Deep vein thrombosis (DVT) is a significant cause of morbidity and mortality throughout the world 1. Risk factors for DVT include major surgery, immobilization, trauma, and cancer, among others 1. Many mouse models of thrombosis have been used to study mechanisms of thrombosis, including those involving small and large veins. In this report from the Animal Models Subcommittee of the ISTH, we summarize the strengths and weaknesses of the inferior vena cava (IVC) stenosis mouse model of venous thrombosis.
History
In this model, the lumen volume of the IVC is reduced by ∼ 90% 2,3. This model was adapted from a similar rat model called the ‘St Thomas Model’, involving IVC stenosis followed by endothelial damage with vascular clamps upstream of the stenosis site 4. Many variants of the St Thomas model have been described. The IVC stenosis mouse model described in this article was first reported in 2011, and does not involve a vascular damage step 2. Since then, several groups have used the model, with slight variations 3,5–9. Thrombosis in this new model is thought to be initiated by the combination of endothelial activation, a reduction in blood flow velocity, and disturbed blood flow upstream of the stenosis site 2,3.
Methods
In this model, a midline laparotomy is performed on anesthetized male mice that are at least 8 weeks of age and 22 g in weight 2,3,6. The IVC is exposed by atraumatic blunt dissection, and the IVC is carefully separated from the abdominal aorta. A spacer is placed on top of the exposed IVC, and a non-reactive permanent narrowing ligature (7.0 or 8.0 monofil polypropylene) is secured around both the IVC and spacer directly below the renal veins. The spacer is then removed, resulting in a ∼ 90% reduction in IVC lumen size at the stenosis site 2. Injury to the IVC wall is avoided during the procedure. Mice with any bleeding from the IVC are excluded. The surgical site is then closed, and mice are allowed to recover under the influence of appropriate analgesia.
Sources of variability
The spacers used for this model range from 0.26 to 0.36 mm in diameter, and consist of guide wires, sutures, or blunted needles 2,3,5–9. The most common spacers used to date are a 0.36-mm guide wire 3,9 and a blunted 30-gauge needle 2,6,7. The effect of spacer diameter and length on thrombosis in this model has not been evaluated. The method of surgical anesthesia also varies, with an isoflurane/oxygen mixture being the most common 2,3,5–9. There is some debate as to whether side branches should be ligated to control for IVC anatomy variation. A concern with side branch ligation is that it may result in endothelial damage and increase the duration of surgery. One group compared the size of thrombi in mice with and without side branch ligation, and found no difference between the two groups 9. This same study, however, found that side branches that were < 1.5 mm from the stenosis site resulted in smaller thrombi 9.
Quantification of thrombus size
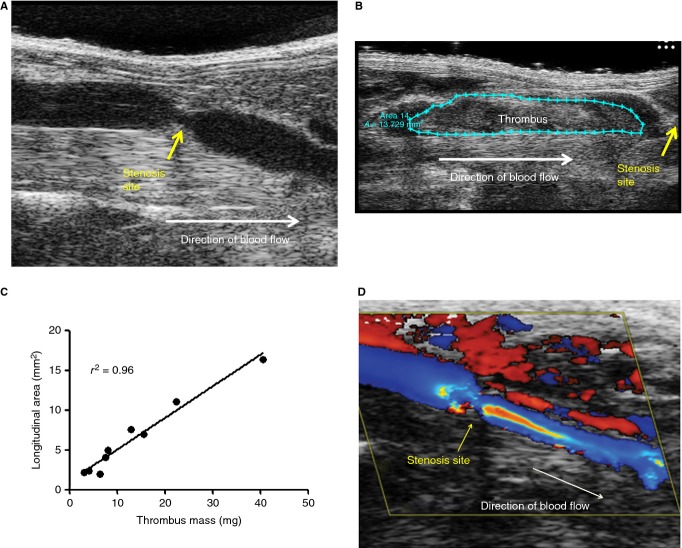
Thrombus size in this model is typically quantified by measuring thrombus mass or size at a given time point after the induction of stenosis. In order to obtain these measurements, mice are euthanized and the IVC is harvested. We and others have extended the utility of this model by using high-frequency ultrasonography to image blood flow and quantify IVC thrombus growth in vivo (Fig.1A–C) 9.
Fig 1.
In vivo imaging of the inferior vena cava (IVC) stenosis model. (A, B) IVC stenosis was performed in C57Bl/6J mice, and ultrasonographic imaging of the stenosis site (A) before and (B) after thrombus formation was performed. (C) Longitudinal thrombus area was measured in mice at 24 h, by ultrasonography. Thrombi were then immediately harvested and weighed. Linear regression analysis for longitudinal thrombus area vs. thrombus mass identified a strong correlation (r2 = 0.96). (D) Color Doppler imaging reveals a pattern of disturbed blood flow around the stenosis site prior to thrombus development.
Strengths and weaknesses of the new model
It has been well documented that human DVT is most often initiated within the valve sinuses of large veins on an intact endothelium 1. A strength of this model is that thrombosis is initiated in a large vein in the mouse in the absence of major vessel damage. Furthermore, although there are no valves in the IVC, the disturbed blood flow occurring around the stenosis site may mimic changes in blood flow that occur over valves and within valve pockets in large veins in humans (Fig.1D). The thrombi that develop in this model are also histologically similar to human venous thrombi 2,3.
The major concern with this model is that thrombus development is variable. The reported incidence of thrombosis at 48 h in wild-type mice ranges from 44% to 100% 2,5,8,9. We have a lot of experience with this model in our laboratory, and followed the development and growth of thrombi in 15 C57Bl/6J mice aged 14–18 weeks over a period of 48 h. High-frequency ultrasonography was used to measure thrombus size in each mouse at 3, 6, 9 and 48 h after the initiation of thrombosis (Table S1). As expected, we found that both thrombus size at each time point and time to thrombus initiation were variable, with thombi appearing in the IVC anywhere from 3 to 48 h after stenosis (thrombus area, incidence: 3 h, 0–8.5 mm2, 13%; 6 h, 0–11.1 mm2, 33%; 9 h, 0–10.1 mm2, 40%; 48 h, 0–11.1 mm2, 53%). Another limitation of this model is that it is performed in a vein that does not have valves.
Conclusions
Like any model, this mouse model of venous thrombosis does not accurately reflect all aspects of human DVT. However, as discussed above, it does have certain strengths over other available models. Primarily, the initiation of thrombosis occurs without the induction of major vascular damage, which more closely mimics human DVT than models triggered by chemical or physical vessel damage. Nevertheless, the high variability in thrombus development is a significant concern that limits its utility for detecting small differences between groups.
Addendum
J. Geddings and N. Mackman wrote the manuscript, with input from M. Aleman, A. Wolberg, M. von Brühl, and S. Massberg.
Acknowledgments
Technical support was provided by M. Rojas in the UNC McAllister Heart Institute surgical models core.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Size and incidence of thrombosis in the IVC stenosis model.
References
- 1.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–6. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuinness C, Humphries J, Smith A, Burnand K. A new model of venous thrombosis. Cardiovasc Surg. 1997;S5:123. [Google Scholar]
- 5.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–52. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brill A, Yesilaltay A, De Meyer SF, Kisucka J, Fuchs TA, Kocher O, Krieger M, Wagner DD. Extrahepatic high-density lipoprotein receptor SR-BI and apoA-I protect against deep vein thrombosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:1841–7. doi: 10.1161/ATVBAHA.112.252130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110:8674–9. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt M, Schonfelder T, Schwenk M, Becker C, Jackel S, Reinhardt C, Stark K, Massberg S, Munzel T, von Bruhl ML, Wenzel P. Deep vein thrombus formation induced by flow reduction in mice is determined by venous side branches. Clin Hemorheol Microcirc. 2013 doi: 10.3233/CH-131680. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Size and incidence of thrombosis in the IVC stenosis model.