Abstract
The primary infection by Pneumocystis of normal, healthy infants is asymptomatic and goes undiagnosed. Microscopy diagnosis of Pneumocystis was sought in lung impression smears (LIS) from two ~3-month-old infants dying unexpectedly in the community. Pneumocystis nuclei and cysts were identified using Hema-Gurr with subsequent Gomori–Grocott staining in the same spot documenting that these stains may be complementary. LIS provide for an observer–dependent, inexpensive, and ready-available method for detection of Pneumocystis in infant lungs.
Keywords: Pneumocystis, Primary infection, Infant, Lung, Microscopy diagnosis
1. Introduction
Normal healthy infants acquire their primary infection by Pneumocystis shortly after birth. The infection has a mostly asymptomatic, sub-clinical, course consistent with a transient colonization state, that goes undiagnosed [1–3]. This fungal infection is probably the most consistent and frequent pulmonary infection during the early pediatric age [2]. Studies in animals document that Pneumocystis is eliminated from the lungs and does not establish long-term latency [4]. The clinical significance of this infection has not been determined. Although, available evidence indicates that infants may play an important role as reservoir of Pneumocystis in the community [5,6]. However, Pneumocystis has been recently associated with increased mucus in infant lungs as a first evidence of Pneumocystis-associated pathology in infants that warrant investigation to detect eventual associations with lung disease [2].
Diagnosis of this fungal organism is difficult as it does not grow in fungal culture media, and to recognize Pneumocystis in tissue samples would be essential to establish whether it has any significance in pulmonary disease of infancy. Autopsy reports are few and document that the sensitivity of detection is highly dependent on the technique used [2,7]. The more sensitive diagnostic techniques are immunofluorescence and nested-PCR in homogenized tissue. They reveal that Pneumocystis can be detected in autopsied lungs of over 80% of infants between 1 and 6 months of age, with a characteristic prevalence peak reaching around 90% between 2 and 5 months; an age period that coincides with increased respiratory morbidity. Immunofluorescence and nested-PCR in homogenized tissue are expensive and require of laborious tissue processing and laboratory infrastructure not widely available. Consequently decreasing the chances to widely recognize the infection. The examination of lung impression smears (touch preps) has about half the sensitivity of nested-PCR or immunofluorescence in lung tissue homogenates, and is observer dependant requiring observation time and experience [2]. However, lung impression smears are readily available, simple, and inexpensive. These smears have been suggested as a useful screening method for diagnosis of Pneumocystis pneumonia in lung biopsies from immunocompromised patients that have a high Pneumocystis burden, and may provide to be of value for Pneumocystis detection in biopsy or autopsy lung specimens in non-immunocompromised infants worldwide [2,8,9].
Infant age is the most consistent, highly significant, risk factor for the primary infection [2,3,7,10]. Therefore, Pneumocystis was sought by using impression smears in two apparently normal infants dying at an age when the primary infection by Pneumocystis is more common [2,3,7,10].
2. Case
2.1. Case 1
A 3-month-10-days-old, 6200 g, 60 cm, previously healthy girl, was found dead in her crib. The mother was healthy and indicated that she did not noticed anything different in her daughter during the days prior to death, including absence of respiratory symptoms. The girl had no siblings and was exclusively breastfed. The autopsy was legally required, and documented a normal and well-nourished infant without gross and microscopic pathological findings. Pleural petechiae were present in both lungs. The cause of death could not be ascertained even after completion of a forensic protocol that considered the medical history, macroscopic examination, and dissection/sampling of major organs for histological examination, and laboratory tests including toxicology. No bacterial or viral cultures were obtained.
2.2. Case 2
A 2-month-24-days-old, 5500 g, 59 cm boy was found dead in his crib. The boy had three healthy sibling, and his parents were also healthy. He had history of prior illnesses, or respiratory symptoms in the previous three weeks. He was partly formula fed, and had an uneventful medical history. His legally required autopsy reported a well-nourished and normal infant without gross and microscopic pathological findings. Few pleural petechiae were present in both lungs. No bacterial or viral cultures were obtained. The cause of death could not be ascertained even after completion of the same forensic protocol.
2.3. Preparation of smears, staining, and observation procedure
Deep lung-tissue biopsies were obtained at autopsy and impression smears were prepared by firmly opposing cruent-cut surfaces of lung tissue against a glass slide. The slides were air dried and fixed in methanol for 5 min. The smear slides were first examined with Hema ‘Gurr®’ cytology stain (VWR International, Leuven, Belgium), recording the time to find the first conclusive Pneumocystis cells, marking their position on the microscopy slide, and taking the first picture. Additional spots were also marked and photographed. Smears were then hydrated using ethanol 100%, 95%, and 75% in 5 min baths and re-stained with Gomori–Grocott methenamine silver stain (GMS). The same slide spots were located and photographed again. Identification of Pneumocystis forms was done using an Olympus B×60 microscope using 400× magnification to locate Pneumocystis. Further observation was done at 1000× magnification (oil) to obtain photographs.
2.4. Results of pneumocystis screening
Pneumocystis trophic and cyst forms were identified in both infants. The time to observe the first conclusive Pneumocystis image for Observer 1 was 5 min in case 1, and 35 min in case 2; for Observer 2 it was 46 and 18 min, respectively; and for Observer 3, 15 and 150 min, respectively. Seven spots were photographed from Case 1, and 2 spots from Case 2. Selected pictures are presented in Fig. 1.
Fig. 1.
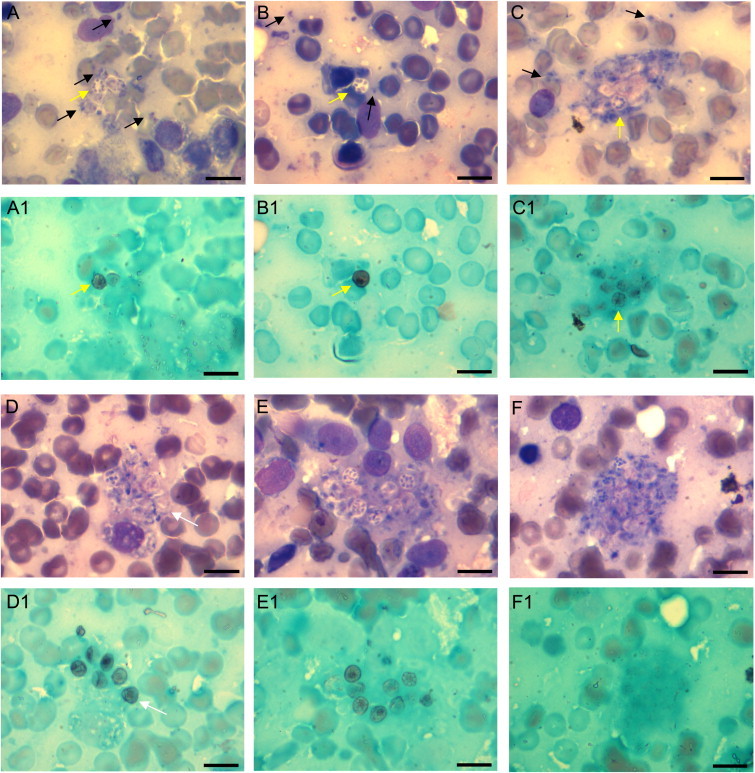
Representative microscopic findings. Lung impression smears of case 1 (A–D) and case 2 (E–F). The same spots are presented as stained with Hema-Gurr (A, B, C, etc.), and GMS (Adjacent below A1, B1, C1, etc.). The Hema-Gurr stained slides allow visualization of trophic forms with their nuclei and cytoplasm (black arrows). They are more abundant than cysts. The GMS stain allows visualization of cyst forms that are easier to see with green contrast. Mature cysts with 8 intracellular bodies as stained with Hema-Gurr (A, B, C) are stained with GMS (A1, B1, C1) (yellow arrows). The Hema-Gurr stain may suggest that cyst forms are present in a given spot. However, cysts were barely detected (C1), or not detected (F1) by GMS stain. This can be explained because cyst walls were either not reached by GMS for impregnation, or the cysts were not yet mature cysts with enough mucopolysaccharide in their wall. Vice versa, an empty cyst whose cell wall is well-stained in D1 is barely suggested in D (white arrows) . (All photos 1000X (Oil); Bar=10 μm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
Reports of Pneumocystis in infant lungs are surprisingly few given the high prevalence of the infection. This lack of reports may be due to the view of Pneumocystis as an infection restricted to the immunocompromised host.
This two-case report illustrates that Pneumocystis can be documented by microscopy in exfoliative cytology preparations of lung specimens from apparently healthy infants whose age is around the peak documented for the primary infection, therefore indicating that finding Pneumocystis in infant lungs is not necessarily an indication of underlying immunosuppression. Both infants were healthy prior to death and without respiratory symptoms illustrating the symptomless nature of this infection. The detection of Pneumocystis in their lungs provides no clue about their cause of death under current knowledge of the Pneumocystis-host interaction in apparently healthy infants. Different autopsy studies demonstrate the strong association of the Pneumocystis infection and young infant age with a typical age peak between 2 and 5 months [2,3,7,10]. The course and duration of this infection is difficult to investigate in infants because the infection is restricted to the lungs and requires of invasive sampling; techniques not warranted for symptomless infections. Understanding of the sensitivity of non-invasive sampling, like nasopharyngeal aspirates, to detect pulmonary infection by Pneumocystis in infants may provide a valuable research tool to clarify this issue. Studies in rodents indicate that non-invasive monitoring of lung infection may be possible [11].
The clinical significance of the primary infection is being actively investigated. Studies in animals show that Pneumocystis increases mucus-related proteins and may impair pulmonary function [12–14]. We have documented an association between Pneumocystis and increased mucus-related proteins in infant lungs [2]. These observations warrant further research before clinical significance of the primary infection can be concluded.
The diagnosis of Pneumocystis poses more difficulty than diagnoses of the majority of other fungal organisms that grow in microbiological culture. The diagnosis relies on microscopy to detect biological forms of the fungus, and on molecular amplification techniques like the polymerase chain reaction (PCR, or nested-PCR) to reveal Pneumocystis DNA. The characteristic appearance of Pneumocystis cysts and trophic forms for microscopy observation is easier to discern and better preserved in lung impression smears than in tissue homogenates [15]. We have previously determined that the sensitivity of Giemsa-like stains as Hema-Gurr is 60% greater when compared with that of the GMS stain to diagnose Pneumocystis in lung impression smears [2]. However, the interpretation of these stains is observer dependent and interpretation of Hema-Gurr stain requires more experience than GMS. Understanding whether use of both stains could increase the sensitivity of microscopy to detect Pneumocystis in lung impression smears had not been considered. The analysis of Hema-Gurr and GMS stained same-spots, as conducted in the lung impression smears of both infants, illustrate that the GMS-stained cysts are not necessarily in the same location that may be suggested by Hema-Gurr stained trophic forms, and that trophic forms in clusters are not stained by GMS as shown in F–F1 (Fig. 1), therefore indicating that these stains may complement each other and possibly increase sensitivity of detection. Microscopy reading time of lung smears will depend on the Pneumocystis burden of the sample, the experience of the observer, and the chance that a group of Pneumocystis touched the smear slide. This infection is highly focal [2,7]. Reporting Pneumocystis in lung homogenates using immunofluorescence will take about 1.4 h; using nested-PCR will take 10.8 h; and in impression smears from infants using Hema-Gurr will take 30 min to 3 h; and using GMS 1.2 h. The time for impression smears is, however, mostly microscopy observation time that may turn this technique as not practical for routine laboratory diagnosis. In addition, lung impression smears in biopsies from immunocompetent individuals beyond the infancy age period may have microscopy-undetectable Pneumocystis burden turning impression smears not useful unless the patient has a Pneumocystis-predisposing condition.
The two biological forms of this fungus stain differently; i. e. Pneumocystis trophic forms are the most abundant biological form, 10 times more abundant than the cyst. This biological form is difficult to distinguish from host cells as may occur with eukaryotic organism when cytologic stains like Hema-Gurr or Giemsa stains are used. The cyst form is stained by cell-coating stains as GMS which are not routinely used in diagnostic pathology. GMS will stain the wall of the pre-cyst and mature cyst forms that are rich in mucopolysaccharides.
In summary, this reports illustrates the recognition of Pneumocystis in infants that have around 3 months of age using microscopy in lung impression smears. This age-related Pneumocystis infection corresponds to the primary infection whose clinical significance has not been established. Therefore implementation of Pneumocystis diagnoses in infants is not currently justified for routine use, especially considering the long microscopy observation time that may be required. Lung impression smears provides a valuable and inexpensive technique that may serve to increase the recognition of this highly prevalent infection of the infancy period and to study potential clinical implications. GMS and Hema-Gurr may be complementary. Lung impression smears may be especially useful in less equipped scenarios where implementation of more sensitive and expensive, non observer dependent techniques can be difficult.
Conflict of interest statement
There are none.
Acknowledgments
This study was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico de Chile (Fondecyt) Grant number 1100225 (SLV), and by the Red Iberoamericana sobre Pneumocystosis (212RT0450), in the framework of The Ibero-American Program for Science, Technology and Development (CYTED).
References
- 1.Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32(6):855–861. doi: 10.1086/319340. (Mar 15) [DOI] [PubMed] [Google Scholar]
- 2.Vargas SL, Ponce CA, Gallo M, Perez F, Astorga JF, Bustamante R. Near-universal prevalence of Pneumocystis and associated increase in mucus in the lungs of infants with sudden unexpected death. Clin Infect Dis. 2013;56(2):171–179. doi: 10.1093/cid/cis870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen HH, von Linstow ML, Lundgren B, Hogh B, Westh H, Lundgren JD. Primary Pneumocystis infection in infants hospitalized with acute respiratory tract infection. Emerg Infect Dis. 2007;13(1):66–72. doi: 10.3201/eid1301.060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Gigliotti F, Harmsen AG. Latency is not an inevitable outcome of infection with Pneumocystis carinii. Infect Immun. 1993;61(12):5406–5409. doi: 10.1128/iai.61.12.5406-5409.1993. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monroy-Vaca EX, de Armas Y, Illnait-Zaragozi MT, Torano G, Diaz R, Vega D. Prevalence and genotype distribution of Pneumocystis jirovecii in Cuban infants and toddlers with whooping cough. J Clin Microbiol. 2014;52(1):45–51. doi: 10.1128/JCM.02381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25(2):297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas SL, Ponce CA, Hughes WT, Wakefield AE, Weitz JC, Donoso S. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin Infect Dis. 1999;29(6):1489–1493. doi: 10.1086/313521. (Dec) [DOI] [PubMed] [Google Scholar]
- 8.Felegie TP, Pasculle AW, Dekker A. Recognition of Pneumocystis carinii by gram stain in impression smears of lung tissue. J Clin Microbiol. 1984;20(6):1190–1191. doi: 10.1128/jcm.20.6.1190-1191.1984. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bii CC, Kose J, Taguchi H, Amukoye E, Ouko TT, Muita LC. Pneumocystis jirovecii and microbiological findings in children with severe pneumonia in Nairobi, Kenya. Int J Tuberc Lung Dis. 2006;10(11):1286–1291. (Nov) [PubMed] [Google Scholar]
- 10.Vargas SL, Ponce CA, Galvez P, Ibarra C, Haas EA, Chadwick AE. Pneumocystis is not a direct cause of sudden infant death syndrome. Pediatr Infect Dis J. 2007;26(1):81–83. doi: 10.1097/01.inf.0000247071.40739.fd. (Jan) [DOI] [PubMed] [Google Scholar]
- 11.Linke MJ, Rebholz S, Collins M, Tanaka R, Cushion MT. Noninvasive method for monitoring Pneumocystis carinii pneumonia. Emerg Infect Dis. 2003;9(12):1613–1616. doi: 10.3201/eid0912.030270. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Novoa B, Bishop L, Logun C, Munson PJ, Elnekave E, Rangel ZG. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol. 2008;84(2):420–430. doi: 10.1189/jlb.1207816. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swain SD, Meissner NN, Siemsen DW, McInnerney K, Harmsen AG. Pneumocystis elicits a STAT6-dependent, strain-specific innate immune response and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2012;46(3):290–298. doi: 10.1165/rcmb.2011-0154OC. (Mar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104(9):1307–1317. doi: 10.1172/JCI6688. (Nov) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savoia D, Belloro S, Calleri G, Caramello P. [Microscopic diagnosis of Pneumocystis carinii. Comparison of various staining technics] G Batteriol Virol Immunol. 1985;78(7-12):178–184. (Jul–Dec) [PubMed] [Google Scholar]


