Background: Pah1p, yeast phosphatidate phosphatase involved in triacylglycerol synthesis, is multiply phosphorylated.
Results: Protein kinase C phosphorylates Pah1p on serine residues and causes a decrease in protein abundance when it is not previously phosphorylated by Pho85p-Pho80p.
Conclusion: Pah1p is regulated for its protein abundance by protein kinase C.
Significance: Unraveling the interrelationship between Pah1p phosphorylations is crucial for understanding the enzyme regulation.
Keywords: Diacylglycerol, Lipid Metabolism, Phosphatase, Phosphatidate, Protein Kinase C (PKC), Triacylglycerol, Yeast
Abstract
Yeast Pah1p is the phosphatidate phosphatase that catalyzes the penultimate step in triacylglycerol synthesis and plays a role in the transcriptional regulation of phospholipid synthesis genes. The enzyme is multiply phosphorylated, some of which is mediated by Pho85p-Pho80p, Cdc28p-cyclin B, and protein kinase A. Here, we showed that Pah1p is a bona fide substrate of protein kinase C; the phosphorylation reaction was time- and dose-dependent and dependent on the concentrations of ATP (Km = 4.5 μm) and Pah1p (Km = 0.75 μm). The stoichiometry of the reaction was 0.8 mol of phosphate/mol of Pah1p. By combining mass spectrometry, truncation analysis, site-directed mutagenesis, and phosphopeptide mapping, we identified Ser-677, Ser-769, Ser-773, and Ser-788 as major sites of phosphorylation. Analysis of Pah1p phosphorylations by different protein kinases showed that prephosphorylation with protein kinase C reduces its subsequent phosphorylation with protein kinase A and vice versa. Prephosphorylation with Pho85p-Pho80p had an inhibitory effect on its subsequent phosphorylation with protein kinase C; however, prephosphorylation with protein kinase C had no effect on the subsequent phosphorylation with Pho85p-Pho80p. Unlike its phosphorylations by Pho85p-Pho80p and protein kinase A, which cause a significant reduction in phosphatidate phosphatase activity, the phosphorylation of Pah1p by protein kinase C had a small stimulatory effect on the enzyme activity. Analysis of phosphorylation-deficient forms of Pah1p indicated that protein kinase C does not have a major effect on its location or its function in triacylglycerol synthesis, but instead, the phosphorylation favors loss of Pah1p abundance when it is not phosphorylated with Pho85p-Pho80p.
Introduction
The PAP2 enzyme catalyzes the Mg2+-dependent dephosphorylation of PA to produce DAG and Pi (1) (see Fig. 1A). The DAG generated in the reaction is used for the de novo synthesis of TAG and the membrane phospholipids phosphatidylcholine and phosphatidylethanolamine (1, 2). The reaction is governed by the DXDX(T/V) catalytic motif present in a conserved haloacid dehalogenase-like domain found in all PAP orthologs (3–7) (see Fig. 1B). In the model eukaryote yeast Saccharomyces cerevisiae, PAP is encoded by PAH1, whereas in mammals, the enzyme is encoded by three genes denoted as Lpin1–3 (LPIN1–3, human) (3, 7). The physiological importance of PAP in yeast is exemplified by pah1Δ phenotypes related to the increased level of PA (e.g. derepression of phospholipid synthesis gene expression and the abnormal expansion of the nuclear/endoplasmic reticulum membrane), decreased levels of DAG and TAG (e.g. susceptibility to fatty acid-induced lipotoxicity and defects in lipid droplet formation), a defect in vacuole homeostasis, respiratory deficiency, and temperature sensitivity (3, 4, 8–12). Many of these phenotypes are attributed to an imbalance of PA and DAG (13) given that some of these defects are suppressed by loss of Dgk1p DAG kinase that catalyzes the conversion of DAG back to PA (see Fig. 1A) (10, 14). The losses of or defects in mammalian lipin PAP enzymes underlie metabolic disorders that include lipodystrophy and insulin resistance, peripheral neuropathy, myoglobinuria, and inflammation (7, 15–26). Together, these observations highlight the importance of PAP in lipid metabolism and cell physiology and the need to understand the genetic and biochemical regulations of the enzyme.
FIGURE 1.
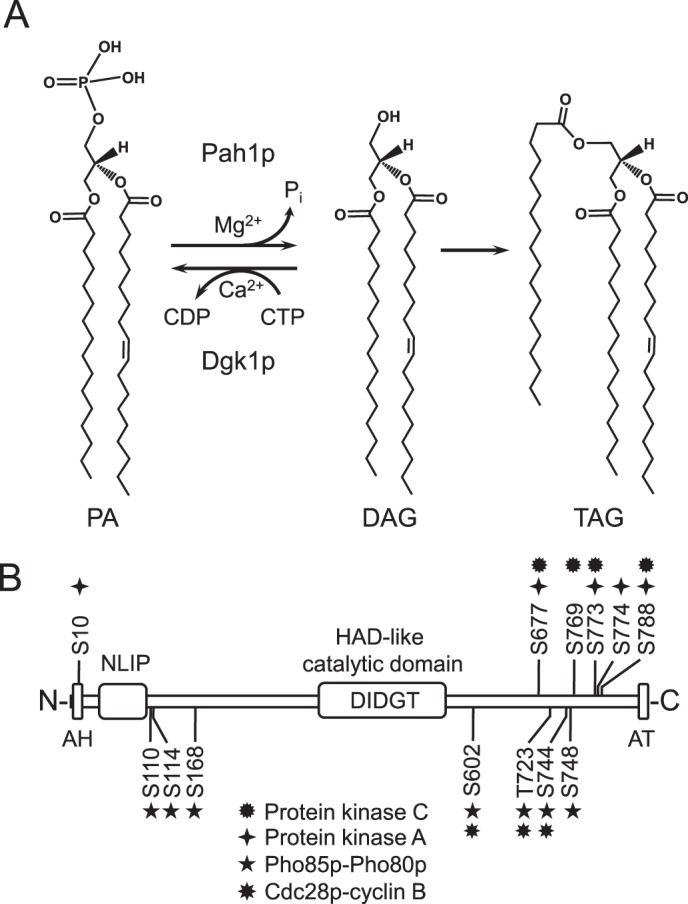
Reactions catalyzed by yeast Pah1p PAP and Dgk1p DAG kinase and the domain structure and phosphorylation sites in Pah1p. A, the reactions catalyzed by Pah1p PAP and Dgk1p DAG kinase are shown. The DAG produced in the PAP reaction is used for the synthesis of TAG (shown) and the synthesis of phosphatidylcholine and phosphatidylethanolamine (not shown). B, the domain structure of Pah1p showing the positions of the amphipathic helix (AH), NLIP domain, the haloacid dehalogenase (HAD)-like domain containing the DXDX(T/V) catalytic motif, acidic tail (AT), and the serine (S) and threonine (T) residues that are phosphorylated by PKC (this study), PKA (38), Pho85p-Pho80p (37), and Cdc28p-cyclin B (36).
Progress in understanding the regulation and function of PAP is facilitated by studies using S. cerevisiae (13, 27, 28). In fact, the discovery that PAH1 codes for a PAP enzyme in yeast (3, 29) made studies on the orthologous enzymes in higher eukaryotes possible (13, 28). In yeast, PAH1 expression is regulated at the transcriptional level by growth phase and nutrient supplementation (30, 31), whereas on a biochemical level, Pah1p function is regulated by lipids (32, 33), nucleotides (34), phosphorylation and dephosphorylation (9, 35–39), and proteasome-mediated degradation (40). The posttranslational modification of phosphorylation may have the greatest impact on Pah1p function in yeast (13, 28). The protein kinases and their sites of phosphorylation that are known to regulate Pah1p are summarized in Fig. 1B. On one hand, phosphorylations by the cyclin-dependent kinases Pho85p-Pho80p and Cdc28p-cyclin B and PKA sequester Pah1p in the cytosol where it does not have access to its membrane-associated substrate PA (35–38). On the other hand, dephosphorylation by the Nem1p-Spo7p protein phosphatase complex facilitates membrane association, PA binding, and catalysis for TAG synthesis (35–37, 39). Some phosphorylations (e.g. by Pho85p-Pho80p and PKA) inhibit PAP activity, whereas dephosphorylation by Nem1p-Spo7p stimulates activity (35, 37, 38). Paradoxically, phosphorylations by Pho85p-Pho80p, Cdc28p-cylcin B, and PKA stabilize Pah1p abundance, whereas the dephosphorylation by Nem1p-Spo7p destabilizes enzyme abundance (36–38). Interestingly, the loss of enzyme abundance, which is mediated by the proteasome (40), occurs as cells progress into the stationary phase of growth when PAH1 expression is induced (40), Pah1p PAP activity is elevated (3, 30, 40), and TAG synthesis increases at the expense of membrane phospholipids (30, 41). Presumably, these mechanisms of regulation work in concert to “fine-tune” Pah1p function to balance the levels of PA and DAG, essential intermediates in lipid metabolism that play signaling roles in several aspects of cell physiology (13, 42, 43).
Previous studies have shown that Pah1p is multiply phosphorylated in vivo (35, 44, 45) and that some of the phosphorylation sites are contained within a PKC consensus motif. PKC is a lipid-dependent protein kinase (46–48), and the enzyme in yeast is required for cell cycle and plays a role in regulating phospholipid synthesis (49–54) and in maintaining cell wall integrity (55–57). In this study, we showed that Pah1p is a bona fide substrate of PKC and identified four major sites of phosphorylation (Fig. 1B). We also examined the interrelationships of Pah1p phosphorylations by PKC, Pho85p-Pho80p, and PKA. Mutational analysis indicated that the phosphorylation by PKC does not have a major effect on its location or on its role in TAG synthesis, but instead, the phosphorylation favors its degradation in the absence of prephosphorylation by Pho85p-Pho80p.
EXPERIMENTAL PROCEDURES
Materials
Difco was the supplier of growth media. DNA gel extraction and plasmid DNA purification kits and nickel-nitrilotriacetic acid-agarose resin were purchased from Qiagen. Enzyme reagents for DNA manipulations, the QuikChange site-directed mutagenesis kit, and carrier DNA for yeast transformation were obtained from New England Biolabs, Stratagene, and Clontech, respectively. Reagents for electrophoresis, Western blotting, protein determination, DNA size ladders, and molecular mass protein standards were obtained from Bio-Rad. Acrylamide solutions and scintillation counting supplies were from National Diagnostics. Radiochemicals were from PerkinElmer Life Sciences. GE Healthcare was the source of PVDF membrane, the enhanced chemifluorescence Western blotting detection kit, and IgG-Sepharose. Conventional PKC (rat brain) and PKA catalytic subunit (bovine heart) were from Promega. Lipids and thin-layer chromatography plates (cellulose and silica gel 60) were purchased from Avanti Polar Lipids and EM Science, respectively. Alkaline phosphatase-conjugated goat anti-rabbit IgG antibodies, alkaline phosphatase-conjugated goat anti-mouse IgG antibodies, and mouse anti-phosphoglycerate kinase antibodies were from Thermo Scientific, Pierce, and Invitrogen, respectively. Oligonucleotides, l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin, protease and phosphatase inhibitors, phosphoamino acid standards, Triton X-100, and bovine serum albumin were obtained from Sigma-Aldrich. All other chemicals were reagent grade or better.
Strains and Growth Conditions
The Escherichia coli and S. cerevisiae strains used in this study are listed in Table 1. E. coli DH5α and BL21(DE3)pLysS were used for the propagation of plasmids and for the expression of yeast His6-tagged Pah1p, respectively. Bacterial cells were grown at 37 °C in LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl (pH 7)). For the selection of cells carrying plasmids, the growth medium was supplemented with ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml) (58). The expression of Pah1p in cells bearing PAH1 derivatives of plasmid pET-15b was induced with 1 mm isopropyl β-d-thiogalactoside (3). The S. cerevisiae pah1Δ, pah1Δ nem1Δ, and app1Δ dpp1Δ lpp1Δ pah1Δ mutants were used for the expression of wild type and phosphorylation-deficient forms of Pah1p. Yeast were generally grown at 30 °C in YEPD medium (1% yeast extract, 2% peptone, and 2% glucose) or in standard synthetic complete medium containing 2% glucose. Appropriate amino acids were omitted from the synthetic complete growth medium to select for cells carrying specific plasmids (59). Cell numbers in liquid cultures were determined spectrophotometrically at an absorbance of 600 nm. The liquid growth medium was supplemented with agar (2% for yeast or 1.5% for E. coli) for growth on plates.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or Ref. |
|---|---|---|
| Strain | ||
| E. coli | ||
| DH5α | F− φ80dlacZΔM15Δ (lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rk− mk+) phoA supE44 l−thi-1 gyrA96 relA1 | 60 |
| BL21(DE3)pLysS | F− ompT hsdSB (rB−mB−) gal dcm (DE3) pLysS | Novagen |
| BL21(DE3) | F− ompT hsdSB (rB−mB−) gal dcm (DE3) | Invitrogen |
| S. cerevisiae | ||
| W303-1A | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | 98 |
| GHY66 | app1Δ::natMX4 dpp1Δ::TRP1/Kanr lpp1Δ::HIS3/Kanr pah1Δ::URA3 derivative of W303-1A | 79 |
| RS453 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-52 | 14 |
| SS1026 | pah1Δ::TRP1 derivative of RS453 | 9 |
| SS1132 | pah1Δ::TRP1 nem1Δ::HIS3 derivative of RS453 | 36 |
| Plasmid | ||
| pET-15b | E. coli expression vector with N-terminal His6 tag fusion | Novagen |
| pGH313 | PAH1 coding sequence inserted into pET-15b | 3 |
| pGH313-18–862 | PAH1 (18–862) derivative of pGH313 | 38 |
| pGH313-1–752 | PAH1 (1–752) derivative of pGH313 | 38 |
| pGH313-1–646 | PAH1 (1–646) derivative of pGH313 | 38 |
| pGH313-235–752 | PAH1 (235–752) derivative of pGH313 | 38 |
| pGH313-S677A | PAH1 (S677A) derivative of pGH313 | 38 |
| pGH313-S769A | PAH1 (S769A) derivative of pGH313 | This study |
| pGH313-S773A | PAH1 (S773A) derivative of pGH313 | 38 |
| pGH313-S788A | PAH1 (S788A) derivative of pGH313 | 38 |
| pGH313-4A | PAH1 (S677A/S769A/S773A/S788A) derivative of pGH313 | This study |
| pGH313-5A | PAH1 (S10A/S677A/S773A/S774A/S788A) derivative of pGH313 | 38 |
| pGH313-7A (pGH332) | PAH1 (S110A/S114A/S168A/S602A/T723A/S744A/S748A) derivative of pGH313 | 37 |
| pRS415 | Low copy E. coli/yeast shuttle vector with LEU2 | 99 |
| pGH315 | PAH1 inserted into pRS415 | 36 |
| pGH315-S677A | PAH1 (S677A) derivative of pGH315 | 38 |
| pGH315-S677D | PAH1 (S677D) derivative of pGH315 | 38 |
| pGH315-S769A | PAH1 (S769A) derivative of pGH315 | This study |
| pGH315-S769D | PAH1 (S769D) derivative of pGH315 | This study |
| pGH315-S773A | PAH1 (S773A) derivative of pGH315 | 38 |
| pGH315-S773D | PAH1 (S773D) derivative of pGH315 | 38 |
| pGH315-S788A | PAH1 (S788A) derivative of pGH315 | 38 |
| pGH315-S788D | PAH1 (S788D) derivative of pGH315 | 38 |
| pGH315-4A | PAH1 (S677A/S769A/S773A/S788A) derivative of pGH315 | This study |
| pGH315-7A (pHC204) | PAH1 (S110A/S114A/S168A/S602A/T723A/S744A/S748A) derivative of pGH315 | 36 |
| pGH315-7A- S769A | PAH1 (S110A/S114A/S168A/S602A/T723A/S744A/S748A/S769A) derivative of pGH315 | This study |
| pGH315-11A | PAH1 (S110A/S114A/S168A/S602A/S677A/T723A/S744A/S748A/S769A/S773A/S788A) derivative of pGH315 | This study |
| EB1164 | PHO85-His6 derivative of pQE-60 | 65 |
| EB1076 | PHO80 derivative of pSBETA | 65 |
DNA Manipulations
Standard methods were used for the isolation of genomic and plasmid DNA, digestion and ligation of DNA, and PCR amplification of DNA (60, 61). The plasmids used in this study are listed in Table 1. Plasmid pGH313 directs the isopropyl β-d-1-thiogalactopyranoside-induced expression of His6-tagged Pah1p in E. coli (3), whereas plasmid pGH315 directs low copy expression of Pah1p in S. cerevisiae (36). The derivatives of pGH313 and pGH315 that contain serine-to-alanine/aspartate mutations were constructed by QuikChange site-directed mutagenesis using appropriate templates and primers. Plasmids containing multiple missense mutations were constructed by the general strategies described previously (36). All mutations were confirmed by DNA sequencing. Plasmid transformations of E. coli (60) and yeast (62) were performed as described previously.
Preparation of Yeast Cell Extracts and Subcellular Fractionation
All steps were performed at 4 °C. Cell extracts were prepared by disruption of cells with glass beads (0.5-mm diameter) using a BioSpec Products Mini-BeadBeater-16 (63). The cell disruption buffer contained 50 mm Tris-HCl (pH 7.5), 0.3 m sucrose, 10 mm 2-mercaptoethanol, 0.5 mm phenylmethanesulfonyl fluoride, 1 mm benzamidine, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml pepstatin, and phosphatase inhibitor mixtures I and II (3). The cytosol (supernatant) and total membrane (pellet) fractions were separated by centrifugation at 100,000 × g for 1 h (63). The membrane pellets were suspended in the cell disruption buffer to the same volume of the cytosolic fraction. Protein concentration was estimated by the method of Bradford (64) using bovine serum albumin as the standard.
Purification of Recombinant Enzymes
His6-tagged wild type and mutant forms of Pah1p expressed in E. coli BL21(DE3)pLysS were purified by affinity chromatography using nickel-nitrilotriacetic acid-agarose (3, 58). The His6-tagged Pho85p-Pho80p protein kinase complex was purified from E. coli BL21(DE3) expressing plasmids EB1164 and EB1076 (65). SDS-PAGE analyses indicated that the enzyme preparations were highly pure.
Phosphorylation Reactions
Phosphorylation reactions were performed in triplicate for 5–20 min at 30 °C in a total volume of 20 μl. The reaction mixture for PKC contained 50 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 10 mm 2-mercaptoethanol, 1.7 mm CaCl2, 500 μm phosphatidylserine, 156 μm DAG, 100 μm [γ-32P]ATP (3,000 cpm/pmol), 50 μg/ml Pah1p, and the indicated amount of PKC (39). The reaction mixture for PKA contained 25 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 2 mm dithiothreitol, 100 μm [γ-32P]ATP (3,000 cpm/pmol), 50 μg/ml Pah1p, and the indicated amount of PKA (38). The reaction mixture for Pho85p-Pho80p contained 25 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 100 μm dithiothreitol, 100 μm [γ-32P]ATP (3,000 cpm/pmol), 50 μg/ml Pah1p, and the indicated amount of Pho85p-Pho80p (37). At the end of the phosphorylation reactions, samples were treated with 4× Laemmli buffer (66), subjected to SDS-PAGE, and transferred to PVDF membranes. Alternatively, SDS-polyacrylamide gels were dried and used for analysis. Phosphorimaging was used to visualize phosphorylated enzyme, and the extent of phosphorylation was quantified with ImageQuant software. A unit of PKC or PKA activity was defined as the amount of enzyme that catalyzes the formation of 1 pmol of phosphorylated product/min. A unit of Pho85p-Pho80p activity was defined as nmol/min.
Mass Spectrometry Analysis of Pah1p Phosphorylation Sites
Mass spectrometry analysis of phosphorylated Pah1p was performed at the Rutgers Biomedical and Health Sciences-New Jersey Medical School Neuroproteomics Core Facility. After trypsin digestion of phosphorylated Pah1p in SDS-polyacrylamide gel slices, peptides were analyzed by matrix-assisted laser desorption ionization tandem time-of-flight mass spectrometry to identify phosphopeptide candidates. Based on the phosphopeptide ion inclusion list, quadrupole time-of-flight and Orbitrap liquid chromatography-mass spectrometry/mass spectrometry were performed to identify phosphorylation sites (36).
Phosphoamino Acid and Phosphopeptide Mapping Analyses
Phosphorylated Pah1p resolved in the SDS-polyacrylamide gel was transferred to a PVDF membrane and subjected to acid hydrolysis with 6 n HCl at 110 °C (for phosphoamino acid analysis) or to proteolytic digestion with l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (for phosphopeptide mapping analysis) (49, 67, 68). Acid hydrolysates of Pah1p were mixed with standard phosphoamino acids and separated by two-dimensional electrophoresis on cellulose thin-layer chromatography plates, whereas tryptic digests of Pah1p were separated by electrophoresis and TLC using cellulose thin-layer chromatography plates (49, 67, 68). Radioactive phosphoamino acids and phosphopeptides were visualized by phosphorimaging analysis, and standard phosphoamino acids were visualized with ninhydrin stain.
SDS-PAGE and Western Blot Analysis
SDS-PAGE (66) and Western blotting (69, 70) with PVDF membrane were performed by standard protocols. Rabbit anti-Pah1p antibodies (36), rabbit anti-phosphatidylserine synthase antibodies (71), and mouse anti-Pgk1p antibodies were used at a concentration of 1 μg/ml. Alkaline phosphatase-conjugated goat anti-rabbit IgG antibodies and goat anti-mouse IgG antibodies were used at a dilution of 1:5,000. Immune complexes were detected using the enhanced chemifluorescence Western blotting detection kit. Fluorimaging was used to acquire images from Western blots, and the signal intensity of the image was analyzed using ImageQuant software. Signals were in the linear range of detectability.
Measurement of PAP Activity
PAP activity was measured by following the release of water-soluble 32Pi from chloroform-soluble [32P]PA (10,000 cpm/nmol) (63). The 32P-labeled PA used for the assay was enzymatically synthesized from DAG and [γ-32P]ATP using E. coli DAG kinase (63). The PAP reaction contained 50 mm Tris-HCl (pH 7.5), 1 mm MgCl2, 0.2 mm PA, 2 mm Triton X-100, and enzyme protein in a total volume of 0.1 ml. All enzyme assays were conducted in triplicate at 30 °C. The average standard deviation of the assays was ±5%. The reactions were linear with time and protein concentration. A unit of PAP activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol of product/min.
Labeling and Analysis of Lipids
Steady-state labeling of lipids with [2-14C]acetate was performed as described previously (72), and lipids were extracted from labeled cells by the method of Bligh and Dyer (73). Lipids were analyzed by one-dimensional thin-layer chromatography on silica gel plates (74). The identity of radiolabeled TAG on TLC plates was confirmed by comparison of its migration with that of a standard after exposure to iodine vapor. Radiolabeled lipids were visualized by phosphorimaging analysis and quantified using ImageQuant software.
Analyses of Data
Statistical analyses were performed with SigmaPlot software. The p values <0.05 were taken as a significant difference. The Enzyme Kinetics module of SigmaPlot software was used to analyze kinetic data.
RESULTS
Pah1p Is a PKC Substrate
In a previous study, Pah1p was shown to be phosphorylated by PKC in vitro (39), and here, we examined the phosphorylation in more detail. We utilized conventional PKC from rat brain and Pah1p expressed in E. coli (3). The rat brain PKC (mixture of α, β, and γ isoforms) (46, 75) with catalytic properties common to yeast PKC (50, 76–78) has been used to phosphorylate several proteins of yeast lipid metabolism (49, 53, 54). The rationale for using the E. coli-expressed Pah1p was to make the PKC substrate free of prior phosphorylations occurring in yeast (35). Phosphorylation was measured by following the incorporation of the radioactive phosphate from [γ-32P]ATP into Pah1p. Phosphorylated Pah1p was resolved by SDS-PAGE and then detected by phosphorimaging (Fig. 2A). Phosphoamino acid analysis showed that Pah1p is phosphorylated by PKC on the serine residue (Fig. 2B). The Pah1p phosphorylation was dependent on the reaction time and the amount of PKC (Fig. 3, A and B, respectively), indicating that it is a bona fide substrate of PKC. In addition, PKC activity followed Michaelis-Menten kinetics with respect to ATP and Pah1p (Fig. 3, C and D, respectively). The Km for Pah1p was 0.75 μm, and this value is higher than that shown by PKA, Pho85p-Pho80p, or Cdc28p-cyclin B (Table 2). At the point of maximum phosphorylation, PKC catalyzed the incorporation of 0.8 mol of phosphate/mol of Pah1p.
FIGURE 2.
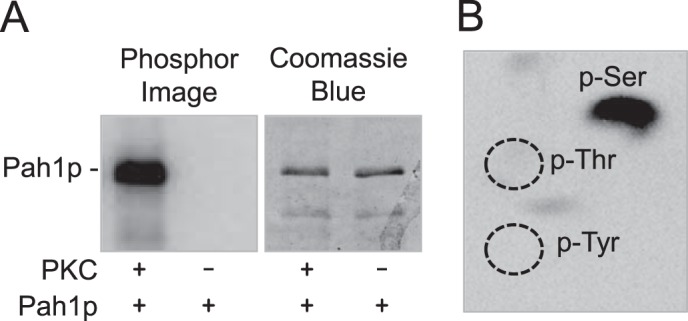
Pah1p is phosphorylated by PKC on the serine residue. Purified recombinant Pah1p (1 μg) was phosphorylated with PKC (2.5 units) and [γ-32P]ATP (1 nmol) for 10 min. Following the reaction, Pah1p was separated from labeled ATP by SDS-PAGE. A, the polyacrylamide gel was dried and subjected to phosphorimaging analysis. After the imaging analysis, the dried gel was swollen in water and stained with Coomassie Blue. B, 32P-labeled Pah1p in the polyacrylamide gel was transferred to a PVDF membrane followed by incubation with 6 n HCl for 90 min at 110 °C. The acid hydrolysates were separated by two-dimensional electrophoresis and subjected to phosphorimaging analysis. The positions of the standard phosphoamino acids phosphoserine (p-Ser), phosphothreonine (p-Thr) (dotted lines), and phosphotyrosine (p-Tyr) (dotted lines) are indicated in the figure. The data shown in A and B are representative of three experiments.
FIGURE 3.
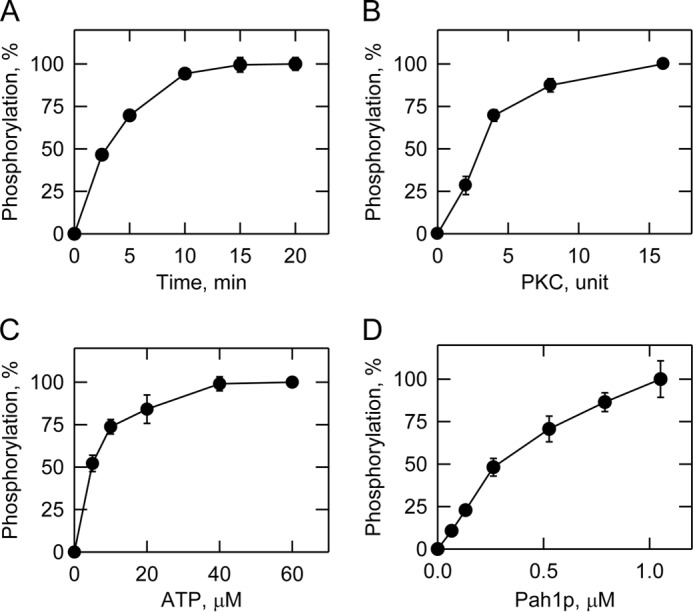
Characterization of PKC activity using Pah1p as a substrate. Phosphorylation of Pah1p by PKC was measured by following the incorporation of the radiolabeled phosphate from [γ-32P]ATP into purified recombinant Pah1p under standard reaction conditions by varying the time (A), amount of PKC (B), and concentrations of ATP (C) and Pah1p (D). Following the reactions, the samples were subjected to SDS-PAGE; the polyacrylamide gels were dried and then subjected to phosphorimaging analysis. The relative amounts of phosphate incorporated into Pah1p were quantified using ImageQuant software. The data shown in A–D are the averages of three experiments ±S.D. (error bars).
TABLE 2.
Kinetic properties of protein kinases that phosphorylate Pah1p
The effect of phosphorylation on Pah1p PAP activity was examined. In this experiment, PAP activity was measured as a function of the surface concentration of PA. As described previously (3), the unphosphorylated Pah1p exhibited positive cooperative kinetics with respect to the PA concentration (Fig. 4). The kinetic behavior of Pah1p PAP activity was moderately affected by PKC (Fig. 4) with 24 and 12% increases in Vmax and Km, respectively (Table 3). The specificity constant of the phosphorylated Pah1p was 10% greater when compared with that of the unphosphorylated enzyme (Table 3).
FIGURE 4.
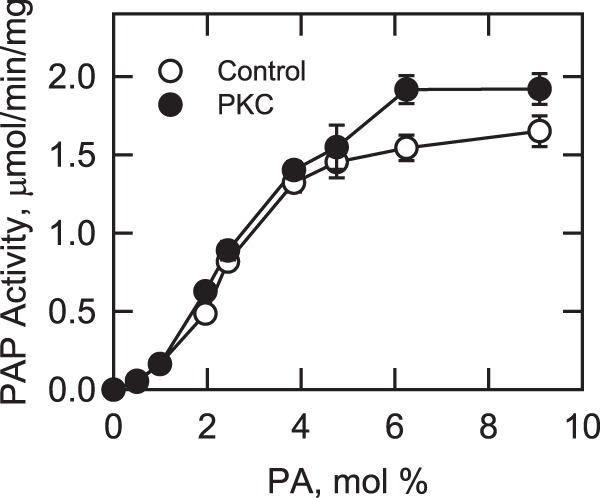
Effect of phosphorylation on Pah1p PAP activity. Purified recombinant Pah1p (0.5 μg) was incubated with and without PKC (10 units) and ATP (2 nmol) for 5 min. The PAP activity of the phosphorylated and unphosphorylated forms of the enzyme was measured as a function of the surface concentration of PA. The molar concentration of PA was held constant at 0.2 mm, and the molar concentration of Triton X-100 was varied to obtain the indicated surface concentrations. The values indicated are the average of three experiments ±S.D. (error bars).
TABLE 3.
Kinetic properties of Pah1p phosphorylated with PKC
The Vmax, Km, and Hill values were determined from the data in Fig. 4 using the Enzyme Kinetics module of SigmaPlot software.
| Pah1p | Vmax | Km | Vmax/Km | Hill |
|---|---|---|---|---|
| μmol min−1 mg−1 | mol % | μmol min−1 mg−1 mol %−1 | n | |
| Control | 1.7 ± 0.05 | 2.5 ± 0.1 | 0.68 | 2.8 |
| PKC | 2.1 ± 0.10 | 2.8 ± 0.2 | 0.75 | 2.3 |
Pah1p Is Phosphorylated by PKC on Ser-677, Ser-769, Ser-773, and Ser-788
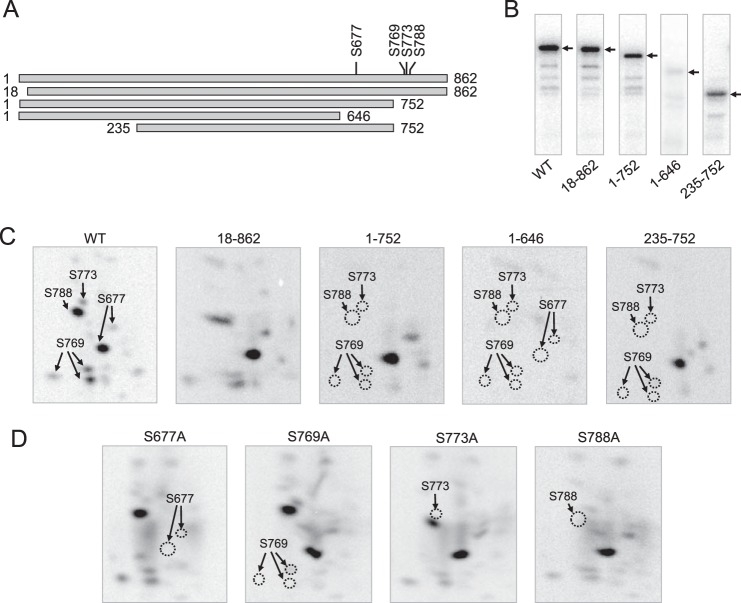
Mass spectrometry, truncation analysis, site-directed mutagenesis, and phosphopeptide mapping were used in combination to determine the PKC phosphorylation sites in Pah1p. Mass spectrometry analysis of phosphorylated full-length Pah1p identified Ser-773 and Ser-788 as the phosphorylation sites. Mutant Pah1p containing S773A or S788A was purified after its expression in E. coli and phosphorylated with PKC. In the phosphopeptide mapping analysis, the S773A and S788A caused the disappearance of specific phosphopeptides (Fig. 5D), confirming that Ser-773 and Ser-788 are target sites of phosphorylation. The presence of other phosphopeptides, however, indicated that additional sites of Pah1p are phosphorylated by PKC (Fig. 5D). In an alternative approach, we examined Pah1p truncations (Fig. 5A) to narrow down the region of PKC target sites. Whereas the N-terminal truncations (e.g. loss of residues 1–17) had little effect on Pah1p phosphorylation, the phosphorylation of the truncations containing residues 1–752, 1–646, and 235–752 was reduced by 65, 92, and 70%, respectively (Fig. 5B). Phosphopeptide mapping analysis of the phosphorylated truncated forms indicated that almost all of the PKC target sites are located between residues 646 and 862 (Fig. 5C). Besides Ser-773 and Ser-788, which were already identified, each of the putative PKC sites in the C-terminal region was mutated to alanine, and the mutational effect was examined by phosphopeptide mapping analysis. Of the mutations examined, only S677A and S769A affected the phosphopeptide map of Pah1p (Fig. 5D), indicating that Ser-677 and Ser-769 are additional phosphorylation sites. By comparing the phosphopeptide maps of S677A, S769A, S773A, and S788A mutants with that of the wild type Pah1p, we could ascribe which site was contained within the phosphopeptide map (Fig. 5D, WT). Multiple phosphopeptides ascribed to phosphorylated Ser-677 and Ser-769 (Fig. 3D, S677A and S769A) are thought to result from incomplete proteolytic digestion at sites that are in close proximity to the phosphorylated serine residues. Overall, four serine residues (Ser-677, Ser-769, Ser-773, and Ser-788) at the C-terminal region of Pah1p were identified as major sites of phosphorylation by PKC.
FIGURE 5.
Identification of Ser-677, Ser-769, Ser-773, and Ser-788 as the PKC phosphorylation sites at the C terminus of Pah1p. A, the diagrams show full-length and truncated forms of purified recombinant Pah1p used for phosphorylation and phosphopeptide mapping analysis. The positions of the phosphorylation sites are indicated in full-length Pah1p. B, samples (1 μg) of the indicated forms of Pah1p were phosphorylated with PKC (2 units) and [γ-32P]ATP (1 nmol) for 20 min. The phosphorylated samples were resolved by SDS-PAGE, transferred to a PVDF membrane, and subjected to phosphorimaging analysis. The arrow indicates the positions of the various forms of Pah1p. C, 32P-labeled Pah1p from the PVDF membrane was digested with l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin. D, the indicated purified recombinant phosphorylation site mutants were phosphorylated with PKC and subjected to proteolytic digestion as described for the truncation mutants. The digested peptides were separated on cellulose thin-layer plates by electrophoresis (from left to right) in the first dimension and by chromatography (from bottom to top) in the second dimension followed by phosphorimaging analysis. The identity of the phosphorylation sites in the radioactive phosphopeptides of full-length wild type Pah1p was determined from the maps of the truncation and phosphorylation site mutant enzymes. The positions of the phosphopeptides that were absent in the mutant enzymes (indicated by the dotted line ellipse) but present in the wild type full-length enzyme are indicated in the figure. The data are representative of three independent experiments.
Effects of PKC Phosphorylation Site Mutations on Pah1p Phosphorylation and Its PAP Activity
We examined the effects of the PKC target site mutations, singly or in combination, on the phosphorylation of Pah1p. S677A, S769A, S773A, and S788A caused a decrease in the phosphorylation of Pah1p by 51, 27, 35, and 59%, respectively (Fig. 6A). This result indicated that the efficiency of phosphorylation on each of the serine residues is different, suggesting that Ser-677 and Ser-788 are better phosphorylated than Ser-769 and Ser-773. The quadruple mutations (i.e. S677A/S769A/S773A/S788A), designated as 4A, reduced the Pah1p phosphorylation by 62% (Fig. 6B). The effects of the combined mutations were not cumulative and were smaller than the effect of the C-terminal truncation (e.g. 1–646) described above. Considering the presence of several minor phosphopeptides of Pah1p (Fig. 5C), this result suggested that the phosphorylation of unidentified PKC sites, which are poorly phosphorylated in wild type Pah1p, is increased in the absence of major phosphorylation sites.
FIGURE 6.
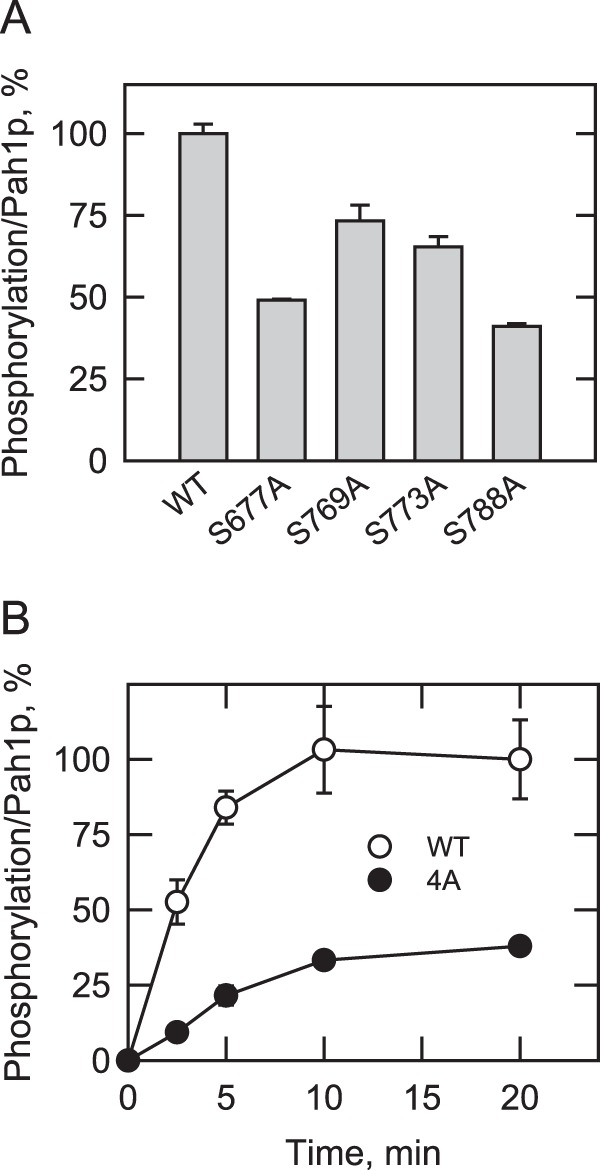
Effects of the PKC phosphorylation site mutations on the phosphorylation of Pah1p. Wild type and the indicated Pah1p phosphorylation site mutants were expressed and purified from E. coli. The Pah1p enzymes were phosphorylated with PKC (2 units) and [γ-32P]ATP (1 nmol) for 20 min (A) or for the indicated time intervals (B). Following the reaction, Pah1p was separated from labeled ATP by SDS-PAGE. The polyacrylamide gel was dried and subjected to phosphorimaging and ImageQuant analysis. To control for loading, the dried gel was swollen with water, stained with Coomassie Blue, and subjected to image analysis. The relative amount of the phosphorylated wild type enzyme was arbitrarily set at 100%. The data reported are the average of three independent experiments ±S.D. (error bars).
We also examined the effects of the phosphorylation site mutations on PAP activity. The recombinant wild type and the phosphorylation-deficient single and 4A mutations were phosphorylated with PKC and then assayed for PAP activity under standard conditions. The enzyme activity without the PKC phosphorylation was used as control activity. This analysis indicated that the phosphorylation site mutations did not have a significant effect on PAP activity. In a second experiment, PAP activity was measured from the extracts of the app1Δ dpp1Δ lpp1Δ pah1Δ mutant expressing the wild type or 4A mutant allele of PAH1 on a low copy plasmid. The quadruple mutant strain lacking all PAP genes was used to eliminate interference from the activity of PAP enzymes encoded by endogenous PAH1 as well as by other PAP genes APP1, DPP1, and LPP1 (79). The PAP activity of cells expressing the phosphorylation-deficient 4A allele was not significantly different from the activity of cells expressing the wild type PAH1 allele (data not shown). These results substantiated the data shown in Fig. 4, indicating that the PAP activity of Pah1p is not significantly affected by its PKC phosphorylation.
Prephosphorylation of Pah1p by PKA or Pho85p-Pho80p Inhibits Its Subsequent Phosphorylation by PKC
In this set of experiments, we questioned whether the prior phosphorylations of Pah1p with PKA or Pho85p-Pho80p affect its phosphorylation with PKC. We did not address phosphorylation interrelationships with Cdc28p-cyclin B because all its target sites are included in the seven sites phosphorylated by Pho85p-Pho80p (Fig. 1B). Pah1p was first phosphorylated with either PKA (38) or Pho85p-Pho80p (37) using unlabeled ATP as described previously. After the first phosphorylations, Pah1p was then phosphorylated with PKC using [γ-32P]ATP, and the extent of radioactive phosphorylation was determined after SDS-PAGE by phosphorimaging and ImageQuant analysis. A similar procedure was utilized to examine phosphorylation with other kinase combinations. The control for these experiments was Pah1p that was not subject to prephosphorylation. When Pah1p was prephosphorylated with PKA, its subsequent phosphorylation with PKC was reduced by 76% (Fig. 7A). PKA phosphorylates Pah1p at five serine residues (38), and coincidently, three of these residues (e.g. Ser-677, Ser-773, and Ser-788) are also sites phosphorylated by PKC (Fig. 1B). This provides an explanation why prephosphorylation with PKA affected the phosphorylation with PKC. This conclusion is further supported by the fact that mutant Pah1p (i.e. 5A) in which all five PKA sites were mutated to alanine residues was reduced for its PKC phosphorylation whether or not it was subject to prior treatment with PKA (Fig. 7A). In a reciprocal experiment, prephosphorylation with PKC caused a 60% reduction in the subsequent phosphorylation with PKA, and the phosphorylation by PKA was similarly reduced in the PKC phosphorylation-deficient 4A mutant whether or not it was subject to pretreatment with PKC (Fig. 7B).
FIGURE 7.
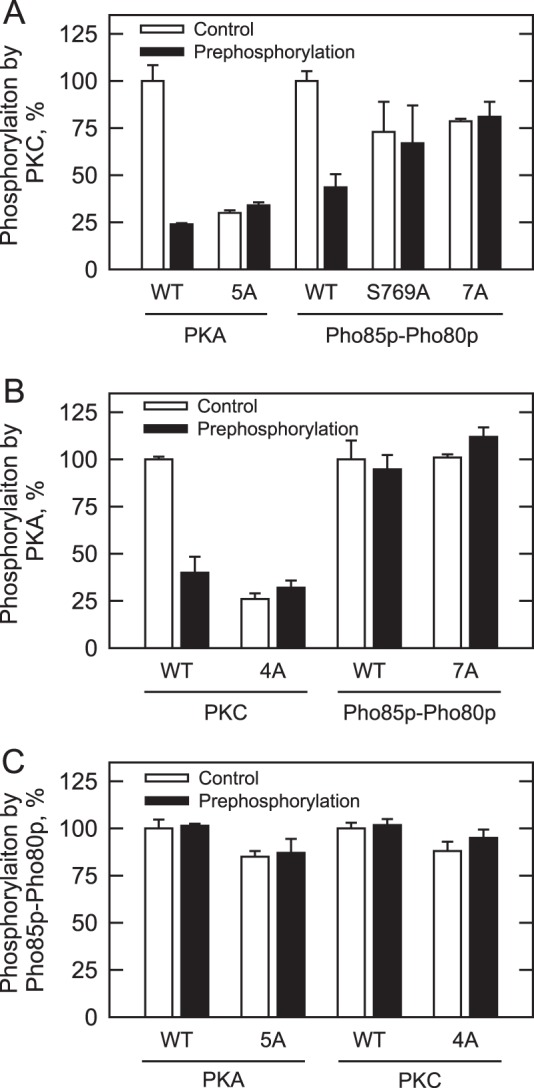
Pah1p phosphorylation by PKA or Pho85p-Pho80p reduces subsequent phosphorylation by PKC. Wild type and the indicated phosphorylation site mutants were expressed and purified from E. coli. The Pah1p enzymes were phosphorylated with PKC (A), PKA (B), or Pho85p-Pho80p (C) using [γ-32P]ATP without or with prephosphorylation by the indicated protein kinases using unlabeled ATP. Following the reactions, Pah1p was separated from labeled ATP by SDS-PAGE. The polyacrylamide gel was dried and subjected to phosphorimaging and ImageQuant analysis. To control for loading, the dried gel was swollen with water, stained with Coomassie Blue, and subjected to image analysis. The relative amount of the phosphorylated wild type enzyme was arbitrarily set at 100%. The data reported are the average of four independent experiments ±S.D. (error bars).
The target phosphorylation sites of Pho85p-Pho80p are distinct from those of PKC and PKA (Fig. 1B). However, prephosphorylation with Pho85p-Pho80p resulted in a 57% decrease in the subsequent PKC phosphorylation (Fig. 7A). In contrast, the prephosphorylation with Pho85p-Pho80p did not affect the phosphorylation with PKA (Fig. 7B). These results suggested that phosphorylation on Ser-769, a unique PKC site that does not overlap with the PKA sites, was affected by prephosphorylation with Pho85p-Pho80p. Indeed, the phosphorylation of the S769A mutant with Pho85p-Pho80p had no effect on the subsequent phosphorylation with PKC (Fig. 7A). The inhibitory effect of Pho85p-Pho80p on the PKC phosphorylation was also confirmed by the fact that mutant Pah1p (i.e. 7A) lacking seven sites of Pho85p-Pho80p phosphorylation did not show a change in the PKC phosphorylation when pretreated with Pho85p-Pho80p (Fig. 7A). In reciprocal experiments, the prephosphorylation of Pah1p (wild type, 4A, or 5A) with PKC or PKA did not affect its phosphorylation by Pho85p-Pho80p (Fig. 7C).
Effects of the PKC and Pho85p-Pho80p Phosphorylation Site Mutations on Cell Growth, Pah1p Abundance and Location, and TAG Synthesis
The function of Pah1p in vivo depends on its dephosphorylation catalyzed by the Nem1p-Spo7p protein phosphatase complex (35–38, 80). The dephosphorylation permits Pah1p to associate with the membrane through a short N-terminal amphipathic helix that facilitates binding to PA and catalysis (80). Thus, a lack of the Nem1p-Spo7p complex (e.g. nem1Δ) results in phenotypes characteristic of the pah1Δ mutant that include temperature sensitivity (36, 38), aberrant expansion of the nuclear/endoplasmic reticulum membrane (36), reduction in TAG content (30, 36, 38), and derepression of phospholipid synthesis genes (9). That alanine mutations of the seven sites (i.e. 7A mutations) phosphorylated by Pho85p-Pho80p bypass the requirement of Nem1p-Spo7p dephosphorylation indicates the importance of phosphorylation/dephosphorylation in regulating Pah1p function (36–38). To examine the effect of the PKC site mutations, we examined the growth of pah1Δ and pah1Δ nem1Δ cells that express Pah1p in which PKC target sites are mutated to alanine residues. The mutant Pah1p containing the alanine mutations, singly or in combination, did not complement the temperature sensitivity of the pah1Δ nem1Δ mutant. This result indicates that Pah1p lacking the PKC phosphorylation does not bypass the requirement of dephosphorylation by Nem1p-Spo7p. The PKC phosphorylation-deficient 4A mutations did not affect the 7A mutations in the complementation of temperature sensitivity shown by pah1Δ nem1Δ cells. The effect of the combined 11A mutations was not significantly different from the effect of the 7A mutations on the growth of pah1Δ nem1Δ cells at 30 or 37 °C. In addition, the phosphorylation mimic (aspartate) mutations of the PKC sites did not complement the temperature sensitivity exhibited by pah1Δ nem1Δ cells.
Previous work has shown that the phosphorylation state of Pah1p affects its abundance (36–38). For example, the level of Pah1p is elevated in the nem1Δ mutant, but its level is reduced by containing the 7A mutations (36, 37). Here, we examined the effect of the PKC phosphorylation site mutations on the level of Pah1p at the late exponential phase of growth. Western blot analysis of pah1Δ and pah1Δ nem1Δ cells expressing Pah1p with the PKC site mutations, singly or in combination, indicated no major effects on the protein level (Fig. 8A). However, the PKC site mutations (4A) reversed the negative effect that the 7A mutations had on Pah1p abundance. The 7A mutations introduced into Pah1p caused a reduction in the protein level by 40%, whereas the 4A mutations introduced into the mutant Pah1p restored its level to that of the wild type control (Fig. 8A). The level of Pah1p containing the 7A/S769A mutations was essentially the same as that containing the 7A mutations, indicating that the other PKC sites also played a role in this regulation (data not shown). The serine-to-aspartate mutations of the PKC sites did not affect Pah1p abundance (data not shown).
FIGURE 8.
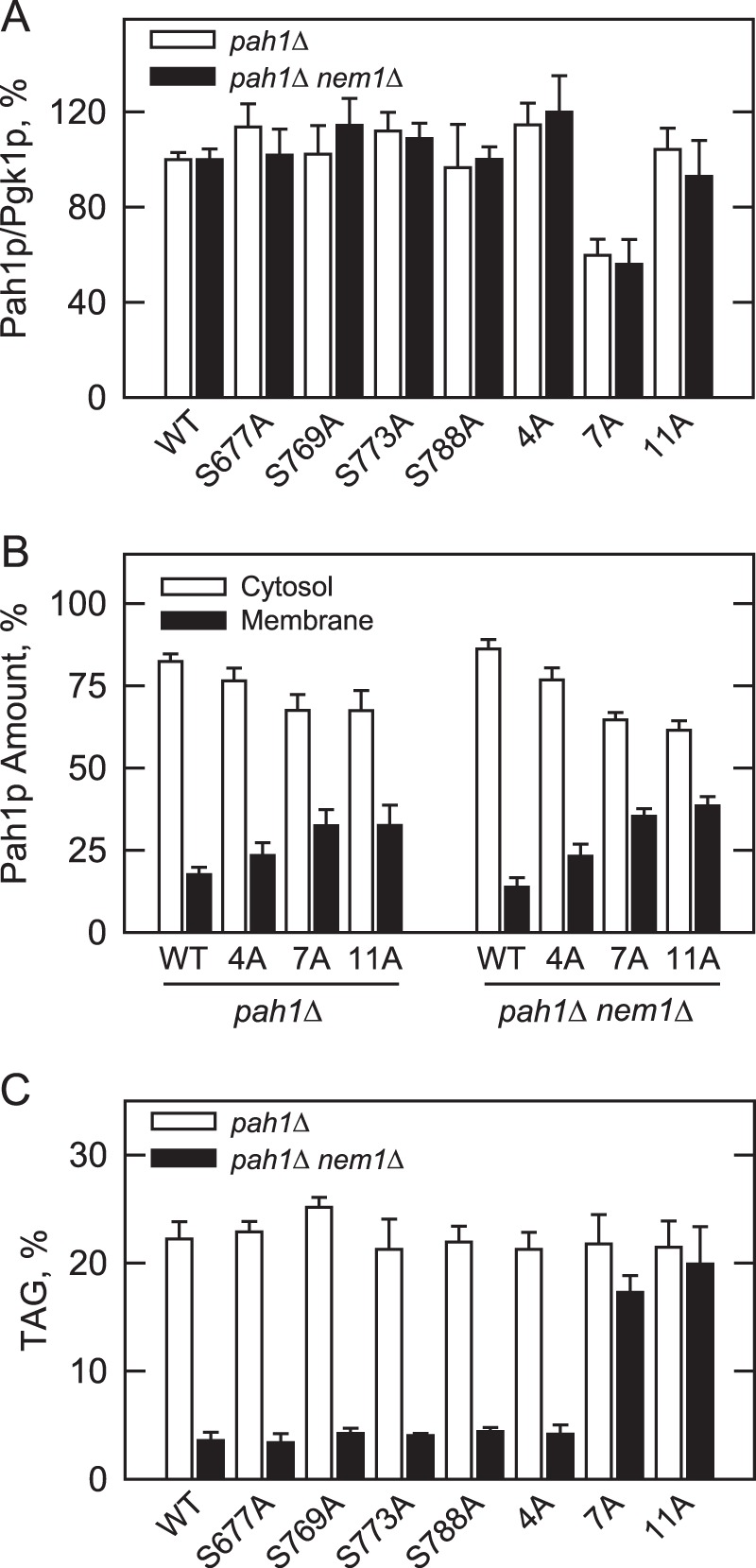
Effects of the PKC and Pho85p-Pho80p phosphorylation site mutations on the abundance and location of Pah1p and TAG content. The indicated wild type and phosphorylation site mutant forms of Pah1p were expressed in pah1Δ and pah1Δ nem1Δ cells. A, cell extracts were prepared from late exponential phase cells (A600 nm ∼ 0.8) and used for Western blot analysis using anti-Pah1p and anti-Pgk1p (loading control) antibodies. The levels of Pah1p and Pgk1p were quantified with ImageQuant software. The relative amount of Pah1p/Pgk1p of the wild type enzyme was arbitrarily set at 100%. B, cell extracts were fractionated into the cytosol and membrane fractions by centrifugation. The membrane fraction was resuspended in the same volume as the cytosol fraction, and equal volumes of the fractions were subjected to Western blot analysis using anti-Pah1p, anti-Pgk1p (cytosol marker), and anti-phosphatidylserine synthase (endoplasmic reticulum marker) antibodies. As described previously (100), the Western blot analysis for the marker proteins indicated highly enriched cytosol and membrane fractions. The relative amounts of cytosolic and membrane-associated Pah1p were determined for the wild type and phosphorylation site mutant forms of the enzyme by ImageQuant analysis of the data. Each data point represents the average of four experiments ±S.D. (error bars). C, cultures were grown to the stationary phase (A600 nm ∼ 3) in the medium containing [2-14C]acetate (1 μCi/ml) to label lipids. The lipids were extracted and separated by one-dimensional TLC, and the phosphorimages were subjected to ImageQuant analysis. The percentages shown for TAG were normalized to the total 14C-labeled chloroform-soluble fraction. Each data point represents the average of three experiments ±S.D. (error bars).
The effects of PKC target site mutations on the localization of Pah1p were examined at the late exponential phase of growth. In the pah1Δ and pah1Δ nem1Δ mutants, the levels of wild type Pah1p associated with the membrane fraction were 17 and 14%, respectively (Fig. 8B). The PKC 4A mutations caused a small but reproducible increase (35% in pah1Δ and 64% in pah1Δ nem1Δ) in membrane association when compared with the wild type control (Fig. 8B). The single mutations of PKC sites, however, did not show a significant effect on the location of Pah1p (data not shown). As described previously (36–38), the 7A mutations caused an increase (135 and 206%, respectively) in the relative proportion of Pah1p associated with the membranes of both pah1Δ and pah1Δ nem1Δ cells (Fig. 8B). Combining the 4A and 7A mutations (i.e. 11A mutations) did not further affect the membrane association of Pah1p over that caused by the 7A mutations (Fig. 8B).
The effects of the PKC phosphorylation-deficient mutations on the role of Pah1p in TAG synthesis were examined in the pah1Δ and pah1Δ nem1Δ mutants at the stationary phase of growth when TAG synthesis dominates over phospholipid synthesis and is most affected by Pah1p PAP activity (3, 12, 30). As described previously (36–38), the nem1Δ mutation introduced into pah1Δ cells expressing wild type Pah1p caused an 84% reduction in the TAG level (Fig. 8C), illustrating the importance of Nem1p-Spo7p in the regulation of Pah1p function in vivo (36). This defect was complemented by replacing the expression of wild type Pah1p with that of the mutant protein lacking the Pho85p-Pho80p target sites (36–38). This complementary effect, however, was not shown by the expression of Pah1p lacking the PKC target sites (singly and in combination) (Fig. 8C). Similarly, the level of TAG in the pah1Δ nem1Δ mutant expressing Pah1p lacking the Pho85p-Pho80p target sites was not significantly affected by introduction of the PKC site mutations into the protein (Fig. 8C).
DISCUSSION
The posttranslational modification of phosphorylation plays a major role in regulating Pah1p function and lipid metabolism in S. cerevisiae (13, 42, 43, 81). Multiple phosphorylation sites (e.g. ∼30) have been identified in Pah1p (35, 82–87), and at least 30 different protein kinases have been shown to phosphorylate the enzyme in vitro (39, 85). The protein kinase-target site relationship, however, has been confirmed for only a few of these kinases, namely Pho85p-Pho80p, Cdc28p-cyclin B, PKA, and now PKC (Fig. 1B). This knowledge, along with the analysis of phosphorylation-deficient forms of the enzyme, has advanced our understanding of the physiological relevance of these phosphorylations (35–38, 80).
In this study, we established that Pah1p is a bona fide substrate for PKC and identified four serine residues (Ser-677, Ser-769, Ser-773, and Ser-788) as major sites of phosphorylation. The phosphopeptide mapping analysis indicated that the four sites in Pah1p were not uniformly phosphorylated. The phosphorylations of Ser-677 and Ser-788 were greater than those of Ser-769 and Ser-773, and Ser-773 was least phosphorylated. These differences suggest that the phosphorylation of one site might inhibit the phosphorylation of another site (88–91), which could explain why the stoichiometry of 0.8 mol of phosphate/mol of Pah1p was lower than the theoretical value of 4. This finding is not uncommon for Pah1p; the stoichiometry values for the phosphorylations mediated by PKA, Cdc28p-cyclin B, and Pho85p-Pho80p are also lower than expected values (Table 2).
The specificity of the PKC phosphorylation was similar to that of the other protein kinases with respect to their relative Km values for ATP, but the Km for Pah1p in the PKC phosphorylation was 3- and 1.7-fold greater than those in the phosphorylations mediated by the cyclin-dependent protein kinases and PKA, respectively (Table 2). Cross-talk between phosphorylations has been shown for several proteins that are multiply phosphorylated by the same or different protein kinases (88–91). Here, we showed that the phosphorylation of Pah1p by PKC, like its phosphorylation by Pho85p-Pho80p (37), Cdc28p-cyclin B (36), and PKA (38), did not require a prior phosphorylation. In addition, a prior phosphorylation by one protein kinase did not stimulate its phosphorylation by another protein kinase. However, the prior phosphorylation with PKA or Pho85p-Pho80p reduced the subsequent phosphorylation with PKC. The inhibitory effect of PKA on PKC phosphorylation may be explained, at least in part, by the overlapping target sites for these kinases (Fig. 1B). Indeed, PKC also reduced the subsequent phosphorylation with PKA. Our data indicated that phosphorylation of Ser-769, a unique PKC site, was inhibited by the prior phosphorylation with Pho85p-Pho80p. The other PKC sites, which are also PKA sites, did not appear to be affected by the prephosphorylation with Pho85p-Pho80p. The negative effect of Pho85p-Pho80p on PKC phosphorylation might be explained by a structural change in Pah1p. For example, one of the sites (e.g. Thr-723) phosphorylated by Pho85p-Pho80p (and Cdc28p-cyclin B) causes a decrease in electrophoretic mobility upon SDS-PAGE (36, 37), suggesting a change in the enzyme structure. Well defined structural studies will be necessary to address this notion more fully.
The effects of the Pah1p phosphorylation by PKC were significantly different from those of Pho85p-Pho80p. The phosphorylation by Pho85p-Pho80p prevents Pah1p from associating with the nuclear/endoplasmic reticulum membrane and inhibits PAP activity and the synthesis of TAG (36, 37, 80). However, the phosphorylation by Pho85p-Pho80p has a stabilizing effect on Pah1p abundance (36, 37). Moreover, 7A mutations allow Pah1p to bypass the Nem1p-Spo7p requirement for TAG synthesis and suppress temperature sensitivity caused by the nem1Δ mutation (36–38). Alanine mutations of the Cdc28p-cyclin B sites only partially mimic the physiological consequences of the 7A mutations (36). Thus, Pho85p-Pho80p is the main regulator in modulating Pah1p function (36, 37). The phosphorylation of the unique PKA site, namely Ser-10, but not the sites that are also sites for PKC, accentuates the effects imparted by the phosphorylation of the Pho85p-Pho80p sites (38). In contrast, the phosphorylation with PKC did not have major effects on Pah1p location, PAP activity, or TAG synthesis. Moreover, the phosphorylation-deficient PKC 4A mutations did not suppress the nem1Δ mutation with respect to TAG synthesis or temperature sensitivity caused by the nem1Δ mutation. However, PKC had a striking effect on Pah1p abundance. Data with the cells expressing the phosphorylation-deficient 4A mutations indicated that PKC had a destabilizing effect on Pah1p abundance but only when the enzyme was not previously phosphorylated on the Pho85p-Pho80p target sites. The destabilizing effect of PKC required the phosphorylation of all four target sites; none of the individual mutations (including the S769A) stabilized Pah1p. Based on the relative Km values for Pah1p (Table 2), the phosphorylation by Pho85p-Pho80p would be favored over PKC. Moreover, the prephosphorylation of Pah1p with Pho85p-Pho80p inhibited the phosphorylation with PKC. Thus, the phosphorylations of the seven sites would eliminate the destabilizing effect of PKC on Pah1p abundance. Alternatively, the phosphorylation by PKC would not be hindered in cells bearing the phosphorylation-deficient 7A mutations, and in fact, these mutations render a reduced level of Pah1p abundance (36, 37). This observation raised the suggestion that the PKC phosphorylations might be responsible for the 7A mutant enzyme loss of abundance. These explanations are supported by the observation that the combination of the PKC 4A mutations with the 7A mutations brought the level of Pah1p abundance back to the level of the wild type control.
The cross-talk regulation of Pah1p abundance by PKC and Pho85p-Pho80p begs the question of when the phosphorylations might occur in vivo. One possibility is that PKC phosphorylation might prevail in the late exponential phase when this kinase is required for cells to enter stationary phase and Pho85p-Pho80p is less active (92, 93). The inhibitory effects (e.g. Pah1p cytosolic location and inhibition of PAP activity) of the phosphorylation by Pho85p-Pho80p correlate with phospholipid synthesis being favored over TAG synthesis in the exponential phase of growth (30, 41). Incidentally, low PAP activity is associated with utilization of PA for the synthesis of membrane phospholipids via the CDP-DAG pathway (3, 4, 42, 43). Elevated PKC activity in the late exponential phase coupled with a reduced level of Pho85p-Pho80p activity correlates with the loss of Pah1p abundance observed in this phase of growth (30, 37, 40). Another possibility is that the phosphorylation by PKC would prevail when the sites phosphorylated by Pho85p-Pho80p are dephosphorylated by the Nem1p-Spo7p phosphatase complex, which is required for Pah1p function and TAG synthesis throughout growth (30, 36, 37). That PKC had little effect on TAG synthesis suggests that Pah1p is phosphorylated and thereby degraded after it is localized to the membrane via its dephosphorylation by Nem1p-Spo7p and exerts its PAP activity. However, at this point in our studies, we cannot discriminate between these two possibilities or whether they are mutually exclusive.
We sought to corroborate the role of PKC phosphorylation in the stability/degradation of Pah1p using the pkc1Δ mutant, which lacks PKC (55). We predicted that if PKC destabilizes Pah1p then the enzyme abundance might be elevated in pkc1Δ mutant cells. Because of the fact that the pkc1Δ mutant exhibits defects in the cell wall integrity pathway, high levels of sorbitol or salts must be supplemented to the medium to sustain growth (56, 94, 95). The results of our experiments were complicated by the fact that 1 m sorbitol or 0.5 m KCl increased Pah1p abundance in the wild type control strain. Thus, the effect of the pkc1Δ mutation on Pah1p abundance could not be interpreted in a meaningful way. Nonetheless, our in vitro and in vivo studies using purified reagents and the PKC phosphorylation-deficient mutations supported the role of PKC in regulating Pah1p PAP. Moreover, we postulate that this regulation is a component of the coordinate regulation that PKC plays in overall lipid metabolism.
PKC is known to phosphorylate and thereby stimulate the activities of Ura7p CTP synthetase (49–52) and Cki1p choline kinase (53). The analysis of cells expressing phosphorylation-deficient forms of these enzymes indicates that the PKC phosphorylations of the enzymes cause the stimulation of phosphatidylcholine synthesis (51–53). These PKC substrates have metabolic relationships with the PAP reaction (42, 43, 96). CTP synthetase and choline kinase provide intermediates (e.g. CTP and phosphocholine, respectively) that lead to the formation of phosphatidylcholine from DAG via the CDP-choline branch of the Kennedy pathway (42, 43, 97). CTP is also utilized along with PA for the formation of CDP-DAG, the liponucleotide intermediate of all membrane phospholipids synthesized via the CDP-DAG pathway (42, 43, 97). PKC also plays a role in the regulation of phospholipid synthesis by phosphorylating the transcriptional repressor Opi1p (54). This phosphorylation attenuates repressor function and stimulates phospholipid synthesis gene expression (54). The Opi1p-mediated regulation of phospholipid synthesis gene expression is governed by a pool of PA that is controlled by Pah1p PAP activity (4, 9, 35, 42, 43, 97). Thus, the multiple phosphorylations by PKC appear to be connected for the coordinate regulation of lipid synthesis throughout growth. However, the PKC phosphorylation of Pah1p is only a component of the multiple protein kinase-mediated phosphorylations that regulate the enzyme.
Acknowledgments
We thank Symeon Siniossoglou for supplying the pah1Δ and pah1Δ nem1Δ mutants and Erin O'Shea for the plasmids to express Pho85p-Pho80p. Mass spectrometry data were obtained from an Orbitrap instrument funded in part by National Institutes of Health Grant NS046593 for the support of the Rutgers Biomedical and Health Sciences-New Jersey Medical School Neuroproteomics Core Facility.
This work was supported, in whole or in part, by National Institutes of Health Grant GM050679 from the United States Public Health Service.
- PAP
- phosphatidate phosphatase
- PA
- phosphatidate
- DAG
- diacylglycerol
- TAG
- triacylglycerol
- 4A
- alanine mutations of PKC phosphorylation sites Ser-677, Ser-769, Ser-773, and Ser-788
- 5A
- alanine mutations of PKA phosphorylation sites Ser-10, Ser-677, Ser-773, Ser-774, and Ser-788
- 7A
- alanine mutations of Pho85p-Pho80p phosphorylation sites Ser-110, Ser-114, Ser-168, Ser-602, Thr-723, Ser-744, and Ser-748
- 11A
- alanine mutations of the PKC plus Pho85p-Pho80p phosphorylation sites.
REFERENCES
- 1. Smith S. W., Weiss S. B., Kennedy E. P. (1957) The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 228, 915–922 [PubMed] [Google Scholar]
- 2. Kennedy E. P. (1961) Biosynthesis of complex lipids. Fed. Proc. 20, 934–940 [PubMed] [Google Scholar]
- 3. Han G.-S., Wu W.-I., Carman G. M. (2006) The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281, 9210–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han G.-S., Siniossoglou S., Carman G. M. (2007) The cellular functions of the yeast lipin homolog Pah1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282, 37026–37035 [DOI] [PubMed] [Google Scholar]
- 5. Koonin E. V., Tatusov R. L. (1994) Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity. Application of an iterative approach to database search. J. Mol. Biol. 244, 125–132 [DOI] [PubMed] [Google Scholar]
- 6. Madera M., Vogel C., Kummerfeld S. K., Chothia C., Gough J. (2004) The SUPERFAMILY database in 2004: additions and improvements. Nucleic Acids Res. 32, D235–D239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Péterfy M., Phan J., Xu P., Reue K. (2001) Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27, 121–124 [DOI] [PubMed] [Google Scholar]
- 8. Irie K., Takase M., Araki H., Oshima Y. (1993) A gene, SMP2, involved in plasmid maintenance and respiration in Saccharomyces cerevisiae encodes a highly charged protein. Mol. Gen. Genet. 236, 283–288 [DOI] [PubMed] [Google Scholar]
- 9. Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S., Siniossoglou S. (2005) The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24, 1931–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adeyo O., Horn P. J., Lee S., Binns D. D., Chandrahas A., Chapman K. D., Goodman J. M. (2011) The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 192, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasser T., Qiu Q. S., Karunakaran S., Padolina M., Reyes A., Flood B., Smith S., Gonzales C., Fratti R. A. (2012) The yeast lipin 1 orthologue Pah1p regulates vacuole homeostasis and membrane fusion. J. Biol. Chem. 287, 2221–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fakas S., Qiu Y., Dixon J. L., Han G.-S., Ruggles K. V., Garbarino J., Sturley S. L., Carman G. M. (2011) Phosphatidate phosphatase activity plays a key role in protection against fatty acid-induced toxicity in yeast. J. Biol. Chem. 286, 29074–29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pascual F., Carman G. M. (2013) Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim. Biophys. Acta 1831, 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han G.-S., O'Hara L., Carman G. M., Siniossoglou S. (2008) An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 283, 20433–20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phan J., Reue K. (2005) Lipin, a lipodystrophy and obesity gene. Cell Metab. 1, 73–83 [DOI] [PubMed] [Google Scholar]
- 16. Lindegaard B., Larsen L. F., Hansen A. B., Gerstoft J., Pedersen B. K., Reue K. (2007) Adipose tissue lipin expression levels distinguish HIV patients with and without lipodystrophy. Int. J. Obes. 31, 449–456 [DOI] [PubMed] [Google Scholar]
- 17. Nadra K., de Preux Charles A.-S., Médard J.-J., Hendriks W. T., Han G.-S., Grès S., Carman G. M., Saulnier-Blache J.-S., Verheijen M. H., Chrast R. (2008) Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22, 1647–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeharia A., Shaag A., Houtkooper R. H., Hindi T., de Lonlay P., Erez G., Hubert L., Saada A., de Keyzer Y., Eshel G., Vaz F. M., Pines O., Elpeleg O. (2008) Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 83, 489–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reue K., Brindley D. N. (2008) Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49, 2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reue K., Dwyer J. R. (2009) Lipin proteins and metabolic homeostasis. J. Lipid Res. 50, (suppl.) S109–S114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donkor J., Zhang P., Wong S., O'Loughlin L., Dewald J., Kok B. P., Brindley D. N., Reue K. (2009) A conserved serine residue is required for the phosphatidate phosphatase activity but not transcriptional coactivator functions of lipin-1 and lipin-2. J. Biol. Chem. 284, 29968–29978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H. B., Kumar A., Wang L., Liu G. H., Keller S. R., Lawrence J. C., Jr., Finck B. N., Harris T. E. (2010) Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol. Cell. Biol. 30, 3126–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grkovich A., Dennis E. A. (2009) Phosphatidic acid phosphohydrolase in the regulation of inflammatory signaling. Adv. Enzyme Regul. 49, 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mul J. D., Nadra K., Jagalur N. B., Nijman I. J., Toonen P. W., Médard J. J., Grès S., de Bruin A., Han G.-S., Brouwers J. F., Carman G. M., Saulnier-Blache J. S., Meijer D., Chrast R., Cuppen E. (2011) A hypomorphic mutation in Lpin1 induces progressively improving neuropathy and lipodystrophy in the rat. J. Biol. Chem. 286, 26781–26793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nadra K., Médard J. J., Mul J. D., Han G.-S., Grès S., Pende M., Metzger D., Chambon P., Cuppen E., Saulnier-Blache J. S., Carman G. M., Desvergne B., Chrast R. (2012) Cell autonomous lipin 1 function is essential for development and maintenance of white and brown adipose tissue. Mol. Cell. Biol. 32, 4794–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michot C., Mamoune A., Vamecq J., Viou M. T., Hsieh L.-S., Testet E., Lainé J., Hubert L., Dessein A. F., Fontaine M., Ottolenghi C., Fouillen L., Nadra K., Blanc E., Bastin J., Candon S., Pende M., Munnich A., Smahi A., Djouadi F., Carman G. M., Romero N., de Keyzer Y., de Lonlay P. (2013) Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim. Biophys. Acta 1832, 2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carman G. M., Han G.-S. (2006) Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 31, 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carman G. M., Han G.-S. (2009) Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 284, 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carman G. M. (2011) The discovery of the fat-regulating phosphatidic acid phosphatase gene. Front. Biol. 6, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pascual F., Soto-Cardalda A., Carman G. M. (2013) PAH1-encoded phosphatidate phosphatase plays a role in the growth phase- and inositol-mediated regulation of lipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 288, 35781–35792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soto-Cardalda A., Fakas S., Pascual F., Choi H. S., Carman G. M. (2012) Phosphatidate phosphatase plays role in zinc-mediated regulation of phospholipid synthesis in yeast. J. Biol. Chem. 287, 968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu W.-I., Carman G. M. (1996) Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by phospholipids. Biochemistry 35, 3790–3796 [DOI] [PubMed] [Google Scholar]
- 33. Wu W.-I., Lin Y.-P., Wang E., Merrill A. H., Jr., Carman G. M. (1993) Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by sphingoid bases. J. Biol. Chem. 268, 13830–13837 [PubMed] [Google Scholar]
- 34. Wu W.-I., Carman G. M. (1994) Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by nucleotides. J. Biol. Chem. 269, 29495–29501 [PubMed] [Google Scholar]
- 35. O'Hara L., Han G.-S., Peak-Chew S., Grimsey N., Carman G. M., Siniossoglou S. (2006) Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 281, 34537–34548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi H.-S., Su W.-M., Morgan J. M., Han G.-S., Xu Z., Karanasios E., Siniossoglou S., Carman G. M. (2011) Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae. Identification of Ser602, Thr723, and Ser744 as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase. J. Biol. Chem. 286, 1486–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi H.-S., Su W.-M., Han G.-S., Plote D., Xu Z., Carman G. M. (2012) Pho85p-Pho80p phosphorylation of yeast Pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism. J. Biol. Chem. 287, 11290–11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su W.-M., Han G.-S., Casciano J., Carman G. M. (2012) Protein kinase A-mediated phosphorylation of Pah1p phosphatidate phosphatase functions in conjunction with the Pho85p-Pho80p and Cdc28p-cyclin B kinases to regulate lipid synthesis in yeast. J. Biol. Chem. 287, 33364–33376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu Z., Su W.-M., Carman G. M. (2012) Fluorescence spectroscopy measures yeast PAH1-encoded phosphatidate phosphatase interaction with liposome membranes. J. Lipid Res. 53, 522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pascual F., Hsieh L.-S., Soto-Cardalda A., Carman G. M. (2014) Yeast Pah1p phosphatidate phosphatase is regulated by proteasome-mediated degradation. J. Biol. Chem. 289, 9811–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor F. R., Parks L. W. (1979) Triacylglycerol metabolism in Saccharomyces cerevisiae relation to phospholipid synthesis. Biochim. Biophys. Acta 575, 204–214 [DOI] [PubMed] [Google Scholar]
- 42. Carman G. M., Han G.-S. (2011) Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Annu. Rev. Biochem. 80, 859–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry S. A., Kohlwein S. D., Carman G. M. (2012) Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 190, 317–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saleem R. A., Rogers R. S., Ratushny A. V., Dilworth D. J., Shannon P. T., Shteynberg D., Wan Y., Moritz R. L., Nesvizhskii A. I., Rachubinski R. A., Aitchison J. D. (2010) Integrated phosphoproteomics analysis of a signaling network governing nutrient response and peroxisome induction. Mol. Cell. Proteomics 9, 2076–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soufi B., Kelstrup C. D., Stoehr G., Fröhlich F., Walther T. C., Olsen J. V. (2009) Global analysis of the yeast osmotic stress response by quantitative proteomics. Mol. Biosyst. 5, 1337–1346 [DOI] [PubMed] [Google Scholar]
- 46. Nishizuka Y. (1984) The role of protein kinase C in cell surface signal transduction and tumor promotion. Nature 308, 693–698 [DOI] [PubMed] [Google Scholar]
- 47. Nishizuka Y. (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258, 607–614 [DOI] [PubMed] [Google Scholar]
- 48. Kamada Y., Qadota H., Python C. P., Anraku Y., Ohya Y., Levin D. E. (1996) Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271, 9193–9196 [DOI] [PubMed] [Google Scholar]
- 49. Yang W.-L., Carman G. M. (1995) Phosphorylation of CTP synthetase from Saccharomyces cerevisiae by protein kinase C. J. Biol. Chem. 270, 14983–14988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang W.-L., Bruno M. E., Carman G. M. (1996) Regulation of yeast CTP synthetase activity by protein kinase C. J. Biol. Chem. 271, 11113–11119 [DOI] [PubMed] [Google Scholar]
- 51. Park T.-S., O'Brien D. J., Carman G. M. (2003) Phosphorylation of CTP synthetase on Ser36, Ser330, Ser354, and Ser454 regulates the levels of CTP and phosphatidylcholine synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 278, 20785–20794 [DOI] [PubMed] [Google Scholar]
- 52. Choi M.-G., Park T. S., Carman G. M. (2003) Phosphorylation of Saccharomyces cerevisiae CTP synthetase at Ser424 by protein kinases A and C regulates phosphatidylcholine synthesis by the CDP-choline pathway. J. Biol. Chem. 278, 23610–23616 [DOI] [PubMed] [Google Scholar]
- 53. Choi M.-G., Kurnov V., Kersting M. C., Sreenivas A., Carman G. M. (2005) Phosphorylation of the yeast choline kinase by protein kinase C. Identification of Ser25 and Ser30 as major sites of phosphorylation. J. Biol. Chem. 280, 26105–26112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sreenivas A., Villa-Garcia M. J., Henry S. A., Carman G. M. (2001) Phosphorylation of the yeast phospholipid synthesis regulatory protein Opi1p by protein kinase C. J. Biol. Chem. 276, 29915–29923 [DOI] [PubMed] [Google Scholar]
- 55. Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. (1990) A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell 62, 213–224 [DOI] [PubMed] [Google Scholar]
- 56. Levin D. E., Bartlett-Heubusch E. (1992) Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kamada Y., Jung U. S., Piotrowski J., Levin D. E. (1995) The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9, 1559–1571 [DOI] [PubMed] [Google Scholar]
- 58. Han G.-S., Sreenivas A., Choi M. G., Chang Y. F., Martin S. S., Baldwin E. P., Carman G. M. (2005) Expression of human CTP synthetase in Saccharomyces cerevisiae reveals phosphorylation by protein kinase A. J. Biol. Chem. 280, 38328–38336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rose M. D., Winston F., Heiter P. (1990) Methods in Yeast Genetics: A Laboratory Course Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 60. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 61. Innis M. A., Gelfand D. H. (1990) in PCR Protocols. A Guide to Methods and Applications (Innis M. A., Gelfand D. H., Sninsky J. J., White T. J., eds) pp. 3–12, Academic Press, Inc., San Diego, CA [Google Scholar]
- 62. Ito H., Fukuda Y., Murata K., Kimura A. (1983) Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carman G. M., Lin Y.-P. (1991) Phosphatidate phosphatase from yeast. Methods Enzymol. 197, 548–553 [DOI] [PubMed] [Google Scholar]
- 64. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 65. Jeffery D. A., Springer M., King D. S., O'Shea E. K. (2001) Multi-site phosphorylation of Pho4 by the cyclin-CDK Pho80-Pho85 is semi-processive with site preference. J. Mol. Biol. 306, 997–1010 [DOI] [PubMed] [Google Scholar]
- 66. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 67. Boyle W. J., van der Geer P., Hunter T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201, 110–149 [DOI] [PubMed] [Google Scholar]
- 68. MacDonald J. I., Kent C. (1994) Identification of phosphorylation sites in rat liver CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 269, 10529–10537 [PubMed] [Google Scholar]
- 69. Burnette W. (1981) Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112, 195–203 [DOI] [PubMed] [Google Scholar]
- 70. Haid A., Suissa M. (1983) Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 96, 192–205 [DOI] [PubMed] [Google Scholar]
- 71. Choi H.-S., Han G.-S., Carman G. M. (2010) Phosphorylation of yeast phosphatidylserine synthase by protein kinase A. Identification of Ser46 and Ser47 as major sites of phosphorylation. J. Biol. Chem. 285, 11526–11536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morlock K. R., Lin Y.-P., Carman G. M. (1988) Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J. Bacteriol. 170, 3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 74. Henderson R. J., Tocher D. R. (1992) in Lipid Analysis (Hamilton R. J., Hamilton S., eds) pp. 65–111, IRL Press, New York [Google Scholar]
- 75. Nishizuka Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334, 661–665 [DOI] [PubMed] [Google Scholar]
- 76. Ogita K., Miyamoto S., Koide H., Iwai T., Oka M., Ando K., Kishimoto A., Ikeda K., Fukami Y., Nishizuka Y. (1990) Protein kinase C in Saccharomyces cerevisiae: comparison with the mammalian enzyme. Proc. Natl. Acad. Sci. U.S.A. 87, 5011–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Simon A. J., Milner Y., Saville S. P., Dvir A., Mochly-Rosen D., Orr E. (1991) The identification and purification of a mammalian-like protein kinase C in the yeast Saccharomyces cerevisiae. Proc. Biol. Sci. 243, 165–171 [DOI] [PubMed] [Google Scholar]
- 78. Watanabe M., Chen C.-Y., Levin D. E. (1994) Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J. Biol. Chem. 269, 16829–16836 [PubMed] [Google Scholar]
- 79. Chae M., Han G.-S., Carman G. M. (2012) The Saccharomyces cerevisiae actin patch protein App1p is a phosphatidate phosphatase enzyme. J. Biol. Chem. 287, 40186–40196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karanasios E., Han G.-S., Xu Z., Carman G. M., Siniossoglou S. (2010) A phosphorylation-regulated amphipathic helix controls the membrane translocation and function of the yeast phosphatidate phosphatase. Proc. Natl. Acad. Sci. U.S.A. 107, 17539–17544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pearlman S. M., Serber Z., Ferrell J. E., Jr. (2011) A mechanism for the evolution of phosphorylation sites. Cell 147, 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chi A., Huttenhower C., Geer L. Y., Coon J. J., Syka J. E., Bai D. L., Shabanowitz J., Burke D. J., Troyanskaya O. G., Hunt D. F. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li X., Gerber S. A., Rudner A. D., Beausoleil S. A., Haas W., Villén J., Elias J. E., Gygi S. P. (2007) Large-scale phosphorylation analysis of α-factor-arrested Saccharomyces cerevisiae. J. Proteome. Res. 6, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 84. Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 [DOI] [PubMed] [Google Scholar]
- 85. Ptacek J., Devgan G., Michaud G., Zhu H., Zhu X., Fasolo J., Guo H., Jona G., Breitkreutz A., Sopko R., McCartney R. R., Schmidt M. C., Rachidi N., Lee S. J., Mah A. S., Meng L., Stark M. J., Stern D. F., De Virgilio C., Tyers M., Andrews B., Gerstein M., Schweitzer B., Predki P. F., Snyder M. (2005) Global analysis of protein phosphorylation in yeast. Nature 438, 679–684 [DOI] [PubMed] [Google Scholar]
- 86. Dephoure N., Howson R. W., Blethrow J. D., Shokat K. M., O'Shea E. K. (2005) Combining chemical genetics and proteomics to identify protein kinase substrates. Proc. Natl. Acad. Sci. U.S.A. 102, 17940–17945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mah A. S., Elia A. E., Devgan G., Ptacek J., Schutkowski M., Snyder M., Yaffe M. B., Deshaies R. J. (2005) Substrate specificity analysis of protein kinase complex Dbf2-Mob1 by peptide library and proteome array screening. BMC Biochem. 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roach P. J. (1991) Multisite and hierarchal protein phosphorylation. J. Biol. Chem. 266, 14139–14142 [PubMed] [Google Scholar]
- 89. Roach P. J. (1990) Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 4, 2961–2968 [PubMed] [Google Scholar]
- 90. Lutterbach B., Hann S. R. (1994) Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol. Cell. Biol. 14, 5510–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cohen P. (2000) The regulation of protein function by multisite phosphorylation—a 25 year update. Trends Biochem. Sci. 25, 596–601 [DOI] [PubMed] [Google Scholar]
- 92. Gray J. V., Petsko G. A., Johnston G. C., Ringe D., Singer R. A., Werner-Washburne M. (2004) “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 68, 187–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Virgilio C. (2012) The essence of yeast quiescence. FEMS Microbiol. Rev. 36, 306–339 [DOI] [PubMed] [Google Scholar]
- 94. Lee K. S., Levin D. E. (1992) Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12, 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nunez L. R., Jesch S. A., Gaspar M. L., Almaguer C., Villa-Garcia M., Ruiz-Noriega M., Patton-Vogt J., Henry S. A. (2008) Cell wall integrity MAPK pathway is essential for lipid homeostasis. J. Biol. Chem. 283, 34204–34217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carman G. M., Kersting M. C. (2004) Phospholipid synthesis in yeast: regulation by phosphorylation. Biochem. Cell Biol. 82, 62–70 [DOI] [PubMed] [Google Scholar]
- 97. Carman G. M., Henry S. A. (2007) Phosphatidic acid plays a central role in the transcriptional regulation of glycerophospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 282, 37293–37297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thomas B. J., Rothstein R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56, 619–630 [DOI] [PubMed] [Google Scholar]
- 99. Sikorski R. S., Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kastaniotis A. J., Autio K. J., Sormunen R. T., Hiltunen J. K. (2004) Htd2p/Yhr067p is a yeast 3-hydroxyacyl-ACP dehydratase essential for mitochondrial function and morphology. Mol. Microbiol. 53, 1407–1421 [DOI] [PubMed] [Google Scholar]