Background: The role of flagellin glycosylation is not well understood.
Results: The Burkholderia cenocepacia flagellin is glycosylated on at least 10 different sites.
Conclusion: The presence of glycan in flagellin significantly impaired the inflammatory response of epithelial cells.
Significance: Flagellin glycosylation reduces recognition of flagellin by host TLR5, providing an evasive strategy to infecting bacteria.
Keywords: Bacteria, Carbohydrate Glycoprotein, Glycoprotein Biosynthesis, Lipopolysaccharide (LPS), Toll-like Receptor (TLR)
Abstract
Burkholderia cenocepacia is an opportunistic pathogen threatening patients with cystic fibrosis. Flagella are required for biofilm formation, as well as adhesion to and invasion of epithelial cells. Recognition of flagellin via the Toll-like receptor 5 (TLR5) contributes to exacerbate B. cenocepacia-induced lung epithelial inflammatory responses. In this study, we report that B. cenocepacia flagellin is glycosylated on at least 10 different sites with a single sugar, 4,6-dideoxy-4-(3-hydroxybutanoylamino)-d-glucose. We have identified key genes that are required for flagellin glycosylation, including a predicted glycosyltransferase gene that is linked to the flagellin biosynthesis cluster and a putative acetyltransferase gene located within the O-antigen lipopolysaccharide cluster. Another O-antigen cluster gene, rmlB, which is required for flagellin glycan and O-antigen biosynthesis, was essential for bacterial viability, uncovering a novel target against Burkholderia infections. Using glycosylated and nonglycosylated purified flagellin and a cell reporter system to assess TLR5-mediated responses, we also show that the presence of glycan in flagellin significantly impairs the inflammatory response of epithelial cells. We therefore suggest that flagellin glycosylation reduces recognition of flagellin by host TLR5, providing an evasive strategy to infecting bacteria.
Introduction
Burkholderia cenocepacia is a Gram-negative bacterium belonging to the B. cepacia complex. This group of opportunistic pathogens poses a health threat to patients with cystic fibrosis (1, 2). Chronic airway infection of these patients with the B. cepacia complex bacteria, particularly B. cenocepacia, accelerates the decay of lung function and in some cases leads to a lethal necrotizing pneumonia known as “cepacia syndrome” (3). B. cepacia complex infections have also been reported in nosocomial outbreaks not related to cystic fibrosis (4–7). Together with Burkholderia multivorans, B. cenocepacia accounts for the majority of B. cepacia complex infections in cystic fibrosis patients (8, 9). B. cenocepacia encompasses at least four phylogenetic lineages, IIIA to IIID, but most of the cystic fibrosis isolates belong to lineage IIIA and IIIB (10, 11). The clonal lineage ET12 belongs to the IIIA group, and these bacteria were responsible for most of the deaths related to cepacia syndrome in the early 1980s (3, 12, 13).
B. cenocepacia K56-2 is an ET12 strain that carries various virulence factors, including lipopolysaccharide (LPS) and flagella. The LPS from K56-2 has been intensively studied in our laboratory (14–18) and consists of lipid A, core oligosaccharide, and polymeric O-antigen (19). The K56-2 O-antigen is a polymer of a trisaccharide-repeating unit containing rhamnose and two N-acetylgalactosamine residues (15). In general, LPS is a potent proinflammatory molecule, and the K56-2 O-antigen influences phagocytosis by human macrophages and interferes with bacterial adherence to bronchial epithelial cells (18, 20).
Flagella are organelles for bacterial motility, but they are also involved in pathogenicity (21) such as adhesion to and invasion of epithelial cells, and biofilm formation (22–26). Flagella consist of a basal body, flagellar hook, and a filament built of flagellin monomers, which are specifically recognized by the innate immune system via the Toll-like receptor 5 (TLR5)2 (26, 27). Toll-like receptors are membrane-bound pattern-recognition receptors in epithelial and immune cells, which play an essential role in initiating innate immune responses (28). TLRs recognize pathogen-derived microbial molecules (pathogen-associated molecular patterns) like LPS (TLR4) or flagellin (TLR5). Engagement of TLR by its specific ligand initiates an intracellular signaling cascade leading to the activation of nuclear factor κB (NF-κB) and members of the MAPK family. These signaling pathways subsequently activate transcription of pro-inflammatory cytokines like interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor α (TNF-α). The TLR5 signaling pathway plays a pivotal role in exacerbating lung inflammation in cystic fibrosis (29), and it is responsible for B. cenocepacia-induced lung epithelial inflammatory response (30). Furthermore, a mutation leading to reduced activating capacity of the TLR5 was associated with reduced organ failure and improved survival in patients infected with Burkholderia pseudomallei, another important pathogen of the genus Burkholderia (31), underscoring the critical role of TLR5 and its ligand in human infection.
B. cenocepacia strains produce two types of flagellin, type I and II, distinguished by the molecular size of the protein and restriction fragment length polymorphism analyses (32). Flagellin in B. cenocepacia K56-2 belongs to type II, and these bacteria carry a single long polar flagellum that contributes to virulence in a mouse infection model and induces host immune responses via TLR5 (26). B. pseudomallei and Burkholderia thailandensis produce glycosylated flagellin (33), but the glycosylation status of flagellin in B. cenocepacia is unknown. In this work, we report that B. cenocepacia flagellin filaments are post-translationally modified by glycosylation at multiple sites with a single glycan residue and identify the key genes responsible for this modification. We also demonstrate that flagellin glycosylation reduces the ability of this protein to trigger TLR5-mediated inflammatory responses in epithelial cells.
EXPERIMENTAL PROCEDURES
Strains and Chemicals
The strains used in this study are listed in Table 1. Bacteria were grown either on 1.5% agar plates or in LB broth (Lennox) at 37 °C. When required, antibiotics were added as follows: trimethoprim, 50 μg ml−1 for Escherichia coli and 100 μg ml−1 for B. cenocepacia; tetracycline, 20 μg ml−1 for E. coli and 100 μg ml−1 for B. cenocepacia; kanamycin, 40 μg ml−1 for E. coli; chloramphenicol, 30 μg ml−1 for E. coli and 150 μg ml−1 for B. cenocepacia. Ampicillin at 200 μg ml−1 was used during triparental mating to selectively eliminate donor and helper E. coli strains. When required, rhamnose was added to a final concentration of 0.4% (w/v). Sucrose plates for the final curing of deletion mutants were prepared with 10 g liter−1 of tryptone, 5 g liter−1 of yeast extract, and 50 g liter−1 of sucrose in 1.5% agar. Antibiotics and chemicals were purchased from Sigma. Growth media were purchased from BD Biosciences. Restriction enzymes, Antarctic phosphatase, and T4 ligase were purchased from New England Biolabs (Ipswich, MA). HEK293-TLR5 cells expressing human TLR5 were purchased from Invivogen (San Diego), and p-P65, p-ERK, ERK, p-P38, P38, p-JNK and JNK antibodies were from Cell Signaling (Danvers, MA). P65 was purchased from Santa Cruz Biotechnology (Dallas, TX) and β-actin antibody from Sigma.
TABLE 1.
Strains and plasmids used in this study
| Name | Description | Source |
|---|---|---|
| Strains | ||
| B. cenocepacia | ||
| K56-2 | ET12 clone related to J2315, cystic fibrosis clinical isolate | BCRRCa (2, 73) |
| RSF44 | K56-2, ΔfliCD | 38 |
| MH1K | K56-2, ΔamrABC (BCAL1674–1676); Gms | 74 |
| ΔBCAL3119–3131 | MH1K, ΔwbiI-wzm | This study |
| ΔBCAL3123–3124 | MH1K, ΔwbxCD | This study |
| ΔBCAL0111 | MH1K, ΔflmQ (flagellin glycan glycosyltransferase) | This study |
| ΔBCAL0110 | MH1K, ΔBCAL0110 (vioA paralogue in the fliC gene cluster) | This study |
| ΔBCAS0105 | MH1K, ΔBCAS0105 (rmlD paralogue in chromosome 3) | This study |
| ΔBCAS0105 pSC200/rmlD | MH1K, ΔBCAS0105, containing Prha::rmlD | This study |
| ΔBCAL3129 | MH1K, ΔvioA | This study |
| XOA10 | K56-2, Prha::BCAL1928 | 17 |
| XOA11 | K56-2, Prha::arnT | 17 |
| MH43 | MH1K, ΔwbxD (BCAL3124) | M. Hamad |
| MH1K pSC200/rmlD | MH1K, Prha::rmlD | This study |
| MH1K pSC200/rmlB | MH1K, Prha::rmlB | This study |
| MH1K pSC200/rmlC | MH1K, Prha::rmlC | This study |
| MH1K pSC200/BCAL0111 | MH1K, Prha::BCAL0111 | This study |
| ΔBCAL3123–3124 pIN62/BCAL3123 | MH1K, ΔwbxCD; wbxC+ | This study |
| E. coli | ||
| GT115 | F− mcrA Δ (mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 rpsL endA1 Δdcm uidA (ΔMluI)::pir-116 ΔsbcC-sbcD; used as donor strain | Laboratory stock |
| SY327 | araD Δ(lac pro) argE(Am) recA56 nalA λ pir; Rifr; used as helper strain | 75 |
| Plasmids | ||
| pRK2013 | Helper plasmid used for bacterial conjugation; Kanr | 76 |
| pGPI-SceI-2 | Suicide vector used for genetic manipulation of B. cenocepacia; Tpr | 14 |
| pDAI-SceI-SacB | Replicative vector expressing I-SceI homing endonuclease; Tetr | 74 |
| pIN62 | Broad host range replicative vector expressing DSRed, Cmr | 40 |
| pGPΩTp | Suicide vector used for genetic manipulation of B. cenocepacia; Tpr | 39 |
| pSC200 | Rhamnose-inducible vector used for depletion experiments; Tpr | 17 |
| pGPI-SceI-2/BCAL3119–3131 | Suicide vector used to delete O-antigen cluster | This study |
| pGPI-SceI-2/BCAL3123–3124 | Suicide vector used to delete wbxC and wbxD | This study |
| pGPI-SceI-2/BCAL0111 | Suicide vector used to delete BCAL0111 | This study |
| pGPI-SceI-2/BCAL0110 | Suicide vector used to delete BCAL0110 | This study |
| pGPI-SceI-2/BCAS0105 | Suicide vector used to delete BCAS0105 | This study |
| pGPI-SceI-2/BCAL3129 | Suicide vector used to delete vioA homologue in O-antigen cluster | This study |
| pGPΩTp/rmlD | Vector used to create gene disruption in rmlD (BCAL3132) | This study |
| pSC200/rmlB | Prha::rmlB (BCAL3135) | This study |
| pSC200/rmlC | Prha::rmlC (BCAL3133) | This study |
| pSC200/rmlD | Prha::rmlD (BCAL3132) | This study |
| pSC200/BCAL0111 | Prha::BCAL0111 | This study |
| pIN62/BCAL3123 | Vector used for complementation of wbxC (BCAL3123) | This study |
a BCRRC, B. cepacia Research and Referral Repository for Canadian CF Clinics; Cmr, chloramphenicol resistance; Tpr, trimethoprim resistance; Tetr, tetracycline resistance; Kanr, kanamycin resistance; DSRed, red fluorescent protein from Discosoma sp.
Isolation of Flagellin
Flagella were isolated as in Brett et al. (34) with some modifications. Briefly, bacteria were grown for 18 h in 400 ml of LB with antibiotics and/or rhamnose as required and centrifuged, and the bacterial pellets were frozen at −20 °C overnight. Thawed pellets were next resuspended in 20 ml of PBS, and flagella were sheared off with a homogenizer (low speed setting for 4 min on ice). Cell debris was removed by centrifugation (6,000 × g, 10 min, 4 °C), and flagella were precipitated overnight from the supernatant with ammonium sulfate (end concentration 5%). The precipitate was centrifuged (12,000 × g, 30 min, 4 °C) and the supernatant discarded. The pellet, containing flagella, was dissolved in 750 μl of PBS of which 250 μl were stored at −20 °C for SDS-PAGE analysis (crude flagellar filaments fraction), and the remaining 500 μl were centrifuged again (16,900 × g, 10 min, 4 °C). Flagellar filaments in the sediment were solubilized with 8 m urea; insoluble debris was removed by centrifugation (10,000 × g, 1 min), and the solubilized flagellin was desalted on a HiTrap ÄKTA FPLC column (GE Healthcare) using either 25 mm ammonium bicarbonate (prior to structural analyses) or PBS (for biological tests) as eluents. Soluble and purified flagellin was either stored at −20 °C or lyophilized.
SDS-PAGE and Western Blot
The purity and the molecular mass of flagellin were assessed in 14% SDS-polyacrylamide gels stained with PageBlue protein staining solution (Thermo Scientific). Bio-Rad Precision Plus Dual Color Protein Standard was used as a molecular weight marker. To visualize glycosylated proteins, the Pro-Q Emerald glycoprotein stain kit was used according to the manufacturer's manual (Molecular Probes). Flagellin was detected on Western blots with primary polyclonal antibody RFFL/ARP42986_P050 provided by AVIVA Systems Biology (San Diego) and with secondary goat anti-rabbit IgG-HRP secondary antibody. The blots were developed with Western Lightning ECL Pro (PerkinElmer Life Sciences).
Mass Spectrometry and Enzymatic Digestion
Flagellin was in-gel digested with trypsin, chymotrypsin, AspN, and a mixture of AspN and trypsin. LC MS/MS mass spectrometry analyses were performed on a Waters QTof global mass spectrometer equipped with a Z-spray (ESI) source and run in positive ion mode (the instrument was run in DDA mode) in combination with a Waters nanoAcquity UPLC, and the results were confirmed with a Thermo Scientific Orbitrap Elite MS (LC-MS/MS). The Peaks software (Bioinformatics Solutions Inc.) was used to analyze the digested samples. Waters QTof Micro with Waters MassLynx 4.1 was used for whole protein analyses. Flagellin was analyzed as an intact protein in 25 mm ammonium bicarbonate. Lyophilized, digested samples were reconstituted in 20 μl of 0.2% formic acid in water, and 10 μl were injected.
Chemical Deglycosylation of Flagellin
Desalted and lyophilized protein (1.5 mg) was chemically deglycosylated by trifluoromethanesulfonic acid (35). Briefly, 100 μl of a 10% toluene/trifluoromethanesulfonic acid mixture was slowly added to the sample in a glass vial placed in a dry ice/ethanol bath. After 2 h, the mixture was carefully neutralized with 300 μl of pyridine solution (pyridine/methanol/water at a ratio of 3:1:1 v/v/v) for 5 min in a dry ice/ethanol bath, and the sample was transferred to wet ice for another 15 min. The mixture was transferred into a plastic 1.5-ml vial, and 400 μl of 25 mm ammonium bicarbonate was added to precipitate the deglycosylated flagellin. After centrifugation (16,900 × g, 10 min), the supernatant was discarded, and the pellet was dissolved in 8 m urea. Further desalting in 25 mm ammonium bicarbonate was performed on a HiTrap column as described above, and the sample was used directly for MS analysis.
GC/MS Analyses and β-Elimination
Methanolysis was used to analyze the glycan moiety of flagellin. Briefly, 400 μg of the lyophilized sample was treated with 0.5 m methanolic HCl (weak methanolysis) and peracetylated, and an aliquot was used to record GC/MS spectra. Next, the same sample was treated with 2 m methanolic HCl (strong methanolysis), peracetylated, and analyzed again. To determine the character of the bound glycosyl residue, another 400 μg of lyophilized flagellin was used for β-elimination. Briefly, 400 μg of lyophilized sample were treated with 0.1 m NaOH containing 0.8 m NaBH4 for 8 h at 37 °C in the dark. Next, the mixture was dried under nitrogen, peracetylated, and analyzed. To confirm the conformation of the sugar, ions detected in GC/MS spectra from B. cenocepacia FliC were compared with GC/MS of the O-antigen sample from Providencia stuartii O43 (kindly provided by J. Knirel and O. Ovchinnikova). The d-configuration of the sugar was determined by octanolysis (36). Mass spectrometric measurements were performed with Agilent Technologies 5975 inert XL MSD equipped with split/splitless injector system with electron ionization under autotune conditions at 70 eV.
General Molecular Techniques and Genetic Manipulation of B. cenocepacia
Plasmid vectors and primers are listed in Tables 1 and 2, respectively. DNA manipulations and cloning were performed as described previously (37). PCRs were performed with HotStar HiFidelity DNA polymerase (Qiagen). Plasmid and genomic DNA were isolated using QiaPrep Spin kit and DNeasy Blood and Tissue kit (Qiagen), respectively. PCR products were purified using a QIAquick PCR purification kit or a QIAquick gel extraction kit (Qiagen). Freshly prepared chemically competent E. coli GT115 cells were transformed by the calcium chloride method. Plasmids were mobilized into B. cenocepacia by triparental mating (14, 38).
TABLE 2.
Primers used in this study (restriction sites are italicized)
| Name | Sequence (5′ to 3′) | Restriction site |
|---|---|---|
| 5235 | gattgatgcggccgcgaagccgccatcggcgcgaacccg | NotI |
| 5236 | gcacctaagatctgccagcatgcgccgtcttgcggg | BglII |
| 5237 | tagctgagatctggcgcaatcggcaatgagggcgaccag | BglII |
| 5238 | aacgtgtctagaagtgtggtggtgtcgctgctgagc | XbaI |
| 5685 | cgtagtgaattcgacggcagcaagcaggcaccttattcgga | EcoRI |
| 5686 | atcatatctagaccggcacgccgttccgcgagggacttc | XbaI |
| 5852 | aatgaagatctcgccgccgtgccccatgctcgacgcctg | BglII |
| 5853 | catatgcggccgcctacaagcacgtgccgctgatggaag | NotI |
| 5888 | gatcgatgcggccgcacttgaaagacgatcattcccacg | NotI |
| 5889 | attgctctagacgttttgatgaacgtttcggact | XbaI |
| 5922 | gcacctaagatctctaccgaaggggcaggccggggctgtt | BglII |
| 5923 | gtagtcgcggccgccgagtcgaggacgtcgagttcggcg | NotI |
| 5924 | cagtactctagagtcgtcggacggggggatacggtggtc | XbaI |
| 5925 | gtagtcgcggccgcccgttacccgacctacacgcccgacgtc | NotI |
| 6021 | tagctacatatgatcctggttacgggcggcgcggg | NdeI |
| 6022 | taacgtctagagaacgtgccgaccacgttggtctggac | XbaI |
| 6023 | tagctgcatatgcgtgaggcaacgatgagctggaaaccg | NdeI |
| 6024 | atatgtctagacgagccgcgcgctgcggcaacgcgtgcc | XbaI |
| 6093 | cgggtgatccgggaagttctggatgaagacctggcggc | None |
| 6094 | aatgaacgagtgcttccgccgacgccaaaacggctttcc | None |
| 6165 | catagcggccgccttctgcccaccattcgtcaaccacgc | NotI |
| 6166 | cgactagatctatctaagcatcggtcaggtcgacacatg | BglII |
| 6167 | catagcggccgcaagcagttcaacgtattcgcgcgtcgc | NotI |
| 6168 | gtcatctagagctgagcgccgtgttgtatgcggcacatg | XbaI |
| Q38 | tcatctagagctcgtcgatttgatcggtacgcgccatac | XbaI |
| Q39 | ccttttgcggccgcaatgcccgtattgcgcgcgccagac | NotI |
| Q89 | tagctgcatatgatgttctcgaccgaactgcccgccac | NdeI |
| Q90 | taacgtctagaccgtttgcccggtgcgatgcagcg | XbaI |
| Q91 | tagctacatatgatggccatccaagtaacggtgacagc | NdeI |
| Q92 | taacgtctagatcgtccgacaggacaacccccacccac | XbaI |
| L3123 US BglII | gactagatctccgtggccattcgtgccacaggcatcc | BglII |
| L3123 US NotI | attagcggccgcatcgcgatgctctggcgagacgagcg | NotI |
| L0110 US XbaI | agtcatctagattgcgtgcacgctgctcagcgtccgcgg | XbaI |
| L0110 US NotI | catagcggccgcgcaagggtgccgttcgcgaacagcgac | NotI |
| L0110 DS NotI | catagcggccgcgtcgcgaaccacgcgtatttcccgatc | NotI |
| L0110 DS BglII | acgcgttcagatctttcgagttcgacaacagcgcgatgg | BglII |
| L3123 XbaI | tagtcatctagattaggccgaccgtttcatcaatggcac | XbaI |
| L3123 NdeI | acgctcatatggattggagtgaatgatggagcgaatcgc | NdeI |
Cloning of B. cenocepacia K56-2 fliC
The fliC gene (BCAL0114) was amplified from B. cenocepacia K56-2 genomic DNA with the primer pair 6093/6094 and sequenced at the Core Molecular Biology Facility, York University, Toronto, Canada. The B. cenocepacia K56-2 fliC sequence was submitted to GenBankTM and is available under accession number KC763156.
Construction of Mutants in B. cenocepacia
Unmarked deletion mutants were constructed as described previously (14, 38). Briefly, the target genes were deleted by allelic exchange using the pGPI-SceI-2 plasmid containing the corresponding upstream and downstream fragments. The resulting deletion plasmids were introduced into B. cenocepacia by triparental mating. Upstream fragments for deletion of the vioA homologue in the O-antigen cluster (BCAL3129), flmQ (BCAL0111), the vioA homologue in the fliC cluster (BCAL0110), the rmlD homologue (BCAS0105), the O-antigen cluster between wbiI and wzm (BCAL3119 to BCAL3131), and wbxC/wbxD (BCAL3123 to BCAL3124) (15) were amplified with primer pairs 6165/6166, 5235/5236, L0110 US XbaI/L0110 US NotI, 5922/5923, 5852/5853, and L3123 US BglII/L3123 US NotI and downstream fragments by 6167/6168, 5237/5238, L0110 DS NotI/L0110 DS BglII, 5924/5925, 5888/5889, and Q38/Q39, respectively (Table 2). The insertional inactivation of rmlD (BCAL3132) was achieved by cloning ∼300-bp internal fragments from BCAL3132 (amplified using primers pair 5685/5686; Table 2) into pGPΩTp. The resulting mutagenesis plasmid pGPΩTp/rmlD was mobilized into B. cenocepacia (39). Conditional mutants in rmlB (BCAL3135), rmlC (BCAL3133), rmlD (BCAL3132), and flmQ (BCAL0111) were constructed using pSC200 (17). The primers used to amplify DNA fragments were as follows: 6021/6022 (rmlB), Q92/Q91 (rmlC), 6023/6024 (rmlD), and Q89/Q90 (flmQ; Table 2). Each amplicon contained the NdeI restriction site in the starting codon of each gene to facilitate cloning into pSC200.
Rhamnose Depletion Assays
Conditional mutants were grown overnight in 5 ml of LB with trimethoprim (100 μg ml−1) and 0.4% rhamnose. The next day, 1 ml of each strain was centrifuged and washed three times with LB without rhamnose. The absorbance (A600) was adjusted to 1.0 in LB without rhamnose, and 3 μl of each dilution of 10−1 to 10−6 were incubated at 37 °C on LB agar with trimethoprim with or without 0.4% rhamnose for 24 h. The essentiality of each respective gene was also assessed in broth. For this, overnight cultures grown in 5 ml of LB with trimethoprim (100 μg ml−1) and 0.4% rhamnose were centrifuged and washed three times in LB without rhamnose. Each strain was diluted to A600 0.03 in LB/trimethoprim with or without rhamnose, and triplicates of 300 μl were incubated for 4 h in honeycomb plates at 37 °C with shaking using a Bioscreen (Oy Growth Curves, Finland). Next, 3 μl of each dilution were transferred to fresh medium with or without rhamnose and incubated for an additional 19 h. The A600 was measured every 30 min. Strains XOA10 (B. cenocepacia K56-2 pSC200/BCAL1928; nonlethal conditional mutant) and XOA11 (B. cenocepacia K56-2 pSC200/arnT; lethal mutation) were used as controls (17).
Complementation Experiments
Plasmid pIN62 (encoding chloramphenicol resistance (40)) was used to complement BCAL3123, which was cloned from B. cenocepacia K56-2 genomic DNA using the L3123 XbaI/L3123 NdeI primer pair (Table 2). The plasmid and PCR product were digested with XbaI and NdeI at 37 °C for 16 h. The digested plasmid DNA was subsequently dephosphorylated using Antarctic phosphatase (37 °C, 30 min), which was then deactivated at 65 °C (2 min). Ligation was performed at 16 °C for 16 h using T4 DNA ligase. Transformation and triparental mating were performed as described previously (see text above). The resulting plasmid pIN62/BCAL3123 (as confirmed by sequencing) was introduced into the appropriate B. cenocepacia strains via triparental mating.
Whole Cell Lysates and LPS Staining
To determine the presence of O-antigen, whole cell lysates were prepared and resolved on 14% SDS-polyacrylamide gels, and LPS was visualized by silver staining as described previously (41), except that instead of citric acid, a mixture of 2.5% sodium carbonate (w/v) with 0.05% formaldehyde (v/v) in water heated to 60 °C was used as developing solution.
Motility Assays and Biofilm Formation
Bacterial motility was analyzed on soft agar plates (1% Bacto Tryptone in 0.3% agar). The A600 of overnight cultures was adjusted to 1.0, and 2 μl of culture were inoculated in the center of agar plate. The growth zone diameter was measured after 24 h of incubation at 37 °C. Biofilm mass was quantified by the crystal violet protocol as described previously (42).
Biological Assays
Flagellin from B. cenocepacia parental strain and the BCAL0111 deletion mutant was purified in PBS as described above. The concentration of FliC was confirmed densitometrically. THP1 cells or HEK293-TLR5 cells were seeded (2 × 105 cells ml−1; 2 ml) in 12-well plates and stimulated with the indicated concentrations of WT and nonglycosylated flagellin for 24 h. Conditioned medium was then measured for levels of TNF-α, IL-6, IL-8, and IL-1β (DuoSet kits; R & D Systems) according to the manufacturer's protocol. For luciferase reporter assays, HEK293-TLR5 cells were seeded (1.5 × 105 cells ml−1; 200 μl) in 96-well plates and transfected with constructs encoding NFκB-regulated firefly luciferase (80 ng) and the TK Renilla luciferase reporter construct (phRL-TK; 20 ng; Promega Biosciences). Cells were treated as indicated, and cell lysates were assayed for firefly luciferase activity and normalized for transfection efficiency using TK Renilla luciferase activity. Cell extracts were also assayed for phosphorylated and total levels of p65 and p38, JNK and ERK MAPKs by Western blotting.
RESULTS
B. cenocepacia Flagellin Is Glycosylated with 4,6-Dideoxy-4-(3-hydroxybutanoylamino)-d-glucose
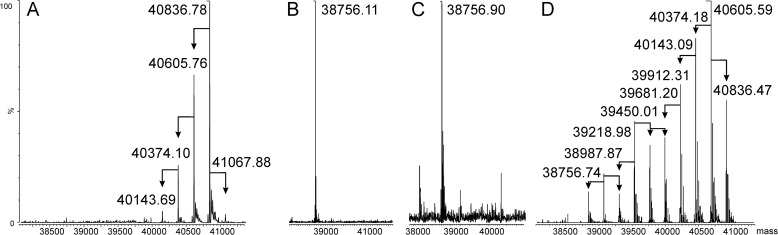
Flagella were sheared off B. cenocepacia cells and solubilized with 8 m urea, as described under “Experimental Procedures” (Fig. 1A). Mass spectrometric analyses of tryptic digests confirmed the identity of the flagellin monomer (FliC). Further MS analyses of native FliC revealed one major molecular ion at 40,836 m/z and minor ions at 40,605, 40,374, 40,143, and 41,067 m/z (Fig. 2A). These masses were compared with the theoretical mass of FliC from B. cenocepacia J2315, which is 38,779.79 Da. Strains J2315 and K56-2 belong to the ET12 clone, but J2315 was the only ET12 strain sequenced at the time of these experiments (43). Thus, the observed mass of the major molecular ion was 2,057 Da larger than expected from the theoretical amino acid sequence. Moreover, the molecular ions differed from each other by 231 m/z, suggesting the presence of at least five modifications. In SDS-polyacrylamide gels, FliC was visualized by Coomassie Blue staining and also reacted with Pro-Q Emerald glycoprotein stain, suggesting that the observed modifications were due to glycosylation. FliC was also detected on Western blot with the primary antibody RFFL/ARP42986_P050 (Fig. 1, B and C).
FIGURE 1.

SDS-PAGE and Western blot analyses of B. cenocepacia FliC. A, Coomassie-stained SDS-PAGE showing crude flagellar filaments (C), supernatant obtained after insoluble flagella were sedimented at 16,000 × g for 10 min (S), and purified flagellin after solubilization with 8 m urea and desalting (P). B, crude flagellar filaments from the B. cenocepacia parental strain (WT) and ΔBCAL0111 (Δ0111) were analyzed by Western blot with the AVIVA RFFL/ARP42986_P050 antibody. C, Coomassie Blue-stained SDS-PAGE of crude flagellar filaments from B. cenocepacia parental strain (WT) and ΔBCAL0111 (Δ0111) from the same preparation used in B. D, Coomassie Blue-stained SDS-PAGE of chemically deglycosylated (dgWT) and native (WT) flagellin. Arrows indicate the corresponding molecular masses of the protein standards in kDa.
FIGURE 2.
Mass spectra of purified flagellin preparations. A, B. cenocepacia flagellin. B, chemically deglycosylated flagellin. C, nonglycosylated flagellin purified from the ΔBCAL0111 mutant strain. D, flagellin purified from strain MH43 (ΔwbxD). Arrows indicate the difference of 231 m/z between ions.
To accurately determine the molecular mass of FliC, purified flagellin was chemically deglycosylated, as indicated under “Experimental Procedures.” The deglycosylation method was optimized to specifically cleave glycosidic bonds without damaging the peptide backbone (35). The MS analysis of the deglycosylated protein showed a single molecular ion of 38,756.90 m/z (Fig. 2B). This result provided additional evidence that FliC was modified by a glycan. Furthermore, MS of the tryptic digest confirmed the identity of the deglycosylated protein as FliC, except that it was 23 Da smaller than expected from the theoretical mass of the J2315 FliC (38,779.79 Da). This suggested that FliC proteins from K56-2 and J2315 were not completely identical. DNA sequencing of the fliC (BCAL0114) gene from K56-2 revealed a single C to A substitution at 1,072 bp, resulting in a histidine to asparagine replacement at position 358 in the K56-2 FliC (H358N) giving a 23-Da difference in molecular mass. The difference in mass between native and chemically deglycosylated FliC was also reflected in SDS-PAGE analyses by Coomassie Blue staining (Fig. 1D). However, deglycosylated FliC still reacted with Pro-Q Emerald, indicating that this stain was not specific for the B. cenocepacia FliC glycan.
Because trypsin digestion alone did not provide sufficient peptide coverage spanning the entire FliC protein, additional digestions were performed with chymotrypsin, AspN, and a mixture of AspN and trypsin. Mass spectra were recorded for all four digested samples separately, and the combined data were analyzed, giving 100% sequence coverage. This strategy allowed us to identify ions matching the peptides with one or two 231 m/z modifications (Table 3). Thus, the localization of single modifications was assigned to peptides 159DLSQSMSAAK168, 177GQTVGTVTGLSLDNNGAYTGSGATITAINVLSDGK211, and 287DISTVSGANVAMVSIDNALQTVNNVQAALGAAQNR321, whereas peptides 212-GGYTFTDQNGGAISQTVAQSVF-233, 234GANATTGTGTAVGNLTLQ251, and 252SGATGAGTSAAQQTAITNAIAQINAVNKPATLVSNL287 carried two modifications. From these combined results, we could clearly identify 9 out of 10 possible modification sites (as determined by MS of the entire FliC (Fig. 2, A and D, and Table 3). The exact position of the modifications in each peptide was not determined.
TABLE 3.
Peptide ions identified after combining MS/MS data from tryptic, chymotryptic, and AspN/tryptic digests of B. cenocepacia FliC
Representative unmodified and modified ions are presented. Ions were confirmed in QTof and Orbitrap Elite analyses; (+231) refers to glycan modification Qui4N(3HOBut); oxidation refers to methionine (+16 Da).
| Start-End |
Mr |
Oxidation (+16) | Sequence | ||
|---|---|---|---|---|---|
| Observed | Calculated | Expected | |||
| m/z | |||||
| 1–36 | 954.2421 | 3812.9393 | 3812.9915 | Yes | MLGINSNINSLVAQQNLNGSQNALSQAITRLSSGKR |
| 37–52 | 773.3992 | 1544.7838 | 1544.7794 | No | INSAADDAAGLAISTR |
| 53–90 | 992.7329 | 3966.9025 | 3966.9011 | Yes (2 x) | MQTQINGLNQGVSNANDGVSMIQTASSALSSLTNSLQR |
| 91–106 | 840.9565 | 1679.8984 | 1679.8512 | Yes | IRQLAVQASTGTMSTT |
| 107–137 | 1154.2551 | 3459.7435 | 3459.7230 | No | DQAALQQEVSQQIQEVNRIASQTTYNGTNIL |
| 138–158 | 1010.5225 | 2019.0304 | 2019.0273 | No | DGSAGIVSFQVGANVGQTISL |
| 159–168 | 519.2255 | 1036.4364 | 1036.485 | No | DLSQSMSAAK |
| 159–168 | 527.2358 | 1052.4570 | 1052.4808 | Yes | DLSQSMSAAK |
| 159–168 | 642.8051 | 1283.5956 | 1283.5677 | Yes | DLSQSMSAAK (+231)a |
| 169–176 | 386.2400 | 770.4654 | 770.4650 | No | IGGGLVQK |
| 177–211 | 1118.2345 | 3351.6817 | 3351.6794 | No | GQTVGTVTGLSLDNNGAYTGSGATITAINVLSDGK |
| 177–211 | 1195.2588 | 3582.7546 | 3582.7663 | No | GQTVGTVTGLSLDNNGAYTGSGATITAINVLSDGK (+231) |
| 187–206 | 1085.5468 | 2169.0790 | 2169.0199 | No | SLDNNGAYTGSGATITAINV (+231) |
| 187–211 | 813.7294 | 2438.1664 | 2438.1925 | No | SLDNNGAYTGSGATITAINVLSDGK |
| 187–211 | 890.7588 | 2669.2546 | 2669.2794 | No | SLDNNGAYTGSGATITAINVLSDGK (+231) |
| 189–208 | 969.9615 | 1937.9084 | 1937.9330 | No | DNNGAYTGSGATITAINVLS |
| 189–208 | 724.0175 | 2169.0307 | 2169.0199 | No | DNNGAYTGSGATITAINVLS (+231) |
| 189–217 | 1032.8304 | 3095.4694 | 3095.4333 | No | DNNGAYTGSGATITAINVLSDGKGGYTFT (+231) |
| 212–233 | 904.7971 | 2709.2692 | 2709.2659 | No | GGYTFTDQNGGAISQTVAQSVF (2× 231)b |
| 234–251 | 1055.0292 | 2108.0438 | 2108.0009 | No | GANATTGTGTAVGNLTLQ (2× 231)a |
| 252–286 | 1258.6530 | 3772.9372 | 3772.9331 | No | SGATGAGTSAAQQTAITNAIAQINAVNKPATVSNL (2× 231) |
| 287–321 | 1176.9285 | 3527.7637 | 3527.7637 | Yes | DISTVSGANVAMVSIDNALQTVNNVQAALGAAQNR |
| 287–321 | 1253.9667 | 3758.8745 | 3758.8745 | Yes | DISTVSGANVAMVSIDNALQTVNNVQAALGAAQNR (+231) |
| 290–321 | 1148.9210 | 3443.7412 | 3443.7076 | Yes | TVSGANVAMVSIDNALQTVNNVQAALGAAQNR (+231) |
| 322–357 | 952.9465 | 3807.7569 | 3807.7381 | Yes | FTAIATSQQAESTDLSSAQSQITDANFAQETANMSK |
| 359–382 | 849.4679 | 2545.3819 | 2545.4592 | No | QVLQQAGISVLAQANSLPQQVLKL |
| 371–384 | 790.4595 | 1578.9044 | 1578.9093 | No | QANSLPQQVLKLLQ |
a Data were obtained with QTof only.
b Data were obtained with Orbitrap Elite only.
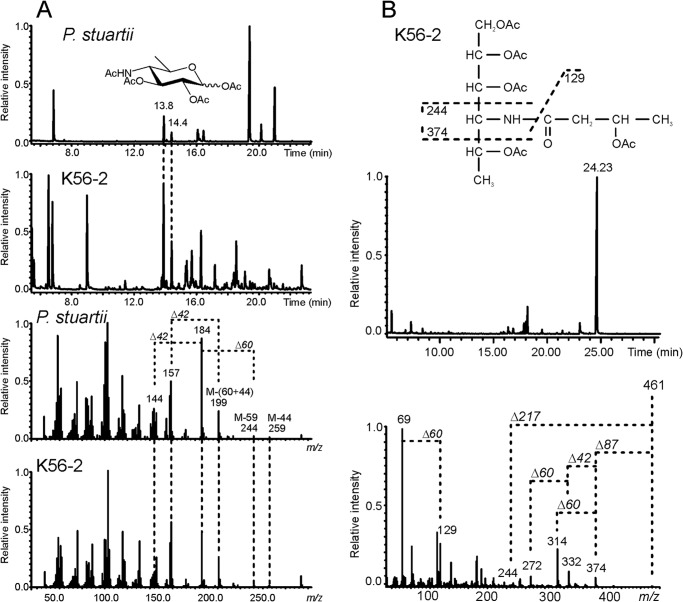
To identify the nature of the FliC glycan, flagellin was analyzed by GC/MS. Combined data collected from GC/MS spectra after weak and strong methanolysis identified a 4,6-dideoxy-4-(3-hydroxybutanoylamino)-hexose. Comparison with GC/MS spectra obtained after similar treatment of P. stuartii O43 O-antigen samples (44), confirmed that the sugar possessed the gluco configuration, representing viosamine with 3-hydroxybutyric acid substituting amino group at C4, referred to as d-Qui4N(3HOBut) (Figs. 3 and 4). We used β-elimination to establish the character of the glycosidic bond between glycan and the FliC peptide backbone. The β-elimination releases glycans that form O-glycosidic bonds with serine or threonine, leaving N-glycosidic bonds intact. d-Qui4N(3HOBut) was the only sugar identified by GC/MS analysis of the sample after β-elimination (Fig. 4), demonstrating that B. cenocepacia FliC was O-glycosylated. The structure of the glycan was also consistent with the measured mass difference of 231 Da (theoretical Mr 249.1212, H2O = 231.1106; Fig. 3).
FIGURE 3.
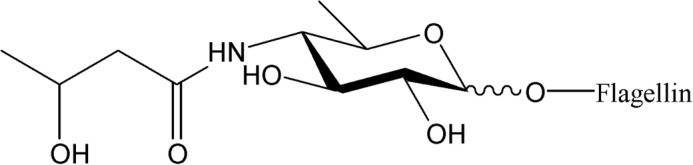
Structure of the B. cenocepacia FliC glycan d-Qui4N(3HOBut).
FIGURE 4.
GC/MS spectra after methanolysis and β-elimination of B. cenocepacia FliC glycan (K56-2) and control sample (O-antigen of P. stuartii O43). A, top two graphs correspond to an overview of entire spectra for P. stuartii O43 O-antigen and B. cenocepacia K56-2 FliC samples. Qui4N peaks at 13.8 and 14.4 (representing α- and β-configured derivatives) are indicated. Additional peaks detected in the O43 spectrum represent other sugars from the O-antigen (44). Additional peaks in the FliC spectrum represent derivatized amino acids released from the FliC protein during methanolysis. The lower two spectra show the characteristic fragmentation pattern of ions at 13.8 min (fragmentation pattern of ion at 14.4 min was identical). M corresponds to molecular weight of derivatized Qui4N (303 Da). B, top graph shows an overview of the GC spectrum of the glycan released from FliC during β-elimination. Inset shows the derivatized glycan (461 Da) with the characteristic fragmentation pattern of the sugar and 3-hydroxybutyric acid. Lower graph shows the MS/MS fragmentation spectrum of the ion at 24.23 min. Differences between fragment ions (Δ) correspond to CH2CO (Δ42), CH3CHO (Δ44), CH3COO− (Δ59), and CH3COOH (Δ60).
Identification of the Genes Involved in FliC Glycosylation
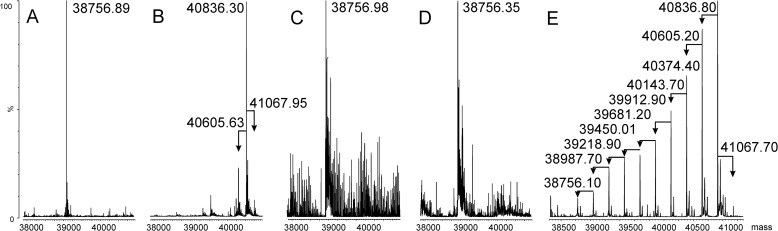
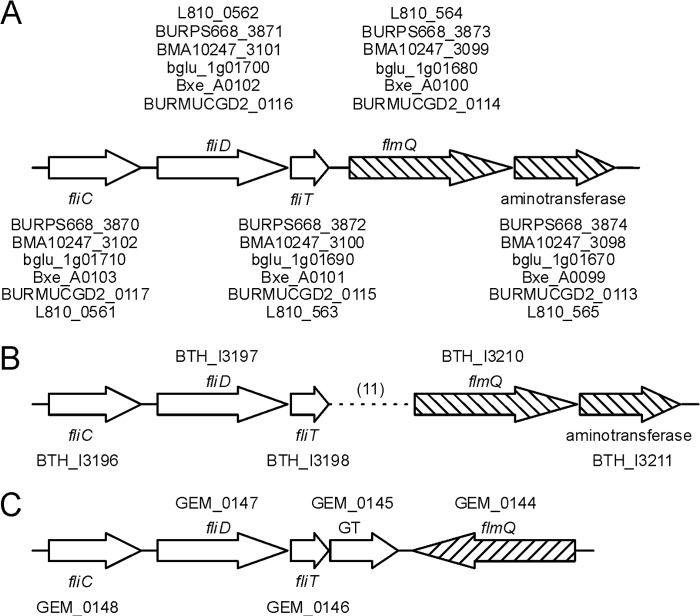
The flagellin gene fliC (BCAL0114) lies upstream of fliD (BCAL0113), fliT (BCAL0112), BCAL0111, and BCAL0110. The fliD and fliT encode the flagellar hook-associated protein and a flagellar chaperone, respectively. BCAL0110 encodes a putative VioA aminotransferase homologue (aminotransferase involved in synthesis of Qui4N; Fig. 5A), and BCAL0111 encodes a predicted protein with homology to the group 1 superfamily of glycosyltransferases and also containing four tetratricopeptide repeats. In silico analysis of BCAL0111 with HHpred revealed a C-terminal domain of 360 amino acids that is structurally homologous to several well characterized glycosyltransferases including the PimB mannosyltransferase from Corynebacterium glutamicum (45), the human UDP-N-acetylglucosamine peptide N-acetylglucosamine transferase (46), and the WaaG lipid A-core biosynthesis glycosyltransferase (47). To investigate whether BCAL0111 plays a role in FliC glycosylation, we constructed a ΔBCAL0111 deletion mutant and analyzed its purified flagellin. Coomassie-stained SDS-PAGE of FliC from ΔBCAL0111 showed a downshift in apparent molecular size (Fig. 1C), which was also evident by Western blotting with the RFFL/ARP42986_P050 antibody (Fig. 1B). Together, these results demonstrated that flagellin biosynthesis can proceed in the absence of glycosylation and that the antibody was specific for B. cenocepacia flagellin regardless of its glycosylation status. The MS spectrum of purified FliC from ΔBCAL0111 confirmed the loss of the glycan, as only a single molecular ion of 38,756.90 m/z corresponding to nonglycosylated flagellin could be detected (Fig. 2C). To confirm that BCAL0111 is required for FliC glycosylation, we placed BCAL0111 under the control of a rhamnose-inducible promoter (Fig. 5A). FliC purified from a culture in rhamnose-containing medium showed the same molecular weight in MS analysis and Coomassie staining as the parental strain. In contrast, flagellin isolated from a culture grown without rhamnose was present only in its nonglycosylated state (Fig. 6, B and C). Hence, we concluded that BCAL0111 is the FliC glycosyltransferase and designated the gene as flmQ for flagellin-modifying protein that transfers d-Qui4N(3OHBut). The deletion of BCAL0110 (vioA homologue) did not cause any detectable defect in FliC glycosylation (see below).
FIGURE 5.
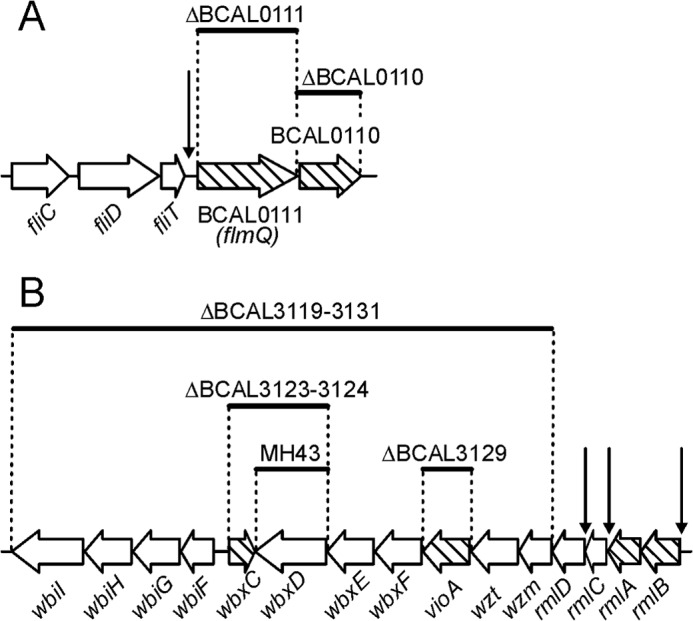
Gene organization of the fliC region (A) and the O-antigen cluster (B) in B. cenocepacia. Deletion mutants are indicated by thick bars. Vertical arrows indicate insertion sites of the rhamnose inducible pSC200 vector. Genes showed as striped arrows encode the predicted enzymes required for FliC glycosylation.
FIGURE 6.
Mass spectra of flagellin from various B. cenocepacia mutant strains. A, ΔBCAL3119–3131; B, MH1K pSC200/BCAL0111 grown in the presence of rhamnose; C, MH1K pSC200/BCAL0111 grown without rhamnose; D, ΔBCAL3123–3124; E, ΔBCAL3123–3124 pIN62/BCAL3123. Arrows indicate the Δmass of 231 Da.
The B. cenocepacia K56-2 LPS contains O-antigen. Glycans from the O-antigen were detected in our sugar analyses. Therefore, we sought to delete the O-antigen genes to avoid this contamination. Repeated attempts to delete genes between wbiI (BCAL3119) and rmlB (BCAL3135; Fig. 5B) (15) failed (see also below). However, a deletion including wbiI and wzm (BCAL3131) was obtained and confirmed by PCR and SDS-PAGE analyses of the LPS profile of the mutant strain (Fig. 7). Analyses of FliC in the ΔwbiI-wzm mutant showed the loss of the flagellin glycan (Fig. 6A). Thus, we concluded that FliC glycosylation requires one or more components of the O-antigen cluster. Genes in the O-antigen cluster that could be involved in the biosynthesis pathway of the FliC glycan are vioA (BCAL3129), a nucleotide sugar aminotransferase from dTDP-d-Qui4N biosynthesis pathway (48) and wbxC (BCAL3123), a putative acetyltransferase. No differences in flagellin glycosylation were detected in ΔBCAL3129 compared with the parental isolate (data not shown). Attempts to generate a single wbxC deletion failed, but it was possible to delete this gene together with the neighboring glycosyltransferase wbxD (BCAL3124). Although the single wbxD deletion did not affect FliC glycosylation (Fig. 2D), MS and SDS-PAGE analyses of ΔwbxCD revealed loss of glycosylation (Fig. 6D). Introducing a functional wbxC on a plasmid (pIN62/wbxC) into ΔwbxCD restored FliC glycosylation (Fig. 6E). From these results we concluded that wbxC is involved in the biosynthesis of dTDP-d-Qui4N(3HOBut), possibly by catalyzing an acetyltransferase step prior to the formation of the 3-hydroxybutyric acid side chain. This interpretation is consistent with the high degree of homology in the primary amino acid sequence of WbxC and the Acinetobacter baumannii WeeI protein, which is an acetyltransferase involved in the biosynthesis of UDP-N,N′-diacetylbacillosamine (49, 50). We did not succeed in any attempts to construct a double deletion mutant eliminating vioA (BCAL3129) and its putative homologue in the fliC region (BCAL0110) despite using the same mutagenic plasmids that were employed to delete both genes separately. However, it was possible to delete BCAL0110 in the ΔwbiI-wzm background and conversely to delete the wbiI-wzm region in the ΔBCAL0110 strain. These results demonstrate that both vioA and its BCAL0110 homologue are nonessential genes (Fig. 5).
FIGURE 7.
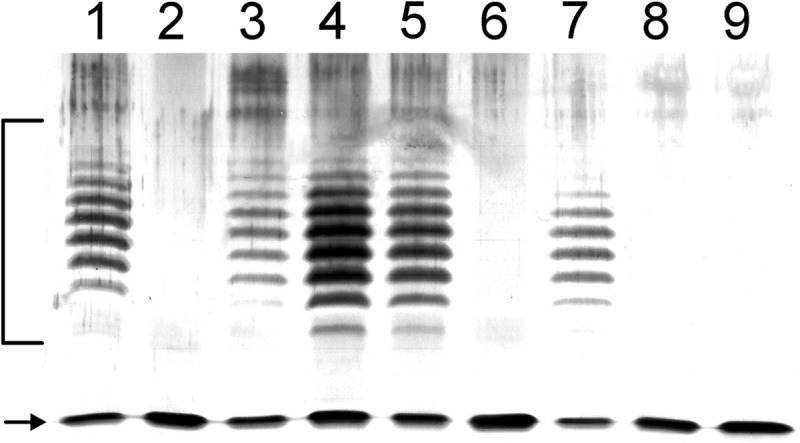
Silver-stained 14% SDS-PAGE of whole cell lysates of B. cenocepacia. Whole cell lysates from B. cenocepacia mutants were analyzed in silver-stained 14% SDS-PAGE. The strains used were as follows: MH1K (lane 1); ΔBCAL3119–3131 (lane 2); ΔBCAL3129 (lane 3); ΔBCAL0110 (lane 4); ΔBCAL0111 (lane 5); ΔBCAL3123–24 (lane 6); ΔBCAS0105 (lane 7); ΔBCAS0105 pGPΩTp/rmlD (lane 8); and MH1K pGPΩTp/rmlD (insertional mutant inactivating the last enzymatic step in dTDP-rhamnose biosynthesis; lane 9). Ladder-like bands (bracket) correspond to LPS-containing lipid A-core covalently linked to O-antigen polysaccharides of varying length. Single bands in the low molecular weight region (arrow) correspond to lipid A-core molecules without O-antigen.
We also investigated the conservation of the genetic organization of the fliC region in other Burkholderia species. A similar gene organization as in J2315, with a putative flmQ (BCAL0111) homologue placed downstream of fliCDT, was observed in B. pseudomallei, Burkholderia mallei, Burkholderia glumae, Burkholderia xenovorans, Burkholderia vietnamiensis, and Burkholderia multivorans (Fig. 8A). B. thailandensis carries 11 additional genes inserted between the flmQ homologue and fliT (Fig. 8B). In all these clusters, the flmQ homologue was placed downstream from fliT and upstream from the putative vioA gene, which was a homologue of BCAL0110; no other vioA homologues were found in these genomes. In B. cepacia, the flagellin cluster has a unique organization (Fig. 8C), where fliT is followed by a gene encoding a glycosyltransferase (GEM_0145) and the flmQ homologue (GEM_0144), but in the reverse orientation. Also in B. cepacia, the only BCAL0110 aminotransferase homologue (GEM_1565) is located outside of the flagellin cluster. Despite the variations among different species, the presence of homologous glycosyltransferase and aminotransferase genes in their flagellin clusters suggests that flagellin glycosylation is common in multiple species of the Burkholderia genus. Indeed, it was reported that B. pseudomallei and B. thailandensis produce glycosylated flagellin, but the glycan described in these strains is different from the one identified here (33).
FIGURE 8.
Gene organization in fliC clusters of other Burkholderia species. The identity to flmQ is indicated in parentheses. A, B. pseudomallei 668 (BURPS668; 49%), B. mallei NCTC 10247 (BMA10247; 49%), B. glumae (bglu_1g; 47%), B. xenovorans LB400 (Bxe_A; 49%), B. multivorans CGD2 (BURMUCGD2; 80%), B. vietnamiensis AU4i (L810; 89%). B, B. thailandensis E264, dotted line represents 11 genes inserted between the putative fliT and flmQ (BCAL0111) homologues. C, B. cepacia GG4. Genes showed as striped arrows represent BCAL0111 (flmQ) homologue, and aminotransferase represents a BCAL0110 homologue. GT, glycosyltransferase.
RmlB Is an Essential Gene in B. cenocepacia
RmlB (dTDP-d-glucose 4,6-dehydratase), one of the enzymes encoded by the B. cenocepacia O-antigen cluster, is needed for the synthesis of dTDP-l-rhamnose, which in turn is required for the assembly of the O-antigen repeating unit (Fig. 5B) (15). RmlB is also responsible for producing the precursor for biosynthesis of dTDP-d-Qui4N (51). In the course of these studies, we noticed that rmlB (BCAL3135) could not be deleted, suggesting the possibility that this gene is essential. To evaluate this notion, we constructed a conditional mutant by placing the rhamnose-inducible promoter upstream from rmlB. All tested strains, including the control strains XOA10 (Prha::BCAL1928; nonlethal conditional mutant) and XOA11 (Prha::arnT; lethal conditional mutant) (17), grew well when incubated on LB agar plates with rhamnose. In contrast, only XOA10 grew well in the absence of rhamnose, whereas XOA11 and the Prha::rmlB strains grew very poorly (data not shown). The effect of rhamnose depletion was much more dramatic in liquid cultures (Fig. 9). The rhamnose-inducible vector was also inserted upstream from rmlC (BCAL3133) and rmlD (BCAL3132), which are downstream from rmlB, to examine their possible essentiality in B. cenocepacia, but rhamnose depletion did not cause any growth alteration in these strains (Fig. 9). Because BCAS0105, a gene located in the third chromosome of B. cenocepacia, encodes a putative RmlD homologue, rhamnose depletion experiments were also performed in a ΔBCAS0105 strain carrying Prha::rmlD. These experiments indicated that ΔBCAS0105/Prha::rmlD is viable under rhamnose-free conditions (Fig. 9), ruling out the possibility that BCAS0105 might have supplied the function of rmlD when this gene was placed under the control of the rhamnose-inducible promoter. Together, these results provide experimental evidence that rmlB is essential in B. cenocepacia K56-2.
FIGURE 9.
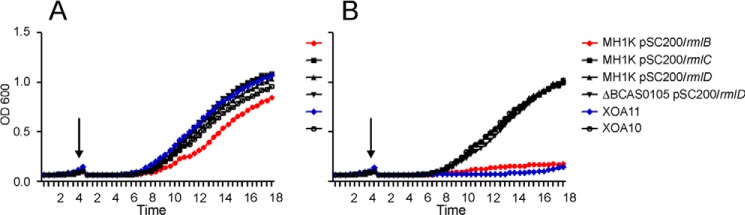
Conditional lethal phenotypes of B. cenocepacia strains. Strains were cultured in LB supplemented with 0.5% (w/v) rhamnose (A) or without rhamnose (B). After initial growth for 4 h (arrow), cultures were diluted 1:100 in fresh medium and incubated for 18 h.
Role of FliC Glycosylation on Bacterial Motility and Biofilm Formation
In natural environments, flagella are bacterial motility organelles. To examine the influence of flagellin glycosylation on B. cenocepacia motility, we tested the motility of the deletion mutants on soft agar by measuring the diameter of bacterial growth after 24 h of incubation at 37 °C. The strain RSF44, which lacks flagella (38), did not migrate from the inoculation spot providing a negative control. Strain ΔBCAL0111, lacking the putative d-Qui4N(3HOBut) transferase flmQ, showed a slight alteration in motility when compared with the parental isolate (Fig. 10A), whereas ΔwbxCD, missing the putative acetyltransferase and an O-antigen glycosyltransferase, had a much stronger effect on motility. The ΔwbiI-wzm mutant, which causes complete loss of O-antigen and the FliC glycan led to an ∼50% decrease in motility. Therefore, we conclude from these results that flagellin glycosylation and a complete O-antigen are required for normal motility of B. cenocepacia. Flagella also contribute to biofilm production. When compared with the parental strain, production of biofilm by ΔBCAL0111 was at a similar level as the flagella lacking strain RSF44 (Fig. 10B), suggesting that the presence of glycosylation and not the flagella alone is required for normal biofilm formation.
FIGURE 10.
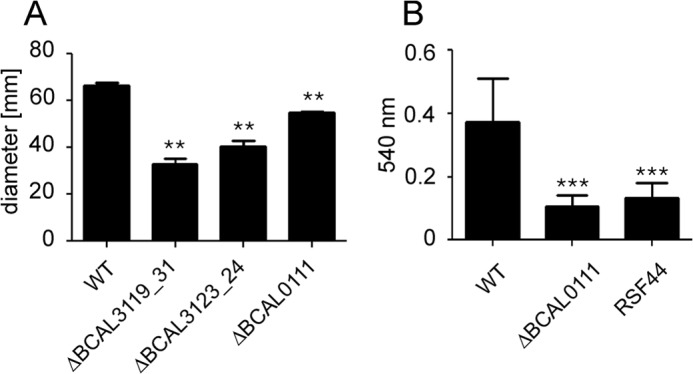
Motility on soft LB agar plates (A) and biofilm formation (B) of B. cenocepacia strains. Data are representative of three independent experiments. Statistical analysis was performed by paired t test using two-tailed p values. Significant differences in comparison with B. cenocepacia parental strain (WT) as control are indicated by ** (p < 0.01) or *** (p < 0.005).
FliC Glycosylation Reduces TLR5-mediated Responses
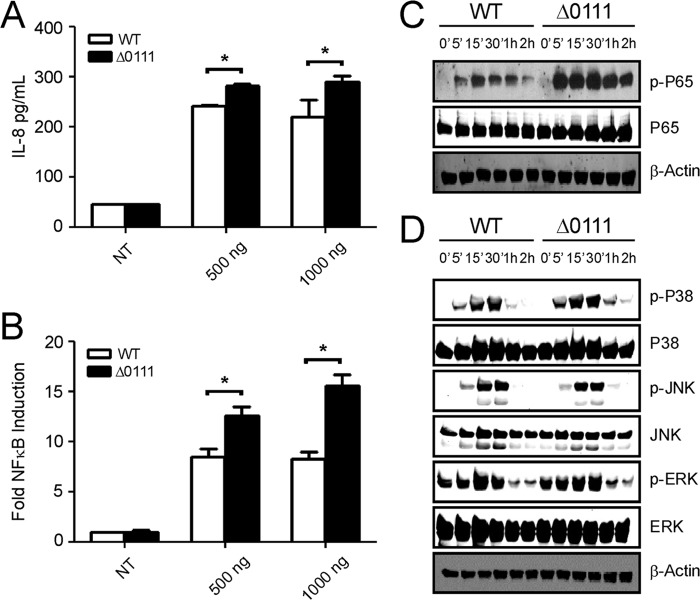
To examine the biological consequence of flagellin glycosylation in innate immune responses, human THP1 monocyte cells were stimulated with purified flagellins obtained from the parental strain (glycosylated FliC) and the ΔBCAL0111 mutant (nonglycosylated FliC). Stimulation of THP1 cells with both proteins resulted in production of the pro-inflammatory cytokines IL-1β (Fig. 11A), TNF-α (Fig. 11B), and IL-6 (Fig. 11C). However, nonglycosylated FliC was significantly more efficacious than the glycosylated counterpart in inducing IL-1β, TNF-α, and IL-6. To eliminate the possibility that LPS contamination in the flagellin preparations could confound these results, additional experiments were performed in HEK293 cells stably expressing TLR5 (HEK293 cells normally lack Toll-like receptors (52, 53)), which specifically recognizes flagellin. Again, the nonglycosylated FliC was more effective in inducing pro-inflammatory cytokine production in TLR5 cells as indicated by increased levels of IL-8 (Fig. 12A). We then looked at intracellular signaling and showed that nonglycosylated FliC is also more effective at activating NFκB (as measured by induction of a transfected NFκB-regulated luciferase reporter gene; Fig. 12B) and the phosphorylation of the NFκB subunit p65 (Fig. 12C). Also, nonglycosylated FliC mediated stronger phosphorylation of p38 MAPK (Fig. 12D). Together, these studies consistently show that nonglycosylated FliC is more effective than the glycosylated protein to stimulate pro-inflammatory signaling by TLR5.
FIGURE 11.
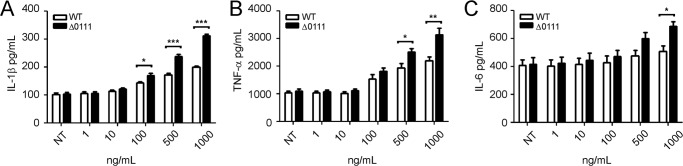
Regulation of pro-inflammatory gene expression in THP1 cells by glycosylated and nonglycosylated forms of flagellin. THP1 cells were stimulated for 24 h in the absence (NT, nontreated) or presence of varying concentrations of fully glycosylated wild-type (WT) or nonglycosylated (Δ0111) forms of flagellin, purified from the B. cenocepacia parental or ΔBCAL0111 strains, respectively. Conditioned media were assayed for expression levels of IL-1β (A), TNF-α (B), and IL-6 (C). Data are representative of three independent experiments. Statistical analysis was performed by paired t test using two-tailed p values. Significant differences between samples from WT and Δ0111-treated cells are indicated by * (p < 0.05), ** (p < 0.01), or ***, (p < 0.001).
FIGURE 12.
Differential stimulation of TLR5 signaling by glycosylated and nonglycosylated forms of flagellin. A, HEK293 cells, stably expressing TLR5, were stimulated for 24 h in the absence (NT, nontreated) or presence of varying concentrations of fully glycosylated wild-type (WT) or nonglycosylated (Δ0111) forms of flagellin purified from the B. cenocepacia parental or ΔBCAL0111 strains, respectively. Conditioned medium was assayed for expression levels of IL-8. B, HEK293 cells, stably expressing TLR5, were transfected with a NFκB-regulated luciferase reporter gene and stimulated for 24 h as indicated above. Cell lysates were assayed for NFκB-regulated firefly luciferase activity, and fold induction levels of NFκB-regulated luciferase are expressed relative to nontreated (NT) cells. Data are representative of three independent experiments. Statistical analysis was performed by paired t test using two-tailed p values. Significant differences between samples from WT and Δ0111-treated cells are indicated by * (p < 0.05). HEK293 cells, stably expressing TLR5, were stimulated for indicated times with WT and Δ0111 flagellin (500 ng/ml). Cell lysates were immunoblotted for phosphorylated (p-) and total levels of p65 (C) and p38 (D), JNK and ERK MAPKs. β-Actin was used as a loading control.
DISCUSSION
Despite the previously described roles for flagella in B. cenocepacia pathogenicity (25, 26), this is the first report describing flagellin glycosylation in this bacterium and identifying the genes involved in the biosynthesis of the glycan. We showed that the B. cenocepacia flagellin is modified with a viosamine (Qui4N) derivative, d-Qui4N(3HOBut), on at least 10 glycosylation sites within the protein. A sugar similar to d-Qui4N(3HOBut) but carrying an additional methyl group at C2 (54) was previously identified in glycosylated flagellin from Pseudomonas syringae pv. tabaci (54, 55), whereas Qui4N itself is a component of the flagellin glycan in Pseudomonas aeruginosa PAK (56). The biosynthesis of dTDP-viosamine requires three enzymatic steps as follows: (i) conversion of d-glucose 1-phosphate into dTDP-d-glucose, catalyzed by RmlA; (ii) formation of dTDP-4-dehydro-6-deoxy-d-glucose, catalyzed by RmlB; and (iii) an amination step catalyzed by the dTDP-4-dehydro-6-deoxy-d-glucose aminotransferase encoded by the vioA gene (57, 58). An additional step involves the acetylation of dTDP-viosamine to yield dTDP-N-acetylviosamine. Homologues of vioA and vioB, encoding the dTDP-viosamine acetyltransferase, have been identified in P. syringae pv. tabaci (54) and P. aeruginosa PAK (56), and both genes are required for the biosynthesis of the modified viosamine in P. syringae pv. tabaci. Despite that in B. cenocepacia there are two vioA homologues (BCAL0110 and BCAL3129), we could not identify a vioB homologue. Instead, we discovered that BCAL3123, encoding a putative acetyltransferase, is necessary for biosynthesis of d-Qui4N(3HOBut). Further experiments are necessary to provide evidence whether BCAL3123 encodes an enzyme catalyzing the direct transfer of 3OHBut or whether there are additional steps with BCAL3123 acting as an N-acetyltransferase prior to the formation of the 3OHBut side chain.
In particular, our results point to a complex link between O-antigen biosynthesis and the biosynthesis of the flagellin glycan. Two genes required for flagellin biosynthesis are located in the fliC gene cluster, whereas the other genes are present in the O-antigen cluster. The flagellin gene cluster contains a vioA homologue, which we show to be functionally redundant, and the flmQ glycosyltransferase gene, which is essential for FliC glycosylation. VioT, the flagellin glycosyltransferase in P. syringae pv. tabaci, has no homologues in B. cenocepacia, and conversely, P. syringae pv. tabaci has no FlmQ homologues. Therefore, despite that both species use similar sugars for flagellin glycosylation, the specific glycosyltransferases involved are unique to each system, perhaps reflecting differences in the FliC acceptor protein in each species. Comparison of fliC biosynthesis clusters in other Burkholderia species indicated the presence of flmQ and vioA homologues just downstream from fliC, with only a few exceptions. Flagellins from B. pseudomallei and B. thailandensis were previously found to be glycosylated by a single glycan (33). Although the structures of the glycans are unknown, their molecular masses are 291 and 342 Da for the B. pseudomallei and B. thailandensis, respectively, suggesting a different sugar than d-Qui4N(3HOBut). Therefore, we conclude that despite a common fliC gene cluster organization in most Burkholderia species, the glycan structure and glycosylation pattern of flagellin is likely species-specific.
The discovery that rmlB is an essential gene in B. cenocepacia was unexpected. In a previous study, Juhas et al. (59) reported 84 candidate essential genes in B. cenocepacia that were not previously described as essential in any other bacteria. One of these genes was rmlD (BCAL3132), located within the O-antigen cluster, but these authors did not report any experimental verification of rmlD essentiality. In our study, we conclusively demonstrate that rmlB (BCAL3135), not rmlD, is essential for B. cenocepacia viability. The B. cenocepacia dTDP-l-rhamnose biosynthesis genes (rmlBACD) form one transcriptional unit with the first 10 genes of O-antigen cluster (15). RmlB is a dTDP-d-glucose 4,6-dehydratase, and its function is required for the biosynthesis of nucleotide sugars like dTDP-d-fucose, dTDP-l-rhamnose, dTDP-d-Qui4N, and several other metabolites (48, 51, 60, 61). In B. cenocepacia, rmlB is involved in the synthesis of O-antigen, which contains rhamnose in its repeating unit (15), and in the synthesis of the d-Qui4N(3HOBut) flagellin glycan, as we show here. However, O-antigen production and flagellin glycosylation are not required for B. cenocepacia viability. To our knowledge, RmlB has not been reported as essential in other bacteria. The rlmB gene could not be deleted in B. thailandensis, but its deletion was possible in B. pseudomallei (33), suggesting it may be essential for at least another Burkholderia species. We speculate that the RmlB function may be required for the synthesis of another sugar nucleotide that may play an essential role in an as yet unidentified metabolic pathway, perhaps becoming a novel attractive candidate for antimicrobial development.
Although the flagellum is important for bacterial motility, colonization, and virulence (21, 62, 63), the functional role of glycosylation in host-bacteria interactions is less clear, and it has only been investigated in a handful of bacterial species. For example, nonglycosylated flagellin mutants of the plant pathogen P. syringae pv. tabaci are much less virulent on tobacco leaves than the wild-type strain (54, 64, 65). In contrast, lack of flagellin glycosylation does not affect the pathogenicity of starfruit pathogen P. syringae pv. averrhoi (66), whereas glycosylated flagellin of Acidovorax avenae elicits a strong immune response in cultured rice cells (67).
Contradictory results have also been reported for P. aeruginosa glycosylated flagellins in their ability to modulate innate immune responses in human epithelial cells (68, 69). Two notorious human pathogens, Campylobacter jejuni and Helicobacter pylori, cannot assemble flagella without glycosylation, and lack of flagella in both strains significantly reduces their virulence (70, 71). It is also not clear whether flagellin glycosylation modulates TLR5 responses. The glycosylated flagellin from C. jejuni is unique in that it fails to stimulate TLR5 (72). Reconstituting a functional TLR5-binding site in the C. jejuni flagellin resulted in the expression of glycosylated flagellin that induces a potent TLR5 response, ruling out a role for flagellin glycosylation in C. jejuni evasion of TLR5 detection (72). The elucidation of the flagellin glycosylation pathway in B. cenocepacia provided us with the opportunity to directly test the role of glycosylation in TLR5/flagellin-mediated inflammatory responses. We show that nonglycosylated flagellin was more pro-inflammatory than its fully glycosylated form. We also demonstrate that glycosylation of flagellin was associated with reduced efficacy with respect to stimulating TLR5-mediated signal transduction and gene expression. These results suggest that the presence of the glycan may alter to some extent flagellin detection by TLR5, although this was not directly examined here. We conclude that flagellin glycosylation could provide B. cenocepacia a strategy to reduce recognition by the innate immune system. However, further experiments are required to assess in vivo the role of flagellin glycosylation in the ability of these bacteria to cause chronic infection in cystic fibrosis patients.
Acknowledgments
We thank Cristina L. Marolda for technical assistance, and Olga Ovchinnikova and Yuriy Knirel for providing us with the d-Qui4N standard.
This work was supported by grants from the Canadian Institutes of Health Research and the United Kingdom Cystic Fibrosis Trust (to M. A. V.) and COST Action BM1003 “Microbial Cell Surface Determinants of Virulence as Targets for New Therapeutics in Cystic Fibrosis” (to A. M. and M. A. V.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) KC763156.
- TLR5
- Toll-like receptor 5
- d-Qui4N(3HOBut)
- 4,6-dideoxy-4-(3-hydroxybutanoylamino)-d-glucose.
REFERENCES
- 1. Mahenthiralingam E., Baldwin A., Dowson C. G. (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J. Appl. Microbiol. 104, 1539–1551 [DOI] [PubMed] [Google Scholar]
- 2. Mahenthiralingam E., Urban T. A., Goldberg J. B. (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3, 144–156 [DOI] [PubMed] [Google Scholar]
- 3. Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. (1984) Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104, 206–210 [DOI] [PubMed] [Google Scholar]
- 4. Graindorge A., Menard A., Neto M., Bouvet C., Miollan R., Gaillard S., de Montclos H., Laurent F., Cournoyer B. (2010) Epidemiology and molecular characterization of a clone of Burkholderia cenocepacia responsible for nosocomial pulmonary tract infections in a French intensive care unit. Diagn. Microbiol. Infect. Dis. 66, 29–40 [DOI] [PubMed] [Google Scholar]
- 5. Katsiari M., Roussou Z., Tryfinopoulou K., Vatopoulos A., Platsouka E., Maguina A. (2012) Burkholderia cenocepacia bacteremia without respiratory colonization in an adult intensive care unit: epidemiological and molecular investigation of an outbreak. Hippokratia 16, 317–323 [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S., Han S. W., Kim G., Song D. Y., Lee J. C., Kwon K. T. (2013) An outbreak of Burkholderia cenocepacia associated with contaminated chlorhexidine solutions prepared in the hospital. Am. J. Infect. Control 2013;41:e93–6 [DOI] [PubMed] [Google Scholar]
- 7. Satpute M. G., Telang N. V., Dhakephalkar P. K., Niphadkar K. B., Joshi S. G. (2011) Isolation of Burkholderia cenocepacia J 2315 from non-cystic fibrosis pediatric patients in India. Am. J. Infect. Control. 39, e21–23 [DOI] [PubMed] [Google Scholar]
- 8. McDowell A., Mahenthiralingam E., Dunbar K. E., Moore J. E., Crowe M., Elborn J. S. (2004) Epidemiology of Burkholderia cepacia complex species recovered from cystic fibrosis patients: issues related to patient segregation. J. Med. Microbiol. 53, 663–668 [DOI] [PubMed] [Google Scholar]
- 9. Novotny L. A., Amer A. O., Brockson M. E., Goodman S. D., Bakaletz L. O. (2013) Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid-binding protein. PLoS One 8, e67629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandamme P., Holmes B., Coenye T., Goris J., Mahenthiralingam E., LiPuma J. J., Govan J. R. (2003) Burkholderia cenocepacia sp. nov.–a new twist to an old story. Res. Microbiol. 154, 91–96 [DOI] [PubMed] [Google Scholar]
- 11. Vandamme P., Mahenthiralingam E. (2003) Strains from the Burkholderia cepacia complex: relationship to opportunistic pathogens. J. Nematol. 35, 208–211 [PMC free article] [PubMed] [Google Scholar]
- 12. De Soyza A., Morris K., McDowell A., Doherty C., Archer L., Perry J., Govan J. R., Corris P. A., Gould K. (2004) Prevalence and clonality of Burkholderia cepacia complex genomovars in UK patients with cystic fibrosis referred for lung transplantation. Thorax 59, 526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drevinek P., Mahenthiralingam E. (2010) Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 16, 821–830 [DOI] [PubMed] [Google Scholar]
- 14. Hamad M. A., Di Lorenzo F., Molinaro A., Valvano M. A. (2012) Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol. Microbiol. 85, 962–974 [DOI] [PubMed] [Google Scholar]
- 15. Ortega X., Hunt T. A., Loutet S., Vinion-Dubiel A. D., Datta A., Choudhury B., Goldberg J. B., Carlson R., Valvano M. A. (2005) Reconstitution of O-specific lipopolysaccharide expression in Burkholderia cenocepacia strain J2315, which is associated with transmissible infections in patients with cystic fibrosis. J. Bacteriol. 187, 1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortega X., Silipo A., Saldías M. S., Bates C. C., Molinaro A., Valvano M. A. (2009) Biosynthesis and structure of the Burkholderia cenocepacia K56-2 lipopolysaccharide core oligosaccharide: truncation of the core oligosaccharide leads to increased binding and sensitivity to polymyxin B. J. Biol. Chem. 284, 21738–21751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ortega X. P., Cardona S. T., Brown A. R., Loutet S. A., Flannagan R. S., Campopiano D. J., Govan J. R., Valvano M. A. (2007) A putative gene cluster for aminoarabinose biosynthesis is essential for Burkholderia cenocepacia viability. J. Bacteriol. 189, 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saldías M. S., Ortega X., Valvano M. A. (2009) Burkholderia cenocepacia O-antigen lipopolysaccharide prevents phagocytosis by macrophages and adhesion to epithelial cells. J. Med. Microbiol. 58, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 19. Raetz C. R., Whitfield C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotrange S., Kopp B., Akhter A., Abdelaziz D., Abu Khweek A., Caution K., Abdulrahman B., Wewers M. D., McCoy K., Marsh C., Loutet S. A., Ortega X., Valvano M. A., Amer A. O. (2011) Burkholderia cenocepacia O polysaccharide chain contributes to caspase-1-dependent IL-1β production in macrophages. J. Leukocyte Biol. 89, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erhardt M., Namba K., Hughes K. T. (2010) Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harbor Perspect. Biol. 2, a000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drake D., Montie T. C. (1988) Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J. Gen. Microbiol. 134, 43–52 [DOI] [PubMed] [Google Scholar]
- 23. Eaves-Pyles T., Murthy K., Liaudet L., Virág L., Ross G., Soriano F. G., Szabó C., Salzman A. L. (2001) Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166, 1248–1260 [DOI] [PubMed] [Google Scholar]
- 24. Feldman M., Bryan R., Rajan S., Scheffler L., Brunnert S., Tang H., Prince A. (1998) Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomich M., Herfst C. A., Golden J. W., Mohr C. D. (2002) Role of flagella in host cell invasion by Burkholderia cepacia. Infect. Immun. 70, 1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urban T. A., Griffith A., Torok A. M., Smolkin M. E., Burns J. L., Goldberg J. B. (2004) Contribution of Burkholderia cenocepacia flagella to infectivity and inflammation. Infect. Immun. 72, 5126–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayashi F., Smith K. D., Ozinsky A., Hawn T. R., Yi E. C., Goodlett D. R., Eng J. K., Akira S., Underhill D. M., Aderem A. (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 [DOI] [PubMed] [Google Scholar]
- 28. Strober W., Murray P. J., Kitani A., Watanabe T. (2006) Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6, 9–20 [DOI] [PubMed] [Google Scholar]
- 29. Blohmke C. J., Victor R. E., Hirschfeld A. F., Elias I. M., Hancock D. G., Lane C. R., Davidson A. G., Wilcox P. G., Smith K. D., Overhage J., Hancock R. E., Turvey S. E. (2008) Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J. Immunol. 180, 7764–7773 [DOI] [PubMed] [Google Scholar]
- 30. de C Ventura G. M., Le Goffic R., Balloy V., Plotkowski M. C., Chignard M., Si-Tahar M. (2008) TLR 5, but neither TLR2 nor TLR4, is involved in lung epithelial cell response to Burkholderia cenocepacia. FEMS Immunol. Med. Microbiol. 54, 37–44 [DOI] [PubMed] [Google Scholar]
- 31. West T. E., Chantratita N., Chierakul W., Limmathurotsakul D., Wuthiekanun V., Myers N. D., Emond M. J., Wurfel M. M., Hawn T. R., Peacock S. J., Skerrett S. J. (2013) Impaired TLR5 functionality is associated with survival in melioidosis. J. Immunol. 190, 3373–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo S. T., Tsuchiya K. (2005) Genotypic characterization of Burkholderia cenocepacia strains by rep-PCR and PCR-RFLP of the fliC gene. FEMS Microbiol. Lett. 245, 19–24 [DOI] [PubMed] [Google Scholar]
- 33. Scott A. E., Twine S. M., Fulton K. M., Titball R. W., Essex-Lopresti A. E., Atkins T. P., Prior J. L. (2011) Flagellar glycosylation in Burkholderia pseudomallei and Burkholderia thailandensis. J. Bacteriol. 193, 3577–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brett P. J., Mah D. C., Woods D. E. (1994) Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect. Immun. 62, 1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr., Weber P. (1981) Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal. Biochem. 118, 131–137 [DOI] [PubMed] [Google Scholar]
- 36. De Castro C., Parrilli M., Holst O., Molinaro A. (2010) Microbe-associated molecular patterns in innate immunity: Extraction and chemical analysis of Gram-negative bacterial lipopolysaccharides. Methods Enzymol. 480, 89–115 [DOI] [PubMed] [Google Scholar]
- 37. Sambrook R., Russell D. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Flannagan R. S., Linn T., Valvano M. A. (2008) A system for the construction of targeted unmarked gene deletions in the genus Burkholderia. Environ. Microbiol. 10, 1652–1660 [DOI] [PubMed] [Google Scholar]
- 39. Flannagan R. S., Aubert D., Kooi C., Sokol P. A., Valvano M. A. (2007) Burkholderia cenocepacia requires a periplasmic HtrA protease for growth under thermal and osmotic stress and for survival in vivo. Infect. Immun. 75, 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vergunst A. C., Meijer A. H., Renshaw S. A., O'Callaghan D. (2010) Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 78, 1495–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marolda C. L., Welsh J., Dafoe L., Valvano M. A. (1990) Genetic analysis of the O7-polysaccharide biosynthesis region from the Escherichia coli O7:K1 strain VW187. J. Bacteriol. 172, 3590–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saldías M. S., Lamothe J., Wu R., Valvano M. A. (2008) Burkholderia cenocepacia requires the RpoN σ factor for biofilm formation and intracellular trafficking within macrophages. Infect. Immun. 76, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holden M. T., Seth-Smith H. M., Crossman L. C., Sebaihia M., Bentley S. D., Cerdeño-Tárraga A. M., Thomson N. R., Bason N., Quail M. A., Sharp S., Cherevach I., Churcher C., Goodhead I., Hauser H., Holroyd N., Mungall K., Scott P., Walker D., White B., Rose H., Iversen P., Mil-Homens D., Rocha E. P., Fialho A. M., Baldwin A., Dowson C., Barrell B. G., Govan J. R., Vandamme P., Hart C. A., Mahenthiralingam E., Parkhill J. (2009) The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 191, 261–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ovchinnikova O. G., Bushmarinov I. S., Kocharova N. A., Toukach F. V., Wykrota M., Shashkov A. S., Knirel Y. A., Rozalski A. (2007) New structure for the O-polysaccharide of Providencia alcalifaciens O27 and revised structure for the O-polysaccharide of Providencia stuartii O43. Carbohydr. Res. 342, 1116–1121 [DOI] [PubMed] [Google Scholar]
- 45. Batt S. M., Jabeen T., Mishra A. K., Veerapen N., Krumbach K., Eggeling L., Besra G. S., Fütterer K. (2010) Acceptor substrate discrimination in phosphatidyl-myo-inositol mannoside synthesis: structural and mutational analysis of mannosyltransferase Corynebacterium glutamicum PimB′. J. Biol. Chem. 285, 37741–37752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lazarus M. B., Jiang J., Gloster T. M., Zandberg W. F., Whitworth G. E., Vocadlo D. J., Walker S. (2012) Structural snapshots of the reaction coordinate for O-GlcNAc transferase. Nat. Chem. Biol. 8, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinez-Fleites C., Proctor M., Roberts S., Bolam D. N., Gilbert H. J., Davies G. J. (2006) Insights into the synthesis of lipopolysaccharide and antibiotics through the structures of two retaining glycosyltransferases from family GT4. Chem. Biol. 13, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 48. Knirel Y. A., Valvano M. A. (eds) (2011) Bacterial Lipopolysaccharides: Structure, Chemical Synthesis, Biogenesis and Interaction with Host Cells, pp. 195–235, Springer-Verlag, Vienna [Google Scholar]
- 49. Morrison M. J., Imperiali B. (2013) Biochemical analysis and structure determination of bacterial acetyltransferases responsible for the biosynthesis of UDP-N,N′-diacetylbacillosamine. J. Biol. Chem. 288, 32248–32260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morrison M. J., Imperiali B. (2013) Biosynthesis of UDP-N,N′-diacetylbacillosamine in Acinetobacter baumannii: biochemical characterization and correlation to existing pathways. Arch. Biochem. Biophys. 536, 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giraud M. F., Naismith J. H. (2000) The rhamnose pathway. Curr. Opin. Struct. Biol. 10, 687–696 [DOI] [PubMed] [Google Scholar]
- 52. Chow J. C., Young D. W., Golenbock D. T., Christ W. J., Gusovsky F. (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692 [DOI] [PubMed] [Google Scholar]
- 53. Kumar Pachathundikandi S., Brandt S., Madassery J., Backert S. (2011) Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS One 6, e19614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nguyen L. C., Yamamoto M., Ohnishi-Kameyama M., Andi S., Taguchi F., Iwaki M., Yoshida M., Ishii T., Konishi T., Tsunemi K., Ichinose Y. (2009) Genetic analysis of genes involved in synthesis of modified 4-amino-4,6-dideoxyglucose in flagellin of Pseudomonas syringae pv. tabaci. Mol. Genet. Genomics 282, 595–605 [DOI] [PubMed] [Google Scholar]
- 55. Toguchi A., Siano M., Burkart M., Harshey R. M. (2000) Genetics of swarming motility in Salmonella enterica serovar typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182, 6308–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schirm M., Arora S. K., Verma A., Vinogradov E., Thibault P., Ramphal R., Logan S. M. (2004) Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa. J. Bacteriol. 186, 2523–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marolda C. L., Feldman M. F., Valvano M. A. (1999) Genetic organization of the O7-specific lipopolysaccharide biosynthesis cluster of Escherichia coli VW187 (O7:K1). Microbiology 145, 2485–2495 [DOI] [PubMed] [Google Scholar]
- 58. Wang Y., Xu Y., Perepelov A. V., Qi Y., Knirel Y. A., Wang L., Feng L. (2007) Biochemical characterization of dTDP-d-Qui4N and dTDP-d-Qui4NAc biosynthetic pathways in Shigella dysenteriae type 7 and Escherichia coli O7. J. Bacteriol. 189, 8626–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Juhas M., Stark M., von Mering C., Lumjiaktase P., Crook D. W., Valvano M. A., Eberl L. (2012) High confidence prediction of essential genes in Burkholderia cenocepacia. PLoS One 7, e40064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu H. W., Thorson J. S. (1994) Pathways and mechanisms in the biogenesis of novel deoxysugars by bacteria. Annu. Rev. Microbiol. 48, 223–256 [DOI] [PubMed] [Google Scholar]
- 61. Thibodeaux C. J., Melançon C. E., 3rd, Liu H. W. (2008) Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Ed. Engl. 47, 9814–9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hitchen P. G., Twigger K., Valiente E., Langdon R. H., Wren B. W., Dell A. (2010) Glycoproteomics: a powerful tool for characterizing the diverse glycoforms of bacterial pilins and flagellins. Biochem. Soc. Trans. 38, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 63. Logan S. M. (2006) Flagellar glycosylation–a new component of the motility repertoire? Microbiology 152, 1249–1262 [DOI] [PubMed] [Google Scholar]
- 64. Taguchi F., Takeuchi K., Katoh E., Murata K., Suzuki T., Marutani M., Kawasaki T., Eguchi M., Katoh S., Kaku H., Yasuda C., Inagaki Y., Toyoda K., Shiraishi T., Ichinose Y. (2006) Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell. Microbiol. 8, 923–938 [DOI] [PubMed] [Google Scholar]
- 65. Taguchi F., Yamamoto M., Ohnishi-Kameyama M., Iwaki M., Yoshida M., Ishii T., Konishi T., Ichinose Y. (2010) Defects in flagellin glycosylation affect the virulence of Pseudomonas syringae pv. tabaci 6605. Microbiology 156, 72–80 [DOI] [PubMed] [Google Scholar]
- 66. Wei C. F., Hsu S. T., Deng W. L., Wen Y. D., Huang H. C. (2012) Plant innate immunity induced by flagellin suppresses the hypersensitive response in non-host plants elicited by Pseudomonas syringae pv. averrhoi. PLoS One 7, e41056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hirai H., Takai R., Iwano M., Nakai M., Kondo M., Takayama S., Isogai A., Che F. S. (2011) Glycosylation regulates specific induction of rice immune responses by Acidovorax avenae flagellin. J. Biol. Chem. 286, 25519–25530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shanks K. K., Guang W., Kim K. C., Lillehoj E. P. (2010) Interleukin-8 production by human airway epithelial cells in response to Pseudomonas aeruginosa clinical isolates expressing type a or type b flagellins. Clin. Vaccine Immunol. 17, 1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verma A., Arora S. K., Kuravi S. K., Ramphal R. (2005) Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 73, 8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guerry P. (2007) Campylobacter flagella: not just for motility. Trends Microbiol. 15, 456–461 [DOI] [PubMed] [Google Scholar]
- 71. Schirm M., Soo E. C., Aubry A. J., Austin J., Thibault P., Logan S. M. (2003) Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 48, 1579–1592 [DOI] [PubMed] [Google Scholar]
- 72. de Zoete M. R., Keestra A. M., Wagenaar J. A., van Putten J. P. (2010) Reconstitution of a functional Toll-like receptor 5 binding site in Campylobacter jejuni flagellin. J. Biol. Chem. 285, 12149–12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mahenthiralingam E., Vandamme P. (2005) Taxonomy and pathogenesis of the Burkholderia cepacia complex. Chron. Respir. Dis. 2, 209–217 [DOI] [PubMed] [Google Scholar]
- 74. Hamad M. A., Skeldon A. M., Valvano M. A. (2010) Construction of aminoglycoside-sensitive Burkholderia cenocepacia strains for use in studies of intracellular bacteria with the gentamicin protection assay. Appl. Environ. Microbiol. 76, 3170–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Miller V. L., Mekalanos J. J. (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Figurski D. H., Helinski D. R. (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U.S.A. 76, 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]