38. Schaefer G., Haber L., Crocker L. M., Shia S., Shao L., Dowbenko D., Totpal K., Wong A., Lee C. V., Stawicki S., Clark R., Fields C., Lewis Phillips G. D., Prell R. A., Danilenko D. M., Franke Y., Stephan J. P., Hwang J., Wu Y., Bostrom J., Sliwkowski M. X., Fuh G., Eigenbrot C. (2011) A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 20, 472–486 [DOI] [PubMed] [Google Scholar]
- 39. Bostrom J., Haber L., Koenig P., Kelley R. F., Fuh G. (2011) High affinity antigen recognition of the dual specific variants of herceptin is entropy-driven in spite of structural plasticity. PLoS One 6, e17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Surati M., Patel P., Peterson A., Salgia R. (2011) Role of MetMAb (OA-5D5) in c-MET active lung malignancies. Exp. Opin. Biol. Ther. 11, 1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schaefer W., Regula J. T., Bähner M., Schanzer J., Croasdale R., Dürr H., Gassner C., Georges G., Kettenberger H., Imhof-Jung S., Schwaiger M., Stubenrauch K. G., Sustmann C., Thomas M., Scheuer W., Klein C. (2011) Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl. Acad. Sci. U.S.A. 108, 11187–11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee H. S., Shu L., De Pascalis R., Giuliano M., Zhu M., Padlan E. A., Hand P. H., Schlom J., Hong H. J., Kashmiri S. V. (1999) Generation and characterization of a novel single-gene-encoded single-chain immunoglobulin molecule with antigen binding activity and effector functions. Mol. Immunol. 36, 61–71 [DOI] [PubMed] [Google Scholar]
- 43. Nagorsen D., Kufer P., Baeuerle P. A., Bargou R. (2012) Blinatumomab: a historical perspective. Pharmacol. Ther. 136, 334–342 [DOI] [PubMed] [Google Scholar]