Abstract
Objective
Integrity of resting-state functional brain networks (RSNs) is important for proper cognitive functioning. In type 1 diabetes mellitus (T1DM) cognitive decrements are commonly observed, possibly due to alterations in RSNs, which may vary according to microvascular complication status. Thus, we tested the hypothesis that functional connectivity in RSNs differs according to clinical status and correlates with cognition in T1DM patients, using an unbiased approach with high spatio-temporal resolution functional network.
Methods
Resting-state magnetoencephalographic (MEG) data for T1DM patients with (n = 42) and without (n = 41) microvascular complications and 33 healthy participants were recorded. MEG time-series at source level were reconstructed using a recently developed atlas-based beamformer. Functional connectivity within classical frequency bands, estimated by the phase lag index (PLI), was calculated within eight commonly found RSNs. Neuropsychological tests were used to assess cognitive performance, and the relation with RSNs was evaluated.
Results
Significant differences in terms of RSN functional connectivity between the three groups were observed in the lower alpha band, in the default-mode (DMN), executive control (ECN) and sensorimotor (SMN) RSNs. T1DM patients with microvascular complications showed the weakest functional connectivity in these networks relative to the other groups. For DMN, functional connectivity was higher in patients without microangiopathy relative to controls (all p < 0.05). General cognitive performance for both patient groups was worse compared with healthy controls. Lower DMN alpha band functional connectivity correlated with poorer general cognitive ability in patients with microvascular complications.
Discussion
Altered RSN functional connectivity was found in T1DM patients depending on clinical status. Lower DMN functional connectivity was related to poorer cognitive functioning. These results indicate that functional connectivity may play a key role in T1DM-related cognitive dysfunction.
Keywords: Resting-state networks, Magnetoencephalography, Functional connectivity, Phase Lag Index (PLI), Oscillations, Type 1 diabetes mellitus
Highlights
-
•
MEG RSN functional connectivity was estimated among T1DM+ and T1DM− patients and controls.
-
•
Lower alpha band in DMN, ECN and SMN significantly differed among groups.
-
•
Functional connectivity may play a key role in T1DM-related cognitive dysfunction.
1. Introduction
Type 1 diabetes mellitus (T1DM) is a chronic disease characterized by failure of insulin secretion, caused by the destruction of pancreatic beta cells, requiring exogenous insulin administration. T1DM patients are exposed to high (hyperglycaemia) and low (hypoglycaemia) blood glucose levels and cumulative hyperglycaemic exposure can lead to microvascular end-organ damage, such as retinopathy and nephropathy (Brownlee, 2005).
Interest is growing into the potential effects dysglycaemia has on the central nervous system. Whereas cortical grey matter seems relatively spared in adult T1DM patients (Lyoo et al., 2012), alterations in white matter tract integrity, functional connectivity and functional networks have been found relative to non-diabetes controls (van Duinkerken et al., 2009, 2012a,b). Furthermore, mild to moderate speed-related cognitive decrements are consistently found (Brands et al., 2005; Jacobson et al., 2007; Wessels et al., 2008). Cumulative hyperglycaemia is hypothesised to be related to T1DM-related cerebral compromise (Jacobson et al., 2007; Wessels et al., 2008). As the retina shares developmental and physiological characteristics with the brain (Lyoo et al., 2012; Patton et al., 2005), proliferative retinopathy, a consequence of long-term hyperglycaemia, is hypothesised to be a marker of cumulative hyperglycaemia on the brain (Jacobson et al., 2010; van Duinkerken et al., 2009, 2012a,b).
In recent years, it has become clear that cognitive functioning strongly depends on the organization of functional brain networks (Bassett and Bullmore, 2009; Brands et al., 2005; Bullmore and Sporns, 2012; Stam and van Straaten, 2012). Neuronal dynamics within segregated functional systems, and their integration, underlie cognitive processing (Friston, 2002; Jacobson et al., 2007; Wessels et al., 2008). The oscillatory properties of these neuronal dynamics are considered a potential means for the implementation of functional communication (Engel et al., 2001). Phase relations between these oscillatory systems are thought to play a key role in such communications, providing the underling mechanism for both local and long-range synchronization (Fell and Axmacher, 2011; Fries, 2005; Sauseng and Klimesch, 2008; Varela et al., 2001). These functional connections between distinct neuronal populations can be measured using different modalities: electroencephalography (EEG), magnetoencephalography (MEG) and functional magnetic resonance imaging (fMRI) (Stam and van Straaten, 2012). This latter technique has established distinct neuronal circuits, so called resting-state networks (RSNs), that exhibit robust temporal correlations in spontaneous brain activity under resting condition (Damoiseaux et al., 2006; Rosazza and Minati, 2011; van den Heuvel and Hulshoff Pol, 2010). Changes in RSN connectivity patterns have been related to cognitive performance: either too much or too little RSN activity in various pathologies (Alzheimer, schizophrenia and epilepsy) has been correlated with cognitive deficits (Broyd et al., 2009), whereas increased RNS activity after resective surgery for glioma correlated with improved cognitive performance (van Dellen et al., 2013).
Earlier EEG/MEG functional connectivity (Cooray et al., 2011; van Duinkerken et al., 2009) and fMRI RSN analyses (van Duinkerken et al., 2012b) have revealed a reduction in functional connectivity measures in T1DM patients with proliferative retinopathy, whereas increased functional connectivity has been found in patients without proliferative retinopathy. This reduction correlated with cognitive performance suggesting that functional connectivity is involved in cognitive functioning (van Duinkerken et al., 2009, 2012b). However, the results of these studies could have been affected by biases in the analysis approach (effects of volume conduction and the use of sensor-level analysis in EEG/MEG; poor temporal resolution of fMRI). Here, we therefore analysed MEG data from a previously described patient cohort (van Duinkerken et al., 2009, 2012b) using an unbiased approach with better spatio-temporal resolution to estimate RSN functional connectivity. In particular: i) A larger cohort is used than in the original MEG study (van Duinkerken et al., 2009) to enhance statistical power; ii) Analyses are performed in source-space instead of sensor-space, in order to enhance the interpretability of the results; iii) A functional connectivity estimator, the phase lag index (PLI), that is insensitive to spurious interactions (Stam et al., 2007) is used, instead of the synchronization likelihood (Montez et al., 2006; Stam and Van Dijk, 2002); iv) Although fMRI allows for the spatially accurate reconstruction of RSNs (van Duinkerken et al., 2012b), it does not capture the rich temporal dynamics of the neuronal activity that underlies the Blood Oxygenation Level Dependent (BOLD) signal. Here, using fMRI literature to define meaningful RSNs (Rosazza and Minati, 2011) in combination with the beamforming technique (Hillebrand et al., 2012), we are able to reconstruct frequency-specific functional connectivity within these RSNs.
Our aim was to test whether functional connectivity in RSNs differs according to clinical status and correlates with cognition in T1DM patients with and without proliferative retinopathy, using an unbiased approach with high spatio-temporal resolution functional network.
2. Methods
2.1. Participants
Forty-two type 1 diabetes mellitus patients with proliferative retinopathy (T1DM+), 41 diabetes mellitus patients without microvascular complications (T1DM−) and 33 healthy control subjects, matched for sex, BMI, and education were recruited in this study. Age range criteria were 18–56 years and participants were excluded if they had a BMI above 35 kg/m2, use of drugs affecting cerebral functioning, current or history of alcohol (men > 21 and women > 14 units a week) or current drug use, psychiatric disorders, anaemia, thyroid dysfunction, use of glucocorticoids, hepatitis, stroke, severe head trauma, epilepsy, pregnancy, or poor visual acuity. For T1DM patients a disease duration of at least 10 years was required.
To control for confounding effects of depression on cognitive performance and functional connectivity, depressive symptoms were assessed using the Centre for Epidemiological Studies scale for Depression (CES-D). To prevent confounding due to current blood glucose level differences, these were measured in T1DM patients before the MEG recording. Blood glucose levels between 4 and 15 mmol/l (72–270 mg/dl) were regarded as appropriate. A detailed description of the inclusion/exclusion criteria for patients and control subjects is provided in our previous work (van Duinkerken et al., 2009), where the MEG data from a sub-set of these participants (n = 15, 29, and 26 for T1DM+, T1DM−, and healthy controls, respectively) were analysed at sensor-level. The original dataset consisted of 148 subjects, but 32 subjects were discarded either because of bad MEG recordings (n = 24) or problems with MRI co-registration (n = 8).
2.2. Structural assessment
Structural MRI scans were performed in order to assess differences in white matter hyperintensities and in whole brain and total grey matter volume.
Magnetic resonance imaging was performed on a 1.5 T whole body MR-scanner (Siemens Sonata, Erlangen, Germany) using an 8-channel phased-array head coil.
SIENAX, which is part of FMRIB's Software Library (FSL, version 5.0.4; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) was used to calculate whole brain volume as well as total grey matter volume. For this analysis a high resolution T1-MPRAGE (repetition time 2.700 ms, echo time 5.17 ms, inversion time 95 ms, flip angle 8°, 248 × 330 mm2 field of view, 1.0 × 1.0 × 1.5 mm voxel size) was used. To increase the reliability of the analyses all scans were first corrected for scanner induced geometric distortion and then excessive neck tissue was removed by registering the Montreal Neurological Institute 152 (MNI152) standard brain to each participant's T1-MPRAGE.
White matter hyperintensities were visually rated by a neuropsychologist trained to assess structural abnormalities on MRI (EvD) according to the Fazekas score (Fazekas et al., 2002). For this rating a 3D-FLAIR sequence (repetition time 6500 ms; echo time 385 ms; variable flip angle (Mugler et al., 2000)) was used.
2.3. Neuropsychological assessment
As described in detail in van Duinkerken et al. (2009) all participants were assessed using a battery of neuropsychological tests to evaluate cognitive performance in six cognitive domains: memory, information processing speed, executive functioning, attention, motor speed and psychomotor speed. For each neuropsychological test z-values were created based on the mean and standard deviation of the controls. These were then grouped to form the cognitive domains (see Appendix A). When necessary, z-values were transformed so that higher z-scores represent better performance. In this study we considered ‘general cognitive ability’ which was obtained by averaging the z-scores over all cognitive domains.
2.4. MEG
MEG data were recorded using a 151-channel whole-head MEG system (CTF Systems; Port Coquitlam, BC, Canada) while participants were in a supine position in a magnetically shielded room (Vacuumschmelze, Hanau, Germany). A third-order software gradient (Vrba et al., 1999) was used with a recording passband of 0.25–125 Hz and a sample frequency of 625 Hz. Magnetic fields were recorded for 2 min in an eyes-open condition, 5 min in an eyes-closed condition, 10 min in a task, and then 3 min in an eyes-closed condition.
At the beginning and end of each of these recordings, the head position relative to the coordinate system of the helmet was determined by leading small alternating currents through three head position coils attached to the left and right preauricular points and the nasion. Changes in head position of < 0.5 cm during a recording were accepted. Here, we only analysed the first (5 min) eyes-closed resting-state condition, which we divided into 45 trials of 6.55 s (4096 samples). Channels and epochs containing artefacts were discarded after careful visual inspection (MD, AH), rejecting on average 3 channels (range: 0–11). A minimum of 25 epochs were selected and considered sufficient for the beamformer analysis (Brookes et al., 2008).
2.5. Beamforming
The structural T1-weighted MRI-scan was used for co-registration as a first step for beamforming. Only data with an estimated co-registration error < 1.0 cm were accepted for further analysis. MRI-data were then spatially normalised to a template MRI using the SEG toolbox in SPM8 (Ashburner and Friston, 2005; Weiskopf et al., 2011), after which anatomical labels were applied (Gong et al., 2009). An atlas-based beamformer approach (Hillebrand et al., 2012) was used to project MEG sensor signals to an anatomical framework consisting of 78 cortical regions (ROIs) (Gong et al., 2009) identified by means of automated anatomical labelling (AAL) (Tzourio-Mazoyer et al., 2002). This resulted in time-series of neuronal activation for all voxels within a ROI, after which a representative voxel was selected (the one with maximum power for a given frequency band (Hillebrand et al., 2012)). The time-series for the 78 ROIs were filtered in the following frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), lower alpha (8–10 Hz), upper alpha (10–13 Hz), beta (13–30 Hz), and lower gamma bands (30–48 Hz). This resulted in a total of 6 sets (one for each frequency band) of 78 time-series (one for each AAL region). As was done in our previous studies, we selected five artefact-free epochs of 4096 samples (6.55 s) from these time-series, based on careful visual inspection (MD) to obtain stable results (Bartolomei et al., 2006; Douw et al., 2013; Olde Dubbelink et al., 2013, 2014; Stam et al., 2009; Tewarie et al., 2013, 2014; van Dellen et al., 2013, 2014). These data were further analysed using Brainwave v0.9.70 [authored by C.S.; available at http://home.kpn.nl/stam7883/brainwave.html].
2.6. Functional connectivity analysis
Functional connectivity between all 78 reconstructed time-series was estimated using the phase lag index (PLI) (Stam et al., 2007) independently for each frequency band. The PLI is a measure of the asymmetry of the distribution of phase differences between two signals. It reflects the consistency of phase relations between two signals, avoiding zero-lag phase coupling and thereby minimizing the influence of volume conduction and field spread. For each subject and epoch, PLI values were computed for each pair of ROIs (i.e. a 78 × 78 adjacency matrix was obtained) and subsequently the mean PLI values were calculated by averaging over the five selected epochs (i.e. a 78 × 78 matrix containing average PLI values per subject was obtained). Using the PLI adjacency matrix it was possible to estimate phase coupling within so-called resting-state networks (RSNs) (Rosazza and Minati, 2011; Tewarie et al., 2013; van Dellen et al., 2013). This was done by averaging the PLI values between the ROIs belonging to a specific resting-state network (see Table 3 in Appendix A for the definition of the RSNs). We estimated the functional connectivity for the auditory, default-mode (DMN), executive control (ECN), left and right frontoparietal, sensorimotor (SMN), temporoparietal and visual resting-state networks.
2.7. Statistical analysis
Participant characteristics were assessed using one-way ANOVA or Student's t-test for continuous variables and chi-square for dichotomous variables.
Group differences for general cognitive ability were evaluated using an ANCOVA, with group and gender as independent variables and age, systolic blood pressure and depressive symptoms as covariates.
For each frequency band independently, a MANCOVA model was used to evaluate differences in functional connectivity between groups. PLI values of resting-state networks were used as dependent variables, with group and gender as independent variables. Functional connectivity values were log-transformed (log10(x ∕ 1 − x)) to obtain normal distributions to allow the use of parametric statistics. This resulted in 6 MANCOVAs. When the overall F-test was significant, post-hoc MANCOVA was used to determine which networks contributed most to the model. In order to correct for possible confounding factors, age, depression symptoms and systolic blood pressure were used as covariates in all statistical tests.
Finally, for those networks that differed between groups, we determined the association with general cognitive ability using stepwise regression analyses for each patient group separately. For this analysis, significant RSN values were used as predictors for ‘general cognitive ability’ z-scores. In order to correct for possible confounding factors, age, depression symptoms, systolic blood pressure, and diabetes duration were entered in the regression. Statistical analyses were performed with SPSS v.19 (IBM-SPSS, Chicago, IL, USA).
2.8. Discriminant function analysis
In order to test if significant group differences enabled group (T1DM+, T1DM− and healthy controls) classification, we performed a supplementary discriminant function analysis with the connectivity values for the significant RSNs. The covariates age, sex, systolic blood pressure, disease duration and depressive symptoms were included in the analysis. The discriminant function analysis was performed using a stepwise procedure in order determine which of the variables could be omitted because of low predictive ability. Wilks' lambda and a summary table of the classification results are reported in Supplementary Material Tables 1–4. All the computations were performed with SPSS v.19 (IBM-SPSS, Chicago, IL, USA).
3. Results
3.1. Subject characteristics
Subject characteristics and structural assessment are summarised in Table 1. There were no differences in gender distribution between groups (p > 0.05). Groups were significantly different for age (F(2,113) = 6.55, p = 0.002), systolic blood pressure (F(2,113) = 4.03, p = 0.02), depressive symptoms (F(2,113) = 5.82, p = 0.004), diabetes duration (t(81) = 6.14, p < 0.001) and diabetes onset age (t(81) = − 3.00, p = 0.004). T1DM+ patients were the oldest, had the highest systolic blood pressure values and had the highest scores on the depressive symptoms assessment. The T1DM− and control groups did not differ on any of these three characteristics. Ten (23.8%) patients with proliferative retinopathy, 8 (19.5%) patients without microvascular complications and 4 (12.1%) of the control participants had a Fazekas score of 1 (small punctiform lesions). This percentage was not statistically different between groups (χ2(2,116) = 1.655 p = 0.437). Fazekas scores 2 and 3 were not present in this sample. There were no between-group differences regarding total grey matter volume (F(2,113) = 1.75; p = 0.178). Total brain volume tended to be altered between groups, with the lowest volume in T1DM+ patients (F(2,113) = 3.02; p = 0.053).
Table 1.
Subject characteristics.
| T1DM+ patients | T1DM− patients | Control subjects | p-Values | |
|---|---|---|---|---|
| N | 42 | 41 | 33 | – |
| Age (years) | 44.7 ± 7.15⁎# | 38.39 ± 9.18 | 38.21 ± 11.09 | 0.002 |
| Gender (m/f) | 19/23 | 17/24 | 15/18 | 0.922 |
| Depressive symptoms (CES-D)a | 12.07 ± 10.56 | 7.00 ± 6.61 | 6.09 ± 7.12 | 0.004 |
| Estimated IQ (NART)b | 110.05 ± 13.69 | 106.29 ± 11.16 | 108.66 ± 12.14 | 0.306 |
| Systolic blood pressure (mm Hg) | 135.42 ± 17.41⁎ | 128.82 ± 13.89 | 126.34 ± 10.78 | 0.020 |
| Diastolic blood pressure (mm Hg) | 77.26 ± 8.62 | 77.68 ± 9.72 | 78.92 ± 6.65 | 0.694 |
| BMI (kg/m2) | 26.04 ± 4.23 | 25.12 ± 3.62 | 24.88 ± 3.40 | 0.365 |
| Hypertension (%)c | 30 (71.4) | 11 (26.8) | – | < 0.001 |
| Diabetes early onset (%)d | 13 (31) | 6 (14.6) | – | 0.077 |
| Diabetes duration (years) | 33.78 ± 7.80 | 21.85 ± 9.78 | – | < 0.001 |
| Diabetes onset age (years) | 10.09 ± 7.47 | 16.53 ± 9.50 | – | 0.004 |
| Lifetime severe hypoglycaemic eventse | 6.09 ± 9.83 | 6.85 ± 11.15 | – | 0.576 |
| Peripheral neuropathy (%)f | 21 (50) | – | – | – |
| Whole brain volume (mL) | 1424 ± 12.0 | 1427 ± 12.2 | 1465 ± 136 | 0.053 |
| Grey matter volume (mL) | 744 ± 7.7 | 752 ± 7.8 | 765 ± 8.7 | 0.178 |
| White matter hyperintensities (%)g | 10 (23.8) | 8 (19.5) | 4 (12.1) | 0.437 |
Subject characteristics for T1DM with proliferative retinopathy (T1DM+), T1DM without complications (T1DM−) and control participants. Data are given as means with SD or absolute numbers with percentage.
Bold values indicate significance at p < 0.05.
Depressive symptoms were measured using the Centre for Epidemiological Studies scale for Depression.
Estimated IQ was measured using the Dutch version of the National Adult Reading Test.
Hypertension was defined as a systolic blood pressure of ≥ 140 mm Hg, a diastolic blood pressure of ≥ 90 mm Hg, or use of antihypertensive drugs.
Diabetes early onset was defined as an onset age below the age of 7 years.
Severe hypoglycaemic events were self-reported and defined as events for which the patient needs assistance from a third person to recuperate as a result of loss of consciousness or seriously deranged functioning, coma, or seizure owing to low glucose levels.
Peripheral neuropathy was based on medical records or, in case they were not available, based on self-report.
White matter hyperintensities were classified according to the Fazekas score. In this sample only Fazekas scores 0 (no lesions) or 1 (small punctiform lesions) were present. Number of patients (and as a percentage of the group) with Fazekas score 1 is given for each group.
Significantly different from controls (p < 0.05).
Significantly different from T1DM− (p < 0.05).
3.2. Neuropsychological assessment
A significant effect of group (F(2,107) = 6.86, p = 0.002) for general cognitive ability was found, but no gender effect or interaction effect, was observed. Post-hoc analysis revealed significantly poorer performance in T1DM+ patients compared with T1DM− patients (MD = − 0.301; 95% CI = [− 0.512, − 0.090]; p = 0.006) and controls (MD = − 0.409; 95% CI = [− 0.636, − 0.182]; p = 0.001).
3.3. MEG results
The MANCOVA model with log-transformed PLI values for the RSNs revealed a significant effect of group for the lower alpha band (F(16,200) = 1.90, p = 0.022; Wilks' Λ = 0.753, partial η2 = 0.132), while the other frequency bands did not show a significant group effect. Neither a main effect of gender nor an interaction effect was found.
Post-hoc MANCOVA revealed significant differences in DMN (F(2,107) = 3.45, p = 0.035, partial η2 = 0.061), ECN (F(2,107) = 5.55, p = 0.005, partial η2 = 0.094) and SMN (F(2,107) = 4.67, p = 0.011, partial η2 = 0.080). Specifically, for every significant sub-network, T1DM+ patients showed the lowest functional connectivity values (Fig. 1), while T1DM− had similar values to, or showed higher functional connectivity values than controls.
Fig. 1.
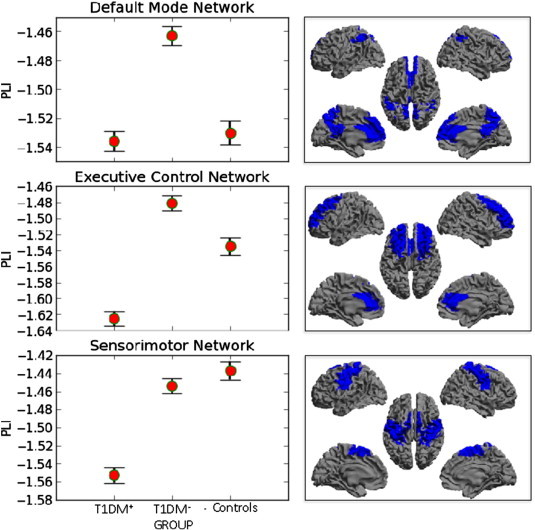
Left panels: Average (and 2 standard errors) connectivity (lower alpha band, log-transformed) within resting-state networks that showed a significant group effect. Note that for all these networks the functional connectivity was significantly lower for the patient group with microvascular complications (T1DM+) than for the patient group without microvascular complications (T1DM−), as well as in the sensorimotor network (SMN) for the T1DM+ group compared to controls. In the default mode network (DMN), the PLI was significantly higher for the T1DM− group than for the controls. Right panels show the areas for the relevant RSN (highlighted in blue) on a template brain (see also Appendix A). Here, cold colours indicate low PLI, hot colours indicate high PLI.
T1DM+ patients compared to T1DM− patients had significantly lower functional connectivity within the DMN (log-transformed MD = − 0.072; 95% CI = [− 1.136, − 0.009]; p = 0.026), ECN (MD = − 0.144; 95% CI = [− 0.230, − 0.058]; p = 0.001) and SMN (MD = − 0.100; 95% CI = [− 0.177, − 0.023]; p = 0.011). For the SMN a significant difference (MD = − 0.117; 95% CI = [− 0.199, − 0.034]; p = 0.006) between T1DM+ and control subjects was found, while in DMN a significant difference (MD = 0.067; 95% CI = [0.005, 0.128]; p = 0.034) was found between the T1DM− group and controls, with functional connectivity being higher for the T1DM− group.
Stepwise regression using gender, age, systolic blood pressure, depressive symptoms and diabetes duration as covariates; and the significant RSN values as predictor, showed that DMN functional connectivity was a significant predictor (Adj. R2 = 0.427, standardized Beta = 0.343, p = 0.013) for general cognitive ability in T1DM+ patients.
3.4. Discriminant function analysis
The three significant RSNs (Default Mode Network (DMN), Executive Control Network (ECN) and Sensorimotor Network (SMN)) of the lower alpha band were added as variables in a discriminant function analysis. Moreover, the covariates age, sex, systolic blood pressure, disease duration and depressive symptoms were included in the analysis.
The results for the classification of the three groups are shown in Supplementary Material Tables 1 and 2, while the classification for the patient groups only is shown in Supplementary Material Tables 3 and 4.
For classification of the three groups, age, depressive symptoms, systolic blood pressure and connectivity for two RSNs (ECN and SMN) were added during the stepwise analysis (Supplementary Material Table 1). However, the overall classification accuracy was low (62.1%) (Supplementary Material Table 2), indicating that despite significant differences in RSN connectivity between the groups, this does not allow for good discrimination. It is worth noting though that functional connectivity in the ECN and SMN adds information that is additional to that provided by the covariates, as these variables survived the stepwise procedure.
Functional connectivity in the SMN, disease duration, depressive symptoms and systolic blood pressure enables classification of the two patient groups with an overall accuracy of 85.5% (Supplementary Material Tables 3 and 4).
4. Discussion
In this study we showed that T1DM+ patients had a reduction of functional connectivity in MEG resting-state networks (RSNs) when compared to T1DM− patients, as well as compared to healthy participants. In contrast, T1DM− patients showed increased DMN functional connectivity relative to controls. The finding of altered functional connectivity was observed specifically in the lower alpha band for the three resting-state networks (DMN, ECN, and SMN). Furthermore, in T1DM+ patients a significant correlation between cognitive decrements and lower DMN functional connectivity in the lower alpha band was observed.
Despite differences in methodologies and/or modality, the connectivity results of this study agree with previous EEG (Cooray et al., 2011), MEG (van Duinkerken et al., 2009) and fMRI (van Duinkerken et al., 2012b) studies in terms of the overall result: T1DM influences functional connectivity. Importantly, our results also expand on our previous MEG and fMRI studies involving the same patient cohort; by using the phase lag index and source-level analysis we avoided spurious estimates of functional connectivity, and obtained results that were easier to interpret in terms of the exact anatomical regions that were involved (van Duinkerken et al., 2009). Moreover, using MEG analysis of RSNs allowed us to utilise the rich temporal dynamics of neuronal activity (van Duinkerken et al., 2012b).
Methodological differences, including a different acquisition method, different epoch time lengths, different resting-state protocol, and different source reconstruction approaches (no source reconstruction versus beamforming; grouping of connections in RSNs) might also explain why the EEG study by Cooray et al. did not find diabetes-related decreases in functional connectivity when using the PLI.
Our current results agree partly with our previous fMRI RSN analysis in this group (van Duinkerken et al., 2012a,b). In that study, connectivity in the sensorimotor and visual networks was increased in patients without complications versus controls, whereas patients with proliferative retinopathy showed lower connectivity relative to their counterparts with uncomplicated T1DM. A similar pattern was found in the current study for the DMN and ECN. Although the pattern of connectivity changes across the groups is similar for both studies, the affected RSNs differed. The exact relationship between functional connectivity estimates obtained through hemodynamic correlations and functional connectivity estimates based on electrophysiological oscillatory activity is still unknown (Logothetis, 2008; Singh, 2012; Tewarie et al., n.d.). This might also explain why our previous fMRI study (van Duinkerken et al., 2012b) revealed a dose–response effect for some RSNs (auditory, frontoparietal, and ventral attention), where functional connectivity varied according to clinical status, whereas in our current and previous MEG studies (van Duinkerken et al., 2009) the level of functional connectivity for the healthy participants was, generally, in between that for the T1DM+ and T1DM− patients.
In the current study, for the lower alpha band, T1DM− patients showed significantly higher functional connectivity in the DMN compared to the control group. This result suggests that differences in functional connectivity are evident even before microvascular complications are manifest. In T1DM+ patients a decreased functional connectivity was observed. This particular pattern of increased connectivity, followed by a breakdown in connectivity, has also been observed in patients with Alzheimer's disease, multiple sclerosis, Parkinson's disease and minimal hepatic encephalopathy (de Haan et al., 2012; Qi et al., 2012; Schoonheim et al., 2013; Stoffers et al., 2008). This indicates that regardless of the type of disease, neurodegeneration in Alzheimer's and Parkinson's disease, neuroinflammation in multiple sclerosis and metabolic in T1DM and hepatic encephalopathy, functional connectivity behaves in similar patterns. The mechanisms leading to first increased and with disease progression decreased functional connectivity are not yet fully understood. One hypothesis suggests that the initial increase in functional connectivity is explained by a loss of inhibition, and that increased functional connectivity in time leads to a decrease due to activity dependent degeneration (de Haan et al., 2012). Alternatively, the increase could be related to loss of functional connectivity in other networks (Seeley, 2011). Lastly, it could represent functional reorganization (Schoonheim et al., 2010) due to subtle cortical structural reorganization, which has been shown in adolescents and young adults with T1DM (Marzelli et al., 2014; Northam et al., 2009).
The only frequency band that showed significant results was the lower alpha band, which is in line with previous studies that have highlighted the relevance of this rhythm in mediating the functional processing, within and between areas, both in healthy subjects and its deviation in pathology (Fingelkurts and Fingelkurts, 2010; Mayhew et al., 2013).
The observed reduced alpha band functional connectivity in the DMN was behaviourally relevant, as it was related to cognitive dysfunction in T1DM+. This is in line with previous work that has shown a relationship between alpha band functional connectivity of RSNs, in particular the DMN (Jann et al., 2009), and the effect of damage to the DMN on cognitive performance (Broyd et al., 2009). The observation that only the functional integrity of the DMN, and not of the other RSNs, was related to cognitive performance may be explained by the finding that the DMN is important for cognition (Broyd et al., 2009) and also contains the strongest functional hubs (Damoiseaux and Greicius, 2009). As was shown by de Haan et al. (2012), these hubs are also most vulnerable to damage, yielding the most profound effects on cognition.
Here, we only looked at general cognition. In our previous fMRI study (van Duinkerken et al., 2012b) we correlated specific cognitive domains (see Table 2, Appendix A) with RSN activity, and found that information processing-speed as well as general cognitive ability correlated with the secondary visual network. As a post-hoc analysis, we therefore explored whether there were correlations between MEG RSNs functional connectivity (lower alpha band only) and specific cognitive domains. However, we did not find any significant correlations. A possible explanation for the lack of correlation between MEG RSNs and specific cognitive domains is that, in contrast to the fMRI study, here we split the patient cohort into two groups.
Structural assessment did not reveal differences in the incidence of white matter lesions between the three groups, nor were there statistically significant differences in total brain or grey matter volume. Despite the absence of overall volumetric differences between groups, more subtle structural brain changes, i.e. loss of white matter integrity, have been found in this study sample (van Duinkerken et al., 2012a). The latter result could suggest that a structural rewiring is taking place from which functional differences may originate. However, our results for the DMN and SMN, where the T1DM− patients showed an increase and decrease in functional connectivity, respectively, indicate that this hypothesis about a direct link between brain volume/white matter integrity and functional changes may be overly simplistic. More extensive analyses, incorporating regional variability in brain volume changes, are required to shed light on this issue.
Our approach is relatively new, yet recent studies support the investigation of MEG functional connectivity within RSNs (Brookes et al., 2011; de Pasquale et al., 2010; Hipp et al., 2012); (Tewarie et al., 2013; van Dellen et al., 2013), where the latter studies revealed that the investigation of MEG RSNs is sensitive in detecting alterations in functional connectivity that are associated with cognition. Furthermore, the employment of an atlas-based beamforming solution and analysis of RSNs allowed for a direct comparison between the current MEG results and the results from our previous fMRI study (van Duinkerken et al., 2012b); this was not possible with the analysis approach used in our previous MEG work (van Duinkerken et al., 2009).
We also explored whether functional connectivity within RSNs enables accurate classification of the three groups, as well as of the two patient groups alone, by means of discriminant function analyses. The patient classification was more accurate compared to the three group classification, probably as direct consequence of the fact that RSN connectivity for the healthy controls was usually in between that for the patient groups. For the three groups the functional connectivity within specific RSNs alone was not informative enough to discriminate accurately. An analysis considering both within and between RSN relationships could perhaps provide more predictive capability. However, the patient classification itself showed a reasonable accuracy, suggesting that different phenomena are evident between patients.
Overall, this analysis showed that functional connectivity within RSNs provides extra discriminatory information, in addition to patient characteristics such as age, depressive symptoms, systolic blood pressure and disease duration.
This study has some limitations: i) We assessed functional connectivity only within RSNs, and interactions between sub-networks were therefore not taken into account. This could be an interesting topic for future investigations, as there is recent evidence that higher-order cognitive functions emerge from dynamic interactions between RSNs (de Pasquale et al., 2012); ii) Our study could be complemented and expanded by a topological assessment of brain functional networks (Rubinov and Sporns, 2010), thereby providing insight in both local and global properties of the functional brain networks, and its relation with cognition; iii) Our results support the hypothesis that electrophysiological changes precede signs of microvascular alterations. Neurophysiological studies have suggested that these changes at the level of the cortex are a result of bottom-up driven rearrangements due to widespread sub-clinical sensory alterations (Várkonyi, 2006; Várkonyi et al., 2002). Unfortunately, neurophysiological measures of alterations at early stages of sensory processing were not included in this study, and we could therefore not relate functional connectivity in primary sensory networks to (possible) changes in early stages of sensory processing.
4.1. Conclusion
In conclusion, our results confirmed that functional sub-networks (resting-state networks such as DMN, ECN and SMN) are affected by T1DM, and these changes are related to cognitive performance. These results indicate that functional connectivity may play a key role in T1DM-related cognitive dysfunction.
Acknowledgements
The authors would like to thank C.J. Stam for his helpful discussions and suggestions. This study was supported by grant 2005.00.006 of the Dutch Diabetes Research Foundation and a grant of the European Foundation for the Study of Diabetes. M. Demuru was supported in part by Assegno di Ricerca related to D.R. 832/2012 University of Cagliari. M. Fraschini was in part supported by the Fondazione Banco di Sardegna (Prot.U7989.2013/AI.713.MGB. Prat.2013.1237). None of the authors have any conflict of interest to report.
Appendix A.
Table 2.
Cognitive domains.
| Cognitive domain | Neuropsychological test |
|---|---|
| Memory | Rey auditory verbal learning test WAIS-III-R digit span forward and backward WAIS-III-R symbol substitution incidental learning test |
| Information processing speed | WAIS-III-R symbol substitution test Stroop color–word test parts 1 and 2 Concept shifting task parts A and B Simple auditory and visual reaction time tests Computerized visual searching task |
| Executive functions | Stroop color–word test part 3, correct for time on parts 1 and 2 Concept shifting task part C, correct for time on parts A and B D2-test total errors Wisconsin cart sorting test Category word fluency task |
| Attention | D2-test range with total correct answers and total span |
| Motor speed | Tapping test Concept shifting task part |
| Psychomotor | Letter Digit Modalities Test |
Table 3.
Resting-state networks.
| Resting-state network | Corresponding AAL atlas ROIs (Rosazza and Minati, 2011) |
Corresponding AAL atlas ROIs (1 ROI overlap) |
|---|---|---|
| Default mode network | Precuneus, posterior cingulate gyrus, inferior parietal gyrus, medial prefrontal gyrus | Precuneus, posterior cingulate gyrus, anterior cingulate gyrus*, inferior parietal gyrus, medial prefrontal gyrus |
| Executive control | Medial frontal cortex, superior frontal gyrus, anterior cingulate gyrus | Medial frontal cortex, superior frontal gyrus, anterior cingulate gyrus |
| Frontoparietal (left/right) | Inferior frontal gyrus pars triangularis, inferior frontal gyrus pars opercularis*, medial frontal gyrus, precuneus*, inferior parietal gyrus, angular gyrus | Inferior frontal gyrus pars triangularis, medial frontal gyrus, inferior parietal gyrus, superior parietal gyrus*, angular gyrus |
Definitions of the analysed RSNs. Data that were presented as main results in the paper were based on a slight modification of the ROI definition of Rosazza and Minati. This definition was proposed by Tewarie (Tewarie et al., 2013) and others, it prevents overlap of connections between RSNs (right column). Our data were analysed using Tewarie's definition. Differences between both definitions were marked with *.
(See Table 3.)
Appendix B. Supplementary data
Supplementary tables. Supplementary tables for Discriminant Function Analysis.
References
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bartolomei F., Bosma I., Klein M., Baayen J.C., Reijneveld J.C., Postma T.J., Heimans J.J., Van Dijk B.W., de Munck J.C., de Jongh A., Cover K.S., Stam C.J. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin. Neurophysiol. 2006;117:2039–2049. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E.T. Human brain networks in health and disease. Curr. Opin. Neurol. 2009;22:340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands A.M.A., Biessels G.J., de Haan E.H.F., Kappelle L.J., Kessels R.P.C. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- Brookes M.J., Vrba J., Robinson S.E., Stevenson C.M. Optimising experimental design for MEG beamformer imaging. NeuroImage. 2008;39:1788–1802. doi: 10.1016/j.neuroimage.2007.09.050. [DOI] [PubMed] [Google Scholar]
- Brookes M.J., Woolrich M., Luckhoo H., Price D., Hale J.R., Stephenson M.C., Barnes G.R., Smith S.M., Morris P.G. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. 2011;108:16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J.S. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;1–14 doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cooray G.K., Hyllienmark L., Brismar T. Decreased cortical connectivity and information flow in type 1 diabetes. Clin. Neurophysiol. 2011;122:1943–1950. doi: 10.1016/j.clinph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Greicius M.D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W., Mott K., van Straaten E.C.W. PLOS Computational Biology: activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput. Biol. 2012;8:e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F., Penna Della S., Snyder A.Z., Lewis C., Mantini D., Marzetti L., Belardinelli P., Ciancetta L., Pizzella V., Romani G.L., Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F., Penna Della S., Snyder A.Z., Marzetti L., Pizzella V., Romani G.L., Corbetta M. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. 2012;74:753–764. doi: 10.1016/j.neuron.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L., de Groot M., van Dellen E., Aronica E., Heimans J.J., Klein M., Stam C.J., Reijneveld J.C., Hillebrand A. Local MEG networks: the missing link between protein expression and epilepsy in glioma patients? NeuroImage. 2013;75:195–203. doi: 10.1016/j.neuroimage.2013.02.067. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Barkhof F., Wahlund L.O., Pantoni L., Erkinjuntti T., Scheltens P., Schmidt R. CT and MRI rating of white matter lesions. Cerebrovasc. Dis. 2002;13:31–36. doi: 10.1159/000049147. [DOI] [PubMed] [Google Scholar]
- Fell J., Axmacher N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fingelkurts Andrew A., Fingelkurts Alexander A. Alpha rhythm operational architectonics in the continuum of normal and pathological brain states: current state of research. Int. J. Psychophysiol. 2010;76:93–106. doi: 10.1016/j.ijpsycho.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. (Regul. Ed.) 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu. Rev. Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Gong G., He Y., Concha L., Lebel C., Gross D.W., Evans A.C., Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R., Bosboom J.L., Berendse H.W., Stam C.J. Frequency-dependent functional connectivity within resting-state networks: an atlas-based MEG beamformer solution. NeuroImage. 2012;59:3909–3921. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp J.F., Hawellek D.J., Corbetta M., Siegel M., Engel A.K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 2012;15:884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A.M., Musen G., Ryan C.M., Silvers N., Cleary P.A., Waberski B.H., Burwood A., Weinger K., Bayless M., Dahms W., Harth J. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A.M., Ryan C.M., Cleary P.A., Waberski B.H., Weinger K., Musen G., Dahms W. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2010;54:245–255. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Dierks T., Boesch C., Kottlow M., Strik W., Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. NeuroImage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Yoon S., Jacobson A.M., Hwang J., Musen G., Kim J.E., Simonson D.C., Bae S., Bolo N., Kim D.J., Weinger K., Lee J.H., Ryan C.M., Renshaw P.F. Prefrontal cortical deficits in type 1 diabetes mellitus: brain correlates of comorbid depression. Arch. Gen. Psychiatry. 2012;69:1267–1276. doi: 10.1001/archgenpsychiatry.2012.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzelli M.J., Mazaika P.K., Barnea-Goraly N., Hershey T., Tsalikian E., Tamborlane W., Mauras N., White N.H., Buckingham B., Beck R.W., Ruedy K.J., Kollman C., Cheng P., Reiss A.L. 2014. Neuroanatomical Correlates of Dysglycemia in Young Children With Type 1 Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew S.D., Ostwald D., Porcaro C., Bagshaw A.P. Spontaneous EEG alpha oscillation interacts with positive and negative BOLD responses in the visual–auditory cortices and default-mode network. NeuroImage. 2013;76:362–372. doi: 10.1016/j.neuroimage.2013.02.070. [DOI] [PubMed] [Google Scholar]
- Montez T., Linkenkaer-Hansen K., Van Dijk B.W., Stam C.J. Synchronization likelihood with explicit time–frequency priors. NeuroImage. 2006;33:1117–1125. doi: 10.1016/j.neuroimage.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Mugler J.P., Bao S., Mulkern R.V., Guttmann C.R., Robertson R.L., Jolesz F.A., Brookeman J.R. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216:891–899. doi: 10.1148/radiology.216.3.r00au46891. [DOI] [PubMed] [Google Scholar]
- Northam E.A., Rankins D., Lin A., Wellard R.M. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes. 2009;32(3):445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Hillebrand A., Twisk J.W., Deijen J.B., Stoffers D., Schmand B.A., Stam C.J., Berendse H.W. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82:263–270. doi: 10.1212/WNL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Stoffers D., Deijen J.B., Twisk J.W.R. Resting-state functional connectivity as a marker of disease progression in Parkinson's disease: a longitudinal MEG study. NeuroImage. 2013;2:612–619. doi: 10.1016/j.nicl.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton N., Aslam T., Macgillivray T., Pattie A., Deary I.J., Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R., Zhang L.J., Xu Q., Zhong J., Wu S., Zhang Z., Liao W., Ni L., Zhang Z., Chen H., Zhong Y., Jiao Q., Wu X., Fan X., Liu Y., Lu G. Selective impairments of resting-state networks in minimal hepatic encephalopathy. PLoS One. 2012;7:e37400. doi: 10.1371/journal.pone.0037400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C., Minati L. Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M., Geurts J., Landi D. Functional connectivity changes in multiple sclerosis patients: a graph analytical study of MEG resting state data. Hum. Brain Mapp. 2013;4(1):52–61. doi: 10.1002/hbm.21424. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim M.M., Geurts J.J.G., Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology. 2010;74:1246–1247. doi: 10.1212/WNL.0b013e3181db9957. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. Divergent network connectivity changes in healthy APOE ε4 carriers: disinhibition or compensation? Arch. Neurol. 2011;68:1107–1108. doi: 10.1001/archneurol.2011.202. [DOI] [PubMed] [Google Scholar]
- Singh K.D. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. NeuroImage. 2012;62:1121–1130. doi: 10.1016/j.neuroimage.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Stam C.J., de Haan W., Daffertshofer A., Jones B.F., Manshanden I., van Cappellen van Walsum A.M., Montez T., Verbunt J.P.A., de Munck J.C., Van Dijk B.W., Berendse H.W., Scheltens P. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132:213–224. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Stam C.J., Nolte G., Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., Van Dijk B.W. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D. 2002;163:236–251. [Google Scholar]
- Stam C.J., van Straaten E.C.W. The organization of physiological brain networks. Clin. Neurophysiol. 2012;123(6):1067–1087. doi: 10.1016/j.clinph.2012.01.011. (June) [DOI] [PubMed] [Google Scholar]
- Stoffers D., Bosboom J., Deijen J.B., Wolters E.C. Increased cortico-cortical functional connectivity in early-stage Parkinson's disease: an MEG study. NeuroImage. 2008;41(2):212–222. doi: 10.1016/j.neuroimage.2008.02.027. (June) [DOI] [PubMed] [Google Scholar]
- Tewarie P., Hillebrand A., Schoonheim M.M., Van Dijk B.W. Functional brain network analysis using minimum spanning trees in Multiple Sclerosis: an MEG source-space study. NeuroImage. 2014;88:308–318. doi: 10.1016/j.neuroimage.2013.10.022. (March) [DOI] [PubMed] [Google Scholar]
- Tewarie, P., Hillebrand, A., van Dellen, E., Schoonheim, M.M., Barkhof, F., Polman, C.H., Beaulieu, C., Gong, G., Van Dijk, B.W., Stam, C.J., n.d. Structural degree predicts functional network connectivity: a multimodal fmri and meg study. [DOI] [PubMed]
- Tewarie P., Schoonheim M.M., Stam C.J., van der Meer M.L., Van Dijk B.W., Barkhof F., Polman C.H., Hillebrand A. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PLoS One. 2013;8:e69318. doi: 10.1371/journal.pone.0069318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Dellen E., de Witt Hamer P.C., Douw L., Klein M., Heimans J.J., Stam C.J., Reijneveld J.C., Hillebrand A. Connectivity in MEG resting-state networks increases after resective surgery for low-grade glioma and correlates with improved cognitive performance. NeuroImage. 2013;2:1–7. doi: 10.1016/j.nicl.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen E., Douw L., Hillebrand A., de Witt Hamer P.C. Epilepsy surgery outcome and functional network alterations in longitudinal MEG: a minimum spanning tree analysis. Neuroimage. 2014;86(1):354–363. doi: 10.1016/j.neuroimage.2013.10.010. (February) [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van Duinkerken E., Klein M., Schoonenboom N.S.M., Hoogma R.P.L.M., Moll A.C., Snoek F.J., Stam C.J., Diamant M. Functional brain connectivity and neurocognitive functioning in patients with long-standing type 1 diabetes with and without microvascular complications: a magnetoencephalography study. Diabetes. 2009;58:2335–2343. doi: 10.2337/db09-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinkerken E., Schoonheim M.M., IJzerman R.G., Klein M., Ryan C.M., Moll A.C., Snoek F.J., Barkhof F., Diamant M., Pouwels P.J.W. Diffusion tensor imaging in type 1 diabetes: decreased white matter integrity relates to cognitive functions. Diabetologia. 2012;55:1218–1220. doi: 10.1007/s00125-012-2488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinkerken E., Schoonheim M.M., Sanz-Arigita E.J., IJzerman R.G., Moll A.C., Snoek F.J., Ryan C.M., Klein M., Diamant M., Barkhof F. Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61(7):1814–1821. doi: 10.2337/db11-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F., Lachaux J.P., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Várkonyi T.T. Severity of autonomic and sensory neuropathy and the impairment of visual- and auditory-evoked potentials in type 1 diabetes: is there a relationship? Diabetes Care. 2006;29:2325–2326. doi: 10.2337/dc06-1174. [DOI] [PubMed] [Google Scholar]
- Várkonyi T.T., Petõ T., Dégi R., Keresztes K., Lengyel C., Janáky M., Kempler P., Lonovics J. Impairment of visual evoked potentials. Diabetes. 2002;25(9):1661–1662. doi: 10.2337/diacare.25.9.1661. [DOI] [PubMed] [Google Scholar]
- Vrba J., Anderson G., Betts K., Burbank M.B., Cheung T., Cheyne D., Fife A.A., Govorkov S., Haibi F., Haid G., Haid V.V., Hoang T., Hunter C., Kubik P.R., Lee S., McCubbin J., McKay J., McKenzie D., Nonis D., Paz J., Reichl E., Ressl D., Robinson S.E., Schroyen C., Sekatchev I., Spear P., Taylor B., Tillotson M., Sutherling W. 151-Channel whole-cortex MEG system for seated or supine positions. Recent Adv. Biomagn. 1999;93–96 [Google Scholar]
- Weiskopf N., Lutti A., Helms G., Novak M., Ashburner J., Hutton C. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT) NeuroImage. 2011;54:2116–2124. doi: 10.1016/j.neuroimage.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A.M., Scheltens P., Barkhof F., Heine R.J. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur. J. Pharmacol. 2008;585:88–96. doi: 10.1016/j.ejphar.2007.11.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables. Supplementary tables for Discriminant Function Analysis.


