Abstract
Objective
Myxofibrosarcoma frequently shows curvilinear extensions of high T2 signal that also enhance on magnetic resonance imaging; these “tails” represent fascial extension of tumor at histopathological examination. This study was performed to determine whether the tail sign is helpful in distinguishing myxofibrosarcoma from other myxoid-containing neoplasms.
Materials and methods
The study group consisted of 44 patients with pathologically proven myxofibrosarcoma; the control group consisted of 52 patients with a variety of other myxoid-predominant tumors. Three musculoskeletal radiologists independently evaluated T2-weighted (and/or short-tau inversion recovery) and post-contrast MR images for the presence of one or more enhancing, high-signal intensity, curvilinear projections from the primary mass. Sensitivity and specificity for the diagnosis of myxofibrosarcoma were calculated for each reader. Interobserver variability was assessed with kappa statistic and percentage agreement.
Results
A tail sign was deemed present in 28, 30, and 34 cases of myxofibrosarcoma and in 11, 9, and 5 of the controls for the three readers respectively, yielding a sensitivity of 64–77 % and a specificity of 79–90 %. The interobserver agreement was moderate-to-substantial (kappa= 0.626).
Conclusion
The tail sign at MRI is a moderately specific and sensitive sign for the diagnosis of myxofibrosarcoma relative to other myxoid-containing tumors.
Keywords: Myxofibrosarcoma, Myxoid, Sarcoma, Magnetic resonance imaging (MRI), Soft tissue tumor
Introduction
Myxoid matrix, a gelatinous, watery substance composed of sulfated (chondroitin sulfate, keratan sulfate) and non-sulfated (hyaluronic acid) glycosaminoglycans [1], is a predominant component of several types of benign and malignant soft tissue tumors, such as ganglion cyst, myxoma, nerve sheath tumors, myxoid liposarcoma, myxofibrosarcoma (MFS), extraskeletal myxoid chondrosarcoma, low-grade fibromyxosarcoma (Evans tumor), and myxoinflammatory fibroblastic sarcoma. The high water content of myxoid matrix manifests as very high signal intensity on fluid-sensitive magnetic resonance (MR) images, similar to (or slightly less than) the signal intensity of water [2]. Unlike cysts or simple fluid collections, however, myxoid-predominant tumors show some enhancement, ranging from lacy internal to diffuse intense enhancement [3]. Owing to the myxoid nature of these tumors, their appearance at magnetic resonance imaging (MRI) is distinct from that of tumors that are predominantly cellular, fibrous, or lipomatous. On T1-weighted MR images, the signal of myxoid-predominant tumors is lower than that of muscle, whereas the signal of cellular and fibrous tumors is similar to that of muscle. The signal of lipomas and well-differentiated lip-osarcomas is similar to that of subcutaneous fat [4–7].
MFS is one of the most common sarcomas in elderly patients, primarily affecting the extremities and limb girdles [8]. Whereas most soft tissue sarcomas grow as discrete round or oval masses, MFS often has an infiltrative border (macroscopically and microscopically) that extends into surrounding tissues for substantial distances along normal anatomical planes, particularly fascial planes, resulting in distant microscopic tumor deposits that predispose to local recurrence after resection. At MRI, this infiltrative spread may manifest as curvilinear projections, or “tails”, that extend from the primary mass-like portion of the MFS. Radiologists need to recognize these tails as representing tumor and to prospectively suggest the diagnosis of MFS with fascial spread to facilitate their complete resection.
Although the tail sign has been reported in recurrent low-grade MFS [3], to our knowledge, no study has assessed the frequency with which the tail sign is evident at MRI in a large number of patients with MFS or whether this sign can be used to distinguish MFS from other myxoid-predominant tumors, i.e., the group of tumors with which MFS could be confused at MRI. We therefore undertook this study to assess the prevalence of the tail sign in MFS and other myxoid-predominant tumors at MRI, and to determine its specificity in making this diagnosis.
Materials and methods
Study population
This retrospective study was approved by our institutional review board, which waived the need for informed consent. A search was performed in our institutional tumor database for all patients with a proven diagnosis of MFS from January 2003 through December 2010. Of the 422 patients identified, 365 were excluded because of the lack of preoperative MRI in our picture archiving and communication system or because preoperative MRI from an outside facility only contained images digitized from sheets of film.
An MRI was considered sufficient for review if it contained at least T2-weighted images with fat suppression or short-tau inversion recovery (STIR) images, and pre- and post-gadolinium T1-weighted fat-suppressed images. Of the remaining 57 patients, 3 patients were excluded because their MRIs lacked fluid-sensitive sequences with fat suppression, and 8 others because the examinations had been performed without administration of gadolinium contrast material. Two of the remaining 46 patients were subsequently excluded because the MR images were of poor quality (severe motion artifacts, or heterogeneous fat suppression around the lesion). The study population thus consisted of 44 patients with MFS (high grade in 39 patients, low grade in 5).
A similar database search was performed to create a control group, consisting of examples of 13 different tumors that could potentially be confused with MFS at MRI by virtue of showing very high T2 signal (visually slightly less than or equal to that of fluid) owing to a predominant myxoid content. Non-myxoid tumors were not included, as they have other features such as fat, hemosiderin, collagen, or fibrous tissue that show different signal patterns at MRI that would not be confused with myxoid material. One of the investigators who was not a reader in the study selected 52 patients with such tumors for the control group. The criteria for inclusion in the control group were the same as for the study group: histologically proven tumor and pre-treatment MRI containing T2-weighted with fat suppression images or STIR images, and pre- and post-gadolinium T1-weighted images with fat suppression. Scans in this group were obtained between January 2003 and December 2010, with a temporal distribution approximating that of the MFS group to minimize differences between the groups based on scanner quality available during different time periods. Approximately half the controls had undergone pretreatment MRI at our institution, and the other half at outside facilities, similar to the study group. Owing to the various sources of the scans, magnets of various field strengths were utilized in the study; most scans performed at our institution were done at 1.5 T. None of the scans in this study was obtained with an open magnet.
After the control group was created, the selecting radiologist reviewed the MRI of each control case to confirm that the appropriate sequences were available, that the images were of reasonable quality, and that a mass with a largest diameter of least 1.5 cm was present on the images (all masses in the MFS study group were also larger than 1.5 cm). If ineligible for the control group for any of the above reasons, a patient was replaced by another randomly selected patient with the same diagnosis. The control group consisted of 52 patients with the following diagnoses: low-grade fibromyxoid sarcoma (n=8); myxoma (n=8; 6 intramuscular, 1 cellular, 1 unspecified); malignant fibrous histiocytoma (undifferentiated pleomorphic sarcoma; n=6); myxoid liposarcoma (n=6); extraskeletal myxoid chondrosarcoma (n=5); schwannoma (n=4); neurofibroma (n=3); ganglion (n=3); acral myxoinflammatory fibroblastic sarcoma (n=3); malignant peripheral nerve sheath tumor (n=2); synovial sarcoma (n=2); undifferentiated pleomorphic leiomyosarcoma (n=1); and low-grade fibrosarcoma with focal myxoid areas (n=1).
Prior to data collection, all three readers attended a training session in which the above definitions were discussed and illustrated with several examples of cases not included in the study.
Data collection
Three radiologists, with 6, 7, and 23 years of experience in musculoskeletal tumor imaging, respectively, independently reviewed the pre-operative MRIs in random order. The readers were blinded with respect to the specific histological diagnoses and other clinical information.
Each radiologist reviewed the T2-weighted or STIR images (or both, if available) in all available planes, and recorded whether the mass was predominantly of fluid signal, defined as very high signal, similar to that of vessels, urine, or joint fluid seen on the same image. T1-weighted pre- and post-contrast images were reviewed to assess the extent of enhancement within each lesion (less than one-third, one-third to two-thirds, or greater than two-thirds of the lesion). The pre- and post-contrast images, in any imaging plane available, were also used to assess for the presence or absence of the tail sign, defined as a well-defined, sharp or tapering, pointed curvilinear projection at least 1.0 cm in length that enhanced (Figs. 1 and 2). If present, the length of the tail was recorded in the axial plane. The location of the tail, when present, was recorded as along the fascia, along neurovascular planes, and/or along muscle planes (defined as within muscles). If the tail extended along the outside of a muscle, it was classified as extending along fascial planes. All available sequences were used to determine the shape of the primary lesion: superficial spreading (extending longitudinally in subcutaneous tissues, without a rounded or oval mass; if pointed, the entire lesion was classified as a tail and measured in the axial plane); mass-like (relatively distinct, single, round or oval mass; internally may contain multiple nodules); or multinodular (more than one separate mass in the same region). The greatest dimension of each tumor mass was recorded in the axial plane, excluding any tail; if the lesion showed superficial spreading, the entire lesion was measured. The location of each tumor relative to the deep fascia was classified as superficial, deep, or both; if deep, the location was also subclassified as extra-muscular, intramuscular, or both.
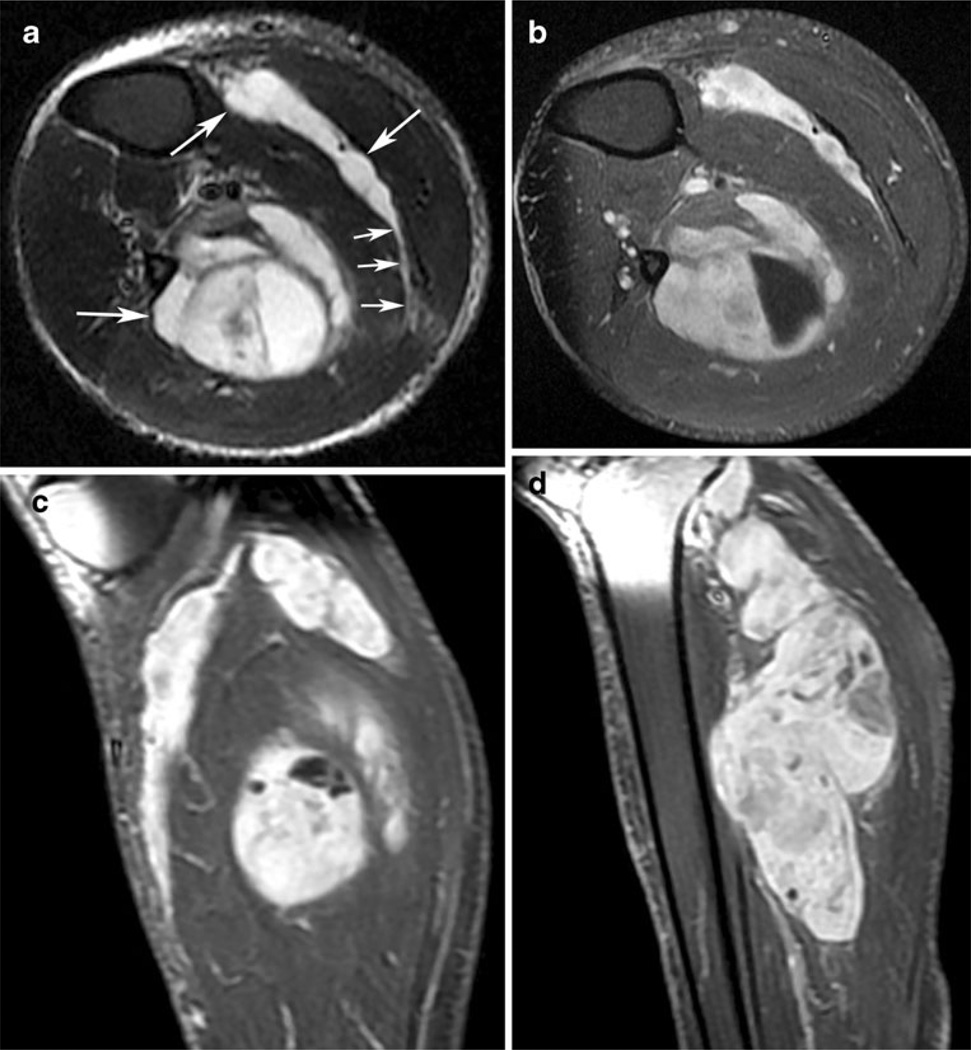
Fig. 1.
A 60-year-old man with high-grade myxofibrosarcoma (MFS). a Axial short-tau inversion recovery image through the proximal calf demonstrates multinodular, very high signal masses (large arrows) with a deep location. Note the prominent, long, high-signal extension (small arrows) in the posterior calf. Such extension along fascial planes, between muscles in this case, is a common imaging manifestation of MFS. High signal in the subcutaneous fat represents edema. b, c Axial and sagittal T1-weighted images with fat suppression obtained after gadolinium contrast administration demonstrate marked enhancement of the masses, including the tail. Tumor contacts the fibular periosteum in b, but no gross osseous invasion was found at pathological examination. d Sagittal T1-weighted post-contrast image with fat suppression shows the multinodular morphology of the largest component of the mass
Fig. 2.
A 53-year-old man with high-grade myxofibrosarcoma. a Axial T2-weighted image with fat suppression demonstrates a multinodular and superficially spreading mass along the dorsum of the right foot. Most of the lesion shows very high T2 signal, including the curvilinear extensions (arrows) on both sides of the main mass. b Axial T1-weighted post-gadolinium image with fat suppression shows that the linear projections from the lesion do not enhance, consistent with edema rather than tumor
Data analysis
All tumor characteristics were examined separately by each reader. Tumor characteristics were summarized by whether or not the tumor was MFS using frequencies and percentages. The proportion of MFS tumors with a tail sign noted was calculated along with a 95 % exact binomial confidence interval (CI). Next, we tested for an association between pathological classification as MFS and the presence of a tail sign using a Chi-squared test. The sensitivity and specificity were determined and 95 % exact binomial CIs were calculated. Interobserver agreement was assessed using kappa statistic and percentage agreement. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Among the 44 patients with MFS, 25 (57 %) were male and 19 (43 %) were female, with a median age of 61 years (range, 29–86 years); 22 patients had tumors located in a lower extremity, 10 in an upper extremity, 7 in the chest wall, and 5 in the pelvis. T2-weighted images were performed in 38 of the 44 pre-operative MRIs for this group (86.4 %), 34 (77.3 %) of which were performed with fat suppression, and 4 (9.1 %) were performed without fat suppression. STIR images were performed in 21 (47.7 %) of the 44 cases. Table 1 shows the characteristics of MFS versus non-MFS tumors, separately for each reader. MFS was classified by the three readers as predominantly of fluid signal in 42–62 % cases on T2-weighted images and 48–63 % on STIR images, compared with 44–60 % and 39–44 %, respectively, for the control cases. The three readers classified MFS as located superficial to the deep muscular fascia in 16–21 % of cases, deep to that fascia in 50–66 %, and both deep and superficial in 14–30 %. MFS demonstrated enhancement throughout a larger proportion of the tumor than did the controls; 50.0–63.6 % of MFS showed enhancement throughout more than two thirds of the mass, compared with 25.5–46.2 % for the controls.
Table 1.
Features of 44 myxofibrosarcomas (MFS) and 52 other myxoid-predominant tumors, by reader
| Characteristic | Reader A |
Reader B |
Reader C |
|||
|---|---|---|---|---|---|---|
| MFS | Non-MFS | MFS | Non-MFS | MFS | Non-MFS | |
| Tumor depth, n (%) | ||||||
| Superficial | 9 (20.5) | 4 (7.7) | 7 (15.9) | 5 (9.6) | 9 (20.5) | 7 (13.5) |
| Deep | 29 (65.9) | 43 (82.7) | 24 (54.5) | 40 (76.9) | 22 (50.0) | 41 (78.8) |
| Both | 6 (13.6) | 5 (9.6) | 13 (29.5) | 7 (13.5) | 13 (29.5) | 4 (7.7) |
| Depth type (if deep), N (%) | ||||||
| Extramuscular | 10 (29.4) | 21 (43.8) | 13 (35.1) | 14 (29.8) | 9 (25.7) | 12 (26.7) |
| Intramuscular | 21 (61.8) | 21 (43.8) | 13 (35.1) | 21 (44.7) | 19 (54.3) | 23 (51.1) |
| Both | 3 (8.8) | 6 (12.5) | 11 (29.7) | 12 (25.5) | 7 (20.0) | 10 (22.2) |
| Primary mass shape, N (%) | ||||||
| Superficial spreading | 1 (2.3) | 0 (0.0) | 4 (9.1) | 0 (0.0) | 3 (6.8) | 0 (0.0) |
| Mass-like | 36 (81.8) | 46 (88.5) | 21 (47.7) | 43 (82.7) | 34 (77.3) | 49 (94.2) |
| Multinodular | 2 (4.5) | 1 (1.9) | 3 (6.8) | 2 (3.8) | 7 (15.9) | 3 (5.8) |
| Superficial spreading and mass-like | 3 (6.8) | 0 (0.0) | 5 (11.4) | 2 (3.8) | 0 (0.0) | 0 (0.0) |
| Mass-like and multinodular | 2 (4.5) | 5 (9.6) | 11 (25.0) | 5 (9.6) | 0 (0.0) | 0 (0.0) |
| Enhancement (% of mass), N (%) | ||||||
| < 1/3 | 4 (9.1) | 13 (25.0) | 3 (7.5) | 17 (33.3) | 3 (6.8) | 11 (21.2) |
| 1/3–2/3 | 12 (27.3) | 15 (28.8) | 17 (42.5) | 21 (41.2) | 14 (31.8) | 17 (32.7) |
| >2/3 | 28 (63.6) | 24 (46.2) | 20 (50.0) | 13 (25.5) | 27 (61.4) | 24 (46.2) |
| Fluid signal on T2-weighted image, N (%) | ||||||
| Yes | 22 (59.5) | 23 (52.3) | 16 (42.1) | 20 (44.4) | 23 (62.2) | 27 (60.0) |
| No | 15 (40.5) | 21 (47.7) | 22 (57.9) | 25 (55.6) | 14 (37.8) | 18 (40.0) |
| Fluid signal on STIR, N (%) | ||||||
| Yes | 11 (55.0) | 8 (44.4) | 10 (47.6) | 8 (44.4) | 12 (63.2) | 7 (38.9) |
| No | 9 (45.0) | 10 (55.6) | 11 (52.4) | 10 (55.6) | 7 (36.8) | 11 (61.1) |
| Tail present, N (%) | ||||||
| Yes | 28 (63.6) | 11 (21.2) | 30 (68.2) | 9 (17.3) | 34 (77.3) | 5 (9.6) |
| No | 16 (36.4) | 41 (78.8) | 14 (31.8) | 43 (82.7) | 10 (22.7) | 47 (90.4) |
| Tail location (if present), N (%) | ||||||
| Fascia | 20 (71.4) | 6 (54.5) | 15 (50.0) | 1 (11.1) | 20 (58.8) | 3 (60.0) |
| Neurovascular planes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 1 (20.0) |
| Muscle planes | 2 (7.1) | 1 (9.1) | 7 (23.3) | 5 (55.6) | 9 (26.5) | 0 (0.0) |
| All | 3 (10.7) | 1 (9.1) | 1 (3.3) | 1 (11.1) | 0 (0.0) | 0 (0.0) |
| Fascia and muscle planes | 1 (3.6) | 0 (0.0) | 6 (20.0) | 1 (11.1) | 0 (0.0) | 1 (20.0) |
| Fascia and neurovascular planes | 2 (7.1) | 3 (27.3) | 0 (0.0) | 0 (0.0) | 3 (8.8) | 0 (0.0) |
| Muscle planes and neurovascular planes | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (11.1) | 1 (2.9) | 0 (0.0) |
| Median length of tail (cm; range) | 4 (1–10) | 3 (1–9) | 2 (1–7) | 1 (1–16) | 2 (1–8) | 2 (1–3) |
A tail was deemed present in 64–77 % of MFS cases by the three readers, with a median maximal length of 1–4 cm (Fig. 3). The three readers recorded that 50– 71 % of tails extended along the fascial planes; 0–3 % along neurovascular planes; 7–27 % along muscular planes; and 12–34 % along some combination of those planes.
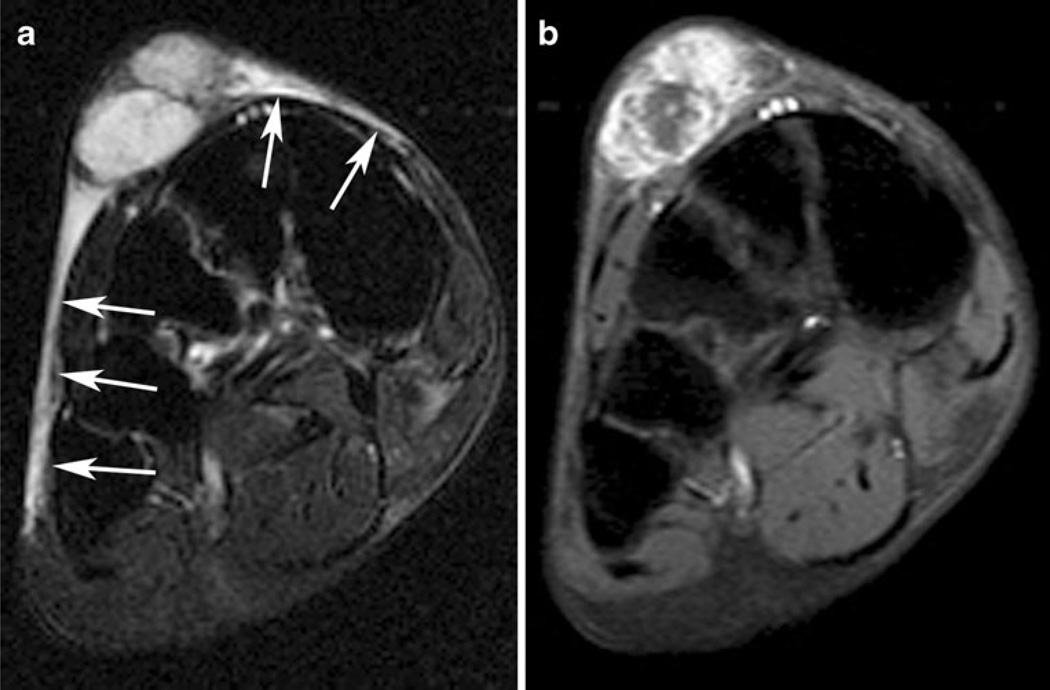
Fig. 3.
A 48-year-old woman with superficial high-grade myxofibrosarcoma. a Axial short-tau inversion recovery image demonstrates an intermediate-to-high signal mass in the subcutaneous tissues of the dorsal forearm. Prominent curvilinear extensions of high signal are present on both sides of the mass extending along the superficial fascial planes (arrow). b Axial T1-weighted post-gadolinium image with fat suppression at the same location demonstrates marked enhancement of tails, as well as of the mass, consistent with linear extension of tumor rather than edema
The control (non-MFS) group consisted of 23 male subjects (44 %) and 29 female subjects (56 %; median age, 53.5 years; range, 4–87 years). T2-weighted images were performed in 45 of the 52 preoperative MRIs (86.5 %), 41 (78.8 %) of which were performed with fat suppression and 4 (7.7 %) were performed without fat suppression. STIR images were performed in 18 of the 52 cases (36.4 %). Table 2 shows the frequency of tails by type of non-MFS tumor separately for each reader. A tail was considered present in 10–21 % of non-MFS tumors in the control group by the three readers, most commonly in undifferentiated pleomorphic sarcoma (50–67 %) and acral myxoinflammatory fibroblastic sarcoma (33–67 %; Fig. 4). No tail was reported to be present in extraskeletal myxoid chondrosarcoma, fibrosarcoma, ganglion, leiomyosarcoma, neurofibroma, or schwannoma.
Table 2.
Presence of tail by tumor type among 52 non-MFS tumors
| Tumor type | Reader A |
Reader B |
Reader C |
|||
|---|---|---|---|---|---|---|
| Tail (%) | No tail (%) | Tail (%) | No tail (%) | Tail (%) | No tail (%) | |
| Acral myxoinflammatory fibroblastic sarcoma (n=3) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) |
| Evans tumor (n=8) | 1 (12.5) | 7 (87.5) | 0 (0.0) | 8 (100) | 0 (0.0) | 8 (100) |
| Extraskeletal myxoid chondrosarcoma (n=5) | 0 (0.0) | 5 (100) | 0 (0.0) | 5 (100) | 0 (0.0) | 5 (100) |
| Fibrosarcoma (n=1) | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) |
| Ganglion (n=3) | 0 (0.0) | 3 (100) | 0 (0.0) | 3 (100) | 0 (0.0) | 3 (100) |
| Leiomyosarcoma (n=1) | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) |
| Malignant peripheral nerve sheath tumor (n=2) | 0 (0.0) | 2 (100) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100) |
| Malignant fibrous histiocytoma (n=6) | 4 (66.7) | 2 (33.3) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 3 (50.0) |
| Myxoid liposarcoma (n=6) | 3 (50.0) | 3 (50.0) | 0 (0.0) | 6 (100) | 0 (0.0) | 6 (100) |
| Myxoma (n=8) | 1 (12.5) | 7 (87.5) | 2 (25.0) | 6 (75.0) | 0 (0.0) | 8 (100) |
| Neurofibroma (n=3) | 0 (0.0) | 3 (100) | 0 (0.0) | 3 (100) | 0 (0.0) | 3 (100) |
| Schwannoma (n=4) | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) | 0 (0.0) | 4 (100) |
| Synovial sarcoma (n=2) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100) |
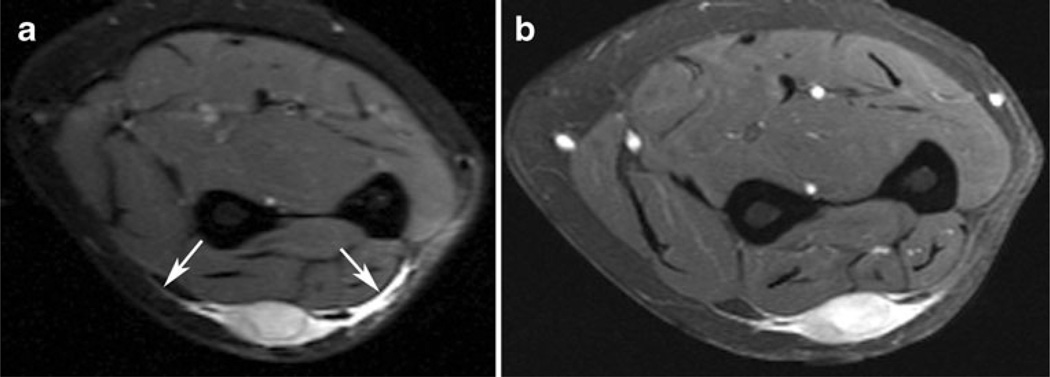
Fig. 4.
A 43-year-old man with malignant fibrous histiocytoma of the anterior thigh that also contained small foci of myxofibrosarcoma constituting less than 5 % of specimens. a Sagittal short-tau inversion recovery image demonstrates a high-signal mass with a small, intermediate-to-high signal curvilinear extension (arrows) arising from the superior aspect of the lesion. b Sagittal T1-weighted post-contrast image with fat suppression demonstrates enhancement of both the mass and the tail
Table 3 shows the association between tail sign and type of tumor separately for each reader. The tail sign was significantly associated with MFS (p<0.001 for all readers). Sensitivity ranged from 64 to 77 %, and specificity from 79 to 90 % for the three readers. All three agreed on the presence or absence of a tail in 73 % of tumors. The overall kappa for inter-reader agreement was 0.626 (95 % CI: 0.510, 0.741), which is typically considered moderate-to-substantial agreement [9].
Table 3.
Sensitivity and specificity of the tail sign for determination of myxofibrosarcoma (MFS) versus non-MFS tumors separately by reader
| Reader A |
Reader B |
Reader C |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MFS | Non-MFS | p* | MFS | Non-MFS | p* | MFS | Non-MFS | p* | |
| Tail present | 28 (63.6) | 11 (21.2) | <0.001 | 30 (68.2) | 9 (17.3) | <0.001 | 34 (77.3) | 5 (9.6) | <0.001 |
| No tail present | 16 (36.4) | 41 (78.8) | 14 (31.8) | 43 (82.7) | 10 (22.7) | 47 (90.4) | |||
| Sensitivity (95 % CI) | 0.64 (0.49, 0.78) | 0.68 (0.54, 0.82) | 0.77 (0.65, | 0.90) | |||||
| Specificity (95 % CI) | 0.79 (0.68, 0.90) | 0.83 (0.72, 0.93) | 0.90 (0.82, | 0.98) | |||||
p value from Chi-squared test
CI confidence interval
Discussion
Soft tissue tumors are a diverse group of neoplasms with a broad range of imaging manifestations on MRI. In many cases it is difficult to make specific diagnoses in soft tissue tumors lacking characteristic features at MRI, but some of these tumors display characteristic features that allow for a specific diagnosis—in some cases even obviating the need for a biopsy. Adipose tissue, for example, can be identified with certainty within lipomas, well-differentiated liposarcomas, many hemangiomas, and elastofibromas, among other tumors. Myxoid-predominant tumors can be identified because they show very high signal on fluid-sensitive MR sequences, but, unlike cysts, their internal stromal and tumor elements enhance. Among the various myxoid-predominant tumors, some possess MRI features that suggest a specific diagnosis, for example the presence of small linear or nodular foci of fat within a myxoid background is pathognomonic for myxoid liposarcoma at MRI, and has been reported to be evident in 42–95 % of cases [10].
The term “myxofibrosarcoma” was originally proposed by Angervall et al. [11] to describe a group of fibroblastic lesions that show a spectrum of cellularity, nuclear pleomorphism, and mitotic activity, ranging from hypocellular lesions with minimal cytological atypia to more cellular lesions bordering on undifferentiated pleomorphic sarcomas [formerly called malignant fibrous histiocytoma (MFH)] [11]. High-grade MFS was formerly classified as the myxoid subtype of MFH (the other subtypes being storiform pleomorphic, inflammatory, and giant cell type). The diagnosis of myxoid MFH was given to high-grade sarcomas with MFH morphology that had a predominantly myxoid component (>50 %). With the evolution in terminology and the proposed phasing out of the MFH terminology, these tumors are currently diagnosed as high-grade MFS [12]. MFS is distinct from other, similar-sounding tumors such as myxoid pleomorphic sarcoma and fibromyxoid sarcoma. Weiss and Enzinger, in their initial description of the myxoid variant of MFH, required at least 50 % of the tumor to be composed of myxoid areas to be classified as MFS [13]. Mentzel et al. required that at least 10 % of the tumor be myxoid for inclusion in their study [14]. At our institution, the pathologists require at least a 30 % myxoid component to classify the tumor as an MFS. The relative ratios of myxoid and cellular components required by various investigators to make the diagnosis of high-grade MFS varies, whereas low-grade MFS invariably shows greater than 75 % myxoid change. A continuum exists between low-grade and high-grade MFS, with some tumors showing both low-grade and high-grade areas and other tumors showing a higher grade in recurrences.
Because of its high myxoid content, MFS, like other myxoid-predominant neoplasms, can show very high signal, similar to the intensity of fluid on fluid-sensitive MR sequences. A myxoid-predominant tumor can occasionally be mistaken for a cyst unless intravenous contrast medium is administered, or if its subtle internal heterogeneity is not recognized. Even after intravenous contrast medium administration, MFS can resemble other myxoid-predominant neoplasms, such as myxoid liposarcoma, Evans tumor, extraskeletal myxoid chondrosarcoma, and intramuscular myxoma. Given the unusual and complex clinical behavior of MFS, it would be desirable to be able to suggest the diagnosis at pre-operative MRI.
Although previous reports have described the infiltrative nature of MFS at MRI and its proclivity to spread along fascial planes in a curvilinear fashion [3, 15], only one study, performed by Kaya et al. [16], analyzed the prevalence of the tail sign at MRI, which was identified in 17 out of 21 cases (81 %). However, none of these studies assessed the usefulness of the tail sign in distinguishing MFS from other myxoid-predominant neoplasms. Tails at MRI are not unique to MFS, but, to our knowledge, tails have not been emphasized as a characteristic feature in other myxoid-predominant tumors, which were the focus of this study. We evaluated the tail sign only on post-contrast fat-suppressed T1-weighted images, as we have observed that MFS enhances avidly and discretely compared with more ill-defined edema [17]—both of which can manifest as high-signal tails on fluid-sensitive images.
A tail was observed in 64–77 % of our 44 MFS patients by three readers in our study, with a sensitivity of 64–77 % and a specificity of 79–90 % among myxoid-predominant neoplasms. The results suggest that for a solid soft tissue mass showing predominantly fluid-like signal at MRI (i.e., myxoid matrix) and an enhancing, curvilinear tail, a presumptive diagnosis of MFS may be suggested with moderate sensitivity and high specificity.
Among the control group, it is perhaps not surprising that acral myxoinflammatory fibroblastic sarcoma, histologically a myxoid tumor with a dense inflammatory infiltrate, would also show tails on MRI, given the frequently curvilinear, infiltrative manifestations of other inflammatory processes on MRI [18, 19]. It is possible that some of the patients with a diagnosis of MFH who were considered to have a tail sign by the readers actually might have had MFS, which has only relatively recently been recognized as a subtype of MFH. In fact, among the MFH cases with tails, one contained a small component of MFS (<5 %), while another lesion was classified as the inflammatory subtype of MFH. Although not formally assessed, the tails associated with MFS anecdotally seemed more prominent and easier to identify than those in the non-MFS group; the former tended to be thicker, slightly longer, and larger relative to the size of the main mass, and showed subjectively higher signal on fluid-sensitive images (Fig. 5).
Fig. 5.
A 45-year-old man with myxoid liposarcoma in the right hip region. a Coronal short-tau inversion recovery image demonstrates a high signal mass with a small, intermediate-to-high signal curvilinear extension arising from the superior aspect of the mass (arrow). b Coronal T1-weighted image with fat suppression after gadolinium administration shows moderate enhancement of the mass and the tail. Note that this tail is relatively small with respect to the overall size of the lesion, compared with the myxofibrosarcoma in Fig. 3, which contains a relatively larger and more-hyperintense tail
The tail sign is not only valuable for suggesting the diagnosis of MFS, but its recognition is also essential in pre-operative planning. The radiologist needs to recognize tails and alert the surgeon that tails must also be resected in order to fully remove the gross tumor (Fig. 6) and thus avoid an increased risk of local tumor recurrence. In a study of 21 MFS patients, Manoso et al. [15] reported that the presence of an infiltrative growth pattern at MRI was strongly associated with local recurrence (local failure rate of 62 % in patients with infiltrative lesions vs 0 % in patients with “centripetal” or non-infiltrative growth). The radiologists who interpreted the pre-operative MRIs in that study reported the “thin film of increased signal along fascial planes” on T2-weighted or STIR images to represent peritumoral edema, rather than tumor extension, as confirmed at histological analysis [15].
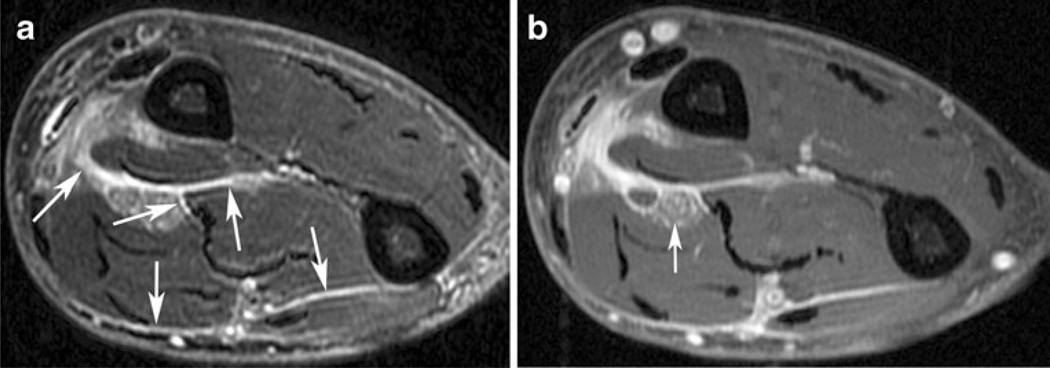
Fig. 6.
A 67-year-old man with high-grade myxofibrosarcoma (MFS) of the right forearm. a Axial T2-weighted image with fat suppression demonstrates curvilinear extensions of high signal infiltrating along the deep muscular fascia between individual muscles (arrows). A discrete mass (not shown; average recorded size, 2.9 cm) was associated with these tails. b Axial T1-weighted post-contrast image with fat suppression demonstrates that these tails enhance avidly. Tumor also encases the median nerve (arrow). This appearance is characteristic of deep-seated MFS, which can spread along multiple fascial planes. Being familiar with the infiltrative nature of MFS, the radiologist who initially interpreted this scan reported that the tumor had spread along multiple fascial planes in the forearm. Based partially on the radiologist’s recommendation, the surgeon subsequently performed an amputation despite the absence of an apparent “mass.” Histopathological examination confirmed the presence of MFS throughout the fascial planes of the forearm, with encasement of neurovascular structures. Amputation resulted in negative surgical margins; the patient has remained disease-free for 18 months
High-grade MFS has metastatic potential, while low-grade lesions are only locally aggressive; however, local recurrences can be higher grade than the original tumor, and low-grade MFS can gain metastatic potential at each recurrence. This is another reason why low-grade lesions should undergo locally aggressive treatment from the outset, along with meticulous postoperative imaging surveillance, in order to minimize the risk of local recurrence [8, 13].
Our study has limitations. First, this was a retrospective study, with resultant selection biases inherent in such studies. The MR images were obtained with a variety of non-standardized technical parameters. However, as MFS represents only a small percentage of all soft tissue masses, a prospective study of a large number of MFS patients could not be performed within a reasonable time frame at a single institution. Another limitation of this retrospective study is that point-by-point radiological–pathological correlation could not be performed. In our experience, based on years of weekly radiology–pathology correlation conferences about musculoskeletal tumors, tails of MFS enhance strongly, whereas edema enhances weakly and/or is poorly defined. We have not yet formally reported this experience, and there is no definitive literature on this issue, to our knowledge. It is possible that edema was misdiagnosed in some cases as tumor tails.
The diagnosis of MFS or other myxoid-predominant tumor was not reconfirmed by a pathologist in this study. It is possible that some of the tumors were misclassified, for example MFS might have been misclassified as MFH with myxoid features. This potential problem is likely not large, however; our mus-culoskeletal tumor pathologists are quite subspecialized and have considerable experience with this particular diagnosis. Nevertheless, even expert musculoskeletal tumor pathologists often disagree on the diagnosis of soft-tissue tumors [20]. Interobserver variability is also an inevitable feature in most imaging studies. The moderate-to-substantial interobserver agreement observed for some elements in our study was not a function of reader experience. For example, one reader recorded tails in three out of six cases of myxoid liposarcoma, whereas the other two readers did not. We re-reviewed those three cases, and can identify what were considered to be thin tails; we attribute this result to differences in reader sensitivity in the diagnosis of tails.
The control subjects were selected by one of the authors (who was not a reader), and the relative proportion of MFS to control cases was enriched relative to actual clinical practice. The control cases that were chosen were those most likely to be confused with MFS at MRI, with the express purpose of creating a particularly challenging set of cases. Also, the readers were not informed of the relative proportions of MFS and non-MFS cases. The control group did not contain all possible tumors that might mimic MFS at MR imaging, but did include a range of the more common myxoid-predominant tumors. In addition to MFS, other types of non-myxoid masses can also demonstrate an infiltrative growth pattern on MRI, including nodular fasciitis, fibromatosis, dermatofibrosarcoma protuberans, and fibrosarcoma. We did not include such lesions in our control group as they generally do not consist predominantly of myxoid substrate and thus can be distinguished from MFS by their more solid (non-fluid) signal intensity patterns, rather than by the presence or absence of a tail. Qualitative assessment of lesion signal intensity was made by comparing the lesion signal with that of vessels, urine, or joint fluid visible on the same image. Such comparison is subjective, and vessels are an imperfect reference standard as their signal intensity is affected by factors such as flow and vessel orientation relative to the magnetic field. Nevertheless, vessels often show the brightest signal on fluid-sensitive images and are thus a reasonable surrogate for fluid.
Accurate measurement of tails was difficult in many cases, owing to their complex, non-linear configurations. In addition, the post-contrast images in most studies, including those performed at our institution, were performed in the axial plane only; thus, we measured the largest dimension of the tails in the axial plane, which was not necessarily the plane that would provide the largest size. However, these measurements were made only to provide a general reflection of their size. In cases in which biopsy was performed prior to MRI, post-procedural edema and hemorrhage might be difficult to distinguish from a tail of MFS. It is likely that the tail would enhance more discretely than edema, but this potential discriminating feature was not assessed in this study. Similarly, after initial resection of MFS, the baseline postoperative MR images often show curvilinear areas of enhancement that can mimic recurrent tumor. Short-interval follow-up MRI can be useful to assess for resolution of such changes. Our study included only five low-grade MFS, precluding meaningful statistical subgroup analysis of tail prevalence by grade. Correlation of the presence of tails in MFS with patient clinical outcome was beyond the scope of our study.
Conclusion
The presence of an enhancing tail extending from a myxoid-containing tumor, especially in an extremity or limb girdle of an elderly patient, is a rather specific and moderately sensitive MRI feature of MFS. Suggesting this diagnosis pre-operatively and describing the full extent of tails are critical for optimal surgical planning; this can facilitate complete surgical resection and minimize the risk of local recurrence, ultimately reducing the potential for metastatic disease and improving patient survival.
Acknowledgements
We would like to thank Roselyn Latorre for her timely assistance with data organization for this project.
Footnotes
Conflict of interest None.
Contributor Information
Robert A. Lefkowitz, Email: lefkowir@mskcc.org, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Weill Medical College of Cornell University, New York, NY, USA.
Jonathan Landa, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Weill Medical College of Cornell University, New York, NY, USA.
Sinchun Hwang, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Weill Medical College of Cornell University, New York, NY, USA.
Emily C. Zabor, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, 305 E. 63rd Street, New York, NY 10065, USA
Chaya S. Moskowitz, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, 305 E. 63rd Street, New York, NY 10065, USA
Narasimhan P. Agaram, Department of Pathology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
David M. Panicek, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA Weill Medical College of Cornell University, New York, NY, USA.
References
- 1.Willems SM, Wiweger M, Graadt van Roggen JF, Hogendoorn PCW. Running GAGs: myxoid matrix in tumor pathology revisited What’s in it for the pathologist? Virchows Arch. 2010;456:181–192. doi: 10.1007/s00428-009-0822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura H, Zhang Y, Ohkuma K, Uchida M, Hayabuchi N, Sun S. MR imaging of soft-tissue masses of the extraperitoneal spaces. Radiographics. 2001;21(5):1141–1154. doi: 10.1148/radiographics.21.5.g01se141141. [DOI] [PubMed] [Google Scholar]
- 3.Waters B, Panicek DM, Lefkowitz RA, Healey JH, Athanasian EA, Brennan MF. Low-grade myxofibrosarcoma; CT and MRI patterns in recurrent disease. AJR. 2007;188:193–198. doi: 10.2214/AJR.05.1130. [DOI] [PubMed] [Google Scholar]
- 4.Sung MS, Kang HS, Suh JS, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. Radiographics. 2000;20:1007–1019. doi: 10.1148/radiographics.20.4.g00jl021007. [DOI] [PubMed] [Google Scholar]
- 5.Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics. 2007;27:173–187. doi: 10.1148/rg.271065065. [DOI] [PubMed] [Google Scholar]
- 6.Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculo-skeletal lipomatous lesions. Radiographics. 2004;24:1433–1466. doi: 10.1148/rg.245045120. [DOI] [PubMed] [Google Scholar]
- 7.Ohguri T, Aoki T, Hisaoka M, et al. Differential diagnosis of benign peripheral lipoma from well-differentiated liposarcoma on MR imaging: is comparison of margins and internal characteristics useful? AJR Am J Roentgenol. 2003;180:1689–1694. doi: 10.2214/ajr.180.6.1801689. [DOI] [PubMed] [Google Scholar]
- 8.Willems SM, Debiec-Rychter M, Szuhai K, Hogendoorn PCW, Sciot R. Local recurrence of myxofibrosarcoma is associated with increase in tumor grade and cytogenetic aberrations, suggesting a multistep tumor progression model. Mod Pathology. 2006;19:407–416. doi: 10.1038/modpathol.3800550. [DOI] [PubMed] [Google Scholar]
- 9.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 10.Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25:1371–1395. doi: 10.1148/rg.255055106. [DOI] [PubMed] [Google Scholar]
- 11.Angervall L, Kindblom LG, Merck CMyxofibrosarcoma. A study of 30 cases. Acta Pathol Microbiol Scand. 1997;85A:127–140. [PubMed] [Google Scholar]
- 12.Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of a efficacy of 3-tier and 4-tier grading system. Hum Pathol. 2004;35:612–621. doi: 10.1016/j.humpath.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977;39:1672–1685. doi: 10.1002/1097-0142(197704)39:4<1672::aid-cncr2820390442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20:391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Manoso MW, Pratt J, Healey JH, Boland PJ, Athanasian EA. Infiltrative MRI pattern and incomplete initial surgery compromise local control of myxofibrosarcoma. Clin Orthop Relat Res. 2006;450:89–94. doi: 10.1097/01.blo.0000229292.98850.14. [DOI] [PubMed] [Google Scholar]
- 16.Kaya M, Wada T, Nagoya S, et al. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skeletal Radiol. 2008;37:1085–1090. doi: 10.1007/s00256-008-0542-4. [DOI] [PubMed] [Google Scholar]
- 17.Erlemann R, Reiser MF, Peters PE, et al. Musculoskeletal neoplasms: static and dynamic Gd-DTPA—enhanced MR imaging. Radiology. 1989;171:767–773. doi: 10.1148/radiology.171.3.2717749. [DOI] [PubMed] [Google Scholar]
- 18.Narváez JA, Martinez S, Dodd LG, Brigman BE. Clinical observations: acral myxoinflammatory fibroblastic sarcomas: MRI findings in four cases. AJR Am J Roentgenol. 2007;188:1302–1305. doi: 10.2214/AJR.05.0141. [DOI] [PubMed] [Google Scholar]
- 19.Tateishi U, Hasegawa T, Onaya H, Satake M, Arai Y, Moriyama N. Myxoinflammatory fibroblastic sarcoma: MR appearance and pathologic correlation. AJR Am J Roentgenol. 2005;184:1749–1753. doi: 10.2214/ajr.184.6.01841749. [DOI] [PubMed] [Google Scholar]
- 20.Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: the frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol. 1986;4:1658–1661. doi: 10.1200/JCO.1986.4.11.1658. [DOI] [PubMed] [Google Scholar]