Background: Two major NADPH dehydrogenase complexes, NDH-1L and NDH-1M, have been identified in cyanobacteria.
Results: NdhP is localized in the NDH-1L complex, and absence of this subunit or its C-terminal tail disassembled NDH-1L to NDH-1M.
Conclusion: C terminus of NdhP is essential to stabilize the NDH-1L complex.
Significance: Our results provide novel insights into the assembly and stabilization of NDH-1L complex.
Keywords: Cyanobacteria, Electron Transfer Complex, Membrane Enzyme, Mutant, Photosynthesis, NDH-1L Complex, NDH-CET, NdhP, Synechocystis 6803, Stabilization
Abstract
Two major complexes of NADPH dehydrogenase (NDH-1) have been identified in cyanobacteria. A large complex (NDH-1L) contains NdhD1 and NdhF1, which are absent in a medium size complex (NDH-1M). They play important roles in respiration, cyclic electron transport around photosystem I, and CO2 acquisition. Two mutants sensitive to high light for growth and impaired in NDH-1-mediated cyclic electron transfer were isolated from Synechocystis sp. strain PCC 6803 transformed with a transposon-bearing library. Both mutants had a tag in sml0013 encoding NdhP, a single transmembrane small subunit of the NDH-1 complex. During prolonged incubation of the wild type thylakoid membrane with n-dodecyl β-d-maltoside (DM), about half of the NDH-1L was disassembled to NDH-1M and the rest decomposed completely without forming NDH-1M. In the ndhP deletion mutant (ΔndhP), disassembling of NDH-1L to NDH-1M occurred even on ice, and decomposition to a small piece occurred at room temperature much faster than in the wild type. Deletion of the C-terminal tail of NdhP gave the same result. The C terminus of NdhP was tagged by YFP-His6. Blue native gel electrophoresis of the DM-treated thylakoid membrane of this strain and Western analysis using the antibody against GFP revealed that NdhP-YFP-His6 was exclusively confined to NDH-1L. During prolonged incubation of the thylakoid membrane of the tagged strain with DM at room temperature, NDH-1L was partially disassembled to NDH-1M and the 160-kDa band containing NdhP-YFP-His6 and possibly NdhD1 and NdhF1. We therefore conclude that NdhP, especially its C-terminal tail, is essential to assemble NdhD1 and NdhF1 and stabilize the NDH-1L complex.
Introduction
Cyanobacterial NADPH dehydrogenase (NDH-1)3 complexes are localized in the thylakoid membrane (1–5) and participate in a variety of bioenergetic reactions, such as respiration, cyclic electron transport around photosystem I, and CO2 acquisition (6–8). Structurally, the cyanobacterial NDH-1 complexes closely resemble energy-converting complex I in eubacteria and the mitochondrial respiratory chain regardless of the absence of homologues of three subunits in cyanobacterial genomes that constitute the catalytically active core of complex I (9–11). Over the past few years, significant achievements have been made in resolving the subunit compositions and functions of the multiple NDH-1 complexes in several cyanobacterial strains (12–15). Four types of NDH-1 have been identified in the cyanobacterium Synechocystis sp. strain PCC 6803 (hereafter Synechocystis 6803), and all four types are involved in NDH-1-dependent cyclic electron transport around photosystem I (NDH-CET) (16). The NDH-CET allows optimal functioning of photosynthesis by increasing the pH gradient and supplying extra ATP for CO2 assimilation. This function would be particularly important under environmental stress conditions, such as high light (5, 17, 18), in which the ATP demand is greatly increased. Moreover, the impairment of cyanobacterial NDH-CET caused by mutation of Ndh subunits would result in high light-sensitive growth phenotypes. Therefore, high light strategy can help in identifying the proteins essential to NDH-CET.
Proteomics studies revealed the presence of two major NDH-1 complexes in cyanobacteria: a large complex (NDH-1L), and a medium size complex (NDH-1M) with molecular masses of about 460 and 350 kDa, respectively (19). NDH-1M consists of 16 subunits, i.e. those constituting a membrane-embedded arm (NdhA to NdhC, NdhE, NdhG, NdhL, NdhP, and NdhQ) and a hydrophilic connecting domain (NdhH to NdhK, NdhM to NdhO, and NdhS). In addition to these subunits, NDH-1L complex contains NdhD1 and NdhF1 (15, 20–22). NDH-1S is another complex of about 200 kDa composed of NdhD3, NdhF3, CupA, and CupS (13). This complex is considered to be associated with NDH-1M in the cells as a functional complex NDH-1MS (3, 22) participating in CO2 uptake and is easily dissociated into NDH-1M and NDH-1S during solubilization of the membranes with detergent (12–15). Among the several copies of ndhD and ndhF genes found in cyanobacterial genomes, ndhD1 and ndhF1 show the highest homology to chloroplast ndhD and ndhF genes, respectively, and CupA and CupS subunits of the cyanobacteria have no counterparts in higher plants.
Recently, a new oxygen photosynthesis-specific small subunit NdhP was identified in Thermosynechococcus elongatus (23). Deletion of ndhP in Synechocystis 6803 led to the cells unable to grow under photoheterotrophic conditions (24). It was suggested that NdhP is involved in the respiratory and cyclic electron flows, but the role of this subunit is not known. We demonstrate in this study that NdhP is exclusively confined to the NDH-1L complex and absence of its C-terminal tail destabilizes the complex, thereby impairing respiration and NDH-CET activities. A possible role of the C terminus of NdhP in stabilizing the NDH-1L complex is discussed.
EXPERIMENTAL PROCEDURES
Culture Conditions
Glucose-tolerant strain of wild type (WT) Synechocystis 6803 and its mutants, ΔndhP, ndhPΔC, and ΔndhB (M55) (6) and WT-NdhP-YFP-His6, ΔndhS-NdhP-YFP-His6, ΔndhD1/D2 (ΔD1/D2)-NdhP-YFP-His6, and M55-NdhP-YFP-His6 were cultured at 30 °C in BG-11 medium (25) buffered with Tris-HCl (5 mm, pH 8.0) and bubbled with 2% (v/v) CO2 in air. Solid medium was BG-11 supplemented with 1.5% agar. The mutant strains were grown in the presence of appropriate antibiotics under illumination by fluorescence lamps at 40 μE m−2 s−1.
Isolation and Construction of Mutants
A cosmid library of Synechocystis 6803 genome was constructed. The library that contained 105 clones with inserts of 35–38.5 kb was subjected to in vitro transposon mutagenesis using EZ-Tn5TM 〈KAN-2〉 insertion kit (Epicenter Biotechnologies, Madison, WI) and was then used to transform the WT cells of Synechocystis 6803. Following transformation, cells were spread on 1.5% BG-11 agar plates (5 μg of kanamycin ml−1), and KamR mutants that grew slowly under high light but normally under growth light were isolated. Genomic DNA isolated from each mutant was digested with HhaI, and after self-ligation, it was used as a template for inverse PCR with primers (supplemental Table 1) complementary to the N- and C-terminal regions of the KamR cassette. The exact position of the cassette in the mutant genome was determined by sequencing the PCR product.
ΔndhP and ndhPΔC mutants were constructed as follows: (i) The upstream and downstream regions of sml0013 (ndhP) were amplified by PCR creating appropriate restriction sites. A DNA fragment encoding a kanamycin resistance (KamR) cassette was also amplified by PCR creating XbaI and KpnI sites using PCR primers, ndhP-C and -D (supplemental Table 1). These three PCR products were ligated into the MCS of pUC19 (Fig. 2A) and was used to transform the WT cells to generate the ΔndhP mutant. (ii) A fragment that contains sml0013 (ndhP), except the C-terminal region (Lys-24 to His-40, see Fig. 5) and its upstream region, was amplified by PCR creating PstI and XbaI sites and was ligated to MCS of pUC19 together with the two PCR products obtained in (i). The plasmid thus constructed was used to transform the WT cells to create the ndhP C-terminal deletion mutant, ndhPΔC (Fig. 2A). The transformants were spread on agar plates containing BG-11 medium and kanamycin (10 μg ml−1) buffered at pH 8.0, and the plates were incubated in 2% (v/v) CO2 in air under illumination by fluorescent lamps at 40 μE m−2 s−1. The mutated ndhP and its C-terminal tail in the transformants were segregated to homogeneity (by successive-streak purification) as determined by PCR amplification and RT-PCR analysis (Fig. 2, B and C).
FIGURE 2.
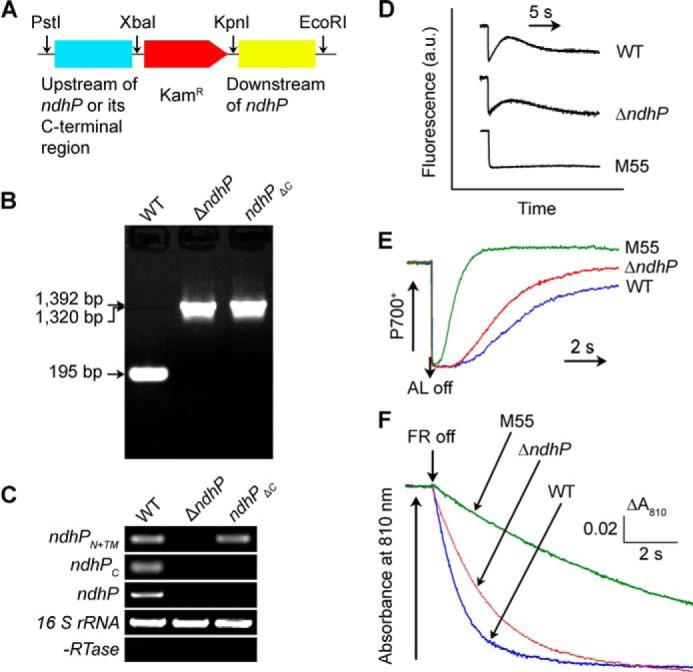
Construction of ndhP deletion and C-terminal deletion mutants. A, construction of plasmid used to generate the ndhP deletion mutant (ΔndhP) and the C-terminal deletion mutant (ndhPΔC). B, PCR segregation analysis of the ΔndhP and ndhPΔC mutants using the ndhP-G and ndhP-H primers (supplemental Table 1). C, transcript levels of ndhPN+TM, ndhPC, and ndhP in the WT, ndhPΔC, and ΔndhP strains. The transcript level of 16 S rRNA in each sample is shown as a control. The absence of contamination of DNA was confirmed by PCR without reverse transcriptase reaction. D, monitoring of NDH-CET activity by chlorophyll fluorescence. E, redox kinetics of P700 after termination of AL illumination (800 μE m−2 s−1 for 30 s) under a background of FR. F, kinetics of the P700+ re-reduction in darkness after turning off FR in the presence of 10 μm DCMU. The chlorophyll a concentration was adjusted to 20 μg/ml before measurement, and curves are normalized to the maximal signal.
FIGURE 5.
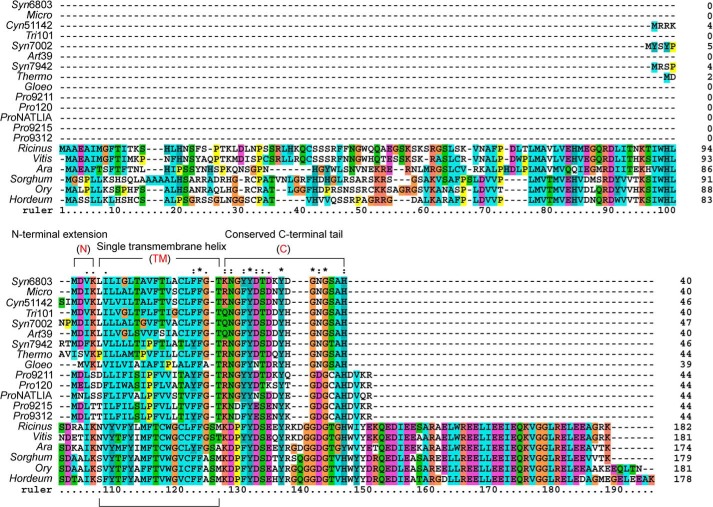
Sequence alignment of NdhP of Synechocystis 6803 and its homologues from other species. The sequence of the NdhP from Synechocystis sp. PCC 6803 (Syn6803) was compared with homologous sequences from M. aeruginosa NIES-843 (Micro); Cyanothece sp. ATCC 51142 (Cyn51142); T. erythraeum IMS101 (Tri101); Synechococcus sp. PCC 7002 (Syn7002); A. platensis NIES-39 (Art39); S. elongatus PCC 7942 (Syn7942); T. elongatus BP-1 (Thermo); G. violaceus PCC 7421 (Gloeo); P. marinus MIT 9211 (Pro9211); P. marinus SS120 (Pro120); P. marinus NATLIA (ProNATLIA); P. marinus MIT 9215 (Pro9215); P. marinus MIT 9312 (Pro9312); R. communis (Ricinus); V. vinifera (Vitis); A. thaliana (Ara); S. bicolor (Sorghum), O. sativa (Ory). and H. vulgare (Hordeum). Membrane domain analysis was performed by the TMHMM software, and N-terminal extension (N), single transmembrane helix (TM), and C-terminal tail (C) were signed, respectively. The sequences were aligned using ClustalX 1.83. Asterisks indicate identical amino acids; colons and dots indicate conserved amino acid substitutions.
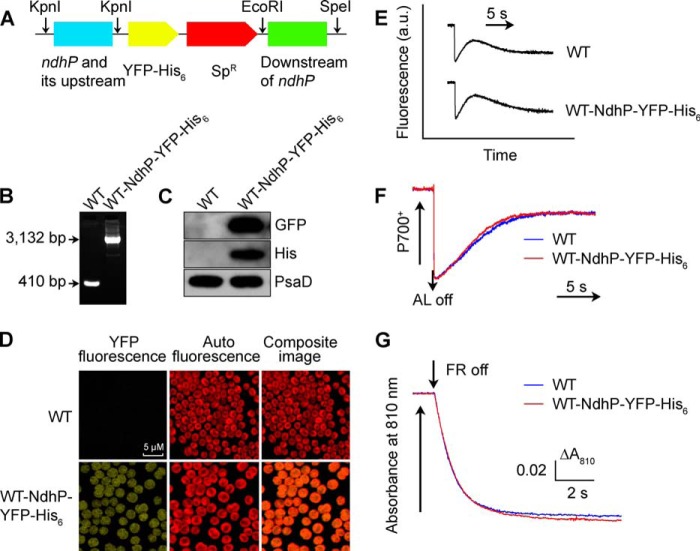
A DNA fragment containing ndhP and its upstream region was amplified by PCR creating a KpnI site on both ends and was ligated to the KpnI site in MCS of the pEYFP-His6-SpR plasmid (26). A fragment containing the downstream region of ndhP was also amplified by PCR creating EcoRI and SpeI sites and was ligated to the downstream of the SpR gene (Fig. 6A). The vector thus constructed was used to transform the WT, ΔndhS, ΔD1/D2, and M55 cells of Synechocystis 6803 to generate the WT-NdhP-YFP-His6, ΔndhS-NdhP-YFP-His6, ΔD1/D2-NdhP-YFP-His6, and M55-NdhP-YFP-His6 mutant strains. The transformation was performed as described previously (27, 28). The yfp and his6 region in the transformants was segregated to homogeneity (by successive-streak purification) as determined by PCR amplification (Figs. 6B and 7D).
FIGURE 6.
Construction and characterization of WT-NdhP-YFP-His6 strain. A, construction of plasmid to generate WT-NdhP-YFP-His6 mutant. A DNA fragment containing ndhP and its upstream region amplified by PCR was ligated to the KpnI site of the pEYFP-His6-SpR plasmid, and a fragment downstream of ndhP was ligated between EcoRI and SpeI sites. The plasmid thus constructed was used to transform WT cells to generate the tagged mutant. B, PCR segregation analysis of the WT-NdhP-YFP-His6 mutant using the ndhP-yfp-his6-E and -F primer sequences (supplemental Table 1). C, Western analysis of proteins from the WT and WT-NdhP-YFP-His6 strains using GFP and His antibodies. Total protein corresponding to 1 μg of chlorophyll a was loaded onto each lane, and PsaD was detected as a loading control. D, confocal microscopy analysis of WT and WT-NdhP-YFP-His6 cells. The scale bar indicates 5 μm. E, monitoring of NDH-CET activity by chlorophyll fluorescence. F, redox kinetics of P700 after termination of AL illumination (800 μE m−2 s−1 for 30 s) under a background of FR. G, kinetics of P700+ re-reduction in darkness after turning off FR with a maximum at 720 nm in the presence of 10 μm DCMU. The chlorophyll a concentration was adjusted to 20 μg/ml before measurement, and curves are normalized to the maximal signal. See “Experimental Procedures” for details.
FIGURE 7.
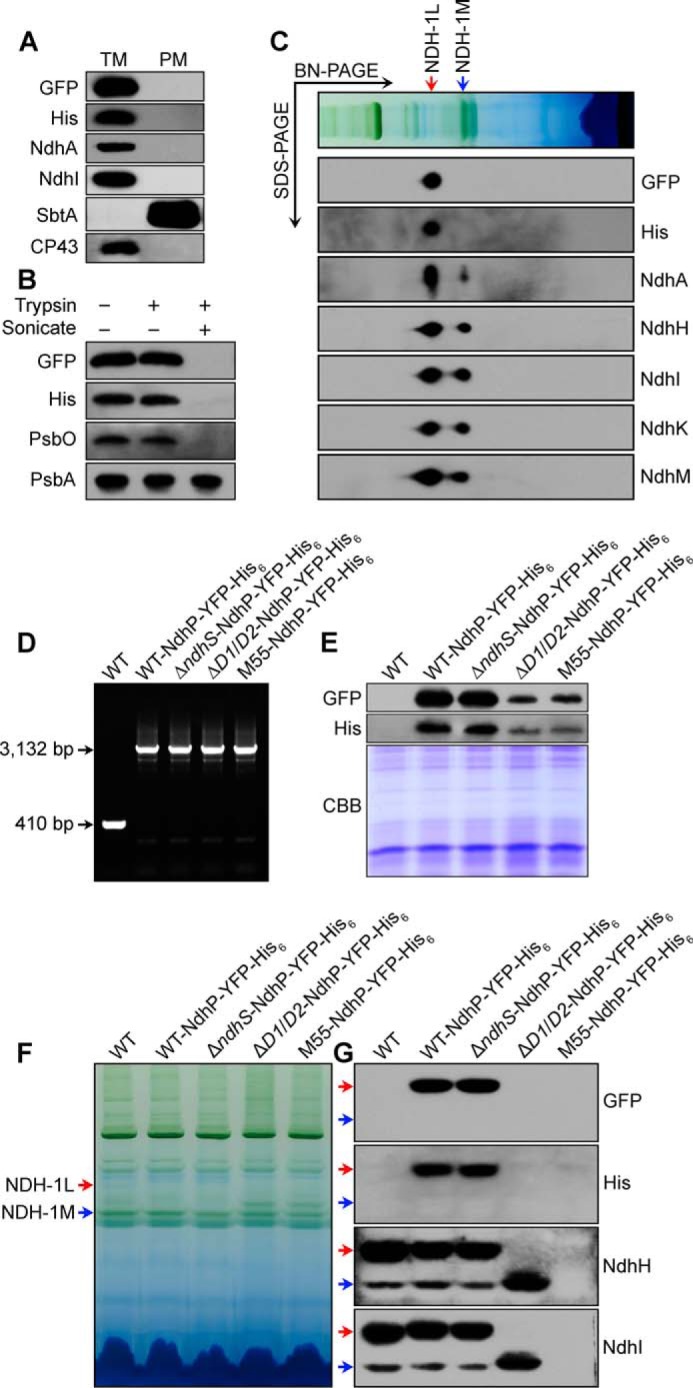
Localization and topology of NdhP. A, Western analyses of the purified thylakoid membrane (TM) and plasma membrane (PM); B, of crude thylakoid membrane before and after treatment with trypsin with or without sonication; and C, of NDH-1L and NDH-1M complexes after separation by two-dimensional gel electrophoresis (BN-PAGE and SDS-PAGE) of DM-solubilized thylakoid membranes. NdhP-YFP-His6 strain was used in these experiments, and Ndh subunits were probed with specific antibodies against GFP, His, NdhA, NdhH, NdhI, NdhK, and NdhM. Antibodies against CP43 and SbtA were used to check the purity of the TM and PM fractions, respectively. PsbO and PsbA were served as controls for the thylakoid lumenal protein and the intrinsic thylakoid membrane protein, respectively. Identification of NdhP-YFP-His6 in various ndh mutants at DNA (D) and protein (E) levels. D, PCR segregation analysis of NdhP-YFP-His6 in WT, ΔndhS, ΔndhD1/D2 (ΔD1/D2), and M55 having NdhP-YFP-His6 using the ndhP-yfp-his6-E and -F primer sequences (supplemental Table 1). E, Western analysis of total proteins of WT and WT, ΔndhS, ΔD1/D2, and M55 strains having NdhP-YFP-His6. Total proteins corresponding to 1 μg of chlorophyll a were loaded onto each lane, and profiles of Coomassie Brilliant Blue (CBB)-stained gel are shown at the bottom of the panel. F, profiles of BN-PAGE of the thylakoid membranes; G, Western analysis of NDH-1L and NDH-1M complexes. WT, ΔndhS, ΔD1/D2, and M55 in which NdhP is tagged with YFP-His6, together with the untagged WT, were used in these experiments. Sample containing 9 μg of chlorophyll a was loaded onto each lane. Red and blue arrows indicate positions of NDH-1L and NDH-1M complexes, respectively.
RNA Extraction and RT-PCR Analysis
Total RNA was isolated and analyzed as described previously (29). RT-PCR was performed using the Access RT-PCR system (Promega) to generate products corresponding to ndhP, ndhPN+TM, ndhPC, and 16 S rRNA, with 0.5 μg of DNase-treated total RNA as starting material. RT-PCR conditions were 95 °C for 5 min followed by cycles of 95, 62, and 72 °C for 30 s each. The reactions were stopped after 25 cycles for 16 S rRNA, and after 35 cycles for ndhP, ndhPN+TM and ndhPC. The primers used are summarized in supplemental Table 1.
Chlorophyll Fluorescence and P700 Analysis
The transient increase in chlorophyll fluorescence after actinic light had been turned off was monitored as described (30). The redox kinetics of P700 was measured according to previously described methods (5, 18). The re-reduction of P700+ in darkness was measured with a Dual-PAM-100 (Walz, Effeltrich, Germany) with an emitter-detector unit ED-101US/MD by monitoring absorbance changes at 830 nm and using 875 nm as a reference. Cells were kept in the dark for 2 min, and 10 μm of DCMU was added to the cultures prior to the measurement. The P700 was oxidized by far-red light with a maximum at 720 nm from LED lamp for 30 s, and the subsequent re-reduction of P700+ in the dark was monitored.
Isolation of Crude Thylakoid Membranes and Total Membranes
The cell cultures (5 liters) were harvested at the logarithmic phase (A730 = 0.6–0.8) and washed twice with 50 ml of fresh BG-11 medium and then thylakoid membranes were isolated according to Gombos et al. (31) with some modifications as follows. Cells were suspended in 5 ml of disruption buffer (10 mm HEPES-NaOH, 5 mm sodium phosphate, pH 7.5, 10 mm MgCl2, 10 mm NaCl, and 25% glycerol (v/v)) and, after adding zirconia/silica beads, broken by vortexing 15 times at the highest speed for 20 s at 4 °C with 5 min cooling on ice between the runs. The crude extract was centrifuged at 5,000 × g for 5 min to remove the glass beads and unbroken cells. The supernatant was centrifuged at 20,000 × g for 30 min to precipitate crude thylakoid membranes or at 150,000 × g for 40 min to obtain total membranes, which were resuspended in storage buffer (20 mm potassium phosphate, pH 7.5).
Determination of NdhP Topology
Tryptic proteolysis experiment was done according to Zhang et al. (32). Crude thylakoid membranes isolated as described above were suspended in 50 mm HEPES-KOH, pH 7.5, 5 mm MgCl2, 10 mm NaCl, and 600 mm sucrose at a final protein concentration of 2.5 mg/ml. Samples were digested on ice for 30 min in the presence of trypsin solution (Promega, Madison, WI). The final concentration of trypsin was 50 μg/ml. The treatments were terminated by adding an equal volume of Laemmli sample buffer and immediately heated at 65 °C for 5 min.
Aqueous Polymer Two-phase Partitioning of Plasma and Thylakoid Membranes
Plasma and thylakoid membranes were isolated from Synechocystis 6803 cells by aqueous polymer two-phase partitioning. In this process, the total Synechocystis 6803 membrane pellet (isolated as described above) was fractionated by two-phase partitioning using the polymers dextran T-500 and PEG 3350 (33, 34). CP43 and SbtA proteins were used as markers of the purity of the thylakoid and the plasma membranes, respectively (3, 33).
Electrophoresis and Immunoblotting
Blue native (BN)-PAGE of Synechocystis 6803 membranes was performed as described previously (35) with slight modifications (5, 18). Isolated membranes were prepared for BN-PAGE as follows. Membranes were washed with 330 mm sorbitol, 50 mm BisTris, pH 7.0, and 0.5 mm PMSF (Sigma) and resuspended in 20% glycerol (w/v), 25 mm BisTris, pH 7.0, 10 mm MgCl2, 0.1 unit RNase-free DNase RQ1 (Promega, Madison, WI) at a chlorophyll a concentration of 0.3 mg ml−1 and 0.5 mm PMSF. The samples were incubated on ice for 10 min, and an equal volume of 3% n-dodecyl β-d-maltoside (DM) was added. Solubilization was performed for 40 min on ice. Insoluble components were removed by centrifugation at 18,000 × g for 15 min. The collected supernatant was mixed with 0.1 volume of sample buffer, 5% Serva Blue G, 100 mm BisTris, pH 7.0, 30% sucrose (w/v), 500 mm ϵ-amino-n-caproic acid, and 10 mm EDTA. Solubilized membranes were then applied to a 0.75-mm-thick, 5–12.5% acrylamide gradient gel (Hoefer Mighty Small mini-vertical unit; San Francisco, CA). Samples were loaded on an equal chlorophyll a basis per lane. Electrophoresis was performed at 4 °C by increasing the voltage gradually from 50 to 200 V during the 5.5-h run. Several lanes of the BN gel were cut out and incubated in Laemmli SDS sample buffer containing 5% β-mercaptoethanol and 6 m urea for 1 h at 25 °C. SDS-PAGE of Synechocystis 6803 crude thylakoid membranes was carried out on 12% polyacrylamide gel with 6 m urea as described earlier (36).
For immunoblotting, the proteins were electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA) and detected by protein-specific antibodies using an ECL assay kit (Amersham Biosciences) according to the manufacturer's protocol. The NDH-1 complexes were detected using the antibodies against NdhA, NdhH, NdhI, NdhK, and NdhM, respectively, which were previously raised in our laboratory (30). Antibody against SbtA was provided from Professor Teruo Ogawa (Bioscience Center, Nagoya University). Antibody against His was purchased from Shanghai Immune Biotech Co., Ltd., and antibodies against GFP, photosystem II subunits (PsbA, PsbO, and CP43), and a photosystem I subunit (PsaD) were purchased from Agrisera Co. (Cännäs, Sweden).
Yeast Two-hybridization
Yeast two-hybridization was performed using the LexA system (Clontech). PCR-amplified ndhP-, ndhPN+TM-, ndhPC-, and ndhI-encoding fragments were cloned in-frame into EcoRI and XhoI or XhoI sites of pLexA to form the bait construct (primers are shown in supplemental Table 1). The fragments containing ndhB, ndhD1, and ndhF1 genes were amplified by PCR and inserted into the EcoRI and XhoI or XhoI sites of pJG4-5 to form the prey construct (primers are shown in supplemental Table 1). The bait and prey constructs together with a reporter vector pSH18-34 were co-transformed into yeast strain EGY48 according to the manufacturer's instructions for the Matchmaker LexA two-hybrid system (Clontech). Transformed yeast was diluted and dropped onto synthetic dropout (SD) media, including X-gal, and then was grown at 30 °C in darkness as described previously (18).
Confocal Laser Scanning Microscopy
Cell imaging was observed using a laser scanning confocal microscope (FV1000, Olympus, Japan) with a 100×/1.4 plan apochromat oil immersion objective and with an 80-μm confocal pinhole. The argon laser line, 515 nm, and the helium laser line, 543 nm, were used for the excitation of YFP and autofluorescence, respectively. YFP fluorescence emission was selected with a 515-nm dichroic mirror and a bandpass filter of 535–590 nm. Autofluorescence was collected through a 545-nm dichroic mirror and a long pass filter of 560 nm.
Accession Numbers
Sequence data from this article can be found in the cyanobase, GenBankTM (www.ncbi.nlm.nih.gov), or EMBL databases under the following accession numbers: Synechocystis sp. PCC 6803, Syn6803, Sml0013 (NdhP); Microcystis aeruginosa NIES-843, Micro, 190192163; Cyanothece sp. ATCC 51142: Cyn51142, 172036397; Trichodesmium erythraeum IMS101, Tri101, 113477709; Synechococcus sp. PCC 7002, Syn7002, 170078200; Arthrospira platensis NIES-39, Art39, 479129538; Synechoccus elongatus PCC 7942, Syn7942, 81299479; Thermosynechococcus elongatus BP-1, Thermo, NdhP; Gloeobacter violaceus PCC 7421, Gloeo, 37523392; Prochlorococcus marinus MIT 9211, Pro9211, 159903221; P. marinus SS120, Pro120, 33240183; P. marinus NATLIA, ProNATLIA, 124025625; P. marinus MIT 9215, Pro9215, 157413267; P. marinus MIT 9312, Pro9312, 78779226; Ricinus communis, Ricinus, 255547361; Vitis vinifera, Vitis, 359497630; Arabidopsis thaliana, Ara, 145335876; Sorghum bicolor, Sorghum, 242066004; Oryza sativa, Ory, 115448639; and Hordeum vulgare, Hordeum, 326490323.
RESULTS
Isolation of NDH-CET-defective Mutants
The NDH-CET has a protective role against high light stress in cyanobacteria (5, 18) and higher plants (17). Under high light conditions, the growth of NDH-CET-defective mutants, such as ΔndhS and Δslr1097 mutants, is markedly retarded compared with the wild type (WT) despite similar growth under moderate light irradiation. To screen for NDH-CET-defective mutants, we transformed WT cells with a transposon-bearing library, thus tagging and inactivating many genes randomly, and then cultured the mutant cells under high light conditions. We isolated two mutants, which grew slowly on the plate under high light but similarly to the WT under growth light (Fig. 1A).
FIGURE 1.
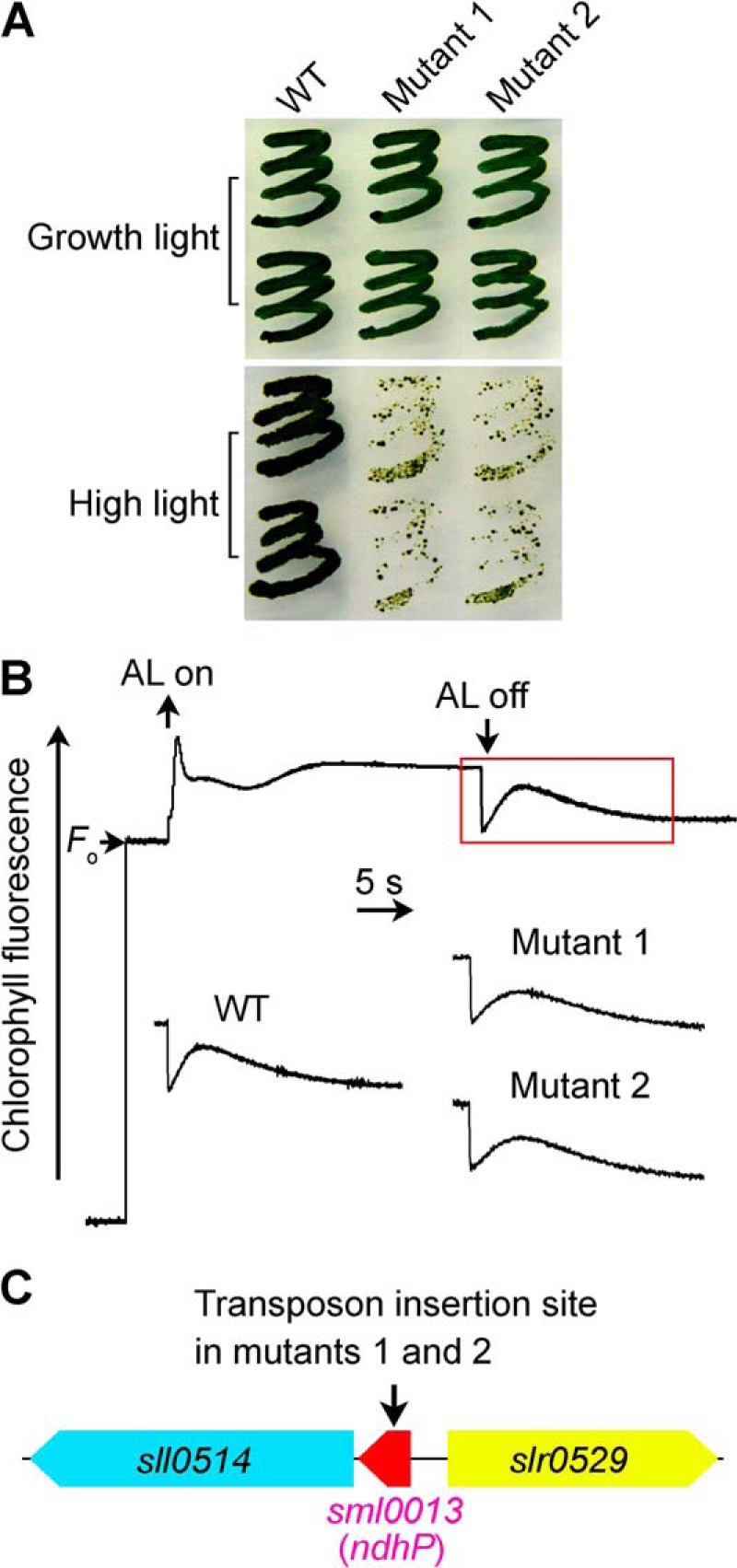
Growth, NDH-CET activity, and transposon insertion site of high light-sensitive mutants of Synechocystis 6803. A, growth of WT and 2 mutants under normal light (40 μE m−2 s−1) and high light (200 μE m−2 s−1). B, monitoring of NDH-CET activity using chlorophyll fluorescence analysis. The top curve shows a typical trace of chlorophyll fluorescence in the WT Synechocystis 6803. See “Experimental Procedures” for experimental details. C, arrow schematically indicates the transposon insertion site in mutants 1 and 2 probed by PCR analysis using the primers listed in supplemental Table 1.
To investigate whether the high light-sensitive growth phenotype of the two mutants resulted from defective NDH-CET, we monitored the post-illumination rise in chlorophyll a fluorescence, which is extensively used to monitor NDH-CET activity in cyanobacteria (5, 18, 30, 37, 38) and higher plants (39–48). As shown in Fig. 1B, the NDH-CET activity in both mutants was lower than that in the WT as judged by the height and relative rate of post-illumination increase in chlorophyll fluorescence. The results indicate that NDH-CET is impaired in mutants 1 and 2.
To identify the genes inactivated by transposon tagging, we analyzed the sites of transposon insertion in both mutants. As shown by the PCR results (Fig. 1C), both mutants were tagged in the same gene sml0013 (ndhP). The transposon insertion occurred at position 3188117 of the Synechocystis 6803 genome (49). This implies that inactivation of ndhP impairs NDH-CET activity, as suggested recently by Schwarz et al. (24).
Deletion of ndhP Impairs NDH-CET Activity
To confirm that inactivation of ndhP impairs NDH-CET activity, we replaced the entire ndhP coding region with a kanamycin resistance marker (KamR) (Fig. 2A). PCR analysis of the ndhP locus confirmed complete segregation of the ΔndhP mutant allele (Fig. 2B). Transcript analysis using a specific primer pair for the ndhP-encoding gene (see supplemental Table 1) demonstrated the absence of gene product in the mutant (Fig. 2C). As expected, the NDH-CET activity, as measured by the post-illumination increase in chlorophyll fluorescence, was lower in ΔndhP than in the WT. However, the activity remained relatively high compared with the M55 mutant (Fig. 2D). A similar result was obtained by measuring the oxidation of P700 by FR after AL illumination. When AL was turned off after a 30-s illumination by AL (800 μE m−2 s−1) supplemented with FR, P700+ was transiently reduced by electrons from the plastoquinone pool, and subsequently P700 was reoxidized by background FR. Operation of the NDH-1 complexes, which transfer electrons from the reduced cytoplasmic pool to plastoquinone, hinders the reoxidation of P700 (5, 18, 39). The reoxidation of P700 was evidently faster in ΔndhP compared with the WT but was much slower than in M55 (Fig. 2E). We also measured the NDH-CET by monitoring the reduction rate of P700+ in darkness after illumination of cells with FR light. The re-reduction of P700+ was markedly slower in ΔndhP compared with that in the WT, although it was still faster than in M55 (Fig. 2F). Furthermore, the growth of ΔndhP under high light intensities was evidently slower than the WT despite similar growth under growth light (data not shown). Taking all these results together, we can conclude that deletion of ndhP impairs NDH-CET activity.
NdhP Is Required for Stabilization of NDH-1L Complex
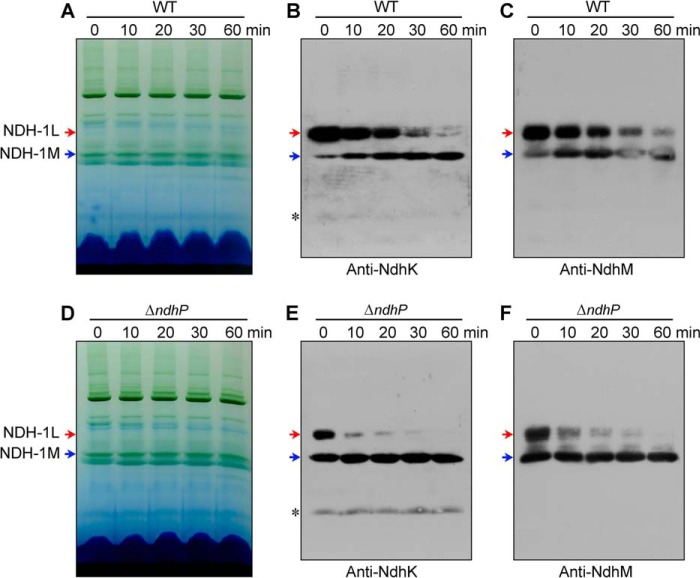
To reveal how deletion of ndhP impairs NDH-CET activity, we separated NDH-1L and NDH-1M complexes from thylakoid membranes of the WT, ΔndhP, and M55 strains and deduced the amount of these complexes from the density of NdhH, NdhI, NdhK, and NdhM bands visualized by Western analyses. Deletion of ndhP did not influence the abundance of NDH-1 subunits in the thylakoid membranes (Fig. 3A). However, the deletion decreased the amount of NDH-1L complex and increased that of NDH-1M complex (Fig. 3, B and C). These results indicate that treatment of the thylakoid membrane of the ΔndhP mutant by DM on ice disassembled the NDH-1L complex to form the NDH-1M complex, possibly by removing NdhD1 and NdhF1 or it was already disassembled in the cells. However, only about half of the NDH-1L complex was disassembled on ice, and the rest was decomposed into small pieces without forming NDH-1M during incubation with DM at room temperature (Fig. 4, D–F). The disassembling of NDH-1L to NDH-1M and decomposition to small pieces occurred even in the WT when thylakoid membrane was treated with DM at room temperature. However, NDH-1L was stable on ice, and the thermal collapse of the complex was much slower in the WT than in the ΔndhP mutant (Fig. 4, A–F). It appears that there are two types of NDH-1L complex; one disassembled to NDH-1M even on ice in the absence of NdhP and the other structurally more stable to the DM treatment but decomposed to small pieces at room temperature. NdhP retards the thermal collapse of the complex and plays an important role in stabilizing the NDH-1L complex in Synechocystis 6803.
FIGURE 3.
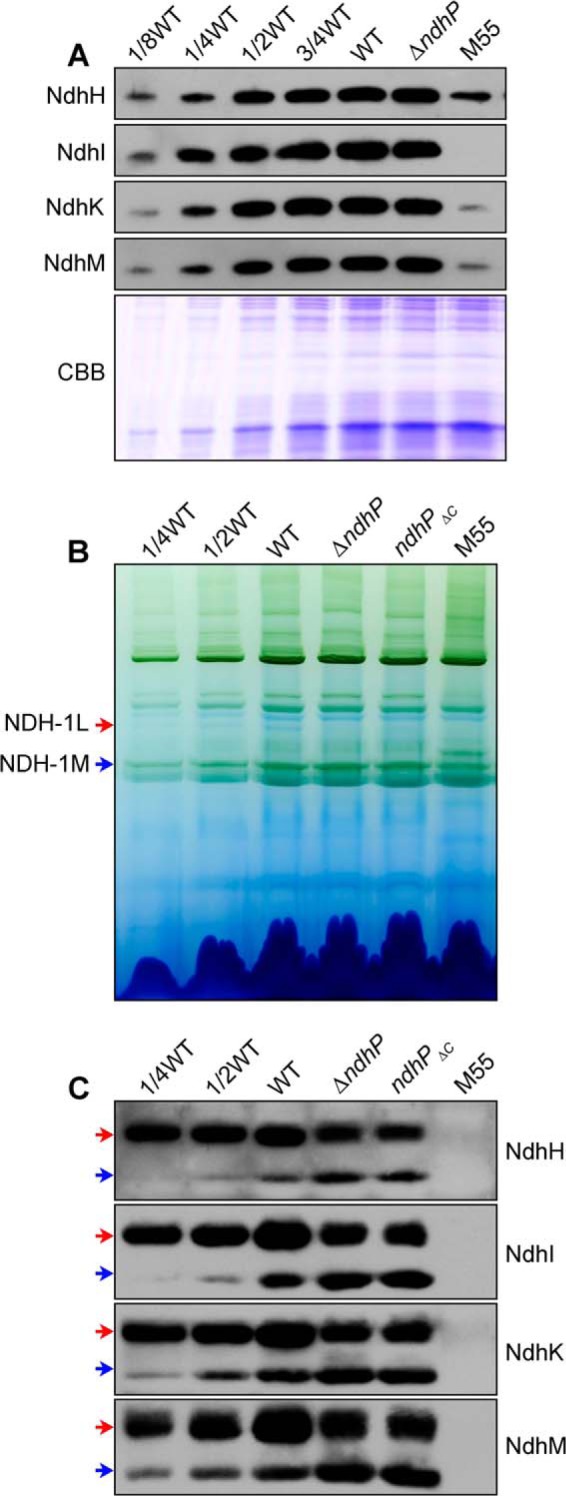
Western analyses of NDH-1L and NDH-1M complexes from the WT, ΔndhP, ndhPΔC, and M55 strains. A, Coomassie Brilliant Blue (CBB)-staining PAGE profiles and Western analysis of total NDH-1 in the thylakoid membranes. B, profiles of BN-PAGE of the thylakoid membranes; C, Western analysis of NDH-1L and NDH-1M complexes (indicated by red and blue arrows, respectively) from the WT, ΔndhP, ndhPΔC, and M55 strains. Sample containing 9 μg of chlorophyll a was loaded onto each lane.
FIGURE 4.
Effect of temperature and incubation time on the stability of NDH-1L and NDH-1M complexes from the WT and ΔndhP strains. A and D, profiles of BN-PAGE of the thylakoid membranes isolated from the WT and ΔndhP strains. The membranes were incubated with 1.5% DM on ice for 60 min (at 25 °C for 0 min) or at 25 °C for 10, 20, 30, and 60 min. Western analyses of NDH-1L (red arrows) and NDH-1M (blue arrows) of the WT (B and C) and ΔndhP (E and F) isolated in A and D, using antibodies against NdhK and NdhM. Asterisk represents nonspecific signals.
C-terminal Tail of NdhP Is Located on the Lumen Side
The NdhP protein consists of 40 amino acids and contains a single transmembrane helix (Fig. 5), as predicted by the TMHMM software. To clarify the localization of NdhP, we constructed a mutant, WT-NdhP-YFP-His6, which has a YFP-His6 tag on the C terminus of NdhP (Fig. 6, A and D). PCR analysis indicated complete segregation of the tagged gene (Fig. 6B). Western analysis of the thylakoid membrane from the tagged strain clearly indicated the presence of NdhP-YFP-His6 (Fig. 6C). The tagging did not influence the activity of NDH-1 complexes (Fig. 6, E–G). Plasma membrane (PM) and thylakoid membrane (TM) were isolated from the tagged cells, and their purity was confirmed by Western analysis using antibodies against SbtA and CP43, which cross-reacted with PM and TM, respectively (Fig. 7A). The NdhP protein as assessed by the protein abundance of YFP and His was detected only in the TM fraction but not in the PM (Fig. 7A) or soluble (data not shown) fractions. Thus, like other Ndh subunits, NdhP is localized in the thylakoid membranes.
To determine the topology of NdhP, thylakoid membranes from the WT-NdhP-YFP-His6 cells were subjected to mild digestion with trypsin. NdhP-YFP-His6 fusion protein was protected from tryptic treatment but was digested after sonication (Fig. 7B), similar to the photosystem II subunit PsbO located on the lumen side of the thylakoids. Thus, we may conclude that the C-terminal tail of NdhP is located on the lumen side of the thylakoid membranes.
NdhP Is Confined to NDH-1L Complex
The thylakoid membrane from the WT-NdhP-YFP-His6 strain was solubilized with DM and subjected to two-dimensional gel analysis (the gel strip obtained by BN-PAGE was subjected to SDS-PAGE in the second dimension). Proteins were transferred onto a PVDF membrane by electroblotting and analyzed using specific antibodies against GFP, His, NdhA, NdhH, NdhI, NdhK, and NdhM. The NdhP-YFP-His6 protein was present only in the NDH-1L complex (Fig. 7C). Thus, NdhP is confined exclusively to NDH-1L.
To confirm that NdhP is confined to the NDH-1L complex, NdhP in the ΔndhS, ΔndhD1/D2 (ΔD1/D2), and ΔndhB (M55) mutants was tagged with YFP-His6. PCR analysis of the ndhP-yfp-his6 locus confirmed the complete segregation of the ndhP-yfp-his6 allele in these mutants (Fig. 7D). Western blot analysis of total protein using GFP and His antibodies demonstrated the expression of NdhP-YFP-His6 protein in all these mutants (Fig. 7E), although the amount of NdhP-YFP-His6 was much lower in the tagged ΔD1/D2 and M55 mutants. Analysis of NDH-1L and NDH-1M complexes indicated that NdhP-YFP-His6 protein was absent in the tagged ΔD1/D2 and M55 mutants but present in the tagged ΔndhS mutant in an amount equivalent to that in the WT (Fig. 7, F and G). NDH-1L was absent in ΔD1/D2 and M55 mutants, and NDH-1M was remarkably increased in the ΔD1/D2 mutant but was absent in M55; however, deletion of ndhS did not affect the amount of these two complexes (Fig. 7G). It is evident that the NdhP-YFP-His6 protein is associated with NDH-1L but not with NDH-1M.
When the thylakoid membrane of the WT-YFP-His6 strain was incubated with DM at room temperature, NDH-1L was broken down gradually to form NDH-1M (Fig. 8), as was observed with the WT strain (Fig. 4, A–C). A band around 160 kDa appeared during breakdown of NDH-1L (Fig. 8D). This band cross-reacted with the antibody against GFP but not with the antibody against NdhK or NdhM (Fig. 8, B and C). Thus, NdhP-YFP-His6 was released from NDH-1L, possibly together with NdhD1 and NdhF1.
FIGURE 8.
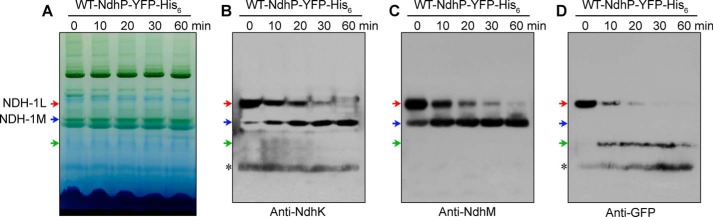
Effect of temperature and incubation time on the stability of NDH-1L and NDH-1M complexes from the WT-NdhP-YFP-His6 strain. A, profiles of BN-PAGE of thylakoid membranes isolated from the WT-NdhP-YFP-His6 strain treated with 1.5% DM on ice for 60 min (at 25 °C for 0 min) or at 25 °C for 10, 20, 30, and 60 min. Red and blue arrows indicate the positions of NDH-1L and NDH-1M, respectively. A band with the apparent molecular mass of about 160 kDa is shown by a green arrow. B–D, Western analyses of protein complexes separated by BN-PAGE using the antibodies against NdhK (B), NdhM (C), and GFP (D). Asterisk represents nonspecific signals.
C-terminal Tail of NdhP Is Essential for Stabilization of NDH-1L Complex
The C-terminal tail of NdhP is highly conserved among cyanobacteria (Fig. 5). Recently, Torres-Bacete et al. (50) demonstrated that the C-terminal tail of NdhD1 and NdhF1 from Escherichia coli is required to stabilize the NDH-1 complex. Based on this fact, we assumed that the C-terminal tail of NdhP is also required for stabilization of the NDH-1L complex in Synechocystis 6803.
To test this possibility, we constructed an ndhP C-terminal deletion mutant (ndhPΔC) (Fig. 2A). The C-terminal tail of NdhP was completely deleted in the mutant, but its absence did not influence the expression of both N-terminal extension and single transmembrane helix (Fig. 2, B and C). Absence of the C-terminal tail of NdhP destabilizes the NDH-1L complex (Fig. 3, B and C), as observed in the ΔndhP mutant. This finding was reinforced by the observation of impaired NDH-CET activity and a high light-sensitive growth phenotype of the ndhPΔC mutant, similarly to the ΔndhP mutant (data not shown). We therefore conclude that the C-terminal tail of NdhP is essential to stabilize the NDH-1L complex.
Interaction of NdhP with NdhD1, NdhF1, and NdhB
Yeast two-hybrid system assay revealed that NdhP interacts with NdhD1, NdhF1, and NdhB (Fig. 9). The interaction was absent between N-terminal and transmembrane regions of NdhP (NdhPN+TM) and NdhB or between the C-terminal region (NdhPC) and NdhF1. This suggests that the C terminus of NdhP is in contact with NdhD1 and NdhB but not with NdhF1.
FIGURE 9.
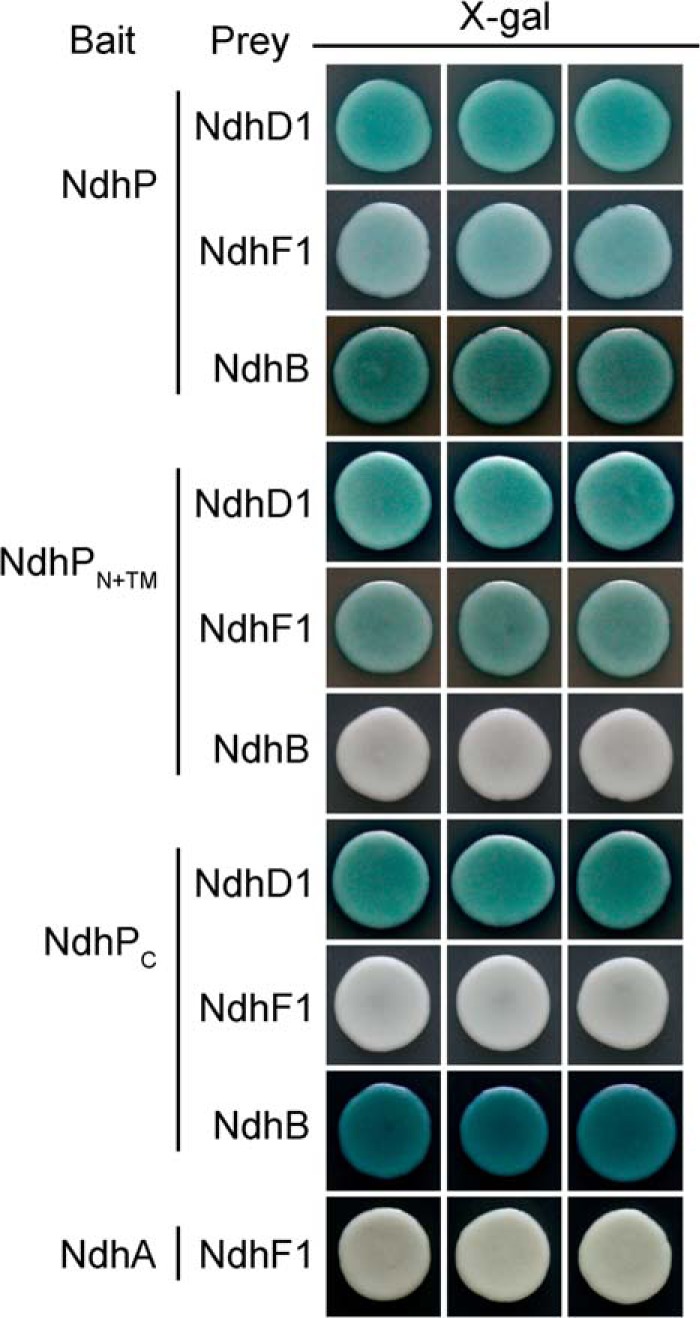
Yeast two-hybrid system for assaying the interaction of NdhP with NdhD1, NdhF1, and NdhB. ndhP, ndhPN+TM, and ndhPC were constructed into bait vector, whereas ndhD1, ndhF1, and ndhB were contracted into prey vector, respectively. Subsequently, they were transformed into the yeast strain EGY48. Transformed yeast was dropped onto X-gal medium. Blue precipitate represents accumulated β-galactosidase activity resulting from the activation of the lacZ reporter gene by protein-protein interaction. The induction plate was incubated at 30 °C for 28 h and then photographed. The interaction of NdhA with NdhF1 was assayed as a negative control. At least six independent experiments were performed, and one representative result is shown on each assay.
DISCUSSION
Over the past few decades, a significant achievement has been made in identifying the composition and function of subunits from the NDH-1 complexes in cyanobacteria (12–15) and higher plants (51–53). Recently, a new oxygen photosynthesis-specific subunit NdhP was identified in T. elongatus (23) and was suggested to be involved in respiration and NDH-CET in Synechocystis 6803 (24). However, how NdhP affected the respiratory and cyclic electron flows is poorly understood. Here, we successfully identified the NdhP subunit in Synechocystis 6803 (Fig. 6C). This subunit is exclusively confined to the NDH-1L complex (Fig. 7, C and G), and absence of its C-terminal tail destabilizes the complex (Figs. 3C and 4, E and F). The NDH-1L complex is involved in respiration and NDH-CET (3), which explains the impairment of these activities in the ΔndhP mutant of Synechocystis 6803 (Figs. 1B and 2, D–F) (24).
There are two types of NDH-1L complex in the WT, one dissociated to NDH-1M and possibly NdhD1/NdhF1/NdhP and the other decomposed to small pieces during incubation with DM at room temperature for 30–60 min (Figs. 4, A–C, and 8D). In the ΔndhP mutant, dissociation of the former type occurred even on ice and decomposition of the other type occurred much faster than in the WT at room temperature (Fig. 4, D–F). Evidently, NdhP is essential to stabilize both types of NDH-1L.
NdhP is ubiquitously distributed among cyanobacteria (24), and its counterpart in higher plants is NDF6 (Fig. 5) (54). Inactivation of the ndf6 gene resulted in complete impairment of NDH-CET and collapse of the chloroplast NDH-1 complex (54, 55). In contrast, deletion of ndhP only partially decreased the NDH-CET activity (Fig. 2, D–F) and disassembled NDH-1L complex (Fig. 3C). This clearly indicates that the role of NdhP on the stability and function of NDH-1 enzyme is significantly different from that of NDF6. This difference was reinforced by the fact that NDF6 of A. thaliana did not rescue the disassembly of NDH-1L complex and the impairment of NDH-CET caused by the deletion of NdhP in Synechocystis 6803 (data not shown). Furthermore, NDF6 is localized in subcomplex B, an exclusive subcomplex for chloroplast NDH-1 (43, 45, 55). By contrast, NdhP is exclusively localized in NDH-1L complex (Fig. 7, C and G), which is a counterpart of subcomplex A and membrane subcomplex in chloroplast NDH-1 (43). The different localizations of NdhP and NDF6 in NDH-1 complexes may interpret the different effects of these two homologous subunits on the stability of NDH-1 complexes.
In addition to NdhD1 and NdhF1, NdhP is the third exclusive subunit of the NDH-1L complex. The interaction of NdhP with NdhD1 and NdhF1 (Fig. 9) suggests that these NDH-1L-specific subunits are clustered. During room temperature incubation of the thylakoid membrane of the NdhP-YFP-His6 strain, NDH-1L complex was gradually disassembled into NDH-1M complex (Fig. 8), and a band with the apparent molecular mass of about 160 kDa containing YFP protein (see green arrow in Fig. 8) was released. This band did not contain NdhK and NdhM and most likely consists of NdhP-YFP-His6, NdhD1, and NdhF1.
NDH-1M is considered to be associated with NDH-1S in the cells as a functional NDH-1MS complex, which is easily dissociated into NDH-1M and NDH-1S during solubilization of the membranes with DM even on ice (3, 22). Such instability of the NDH-1MS is consistent with the absence of NdhP in this complex.
Analysis of a crystal structure of the hydrophobic domain of complex I from T. thermophilus showed a long amphipathic helix formed by a C terminus of NuoL (corresponding to cyanobacterial NdhF1) that runs along almost the entire length of the membrane domain (56–58). Absence of this long amphipathic helix resulted in a complete collapse of complex I (50). The C-terminal tail of NdhP extends toward the lumen (Fig. 7B) and is required to stabilize the NDH-1L complex (Fig. 3C). The C terminus of NdhP but not its N-terminal extension and single transmembrane helix interacts with NdhB (Fig. 9), regardless of the interaction of the N terminus and membrane helix of NdhP with NdhD1 and NdhF1 (Fig. 9). This suggests that the C terminus of NdhP may also be involved in stabilizing NdhB, especially in the NDH-1L completely decomposed to small pieces by DM treatment at room temperature. Destabilization of NdhB might result in complete collapse of the complex, as observed in M55 by deletion of NdhB (Fig. 3, A–C). The C terminus of NdhP also interacts with NdhD1 but not with NdhF1 (Fig. 9). We may propose a model of NDH-1L as depicted in Fig. 10, where NdhP is located between NdhD1 and NdhF1, and the C-terminal tails of NdhP and NdhF1 located on the lumen and cytoplasm sides as shown by the green and pink stripes, respectively, extend to NdhB. This may explain the disassembly of NDH-1L complex in the absence of the C terminus of NdhP.
FIGURE 10.
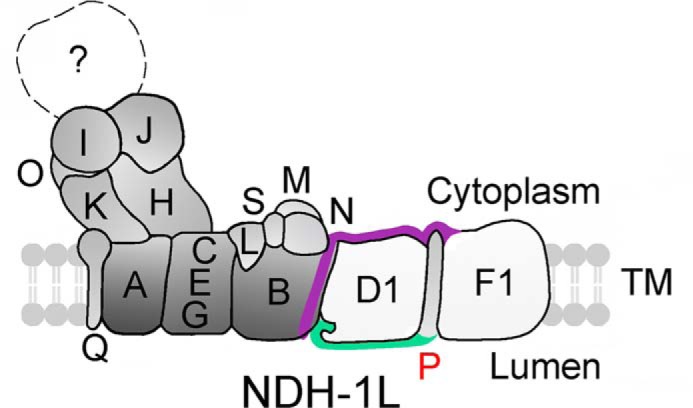
Model schematically represents how the C terminus of NdhP stabilizes the NDH-1L complex. NdhP and NdhF1 stabilize the NDH-1L complex by their C-terminal tails at the lumen and cytoplasm sides, respectively. The single transmembrane helix of NdhP spans the membrane between NdhD1 and NdhF1 and its C-terminal tail extends to NdhB interacting with NdhD1. The C-terminal tails of NdhP and NdhF1 are indicated by green and dark pink stripes, respectively.
Supplementary Material
Acknowledgments
We thank Professors Hualing Mi (Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, China) for kindly providing NdhI antibody of Synechocystis 6803 and Hongquan Yang (Shanghai Jiaotong University, China) for supplying the yeast two-hybrid system. We also thank Xiaoshu Gao (Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, China) for excellent technical support for the confocal microscopy measurement.
This work was supported in part by National Natural Science Foundation of China Grant 31370270, National Basic Research Program of China Grant 2009CB118500, and Project of Shanghai Education Committee Grant 12ZZ132.

This article contains supplemental Table 1.
- NDH-1
- type I NADPH dehydrogenase
- NDH-1L
- large size NDH-1 complex
- NDH-1M
- medium size NDH-1 complex
- NDH-1S
- small size NDH-1 complex
- Synechocystis 6803
- Synechocystis sp. PCC 6803
- CET
- cyclic electron transfer
- NDH-CET
- NDH-1-mediated CET
- FR
- far-red light
- AL
- actinic light
- DM
- n-dodecyl β-d-maltoside
- PM
- plasma membrane
- TM
- thylakoid membrane
- BN
- Blue-Native
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MCS
- multiple cloning site
- DCMU
- 3-(3,4-dichlorophenyl)-1,1-dimethylurea.
REFERENCES
- 1. Ohkawa H., Sonoda M., Shibata M., Ogawa T. (2001) Localization of NAD(P)H dehydrogenase in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183, 4938–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohkawa H., Sonoda M., Hagino N., Shibata M., Pakrasi H. B., Ogawa T. (2002) Functionally distinct NAD(P)H dehydrogenases and their membrane localization in Synechocystis sp. PCC6803. Funct. Plant Biol. 29, 195–200 [DOI] [PubMed] [Google Scholar]
- 3. Zhang P., Battchikova N., Jansen T., Appel J., Ogawa T., Aro E. M. (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp. PCC 6803. Plant Cell 16, 3326–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu M., Ogawa T., Pakrasi H. B., Mi H. (2008) Identification and localization of the CupB protein involved in constitutive CO2 uptake in the cyanobacterium, Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 49, 994–997 [DOI] [PubMed] [Google Scholar]
- 5. Battchikova N., Wei L., Du L., Bersanini L., Aro E. M., Ma W. (2011) Identification of a novel Ssl0352 protein (NdhS), essential for efficient operation of cyclic electron transport around photosystem I, in NADPH:plastoquinone oxidoreductase (NDH-1) complexes of Synechocystis sp. PCC 6803. J. Biol. Chem. 286, 36992–37001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogawa T. (1991) A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 88, 4275–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mi H., Endo T., Schreiber U., Ogawa T., Asada K. (1992) Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 33, 1233–1237 [Google Scholar]
- 8. Ohkawa H., Pakrasi H. B., Ogawa T. (2000) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J. Biol. Chem. 275, 31630–31634 [DOI] [PubMed] [Google Scholar]
- 9. Friedrich T., Steinmüller K., Weiss H. (1995) The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett. 367, 107–111 [DOI] [PubMed] [Google Scholar]
- 10. Friedrich T., Scheide D. (2000) The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett. 479, 1–5 [DOI] [PubMed] [Google Scholar]
- 11. Arteni A. A., Zhang P., Battchikova N., Ogawa T., Aro E. M., Boekema E. J. (2006) Structural characterization of NDH-1 complexes of Thermosynechococcus elongatus by single particle electron microscopy. Biochim. Biophys. Acta 1757, 1469–1475 [DOI] [PubMed] [Google Scholar]
- 12. Battchikova N., Aro E. M. (2007) Cyanobacterial NDH-1 complexes: multiplicity in function and subunit composition. Physiol. Plant. 131, 22–32 [DOI] [PubMed] [Google Scholar]
- 13. Ogawa T., Mi H. (2007) Cyanobacterial NADPH dehydrogenase complexes. Photosynth. Res. 93, 69–77 [DOI] [PubMed] [Google Scholar]
- 14. Ma W. (2009) Identification, regulation and physiological functions of multiple NADPH dehydrogenase complexes in cyanobacteria. Front. Biol. China 4, 137–142 [Google Scholar]
- 15. Battchikova N., Eisenhut M., Aro E. M. (2011) Cyanobacterial NDH-1 complexes: novel insights and remaining puzzles. Biochim. Biophys. Acta 1807, 935–944 [DOI] [PubMed] [Google Scholar]
- 16. Bernát G., Appel J., Ogawa T., Rögner M. (2011) Distinct roles of multiple NDH-1 complexes in the cyanobacterial electron transport network as revealed by kinetic analysis of P700+ reduction in various ndh-deficient mutants of Synechocystis sp. strain PCC6803. J. Bacteriol. 193, 292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endo T., Shikanai T., Takabayashi A., Asada K., Sato F. (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett. 457, 5–8 [DOI] [PubMed] [Google Scholar]
- 18. Dai H., Zhang L., Zhang J., Mi H., Ogawa T., Ma W. (2013) Identification of a cyanobacterial CRR6 protein, Slr1097, required for efficient assembly of NDH-1 complexes in Synechocystis sp. PCC 6803. Plant J. 75, 858–866 [DOI] [PubMed] [Google Scholar]
- 19. Herranen M., Battchikova N., Zhang P., Graf A., Sirpiö S., Paakkarinen V., Aro E. M. (2004) Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiol. 134, 470–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prommeenate P., Lennon A. M., Markert C., Hippler M., Nixon P. J. (2004) Subunit composition of NDH-1 complexes of Synechocystis sp. PCC 6803: identification of two new ndh gene products with nuclear-encoded homologues in the chloroplast Ndh complex. J. Biol. Chem. 279, 28165–28173 [DOI] [PubMed] [Google Scholar]
- 21. Battchikova N., Zhang P., Rudd S., Ogawa T., Aro E. M. (2005) Identification of NdhL and Ssl1690 (NdhO) in NDH-1L and NDH-1M complexes of Synechocystis sp. PCC 6803. J. Biol. Chem. 280, 2587–2595 [DOI] [PubMed] [Google Scholar]
- 22. Zhang P., Battchikova N., Paakkarinen V., Katoh H., Iwai M., Ikeuchi M., Pakrasi H. B., Ogawa T., Aro E. M. (2005) Isolation, subunit composition and interaction of the NDH-1 complexes from Thermosynechococcus elongatus BP-1. Biochem. J. 390, 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nowaczyk M. M., Wulfhorst H., Ryan C. M., Souda P., Zhang H., Cramer W. A., Whitelegge J. P. (2011) NdhP and NdhQ: two novel small subunits of the cyanobacterial NDH-1 complex. Biochemistry 50, 1121–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarz D., Schubert H., Georg J., Hess W. R., Hagemann M. (2013) The gene sml0013 of Synechocystis species strain PCC 6803 encodes for a novel subunit of the NAD(P)H oxidoreductase or complex I that is ubiquitous distributed among cyanobacteria. Plant Physiol. 163, 1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen M. M. (1968) Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 4, 1–4 [DOI] [PubMed] [Google Scholar]
- 26. Birungi M., Folea M., Battchikova N., Xu M., Mi H., Ogawa T., Aro E. M., Boekema E. J. (2010) Possibilities of subunit localization with fluorescent protein tags and electron microscopy exemplified by a cyanobacterial NDH-1 study. Biochim. Biophys. Acta 1797, 1681–1686 [DOI] [PubMed] [Google Scholar]
- 27. Williams J. G., Szalay A. A. (1983) Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 24, 37–51 [DOI] [PubMed] [Google Scholar]
- 28. Long Z., Zhao J., Zhang J., Wei L., Wang Q., Ma W. (2011) Effects of different light treatments on the natural transformation of Synechocystis sp. strain PCC 6803. Afr. J. Microbiol. Res. 5, 3603–3610 [Google Scholar]
- 29. McGinn P. J., Price G. D., Maleszka R., Badger M. R. (2003) Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 132, 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma W., Mi H. (2005) Expression and activity of type-1 NAD(P)H dehydrogenase at different growth phases of cyanobacterium, Synechocystis PCC 6803. Physiol. Plant. 125, 135–140 [Google Scholar]
- 31. Gombos Z., Wada H., Murata N. (1994) The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc. Natl. Acad. Sci. U.S.A. 91, 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang P., Eisenhut M., Brandt A. M., Carmel D., Silén H. M., Vass I., Allahverdiyeva Y., Salminen T. A., Aro E. M. (2012) Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24, 1952–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Norling B., Zak E., Andersson B., Pakrasi H. (1998) 2D-isolation of pure plasma and thylakoid membranes from the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 436, 189–192 [DOI] [PubMed] [Google Scholar]
- 34. Jansén T., Kanervo E., Aro E. M., Mäenpää P. (2002) Localization and processing of the precursor form of photosystem II protein D1 in Synechocystis 6803. J. Plant Physiol. 159, 1205–1211 [Google Scholar]
- 35. Kügler M., Jänsch L., Kruft V., Schmitz U. K., Braun H. P. (1997) Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 53, 35–44 [Google Scholar]
- 36. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 37. Mi H., Endo T., Ogawa T., Asada K. (1995) Thylakoid membrane-bound pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 36, 661–668 [Google Scholar]
- 38. Deng Y., Ye J., Mi H. (2003) Effects of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem I in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 44, 534–540 [DOI] [PubMed] [Google Scholar]
- 39. Shikanai T., Endo T., Hashimoto T., Yamada Y., Asada K., Yokota A. (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc. Natl. Acad. Sci. U.S.A. 95, 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burrows P. A., Sazanov L. A., Svab Z., Maliga P., Nixon P. J. (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 17, 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto M., Endo T., Peltier G., Tasaka M., Shikanai T. (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 36, 541–549 [DOI] [PubMed] [Google Scholar]
- 42. Wang P., Duan W., Takabayashi A., Endo T., Shikanai T., Ye J. Y., Mi H. (2006) Chloroplastic NAD(P)H dehydrogenase in tobacco leave functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 141, 465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng L., Fukao Y., Fujiwara M., Takami T., Shikanai T. (2009) Efficient operation of NAD(P)H dehydrogenase requires the supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 21, 3623–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng L., Fukao Y., Myouga F., Motohashi R., Shinozaki K., Shikanai T. (2011) A chaperonin subunit with unique structures is essential for folding of a specific substrate. PLoS Biol. 9, e1001040. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Peng L., Fukao Y., Fujiwara M., Shikanai T. (2012) Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis. Plant Cell 24, 202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sirpiö S., Allahverdiyeva Y., Holmström M., Khrouchtchova A., Haldrup A., Battchikova N., Aro E. M. (2009) Novel nuclear-encoded subunits of the chloroplast NAD(P)H dehydrogenase complex. J. Biol. Chem. 284, 905–912 [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto H., Peng L., Fukao Y., Shikanai T. (2011) An Src homology 3 domain-like fold protein forms a ferredoxin-binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23, 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Armbruster U., Rühle T., Kreller R., Strotbek C., Zühlke J., Tadini L., Blunder T., Hertle A. P., Qi Y., Rengstl B., Nickelsen J., Frank W., Leister D. (2013) The PHOTOSYNTHESIS AFFECTED MUTANT68-LIKE protein evolved from a PSII assembly factor to mediate assembly of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell 25, 3926–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., Kimura T., Hosouchi T., Matsuno A., Muraki A., Nakazaki N., Naruo K., Okumura S., Shimpo S., Takeuchi C., Wada T., Watanabe A., Yamada M., Yasuda M., Tabata S. (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3, 109–136 [DOI] [PubMed] [Google Scholar]
- 50. Torres-Bacete J., Sinha P. K., Matsuno-Yagi A., Yagi T. (2011) Structural contribution of C-terminal segments of NuoL (ND5) and NuoM (ND4) subunits of complex I from Escherichia coli. J. Biol. Chem. 286, 34007–34014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suorsa M., Sirpiö S., Aro E. M. (2009) Towards characterization of the chloroplast NAD(P)H dehydrogenase complex. Mol. Plant 2, 1127–1140 [DOI] [PubMed] [Google Scholar]
- 52. Ifuku K., Endo T., Shikanai T., Aro E. M. (2011) Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant Cell Physiol. 52, 1560–1568 [DOI] [PubMed] [Google Scholar]
- 53. Peng L., Yamamoto H., Shikanai T. (2011) Structure and biogenesis of the chloroplast NAD(P)H dehydrogenase complex. Biochim. Biophys. Acta 1807, 945–953 [DOI] [PubMed] [Google Scholar]
- 54. Ishikawa N., Takabayashi A., Ishida S., Hano Y., Endo T., Sato F. (2008) NDF6: a thylakoid protein specific to terrestrial plants is essential for activity of chloroplastic NAD(P)H dehydrogenase in Arabidopsis. Plant Cell Physiol. 49, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 55. Yabuta S., Ifuku K., Takabayashi A., Ishihara S., Ido K., Ishikawa N., Endo T., Sato F. (2010) Three PsbQ-like proteins are required for the function of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol. 51, 866–876 [DOI] [PubMed] [Google Scholar]
- 56. Efremov R. G., Baradaran R., Sazanov L. A. (2010) The architecture of respiratory complex I. Nature 465, 441–445 [DOI] [PubMed] [Google Scholar]
- 57. Efremov R. G., Sazanov L. A. (2011) Structure of the membrane domain of respiratory complex I. Nature 476, 414–420 [DOI] [PubMed] [Google Scholar]
- 58. Baradaran R., Berrisford J. M., Minhas G. S., Sazanov L. A. (2013) Crystal structure of the entire respiratory complex I. Nature 494, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.