Abstract
Inflammaging refers to a continuous, low-grade inflammation associated with aging. Such chronic inflammatory response could build up with time and gradually causes tissue damage. It is considered as one of the driving forces for many age-related diseases such as diabetes, atherosclerosis, age-related macular degeneration (AMD), and skin aging. There is mounting evidence that indicates aging is driven by the pro-inflammatory cytokines and substances produced by our body’s innate immune system. The macrophage and complement system, two important components of innate immune system, have attracted more and more attention since they appear to be involved in the pathogenesis of several inflammaging-associated diseases, such as AMD and atherosclerosis. This paper will review what we know about these two innate immune systems in the pathogenesis of AMD, atherosclerosis and skin aging.
Keywords: Age-related macular disease, atherosclerosis, complement, inflammaging, innate immune system, macrophage, skin.
INTRODUCTION
Chronic low-grade inflammation has been referred to as the “silent killer” since it induces a wide range of unforeseen internal tissue damage difficult to be diagnosed at early stage [1]. In the western world alone, at least 5-7% of the population is impacted by immune-mediated inflammatory diseases [2] and the prevalence of inflammatory conditions has steadily been on the rise [3].
The term “inflammaging” was coined more than a decade ago [4] and since then, numerous research studies provided convincing evidence to support this concept. Inflammaging is the low-grade, asymptomatic, chronic inflammation which occurs during physiological aging and is recognized as a pathogenic factor in the development of several age-associated diseases, such as atherosclerosis [5], diabetes [6], and Alzheimer’s disease [7]. Studies reveal that innate immunity is a major player in the inflammaging process [6]. Mononuclear phagocytes such as macrophages play pivotal role in innate immunity and its mediated inflammation is implicated in several aging related diseases, such as atherosclerosis [8], cancers [9] and age-related macular degeneration (AMD) [10]. The complement system is an old innate immune component which is now recognized to be the central player in innate immunity. The complement system has long been linked to AMD [11]. Lately, its role in atherosclerosis is also becoming evident [12]. Recently, skin aging has also been associated with inflammation and innate immunity [13]. Although complement dysregulation is found in various skin diseases [14], its role in skin aging is not yet clear. This review will summarize the role of both macrophage and complement system in the pathogenesis of AMD and atherosclerosis and also the impact on skin aging including formation of solar lentigo (or age spots).
COMPLEMENT SYSTEM
The complement system was first thought to play, as its name implies, a complementary role in the body immune system. However, recently it is recognized as a central player of the innate immune system [15]. It not only defends the host against pathogen infection, but also coordinates various events during inflammation and bridges innate and adaptive immune response [16].
There are three complement pathways: the classic pathway, the lectin pathway and the alternative pathway. All three pathways converge at the formation of the C3 convertase which cleaves C3, and trigger a cascade of events leading to the formation of a membrane attack complex (MAC), which destroy pathogens or damaged “self” cell by opsonisation and lytic destruction [16].
The classic pathway is activated by antibody binding to the pathogen; the lectin pathway is activated by polysaccharides on microbial surfaces; The alternative pathway is unique due to the fact that it is in a continuous low state of activation (‘tickover’) characterized by the spontaneous hydrolysis of C3 into C3a and C3b fragments. The alternative pathway therefore serves as a “surveillance mechanism”, killing invading pathogens and clearing damaged “self” cells when they first appear [16]. Due to these activities, the alternative pathway is most likely to be the key player in inflammageing.
AGE-RELATED MACULAR DEGENERATION (AMD)
Age-related macular degeneration is a medical condition affecting the human macula. It is the leading cause of blindness in people over 50 years of age [17]. The risk factors for AMD include obesity, smoking, light exposure, and fat in-take. AMD, like atherosclerosis, is associated with aging and inflammation [18].
In aging retina, it is said that oxidized lipoproteins and free radicals trigger the para-inflammation. The para-inflammation in the aging retina is manifested in many ways. At the molecular level, upregulation of numerous genes associated with inflammation and immune response, such as inflammatory cytokines and chemokines, complement system components, and proinflammatory enzymes, were found using both microarray [19] and proteomic studies [20]. At the tissue level, microglial activation and subretinal migration are seen in the neuroretina; complement activation is seen in Bruch’s membrane and RPE cells; increased thickness of choroid, increased number of CD45+CRIg+ macrophages, abnormal morphology of choroidal melanocytes, and fibrosis are seen in the choroidal tissue [19]. It appears that in AMD, many of these changes are exacerbated.
A hallmark of early stage of AMD is the formation of drusen which are yellow or white extracellular deposits that build up between the retinal pigment epithelium (RPE) and Bruch’s membrane, an extracellular matrix complex that separates the neural retina from the capillary network in the choroid [21, 22]. While a few small hard drusen can be found in most people over 40 years old, the large and numerous hard drusen are commonly found in AMD patients [22].
The composition of the drusen includes cell debris, lipid, lipoprotein and complement components, among others [21, 23]. The lipid accumulation in Bruch’s membrane is universally observed during retinal aging. However, there is a difference in composition of the lipids between the macular and peripheral region [24]. In macular Bruch’s membrane, there is a significantly higher amount of esterified cholesterol than in peripheral Bruch’s membrane [25]. This may explain why AMD affects the macula but not the periphery. It is now believed the lipid accumulated in Bruch’s membrane come from RPE [26]. This is supported by finding that RPE grown in vitro deposits basal debris that react with complement. If this reflects the normal physiological process in vivo, it is likely that in AMD, the debris clearance process deteriorates over time and the complement system is over activated.
Complement in AMD
Abundant studies show that a large number of complement and its related proteins are molecular constituents of drusen [11, 23, 27, 28]. During the past decade, compelling evidence indicates that complement plays an essential role in the biogenesis of drusen and pathogenesis of AMD. This also leads to the recognition that AMD is a disease of inflammaging.
Numerous genome wide association studies found strong correlation of AMD with sequence variants of genes encoding for molecules involved in the activities of the complement pathways, especially those of the alternative pathway [11]. They include complement factor H (CFH) [29], complement factor B [30], C3 [31] and C2 [32]. The common or rare polymorphisms in these genes can either increase or reduce the risk for AMD late in life.
Whitcup et al. [33] pointed out that in general, protective variants result in less alternative pathway activity, whereas risk variants result in more alternative pathway activity. For example, a CFH polymorphism, which resulted in an amino acid substitution of histidine for tyrosine (Y402H), is strongly associated with the risk of AMD [29, 34, 35]. CFH functions as a complement inhibitor which blocks alternative pathway in response to tissue injury. CFH itself shows increased expression in the retina during aging [36]. Complement components seem to increase during aging. The production of CFB increases with age accompanied by complement activation [37]. Increased C3 and C3d depositions have been shown in several retinal layers of aged mice [37]. Interestingly, systemic increases in complement activation have been observed in patients with AMD [38]. Therefore, it can be postulated that during aging, complement system gets activated and the upregulation of CFH is probably the body’s attempt to tame the overactivated complement system. Moreover, complement dysregulation is likely an inflammatory driving force in AMD.
It was once thought complement proteins are synthesized solely in the liver and released into circulation. It was recently shown that the complement system exists locally in the retina-choroid complex [39, 40]. In fact, qPCR studies indicate the cells in the human RPE-choroid complex express a complete set of transcripts of classic and alternative pathways [11]. More importantly, Chen et al. [37] have shown there is a constitutive low level of complement activation continually in process at the retinal/choroid interface and the level of the complement activation at this site increases with age.
Another feature of aged RPE is accumulation of lipofuscin, the byproducts of photoreceptor outer segment turnover. Lipofuscin typically appears in RPE after age 40. It has been shown that one of main lipofuscin fluorophores, A2E, is able to induce activation of the complement system in vitro [41, 42], providing a link to AMD pathogenesis.
Macrophages and Microglia
Beside complement system, macrophage seems to play an important role in AMD pathogenesis. Much like the presence of macrophages at atherosclerosis sites, macrophages are found at the sites of RPE atrophy, breakdown of Bruch’s membrane, and choroidal neovascularization [43, 44]. With the lifelong accumulation of cell damage and oxidative stress, macrophages may be overburdened, much like the overload of lipids in foam cells in atherosclerosis. In addition, the macrophage itself is undergoing aging and loses the phagocytosis capacity, which is evidenced by less MHC class II expression [45]. On the other hand, increased number of macrophages in choroid expresses complement receptor CRIg [19], which is involved complement-mediated phagocytosis [46]. This may reflect the increased apoptotic cells in aged retina and the cell’s attempt to clear them with the complement system. Cao et al. [47] also show that with aging, there is a shift of M1 macrophage to M2 macrophage. However, in AMD patient, there is a reverse shift of macrophages to a younger phenotype. Macrophage with M1 phenotypes are able to engulf and digest damaged cells. They produce pro-inflammatory factors and generate reactive oxygen species (ROS), which is also similar to foam cells in atherosclerosis (see the atherosclerosis section below).
Microglia are residential macrophages of the central nervous system that function to maintain homeostasis of the tissue with nervous system components. During aging, there is an accumulation of microglia at subretinal region [19]. The accumulation of microglia has been suggested to be a result of accumulation of lipofuscin constituent A2E within microglia. Lipofuscin is capable of reduce microglial migratory capabilities, increased deposition of CFB, and reduce expression of CFH [48]. As with aged macrophages in retina, the microglia show diminished function upon aging, evidenced by reduced size of dendritic arbors, slow to migrate to site of tissue stress and slow to leave the site afterwards [19]. Therefore, the accumulation of microglia can be the result of dysfunctional microglia overburdened with lifelong accumulation of debris in the aged eye.
Based on above findings, we would like to propose a hypothesis on how complement and macrophages drive AMD. The alternative pathway of complement system is activated by either modified lipids, LDL or damaged cells, along with the activation of macrophages and resident microglia. Inflammation is triggered. If the complement activation is not tamed, either due to the mutation of the complement inhibitors, such as CFH, or the overwhelming high LDL due to high fat diet, the parainflammation will become more severe chronic inflammation and disease ensues. In addition, with the lifelong accumulation of the cell damage and oxidative stress, complement system maybe overactivated and macrophage/microglia may be overburdened, much like the overload of lipids in foam cells in atherosclerosis. These will cause further damage to the retina.
ATHEROSCLEROSIS
Atherosclerosis is now known to be caused by chronic inflammation [49]. It starts with lipid infiltrating the artery wall of the blood vessel. Lipids retained in the vessel wall (intima) undergo oxidative modifications and become oxidized LDL. The modified LDL particles induce expression of the leukocyte adhesion molecules VCAM-1 and ICAM-1, which help monocyte enter the vessel wall. The monocytes will differentiate into macrophages and take up the oxidized lipids. Lipid-filled macrophages are called foam cells, which is the hallmark of atherosclerosis. The dysregulated lipid metabolism in the foam cells alters macrophage phenotype and makes them dysfunctional. They release ROS and a variety of pro-inflammatory cytokines and chemokines, therefore enhancing the local inflammatory response within the arterial wall and hence the chronic inflammation.
Recent evidence suggests the complement system, like in AMD, plays a crucial role in the pathogenesis of atherosclerosis. As with AMD, increased C3 deposition is found in the intima of human atherosclerotic lesions as compared to normal vessel intima [50]. In addition, the C5b-9 is found in the atherosclerotic plaque from the earliest to advanced lesions [51], indicating full complement activation exists in the lesion and suggesting an active contribution of complement to intravascular inflammation and atherosclerosis. The finding that increased expression of complement mRNA in atherosclerotic plaque compared with normal tissue [52] indicates there is localized complement synthesis, like in the aging retina.
Complement polymorphism is also found to be associated with atherosclerosis. Hoke et al. [53] found that C5 polymorphism rs17611 GG genotype is a risk factor for adverse cardiovascular outcome with atherosclerosis. Boiocchi et al. [54] demonstrated that complement receptor I (CR1) gene polymorphisms may be involved in the predisposition to the development of the disease. More interestingly, the same CFH Y402H polymorphism that is associated with the risk of AMD, is found to be associated with increased incidence of atherosclerosis in hypertensive white individuals [55].
Complement’s influences on processes involved in the development and progression of atherosclerosis are also evidenced by numerous in vitro studies. For example, endothelial cells can be induced by C5a and C5b-9 to release cellular adhesion molecules including P-selectin, E-selectin, ICAM-1, VCAM-1, all of which contribute to endothelial cell activation and promotion of leukocyte infiltration into the vessel wall [56].
So what causes the activation of the complement system in atherosclerosis? It has long been known that alternative complement pathway can be activated by lipids components isolated from atherosclerosis lesion [57]. Seifert et al. [57] isolated C5b-9 complement complex from early adult human atherosclerotic lesion along with a lipoprotein derivative that had complement-activating properties. Subsequent analyses of this lipoprotein derivative showed it to be enzymatically modified LDL (eLDL) with potent complement activating capacity [58]. This is further supported by the immunohistochemistry study showing co-localization of eLDL and terminal complement complex in the early atherosclerosis lesion [51]. These studies indicate that complement system plays a role in the earliest stages of atherogenesis.
Based on the above findings, a new hypothesis with regard to the atherogenesis was formed recently [59]. According to this hypothesis, the insudation of LDL into the vessel wall is efficiently removed either by HDL-dependent reverse transport pathway or by macrophages. However, under hypercholesterolemic conditions, these two removal systems are overburdened, resulting in excessive tissue-stranded LDL with subsequent enzymatic modification. This triggers the complement system and different subsets of monocyte-derived macrophages. However, we want to point out that this hypothesis does not take into account aging, which is one of the main factor that is closely associated with atherosclerosis. It is known that during aging, oxidative stress accumulates and more damaged cells with peroxidized phospholipids occur. This leads to the formation of oxidation-specific epitopes or OSE, which are present on oxidized LDL, apoptotic cells, cell debris and modified proteins in the vessel wall [60]. In fact, the OSE can be recognized by innate immune system including macrophages and complement factors, leading to cytokine, chemokine, and adhesion molecule synthesis [61]. This is likely how aging plays a role in atherogenesis.
INFLAMMAGING IN SKIN
With skin, there is intrinsic (or chronological) aging and extrinsic UV-induced aging (or photoaging). Intrinsic aging is accompanied by cell loss, thinning of epidermis, flattening of DEJ (dermal-epidermal junction) and fine lines of wrinkles. Photoaged skin is coarsely wrinkled and associated with dyspigmentation [62, 63]. Inflammation and accumulation of ROS are now believed to be the causative factors in both types of skin aging [64, 65]. Several global gene expression profiling studies all linked immune system and inflammation genes with photoaging, regardless of ethnic type [66-68].
UV-induced photoaging can be viewed as premature skin aging. UV induces an array of events that can lead to inflammation: 1) UV radiation can induce the epidermal keratinocytes to release inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-a) [69], 2) UV can induce ROS generation and is known to reduce cellular anti-oxidant mechanism [70], 3) UV can induce mast cells to generate prostaglandins and other inflammatory mediators such as histamine and leucotrines [71], 4) skin cell death [72], and 5) peroxidation of the membrane lipids [73].
Acute UV exposure results in the infiltration of the neutrophils within the epidermis and dermis [74]. The activation of neutrophils is used for clearance of UV-induced apoptotic cells, and to kill skin cells with oxidized surface lipids. It has been suggested that the enzymes they release, including neutrophil elastase, matrix metalloproteinase-1 (MMP-1) and matrix metalloproteinase-9 (MMP-9) contri-bute to the process of photoaging [74]. It is likely mono-cytes/macrophages actually play more important role in this process because they dominate the infiltrates after a few hours to clear up apoptotic cells and oxidized lipids [75].
There are two sources of macrophages; one from the blood stream (through recruitment) and expansion of the pre-existing monocyte precursors in the dermis [76]. Macrophages express various matrix metalloproteinases [77], which can help them migrate within the skin by degrading ECM. Macrophages also generate ROS [78], which can induce the transcription of matrix metalloprotei-nase (MMP) in dermal fibroblasts [70]. Repeated UV damage to the skin results in repeated cycles of macrophage infiltration after each exposure. The repeated macrophage infiltration will cause repeated damage to the ECM of the dermis due to MMP and ROS release. This is dampened in aged skin, where both the number and function of fibroblasts decrease and therefore, can-not efficiently repair or regenerate ECM. This is evident in histological studies, which show chronically sun-exposed skin contain more infiltrating mononuclear cells [79] and abnormal elastic fibers in the dermis than sun-protected skin [80]. The known key inflammatory mediators during skin inflammaging are summarized in Table 1.
Table 1.
Known Key Inflammatory Factors and their Role in Skin Aging
| (Pro-) Inflammatory Factors | Pathway and Potential Skin Damage | References |
|---|---|---|
| Reactive oxygen species (ROS) | Cause skin cell damage; generate oxidized lipids; induce MMP expression in dermal fibroblast | [62, 78, 81, 82] |
| TNF-a, IL-1 (“Primary” pro-inflammatory cytokines) | Initiate inflammatory responses in skin; induce the synthesis and release of other pro-inflammatory cytokines | [69, 83- 86] |
| IL-6, IL-8 etc. (Other pro-inflammatory cytokines) | Recruit neutrophils and macrophages; activate dermal fibroblasts to secret MMPs | [81, 87] |
| Neutrophils | Release elastase and MMPs that cause ECM degradation | [74, 81, 88] |
| Matrix metalloproteinases (MMPs) | Cause ECM degradation, thus cause damage to dermis connective tissue and skin aging | [80, 81] |
| Complement system | Activate macrophage; induced by UV and deposits on dermal-epidermal junction | [89, 90] |
| Macrophages | Infiltrate skin after UV-exposure; generate ROS and MMPs that cause ECM degradation | [89, 91, 92] |
Complement in Skin
Complement is known to be expressed in a variety of skin cells, such as mast cells [93], macrophages [94], keratinocytes [95] and fibroblasts [96]. This suggests skin has its own complement synthesis system, like retina and vessel wall (see above). UV induces complement synthesis in skin [90, 97]. Epidermal keratinocytes express and produce C3, CFB and UV irradiation induced C3 production in keratinocytes possibly through alternative pathway [97]. It is possible that UV-induce oxPL (oxidized phospholipid) can be recognized by also UV-induced complement, leading to inflammation, as in atherosclerosis. As mentioned above, the complement pathway can be activated by oxidation-specific epitopes or damaged cells, which are known to be generated by UV. Indeed, the activation of complement C3 into iC3b ligates the monocytes’ beta2-integrin receptors, leading to the differentiation of CD11b+ monocytic cells into activated macrophages with induced MMPs and ROS-generating activity [89]. Complement iC3b is found to be deposited onto DEJ region after UV exposure, and macrophages are observed to be in physical opposition with the fine granular deposits of C3 along the DEJ. The macrophages infiltrated into epidermis region after UV exposure are found next to damaged keratinotyes which are C3 positive [90]. Therefore, the complement-damaged cell complex formation seems to serve as a signal to activate and recruit macrophages. In addition, the activated macrophage can produce ROS and exacerbate the oxidative damage.
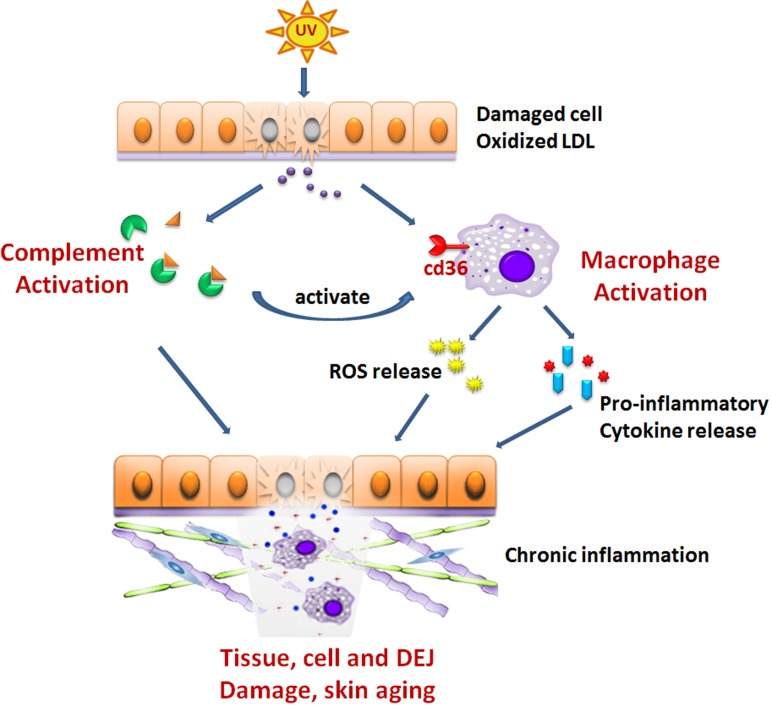
It is tempting to postulate a similar scenario like the events that occur in the pathogenesis of atherosclerosis and AMD, which is described as follows. UV exposure induces oxidative stress, leading to accumulation of the damaged cell and OSEs. They are recognized by complement system, which will trigger inflammation, leading to the macrophage infiltration. Macrophages release MMPs, which can degrade extracellular matrix (ECM). With repeated UV insult and build-up of wastes, the complement system is over-activated and macrophages are overburdened with oxidized lipids with its function compromised. Overactive complement system can cause the damage of DEJ, where they deposit. Much like the case in AMD and atherosclerosis, overburdened macrophages release ROS and pro-inflammatory cytokines and cause further damage to the skin dermis. In addition, aged macrophage may lose their migration ability. This may lead to the retention of the macrophages at the dermis and therefore exacerbate the damage. Schematic description is shown in Fig. (1). It would be important to determine if an increased number of pro-inflammatory macrophages are present in photo-aged skin. It will be also interesting to see if activation of alternative complement pathway or polymorphism of complement components occurs during skin aging.
Fig. (1).
Complement and macrophage in UV-induced skin inflammaging. UV exposure induces oxidative stress, leading to accumulation of the damaged cell and oxidized lipids. They are recognized by complement system, which will trigger inflammation, leading to the macrophage infiltration. Macrophage release MMPs, which can degrade extracellular matrix (ECM). With the repeated UV insult and the build-up of these wastes, the complement system is over-activated and macrophages are overburdened with oxidized lipids with its function compromised. Over-active complement can cause the damage of DEJ, where they deposit. Overburdened macrophages release ROS and pro-inflammatory cytokines and cause chronic inflammation and further damage to the skin dermis.
Age Spot
Another type of skin disorder associated with UV is senile lentigo or age spot [98]. Age spots are hyperpigmented macules with irregular shape on the skin. They are associated with exposure of UV since they mostly occur on UV-exposed areas such as forearm, back of the hand and face. They are also associated with age since they only occur after age of 40 [99]. Histological studies show that, compared to peri-lesion area, they show hyperpigmented basal layer, increased amount of melanocytes and melanosomal protein, elongated rete ridges with increased melanin at the tips, altered keratinocyte differentiation and increased inflammatory response [100]. In the dermis, increased melanophages is observed. This suggests damaged melanocytes probably also due to life-long oxidative stress. This also suggests that macrophages play an important role in age-spot formation. It has been shown lately that macrophages are necessary for UV-induced melanocyte proliferation in the basal layer of epidermis [92], confirming that the innate immune system can affect melanocyte function.
Aoki et al. [101] conducted a gene profiling study and found that in addition to melanocyte-related genes, genes related to inflammation and fatty acid metabolism are also induced. They also found fewer cycling epidermal cells in the lesional skin of solar lentigo. This is consistent with the finding that inflammation can deplete epidermal stem cells [102]. Since age spot is an inflammation and age-related disease, the complement system is very likely to play a role in its pathogenesis. This can be supported by the observation that the DEJ in lesional skin is severely abnormal [100] and complement deposits onto DEJ after UV exposure [90]. Therefore, an over-activated complement system is likely to cause damage to DEJ. The composition of the age spot needs to be examined to determine whether complement and lipoproteins are the constituent of the age spot.
CONCLUSION
Age-associated low grade inflammation (inflammaging) is now recognized to be the driving force of many age-associated diseases. The research of the past decade has greatly improved our understanding on its mechanism. While the studies of complement and macrophage with regard to their role in AMD and atherosclerosis are abundant, the studies on the roles they play in skin aging and aging-related skin disease such as solar lentigo are much fewer. Both clinicians and researchers studying skin inflammaging should consider to pay more attention to these two important players. The learnings from research on AMD, atherosclerosis and other diseases can help us advance our understandings of inflammaging in skin. On the other hand, we also need to recognize the fundamental differences between skin and other tissues of the body since skin is a unique and dynamic organ, with highly active immune capability and constantly being challenged by UV and oxidative stress. Understanding the mechanism as to how complement and macrophage drive the skin aging and aging-related skin diseases will help provide new paths for dermatologist and researchers to develop better therapeutics for these conditions.
ACKNOWLEDGEMENTS
We thank Dr. Leopoldo Luistro for his careful and critical reading of the paper.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Gorman C, Park A, Dell K. The fires within. Time: 2004 [Google Scholar]
- 2.Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr. Biol. 2012;22 (17):R733–740. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Gabalawy H, Guenther L C, Bernstein C N. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J. Rheumatol. Suppl. 2010;85:2–10. doi: 10.3899/jrheum.091461. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging.An evolutionary perspective on immunosenescence. . Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Okamoto Y, Rocha V Z, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ. J. 2010;74 (2):213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 6.Goto M. Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci. Tends. 2008;2 (6):218–230. [PubMed] [Google Scholar]
- 7.Morales I, Farias G, Maccioni R B. Neuroimmunomodulation in the pathogenesis of Alzheimer's disease. Neuroimmunomodultion. 2010;17 (3):202–204. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- 8.Moore K J, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145 (3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura Y N, Hamano S, Sonoda K H, Saijo Y, Nukiwa T, Strieter R M, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J. Clin. Invest. 2005;115 (11):2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apte R S, Richter J, Herndon J, Ferguson T A. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3 (8):e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson D H, Radeke M J, Gallo N B, Chapin E A, Johnson P T, Curletti C R, Hancox L S, Hu J, Ebright J N, Malek G, Hauser M A, Rickman C B, Bok D, Hageman G S, Johnson L V. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye. Res. 2010;29 (2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torzewski M, Bhakdi S. Complement and atherosclerosis-united to the point of no return?. Clin. Biochem. 2013;46 (1-2):20–25. doi: 10.1016/j.clinbiochem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M F, Robinson M K, Baron E D, Cooper K D. Skin immune systems and inflammation: protector of the skin or promoter of aging?. J. Invest. Dermatol. Symp. Proc. 2008;13 (1):15–19. doi: 10.1038/jidsymp.2008.3. [DOI] [PubMed] [Google Scholar]
- 14.Kotnik V. Complement in skin diseases. Acta. Dermatovenerol. Alp. Panonica. Adriat. 2011;20 (1):3–11. [PubMed] [Google Scholar]
- 15.Qu H, Ricklin D, Bai H, Chen H, Reis E S, Maciejewski M, Tzekou A, DeAngelis R A, Resuello R R, Lupu F, Barlow P N, Lambris J D. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218 (4):496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markiewski M M, Lambris J D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007;171 (3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman D S, O'Colmain B J, Munoz B, Tomany S C, McCarty C, de Jong P T, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research, G.Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122 (4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 18.Lim L S, Mitchell P, Seddon J M, Holz F G, Wong T Y. Age-related macular degeneration. Lancet. 2012;379 (9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Chen M, Forrester J V. Para-inflammation in the aging retina. Prog. Retin. Eye. Res. 2009;28 (5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Lin T, Walker G B, Kurji K, Fang E, Law G, Prasad S S, Kojic L, Cao S, White V, Cui J Z, Matsubara J A. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62 (3):369–381. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins R F, Russell S R, Anderson D H, Hageman G S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14 (7):835–846. [PubMed] [Google Scholar]
- 22.Anderson D H, Mullins R F, Hageman G S, Johnson L V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002;134 (3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 23.Crabb J W, Miyagi M, Gu X, Shadrach K, West K A, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn M E, Salomon R G, Hollyfield J G. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2002;99 (23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulcan H G, Alvarez R A, Maude M B, Anderson R E. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Invest. Ophthalmol. Vis. Sci. 1993;34 (11):3187–3193. [PubMed] [Google Scholar]
- 25.Curcio C A, Millican C L, Bailey T, Kruth H S. Accumulation of cholesterol with age in human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 2001;42 (1):265–274. [PubMed] [Google Scholar]
- 26.Curcio C A, Johnson M, Rudolf M, Huang J D. The oil spill in ageing Bruch membrane. Br. J. Ophthalmol. 2011;95 (12):1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson L V, Leitner W P, Staples M K, Anderson D H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye. Res. 2001;73 (6):887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 28.Johnson P T, Betts K E, Radeke M J, Hageman G S, Anderson D H, Johnson L V. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc. Natl. Acad. Sci. U. S. A. 2006;103 (46):17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R J, Zeiss C, Chew E Y, Tsai J Y, Sackler R S, Haynes C, Henning A K, SanGiovanni J P, Mane S M, Mayne S T, Bracken M B, Ferris F L, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308 (5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer K L, Hauser M A, Olson L M, Schmidt S, Scott W K, Gallins P, Agarwal A, Postel E A, Pericak-Vance M A, Haines J L. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum. Mol. Genet. 2007;16 (16):1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 31.Despriet D D, van Duijn C M, Oostra B A, Uitterlinden A G, Hofman A, Wright A F, ten Brink J B, Bakker A, de Jong P T, Vingerling J R, Bergen A A, Klaver C C. Complement component C3 and risk of age-related macular degeneration. Ophthalmology. 2009;116 (3):474–480 e472. doi: 10.1016/j.ophtha.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 32.Gold B, Merriam J E, Zernant J, Hancox L S, Taiber A J, Gehrs K, Cramer K, Neel J, Bergeron J, Barile G R, Smith R T, Group A M D G C S, Hageman G S, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38 (4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitcup S M, Sodhi A, Atkinson J P, Holers V M, Sinha D, Rohrer B, Dick A D. The role of the immune response in age-related macular degeneration. Int. J. Inflam. 2013;2013:348092. doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haines J L, Hauser M A, Schmidt S, Scott W K, Olson L M, Gallins P, Spencer K L, Kwan S Y, Noureddine M, Gilbert J R, Schnetz-Boutaud N, Agarwal A, Postel E A, Pericak-Vance M A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308 (5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 35.Edwards A O, Ritter R, 3rd; Abel K J, Manning A, Panhuysen C, Farrer L A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308 (5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 36.Mandal M N, Ayyagari R. Complement factor H: spatial and temporal expression and localization in the eye. Invest. Ophthalmol. Vis. Sci. 2006;47 (9):4091–4097. doi: 10.1167/iovs.05-1655. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Muckersie E, Robertson M, Forrester J V, Xu H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp. Eye. Res. 2008;87 (6):543–550. doi: 10.1016/j.exer.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Scholl H P, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Borncke F, Fritsche L G, Chong N V, Fimmers R, Wienker T, Holz F G, Weber B H, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3 (7):e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Forrester J V, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp. Eye. Res. 2007;84 (4):635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y H, He S, Kase S, Kitamura M, Ryan S J, Hinton D R. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes. Arch. Clin. Exp. Ophthalmol. 2009;247 (5):651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparrow J R. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv. Exp. Med. Biol. 2010;703:63–74. doi: 10.1007/978-1-4419-5635-4_5. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Jang Y P, Kim S R, Sparrow J R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 2006;103 (44):16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman H R, Chan C C, Ferris F L, 3rd; Chew E Y. Age-related macular degeneration. Lancet. 2008;372 (9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dastgheib K, Green W R. Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch. Ophthalmol. 1994;112 (6):813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- 45.Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor A F, Lloberas J, Celada A. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp. Gerontol. 2002;37 (2-3):389–394. doi: 10.1016/s0531-5565(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 46.Gorgani N N, He J Q, Katschke K J, Jr., Helmy K Y, Xi H, Steffek M, Hass P E, van Lookeren Campagne M. Complement receptor of the Ig superfamily enhances complement-mediated phagocytosis in a subpopulation of tissue resident macrophages. J. Immunol. 2008;181 (11):7902–7908. doi: 10.4049/jimmunol.181.11.7902. [DOI] [PubMed] [Google Scholar]
- 47.Cao X, Shen D, Patel M M, Tuo J, Johnson T M, Olsen T W, Chan C. C.Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol. Int. 2011;61 (9):528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma W, Coon S, Zhao L, Fariss R N, Wong W T. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol. Aging. 2013;34 (3):943–960. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340 (2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 50.Meuwissen M, van der Wal A C, Niessen H W, Koch K T, de Winter R J, van der Loos C M, Rittersma S Z, Chamuleau S A, Tijssen J G, Becker A E, Piek J J. Colocalisation of intraplaque C reactive protein, complement, oxidised low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J. Clin. Pathol. 2006;59 (2):196–201. doi: 10.1136/jcp.2005.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, Gabbert H E, Bhakdi S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler. Thromb. Vasc. Biol. 1998;18 (3):369–378. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
- 52.Yasojima K, Schwab C, McGeer E G, McGeer P L. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am. J. Pathol. 2001;158 (3):1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoke M, Speidl W, Schillinger M, Minar E, Zehetmayer S, Schonherr M, Wagner O, Mannhalter C. Polymorphism of the complement 5 gene and cardiovascular outcome in patients with atherosclerosis. Eur. J. Clin. Invest. 2012;42 (9):921–926. doi: 10.1111/j.1365-2362.2012.02669.x. [DOI] [PubMed] [Google Scholar]
- 54.Boiocchi C, Zorzetto M, Sbarsi I, Pirotta A, Schirinzi S, Falcone C, Cuccia M. CR1 genotype and haplotype involvement in coronary artery disease: the pivotal role of hypertension and dyslipidemia. Int. J. Mol. Med. 2009;24 (2):181–187. doi: 10.3892/ijmm_00000221. [DOI] [PubMed] [Google Scholar]
- 55.Volcik K A, Ballantyne C M, Braun M C, Coresh J, Mosley T H, Boerwinkle E. Association of the complement factor H Y402H polymorphism with cardiovascular disease is dependent upon hypertension status: The ARIC study. Am. J. Hypertens. 2008;21 (5):533–538. doi: 10.1038/ajh.2007.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tedesco F, Fischetti F, Pausa M, Dobrina A, Sim R B, Daha M R. Complement-endothelial cell interactions: pathophysiological implications. Mol. Immunol. 1999;36 (4-5):261–268. doi: 10.1016/s0161-5890(99)90054-8. [DOI] [PubMed] [Google Scholar]
- 57.Seifert P S, Hugo F, Tranum-Jensen J, Zahringer U, Muhly M, Bhakdi S. Isolation and characterization of a complement-activating lipid extracted from human atherosclerotic lesions. J. Exp. Med. 1990;172 (2):547–557. doi: 10.1084/jem.172.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhakdi S, Dorweiler B, Kirchmann R, Torzewski J, Weise E, Tranum-Jensen J, Walev I, Wieland E. On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J. Exp. Med. 1995;182 (6):1959–1971. doi: 10.1084/jem.182.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhakdi S, Lackner K J, Han S R, Torzewski M, Husmann M. Beyond cholesterol: the enigma of atherosclerosis revisited. Thromb. Haemost. 2004;91 (4):639–645. doi: 10.1160/TH03-12-0733. [DOI] [PubMed] [Google Scholar]
- 60.Leibundgut G, Witztum J L, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr. Opin. Pharmacol. 2013;13 (2):168–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12(8):1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138 (11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 63.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging state of the art. Ann N.Y. Acad Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 64.Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun HC, Chung JH. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J. Lipid Res. 2006;47(5):921–930. doi: 10.1194/jlr.M500420-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Polte T, Tyrrell RM. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic. Biol. Med. 2004;36 (12):1566–1574. doi: 10.1016/j.freeradbiomed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Robinson M K, Binder R L, Griffiths C E. Genomic-driven insights into changes in aging skin. J. Drugs Dermatol. 2009;8 (7 Suppl):s8–11. [PubMed] [Google Scholar]
- 67.Urschitz J, Iobst S, Urban Z, Granda C, Souza K A, Lupp C, Schilling K, Scott I, Csiszar K, Boyd C D. A serial analysis of gene expression in sun-damaged human skin. J. Invest. Dermatol. 2002;119 (1):3–13. doi: 10.1046/j.1523-1747.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 68.Yan W, Zhang L L, Yan L, Zhang F, Yin N B, Lin H B, Huang C Y, Wang L, Yu J, Wang D M, Zhao Z M. Transcriptome analysis of skin photoaging in chinese females reveals the involvement of skin homeostasis and metabolic changes. PLoS One. 2013;8 (4):e61946. doi: 10.1371/journal.pone.0061946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood L C, Elias P M, Calhoun C, Tsai J C, Grunfeld C, Feingold K R. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J. Invest. Dermatol. 1996;106 (3):397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- 70.Kawaguchi Y, Tanaka H, Okada T, Konishi H, Takahashi M, Ito M, Asai J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1996;288 (1):39–44. doi: 10.1007/BF02505041. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez S, Pathak M A. Inhibition of ultraviolet-induced formation of reactive oxygen species, lipid peroxidation, erythema and skin photosensitization by polypodium leucotomos. Photodermatol. Photoimmunol. Photomed. 1996;12 (2):45–56. doi: 10.1111/j.1600-0781.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 72.Bielenberg D R, Bucana C D, Sanchez R, Donawho C K, Kripke M L, Fidler I J. Molecular regulation of UVB-induced cutaneous angiogenesis. J. Invest. Dermatol. 1998;111 (5):864–872. doi: 10.1046/j.1523-1747.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 73.Hruza L L, Pentland A P. Mechanisms of UV-induced inflammation. J. Invest. Dermatol. 1993;100 (1):35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 74.Rijken F, Kiekens R C, Bruijnzeel P L. Skin-infiltrating neutrophils following exposure to solar-simulated radiation could play an important role in photoageing of human skin. Br. J. Dermatol. 2005;152 (2):321–328. doi: 10.1111/j.1365-2133.2004.06335.x. [DOI] [PubMed] [Google Scholar]
- 75.Kang K, Gilliam A C, Chen G, Tootell E, Cooper K D. In human skin, UVB initiates early induction of IL-10 over IL-12 preferentially in the expanding dermal monocytic/macrophagic population. J. Invest. Dermatol. 1998;111 (1):31–38. doi: 10.1046/j.1523-1747.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 76.Meunier L, Bata-Csorgo Z, Cooper K D. In human dermis, ultraviolet radiation induces expansion of a CD36+ CD11b+ CD1- macrophage subset by infiltration and proliferation; CD1+ Langerhans-like dendritic antigen-presenting cells are concomitantly depleted. J. Invest. Dermatol. 1995;105 (6):782–788. doi: 10.1111/1523-1747.ep12326032. [DOI] [PubMed] [Google Scholar]
- 77.Goetzl E J, Banda M J, Leppert D. Matrix metalloproteinases in immunity. J. Immunol. 1996;156 (1):1–4. [PubMed] [Google Scholar]
- 78.Katiyar S K, Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001;69 (5):719–726. [PubMed] [Google Scholar]
- 79.Bosset S, Bonnet-Duquennoy M, Barre P, Chalon A, Kurfurst R, Bonte F, Schnebert S, Le Varlet B, Nicolas J F. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatol. 2003;149 (4):826–835. doi: 10.1046/j.1365-2133.2003.05456.x. [DOI] [PubMed] [Google Scholar]
- 80.Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008;7 (2 Suppl):s12–16. [PubMed] [Google Scholar]
- 81.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int. J. Cosmet. Sci. 2005;27 (1):17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 82.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 83.Kupper T S, Groves R W. The interleukin-1 axis and cutaneous inflammation. J. Invest. Dermatol. 1995;105 (1 Suppl):62S–66S. doi: 10.1111/1523-1747.ep12316087. [DOI] [PubMed] [Google Scholar]
- 84.Shreedhar V, Giese T, Sung V W, Ullrich S E. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J. Immunol. 1998;160 (8):3783–3789. [PubMed] [Google Scholar]
- 85.Takashima A, Bergstresser P R. Impact of UVB radiation on the epidermal cytokine network. Photochem. Photobiol. 1996;63 (4):397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x. [DOI] [PubMed] [Google Scholar]
- 86.Hirao T, Aoki H, Yoshida T, Sato Y, Kamoda H. Elevation of interleukin 1 receptor antagonist in the stratum corneum of sun-exposed and ultraviolet B-irradiated human skin. J. Invest. Dermatol. 1996;106 (5):1102–1107. doi: 10.1111/1523-1747.ep12340143. [DOI] [PubMed] [Google Scholar]
- 87.Fagot D, Asselineau D, Bernerd F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem. Photobiol. 2004;79 (6):499–505. doi: 10.1562/yg-03-11-r1.1. [DOI] [PubMed] [Google Scholar]
- 88.Rijken F, Bruijnzeel P L. The pathogenesis of photoaging: the role of neutrophils and neutrophil-derived enzymes. J. Investig. Dermatol. Symp. Proc. 2009;14 (1):67–72. doi: 10.1038/jidsymp.2009.15. [DOI] [PubMed] [Google Scholar]
- 89.Takahara M, Kang K, Liu L, Yoshida Y, McCormick T S, Cooper K D. iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J. Invest. Dermatol. 2003;120 (5):802–809. doi: 10.1046/j.1523-1747.2003.12136.x. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida Y, Kang K, Berger M, Chen G, Gilliam A C, Moser A, Wu L, Hammerberg C, Cooper K D. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J. Immunol. 1998;161 (11):5873–5879. [PubMed] [Google Scholar]
- 91.Hammerberg C, Duraiswamy N, Cooper K D. Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating class II MHC+ CD11bbright monocytic/macrophagic cells. J. Immunol. 1994;153 (11):4915–4924. [PubMed] [Google Scholar]
- 92.Handoko H Y, Rodero M P, Boyle G M, Ferguson B, Engwerda C, Hill G, Muller H K, Khosrotehrani K, Walker G J. UVB-induced melanocyte proliferation in neonatal mice driven by CCR2-independent recruitment of Ly6c(low)MHCII(hi) macrophages. J. Invest. Dermatol. 2013;133 (7):1803–1812. doi: 10.1038/jid.2013.9. [DOI] [PubMed] [Google Scholar]
- 93.Fukuoka Y, Hite MR, Dellinger AL, Schwartz LB. Human skin mast cells express complement factors C3 and C5. J. Immunol. 2013;191(4):1827–1834. doi: 10.4049/jimmunol.1202889. [DOI] [PubMed] [Google Scholar]
- 94.Colten HR, Strunk RC, Perlmutter DH, Cole FS. Regulation of complement protein biosynthesis in mononuclear phagocytes. Ciba. Found Symp. 1986;118:141–154. doi: 10.1002/9780470720998.ch10. [DOI] [PubMed] [Google Scholar]
- 95.Pasch M C, Van Den Bosch N H, Daha M R, Bos J D, Asghar S S. Synthesis of complement components C3 and factor B in human keratinocytes is differentially regulated by cytokines. J. Invest. Dermatol. 2000;114 (1):78–82. doi: 10.1046/j.1523-1747.2000.00841.x. [DOI] [PubMed] [Google Scholar]
- 96.Katz Y, Nadiv O, Rapoport M J, Loos M. IL-17 regulates gene expression and protein synthesis of the complement system, C3 and factor B, in skin fibroblasts. Clin. Exp. Immunol. 2000;120 (1):22–29. doi: 10.1046/j.1365-2249.2000.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rauterberg A, Jung E G, Rauterberg E W. Complement deposits in epidermal cells after ultraviolet B exposure. Photodermatol. Photoimmunol. Photomed. 1993;9 (4):135–143. [PubMed] [Google Scholar]
- 98.Bastiaens M, Hoefnagel J, Westendorp R, Vermeer B J, Bouwes Bavinck J N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17 (3):225–229. doi: 10.1111/j.1600-0749.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 99.Hodgson C. Senile lentigo. Arch. Dermatol. 1963;87:197–207. doi: 10.1001/archderm.1963.01590140059010. [DOI] [PubMed] [Google Scholar]
- 100.Noblesse E, Nizard C, Cario-Andre M, Lepreux S, Pain C, Schnebert S, Taieb A, Kurfurst R. Skin ultrastructure in senile lentigo. Skin Pharmacol. Physiol. 2006;19 (2):95–100. doi: 10.1159/000091976. [DOI] [PubMed] [Google Scholar]
- 101.Aoki H, Moro O, Tagami H, Kishimoto J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br. J. Dermatol. 2007;156 (6):1214–1223. doi: 10.1111/j.1365-2133.2007.07830.x. [DOI] [PubMed] [Google Scholar]
- 102.Doles J, Storer M, Cozzuto L, Roma G, Keyes W M. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26 (19):2144–2153. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]