Abstract
Objectives
Stroke is elevated in people of black African descent, but evidence for excess subclinical cerebrovascular disease is conflicting, and the role of risk factors in determining any ethnic differences observed unexplored.
Methods
We compared prevalence of brain infarcts, and severe white matter hyperintensities (WMHs) on cerebral MRI, in a community-based sample of men and women aged 58–86 of African Caribbean (214) and European (605) descent, in London, UK. Resting, central and ambulatory blood pressure (BP) were measured; diabetes was assessed by blood testing and questionnaire.
Results
Mean age was 70. Multiple (≥4) brain infarcts and severe WMH occurred more frequently in African Caribbeans (18/43%), than Europeans (7/33%, P = 0.05/0.008). Separately, clinic and night-time ambulatory BP were significantly associated with severe WMH in both ethnic groups; when both were entered into the model, the association for clinic SBP was attenuated and lost statistical significance [1.00 (0.98–1.02) P = 0.9 in Europeans, 1.00 (0.97–1.04) P = 0.9 in African Caribbeans], whereas the association for night-time SBP was retained [1.04 (1.02–1.07) P < 0.001 in Europeans, 1.08 (1.03–1.12), P = 0.001 in African Caribbeans]. The greater age-adjusted and sex-adjusted risk of severe WMH in African Caribbeans compared with Europeans [2.08 (1.15–3.76) P = 0.02], was attenuated to 1.45 [(0.74–2.83) P = 0.3] on adjustment for clinic and night-time systolic pressure, antihypertensive medication use and glycated haemoglobin.
Conclusion
African Caribbeans have a greater burden of subclinical cerebrovascular disease than Europeans. This excess is related to elevated clinic and ambulatory BP, and to hyperglycaemia.
Keywords: brain infarcts, diabetes, epidemiology, ethnicity, subclinical cerebrovascular disease, white matter hyperintensities
INTRODUCTION
Stroke incidence is approximately two-fold greater in migrants of black African descent to high-income countries than comparator European populations [1-6]. We have shown that although clinic blood pressure (BP) is elevated in UK African Caribbeans, it is not sufficient to account for the observed doubling in stroke mortality [7,8]. Excess stroke mortality in African Caribbeans is more marked in the presence of diabetes, and stroke mortality in the presence of hyperglycaemia is elevated to a greater extent in African Caribbeans than Europeans [8]. However, it has not been possible to establish whether this was because of poorer survival following an event or increased stroke incidence, or to establish mechanisms to account for these differences [8].
Stroke events are relatively infrequent in the general population, and studying people with established stroke to determine cause is problematic, as efforts to modify risk factors, such as BP, will have been made. Assessment of subclinical cerebral MRI disease, specifically brain infarcts and white matter hyperintensities (WMHs) in population studies can further our understanding of explanations for ethnic differences in stroke, as these are determined by classical stroke risk factors and in turn predict cerebrovascular events [9]. Yet previous studies on ethnic differences in subclinical cerebrovascular disease are conflicting. The Cardiovascular Health Study (CHS) [10] reported no ethnic difference in lacunes, whereas both the Atherosclerosis Risk in Communities (ARIC), and the North Manhattan Study (NOMAS) reported an excess of brain infarct in African–Americans [11,12]. Findings for WMH are more consistent, being more prevalent in African–Americans than US whites [13,14]. Previous studies have not comprehensively explored key determinants of brain infarct and severe WMH, nor sought explanations for ethnic differences in subclinical cerebrovascular disease.
We compared the prevalence of subclinical cerebrovascular disease in a community-based cohort of people of African Caribbean and European descent in the UK, and hypothesized that both elevated BP and hyperglycaemia would account for any ethnic differences observed.
METHODS
Southall and Brent REvisited (SABRE) is a population-based tri-ethnic cohort (Europeans, Indian Asians and African Caribbeans), first studied between 1988 and 1990. Full details have been published [8]. In brief, a representative community-based sample of men and women were recruited with no exclusions. Participants were aged between 40 and 69 years. African Caribbeans in the UK were first generation migrants predominantly (92%) from the Caribbean. Migration largely occurred in the 1950s and 1960s. The original study design meant that for Europeans, more men than women were recruited. Ethnicity was self-assigned, and confirmed by country of birth of all four grandparents.
Cohort survivors were invited to attend clinic between 2008 and 2011. Out of 2346 Europeans and 810 African Caribbeans studied at baseline, 2205 (94%) and 680 (85%), respectively, could be traced, and of these, 1629 (74%) and 446 (66%) had some form of follow-up data, including deaths (supplemental figure, http://links.lww.com/HJH/A275). Clinic attendance was achieved for 684 (68% of survivors) Europeans and 232 (73% of survivors) African Caribbeans. Ethics approval was granted by St Mary’s Hospital Research Ethics Committee (07/H0712/109), and written informed consent obtained. A questionnaire was completed detailing comorbidity, risk factors, health behaviours and medications. Prevalent stroke was reported by the participant and confirmed by health records. Prevalent coronary heart disease (CHD) included previous myocardial infarction or intervention. Sitting clinic BP was measured three times after 15-min rest with an Omron CEP 7050; the mean of the final two readings was used in analysis. Hypertension was defined as use of BP-lowering medication, a clinic systolic at least 140 mmHg or a clinical diastolic at least 90 mmHg. Participants who agreed were fitted with an ambulatory blood pressure monitor (Spacelabs 90217; Spacelabs, Hertford, UK) to record BP every 30 min throughout the day, and every hour during the night for 24 h. Daytime was defined as 0900 to 2100 h, and night-time as 0100 to 0900 h [15]. Ambulatory blood pressure monitoring (ABPM) was considered satisfactory if at least two-thirds of SBP and DBP measurements were recorded [15]. Percentage of nocturnal BP dipping was calculated. Central BP (Sphygmocor; Atcor, Sydney, Australia) was measured noninvasively [16]. Transthoracic two-dimensional and Doppler echocardiography were performed on a Philips iE33 machine as described previously [17]. Total arterial compliance was calculated as stroke volume/brachial pulse pressure [18]; stroke volume was calculated from end-systolic and diastolic diameters on echocardiography. An oral glucose tolerance test was performed, and fasting and postload bloods drawn for glucose (hexokinase method), insulin (immunoassay on a clinically validated automated platform; (Elecsys 2010; Roche, Burgess Hill, UK), cholesterol (enzymatic method) and glycated haemoglobin (high-pressure liquid chromatography). Known diabetes was based on patient report, confirmed by health records. WHO 1999 criteria were used to define newly diagnosed diabetes in clinic [19], and the homeostasis model assessment calculator was used to estimate insulin resistance (HOMA-IR) [20]. An early morning urine sample was collected to measure urinary albumin (enzyme immunoassay) and creatinine (kinetic Jaffé method) and the urine albumin/creatinine ratio (ACR) calculated.
Full details of the CHS MRI protocol used in this study have been published [21]. Views included standard sagittal T1-weighted images and axial T1-weighted, proton density, and T2-weighted images of 5-mm thickness with no gaps. Thin section 3-mm axial fluid attenuated inversion recovery (FLAIR) and coronal 1.5-mm three-dimensional T1-weighted gradient echo images were also obtained. A third of the scans were performed on a General Electric 1.5T scanner, the rest on a General Electric 3T scanner. DS and JH read all the scans, masked to all participant information [22]. Assessment of white matter, ventricular and sulcal grades [13] was performed by one grader (JH). Only lesions at least 3 mm were assessed, as previous experience suggests poor reproducibility for smaller lesions. A large infarct was defined as at least 20 mm. Lacunar infarcts were defined as brain infarct at least 3 mm and less than 20 mm and located in the basal ganglia, internal capsules, thalamus and deep cerebral white matter. Severity of WMH was scored on a 10-point scale using CHS standards, which combine periventricular and subcortical foci. White matter grades were grouped into the following categories: none for grade 0, mild for grade 1, moderate for grade 2 and severe for grades at least 3.
Interobserver and intraobserver agreement was performed on 44 scans and a kappa statistic calculated. For presence or absence of brain infarct, interobserver agreement was 0.68 and intraobserver 0.79. For WMH, intraobserver κ was 0.78. Twelve individuals were scanned on both the 1.5 and 3T scanners and graded by a single observer for agreement. The κ for brain infarct was 0.68, indicating good agreement, enabling us to combine data obtained on the 1.5 and 3T scanners.
There were no ethnic differences in reasons for missing MRI data, which included claustrophobia (39), refusal (29), metal implant or other contraindication for MRI (20), scanner malfunction (5) and too large for scanner (4).
Statistical methods and sample size
ARIC [11] reported a 10% prevalence of brain infarct in US whites, and 20% in African–Americans. By design, there were approximately three times as many Europeans as African Caribbeans at baseline. Assuming a similar 3: 1 participation ratio at follow-up, we calculated that we would require at least 411 Europeans and 137 African Caribbeans to attend to detect this magnitude of difference in brain infarct prevalence with 80% power at the 5% significance level. Actual attendance numbers were larger than this. A Student’s t-test was used for significance testing of continuous variables (after log transformation, if required), and the χ2 or Fisher’s exact test for categorical variables. Logistic regression was used to determine associations between risk factors and disease on MRI, and to explore determinants of any ethnic differences. There were no sex differences in the association between ethnicity and subclinical disease, so sexes were combined. Additionally, there was no evidence of age or sex interactions in key associations between risk factors and outcomes. Age and sex were forced into all regression models, and choice of subsequent variables for testing was based on a priori knowledge of their role in stroke aetiology, or observed univariate associations with subclinical disease. The choice of which single variable of a series of related variables (for example which BP measurement, systolic, diastolic, mean or pulse pressure) to be included in multivariate models depended upon which most attenuated or accounted for the ethnic difference in severe WMH. STATA version 11.0 (StataCorp, College Station, Texas, USA) was used for analyses.
RESULTS
MRI data were available on 88% of Europeans (605/684) and 92% of African Caribbeans (214/232). Compared with the whole clinic sample, mean BMI was 2 kg/m2 lower in Europeans and 3.6 kg/m2 lower in African Caribbeans who underwent MRI; however, this difference did not differ by ethnicity (P = 0.2), and there were no other differences between those who did and did not obtain an MRI scan. Daytime and night-time ABPM data were available on 233 of 605 (39%) Europeans, and 89 of 214 (42%) African Caribbeans. Key characteristics of this subsample were similar to those observed in all those who underwent MRI.
There was no ethnic difference in age (69.8 years in Europeans and 70.5 years in African Caribbeans, Table 1). Europeans were more likely to be men, reflecting the original sampling scheme. BPs were generally higher in African Caribbeans than Europeans, with greater clinic and nocturnal systolic pressure, greater loss of nocturnal dipping, greater use of antihypertensive medication and greater prevalence of hypertension. Total arterial compliance was also poorer in African Caribbeans. African Caribbeans were twice as likely to report previous stroke, and had a greater prevalence of diabetes, but urine ACR was similar. In contrast, African Caribbeans had half the prevalence of CHD, a third the prevalence of atrial fibrillation, were more likely to be never smokers and reported less alcohol consumption than Europeans.
TABLE 1. Demographic, haemodynamic, biochemical and health behaviour characteristics by ethnicity.
| Variable | European (n = 605) | African Caribbean (n = 214) | P |
|---|---|---|---|
| Age (years) | 69.8 ± 6.2 | 70.5 ± 5.8 | 0.1 |
|
| |||
| Men % (n) | 78% (477) | 52% (111) | <0.0001 |
|
| |||
| Diabetes (known and newly diagnosed) % (n) | 19% (113) | 41% (87) | <0.0001 |
|
| |||
| HbA1c(%) | 6.0±0.7 | 6.5±1.1 | <0.0001 |
|
| |||
| Fasting plasma glucose (mmol/l) | 5.1 (4.8–5.6) | 5.2 (4.8–5.8) | 0.1 |
|
| |||
| Fasting plasma insulin (μU/ml) | 8.8 (5.5–13.5) | 7.8 (5.3–11.4) | 0.1 |
|
| |||
| HOMA2-IR | 1.1 (0.7–1.8) | 1.0 (0.7–1.5) | 0.1 |
|
| |||
| BMI (kg/m2) | 27.8±4.8 | 28.9±4.9 | 0.005 |
|
| |||
| Ever smoker % (n) | 62% (375) | 31% (67) | <0.0001 |
|
| |||
| Alcohol at least once weekly | 70% (417) | 38% (81) | <0.0001 |
|
| |||
| Urine ACR (mg/mmol) | 0.45 (0.26–0.84) | 0.42 (0.24–0.83) | 0.7 |
|
| |||
| Stroke history % (n) | 5% (28) | 9% (20) | 0.01 |
|
| |||
| Atrial fibrillation % (n) | 9% (52) | 3% (7) | 0.01 |
|
| |||
| Prevalent coronary disease % (n) | 14% (85) | 8% (17) | 0.02 |
|
| |||
| Total cholesterol (mmol/l) | 4.9±1.1 | 4.7±1.1 | 0.06 |
|
| |||
| HDL cholesterol (mmol/l) | 1.39±0.35 | 1.50±0.37 | 0.0004 |
|
| |||
| Antihypertensive medication [% (n)] | 54% (325) | 76% (163) | <0.0001 |
|
| |||
| Brachial systolic clinic (mmHg) | 138±17.2 | 142±17.3 | 0.005 |
|
| |||
| Brachial diastolic clinic (mmHg) | 77±9.7 | 78±9.0 | 0.5 |
|
| |||
| Brachial mean arterial pressure (mmHg) | 98±10.7 | 99±10.5 | 0.05 |
|
| |||
| Brachial pulse pressure (mmHg) | 61±14.5 | 64±14.0 | 0.004 |
|
| |||
| Hypertension % (n) | 71% (428) | 86% (185) | <0.0001 |
|
| |||
| Central systolic clinic (mmHg) | 132±16.2 | 136±15.6 | 0.007 |
|
| |||
| Total arterial compliance (ml/mmHg) | 1.01±0.39 | 0.92±0.29 | 0.002 |
|
| |||
| ABPM subgroup | European (n = 233) | African Caribbean (n = 89) | |
| ABPM day systolic (mmHg) | 129±13.5 | 132±13.7 | 0.1 |
| ABPM day diastolic (mmHg) | 77±8.4 | 78±8.9 | 0.5 |
| ABPM night systolic (mmHg) | 113±15.0 | 118±13.9 | 0.002 |
| ABPM night diastolic (mmHg) | 65±9.3 | 69±9.1 | 0.001 |
| ABPM systolic dipping (%) | 12.8±8.1 | 10.0±8.0 | 0.007 |
| ABPM diastolic dipping (%) | 15.3±9.6 | 11.6±9.8 | 0.002 |
Mean±SD, median (25th, 75th percentile) for skewed variables or % (n). ABPM, ambulatory blood pressure monitoring; ACR, albumin-creatinine ratio; HOMA-IR, homeostatic model assessment of insulin resistance.
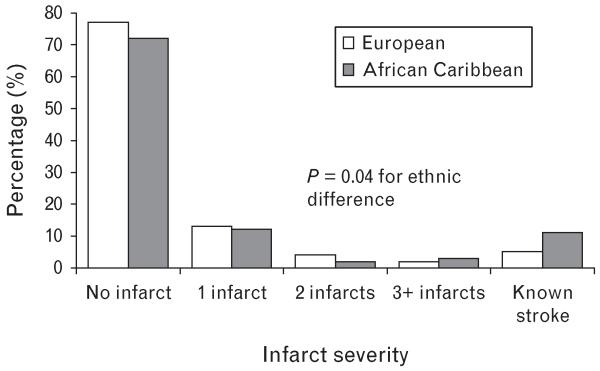
A fifth of participants had brain infarct, with no ethnic difference in overall prevalence or proportion of large infarcts (Table 2). Infarcts were more frequently lacunar in African Caribbeans, though this did not reach conventional statistical significance (72 versus 56%, P = 0.06). Overall, there was no ethnic difference in brain infarct number, but when more severe disease was examined, at least four infarcts occurred more frequently in African Caribbeans (18%) than Europeans (7%, P = 0.05). Multiple infarcts were markedly more frequent in those with a stroke history in both ethnic groups: Europeans four of 15 (27%) in those with, versus five of 111 (5%) in those without known stroke (P = 0.002); African Caribbeans five of nine (56%) and three of 38 (8%), P = 0.001, respectively. As established stroke, regardless of MRI appearance, represents the most severe disease, we compared infarct severity assigning all those with known stroke to the most severe category (Fig. 1). African Caribbeans were more likely to have severe disease (P = 0.04). Similarly, African Caribbeans were more likely to have severe WMH than Europeans (Table 2, P = 0.02).
TABLE 2. Prevalence of brain infarcts and white matter hyperintensities by ethnicity.
| European (n = 605) | African Caribbean (n = 214) | P for ethnic difference | |
|---|---|---|---|
| Infarct present, % (n) | 21% (126) | 22% (47) | 0.8 |
|
| |||
| Large infarcts (≥20mm) as % of all infarcts, % (n) | 10% (12) | 9% (4) | 0.7 |
|
| |||
| Lacunar infarct as % of all infarcts, % (n) | 56% (71) | 72% (34) | 0.06 |
|
| |||
| Number of infarcts | |||
| One, % (n) | 65% (82) | 60% (28) | |
| Two, % (n) | 20% (25) | 15% (7) | |
| Three, % (n) | 8% (10) | 9% (4) | |
| Four or more, % (n) | 7% (9) | 18% (8) | 0.3 |
|
| |||
| Grade of white matter hyperintensities | |||
| None, % (n) | 1% (5) | 1% (3) | |
| Mild, % (n) | 33% (197) | 31% (67) | |
| Moderate, % (n) | 34% (204) | 24% (52) | |
| Severe, % (n) | 33% (199) | 43% (92) | 0.02 |
FIGURE 1.
Infarct severity by ethnicity.
Key associates of brain infarct and severe WMH in both ethnic groups included age, BP (clinic, central and ABPM measures), total arterial compliance, antihypertensive medication, hypertension and cardiovascular disease history (Table 3). Presence of brain infarct was strongly associated with severe WMH and vice versa. Glycated haemoglobin and urine ACR were associated with severe WMH; other measures of the metabolic syndrome, such as HOMA-IR, had no association.
TABLE 3. Associations (odds ratios and 95% confidence interval) between risk factors and brain infarct, and severe white matter hyperintensity by ethnicity.
| Brain infarct |
Severe white matter hyperintensity |
|||||||
|---|---|---|---|---|---|---|---|---|
| European |
African Caribbean |
European |
African Caribbean |
|||||
| Variable | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age (years) | 1.08 (1.05–1.12) | <0.0001 | 1.11 (1.05–1.18) | 0.001 | 1.14 (1.11–1.18) | <0.0001 | 1.15 (1.09–1.21) | <0.0001 |
|
| ||||||||
| Sex (men versus women) | 0.90 (0.55–1.47) | 0.7 | 1.04 (0.55–1.99) | 0.9 | 1.09 (0.72–1.64) | 0.7 | 0.78 (0.45–1.34) | 0.4 |
|
| ||||||||
| Clinic SBP (mmHg) | 1.02 (1.00–1.03) | 0.009 | 1.03 (1.01–1.05) | 0.001 | 1.02 (1.01–1.03) | <0.0001 | 1.03 (1.01–1.04) | 0.002 |
|
| ||||||||
| Clinic DBP (mmHg) | 0.98 (0.96–1.00) | 0.1 | 1.02 (0.98–1.05) | 0.4 | 0.99 (0.98–1.01) | 0.3 | 1.00 (0.97–1.03) | 0.9 |
|
| ||||||||
| Clinic MAP (mmHg) | 1.00 (0.99–1.02) | 0.6 | 1.04(1.01–1.07) | 0.02 | 1.01 (1.00–1.03) | 0.07 | 1.02 (1.00–1.05) | 0.09 |
|
| ||||||||
| Clinic PP (mmHg) | 1.03 (1.01–1.04) | <0.0001 | 1.05 (1.02–1.07) | <0.0001 | 1.04 (1.02–1.05) | <0.0001 | 1.04 (1.02–1.07) | <0.0001 |
|
| ||||||||
| Central SBP (mmHg) | 1.02 (1.01–1.03) | 0.008 | 1.03 (1.01–1.06) | 0.003 | 1.02 (1.00–1.03) | 0.006 | 1.02 (1.00–1.04) | 0.06 |
|
| ||||||||
| Central PP (mmHg) | 1.02 (1.00–1.03) | 0.01 | 1.05 (1.02–1.08) | 0.001 | 1.02 (1.01–1.04) | 0.001 | 1.02 (1.00–1.05) | 0.06 |
|
| ||||||||
| TAC (ml/mmHg) | 0.41 (0.21–0.78) | 0.006 | 0.21 (0.06–0.73) | 0.01 | 0.27 (0.15–0.48) | <0.0001 | 0.33 (0.12–0.87) | 0.03 |
|
| ||||||||
| Antihypertensive medication | 2.26 (1.49–3.43) | <0.0001 | 5.92 (1.75–20.0) | 0.004 | 2.17 (1.53–3.09) | <0.0001 | 3.13 (1.53–6.41) | 0.002 |
|
| ||||||||
| Hypertension | 1.99 (1.23–3.24) | 0.005 | 4.33 (0.99–18.97) | 0.05 | 1.86 (1.25–2.77) | 0.002 | 3.33 (1.30–8.56) | 0.01 |
|
| ||||||||
| History of stroke | 4.84 (2.24–10.47) | <0.0001 | 3.36 (1.30–8.68) | 0.01 | 2.12 (0.99–4.54) | 0.05 | 3.47 (1.28–9.42) | 0.02 |
|
| ||||||||
| Atrial fibrillation | 1.98 (1.07–3.66) | 0.03 | - | - | 1.56 (0.88–2.78) | 0.1 | - | - |
|
| ||||||||
| Prevalent CHD | 1.85 (1.11–3.08) | 0.02 | 3.60 (1.31–9.93) | 0.01 | 1.28 (0.79–2.05) | 0.3 | 2.00 (0.73–5.48) | 0.2 |
|
| ||||||||
| Brain infarct on MRI | - | - | - | - | 5.03 (3.32–7.62) | <0.0001 | 6.49 (3.07–13.70) | <0.0001 |
|
| ||||||||
| Severe WMH on MRI | 5.03 (3.32–7.62) | <0.0001 | 6.49 (3.07–13.70) | <0.0001 | - | - | - | - |
|
| ||||||||
| Diabetes | 1.17 (0.72–1.91) | 0.5 | 2.15 (1.12–4.14) | 0.02 | 1.32 (0.87–2.02) | 0.2 | 2.52 (1.44–4.41) | 0.001 |
|
| ||||||||
| HbA1c(%) | 1.23 (0.96–1.58) | 0.1 | 0.99 (0.74–1.32) | 0.9 | 1.24 (0.99–1.56) | 0.06 | 1.31 (1.02–1.69) | 0.04 |
|
| ||||||||
| Glucose (mmol/l)a | 1.10 (0.96–1.26) | 0.2 | 1.14(0.95–1.38) | 0.2 | 2.17 (0.91–5.17) | 0.08 | 2.38 (0.72–7.89) | 0.2 |
|
| ||||||||
| Fasting insulin (μU/ml)a | 1.17 (0.86–1.58) | 0.3 | 0.95 (0.58–1.54) | 0.8 | 1.10 (0.85–1.43) | 0.5 | 1.11 (0.74–1.67) | 0.6 |
|
| ||||||||
| HOMA-IRa | 1.19 (0.88–1.61) | 0.3 | 0.96 (0.59–1.55) | 0.9 | 1.12 (0.82–1.45) | 0.4 | 1.12 (0.75–1.69) | 0.6 |
|
| ||||||||
| Urine ACR (mg/mmol)a | 1.35 (1.15–1.59) | <0.0001 | 1.10(0.84–1.45) | 0.5 | 1.36 (1.17–1.58) | <0.0001 | 1.38 (1.08–1.76) | 0.01 |
|
| ||||||||
| Cholesterol (mmol/l) | 0.86 (0.72–1.03) | 0.1 | 0.99 (0.73–1.34) | 1.0 | 0.81 (0.69–0.95) | 0.01 | 0.85 (0.66–1.10) | 0.2 |
|
| ||||||||
| HDL cholesterol (mmol/l) | 0.75 (0.42–1.34) | 0.3 | 1.29 (0.55–3.04) | 0.6 | 1.37 (0.84–2.22) | 0.2 | 0.87 (0.42–1.82) | 0.7 |
|
| ||||||||
| Ever smoker | 1.47 (0.69–3.13) | 0.3 | 4.41 (1.20–16.14) | 0.03 | 0.73 (0.51–1.04) | 0.08 | 1.19 (0.66–2.14) | 0.6 |
|
| ||||||||
| Alcohol at least once weekly versus not |
0.70 (0.42–1.17) | 0.2 | 0.81 (0.38–1.72) | 0.6 | 0.90 (0.50–1.64) | 0.7 | 0.80 (0.38–1.72) | 0.6 |
|
| ||||||||
| ABPM subgroup | Brain infarct | Severe white matter hyper intensity | ||||||
|
| ||||||||
| European | African Caribbean | European | African Caribbean | |||||
|
| ||||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
|
| ||||||||
| Daytime SBP (mmHg) | 1.02 (1.00–1.05) | 0.07 | 0.99 (0.95–1.02) | 0.4 | 1.03 (1.00–1.05) | 0.02 | 1.03 (1.00–1.07) | 0.07 |
|
| ||||||||
| Daytime DBP (mmHg) | 1.00 (0.96–1.03) | 0.8 | 0.95 (0.90–1.01) | 0.1 | 0.99 (0.95–1.02) | 0.4 | 1.00 (0.95–1.05) | 1.0 |
|
| ||||||||
| Night-time SBP (mmHg) | 1.03 (1.01–1.05) | 0.005 | 1.02 (0.98–1.05) | 0.3 | 1.04 (1.02–1.07) | <0.0001 | 1.08 (1.04–1.12) | <0.0001 |
|
| ||||||||
| Night-time DBP (mmHg) | 1.02 (0.99–1.05) | 0.3 | 1.00 (0.95–1.06) | 0.9 | 1.03 (1.00–1.06) | 0.06 | 1.07 (1.01–1.13) | 0.01 |
|
| ||||||||
| Systolic dip (%) | 0.96 (0.92–1.00) | 0.03 | 0.94 (0.88–1.00) | 0.05 | 0.94 (0.90–0.97) | 0.001 | 0.93 (0.88–0.99) | 0.02 |
|
| ||||||||
| Diastolic dip (%) | 0.97 (0.94–1.01) | 0.1 | 0.95 (0.90–1.00) | 0.04 | 0.95 (0.92–0.98) | 0.003 | 0.94 (0.89–0.99) | 0.01 |
Risks of subclinical cerebrovascular disease in association with atrial fibrillation in African Caribbeans are not presented as numbers are too small. ABPM, ambulatory blood pressure monitoring; CHD, coronary heart disease; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; MAP, mean arterial pressure; OR, odds ratio; PP, pulse pressure; TAC, total arterial compliance.
Log-transformed variable.
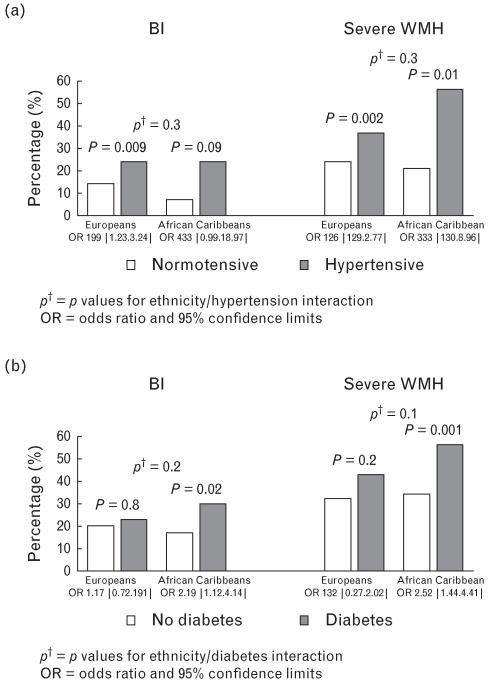
A greater prevalence of brain infarct and severe WMH was observed in those with hypertension, or in those with diabetes, compared with those without. This association appeared more marked in African Caribbeans than Europeans (Fig. 2); however, there was no evidence of an ethnicity/disease interaction, and no evidence for a BP/glycated haemoglobin interaction for either brain infarct (P = 0.3) or severe WMH (P = 0.4).
FIGURE 2.
Prevalence of brain infarcts and severe white matter hyperintensities (WMHs) comparing people with and without (a) hypertension and (b) diabetes by ethnicity. CI, confidence interval; OR, odds ratio.
There was no ethnic difference in brain infarct overall [age-adjusted and sex-adjusted odds ratio (OR) 1.01, 95% confidence interval (CI) 0.68–1.51, P = 1.0]. In contrast, the age-adjusted and sex-adjusted risk of severe WMH was 1.46 (1.02–2.08, P = 0.04) times higher in African Caribbeans than Europeans (Table 4). Addition of covariates singly to a model with age, sex and ethnicity suggested that clinic systolic pressure, antihypertensive medication and HbA1c individually provided the greatest attenuation of the ethnic difference in severe WMH. These factors together markedly attenuated the African Caribbean excess of severe WMH to 1.12 [(0.77–1.63) P = 0.6 (Table 4)]. Although the contribution of HbA1c to this multivariate model did not reach conventional statistical significance (P = 0.08), without it, the ethnic difference was 1.22 [(0.84–1.76), P = 0.3]; thus, HbA1c was retained in the multivariate model.
TABLE 4. Ethnic differences (odds ratios and 95% confidence interval) for severe white matter hyperintensity comparing African Caribbeans with Europeans.
| Modela | Variable | OR (95% CI) | P |
|---|---|---|---|
| Model 1 | Ethnicity | 1.46 (1.02–2.08) | 0.04 |
| Model 2 | Ethnicity | 1.43 (1.00–2.04) | 0.049 |
| History of stroke | 1.66 (0.87–3.14) | 0.1 | |
| Model 3 | Ethnicity | 1.29 (0.90–1.86) | 0.2 |
| Antihypertensive therapy | 1.81 (1.29–2.54) | 0.001 | |
| Model 4 | Ethnicity | 1.34 (0.93–1.92) | 0.1 |
| Clinic SBP (mmHg) | 1.02 (1.01–1.03) | <0.0001 | |
| Model 5 | Ethnicity | 1.46 (1.02–2.10) | 0.04 |
| TAC (ml/mmHg) | 0.47 (0.28–0.79) | 0.03 | |
| Model 6 | Ethnicity | 1.28 (0.89–1.85) | 0.2 |
| HbA1c (%) | 1.25 (1.04–1.49) | 0.02 | |
| Model 7 | Ethnicity | 1.35 (0.94–1.94) | 0.1 |
| Diabetes | 1.46 (1.03–2.09) | 0.04 | |
| Model 8 | Ethnicity | 1.49 (1.04–2.14) | 0.03 |
| Urine ACR (mg/mmol)b | 1.36 (1.16–1.59) | <0.0001 | |
| Model 9 – final multivariate | Ethnicity | 1.12 (0.77–1.63) | 0.6 |
| Clinic SBP (mmHg) | 1.02 (1.01–1.03) | <0.0001 | |
| HbA1c (%) | 1.18 (0.98–1.42) | 0.08 | |
| Antihypertensive treatment | 1.54 (1.09–2.19) | 0.02 | |
| ABPM subgroup (n = 322) | |||
| Model 1 | Ethnicity | 2.08 (1.15–3.76) | 0.02 |
| Model 2 – final multivariate | Ethnicity | 1.45 (0.74–2.83) | 0.3 |
| Clinic SBP (mmHg) | 1.00 (0.98–1.02) | 0.7 | |
| Night-time SBP (mmHg) | 1.05 (1.02–1.07) | <0.0001 | |
| HbA1c (%) | 1.14 (0.82–1.60) | 0.4 | |
| Antihypertensive treatment | 1.94 (1.04–3.62) | 0.04 |
ABPM, ambulatory blood pressure monitoring; CI, confidence interval; OR, odds ratio.
All models have age and sex forced into the model.
Log-transformed.
In the subgroup with ABPM, night-time SBP in particular was associated with severe WMH (Table 3). When both clinic and night-time BP were entered into the model, the association between clinic BP and severe WMH was attenuated and lost statistical significance in both Europeans [1.00 (0.98–1.02) P = 0.9] and African Caribbeans [1.00 (0.97–1.04), P = 0.9], whereas the association for night-time SBP was retained [1.04 (1.02–1.07), P <0.0001 in Europeans, 1.08 (1.03–1.13), P = 0.001 in African Caribbeans]. In those with ABPM data, the excess of severe WMH was 2.08 [(1.15–3.76), P = 0.02 (Table 4)] in African Caribbeans compared with Europeans. Multivariate adjustment for clinic systolic, antihypertensive medication and HbA1c attenuated this to 1.63 [(0.87–3.05), P = 0.1], but additional inclusion of nighttime systolic pressure attenuated the African Caribbean excess still further to 1.45 [(0.74–2.83), P = 0.3 (Table 4)]. Statistical significance of night-time systolic pressure in this multivariate model was retained (P <0.001), whereas for clinic systolic, it was lost (P = 0.7). No other variables contributed further to this model, and substitution of other measures of clinic or ABPM performed less well than those presented.
DISCUSSION
Older people of African Caribbean origin have a greater burden of multiple brain infarcts, and more severe white matter disease on cerebral MRI than Europeans. African Caribbeans also have a greater prevalence of hypertension, higher clinic and ambulatory BP and higher prevalence of diabetes than Europeans. The strong association between clinic BP and severe WMH was abolished when nighttime ambulatory BP was included in the same model. The greater burden of severe WMH in African Caribbeans compared with Europeans was markedly attenuated when clinic and nocturnal BP, and glycated haemoglobin were accounted for.
A doubling of brain infarct in African–Americans compared with US whites was reported in ARIC, a population 6–7 years younger than those in SABRE [11]. NOMAS also reported marked ethnic differences in younger individuals (<65 years), but not in those aged at least 75 [12]. CHS, whose participants were of a similar age to ours, also did not observe marked ethnic differences in infarcts, but noted a nonsignificant excess of multiple lacunar infarcts in African–Americans [10]. Thus, our findings of no ethnic difference in brain infarct overall in this older population, but a greater burden of multiple brain infarcts in African Caribbeans, are broadly consistent with previous observations. We suggest that the greater stroke susceptibility of people of black African descent is noted most strikingly at younger ages [1], and is associated with a more rapid progression of subclinical disease from single to multiple brain infarcts, such that ethnic differences in early disease are more prominent in youth, and severe, multiple disease more marked in older age. Multiple brain infarcts approximately doubles the future risk of stroke, even when other risk factors are accounted for [23]; thus, African Caribbeans would be expected to remain at higher risk of stroke, even though the total proportion of individuals with any brain infarct might not differ from Europeans. The greater burden of prevalent stroke in African Caribbeans at this investigation, and the higher prevalence of multiple infarcts in those with a history of stroke, support this suggestion.
ARIC reported similar proportions of African–Americans and US whites with evidence of any WMH, as we do here [14]. But like us and like CHS [13], they report an excess of severe WMH in people of black African descent. It is notable that WMH predicts increased stroke risk independently of brain infarct [9], and may further contribute to excess stroke in African Caribbeans.
Previous studies have not explored explanations for ethnic differences in subclinical cerebrovascular disease. Hypertension is known to be more prevalent in populations of black African descent; measured resting pressures, use of antihypertensive medication and prevalence of hypertension were higher in African Caribbeans than Europeans. Medication use was associated with an enhanced risk of both brain infarct and WMH, possibly reflecting inadequate long-term control of BP [24] and/or the result of untreated antecedent high BP [25]. In the subsample with ABPM recordings, we noted that adjustment for night-time ambulatory pressure had a greater and independent impact in accounting for the excess of severe WMH in African Caribbeans than resting clinic BP. Loss of nocturnal BP dipping independently increases stroke risk by 2.5-fold in some studies [26,27]. We and others have shown that African Caribbeans are more prone to reduced nocturnal BP dipping [28], possibly as a result of alterations in blood volume regulation and abnormalities in the renin–angiotensin–aldosterone system [29-31]. One of the proposed mechanisms of stroke is failure of cerebral autoregulation to buffer acute BP changes [32]. Hyperglycaemia is associated both with loss of autoregulation and alterations in autonomic function [26] that result in attenuation of diurnal BP patterns. Others show that multiple infarcts are more likely to occur in people with diabetes, and that 24-h ambulatory BP is more strongly associated with multiple than with single infarct [33]. It seems plausible that higher night-time BP and impaired cerebrovascular autoregulation due to hyperglycaemia act together to account for the increased risk of cerebrovascular disease and stroke in African Caribbeans.
There are limitations to this analysis. The number of individuals with multiple infarcts was small, precluding more detailed analysis. ABPM was unpopular and poorly tolerated by the study population; consequently, adequate quality ABPM data were available in slightly less than half of participants. However, there were no marked differences between those who did comply with ABPM and the whole study population suggesting that these data may not be unrepresentative. We applied set time cut-points to define daytime and night-time for ABPM, in line with recommendations from recent guidelines [34]. These guidelines do not prescribe one set of cut-points, and it is clear that set times will not correctly identify daytime and night-time for each individual, yet this will have introduced nondifferential noise into the analysis, so that actual associations are likely if anything to be stronger than those observed here. Further, it is unlikely that this will have been biased by ethnicity. This is a cross-sectional analysis; conclusions regarding causality cannot be made, yet it is unlikely that WMH, for example could cause diabetes or abnormalities in 24-h BP patterns.
Our findings have important implications. First, our data suggest an important role for ABPM and appropriate BP control over the 24-h period, in stroke prevention in people of black African descent, especially because they also have a greater prevalence of diabetes and hyperglycaemia. Second, we show that people on antihypertensive medication are more likely to have subclinical cerebrovascular disease than those who are not independent of their current BP. This suggests that antihypertensive medication may be inadequate, and/or instituted too late, to reverse subclinical cerebrovascular disease with potentially important consequences for healthy ageing and cognitive function in later life.
ACKNOWLEDGEMENTS
The authors would like to thank the participants and primary care practitioners for their kind participation and support.
The study was funded by a joint programme grant from the Wellcome Trust (082464/2/07/Z) and the British Heart Foundation (SP/07/001/23603).
Abbreviations
- ABPM
ambulatory blood pressure monitoring
- ACR
albumin/creatinine ratio
- ARIC
Atherosclerosis Risk in Communities
- BPb
lood pressure
- CHS
Cardiovascular Health Study
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HOMA-IR
Homeostasis model assessment of insulin resistance
- NOMAS
North Manhattan Study
- PWV
pulse wave velocity
- SABRE
Southall And Brent REvisited
- WMH
white matter hyperintensity
Footnotes
Conflicts of interest
No author declares a conflict of interest.
REFERENCES
- 1.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 2.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks. The National Longitudinal Mortality Study. Stroke. 1994;25:2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 4.Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001-2003. J Public Health (Oxf) 2007;29:191–198. doi: 10.1093/pubmed/fdm010. [DOI] [PubMed] [Google Scholar]
- 5.Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR) Stroke. 2008;39:2204–2210. doi: 10.1161/STROKEAHA.107.507285. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe CDA, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, et al. Incidence and case fatality rates of stroke subtypes in a multi-ethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72:211–216. doi: 10.1136/jnnp.72.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N. The role of diabetes and components of the metabolic syndrome in stroke and coronary heart disease mortality in U.K. white and African-Caribbean populations. Diabetes Care. 2006;29:2127–2129. doi: 10.2337/dc06-0779. [DOI] [PubMed] [Google Scholar]
- 8.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N. Southall And Brent REvisited: cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41:33–42. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeer SE, Hollander M, Van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 11.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR Am J Neuroradiol. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longstreth WTJ, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study [see comments] Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 14.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC study. Neuroepidemiology. 1997;16:149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 16.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De BT, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 17.Park CM, March K, Ghosh AK, Jones S, Coady E, Tuson C, et al. Left-ventricular structure in the Southall And Brent REvisited (SABRE) study: explaining ethnic differences. Hypertension. 2013;61:1014–1020. doi: 10.1161/HYPERTENSIONAHA.111.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randall OS, Westerhof N, Van den Bos GC, Alexander B. Reliability of stroke volume to pulse pressure ratio for estimating and detecting changes in arterial compliance. J Hypertens Suppl. 1986;4:S293–S296. [PubMed] [Google Scholar]
- 19.World Health Organisation . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. WHO; Geneva: 1999. [Google Scholar]
- 20.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 21.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the Cardiovascular Health Study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 22.Longstreth WT, McGuire V, Koepsell TD, Wang Y, van Belle G. Risk of amyotrophic lateral sclerosis and history of physical activity: a population-based case-control study. Arch Neurol. 1998;55:201–206. doi: 10.1001/archneur.55.2.201. [DOI] [PubMed] [Google Scholar]
- 23.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, Price T. Silent MRI infarcts and the risk of future stroke: the Cardiovascular Health Study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 24.Falaschetti E, Chaudhury M, Mindell J, Poulter N. Continued improvement in hypertension management in England: results from the Health Survey for England 2006. Hypertension. 2009;53:480–486. doi: 10.1161/HYPERTENSIONAHA.108.125617. [DOI] [PubMed] [Google Scholar]
- 25.Seshadri S, Wolf PA, Beiser A, Vasan RS, Wilson PW, Kase CS, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med. 2001;161:2343–2350. doi: 10.1001/archinte.161.19.2343. [DOI] [PubMed] [Google Scholar]
- 26.Shimada K, Kawamoto A, Matsubayashi K, Nishinaga M, Kimura S, Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 27.Phillips RA, Sheinart KF, Godbold JH, Mahboob R, Tuhrim S. The association of blunted nocturnal blood pressure dip and stroke in a multiethnic population. Am J Hypertens. 2000;13:1250–1255. doi: 10.1016/s0895-7061(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi N, McKeigue PM, Marmot MG. Resting and ambulatory blood pressure differences in Afro-Caribbeans and Europeans. Hypertension. 1993;22:90–96. doi: 10.1161/01.hyp.22.1.90. [DOI] [PubMed] [Google Scholar]
- 29.Takakuwa H, Ise T, Kato T, Izumiya Y, Shimizu K, Yokoyama H, Kobayashi KI. Diurnal variation of hemodynamic indices in nondipper hypertensive patients. Hypertens Res. 2001;24:195–201. doi: 10.1291/hypres.24.195. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun DA, Oparil S. Racial differences in the pathogenesis of hypertension. Am J Med Sci. 1995;310(Suppl 1):S86–S90. doi: 10.1097/00000441-199512000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Grim CE, Cowley AW, Jr, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45:766–772. doi: 10.1161/01.HYP.0000154364.00763.d5. [DOI] [PubMed] [Google Scholar]
- 32.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- 33.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34:2471–2474. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 34.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]