Abstract
The synthesis of several sulfates of trichothecene mycotoxins is presented. Deoxynivalenol (DON) and its acetylated derivatives were synthesized from 3-acetyldeoxynivalenol (3ADON) and used as substrate for sulfation in order to reach a series of five different DON-based sulfates as well as T2-toxin-3-sulfate. These substances are suspected to be formed during phase-II metabolism in plants and humans. The sulfation was performed using a sulfuryl imidazolium salt, which was synthesized prior to use. All protected intermediates and final products were characterized via NMR and will serve as reference materials for further investigations in the fields of toxicology and bioanalytics of mycotoxins.
Keywords: Deoxynivalenol, T2-toxin, Masked mycotoxins, Sulfation, Trichothecenes
Graphical abstract
1. Introduction
Deoxynivalenol (DON), its acetylated derivatives, which occur as 3-acetyldeoxynivalenol (3ADON), 15-acetyldeoxynivalenol (15ADON), and 3,15-diacetyldeoxynivalenol (3,15-diADON) as well as T2-toxin (Fig. 1) are very common and widespread trichothecene mycotoxins. They are predominantly produced by different Fusarium species and can contaminate food and feed. They are known to act as a protein biosynthesis inhibitor,1,2 neurotoxin, immunosuppressive or nephrotoxin3 and can cause acute and chronic symptoms4 after uptake. Based on this knowledge, regulatory limits regarding the toxin concentration for food and feed were established to minimize the daily uptake.
Fig. 1.
Structures of DON, its derivatives, and T2-toxin.
Although these limits cover the toxins themselves, there is still a lack of information regarding the occurrence and toxicity of their conjugated forms. DON, for example, can undergo glycosylation during late phase-II metabolism in the plant to end up as DON-3-β-d-O-glucoside, which is classified as masked mycotoxin.5 A similar conjugation leads to the corresponding T2-toxin glucoside, which was recently discovered.6 These masking mechanisms can also lead to di- and triglycosides, which are difficult to investigate due to a lack of authentic reference standards. The main concern regarding these masked forms is the fact that the occurring conjugates can be cleaved in the stomach after uptake, whereby releasing the parent toxin. In addition to the stomach, this cleavage could also occur within the process of malting,7 leading to an increase of free DON. Besides the formation of glycosides, other masking processes like sulfation or thia-michael addition can take place. Zearalenone is another prominent mycotoxin produced by Fusarium species and its metabolite zearalenone-14-sulfate8 was previously synthesized9 in order to serve as reference material for contamination and toxicity studies. Additionally, the synthesis of different Alternaria toxin sulfates10 was recently published showing the emerging research interest within the field of mycotoxin sulfur conjugates. Besides the occurrence of different masked mycotoxins in plants, many parent toxins also undergo phase-II metabolism in living organisms, leading mainly to their corresponding glucuronides11 but also to their sulfates.12,13 Besides the occurring glucuronides, which are accessible14 and were already used for the successful development of a biomarker method15–17 for human deoxynivalenol exposure estimation, little is known about possible occurring trichothecene sulfates. Considering that sulfate conjugates are described for different substance classes like steroids,18 pollutants,19 and drugs,20,21 we assume that there might be even more trichothecene-derived sulfates occurring during metabolism.
The objective of this work was the development of a reliable synthetic strategy for the sulfation of trichothecenes to access all possible DON-sulfates incorporating their acetylated derivatives and T2-toxin-3-sulfate. Since the reactivity of the three hydroxyl groups in deoxynivalenol is somewhat different in the order 15>3>>7 (Fig. 2), we expected all kinds of 3- and 15-sulfates to occur. However, we were interested in evaluating the possibility to obtain also some C7-sulfates.
Fig. 2.
Structural numbering of trichothecenes.
3ADON was isolated and crystallized22 from an extract of Fusarium graminearum and was directly used after comparison with a 3ADON standard. Deprotection and the following acetylation of DON23 led us to the four desired structures, which we wanted to utilize for sulfation (Scheme 1) to access a series of different sulfates.
Scheme 1.
Synthetic strategies toward all possible sulfate conjugates. S=sulfation, D=deprotection, deAc=deacetylation.
Regarding sulfation itself, numerous synthetic methods24 can be found within the literature mainly based on the application of commercially available sulfur trioxide complexes. Since these methods are limited concerning possible chemical modifications after installation of the sulfate group as well as yield, reproducibility, and regioselectivity, several newer protective groups for the sulfation of organic molecules are described in literature. Besides the fact that we already made good experiences in our group regarding the separation of intermediates and yields by the use of 2,2,2-trichloroethyl (TCE) protective groups25 within chemical sulfation and glycosylation reactions, the use of this protective group also offers the possibility of mild deprotection conditions. The cleavage could therefore be done via catalytic transfer hydrogenation (Pd/C) or via different mild reductive methods including ammonium formate or Zn/ammonium formate.
In addition, a very mild protocol using sulfuryl imidazolium salts was recently employed in the synthesis of trichloroethyl-protected sulfates on carbohydrates and offered a good possibility of the sulfation of the fragile trichothecene scaffold.
2. Results and discussion
After synthesis of the proper sulfuryl imidazolium salt26 (Scheme 2a, 26), the method evaluation was done by the use of a simple mimic27 (Scheme 2b, 27), which was selected because of an incorporated primary alcohol group, a labile acetic ester group (like T2-toxin), and its UV activity.
Scheme 2.
Synthesis of the protected sulfate donor (A, 26) and method evaluation with a simple mimic (B, 27). Conditions: (a) pyridine, SO2Cl2, −80 °C to rt, 24 h, 97%, (b) 2-methyl imidazole, 2 h, 99%, (c) Et2O, 0 °C, MeOTf, 6 h, 94%, (d), 1,2-dimethylimidazole, 0 °C, DCM, 24 h, 82%, (e) MeOH, HCOONH4, Zn, 20 min, 81%, (f) MeOH, NaOMe, 1 h, 42%.
The reaction of 26 and 27 to 28 proceeds smoothly if freshly sublimed dimethylimidazole is used. The deprotection toward 29 was done in an ultrasonic bath at room temperature, which shortened the reaction time to 20 min. This is a crucial achievement since trichothecenes often own labile ester groups, which are rapidly cleaved in different solvents. The deprotection toward 30 was done via NaOMe and was carried out as a proof of concept regarding the deprotection of acetylated sulfates.
The reactions toward the different acetylated DON derivates (Scheme 3) were carried out as described in literature23 and led to all desired products.
Scheme 3.
Synthesis of different deoxynivalenol derivatives. Conditions: (a) NaOMe, MeOH, 2 h, 94%, (b) Ac2O, DMAP, 18 h, 47% for 3, 23% for 4, sum=70%.
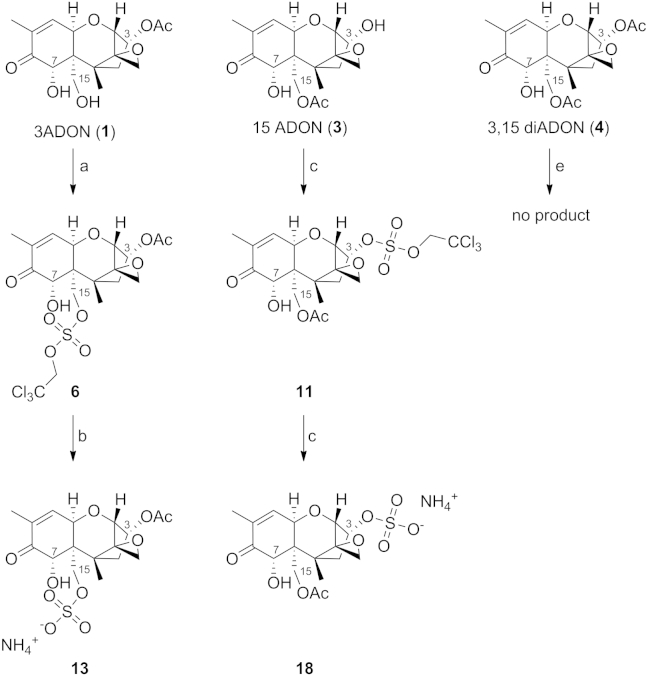
The sulfation of 1 yielded (as expected) only one product, which was identified as the protected 3ADON-15-sulfate, and sulfation of 3 yielded only the protected 15ADON-3-sulfate. Both substances were successfully deprotected to the free sulfates as ammonium salt. In case of 4 no reaction was observed at all, even with 4 equiv of 26 and prolonged reaction time (Scheme 4).
Scheme 4.
Synthesis of different acetyldeoxynivalenol-derived sulfates. Conditions: (a) DCM, 2.5 equiv 1,2-dimethylimidazole, 1.25+0.75 equiv 26, 0 °C, 18 h, 52%, (b) MeOH, 9 equiv HCOONH4, 3 equiv Zn, 20 min, 72%, (c) DCM, 2.7 equiv 1,2-dimethylimidazole, 1.35 equiv 26, 0 °C, 18 h, 34%, (d) MeOH, 9 equiv HCOONH4, 3 equiv Zn, 20 min, 89%.
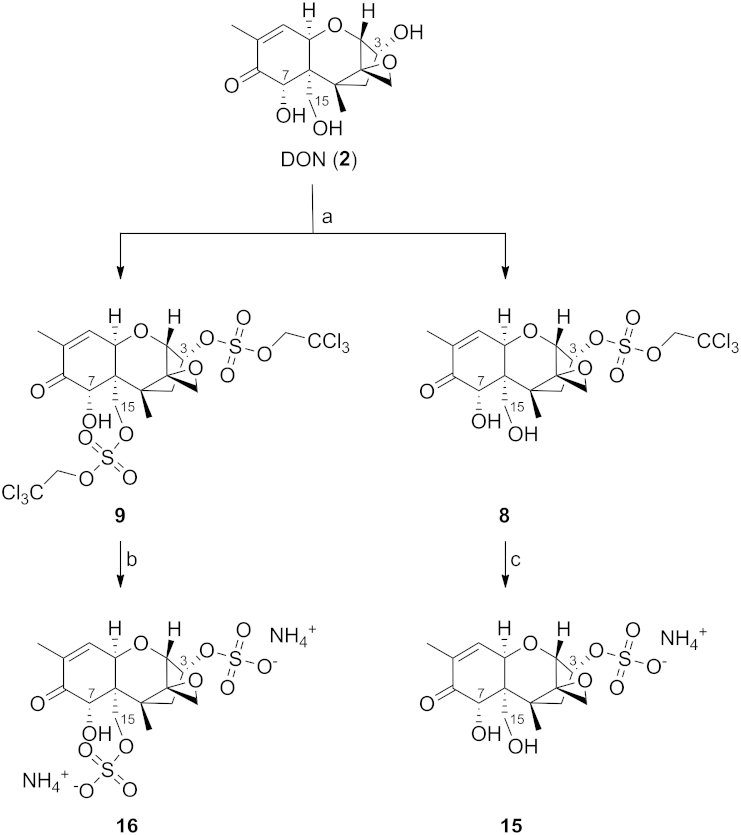
In case of 2 we isolated protected DON-3,15-disulfate surprisingly together with DON-3-sulfate instead of the expected 15-sulfate (Scheme 5). Additionally, these two products were the only ones, which were isolated. In general, we were expecting position 7 to be somewhat unreactive due to steric hindrance and poor nucleophilicity. Nevertheless, since acetylation resulted in a 2:1 mixture of the 15- to the 3-product, we also expected a similar regioselectivity and ratio of the sulfation reaction. The reaction itself has not undergone any decomposition (observed via TLC) during workup, so we could also exclude the theory of degradation from the disulfate species toward the monosulfate. Deprotection of both substances yielded DON-3-sulfate as ammonium salt and DON-3,15-disulfate as the corresponding diammonium salt.
Scheme 5.
Synthesis of DON-3-sulfate and DON-3,15-disulfate as their ammonium salts. Conditions: (a) DCM, 4 equiv 1,2-dimethylimidazole, 2 equiv 26, 0 °C, 18 h, 24% for 9, 30% for 8, (b) MeOH, 18 equiv HCOONH4, 6 equiv Zn, 2 h, 54%, (c) MeOH, 9 equiv HCOONH4, 3 equiv Zn, 30 min, 98%.
Position 7 was not sulfated during any reaction. Of the remaining five theoretically possible sulfates, only DON-15-sulfate was not isolated after direct sulfation (Scheme 1). For this reason we used deacetylation of 3ADON-15-sulfate (Scheme 6) to complete the set of sulfates.
Scheme 6.
Synthesis of DON-15-sulfate as ammonium salt. Conditions: (a) MeOH, NaOMe, 2 h, 69%.
Having proven that the method is well working for trichothecenes, we aimed also for the sulfation of T2-toxin, which was carried out in a similar way, whereby leading to the corresponding 3-sulfate as ammonium salt (Scheme 7).
Scheme 7.
Synthesis of T2-toxin-3-sulfate as ammonium salt. Conditions: (a) DCM, 4 equiv 1,2-dimethylimidazole, 2 equiv 26, 0 °C, 18 h, 49%, (b) MeOH, 9 equiv HCOONH4, 3 equiv Zn, 1 h, 29%.
Finally, all isolated protected intermediates as well as all ammonium sulfates were characterized via NMR, and 1H shifts were assigned using several NMR references28,29 for DON and its derivatives. For the assignment of the 1H shifts of T2-toxin-3-sulfate, COSY and HMBC spectra were recorded. We tried to purify all intermediates very quickly over short columns (10–15 g silica), since we have encountered deacetylation of all types of trichothecenes during column chromatography (silica as well as RP). Therefore, we ended up with traces of 1,2-dimethylimidazole within the protected intermediates. After the deprotection step, column chromatography was done with very polar solvents (DCM/MeOH/NH4OH=10:4:1 or 10:2.5:0.5), which led to HCOONH4 impurities in our products. Because of this we usually made a second column chromatography, followed by lyophilization, and continued drying for several days to remove remaining HCOONH4. All NMR spectra and 1H chemical shift assignments can be found in the Supplementary data.
3. Conclusion
Considering the proven unreactivity of the C7 position to chemical sulfation, we have synthesized, isolated, and characterized all possible DON-sulfates including its acetylated derivatives. In case of T2-toxin we were also able to synthesize the desired T2-toxin-3-sulfate. Therefore, we have proven that the utilized method is well working for trichothecenes and provides a good way to access the class of trichothecene sulfates via sulfation of the parent toxins including their acetylated derivatives. Separation of the protected intermediates was done using column chromatography, followed by fast deprotection by the use of Zn/HCOONH4 within an ultrasonic bath. All products and intermediates were characterized by 1H and 13C NMR, and all 1H chemical shifts were assigned to the substances. The gathered 1H NMR information will serve as a valuable reference for naturally isolated material. Finally, all standards will be used for identification and quantification of their occurrence and formation within human and plant metabolism.
4. Experimental section
4.1. General remarks
CAUTION: All used toxins are strong protein biosynthesis inhibitors and can cause a series of acute and chronic symptoms. Therefore, we strongly recommend considering their toxicity within all reactions!
All reactions were carried out under an argon atmosphere and the progress of all reactions was monitored using thin-layer chromatography (TLC) over silica gel 60F254 (Merck, Germany). All chromatograms were visualized by heat staining using ceric ammonium molybdate/Hanessian's stain30 in ethanol/sulfuric acid. Chromatographic separation was done on silica gel 60 (40–63 μm) using a SepacoreTM Flash System (Büchi, Switzerland) or glass columns. All samples were measured via LC–ESI-MS/MS and LC–APCI-MS/MS and in a negative ionization mode. These measurements were performed on an HCT ion trap mass spectrometer (Bruker, Germany). A TLC–MS interface (Camag, Germany) was used for ESI-MS analysis after TLC. 1H and 13C spectra were recorded upon using a Bruker DPX-200 spectrometer as well as an Avance DRX-400 MHz spectrometer (both Bruker, Germany). Data were recorded and evaluated using TOPSPIN 1.3 (Bruker Biospin). All chemical shifts are given in parts per million relative to tetramethylsilane. The calibration was done using residual solvent signals.31 Multiplicities are abbreviated as s (singlet), d (doublet), t (triplet), q (quartet), and br (broad signal). 3-ADON was obtained from BOKU, Dept. for Agrarbiotechnology (IFA-Tulln) as crude fermentation extract, purified via column chromatography, and used after the 1H NMR purity check. All other chemicals were purchased from ABCR (Germany) and Sigma–Aldrich (Austria/Germany).
4.2. 2,2,2-Trichloroethyl 2-(4-acetoxyphenyl)ethylsulfate (28)
Compound 27 (121.8 mg, 0.68 mmol, 1.0 equiv) was dissolved in 3 mL DCM and a solution of 1,2-dimethylimidazole (194.9 mg, 2.03 mmol, 3.0 equiv) in 1 mL DCM was added at 0 °C. Then, 26 (464.0 mg, 1.01 mmol, 1.5 equiv) was added in one portion and the reaction was allowed to warm to room temperature over night. After TLC indicated full conversion the reaction was directly purified via column chromatography (hexane/EtOAc=1:1) to yield 28 (215.8 mg, 82%) as white solid. 1H NMR (200 MHz, CDCl3) δ 7.28 (s, 4H), 4.82 (s, 2H), 4.27 (t, J=6.8 Hz, 2H), 2.95 (t, J=6.8 Hz, 2H), 2.02 (s, 3H); 13C NMR (50 MHz, CDCl3) δ 171.0 (s, 1C), 148.9 (s, 1C), 138.1 (s, 1C), 130.6 (d, 2C), 121.2 (d, 2C), 92.5 (s, 1C), 80.5 (t, 1C), 64.5 (t, 1C), 34.5 (t, 1C), 21.0 (q, 1C). HRMS m/z calcd for [M+H]+, 390.9571, found 390.9578.
4.3. 2-(4-Acetoxyphenyl)ethylsulfate, ammonium salt (29)
Compound 28 (190.0 mg, 0.49 mmol, 1.0 equiv) was dissolved in 5 mL MeOH. Ammonium formate (276.5 mg, 4.39 mmol, 9.0 equiv) and Zn dust (95.6 mg, 1.46 mmol, 3.0 equiv) were added and the reaction was placed in an ultrasonic bath. After 20 min, TLC revealed complete conversion of the starting material and the reaction mixture was filtered through Celite and concentrated to 1 mL. Direct purification of this solution using column chromatography (DCM/MeOH/NH4OH=10:2.5:0.5) yielded 29 (109.5 mg, 81%) as white solid. 1H NMR (200 MHz, methanol-d4) δ=7.22 (br, 4H), 4.93 (br, H2O), 4.23 (t, J=6.9 Hz, 2H), 2.91 (t, J=6.9 Hz, 2H), 2.00 (s, 3H); 13C NMR (50 MHz, methanol-d4) δ 172.9 (s, 1C), 152.5 (s, 1C), 136.0 (s, 1C), 130.6 (d, 2C), 122.5 (d, 2C), 66.2 (t, 1C), 35.3 (t, 1C), 20.8 (q, 1C). HRMS m/z calcd for C10H11O6S− [M−NH4]−, 259.0282, found 259.0278.
4.4. 2-(4-Hydroxyphenyl)ethylsulfate, ammonium salt (30)
Compound 29 (69.0 mg, 0.25 mmol, 1.00 equiv) was dissolved in 2 mL dry MeOH and NaOMe (14.1 mg, 0.26 mmol, 1.05 equiv) was added. Since no reaction occurred after 30 min we added the same amount of NaOMe again. After stirring for 30 min, TLC indicated full conversion and the reaction was directly purified via column chromatography (DCM/MeOH/NH4OH=10:2.5:0.5) to yield 9 (28.7 mg, 42%) as white solid. 1H NMR (200 MHz, methanol-d4) δ=7.21 (s, 4H), 4.90 (br, H2O), 3.73 (t, J=6.9 Hz, 2H), 2.80 (t, J=6.9 Hz, 2H); 13C NMR (50 MHz, methanol-d4) δ 152.3 (s, 1C), 137.0 (s, 1C), 130.6 (d, 2C), 122.5 (d, 2C), 64.2 (t, 1C), 39.5 (t, 1C). HRMS m/z calcd for C8H9O5S− 217.0176, found 217.0174.
4.5. 15-ADON (3) and 3,15-diADON (4)
3-ADON (1) (95.6 mg, 0.28 mmol, 1.0 equiv) was dissolved in 5 mL methanol, followed by the addition of NaOMe (13.7 mg, 0.25 mmol, 0.9 equiv). After 2 h, TLC showed full conversion of the 1. The reaction was concentrated to 1 mL and directly purified by the use of column chromatography (CHCl3/MeOH=9:1), which yielded deoxynivalenol (2, 79.0 mg, 94%) as white solid. The reaction product was proven to be identical to an authentic sample by TLC and, thus, was directly used for acetylation. For this purpose, 2 (79.0 mg, 0.27 mmol) was dissolved in 50 mL dry dichloromethane. Pyridine (1 mL) and 4-DMAP (app. 10 mg) were added, followed by the dropwise addition of acetic anhydride (27.2 mg, 0.27 mmol). The reaction was stirred over night, treated with 20 mL 2 N HCl, and extracted with dichloromethane. After drying with Na2SO4, filtration, and evaporation of the solvent, the remaining residue was subjected to column chromatography (CHCl3/MeOH=95:5) to yield 3 (42.0 mg, 47%) and 4 (23.5 mg, 23%) as white solid. Total yield=70%, 93% conversion. 15-ADON (3): 1H NMR (200 MHz, CDCl3) δ 6.61 (dq, J=5.7, 1.6 Hz, 1H), 4.89 (d, J=5.7 Hz, 1H), 4.83 (d, J=1.6 Hz, 1H), 4.52 (dt, J=10.2, 4.7 Hz, 1H), 4.24 (s, 2H), 3.78 (d, J=1.8 Hz, 1H), 3.63 (d, J=4.5 Hz, 1H), 3.13 (d, J=4.3 Hz, 1H), 3.08 (d, J=4.3 Hz, 1H), 2.22 (dd, J=14.8, 4.7 Hz, 1H), 2.08 (dd, J=14.7, 10.4 Hz, 1H), 1.88 (s, 3H), 1.87 (s, 3H), 1.07 (s, 3H); 13C NMR (50 MHz, CDCl3) δ=199.6 (s), 170.3 (s), 138.8 (d), 135.6 (s), 80.7 (d), 73.5 (d), 70.1 (d), 68.9 (s), 65.5 (s), 62.2 (t), 51.4 (s), 47.4 (t), 46.3 (s), 43.3 (t), 20.7 (q), 15.4 (q), 13.8 (q). 3,15-diADON (4): 1H NMR (200 MHz, CDCl3) δ 6.56 (dq, J=5.8, 1.4 Hz, 1H), 5.20 (dt, J=10.9, 4.6 Hz, 1H), 4.80 (d, J=2.0 Hz, 1H), 4.69 (d, J=5.8 Hz, 1H), 4.27 (d, J=12.1 Hz, 1H), 4.20 (d, J=12.1 Hz, 1H), 3.89 (d, J=4.3 Hz, 1H), 3.80 (d, J=2.0 Hz, 1H), 3.14 (d, J=4.3 Hz, 1H), 3.09 (d, J=4.3 Hz, 1H), 2.31 (dd, J=15.2, 4.8 Hz, 1H), 2.15 (dd, J=15.2, 10.9 Hz, 1H), 2.12 (s, 3H), 1.88 (s, 3H), 1.87 (s, 3H), 1.08 (s, 3H); 13C NMR (50 MHz, CDCl3) δ 199.3 (s), 170.3 (s), 170.2 (s), 138.4 (d), 135.6 (s), 78.9 (d), 73.4 (d), 71.1 (d), 70.1 (d), 64.9 (s), 62.1 (t), 51.5 (s), 47.4 (t), 45.8 (s), 40.3 (t), 21.0 (q), 20.6 (q), 15.3 (q), 13.6 (q).
4.6. 2,2,2-Trichloroethyl 3-acetyl-DON-15-sulfate (6)
Compound 1 (45.1 mg, 133 μmol, 1.0 equiv) was dissolved in 2.5 mL of DCM and cooled to 0 °C and 1,2-dimethylimidazole (32.0 mg, 333 μmol, 2.5 equiv) in 1 mL DCM was added to the reaction. Then, 26 (76.2 mg, 167 μmol, 1.25 equiv) was added and the reaction was allowed to reach room temperature over night. Since TLC showed remaining starting material after 18 h, the reaction was cooled again to 0 °C and another 0.75 equiv of 26 (45.7 mg, 100 μmol) were added. After another 48 h, TLC showed still starting material, but also the formation of substantial amounts of product. The reaction was directly used for column chromatography (CHCl3/MeOH=95:5), yielding 6 (37.8 mg, 52%) as white solid. 1H NMR (200 MHz, CDCl3) δ 6.66 (dq, J=5.9, 1.4 Hz, 1H), 5.24 (ddd, J=9.5, 6.0, 4.5 Hz, 1H), 4.89 (d, J=1.4 Hz, 1H), 4.80 (d, J=5.9 Hz, 1H), 4.64 (d, J=10.8 Hz, 1H), 4.57 (d, J=10.8 Hz, 1H), 4.56 (d, J=10.6 Hz, 1H), 4.43 (d, J=10.6 Hz, 1H), 3.95 (d, J=4.5 Hz, 1H), 3.84 (d, J=1.4 Hz, 1H), 3.16 (d, J=4.1 Hz, 1H), 3.13 (d, J=4.1 Hz, 1H), 2.10–2.36 (m, 2H), 2.16 (s, 3H), 1.91 (br, 3H), 1.11 (s, 3H); 13C NMR (50 MHz, CDCl3) δ 198.8 (s, 1C), 170.2 (s, 1C), 138.7 (d, 1C), 136.1 (s, 1C), 92.4 (s, 1C), 79.7 (t, 1C), 78.8 (d, 1C), 73.0 (d, 1C), 71.6 (t, 1C), 70.7 (d, 1C), 69.1 (d, 1C), 64.6 (s, 1C), 51.3 (s, 1C), 47.4 (t, 1C), 45.8 (s, 1C), 40.4 (t, 1C), 20.9 (q, 1C), 15.2 (q, 1C), 13.5 (q, 1C); HRMS m/z calcd for [M+H]+, 549.0150, found 549.0160.
4.7. 2,2,2-Trichloroethyl 15-acetyl-DON-3-sulfate (11)
Compound 3 (76.5 mg, 226 μmol, 1.0 equiv) was dissolved in 3 mL of DCM and cooled to 0 °C and 1,2-dimethylimidazole (58.7 mg, 610 μmol, 2.7 equiv) in 1 mL DCM was added to reaction. Then 26 (139.7 mg, 305 μmol, 1.35 equiv) was added and the reaction was allowed to reach room temperature over night. TLC showed substantial amounts of product and the reaction was directly used for column chromatography (DCM/MeOH=95:5) yielding 11 (42.6 mg, 34%) as white solid. 1H NMR (200 MHz, CDCl3) δ 6.60 (dq, J=5.8, 1.5 Hz, 1H), 5.31 (dt, J=11.1, 4.3 Hz, 1H), 4.81 (s, 1H), 4.79 (s, 2H), 4.70 (d, J=5.8 Hz, 1H), 4.27 (d, J=12.1 Hz, 1H), 4.18 (d, J=12.1 Hz, 1H), 4.00 (d, J=4.3 Hz, 1H), 3.71 (s, 1H), 3.21 (d, J=4.1 Hz, 1H), 3.15 (d, J=4.1 Hz, 1H), 2.64 (dd, J=15.7, 4.3 Hz, 1H), 2.29 (dd, J=15.7, 11.1 Hz, 1H), 1.93 (s, 3H), 1.91 (br, 3H), 1.12 (s, 3H); 13C NMR (50 MHz, CDCl3) δ 198.9 (s, 1C), 170.2 (s, 1C), 137.9 (d, 1C), 136.1 (s, 1C), 92.7 (s, 1C), 80.4 (d, 1C), 80.0 (t, 1C), 78.9 (d, 1C), 73.5 (d, 1C), 70.1 (d, 1C), 64.5 (s, 1C), 62.0 (t, 1C), 51.2 (s, 1C), 47.4 (t, 1C), 46.1 (s, 1C), 40.2 (t, 1C), 20.8 (q, 1C), 15.4 (q, 1C), 13.7 (q, 1C); HRMS m/z calcd for [M+H]+, 549.0150, found 549.0146.
4.8. 2,2,2-Trichloroethyl DON-3-sulfate (8) and bis(2,2,2-trichloroethyl) DON-3,15-disulfate (9)
Compound 2 (40.6 mg, 137 μmol, 1.0 equiv) was dissolved in 5 mL of DCM and cooled to 0 °C and 1,2-dimethylimidazole (52.7 mg, 548 μmol, 4.0 equiv) in 1 mL DCM was added to the reaction. Then, 26 (125.4 mg, 274 μmol, 2.0 equiv) was added and the reaction was allowed to reach room temperature over night. TLC after 24 h showed nearly full conversion of the starting material and the reaction was directly used for column chromatography (DCM/MeOH gradient from 100:0→95:5), yielding 8 (22.4 mg, 30%) and 9 (23.9 mg, 24%) as white solid. 1H NMR of 8 (200 MHz, CDCl3) δ 6.62 (dq, J=5.9, 1.4 Hz, 1H), 5.31 (dt, J=11.2, 4.4 Hz, 1H), 4.81 (s, 1H), 4.78 (s, 2H), 4.74 (d, J=5.9 Hz, 1H), 3.99 (d, J=4.5 Hz, 1H), 3.86 (d, J=11.5 Hz, 1H), 3.77 (br, 1H), 3.76 (d, J=11.5 Hz, 1H), 3.22 (d, J=4.1 Hz, 1H), 3.14 (d, J=4.1 Hz, 1H), 2.80 (dd, J=15.7, 4.3 Hz, 1H), 2.25 (dd, J=15.7, 11.2 Hz, 1H), 1.91 (br, 3H), 1.71 (br, 1H), 1.17 (s, 3H); 13C NMR of 8 (50 MHz, CDCl3) δ 199.6 (s, 1C), 138.0 (d, 1C), 136.3 (s, 1C), 92.7 (s, 1C, –CCl3 tiny signal!), 80.8 (d, 1C), 79.9 (t, 1C), 79.1 (d, 1C), 74.5 (d, 1C), 70.1 (d, 1C), 64.8 (s, 1C), 62.0 (t, 1C), 51.8 (s, 1C), 47.7 (t, 1C), 46.1 (s, 1C), 40.3 (t, 1C), 15.4 (q, 1C), 14.1 (q, 1C); HRMS m/z calcd for [M−H+]−, 504.9899, found 504.9878. 1H NMR of 9 (200 MHz, CDCl3) δ 6.69 (dq, J=5.9, 1.5 Hz, 1H), 5.32 (dt, J=11.1, 4.4 Hz, 1H), 4.88 (s, 1H), 4.81 (dt, J=5.7, 1.5 Hz, 1H), 4.79 (s, 2H), 4.66 (d, J=11.0 Hz, 1H), 4.60 (d, J=11.0 Hz, 1H), 4.48 (s, 2H), 4.04 (d, J=4.4 Hz, 1H), 3.83 (br, 1H), 3.21 (d, J=4.1 Hz, 1H), 3.17 (d, J=4.1 Hz, 1H), 2.57 (dd, J=15.7, 4.2 Hz, 1H), 2.33 (dd, J=15.7, 11.1 Hz, 1H), 1.93 (br, 3H), 1.15 (s, 3H); 13C NMR of 9 (50 MHz, CDCl3) δ 198.5 (s, 1C), 138.2 (d, 1C), 136.6 (s, 1C), 2×80.0 (d, 1C/t, 1C), 79.9 (t, 1C), 78.9 (d, 1C), 73.3 (d, 1C), 71.5 (t, 1C), 69.2 (d, 1C), 64.3 (s, 1C), 51.3 (s, 1C), 47.7 (t, 1C), 46.2 (s, 1C), 40.3 (t, 1C), 15.3 (q, 1C), 13.6 (q, 1C), 2× CCl3 between 92 and 93 ppm are missing, but the corresponding CH2 groups are located at 80.0 and 79.9; HRMS m/z calcd for [M−TCE]−, 584.9467, found 584.9505.
4.9. General deprotection procedure
The protected intermediate was dissolved in MeOH (1 mL/10 μmol starting material). HCOONH4 (3 equiv) as well as Zn dust (9 equiv) were added and the reaction was placed in an ultrasonic bath at room temperature. The reaction was followed via TLC until substantial amounts of products were formed (20–90 min). After filtration through Celite the remaining residue was subjected to column chromatography to end up with the corresponding sulfates as ammonium salts. For all acetylated DON derivatives and T2-toxin, DCM/MeOH/NH4OH=10:2.5:0.5 was used for purification. In case of DON-3- and 15-sulfate and 3,15-disulfate, a mixture of DCM/MeOH/NH4OH=10:4:1 was used. Since all products contained accompanying HCOOHNH4, we tried to purify some products via a second and third column chromatography as well as via lyophilization. Nevertheless, we obtained all desired products as a white misty veil.
4.10. 3-Acetyl-DON-15-sulfate, ammonium salt (13)
Following the general deprotection procedure, 6 (34.0 mg, 62 μmol) was converted into 13 (19.3 mg, 72%). 1H NMR (200 MHz, methanol-d4) δ 6.63 (dq, J=6.1, 1.4 Hz, 1H), 5.11 (dt, J=11.3, 4.4 Hz, 1H), 4.92 (d, J=6.1 Hz, 1H), 4.89 (s, 1H), 4,87 (br, H2O), 4.27 (d, J=11.0 Hz, 1H), 3.94 (d, J=11.1 Hz, 1H), 3.85 (d, J=4.5 Hz, 1H), 3.16 (d, J=4.3 Hz, 1H), 3.12 (d, J=4.3 Hz, 1H), 2.79 (dd, J=15.3, 4.3 Hz, 1H), 2.08 (dd, J=15.3, 11.3 Hz, 1H), 2.13 (s, 3H), 1.85 (br, 3H), 1.18 (s, 3H); 13C NMR (50 MHz, methanol-d4) δ 201.0 (s, 1C), 172.5 (s, 1C), 139.4 (d, 1C), 137.1 (s, 1C), 80.5 (d, 1C), 75.8 (d, 1C), 72.7 (d, 1C), 70.7 (d, 1C), 67.1 (t, 1C), 66.3 (s, 1C), 52.3 (s, 1C), 48.4 (t, 1C), 46.8 (s, 1C), 41.7 (t, 1C), 20.8 (q, 1C), 15.3 (q, 1C), 14.4 (q, 1C); HRMS m/z calcd for C17H21O10S− 417.0861, found 417.0834.
4.11. 15-Acetyl-DON-3-sulfate, ammonium salt (18)
Following the general deprotection procedure, 11 (13.1 mg, 24 μmol) was converted into 18 (9.2 mg, 89%). 1H NMR (400 MHz, methanol-d4) δ 6.65 (dq, J=5.9, 1.5 Hz, 1H), 4.70–5.10 (m, C3–H, C7–H, C11–H, H2O), 4.30 (d, J=12.1 Hz, 1H), 4.23 (d, J=12.1 Hz, 1H), 3.82 (d, J=4.5 Hz, 1H), 3.12 (s, 2H), 2.63 (dd, J=15.3, 4.3 Hz, 1H), 2.11 (dd, J=15.3, 11.2 Hz, 1H), 1.90 (s, 3H), 1.85 (br, 3H), 1.11 (s, 3H); 13C NMR (100 MHz, methanol-d4) δ 201.1 (s, 1C), 171.9 (s, 1C), 139.7 (d, 1C), 137.0 (s, 1C), 81.2 (d, 1C), 75.2 (d, 1C), 75.0 (d, 1C), 71.3 (d, 1C), 65.9 (s, 1C), 63.3 (t, 1C), 52.6 (s, 1C), 48.2 (t, 1C), 47.0 (s, 1C), 42.5 (t, 1C), 20.6 (q, 1C), 15.4 (q, 1C), 14.4 (q, 1C); HRMS m/z calcd for C17H21O10S− 417.0861, found 417.0824.
4.12. DON-3-sulfate, ammonium salt (15)
Following the general deprotection procedure, 8 (18.8 mg, 37 μmol) was converted into 15 (14.3 mg, 98%). 1H NMR (400 MHz, methanol-d4) δ 6.61 (dq, J=6.1, 1.4 Hz, 1H), 4.75–4.95 (m, C3–H, C11–H, H2O), 4.79 (s, 1H), 3.80 (d, J=4.4 Hz, 1H), 3.78 (d, J=12.3 Hz, 1H), 3.68 (d, J=12.3 Hz, 1H), 3.12 (d, J=4.5 Hz, 1H), 3.09 (d, J=4.5 Hz, 1H), 2.75 (dd, J=15.2, 4.4 Hz, 1H), 2.06 (dd, J=15.2, 11.4 Hz, 1H), 1.83 (br, 3H), 1.12 (s, 3H); 13C NMR (100 MHz, methanol-d4) δ 201.7 (s, 1C), 139.4 (d, 1C), 137.0 (s, 1C), 81.2 (d, 1C), 75.8 (d, 1C), 75.4 (d, 1C), 71.6 (d, 1C), 66.3 (s, 1C), 61.8 (t, 1C), 53.6 (s, 1C), 48.2 (t, 1C), 46.7 (s, 1C), 42.5 (t, 1C), 15.4 (q, 1C), 14.6 (q, 1C); HRMS m/z calcd for C15H19O9S− 375.0755, found 375.0741.
4.13. DON-15-sulfate, ammonium salt (14)
Compound 13 (12.0 mg, 28 μmol, 1.0 equiv) was dissolved in 5 mL MeOH and NaOMe (3.0 mg, 55 mmol, 2.0 equiv) was added. After stirring for 2 h, TLC indicated full conversion of the starting material and the reaction was subjected to column chromatography (DCM/MeOH/NH4OH=10:4:1), yielding 14 (7.5 mg, 69%) as white solid. 1H NMR (200 MHz, methanol-d4) δ 6.65 (dq, J=6.1, 1.4 Hz, 1H), 5.04 (d, J=6.1 Hz, 1H), 4.85–4.95 (m, C7–H, H2O), 4.76 (dt, J=11.1, 4.5 Hz, 1H), 4.25 (d, J=11.0 Hz, 1H), 3.96 (d, J=11.0 Hz, 1H), 3.55 (d, J=4.5 Hz, 1H), 3.12 (d, J=4.5 Hz, 1H), 3.06 (d, J=4.5 Hz, 1H), 2.57 (dd, J=14.8, 4.4 Hz, 1H), 1.99 (dd, J=14.8, 11.1 Hz, 1H), 1.84 (br, 3H), 1.14 (s, 3H); 13C NMR (50 MHz, methanol-d4) δ 201.1 (s, 1C), 139.9 (d, 1C), 136.9 (s, 1C), 82.3 (d, 1C), 75.9 (d, 1C), 70.7 (d, 1C), 69.6 (d, 1C), 67.2 (s, 1C), 66.7 (t, 1C), 52.5 (s, 1C), 48.2 (t, 1C), 47.5 (s, 1C), 45.0 (t, 1C), 15.3 (q, 1C), 14.4 (q, 1C); HRMS m/z calcd for C15H19O9S− 375.0755, found 375.0746.
4.14. DON-3,15-disulfate, diammonium salt (16)
Following the general deprotection procedure, twice the amount of HCOONH4 (6 equiv) and Zn dust (18 equiv) were used to convert 9 (20.0 mg, 28 μmol) into 16 (7.4 mg, 54%). 1H NMR (400 MHz, methanol-d4) δ 6.67 (dq, J=6.0, 1.5 Hz, 1H), 4.80–5.00 (m, C3–H, C7–H, C11–H, H2O), 4.24 (d, J=10.9 Hz, 1H), 3.98 (d, J=10.9 Hz, 1H), 3.84 (d, J=4.5 Hz, 1H), 3.15 (d, J=4.3 Hz, 1H), 3.10 (d, J=4.3 Hz, 1H), 2.97 (dd, J=15.3, 4.5 Hz, 1H), 2.10 (dd, J=15.3, 11.4 Hz, 1H), 1.85 (br, 3H), 1.16 (s, 3H); 13C NMR (100 MHz, methanol-d4) δ 201.3 (s, 1C), 139.6 (d, 1C), 137.2 (s, 1C), 81.3 (d, 1C), 75.8 (d, 1C), 75.7 (d, 1C), 70.5 (d, 1C), 67.4 (s, 1C), 66.1 (t, 1C), 52.4 (s, 1C), 48.4 (t, 1C), 47.1 (s, 1C), 42.4 (t, 1C), 15.3 (q, 1C), 14.5 (q, 1C); HRMS m/z calcd for 455.0323, found 455.0300.
4.15. 2,2,2-Trichloroethyl T2-toxin-3-sulfate (21)
Compound 5 (37.4 mg, 80 μmol, 1.0 equiv) was dissolved in 3 mL of DCM, cooled to 0 °C, and 1,2-dimethylimidazole (30.8 mg, 321 mmol, 4.0 equiv) in 1 mL DCM was added to the reaction. Then, 26 (73.4 mg, 160 mmol, 2.00 equiv) was added and the reaction was allowed to reach room temperature over night. After 18 h, TLC showed substantial amounts of product and the reaction was directly used for column chromatography (DCM/MeOH=95:5), yielding 21 (26.4 mg, 49%) as white solid. 1H NMR (200 MHz, CDCl3) δ 6.16 (d, J=3.1 Hz, 1H), 5.77 (dt, J=5.7, 1.4 Hz, 1H), 5.28 (d, J=5.5 Hz, 1H), 5.10 (dd, J=4.9, 3.1 Hz, 1H), 4.80 (s, 2H), 4.31 (d, J=12.7 Hz, 1H), 4.25 (d, J=5.7 Hz, 1H), 4.10 (d, J=12.7 Hz, 1H), 3.96 (d, J=4.9 Hz, 1H), 3.09 (d, J=3.9 Hz, 1H), 2.85 (d, J=3.9 Hz, 1H), 2.35 (dd, J=15.2, 6.0 Hz, 1H), 2.11 (s, 3H), 2.09 (s, 3H), 2.00–2.25 (m, 3H), 1.80 (d, J=16.6 Hz, 1H), 1.76 (s, 3H), 0.96 (d, J=6.3 Hz, 3H), 0.95 (d, J=6.3 Hz, 3H), 0.74 (s, 3H); 13C NMR (50 MHz, CDCl3) δ 172.8 (s, 1C), 170.4 (s, 1C), 170.2 (s, 1C), 137.1 (s, 1C), 123.0 (d, 1C), 92.6 (s, 1C), 87.1 (d, 1C), 80.1 (t, 1C), 78.4 (d, 1C), 77.4 (d, 1C), 67.7 (d, 1C), 67.5 (d, 1C), 64.6 (t, 1C), 63.8 (s, 1C), 48.7 and 47.5 (1t, 1s, 2× 1C), 43.7 (t, 1C), 43.1 (s, 1C), 28.4 (t, 1C), 25.9 (d, 1C), 22.6 (q, 1C), 22.5 (q, 1C), 21.2 (q, 1C), 20.8 (q, 1C), 20.4 (q, 1C), 6.5 (q, 1C); HRMS m/z calcd for C24H33O12S− [M−TCE-group]−, 545.1698, found 545.1679.
4.16. T2-toxin-3-sulfate, ammonium salt (22)
Following the general deprotection procedure, 21 (20.8 mg, 31 μmol) was converted into 22 (5.0 mg, 29%). 1H NMR (400 MHz, methanol-d4) δ 6.03 (d, J=3.1 Hz, 1H), 5.78 (dt, J=5.5 Hz, 1H), 5.33 (d, J=5.5 Hz, 1H), 4.80–4.94 (m, C3–H, H2O), 4.33 (d, J=12.1 Hz, 1H), 4.32 (d, J=5.5 Hz, 1H), 4.16 (d, J=12.1 Hz, 1H), 3.79 (d, J=5.1 Hz, 1H), 3.04 (d, J=3.9 Hz, 1H), 2.87 (d, J=3.9 Hz, 1H), 2.38 (dd, J=15.3, 5.5 Hz, 1H), 2.13–2.18 (m, 2H), 2.07 (s, 3H), 2.06 (s, 3H), 2.00–2.12 (m, 1H), 1.92 (d, J=15.3 Hz, 1H), 1.74 (s, 3H), 0.97 (d, J=6.7 Hz, 3H), 0.96 (d, J=6.7 Hz, 3H), 0.72 (s, 3H); 13C NMR (100 MHz, methanol-d4) δ 174.0 (s, 1C), 172.3 (s, 1C), 172.2 (s, 1C), 137.2 (s, 1C), 125.1 (d, 1C), 82.3 (d, 1C), 81.5 (d, 1C), 79.7 (d, 1C), 69.4 (d, 1C), 68.5 (d, 1C), 65.8 (t, 1C), 65.0 (s, 1C), 50.1 and 47.8 (1t, 1s, 2× 1C), 44.5 (t, 1C), 44.3 (s, 1C), 28.8 (t, 1C), 26.9 (d, 1C), 22.8 (q, 1C), 22.7 (q, 1C), 21.3 (q, 1C), 20.7 (q, 1C), 20.4 (q, 1C), 6.9 (q, 1C); HRMS m/z calcd for C24H33O12S− 545.1698, found 545.1682.
Acknowledgements
The graduate school program Applied Bioscience Technology of the VUT (Vienna University of Technology) in cooperation with the BOKU Vienna is gratefully acknowledged for financial support. This work was also funded by the Vienna Science and Technology Fund (WWTF LS12-021) and the Austrian Science Fund (SFB Fusarium F3702 and F3706).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplementary data
NMR spectra of all protected and isolated sulfates as well as tables for the 1H chemical shifts of all substances. The following is the Supplementary data related to this article:
Mol Files
The following ZIP file contains the MOL files of the most important compounds referred to in this article.
ZIP file containing the MOL files of the most important compounds in this article.
References and notes
- 1.Cundliffe E., Cannon M., Davies J. Proc. Natl. Acad. Sci. U.S.A. 1974;71:30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pace J.G., Watts M.R., Canterbury W.J. Toxicon. 1988;26:77–85. doi: 10.1016/0041-0101(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 3.Rotter B.A. J. Toxicol. Environ. Health. 1996;48:1–34. doi: 10.1080/009841096161447. [DOI] [PubMed] [Google Scholar]
- 4.D'Mello J.P.F., Macdonald A.M.C. Anim. Feed Sci. Technol. 1997;69:155–166. [Google Scholar]
- 5.Berthiller F., Dall'Asta C., Schuhmacher R., Lemmens M., Adam G., Krska R. J. Agric. Food Chem. 2005;53:3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- 6.Busman M., Poling S.M., Maragos C.M. Toxins. 2011;3:1554–1568. doi: 10.3390/toxins3121554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostelanska M., Hajslova J., Zachariasova M., Malachova A., Kalachova K., Poustka J., Fiala J., Scott P.M., Berthiller F., Krska R. J. Agric. Food Chem. 2009;57:3187–3194. doi: 10.1021/jf803749u. [DOI] [PubMed] [Google Scholar]
- 8.Berthiller F., Crews C., Dall'Asta C., Saeger S.D., Haesaert G., Karlovsky P., Oswald I.P., Seefelder W., Speijers G., Stroka J. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikula H., Sohr B., Skrinjar P., Weber J., Hametner C., Berthiller F., Krska R., Adam G., Fröhlich J. Tetrahedron Lett. 2013;54:3290–3293. [Google Scholar]
- 10.Mikula H., Skrinjar P., Sohr B., Ellmer D., Hametner C., Fröhlich J. Tetrahedron. 2013;69:10322–10330. [Google Scholar]
- 11.Uhlig S., Ivanova L., Fæste C.K. J. Agric. Food Chem. 2013;61:2006–2012. doi: 10.1021/jf304655d. [DOI] [PubMed] [Google Scholar]
- 12.Prelusky D.B., Veira D.M., Trenholm H.L., Foster B.C. J. Environ. Sci. Health, B. 1987;22:125–148. doi: 10.1080/03601238709372550. [DOI] [PubMed] [Google Scholar]
- 13.Wan D., Huang L., Pan Y., Wu Q., Chen D., Tao Y., Wang X., Liu Z., Li J., Wang L., Yuan Z. J. Agric. Food Chem. 2013;62:288–296. doi: 10.1021/jf4047946. [DOI] [PubMed] [Google Scholar]
- 14.Fruhmann P., Warth B., Hametner C., Berthiller F., Horkel E., Adam G., Sulyok M., Krska R., Fröhlich J. World Mycotoxin J. 2012;5:127–132. [Google Scholar]
- 15.Warth B., Sulyok M., Berthiller F., Schuhmacher R., Krska R. Toxicol. Lett. 2013;220:88–94. doi: 10.1016/j.toxlet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Warth B., Sulyok M., Fruhmann P., Berthiller F., Schuhmacher R., Hametner C., Adam G., Fröhlich J., Krska R. Toxicol. Lett. 2012;211:85–90. doi: 10.1016/j.toxlet.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Warth B., Sulyok M., Fruhmann P., Mikula H., Berthiller F., Schuhmacher R., Hametner C., Abia W.A., Adam G., Fröhlich J., Krska R. Rapid Commun. Mass Spectrom. 2012;26:1533–1540. doi: 10.1002/rcm.6255. [DOI] [PubMed] [Google Scholar]
- 18.Strahm E., Baume N., Mangin P., Saugy M., Ayotte C., Saudan C. Steroids. 2009;74:359–364. doi: 10.1016/j.steroids.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Dhakal K., He X., Lehmler H.-J., Teesch L.M., Duffel M.W., Robertson L.W. Chem. Res. Toxicol. 2012;25:2796–2804. doi: 10.1021/tx300416v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Court M.H., Duan S.X., Hesse L.M., Venkatakrishnan K., Greenblatt D.J. Anesthesiology. 2001;94:110–119. doi: 10.1097/00000542-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Simons P.J., Cockshott I.D., Douglas E.J., Gordon E.A., Hopkins K., Rowland M. Xenobiotica. 1988;18:429–440. doi: 10.3109/00498258809041679. [DOI] [PubMed] [Google Scholar]
- 22.Altpeter F., Posselt U.K. Appl. Microbiol. Biotechnol. 1994;41:384–387. [Google Scholar]
- 23.Grove J.F., McAlees A.J., Taylor A. J. Org. Chem. 1988;53:3860–3862. [Google Scholar]
- 24.Gilbert E.E. Chem. Rev. 1962;62:549–589. [Google Scholar]
- 25.Liu Y., Lien I.F.F., Ruttgaizer S., Dove P., Taylor S.D. Org. Lett. 2003;6:209–212. doi: 10.1021/ol036157o. [DOI] [PubMed] [Google Scholar]
- 26.Ingram L.J., Desoky A., Ali A.M., Taylor S.D. J. Org. Chem. 2009;74:6479–6485. doi: 10.1021/jo9014112. [DOI] [PubMed] [Google Scholar]
- 27.Shi T., Chen H., Jing L., Liu X., Sun X., Jiang R. Synth. Commun. 2011;41:2594–2600. [Google Scholar]
- 28.Blackwell B.A., Greenhalgh R., Bain A.D. J. Agric. Food Chem. 1984;32:1078–1083. [Google Scholar]
- 29.Savard M.E., Blackwell B.A., Greenhalgh R. Can. J. Chem. 1987;65:2254–2262. [Google Scholar]
- 30.Pirrung M.C. John Wiley & Sons; Hoboken, NJ, USA: 2006. The Synthetic Organic Chemist's Companion; pp. 171–172. [Google Scholar]
- 31.Fulmer G.R., Miller A.J.M., Sherden N.H., Gottlieb H.E., Nudelman A., Stoltz B.M., Bercaw J.E., Goldberg K.I. Organometallics. 2010;29:2176–2179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZIP file containing the MOL files of the most important compounds in this article.