Background: Catalytic mechanisms of GTSAG motif subfamily enzymes of the bacterial hormone-sensitive lipases (HSLs) family are largely unknown.
Results: E25, a GTSAG motif subfamily esterase, adopts a novel dimerization pattern. Dimerization keeps the catalytic Asp282 orientation for E25 catalysis.
Conclusion: Dimerization and some catalytic profiles of E25 are distinctive from other HSLs.
Significance: Our study sheds light on protein folding and evolution of HSLs.
Keywords: Carboxylesterase, Catalysis, Crystal Structure, Enzyme Mechanism, Protein Motif, Dimerization
Abstract
Hormone-sensitive lipases (HSLs) are widely distributed in microorganisms, plants, and animals. Microbial HSLs are classified into two subfamilies, an unnamed new subfamily and the GDSAG motif subfamily. Due to the lack of structural information, the detailed catalytic mechanism of the new subfamily is not yet clarified. Based on sequence analysis, we propose to name the new subfamily as the GTSAG motif subfamily. We identified a novel HSL esterase E25, a member of the GTSAG motif subfamily, by functional metagenomic screening, and resolved its structure at 2.05 Å. E25 is mesophilic (optimum temperature at 50 °C), salt-tolerant, slightly alkaline (optimum pH at 8.5) for its activity, and capable of hydrolyzing short chain monoesters (C2–C10). E25 tends to form dimers both in the crystal and in solution. An E25 monomer contains an N-terminal CAP domain, and a classical α/β hydrolase-fold domain. Residues Ser186, Asp282, and His312 comprise the catalytic triad. Structural and mutational analyses indicated that E25 adopts a dimerization pattern distinct from other HSLs. E25 dimer is mainly stabilized by an N-terminal loop intersection from the CAP domains and hydrogen bonds and salt bridges involving seven highly conserved hydrophilic residues from the catalytic domains. Further analysis indicated that E25 also has some catalytic profiles different from other HSLs. Dimerization is essential for E25 to exert its catalytic activity by keeping the accurate orientation of the catalytic Asp282 within the catalytic triad. Our results reveal the structural basis for dimerization and catalysis of an esterase from the GTSAG motif subfamily of the HSL family.
Introduction
Lipolytic enzymes, including esterases and lipases, have been widely used in food, pharmaceutical, and fine chemical industries (1, 2). Esterases prefer short to medium chains of monoesters, whereas lipases hydrolyze water-insoluble long-chain triglycerides (3). Based on amino acid similarities and the presence of different conserved motifs, lipolytic enzymes are classified into four blocks: C, H, L, and X (4). Hormone-sensitive lipases (HSLs)2 are affiliated with block H. HSLs exist widely in microorganisms, plants, and animals. Most microbial HSLs are esterases. Arpigny and Jaeger (5) classified microbial lipolytic enzymes into eight families, families I–VIII, and microbial HSLs belong to family IV.
Recently, microbial HSLs were classified into two subfamilies, an unnamed new subfamily and the GDSAG motif subfamily, based on different conserved motifs (6). Although most microbial HSLs so far described are from the GDSAG motif subfamily, only several of the esterases of the new subfamily have been reported (6). To date, various structures from 21 GDSAG motif subfamily enzymes have been resolved. Structural analysis reveals that microbial HSL esterases consist of two domains, a CAP domain and a catalytic domain (7–9). The CAP domain is comprised mainly of two N-terminal α-helices and participates in substrate binding. The CAP domain of EST2 has been shown to play an important role in maintaining enzyme activity, stability, and specificity (10). The catalytic domain possesses the classic α/β hydrolase fold, with a central, eight-stranded, mixed parallel β-sheet surrounded by several α-helices (11). The catalytic domain contains a catalytic triad formed by Ser, Asp/Glu, and His. The key nucleophile Ser typically appears in a conserved GDSAG motif, which forms a sharp elbow in the center of the catalytic domain (12, 13).
Many HSL esterases from the GDSAG motif subfamily are capable of forming dimers or larger oligomers (7–9, 14, 15). Structural analyses of these HSL oligomers reveal that multiple hydrogen bonds and hydrophobic interactions involving the central β-sheet in the catalytic domain contribute to their dimerization, without the involvement of the CAP domain (8, 9, 14). The recently resolved structure of Aes, a HSL esterase from Escherichia coli, implies that the two α-helices within the catalytic domain are critical for its dimerization (16). For reported HSLs, oligomerization is supposed not to be essential for their catalytic activities, because both the substrate binding pocket and the active site are far from the contact area (8, 9, 14). Dimeric EstE1 become monomeric by mutating only one residue, and the resulting mutant is still active, although with considerable reduction in thermostability (8). As dimerization often contributes to enzyme stabilization (17, 18), HSL enzymes that are capable of forming dimers or larger oligomers are usually thermostable (14, 19), making them qualified for biotechnological and industrial applications.
In 2012, Jeon et al. (6) reported three HSL esterases, EstKT4, EstKT7, and EstKT9. Sequence analysis showed that these esterases have a catalytic triad formed by Ser, Asp, and His, and the conserved GT(S)SA(G)G motif encompassing the catalytic Ser residue is different from the corresponding motif conserved in the GDSAG motif subfamily. These esterases also form a distinct branch in the phylogenetic tree of microbial HSL enzymes. Based on these sequence characteristics, Jeon et al. (6) classified these esterases into a new subfamily of the HSL family. Although EstKT4, EstKT7, and EstKT9 have been biochemically characterized as mesophilic (optimum temperatures at 35–45 °C), salt-tolerant, and slightly alkaline (optimum pHs at 8.0–8.5) esterases, it is still unclear whether the esterases from the new subfamily are capable of forming dimers. Also, the detailed catalytic mechanism of enzymes from the new subfamily is not as yet clarified due to the lack of structural information.
In this study, based on massive sequence alignment, we propose to name the new subfamily as the GTSAG motif subfamily. We previously constructed a metagenomic library containing 10,652 fosmid clones from the surface sediment sample E505 from the South China Sea (20). Here, we isolated the novel HSL esterase E25 belonging to the GTSAG motif subfamily from the metagenomic library. We biochemically characterized this enzyme, and resolved its crystal structure. Structural and functional analyses reveal that E25 adopts a dimerization pattern distinctive from known HSL enzymes, and that dimerization is essential for its catalytic function. Our results reveal the structural basis for dimerization and catalysis of an esterase from the GTSAG motif subfamily of the HSL family.
EXPERIMENTAL PROCEDURES
Screening and Sequence Analysis of Lipolytic Enzymes from a Metagenomic Library
A metagenomic library containing 10,652 fosmid clones was previously constructed from surface sediment sample E505 from the South China Sea (20). Fosmid clones showing lipolytic activity were identified on tributyrin agar plates. Fosmid DNA was extracted from positive clones and partially digested by the restriction enzyme Sau3AI. DNA fragments of 1.5 to 5 kb were isolated, end-repaired, and ligated with pUC19 pretreated by BamHI and bacterial alkaline phosphatase. The ligated DNAs were introduced into E. coli TOP10 cells, which were then plated onto LB agar plates containing 100 μg/ml of ampicillin and 1% (v/v) tributyrin. After incubation at 37 °C for 20 h, transformants exhibiting a clear halo were selected and sequenced. Open reading frames (ORFs) in the sequence were predicted by the GeneMark program. The genes encoding lipolytic enzymes were predicted by BLASTX against the NCBI non-redundant protein database (nr). Multiple sequence alignment was carried out using MUSCLE (21). Phylogenetic analysis was performed using the MEGA 4.0 (22). SignalP 4.0 (23) was used to identify the potential signal peptide sequence.
Gene Cloning, Mutation, Protein Expression, and Purification
The encoding sequence of E25 was amplified from fosmid DNA and cloned into expression vector pET-28a. All of the site-directed mutations and the truncated mutations in E25 were introduced using PCR-based methods and verified by DNA sequencing. The wild-type E25 protein and all mutants were expressed in E. coli BL21(DE3) cells and their expression was induced by the addition of 1 mm isopropyl β-d-thiogalactopyranoside at 20 °C for 20 h. Cells were collected and disrupted by sonication in 50 mm Tris-HCl buffer (pH 8.0). The resulting extract was first purified by nickel-nitrilotriacetic acid resin (Qiagen) and then by ion-exchange chromatography on a Source 15Q column (GE Healthcare, Sweden) with a linear gradient of 0 to 0.6 m NaCl. The eluted enzyme fractions were further purified by gel filtration chromatography on a Superdex-200 column (GE Healthcare) with 10 mm Tris-HCl buffer (pH 8.0) containing 100 mm NaCl. The Superdex-200 column was calibrated in the same buffer with protein size markers, ovalbumin (43 kDa) and conalbumin (75 kDa) from GE Healthcare.
Biochemical Characterization
The standard reaction system for E25 activity determination contained 50 mm Tris-HCl buffer (pH 8.0), 0.02 ml of 10 mm p-nitrophenyl (pNP) butyrate, and 0.02 ml of enzyme in a final volume of 1 ml. After incubation at 50 °C for 5 min, the reaction was terminated by addition of 0.1 ml of 20% (w/v) SDS, and then absorbance of the reaction mixture at 405 nm was measured. One unit of enzyme (U) is defined as the amount of enzyme required to liberate 1 μmol of pNP per min. Substrate specificity assays were performed with the following pNP derivatives: pNP acetate (pNPC2), pNP butyrate (pNPC4), pNP caproate (pNPC6), pNP caprylate (pNPC8), pNP decanoate (pNPC10), pNP laurate (pNPC12), pNP myristate (pNPC14), and pNP palmitate (pNPC16) (Sigma). The optimum temperature for E25 activity was determined ranging from 0 to 80 °C at pH 8.0. For the thermal stability assay, the enzyme was preincubated at temperatures ranging from 0 to 70 °C for 1 h, and the residual activity was measured at 50 °C. The optimum pH for E25 activity was determined ranging from pH 4 to 12 with Britton-Robinson buffer at 50 °C. For the pH stability assay, the enzyme was preincubated in buffers with pH values ranging from 4 to 12 at 20 °C for 1 h, and then the residual activity was measured at pH 8.0 and 50 °C. The effect of NaCl on E25 activity was determined at NaCl concentrations ranging from 0 to 4.8 m. The effects of metal ions and potential inhibitors on E25 activity were determined by addition of various chemical agents to the reaction mixture.
Enzyme kinetic assays were carried out using pNPC4 at concentrations from 0.2 to 5.0 mm. Kinetic parameters were calculated by non-linear regression fit directly to the Michaelis-Menten equation using the Origin8 software. The overall secondary structures of wild-type E25 and its mutants were investigated using a J-810 circular dichroism (CD) spectropolarimeter (JASCO, Japan) at 25 °C. CD spectra were collected from 200 to 250 nm at a scanning rate of 200 nm/min with a path length of 0.1 cm. All proteins for CD spectroscopy assays were at a concentration of 8 μm in 50 mm Tris-HCl buffer (pH 8.0).
Crystallization, Data Collection, and Structure Determination
Native and SeMet-E25 for crystallization were diluted to 3.0 mg/ml in 10 mm Tris-HCl (pH 8.0) containing 100 mm NaCl, respectively. Crystals suitable for x-ray diffraction were obtained after 2 weeks, using the hanging-drop vapor diffusion method at 20 °C. E25 crystals grew in buffer containing 0.2 m NaAC·3H2O, 0.1 m sodium cacodylate trihydrate (pH 6.5), and 17% (w/v) PEG 8,000. SeMet-E25 crystals grew in buffer containing 0.2 m NaAC·3H2O (pH 7.8) and 28% (w/v) PEG 3,350. X-ray diffraction data were collected on the BL17U1 beamline at Shanghai Synchrotron Radiation Facility using Area Detector Systems Corporation Quantum 315r. The initial diffraction data sets were processed by the HKL2000 program (24). The E25 crystal belongs to the P31 space group. The E25 structure was determined by molecular replacement using SeMet-E25 as the starting model. Refinement of the E25 structure was performed using Coot (25) and Phenix (26). All structure figures were generated using PyMOL. The protein interactions, surfaces, and assemblies (PISA) server (27) was used to deduce the dimerization interface of E25.
Accession Code
The nucleotide sequence encoding E25 has been deposited in the GenBankTM database with accession number KJ624992. The structure of E25 has been deposited in the Protein Data Bank (PDB) with accession number 4Q05.
RESULTS AND DISCUSSION
Screening and Sequence Analysis of Lipolytic Enzyme E25 from a Metagenomic Library
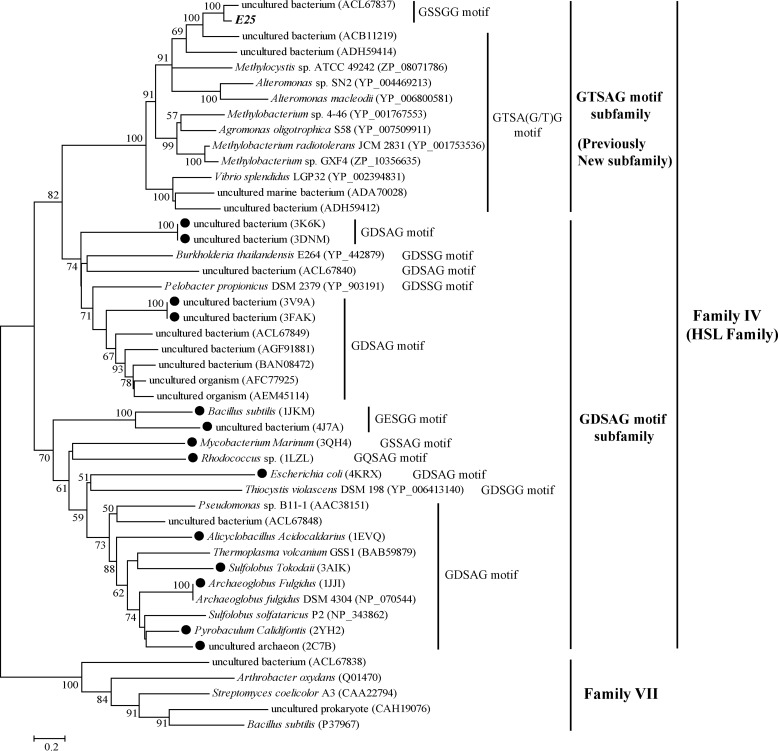
Seven clones showing lipolytic activities were screened from the E505 fosmid library. Five different genes encoding lipolytic enzymes were identified from these fosmids by construction of subcloning libraries and subsequent sequencing. Sequence analysis indicated that one of the identified genes belongs to the HSL family, which was named E25. E25 is 1,023 bp in length, encoding a protein with 340 amino acid residues. Sequence analysis using the SignalP 4.0 program suggested that E25 might lack an N-terminal signal peptide sequence. Phylogenetic analysis showed that E25 belongs to the unnamed new subfamily of the HSL family (Fig. 1). Sequence analysis suggested that E25 has a catalytic triad formed by Ser186, Asp282, and His312 (Fig. 2). The catalytic Ser186 is located in the conserved GT(S)SA(G)G motif. Analysis of 100 protein sequences from this new subfamily revealed that, among the pentapeptide motifs containing the catalytic Ser in these sequences, GTSAG is the highest hit (Table 1). Based on this, we propose to name the new subfamily (6) within the HSL family as the GTSAG motif subfamily, in correspondence with the GDSAG motif subfamily. We thereafter use the GTSAG motif subfamily to refer to the new subfamily in the text. Except for the reported GT(S)SA(G)G motif, we found that enzymes of the GTSAG motif subfamily also contain other conserved residues and regions, such as DYR/AXPP, PAA/GXXD, GTPXXD, and GTRDXXLS motifs (Fig. 2), which may play important roles in maintaining the function of these enzymes. The catalytic Asp282 of E25 is located in the conserved GTRDXXLS motif.
FIGURE 1.
Phylogenetic tree of representative lipolytic sequences from the HSL family. The tree was built by the Neighbor Joining method with a JTT matrix-based model using 229 amino acid positions. Bootstrap analysis of 100 replicates was conducted, and values above 50% are shown. Homologs from Family VII were used as outgroups. Sequences having crystal structures are indicated by solid circles. The conserved pentapeptide motif containing the catalytic Ser residue is also shown for each sequence.
FIGURE 2.
Multiple sequence alignment of E25 and its homologs. Identical and similar amino acids are shaded in black and gray, respectively. Circles indicate amino acid residues belonging to the catalytic triad, the square indicates residues involved in the oxyanion hole, stars indicate hydrophilic residues from the catalytic domain involved in E25 dimerization, and triangles indicate conserved hydrophobic residues within the dimerization interface between the catalytic domains of the E25 dimer.
TABLE 1.
Statistics on the frequency of corresponding residues/regions of E25 in the first 100 hits in NCBI nr database using BLASTP
Sequence identities shared by E25 and its 100 homologs range from 35 to 80%. Based on sequence alignment, the frequency of corresponding residues/regions of E25 in its 100 closest homologs was calculated.
| Domain | Residue/region types | Residue/motifa | Frequencyb |
|---|---|---|---|
| % | |||
| Catalytic domain | Catalytic residues | Ser186 | 100 |
| Asp282 | 99 (1 for Glu) | ||
| His312 | 100 | ||
| Motif having catalytic Ser186 | GXSXG | 100 | |
| GTSAG | 33 | ||
| GTSTG | 16 | ||
| GRSAG | 25 | ||
| Motif having catalytic Asp282 | GTRDXXLS | 99 | |
| Hydrophilic residues involved in E25 dimerization | Asp224 | 98 | |
| Thr280 | 99 | ||
| Arg281 | 100 | ||
| Ser286 | 100 | ||
| Arg296 | 87 (5 for Lys) | ||
| Glu308 | 92 (8 for Asp) | ||
| Glu330 | 75 (9 for Asp) | ||
| Hydrophobic residues within the dimerization interface of E25 | Leu285 | 100 | |
| Val289 | 91 | ||
| Leu304 | 97 (3 for Ile) | ||
| Val306 | 85 (12 for Ile) | ||
| CAP domain | A long N-terminal sequence like E25 | 93 |
a All 100 hits to E25 from NCBI nr have a catalytic triad composed of Ser, Asp/Glu, and His, and the catalytic Ser appears in the typical GXSXG motif of lipolytic enzymes, suggesting that all these E25 homologs are potential lipolytic enzymes. Phylogenetic analysis suggested that these homologs and E25 are belonging to the same subfamily of the HSL family.
b The frequency for similar residue is shown in parentheses.
Expression and Characterization of Esterase E25
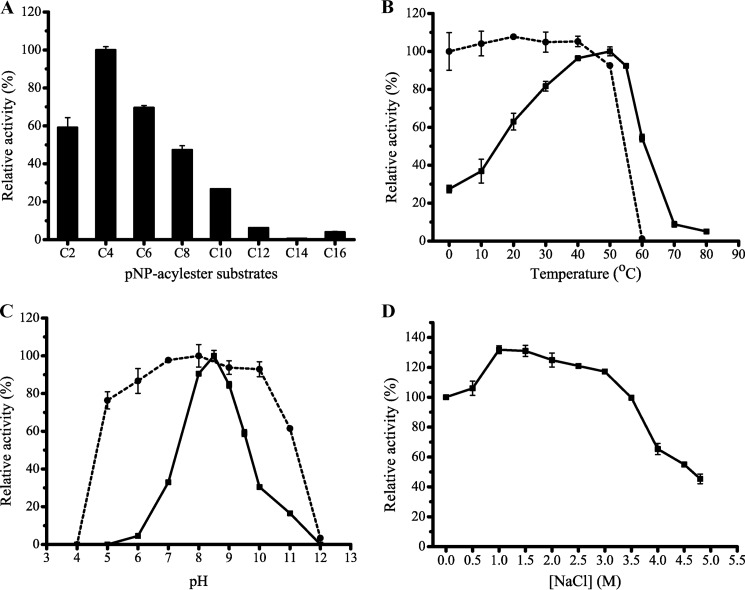
E25 was over-expressed in E. coli BL21(DE3) and purified. Recombinant E25 efficiently hydrolyzed short-chain pNP-esters (C2–C10) with the highest activity toward pNPC4 (9.99 units/mg), but had little activity toward pNP-esters with acyl chain lengths of >10 carbon atoms (Fig. 3A), indicating that E25 is an esterase. The optimal temperature for E25 activity was 50 °C. E25 retained 93% activity after 1 h incubation at 50 °C (Fig. 3B). E25 had the highest activity at pH 8.5 (Fig. 3C) and showed good tolerance over a wide pH range, retaining over 76% activity at pH 5–10. The E25 activity did not decrease in 3.5 m NaCl, indicating that E25 has good salt tolerance (Fig. 3D). Therefore, E25 is mesophilic, salt tolerant, and slightly alkaline for its activity, consistent with the three reported enzymes from the GTSAG motif subfamily (6).
FIGURE 3.
Biochemical characterization of E25 esterase. A, substrate specificity of E25. B, effect of temperature on the activity (solid line) and stability (dashed line) of E25. C, effect of pH on the activity (solid line) and stability (dashed line) of E25. D, effect of NaCl of different concentrations on the activity of E25.
The effect of various metal ions and chemicals on E25 activity was also investigated (Table 2). E25 activity was partly inhibited by Li+, Co2+, Zn2+, and Mn2+ at 10 mm concentration, and was strongly inhibited by Cu2+, Fe2+, and Ni2+ at 1 mm concentration. K+, Ca2+, or Mg2+ had no significant effect on E25 activity. E25 was significantly inhibited by PMSF, indicating that E25 is a serine hydrolase. E25 activity was not affected by EDTA, suggesting that E25 may not require metal ions for catalysis.
TABLE 2.
Effect of metal ions and potent inhibitors on the E25 activity
| Compound | Relative/residual activity |
|
|---|---|---|
| 1 mm | 10 mm | |
| % | ||
| Li+ | 83.6 ± 7.5 | 75.6 ± 6.4 |
| K+ | 101 ± 1.9 | 94.1 ± 2.0 |
| Ca2+ | 86.3 ± 5.2 | 98.7 ± 2.9 |
| Co2+ | 101 ± 2.1 | 69.8 ± 3.9 |
| Cu2+ | 26.6 ± 1.5 | 2.9 ± 0.1 |
| Fe2+ | 10.8 ± 1.9 | 0.4 ± 0.1 |
| Mg2+ | 94.5 ± 2.4 | 97.2 ± 2.1 |
| Mn2+ | 105 ± 3.6 | 79.8 ± 2.4 |
| Ni2+ | 64.9 ± 3.4 | 31.5 ± 3.2 |
| Zn2+ | 91.9 ± 1.6 | 52.8 ± 7.0 |
| PMSF | 41.4 ± 2.2 | 3.1 ± 0.2 |
| EDTA | 96.7 ± 3.7 | 109 ± 2.1 |
Overall Structural Analysis of Esterase E25
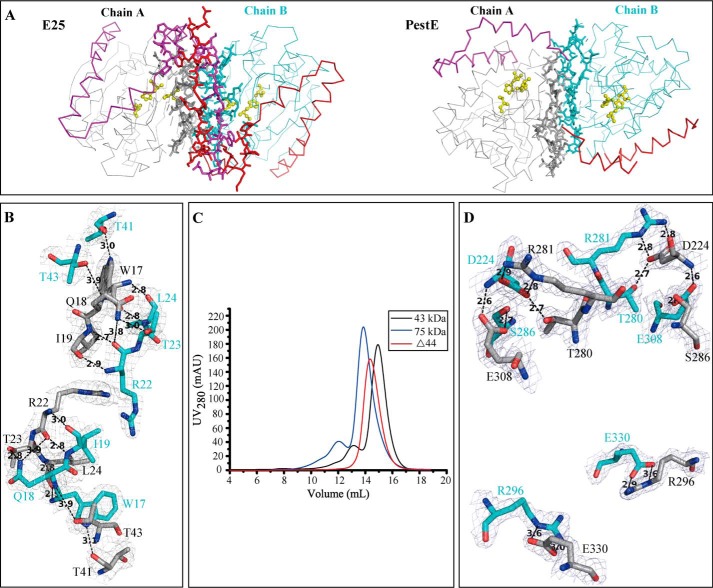
A similarity search using BLASTP against PDB database revealed that, the closest homolog to E25 is a lipolytic enzyme (PDB 3V9A) from the GDSAG motif subfamily of the HSL family, with a sequence identity of only 29%, covering 75% of the E25 sequence. This indicates that no structure in the database can be used as a suitable model for E25 structure construction. Therefore, we resolved the structures of both native and SeMet-E25. The structure of native E25 was resolved at 2.05-Å resolution (Fig. 4A) by molecular replacement using the SeMet-E25 at 3.0-Å resolution as the starting model. The statistics for refinement are summarized in Table 3. The crystal of E25 belongs to the P31 space group with one dimer per asymmetric unit. The N-terminal 13 residues (Met1-Gln13) lack electron density. Structural analysis revealed that E25 is a homodimer, consistent with the result of gel filtration (Fig. 4B). The overall structure of the E25 monomer is similar to those of resolved HSL esterases, containing a CAP domain and a catalytic domain (Fig. 4C). The E25 monomer structure shows a root mean square deviation of 1.735 Å (for 226 Cα positions) to the closest structure 3V9A.
FIGURE 4.
Structural analysis of E25. A, dimer structure of E25. B, gel filtration analysis of recombinant E25 and markers. E25 monomer has a calculated molecular mass of 38.9 kDa. Two protein size markers are ovalbumin (43 kDa) and conalbumin (75 kDa). C, monomer structure of E25. The catalytic domain is in gray and the CAP domain in pink. The nucleophile elbow is colored in red. D, the structure of the active site of E25 with the corresponding 2Fo − Fc electron density map at 1.5 σ. The residues forming the catalytic triad and oxyanion hole are indicated.
TABLE 3.
Diffraction data and refinement statistics of E25
| Parameters | E25 |
|---|---|
| Diffraction data | |
| Space group | P31 |
| Unit cell | |
| a, b, c (Å) | 138.80, 138.80, 49.25 |
| α, β, γ (°) | 90.00, 90.00, 120.00 |
| Resolution range (Å) | 50.00–2.05 (2.12–2.05)a |
| Redundancy | 4.6 (4.6) |
| Completeness (%) | 97.4 (98.3) |
| Rmerge (%)b | 13.0 (47.7) |
| I/σI | 34.39 (5.65) |
| Refinement statistics | |
| R-factor (%) | 16.8 |
| Free R-factor (%) | 19.0 |
| Root mean square deviation from ideal geometry | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 1.04 |
| Ramachandran plot (%) | |
| Favored | 94.88 |
| Allowed | 4.81 |
| Outliers | 0.31 |
| Overall B-factors (Å2) | 33.50 |
a Numbers in parentheses refer to data in the highest resolution shell.
b Rmerge = ΣhklΣi|I(hkl)i − 〈I(hkl)〉|/ΣhklΣi 〈I(hkl)i〉.
The N-terminal CAP domain (Glu14–Ser83) of E25 is composed of four α-helices and four loops, which is much longer than that of the GDSAG motif subfamily enzymes (∼50 residues). The CAP domain of E25 shares homology with only four proteins from the GTSAG motif subfamily, including the mesophilic lipolytic enzyme FLS1 from an uncultured bacterium (69%, ACL67837) (28), the uncharacterized lipolytic enzyme from a metagenomic library (42%, ACB11219), and esterases EstKT7 (38%, ADH59414) and EstKT4 (32%, ADH59412) (6). The catalytic domain (Val84–Arg340) of E25 shows a typical α/β hydrolase fold, with a central, eight-stranded, predominantly parallel β-sheet surrounded by eight α-helices. Ser186, Asp282, and His312 comprise the catalytic triad (Fig. 4D). The key nucleophile Ser186 is located on the apex of the nucleophilic elbow between β5 and α8. Asp282 is located in the loop between β7 and α11, and His312 in the loop between β8 and α12. The hydrogen bond distance within the catalytic triad is 3.8 Å from Ser186-Oγ to His312-Nϵ2 and 3.7 Å from His312-Nδ1 to Asp282-Oδ1. The oxyanion hole is above the active site, composed of Gly118 and Gly119 within the conserved HGG motif (residues 117–119). The substrate binding pocket is a tunnel-shaped cavity, encompassed by the oxyanion hole, the key nucleophile Ser186, and other ambient residues. Unlike the CAP domain, the catalytic domain of E25 has extensive homology (38–84%) with other HSL enzymes, especially to the enzymes of the GTSAG motif subfamily.
Esterase E25 Adopts a New Pattern of Dimerization
HSL enzymes including BFAE (PDB 1JKM) (13), Cest-2923 (PDB 4BZW) (9), Est25 (PDB 4J7A) (29), EstE1 (PDB 2C7B) (8), HerE (PDB 1LZL) (7), PestE (PDB 3ZWQ) (14), Sto-Est (PDB 3AIK) (30) and so on have been identified as dimers or larger oligomers in both solution and the crystals. These oligomers are stabilized mainly by hydrogen bonds and hydrophobic interactions formed between two antiparallel β8 from the catalytic domains of the interactive monomers, without the involvement of the CAP domains (8, 9, 14). However, for E25, both the CAP domain and the catalytic domain may be involved in dimer formation based on its structure.
Structural analysis revealed that the E25 dimer has a long loop intersection at its N-terminal region, which is absent from other HSL oligomers (Fig. 5A). The two intersecting loops are, respectively, from the N terminus (Glu14–Gly29) of the CAP domains in E25 dimer. Hydrogen bonds are formed between these intersecting loops, playing a key role in stabilizing E25 dimer (Fig. 5B). The hydrophobic residues, Ile19, Pro (21, 25, 27), and Leu24, in these two N-terminal loops also form hydrophobic interactions to stabilize the E25 dimer. Moreover, PISA analysis suggested that residues Thr41 and Thr43, located in the loop between α1 and α2 of the CAP domain, are also involved in E25 dimerization (Fig. 5B). Then N-terminal truncation mutants Δ29 without N-terminal residues 1–29 (the intersecting loop) and Δ44 without N-terminal residues 1–44 were generated. Both mutants were inactive, but could form dimers in solution (Fig. 5C). This result indicated that, besides the CAP domain, the catalytic domain also contributes to E25 dimerization.
FIGURE 5.
Dimerization pattern of E25. A, a comparison of dimerization patterns of E25 and PestE (PDB 3ZWQ). In monomer A, the catalytic domain is shown in gray and the CAP domain in magenta. In monomer B, the catalytic domain is shown in cyan and the CAP domain in red. Residues on the dimerization interface predicted by PISA are shown as sticks. The catalytic triads are in ball-and-stick representation and in yellow. B, the hydrogen-bond network between the interactive CAP domains of E25 dimer. The corresponding 2Fo − Fc electron density map is shown at 1.0 σ. C, gel filtration analysis of Δ44 mutant and markers. Δ44 monomer has a calculated molecular mass of 34.3 kDa. D, the hydrogen-bond network between the interactive catalytic domains of E25 dimer. The corresponding 2Fo − Fc electron density map is shown at 1.5 σ. In B and D, residues in monomer A are shown in gray, and residues in monomer B in cyan.
The oligomers of reported HSL enzymes are stabilized mainly by hydrogen bonds and hydrophobic interactions between two antiparallel β8 from the catalytic domains (8, 9, 14). However, in the E25 dimer, the two β8 strands are not antiparallel, but rotate ∼280°. PISA analysis indicated that the E25 dimer is mainly stabilized by hydrogen bonds and salt bridges formed by hydrophilic residues in the loops or α-helices from the interactive catalytic domains, including Asp224 (α9), Thr280 (the loop between β7 and α11), Arg281 (the loop between β7 and α11), Ser286 (α11), Arg296 (α11), Glu308 (the loop between β8 and α12), and Glu330 (α12) (Fig. 5D). Compared with other HSL oligomers, E25 has the largest hydrophilic interface, harboring 35 hydrogen bonds and 16 salt bridges (Table 4). Besides this, hydrophobic interactions within the dimerization interface between the catalytic domains may also contribute to stabilize the E25 dimer, involving hydrophobic residues Leu285 (α11), Val289 (α11), Ile293 (α11), Leu304 (β8), and Val306 (β8).
TABLE 4.
Comparison of HSL oligomers
| Esterasea | Source | Oligomer in solution | Dimer interface (PISA) |
PDB enrty | References | ||
|---|---|---|---|---|---|---|---|
| BSAb | HBc | SBd | |||||
| % | |||||||
| E25 | Metagenomic | Dimer | 16.6 | 35 | 16 | 4Q05 | In this paper |
| BFAE | Bacillus subtilis | Dimer | 12.2 | 25 | 10 | 1JKM | 13 |
| PestE | Pyrobaculum calidifonti | Dimer | 10.6 | 22 | 6 | 3ZWQ | 14 |
| HerE | Rhodococcus sp. | Dimer | 9.4 | 20 | 16 | 1LZL | 7 |
| Sto-Est | Sulfolobus tokodaii | Dimer | 8.5 | 16 | 6 | 3AIK | 30 |
| Est25 | Metagenomic | Dimer | 8.5 | 14 | 2 | 4J7A | 29 |
| Aes | Escherichia coli | Dimer | 7.1 | 14 | − | 4KRX | 16 |
| EstE1 | Metagenomic | Dimer | 6.8 | 10 | 6 | 2C7B | 8 |
| Cest-2923 | Lactobacillus plantarum | Monomer, dimer, tetramer | 12.7 | 6 | − | 4BZW | 9 |
| EstE7 | Metagenomic | Monomer, dimer | 8.2 | 6 | 2 | 3DNM | 15 |
a Of all the HSL structures, E25 is from the GTSAG motif subfamily, and the others all from the GDSAG motif subfamily. Reported values correspond to the A/A′ dimers of HerE, EstE1, and Cest-2923, B/B′ dimer of EstE7, A/D dimers of Sto-Est and Est25, and A/B dimers for other HSL oligomers.
b BSA, the percentage of buried surface area in the total surface area based on PISA analysis.
c HB, hydrogen bond.
d SB, salt bridge; −, no salt bridges detected.
In addition, a weak interaction is also found between the CAP domain from one monomer and the catalytic domain from the other monomer, involving Arg22 in one monomer and Ile228 or Asp230 in the other monomer. Taken together, the E25 dimer adopts a new pattern of dimerization involving both the CAP domain and the catalytic domain, different from other reported HSL enzymes.
Dimerization Is Essential for the Catalytic Function of Esterase E25
For reported HSL oligomers, oligomerization is supposed not to be essential for their catalytic activities (8, 9, 14). In these oligomers, both the substrate binding pocket and the active site are far away from the contact area, as shown in Fig. 5A. However, in the E25 dimer, the catalytic triad is adjacent to the interface, especially the catalytic residue Asp282 (Figs. 5A and 6A), which suggests that dimerization might be important for E25 activity. Immediately upstream of Asp282, residues Arg281 and Thr280 from monomer A form hydrogen bonds and salt bridges with Asp224 from monomer B to maintain the E25 dimer structure (Fig. 6A). Mutating Asp224 to Ala or similar residues, Asn/Ser, resulted in soluble but inactive mutants (Fig. 6B). We also mutated the other residues in the catalytic domain involved in formation of the E25 dimer to Ala (Fig. 6B). Mutants S286A and E308A had no detectable activity. Mutant R296A retained 19.0% and E330A retained 82.5% of the wild-type E25 activity. Although both R296A and E330A mutations destroyed the hydrogen bonds and salt bridges between Arg296A and Asp330B, Arg296 located in α11 is closer to the catalytic Asp282 than Asp330, and therefore, it might be more important for maintaining E25 activity than Asp330. In addition, the mutations in the CAP domain involved in E25 dimer formation, including Δ29, Δ44, and R22A, all resulted in inactive mutants, indicating that the CAP interaction is also necessary for E25 activity. Our results also showed that these mutants were still in the form of dimers or larger oligomers in solution although they lost all or part of the activity. CD spectroscopy analysis showed that the secondary structures of the mutants exhibited little deviation from that of wild-type E25 (Fig. 6C), indicating that the decrease in enzymatic activity of the mutants resulted from residue replacement or deletion rather than structural changes. Therefore, these mutational results indicated that the whole dimerization interface is essential for E25 catalysis.
FIGURE 6.

Impact of dimerization on the catalytic function of E25. A, dimerization interface adjacent to the catalytic triad of monomer A in E25 dimer. Monomer A is shown in gray, and monomer B in cyan. The corresponding 2Fo − Fc electron density map is shown at 1.5 σ. Dimerization interface adjacent to the catalytic triad of monomer B is not shown. In two monomers of E25, the substrate binding pockets, and active sites were indicated by black and pink ellipses, respectively. The substrate binding pocket is composed of the key nucleophile Ser186, catalytic His312, and residues Gly118 and Gly119 forming the oxyanion hole. The active site is composed of catalytic residues Ser186, Asp282, and His312. B, enzymatic activities of the mutants of E25. The activity of wild-type E25 was defined as 100%. C, CD spectra of wild-type E25 and its mutants.
Structural analysis of the E25 dimer revealed that the substrate binding pocket is far away from the dimerization interface, implying that the interface may have little impact on substrate binding (Figs. 5A and 6A). To verify this hypothesis, kinetic parameters of wild-type E25, R296A and E330A mutants were compared (Table 5). The Km values of R296A and E330A were similar to that of wild-type E25, indicating that these mutations had little effect on substrate binding of E25. In contrast, the kcat value was reduced by 82% for R296A, and 27% for E330A, comparable with the reduction in their specific activities (Table 5). Therefore, mutational analysis in combination with enzyme kinetic analysis indicated that dimerization is essential for E25 function, mainly influencing its catalysis, rather than its substrate binding.
TABLE 5.
Kinetic parameters of E25 and its mutants
Reactions were conducted in triplicate in 50 mm Tris-HCl buffer (pH 7.5) at 50 °C using pNPC4 as substrate over a concentration range of 0.2–5.0 mm.
| Enzyme | Vmax | Km | kcat | kcat/Kma |
|---|---|---|---|---|
| μm/min/mg | mm | s−1 | mm−1 s−1 | |
| E25 wild-type | 60 ± 1.3 | 0.74 ± 0.03 | 39 ± 1.2 | 52 (100%) |
| R296A | 11 ± 0.51 | 0.79 ± 0.05 | 7.3 ± 0.46 | 9.3 (18%) |
| E330A | 47 ± 1.2 | 0.80 ± 0.03 | 31 ± 1.1 | 38 (73%) |
a Percentages in parentheses were calculated relative to wild-type E25.
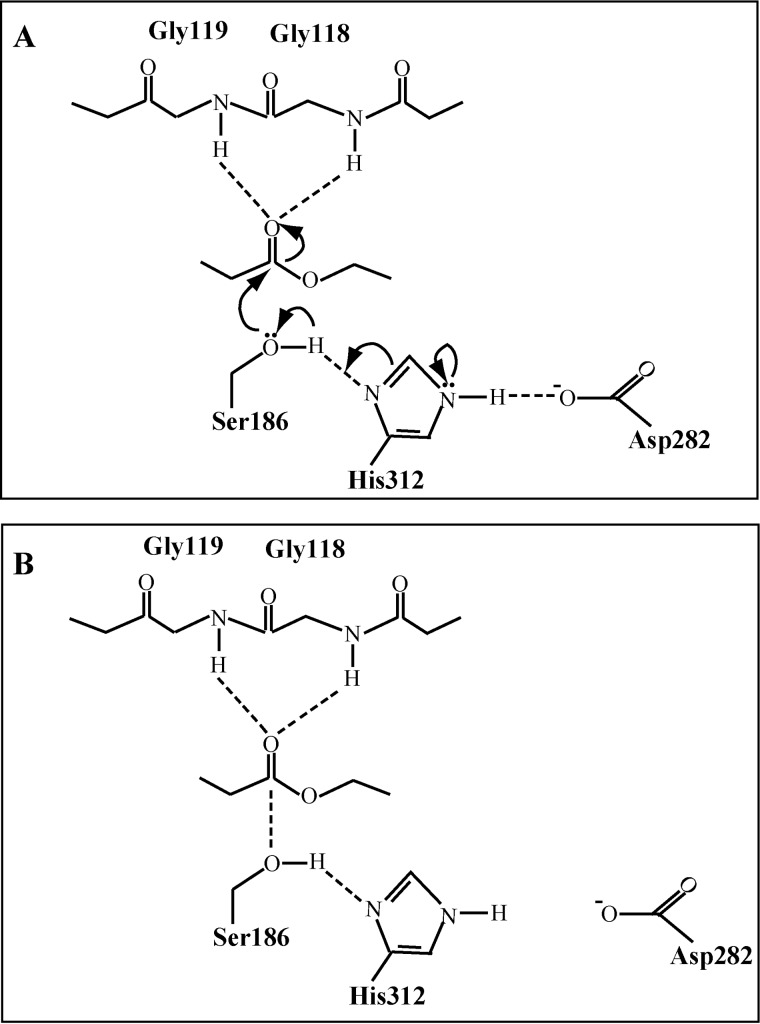
It has been demonstrated in esterase Ape1a that mutation on the catalytic Asp reduced the catalytic efficiency without impact on substrate binding, whereas mutation on its catalytic Ser/His resulted in reduction in both substrate binding and catalytic efficiency (31). For E25, R296A and E330A mutations reduced the catalytic efficiency but had little impact on substrate binding, which suggests that these mutations may disturb the catalytic Asp282. In the E25 dimer, because Asp282 is adjacent to the dimerization interface (Figs. 5A and 6A), the interface may have significant impact on the accurate orientation of the catalytic Asp282 in the active site. E25 has a much larger hydrogen bond distance from His312-Nδ1 to Asp282-Oδ1 (3.7 Å) compared with other HSL enzymes (<3 Å) (7–9, 16, 32). Thus, any damage to the dimerization interface of E25 probably makes Asp282 likely to escape from His312, which will cause partial or complete disruption of the interaction between Asp282 and His312, and therefore, lead to part or all activity loss of E25. Our mutational results support this hypothesis. Besides R296A and E330A, other mutations that are able to disturb the dimerization interface of E25 also could inactivate the enzyme (Fig. 6B). Thus, based on these results, we propose a model for the catalytic mechanism of E25 dimer. The dimerization interface ensures Asp282 in an appropriate distance from His312 and an effective interaction between these two residues, which facilitates proton transfer from the catalytic Ser186 to His312, and therefore enhances the nucleophilicity of Ser186 to attack the carbonyl carbon of the susceptible ester (Fig. 7).
FIGURE 7.
Proposed catalytic mechanisms of wild-type E25 (A) and R296A mutant (B). In wild-type E25, the nucleophile Ser186 attacks the carbonyl carbon of the susceptible ester. The dimerization interface keeps Asp282 in an appropriate distance from His312, which facilitates the proton transfer from catalytic Ser186 to His312, and thus enhances the nucleophilicity of the attacking Ser186. In the R296A mutant, the hydrogen bonds and salt bridges formed by Arg296A and Glu330B are destroyed, resulting in disruption of the interaction between Asp282 and His312. Therefore, although the R296A mutant could bind to the substrate stabilized by the oxyanion hole, the ability of its nucleophile Ser186 to attack the carbonyl carbon of the ester is significantly decreased.
In some HSL enzymes from the GDSAG motif subfamily, the catalytic residue Asp is replaced by Glu (15, 33). However, sequence analysis suggests that enzymes with the catalytic Asp replaced by Glu are quite less in the GTSAG motif family, only one sequence (WP_010127891) in the first 100 hits to E25 in NCBI nr database (Table 1), which is predicted from the genome sequence of Sphingomonas sp. KC8 with no functional study. In addition, mutation of the catalytic Asp282 to Glu in E25 abolished the enzyme activity completely (Fig. 6B). This mutation may result in complete disruption of the interaction between the catalytic residues, Glu282 and His312, thereby leading to the inactivation of E25. These results indicate that the catalytic Asp may be strictly conserved in the GTSAG motif subfamily.
The E25 Dimerization Pattern May Be Common in the GTSAG Motif Subfamily of the HSL Family
Although the CAP domains of enzymes from the GTSAG motif subfamily show low similarity in sequence, nearly all E25 homologs have a long N-terminal sequence like E25 (Fig. 2 and Table 1), which implies that these enzymes may be capable of forming an N-terminal loop intersection between two monomers, similar to E25. In addition, all hydrophilic residues in the catalytic domain involved in E25 dimer formation are highly conserved in the GTSAG motif subfamily, including Asp224, Thr280, Arg281, Ser286, Arg296, and Glu (308 and 330) (Fig. 2 and Table 1). The hydrophobic residues Leu285, Val289, Leu304, and Val306 within the dimerization interface between the catalytic domains are also conserved in this subfamily (Fig. 2 and Table 1). This suggests that other members of the GTSAG motif subfamily probably form a similar interface between the catalytic domains of two monomers as E25. Taken together, these common structural characteristics imply that enzymes of the GTSAG motif subfamily may form oligomers with a similar dimerization pattern as E25.
Conclusions
We identified a novel esterase E25 from a marine metagenomic library, which belongs to the GTSAG motif subfamily of the HSL family. Biochemical characterization showed that E25 is mesophilic, salt-tolerant, and slightly alkaline for its activity, and can effectively hydrolyze short-chain monoesters (C2–C10). E25 forms dimers both in the crystal and in solution. An E25 monomer contains an N-terminal CAP domain, and a classical α/β hydrolase-fold domain, and residues Ser186, Asp282, and His312 comprise the catalytic triad. Structural and mutational analyses indicated that E25 adopts a dimerization pattern distinct from other HSL oligomers, which involves both the CAP domain and the catalytic domain. Different from other HSL enzymes, dimerization is essential for E25 to exert its catalytic function, by keeping the accurate orientation of the catalytic Asp282 in the active site. Because most of the residues involved in E25 dimer formation are conserved in the members of the GTSAG motif subfamily, the E25 dimerization pattern may be common in this subfamily of the HSL family. Our results first reveal the structural basis for dimerization and catalysis of an esterase from the GTSAG motif subfamily of the HSL family, which provide a better understanding of protein folding and evolution of the HSL family lipolytic enzymes.
This work was supported by National Natural Science Foundation of China Grants 91228210, 31290230, 31290231, and 31025001, Hi-Tech Research and Development Program of China Grants 2012AA092105 and 2012AA092103, China Ocean Mineral Resources R & D Association (COMRA) Special Foundation Grant DY125-15-T-05, and Program of Shandong for Taishan Scholars Grant 2008BS02019.
The nucleotide sequence(s) reported in this paper has been submitted to the DDBJ/GenBankTM/EBI Data Bank with accession number(s) KJ624992.
The atomic coordinates and structure factors (code 4Q05) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- HSL
- hormone-sensitive lipase
- nr
- non-redundant protein database
- pNP
- p-nitrophenyl
- PISA
- protein interactions, surfaces, and assemblies
- PDB
- Protein Data Bank
- SeMet
- selenomethionine.
REFERENCES
- 1. Jaeger K. E., Eggert T. (2002) Lipases for biotechnology. Curr. Opin. Biotechnol. 13, 390–397 [DOI] [PubMed] [Google Scholar]
- 2. Bornscheuer U. T. (2002) Methods to increase enantioselectivity of lipases and esterases. Curr. Opin. Biotechnol. 13, 543–547 [DOI] [PubMed] [Google Scholar]
- 3. Jaeger K. E., Dijkstra B. W., Reetz M. T. (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu. Rev. Microbiol. 53, 315–351 [DOI] [PubMed] [Google Scholar]
- 4. Lenfant N., Hotelier T., Velluet E., Bourne Y., Marchot P., Chatonnet A. (2013) ESTHER, the database of the α/β-hydrolase fold superfamily of proteins: tools to explore diversity of functions. Nucleic Acids Res. 41, D423–D429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arpigny J. L., Jaeger K. E. (1999) Bacterial lipolytic enzymes: classification and properties. Biochem. J. 343, 177–183 [PMC free article] [PubMed] [Google Scholar]
- 6. Jeon J. H., Lee H. S., Kim J. T., Kim S. J., Choi S. H., Kang S. G., Lee J. H. (2012) Identification of a new subfamily of salt-tolerant esterases from a metagenomic library of tidal flat sediment. Appl. Microbiol. Biotechnol. 93, 623–631 [DOI] [PubMed] [Google Scholar]
- 7. Zhu X., Larsen N. A., Basran A., Bruce N. C., Wilson I. A. (2003) Observation of an arsenic adduct in an acetyl esterase crystal structure. J. Biol. Chem. 278, 2008–2014 [DOI] [PubMed] [Google Scholar]
- 8. Byun J. S., Rhee J. K., Kim N. D., Yoon J., Kim D. U., Koh E., Oh J. W., Cho H. S. (2007) Crystal structure of hyperthermophilic esterase EstE1 and the relationship between its dimerization and thermostability properties. BMC Struct. Biol. 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benavente R., Esteban-Torres M., Acebrón I., de Las Rivas B., Muñoz R., Alvarez Y., Mancheño J. M. (2013) Structure, biochemical characterization and analysis of the pleomorphism of carboxylesterase Cest-2923 from Lactobacillus plantarum WCFS1. FEBS J. 280, 6658–6671 [DOI] [PubMed] [Google Scholar]
- 10. Mandrich L., Merone L., Pezzullo M., Cipolla L., Nicotra F., Rossi M., Manco G. (2005) Role of the N terminus in enzyme activity, stability and specificity in thermophilic esterases belonging to the HSL family. J. Mol. Biol. 345, 501–512 [DOI] [PubMed] [Google Scholar]
- 11. Ollis D. L., Cheah E., Cygler M., Dijkstra B., Frolow F., Franken S. M., Harel M., Remington S. J., Silman I., Schrag J. (1992) The α/β hydrolase fold. Protein Eng. 5, 197–211 [DOI] [PubMed] [Google Scholar]
- 12. Heikinheimo P., Goldman A., Jeffries C., Ollis D. L. (1999) Of barn owls and bankers: a lush variety of α/β hydrolases. Structure 7, R141–146 [DOI] [PubMed] [Google Scholar]
- 13. Wei Y., Contreras J. A., Sheffield P., Osterlund T., Derewenda U., Kneusel R. E., Matern U., Holm C., Derewenda Z. S. (1999) Crystal structure of brefeldin A esterase, a bacterial homolog of the mammalian hormone-sensitive lipase. Nat. Struct. Biol. 6, 340–345 [DOI] [PubMed] [Google Scholar]
- 14. Palm G. J., Fernández-Álvaro E., Bogdanovi X., Bartsch S., Sczodrok J., Singh R. K., Böttcher D., Atomi H., Bornscheuer U. T., Hinrichs W. (2011) The crystal structure of an esterase from the hyperthermophilic microorganism Pyrobaculum calidifontis VA1 explains its enantioselectivity. Appl. Microbiol. Biotechnol. 91, 1061–1072 [DOI] [PubMed] [Google Scholar]
- 15. Nam K. H., Kim M. Y., Kim S. J., Priyadarshi A., Kwon S. T., Koo B. S., Yoon S. H., Hwang K. Y. (2009) Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins 74, 1036–1040 [DOI] [PubMed] [Google Scholar]
- 16. Schiefner A., Gerber K., Brosig A., Boos W. (2014) Structural and mutational analyses of Aes, an inhibitor of MalT in Escherichia coli. Proteins 82, 268–277 [DOI] [PubMed] [Google Scholar]
- 17. Vonrhein C., Bönisch H., Schäfer G., Schulz G. E. (1998) The structure of a trimeric archaeal adenylate kinase. J. Mol. Biol. 282, 167–179 [DOI] [PubMed] [Google Scholar]
- 18. Singleton M., Isupov M., Littlechild J. (1999) X-ray structure of pyrrolidone carboxyl peptidase from the hyperthermophilic archaeon Thermococcus litoralis. Structure 7, 237–244 [DOI] [PubMed] [Google Scholar]
- 19. Rhee J. K., Kim D. Y., Ahn D. G., Yun J. H., Jang S. H., Shin H. C., Cho H. S., Pan J. G., Oh J. W. (2006) Analysis of the thermostability determinants of hyperthermophilic esterase EstE1 based on its predicted three-dimensional structure. Appl. Environ. Microbiol. 72, 3021–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li P. Y., Xie B. B., Zhang X. Y., Qin Q. L., Dang H. Y., Wang X. M., Chen X. L., Yu J., Zhang Y. Z. (2012) Genetic structure of three fosmid-fragments encoding 16S rRNA genes of the Miscellaneous Crenarchaeotic Group (MCG): implications for physiology and evolution of marine sedimentary archaea. Environ. Microbiol. 14, 467–479 [DOI] [PubMed] [Google Scholar]
- 21. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamura K., Dudley J., Nei M., Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 [DOI] [PubMed] [Google Scholar]
- 23. Petersen T. N., Brunak S., von Heijne G., Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 24. Jensen L. H. (1997) Refinement and reliability of macromolecular models based on X-ray diffraction data. Methods Enzymol. 277, 353–366 [DOI] [PubMed] [Google Scholar]
- 25. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 26. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 27. Krissinel E., Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 28. Hu Y., Fu C., Huang Y., Yin Y., Cheng G., Lei F., Lu N., Li J., Ashforth E. J., Zhang L., Zhu B. (2010) Novel lipolytic genes from the microbial metagenomic library of the South China Sea marine sediment. FEMS Microbiol. Ecol. 72, 228–237 [DOI] [PubMed] [Google Scholar]
- 29. Ngo T. D., Ryu B. H., Ju H., Jang E., Park K., Kim K. K., Kim T. D. (2013) Structural and functional analyses of a bacterial homologue of hormone-sensitive lipase from a metagenomic library. Acta Crystallogr. D Biol. Crystallogr. 69, 1726–1737 [DOI] [PubMed] [Google Scholar]
- 30. Angkawidjaja C., Koga Y., Takano K., Kanaya S. (2012) Structure and stability of a thermostable carboxylesterase from the thermoacidophilic archaeon Sulfolobus tokodaii. FEBS J. 279, 3071–3084 [DOI] [PubMed] [Google Scholar]
- 31. Pfeffer J. M., Weadge J. T., Clarke A. J. (2013) Mechanism of action of Neisseria gonorrhoeae O-acetylpeptidoglycan esterase, an SGNH serine esterase. J. Biol. Chem. 288, 2605–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Simone G., Menchise V., Manco G., Mandrich L., Sorrentino N., Lang D., Rossi M., Pedone C. (2001) The crystal structure of a hyper-thermophilic carboxylesterase from the archaeon Archaeoglobus fulgidus. J. Mol. Biol. 314, 507–518 [DOI] [PubMed] [Google Scholar]
- 33. Nam K. H., Kim M. Y., Kim S. J., Priyadarshi A., Lee W. H., Hwang K. Y. (2009) Structural and functional analysis of a novel EstE5 belonging to the subfamily of hormone-sensitive lipase. Biochem. Biophys. Res. Commun. 379, 553–556 [DOI] [PubMed] [Google Scholar]