Abstract
Background: The role of inflammation and anti-cyclic citrullinated peptide antibodies (anti-CCP) in the pathogenesis of cardiovascular disease in early rheumatoid arthritis (RA) remains unclear. Previous studies have suggested that both disease activity and disease duration are associated with atherosclerosis and a higher mortality rate caused primarily by coronary artery disease. Objective: We investigated how disease activity, anti-CCP status and coronary calcium score in treatment-naive early RA impacts left ventricular (LV) systolic function. Methods: Fifty-tree patients (30 women) with mean age 58.3±1.3 years and steroid- and disease-modifying antirheumatic drug (DMARD)-naive early RA were included. Disease activity was scored by the use of the Danish national DANBIO registry (number of swollen joints (NSJ (28)), number of tender joints (NTJ (28)), C-reactive protein (CRP) and Health Assessment Questionnaire (HAQ)). Pain, fatigue, patient and physician global assessment and a composite disease activity score (DAS28-CRP) were assessed by visual analog scales (VAS) 0-100. IgM rheumafactor (IgM-RF) and anti-CCP titers were evaluated by standardized techniques. Coronary calcium score was estimated by computed tomography by calculating the Agaston score. One experienced senior rheumatologist and one experienced cardiologist performed all the clinical assessments as well as all the transthoracic echocardiography (TTE) and coronary CT analysis. Results: Disease activity scores before treatment at baseline were: NSJ (28) 7.1±2.7, NTJ (28) 8.5±3.5, CRP 11.7±12.9 mmol/l, HAQ 0.71±0.6, pain VAS 51.1±23.7, fatigue VAS 49.3±24.9, physician global assessment 54.2±15.0 and DAS28-CRP 4.8±0.7. Twenty-three (43%) patients were IgM-RF positive and 33 (62%) were anti-CCP positive. We found LV systolic function by conventional ejection fraction (EF) to be 54.1±9.2% and to be non-significant correlated to disease activity (CRP: r=0.07, p=0.64; baseline NSJ: r=-0.13, p=0.33; NTJ: r=-0.08, p=0.58; HAQ: r=0.23, p=0.1; pain VAS: r=-0.05, p=0.74; fatigue VAS: r=0.03, p=0,83; physician global assessment: r=-0.09, p=0.54 and DAS28: r=-0.03, p=0.84). However, using a more sensitive measurement of the LV function by global longitudinal systolic strain (GLS), we found a significant correlation: HAQ (r=0.29; p=0.037), patient global assessment by VAS (r=0.35; p=0.011), patient fatigue assessment by VAS (r=0.3; p=0.03) and DAS28-CRP (r=0.28; p=0.043); all corrected for relevant confounders (age, gender, pulse and blood pressure). Furthermore, anti-CCP was highly significantly correlated with GLS (r=-0.44; p=0.001) in univariate analysis. In multivariate analysis, it still remained significantly correlated (p=0.018), after correction for age, gender, pulse, and blood pressure. Using strain analysis of LV function, we found a significant difference in GLS in patients with high values of anti-CCP (titers ≥340) compared to patients with anti-CCP (titers <340); (-19.9±2.1% vs. -16.4±2.8%; p=0.0001). For patients with high IgM-RF, results were non-significant. Conclusions: We observed a significant correlation between increased disease activity and cardiac function in treatment-naive early RA.
Keywords: Steroid and DMARD-naive rheumatoid arthritis, speckle tracking, left ventricular function
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disorder that affects ~1% of adults and frequently results in significant joint deformity and disability. RA is associated with an increased risk of cardiovascular disease (CVD) in general and especially acute myocardial infarction (AMI) [1] and chronic heart failure [2,3]. The risk of CVD in RA is significantly elevated compared with the general population and comparable with the magnitude of risk in type 2 diabetes mellitus [4].
The increased risk of CVD could be explained by the chronic inflammation; however, several other factors like disease duration, disease activity and immunosuppressive therapy may be associated with the pathogenesis of CVD in RA in addition to conventional risk factors. Previous studies have suggested that both disease activities as well as disease duration are associated with atherosclerosis and a higher mortality rate caused by coronary artery disease [5-7]. The subclinical cardiovascular involvement seems to begin soon after the onset of the disease, at a younger age than in the general population [8,9]. Recently, Sitia et al [8]. demonstrated that left ventricular (LV) longitudinal systolic strain was reduced in RA patients after mean disease duration of 34 months despite the presence of a normal standard echocardiography. However they investigated a rather small number of RA patients and almost 3 years after disease onset. Furthermore, Fine NM et al [10]. recently found global longitudinal systolic strain (GLS) to be reduced in patients with long-standing RA compared to controls. It would be of great interest to detect even earlier evidence of subtle cardiac injury.
We therefore investigated LV function by speckle-tracking echocardiography and estimated the degree of coronary calcification by cardiac computer tomography (CCT) in patients with very early state RA shortly after diagnosis.
Material and methods
Patient characteristics, assessment of disease activity and treatment strategy
53 patients aged ≥18 years and with steroid- and disease-modifying antirheumatic drug (DMARD)-naive early RA were enrolled fulfilling the ACR/EULAR criteria 2010 [11]. No patients had any history of prior AMI or heart failure. Disease activity was scored by the use of the Danish national DANBIO registry (number of swollen joints (NSJ (28)), number of tender joints (NTJ (28)), C-reactive protein (CRP) and Health Assessment Questionnaire (HAQ). Visual analog scales (VAS) 0-100 were used to assess pain, fatigue, patient and physician global assessment and the composite score Disease Activity Score in 28 joints (DAS28-CRP). IgM-RF and anti-cyclic citrullinated peptide antibodies (anti-CCP) were evaluated by standardized techniques. One experienced senior rheumatologist undertook all the clinical assessments.
Treatment strategy was oral methotrexate 15 mg initiated at baseline (time at diagnosis) and increased at week 6 to 20 mg per week. If DAS28-CRP at this point was higher or equal to 3.2 and one or more swollen joints were present, oral methotrexate was discontinued and subcutaneous Metoject 25 mg per week was initiated together with oral salazopyrin 2000 mg per day and oral hydroxychloroquine 200 mg per day. In addition, intra-articular glucocorticoid injections (triamcinolonacetonid (40 mg/ml)) were given in all swollen joints (maximum 4 joints or 4 ml per visit, in agreement with the CIMESTRA algorithm previously published [12]). If disease activity, evaluated every third month, subsequently reached a level higher than DAS28-CRP 3.2, and synovitis was present at two visits, initiation and treatment with biologics was applied according to national guidelines. Oral glucocorticoids were not allowed. All patients received folic acid as well as calcium and vitamin-D supplementation; paracetamol was used as an analgesic on demand.
Transthoracic chocardiography (TTE)
Comprehensive TTE was performed and interpreted in a blinded fashion. LV dimensions, volumes, ejection fraction (EF) and GLS were measured offline using standard methods. The LV EF was calculated by a modified biplane Simpson´s method from apical 4- and 2-chamber views. Early (E) and late (A) transmitral flow velocities, and deceleration time of early filling were measured by pulsed-wave Doppler. The mitral annular early diastolic velocity (e´) by pulsed-wave tissue Doppler imaging was obtained in medial, lateral, anterior and posterior positions and averaged. This was used to calculate the E/e´-ratio. The longitudinal left ventricular fiber velocity (S´) was also measured from the pulsed-wave tissue Doppler imaging in apical 4-chamber (measured in septum and lateral wall) and apical 2-chamber views (measured anterior and posterior). GLS was measured by speckle-tracking echocardiography. This was obtained from 2D gray scale images of the apical 4-chamber, 2-chamber and long-axis view with optimized focus on the LV and frame rate ≥60 frames/sec. Duration of systole was defined in the 5-chamber apical view by marking aorta valve opening and closure from the continuous wave Doppler curve. Strain analyses were done in EchoPAC version 1.12.0 (GE, Vingmed). The LV borderline was manually traced in each apical plane, and tracking of motion was automatically done by the software. Peak systolic strain was determined. Global strain for the LV was provided by the software as the average value of the peak systolic longitudinal strain of the three apical views.
One experienced cardiologist performed all the clinical assessments. All TTE examinations as well as the calculation of the Agatston Score were analyzed and calculated in a blinded fashion according to clinical assessments.
Coronary computer tomography
A dual-source CT scanner was used (Aquilion One, Siemens Medical Solutions, Forchheim, Germany).
A non-contrast-enhanced calcium score was performed, using prospective electrocardiogram-triggering, starting 15 mm cranial of the most superior coronary artery to 15 mm caudal of the inferior border of the heart. Calcium score calculation was performed according to Agatston [13]. Calcium scoring was based on scout radiographs. A beta-blocker (atenolol 50-100 mg, depending on heart rate) was administered orally to patients with a heart rate over 60 bpm, 1-2 hours before the scan.
Analysis of calcium scoring was performed by the attending cardiologist.
Statistical analysis
Continuous variables with normal and non-normal distributions were expressed as mean ± SD and median (interquartile range), respectively. Categorical variables were expressed as numbers and percentages of patients. Group means for continuous variables with normal and non-normal distributions were compared using Student’s t tests and Mann-Whitney U tests, respectively. Non-normally distributed variables were log-transformed for correlation and multivariable regression analyses. Linearity of the regression models was judged based on histograms and scatter plots. Categorical variables were compared using chi-square tests or Fischer’s exact test, as appropriate. Finally, a multiple linear regression analysis was conducted using the continuous variable of anti-CCP as the dependent variable to assess the relation between the GLS levels and the degree of LV dysfunction.
Results
Fifty-three patients (30 women, mean age 58.3±11.3 years) with steroid- and DMARD-naive early RA were enrolled.
Baseline characteristics of the total population
Table 1 shows the baseline rheumatologic parameters (mean values) in the total population. As seen in Table 1, disease activity before treatment at baseline NSJ (28) was 7.1±2.7, NTJ (28) 8.5±3.5, CRP 11.7±12.9 mg/l, HAQ 0.71±0.60, pain VAS 51.1±23.7, fatigue VAS 49.3±24.9, physician global assessment 54.2±15.0 and DAS28-CRP 4.8±0.7. Twenty-three (43.4%) patients were rheumatoid factor positive and 33 (62.3%) were anti-CCP positive.
Table 1.
Baseline characteristics of the DMARD-naive early RA patients
| Baseline characteristics | |
|---|---|
| Age, years | 58.3±11.3 |
| Female, % | 56.6 |
| Height, cm | 171.9±9.0 |
| Weight, kg | 78.6±16.3 |
| Systolic blood pressure, mmHg | 142.5±23.4 |
| Diastolic blood pressure, mmHg | 90.0±17.0 |
| Heart rate, bpm | 65.2±10.0 |
| Creatinine, mmol/L | 65.2±12.9 |
| Total cholesterol, mmol/L | 4.86±0.96 |
| HDL, mmol/L | 1.55±0.44 |
| LDL, mmol/L | 2.82±0.87 |
| Triglyceride, mmol/L | 1.17±0.77 |
| Plasma glucose, mmol/L | 5.9±1.0 |
| Haemoglobin, mmol/L | 8.5±0.7 |
| Serum CRP, mg/L | 11.7±12.9 |
| IgM-RF positive, % | 43.4 |
| Anti-CCP positive, % | 62.3 |
| Rheumatologic parameters | |
| No. of swollen joints (28 joint score), n | 7.1±2.7 |
| No of tender joints (28 joint score), n | 8.5±3.5 |
| HAQ score | 0.71±0.60 |
| Physician global assessment, (0-100 mm VAS) | 54.2±15.0 |
| Patient global assessment, (0-100 mm VAS) | 53.6±26.2 |
| Patient assessment of pain, (0-100 mm VAS) | 51.1±23.7 |
| Patient assessment of fatique, (0-100 mm VAS) | 49.3±24.9 |
| DAS28CRP score | 4.8±0.7 |
Abbreviations: HDL: High density lipoprotein; LDL: low density lipoprotein; CRP: C-reactive protein; IgM-RF: IgM rheumatoid factor; anti-CCP: anti-cyclic citrullinated peptide antibodies (in serum); HAQ: Health Assessment Questionnaire; VAS: Visual analog scale; DAS28: Disease Activity Score in 28 joints and CRP.
Echocardiographic parameters
The echocardiographic parameters are shown in Table 2. By using the conventional estimation of LV function by EF we found no significant correlation with disease activity (CRP: r=0.07, p=0.64; baseline NSJ: r=-0.13, p=0.33; NTJ: r=-0.08, p=0.58; HAQ: r=0.23, p=0.1; pain VAS: r=-0.05, p=0.74; fatigue VAS: r=0.03, p=0.83; physician global assessment: r=-0.09, p=0.54 and DAS28-CRP: r=-0.03, p=0.84).
Table 2.
Baseline echocardiographic parameters
| Echocardiographic parameters | |
|---|---|
| Systolic parameters | |
| EF, % | 54.1±9.2 |
| LV end-diastolic volume, ml | 123.0±33.1 |
| LV end-systolic volume, ml | 58.2±24.0 |
| GLS, % | -17.53±3.27 |
| S´ global, cm/s | 8.28±1.84 |
| Diastolic parameters | |
| E/é-ratio | 8.77±3.38 |
| E/A-ratio | 1.08±0.39 |
| LA-volume, ml | 65.5±24.5 |
| E-deceleration time, m/s | 207.3±60.9 |
Abbreviations: EF: Ejection Fraction; LV: Left ventricular; GLS: global longitudinal systolic strain; S´: longitudinally left ventricular fiber velocity; E: Early transmitral flow velocity; e´: mitral annular early diastolic velocity; A: Late transmitral flow velocity; LA: left atrium.
However using a more sensitive measurement of the LV by GLS we found a significant correlation: HAQ (r=0.29; p=0.037), patient global assessment VAS (r=0.35; p=0.011), patients fatigue assessment VAS (r=0.3; p=0.03) and DAS28-CRP (r=0.28; p=0.043); all corrected for relevant confounders (age, gender, pulse and blood pressure). Furthermore, anti-CCP was highly significantly correlated with GLS (r=-0.44; p=0.001) in uni-variate analysis. In multi-variate analysis it still remained significantly correlated (p=0.018) after correction for age, gender, pulse, and blood pressure.
Coronary calcium score evaluated by CCT did not correlate with any of the echocardiographic or rheumatologic parameters. Furthermore, it did not correlate with the duration of disease.
Rheumatoid arthritis disease activity and severity in relation to TTE and calcium score at presentation
We dichotomized patients according to anti-CCP levels in three groups: normal (<7), intermediate (≥7 and <340) and high levels of anti-CCP (≥340). Parameters according to the dichotomization are seen in Table 3. It shows that patients with increased anti-CCP had more swollen joints and had lower systolic blood pressure.
Table 3.
Parameters according to anti-CCP levels
| Anti-CCP negative (n=21) | Anti-CCP (≥7 and <340) (n=18) | P-value1 | Anti-CCP <340 (n=39) | Anti-CCP ≥340 (n=14) | P-value2 | |
|---|---|---|---|---|---|---|
| Age, years | 60.8±9.9 | 58.2±12.7 | 0.47 | 59.6±11.1 | 54.5±11.3 | 0.15 |
| Female, % | 42.9 | 50 | 0.66 | 46.2 | 85.7 | 0.01 |
| Height, cm | 171.7±8.2 | 174.5±8.8 | 0.31 | 173.0±8.5 | 168.8±9.8 | 0.14 |
| Weight, kg | 81.1±12.9 | 80.3±19.8 | 0.88 | 80.7±16.2 | 72.6±15.6 | 0.11 |
| SBP, mmHg | 150±18.8 | 145.4±22.8 | 0.5 | 148.2±20.3 | 127.1±25.0 | 0.003 |
| DBP, mmHg | 93.5±13.3 | 86.4±15.1 | 0.13 | 90.7±14.3 | 88.1±23.5 | 0.63 |
| Pulse, bpm | 64.2±10.42 | 64±8.7 | 0.96 | 64.1±9.5 | 68.3±10.8 | 0.18 |
| Creatinine, mmol/L | 63.6±13.5 | 66.9±13.2 | 0.45 | 65.1±13.3 | 65.4±12.2 | 0.94 |
| Total cholesterol, mmol/L | 4.92±1.04 | 4.62±0.92 | 0.34 | 4.8±1.0 | 5.1±0.9 | 0.3 |
| HDL, mmol/L | 1.49±0.43 | 1.51±0.44 | 0.89 | 1.5±0.43 | 1.69±0.46 | 0.18 |
| LDL, mmol/L | 2.89±0.97 | 2.59±0.83 | 0.32 | 2.8±0.9 | 3±0.79 | 0.36 |
| Triglyceride, mmol/L | 1.33±1 | 1.19±0.69 | 0.62 | 1.27±0.86 | 0.88±0.27 | 0.1 |
| Plasma glucose, mmol/L | 6.07±0.79 | 6.08±1.3 | 0.97 | 6.08±1.04 | 5.56±0.75 | 0.09 |
| Haemoglobin, mmol/L | 8.6±0.7 | 8.5±0.7 | 0.94 | 8.6±0.7 | 8.2±0.6 | 0.08 |
| CRP, mg/L | 13.6±11.8 | 9.2±10.6 | 0.23 | 11.6±11.4 | 12.1±12.1 | 0.87 |
| Rheumatoid factor titer | 3±7.6 | 96.1±83 | <0.0001 | 45.3±73.2 | 64.9±74.9 | 0.4 |
| Disease activity | ||||||
| No. of swollen joints, n | 7±1.8 | 6.67±1.9 | 0.58 | 6.8±2.3 | 8.5±1.9 | 0.022 |
| No. of tender joints, n | 8.8±3.7 | 8.4±3.5 | 0.72 | 8.6±3.6 | 8.8±3.2 | 0.8317 |
| HAQ score | 0.69±0.7 | 0.7±0.46 | 0.95 | 0.7±0.6 | 0.8±0.5 | 0.57 |
| Physician global assessment* | 52.6±15.7 | 57.1±14.8 | 0.37 | 54.7±15.3 | 52.7±14.6 | 0.68 |
| Patient global assessment* | 54.8±28.1 | 56.2±24 | 0.87 | 55.4±26.0 | 48.6±27.1 | 0.41 |
| Patient assessment of pain* | 54.3±22.4 | 51.5±24.4 | 0.71 | 53±23.1 | 45.7±25.5 | 0.33 |
| Patient assessment of fatique* | 52.6±24.1 | 51.9±22.8 | 0.93 | 52.3±23.2 | 41±28.2 | 0.15 |
| DAS28-CRP score | 4.89±0.79 | 4.72±0.68 | 0.48 | 4.8±0.7 | 4.7±0.8 | 0.63 |
| Systolic echocardiographic parameters | ||||||
| Ejection Fraction, % | 56.3±8.7 | 50.4±9.9 | 0.06 | 53.6±9.7 | 55.6±7.7 | 0.47 |
| LV end-diastolic volume, ml | 129.8±33.3 | 127.6±33.5 | 0.83 | 128.8±33.0 | 106.9±28.5 | 0.032 |
| LV end-systolic volume, ml | 57.7±20.8 | 67.1±30.2 | 0.26 | 62.1±25.7 | 47.3±14.2 | 0.047 |
| GLS, % | -16.7±2.5 | -16.1±3.2 | 0.5 | -16.4±2.8 | -19.9±2.1 | 0.0001 |
| S´ global, cm/s | 7.8±1.6 | 8.5±2.1 | 0.28 | 8.1±1.9 | 8.8±1.8 | 0.25 |
| Diastolic echocardiographic parameters | ||||||
| E/é-ratio | 9.8±3.7 | 8.5±3.1 | 0.26 | 9.2±3.5 | 7.54±2.9 | 0.11 |
| E/A-ratio | 1±0.2 | 1.15±0.5 | 0.12 | 1.04±0.4 | 1.2±0.4 | 0.2 |
| LA-volume, ml | 65.8±27.2 | 69.1±21. | 0.68 | 67.3±24.3 | 60.3±25.3 | 0.36 |
| E-deceleration time, m/s | 210.2±75.7 | 216.4±53.8 | 0.77 | 213.1±65.8 | 191.2±42.5 | 0.25 |
| Cardiac Tomography | ||||||
| Calcium score (Agatston Score) | 74.4±123.3 | 67.1±111 | 0.85 | 71.1±115.9 | 29.3±57.4 | 0.2 |
Abbreviations: SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: High density lipoprotein; LDL: Low density lipoprotein; CRP: C-reactive protein; HAQ: Health Assessment Questionnaire; VAS: Visual analog scale; DAS28: Disease Activity Score in 28 joints; GLS: global longitudinal systolic strain; S´: longitudinal left ventricular fiber velocity; E: Early transmitral flow velocity; e´: mitral annular early diastolic velocity; A: Late transmitral flow velocity; LA: left atrium.
p-value between patients with normal anti-CCP and intermediate group (≥7 and <340).
P-value between patients with anti-CCP <340 versus anti-CCP ≥340.
(0-100 mm VAS).
By using strain analysis of LV function, we found a significant difference in GLS values in 14 (26.4%) patients with high values of anti-CCP (values ≥340 (GLS: -19.9% vs. -16.4%) in 39 (73.6%) patients with anti-CCP <340; p=0.0001, Figure 1. No significant values were obtained in patients with high rheumatoid factor.
Figure 1.
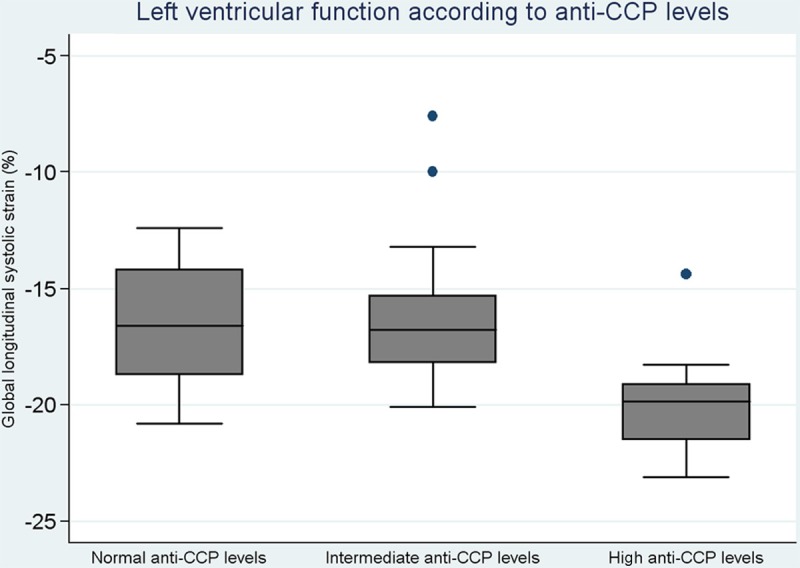
Left ventricular function according to anti-CCP levels in early diagnosed treatment-naive RA patients.
Discussion
To our knowledge this is the first and largest study reporting LV cardiac function in patients with early treatment-naive RA without known CVD. We found that patients with high disease activity (evaluated by HAQ, patient global assessment and DAS28-CRP) and increased high anti-CCP titers (>340) had an increased LV function (evaluated by GLS) compared to patients with fewer symptoms and less disease activity in the very early phase of RA disease. These findings were evident despite equal amounts of coronary calcification.
Our findings add important evidence to the growing body of research that suggests an intrinsic change and altered function in LV function in RA patients. Growing evidence supports the presence of subclinical heart disease in these patients [8,10]; however, all published studies have investigated patients with long disease duration. Several mechanisms may be responsible for longitudinal systolic dysfunction in RA. There is considerable evidence suggesting that long-term chronic inflammation impairs endothelial and microcirculatory function, leading to accelerated early atherosclerosis in patients with RA [14-17]. This chronic inflammation may lead to oxidative stress, myocyte dysfunction and to a cytokine-induced increase in fibroblast activity causing myocardial collagen deposition and interstitial fibrosis. All these actions are observed in the long-term follow-up of patients with RA. Furthermore, the accelerated atherosclerosis seen in RA has been associated with IgM-RF sero-positivity [18,19] and anti -CCP positivity [20]. However, we observed an increased LV function in the very early phase of the RA continuum. The difference in GLS that we observed did not seem to reflect the degree of coronary calcifications. It could be speculated whether the present findings are a sign of the “acute” inflammatory process that is present in the disease continuum and the early adaptive cardiac “response”. During the disease continuum this might progress along with the disease duration and “exhaust” the myocardial function in conjunction with the increased coronary calcification, as recently proposed by Chung et al [21].
The linkage to anti-CCP positivity/high anti-CCP positivity and the development of increased intima-medial thickness of common carotid arteries and decreased EF and left ventricular diastolic dysfunction are documented in uni- as well as multi-variate analysis in comparison to anti-CCP negative patients; however, in contrast to our population these patients had a disease duration of at least 3 years [22]. Hjeltnes et al [23]. found that the presence of anti-CCP antibodies was related to impaired endothelial function independent of other cardiovascular risk factors in RA patients.
In future studies it would be of special interest to monitor LV function and the possibly increasing calcium load and degree of anti-CCP positivity in a serial follow-up to trace the RA continuum and to observe these predictive markers of cardiovascular co-morbidity.
Limitations
Although our observations are based on the largest reported cohort of early RA patients evaluated by speckle-tracking echocardiography, the relative low number of patients is a limitation of our study.
Conclusion
We observed a significant correlation between increased disease activity and cardiac function evaluated by speckle-tracking in treatment-naive early rheumatoid arthritis.
Acknowledgements
We thank study nurse Elsebet Løkke for her help with patients.
Disclosure of conflict of interest
None.
Abbreviations
- RA
Rheumatoid arthritis
- CVD
Cardiovascular disease
- LV
Left ventricular
- GLS
Global longitudinal systolic strain
- CCT
Cardiac computer tomography
- DMARD
Disease-modifying antirheumatic drug
- AMI
Acute myocardial infarction
- NSJ (28)
Number of swollen joints
- NTJ (28)
Number of tender joints
- CRP
C-reactive protein
- HAQ
Health Assessment Questionnaire
- VAS
Visual analog scales
- DAS28-CRP
Disease Activity Score in 28 joints
- IgM-RF
IgM rheumatoid factor
- Anti-CCP
Anti-cyclic citrullinated peptide antibodies
- TTE
Transthoracic echocardiography
- EF
Ejection fraction
- E
Early transmitral flow velocity
- A
Late transmitral flow velocity
- e´
Mitral annular early diastolic velocity
- S´
Longitudinal left ventricular fiber velocity
References
- 1.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, Gabriel SE. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Liang KP, Myasoedova E, Crowson CS, Davis JM, Roger VL, Karon BL, Borgeson DD, Therneau TM, Rodeheffer RJ, Gabriel SE. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665–1670. doi: 10.1136/ard.2009.124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters MJ, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, Visser M, Stehouwer CD, Dekker JM, Nijpels G, Heine R, Dijkmans BA, Nurmohamed MT. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571–1579. doi: 10.1002/art.24836. [DOI] [PubMed] [Google Scholar]
- 5.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 6.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 7.Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–2571. [PubMed] [Google Scholar]
- 8.Sitia S, Tomasoni L, Cicala S, Atzeni F, Ricci C, Gaeta M, Sarzi-Puttini P, Turiel M. Detection of preclinical impairment of myocardial function in rheumatoid arthritis patients with short disease duration by speckle tracking echocardiography. Int J Cardiol. 2012;160:8–14. doi: 10.1016/j.ijcard.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Sitia S, Atzeni F, Sarzi-Puttini P, Di BV, Tomasoni L, Delfino L, Antonini-Canterin F, Di Salvo G, De Gennaro Colonna V, La Carrubba S, Carerj S, Turiel M. Cardiovascular involvement in systemic autoimmune diseases. Autoimmun Rev. 2009;8:281–286. doi: 10.1016/j.autrev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203314. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 12.Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Ellingsen T, Andersen LS, Hansen I, Skjødt H, Pedersen JK, Lauridsen UB, Svendsen A, Tarp U, Pødenphant J, Hansen G, Lindegaard H, de Carvalho A, Østergaard M, Hørslev-Petersen K CIMESTRA Study Group. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis: an investigator-initiated, multicenter, randomized, double-blind, parallel-group, placebo-controlled study. Arthritis Rheum. 2006;54:1401–1409. doi: 10.1002/art.21796. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Ciftci O, Yilmaz S, Topcu S, Caliskan M, Gullu H, Erdogan D, Pamuk BO, Yildirir A, Muderrisoglu H. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198:332–337. doi: 10.1016/j.atherosclerosis.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 17.Turiel M, Atzeni F, Tomasoni L, de PS, Delfino L, Bodini BD, Longhi M, Sitia S, Bianchi M, Ferrario P, Doria A, De Gennaro Colonna V, Sarzi-Puttini P. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009;48:834–839. doi: 10.1093/rheumatology/kep082. [DOI] [PubMed] [Google Scholar]
- 18.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 19.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Longo FJ, Oliver-Miñarro D, de la Torre I, González-Díaz de Rábago E, Sánchez-Ramón S, Rodríguez-Mahou M, Paravisini A, Monteagudo I, González CM, García-Castro M, Casas MD, Carreño L. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:419–424. doi: 10.1002/art.24390. [DOI] [PubMed] [Google Scholar]
- 21.Chung CP, Giles JT, Kronmal RA, Post WS, Gelber AC, Petri M, Szklo M, Detrano R, Budoff MJ, Blumenthal RS, Ouyang P, Bush D, Bathon JM. Progression of coronary artery atherosclerosis in rheumatoid arthritis: comparison with participants from the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2013;15:R134. doi: 10.1186/ar4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnab B, Biswadip G, Arindam P, Shyamash M, Anirban G, Rajan P. Anti-CCP antibody in patients with established rheumatoid arthritis: Does it predict adverse cardiovascular profile? J Cardiovasc Dis Res. 2013;4:102–106. doi: 10.1016/j.jcdr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjeltnes G, Hollan I, Forre O, Wiik A, Mikkelsen K, Agewall S. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40:422–427. doi: 10.3109/03009742.2011.585350. [DOI] [PubMed] [Google Scholar]


