Abstract
Objectives:
Airway dilator muscles play an important role in the analysis of breathing-related symptoms, such as obstructive sleep apnoea. Texture analysis (TA) provides a new non-invasive method for analysing airway dilator muscles. In this study, we propose a TA methodology for airway dilator muscles and prove the robustness of this method.
Methods:
15 orthognathic surgery patients underwent 3-T MRI. Computerized TA was performed on 20 regions of interest (ROIs) in the patients' airway dilator muscles. 53 texture parameters were calculated for all ROIs. The robustness of the TA method was analysed by altering the locations, sizes and shapes of the ROIs.
Results:
Our study shows that there is significant difference in TA results as the size or shape of ROI changes. The change of location of the ROI inside the studied muscle does not affect the TA results.
Conclusions:
The TA method is valid for airway dilator muscles. We propose a methodology in which the number of co-occurrence parameters is reduced by using mean values from four different directions (0°, 45°, 90° and 135°) with pixel spacing of 1 pixel.
Keywords: Computer-assisted image analysis, magnetic resonance imaging, pharyngeal muscles, pterygoid muscles, palatal muscles
Introduction
Airway dilator muscles play an important role in the analysis of breathing-related symptoms, such as obstructive sleep apnoea. There is insufficient knowledge about the specificity of airway dilator muscles in obstructive sleep apnoea patients. In this article, we propose a texture analysis (TA) methodology for airway dilator muscles and provide a study of the robustness of the method.
Airway dilator muscles
The pharyngeal airway extends from the nasal choanae to the epiglottis and has no bony structures supporting it except from the posterior pharyngeal wall. The airway can be considered as a collapsible tube, meaning that the airflow is determined by the possible collapsibility of the tube wall, or the patency of the pharyngeal wall.1 The upper airway may be anatomically small,2,3 but there are also anatomical factors influencing the volume of the upper airway, such as excess body weight, the amount of fat tissue and craniofacial morphology.4 The upper airway tends to be sucked closed during inspiration by the negative pharyngeal pressure. The activation of the pharyngeal dilator muscles counteracts this. During sleep, an increased pharyngeal resistance takes place in all individuals, most likely owing to diminished activity of the tonically active muscles.1
There are several muscles surrounding the pharyngeal airway that dilate and open it. A detailed revision of those muscles has previously been made by several authors.5–7 Jordan and White8 have stated that the most important muscles are tensor veli palatini, levator veli palatini, musculus uvulae, palatoglossus and palatopharyngeus (the palatal muscles), genioglossus, the pharyngeal constrictor muscles and the hyoid muscles sternohyoideus, geniohyoideus, thyrohyoideus and mylohyoideus.
The largest upper airway control muscle is the genioglossus. It widens the oropharyngeal airway in the anterioposterior dimension if contracted.9 During inspiration, the negative pressure may cause pharyngeal collapse, and this is thought to be prevented by contraction of the genioglossus. If the posterior part of the tongue comes in contact with the posterior pharyngeal wall during sleep, it causes obstruction. The genioglossus also plays a role in several other functions, for example, in swallowing.
Pharyngeal constrictor muscles are three separate muscles, the superior, middle and inferior, and their primary function is to promote pharyngeal closure during swallowing. The posterior pharyngeal wall is also stiffened by these muscles.
The position and stiffness of the palate is controlled by the palatal muscles. The tensor veli palatini controls the stiffness of the palate, and the levator veli palatini controls the position of the palate.
The hyoid bone position is influenced by the hyoid muscles. The hyoid bone is moved upward and forward when the geniohyoideus and mylohyoideus are activated. The sternohyoideus and thyrohyoideus move the hyoid bone caudally. Airway dilatation is thought to be the result of simultaneous activation of both muscle groups.10
Texture analysis
TA is a quantitative method to classify textures in medical images. Textures describe the relationship between image grey levels. Textures have characteristics such as brightness, colour, shape and size.11–13 TA can be performed using three different methods: syntactic, statistical or spectral TA. The statistical TA is the most reported in the medical field, and we applied it as well. The statistical TA examines the spatial distribution of grey values. Statistical texture parameters are extracted from the histogram, the gradient and run-length and co-occurrence matrices.13
The muscle has been considered a good candidate for TA in MRI because it is possible to examine fairly large areas of uniform tissue with no partial volume effects.11 Only a few studies have been published related to the classification of muscles by TA.14–17 To the best of our knowledge, this is a novel study of a TA method for airway dilator muscles.
Studies by Herlidou et al14 compared TA to normal visual analysis by radiologists for the diagnosis of skeletal muscle dystrophy. According to their studies, the TA method discriminated healthy volunteers and patients with a sensitivity of 70% and a specificity of 86%, and they concluded that TA can provide useful information that contributes to the diagnosis of skeletal muscle disease.
Mahmoud-Ghoneim et al15 introduced a TA method for muscle investigation to discriminate three right-hind limb calf muscle conditions (normal, atrophy and regeneration) in rats. Quantitative TA results were statistically consistent with histology, and therefore the TA method can be considered a potentially interesting, non-destructive method for muscle examination during atrophy and regeneration.
Skoch et al16 evaluated a TA method for the description of MR images of healthy and diseased calf muscles. They also compared the TA method to standard radiological evaluation. As results, they found the classification by TA to be in 80% agreement with the categorization made by the radiological diagnosis, and in some cases, TA was able to describe changes not apparent by visual inspection.
Sikiö et al17 studied the influence of exercise loading on MR image texture of soft tissues in the thighs of athletes. Five thigh muscles were analysed by TA at two anatomical levels of the dominant leg. Significant differences were found, especially between the control and high-impact athlete groups, as well as between the control and odd-impact athlete groups. To conclude, exercise loading was associated with textural variation in the MR images of soft thigh tissues, and TA proved to be a potential method for detecting apparent structural differences in the muscle, fat and bone marrow.
Effect of region of interest size and location
The selection of the region of interest (ROI) is an important and delicate part of the TA process, especially when considering the clinical implementation of the TA method in radiology. Only a very few studies related to the ROI size, shape and location have been published. Li et al18 performed a computerized analysis on the effect of ROI size and location in studying mammographic parenchymal patterns. Lee et al19 carried out performance testing of several classifiers for differentiating obstructive lung diseases based on TA. To implement the TA method in clinical radiology, it has to be robust, and results have to be independent of intra-observer and interobserver variations. Eventually, the ROI selection has to become an automated or a semi-automated process.
The purpose of our study
The purpose of our study is to propose a TA methodology for airway dilator muscles and to prove the robustness of this method.
Methods and Materials
Patient material
15 patients (11 females and 4 males) with a mean age of 37.2 years (standard deviation, 12.7 years; range, 19–61 years) were included in this study.20 The subjects of the study were individuals from the study “Effectiveness of orthognathic surgical treatment” made jointly by the University Hospitals of Tampere, Tampere, Finland and University Hospital of Turku, Turku, Finland (supportive statement received from the Ethics Committee of the Hospital District of Southwest Finland and written consent received from all the patients). The subjects were Finnish-speaking adult patients referred for orthognathic surgical treatment to the Oral and Maxillofacial Unit of Tampere University Hospital, Tampere, Finland, during the year 2010.
MRI protocol
MRI was performed with a 3T Siemens Trio (Siemens Healthcare, Erlangen, Germany). The MRI sequence used in this study was sagittal T2 weighted 3D-SPACE (sampling perfection with application-optimized contrasts using different flip angle evolution), with an isotropic resolution of 0.9 mm, a repetition time of 3200 ms and an echo time of 407 ms. The MRI study focused on temporomandibular joints and upper airways.
Texture analysis
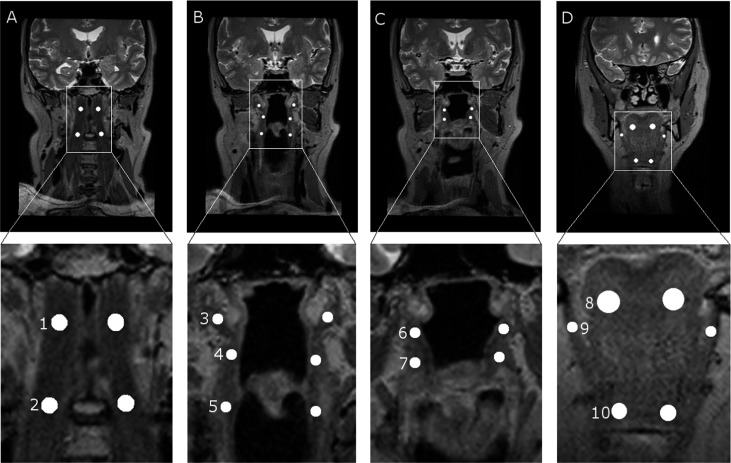
In this study, the following 20 ROIs were located in five airway dilator muscles or muscle groups: four in the longus capitis, six in pharyngeal constrictor muscles, four in palatal muscles, two in the genioglossus, two in the geniohyoid and two in the mylohyoid. All the ROIs are illustrated in Figure 1. These muscles were selected based on the experience of radiologists and on the study by Jordan and White;8 these muscles can be detected accurately in the MR images of all the patients; and large enough ROIs can be drawn into them. ROIs were paired to locate on the left and right sides of the pharynx. No free-hand ROIs were used in this study, as they are too laborious to draw in clinical use.
Figure 1.
Region of interest (ROI) locations in MR images. The upper row represents the original images, and the lower row represents the magnifications of ROI locations. (a) ROIs in longus capitis: 1, superior; and 2, inferior. (b) ROIs in pharyngeal muscles: 3, superior; 4, middle; and 5, inferior. (c) ROIs in palatal muscles: 6, tensor veli palatini; and 7, levator veli palatini. (d) ROIs in hyoid muscles: 8, genioglossus; 9, mylohyoideus; and 10, geniohyoideus.
Image orientation and slice thickness were individually reconstructed from three-dimensional image information. Image orientation was coronal with minor individual variations, depending on the orientation of the pharynx. The slice thickness was 1 mm for all patients. Image levels and ROI locations were selected by an operator (PK) with the supervision of two experienced radiologists (JJ and PD). The ROI size depended on the airway dilator muscle in which it was located. ROI sizes varied between 5 × 5 and 25 × 25 pixels. Most of the ROIs were small, from 5 × 5 to 8 × 8 pixels, owing to the small size of the studied airway dilator muscles. ROI sizes are presented in Table 1.
Table 1.
Region of interest (ROI) set characteristics
| Characteristics | A | B | C | D | E | |
|---|---|---|---|---|---|---|
| ROI shape | Circle | Circle | Circle | Circle | Square | |
| Longus capitis | 8 | 8 | 8 | 8–20 | 8 × 8 | |
| Pharyngeal muscles | 5 | 5 | 5 | 5–9 | 5 × 5 | |
| ROI diameter (circle, pixels)/size in pixels (square) | Palatal muscles | 5 | 5 | 5 | 5–8 | 5 × 5 |
| Genioglossus | 10 | 10 | 10 | 10–25 | 10 × 10 | |
| Geniohyoid | 7 | 7 | 7 | 7–13 | 7 × 7 | |
| Mylohyoid | 5 | 5 | 5 | 5–8 | 5 × 5 | |
| ROI location | I | II | III | I | I |
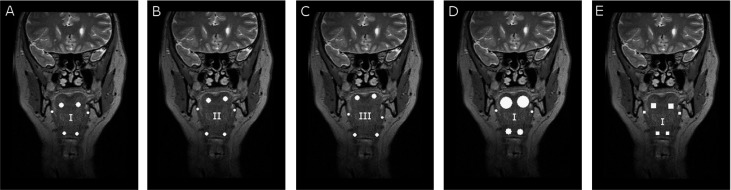
For validation of the TA method for airway dilator muscles, five different sets of ROIs were selected. In each ROI set, the location, size or shape of the ROI were altered. ROI locations, sizes and shapes varied in the following manner (presented also in Table 1):
A: Circular ROIs and ROI size depending on the muscle (all the patients have ROIs of the same size in the corresponding muscle).
B and C: Circular ROIs of the same sizes as in A, but the exact location inside the muscles varies.
D: ROI locations the same as in A, but ROI sizes vary among patients so that the circular ROI is always as large as possible.
E: ROI dimensions and locations the same as in A, but the ROI shape is a square.
An example of ROI sets is illustrated in Figure 2. The number of ROI sets is five; because most of the studied airway dilator muscles are so small, more than five clearly different ROIs cannot be located.
Figure 2.
Illustration of five region of interest (ROI) sets in MR images of hyoid muscles (Figure 1d) to illustrate ROI sets. (a) Circular ROIs and ROI size depending on the muscle. All the patients have ROIs of the same size in the corresponding muscle. ROI location (I). (b and c) Circular ROIs of the same sizes as in (a), but the exact location (II and III) inside the muscles varies. (d) ROI locations the same as in (a) (I), but ROI sizes vary among patients so that the circular ROI is always as large as possible. (e) ROI dimensions and locations (I) the same as in (a), but ROI shape is a square.
Altogether, 53 texture parameters were calculated for all ROIs using MaZda (Technical University of Lodz, Lodz, Poland),21 the TA software created by the European COST project (co-operation in the field of scientific and technical research).11 The parameters included grey-level histogram parameters (the first-order parameters) and a grey-level co-occurrence matrix (the second-order parameters) and were calculated for each ROI as described by Haralick et al.22 The histogram-based parameters that were calculated included mean, variance, skewness, kurtosis and percentiles. Co-occurrence parameters from the four directions (0°, 45°, 90° and 135°) with pixel spacing of 1 pixel were averaged to represent one texture value for each parameter (mean angular second moment, mean contrast, mean correlation, mean sum of squares, mean inverse difference moment, mean sum average, mean sum variance, mean sum entropy, mean entropy, mean difference variance and mean difference entropy). Mathias et al23 and Kassner et al24 used this co-occurrence parameter reduction method in their studies, and we used the same method, as the muscles and ROIs studied are small, and we consider the pixel spacing of 1 pixel the most relevant method to reduce the amount of textural parameters in studying airway dilator muscles. In clinical use, the reduction of textural parameters is essential.
Image grey-level normalization to µ ± 3σ for each ROI was applied, where µ represents the mean grey level and σ represents the standard deviation. This grey-level normalization scheme removes the dependency on the shift of the mean value and on multiplicative change in the image intensity. Studies by Collewet et al25 showed that this normalization method provides the best classification results and enhances the differences between two classes.
Statistical analysis
Statistical analysis was performed using SPSS® software (Windows v. 20.0; SPSS Inc., Chicago, IL). For validation of the TA method in the analysis of airway dilator muscles, the differences of TA results from different ROI sets were analysed using analysis of variance (ANOVA). ANOVA provides a statistical test of whether the means of several groups are all equal and therefore generalizes t-tests to more than two groups. Post hoc tests of ANOVA examine whether TA results from two different ROI sets show statistically significant differences. Results differ significantly if the p-value is <0.05. Bonferroni correction was also taken into account in the ANOVA results. To study the reasons for significant differences in TA results of ROIs of different size and shape, a Pearson's correlation of similarity was performed. All the pairs of ROI sets were correlated to find all correlation coefficients and to obtain a similarity matrix.
Results
The change of location of ROIs inside the studied airway dilator muscles did not result in significant differences in TA results, as significant differences were found in <5% of the ROIs. The change of ROI size or shape resulted in significant differences in TA results, as significant differences were found in >50% of the ROIs among the following TA parameters: angular second moment, difference entropy, entropy and sum entropy. Results are presented in Table 2. A–E in the table are introduced in Figure 2.
Table 2.
A summary of analysis of variance (ANOVA) results
| ANOVA | A vs B | A vs C | B vs C | A vs D | A vs E | B vs D | B vs E | C vs D | C vs E | D vs E |
|---|---|---|---|---|---|---|---|---|---|---|
| Angular second moment | ••• | ••• | ••• | •• | ••• | ••• | • | |||
| Diffusion entropy | ••• | •• | ••• | |||||||
| Entropy | •• | ••• | ••• | •• | ••• | •• | • | |||
| Sum entropy | •• | ••• | ••• | • | ••• | •• | • |
ROI, region of interest.
Statistically significant differences in texture analysis results are presented with dots in table.
•p < 0.05 (found in ≥50% of the ROIs).
••p < 0.05 (found in ≥70% of the ROIs).
•••p < 0.05 (found in ≥90% of the ROIs).
The range of variation between different patients and mean values for all the correlation coefficients of different pairs of the ROI sets are presented in Table 3. All the correlation coefficients were >0.95, so we can conclude that all the TA results correlate and significant differences in the TA results of ROIs of different sizes and shapes can be explained by the variation of the ROI area.
Table 3.
A summary of correlation coefficients
| Sets | Range of variation | Average |
|---|---|---|
| A vs B | 0.96–0.99 | 0.97 |
| A vs C | 0.96–0.99 | 0.97 |
| A vs D | 0.98–1.00 | 0.99 |
| A vs E | 0.97–1.00 | 0.98 |
| B vs C | 0.95–0.98 | 0.97 |
| B vs D | 0.94–0.99 | 0.97 |
| B vs E | 0.95–0.99 | 0.97 |
| C vs D | 0.96–0.99 | 0.97 |
| C vs E | 0.97–0.98 | 0.96 |
| D vs E | 0.98–0.99 | 0.99 |
Discussion
In this study, we proposed a methodology for examining airway dilator muscles and proved the robustness of the TA method. In the proposed methodology, the number of co-occurrence parameters is reduced by using mean values from four directions (0°, 45°, 90° and 135°) with pixel spacing of 1 pixel. We studied TA results with different ROI locations, sizes and shapes. The change of location of ROIs inside the studied airway dilator muscles did not result in significant differences in TA results, whereas the change of ROI size or shape resulted in significant differences in TA results. The significant differences were explained by the variation of ROI area and by the means of correlations.
Cobo et al26 evaluated the feasibility and reproducibility of foetal lung TA using automatic ultrasound analysis to assess gestational age. As a result, the association between the weeks of gestation and foetal lung texture presented a Pearson's correlation of 0.97. The association was not influenced by the ROI localization or ROI size.
We obtained similar results as Cobo et al,25 as the association was not influenced by ROI localization or ROI size, and the magnitude of the Pearson's correlation was similar in both studies. In future studies, we could also evaluate the feasibility and reproducibility of the TA method in differentiating patient groups by TA of airway dilator muscles.
Li et al18 performed a computerized analysis on the effect of ROI size and location in studying mammographic parenchymal patterns. Their results showed that there was a statistically significant decrease in performance of the computerized texture features, as the ROI location was varied, but no significant decrease in performance as the ROI size decreased. Lee et al19 carried out performance testing of several classifiers for differentiating obstructive lung diseases based on TA. They studied each ROI location with various ROI sizes. They found that ROI size had a significant effect on the overall accuracy in classification of three different lung diseases and healthy lungs: the overall accuracy increased as the ROI size increased. Li et al18 found that in the case of TA of mammographic parenchymal patterns, the decrease of the ROI size did not decrease the performance of the TA method, whereas Lee et al19 found that in the case of TA for differentiating obstructive lung diseases, the overall accuracy increased as the ROI size increased. These studies suggest that in various anatomical sites, the size, shape and location of ROIs affect TA results in distinctive ways, as we presumed. In summary, TA protocol in different anatomical sites needs to be validated carefully before the TA process.
In our study of airway dilator muscles, the exact ROI location inside the studied airway dilator muscle did not result in significant differences in TA results. When the sizes or shapes of ROIs studied are different, the results differ significantly. This finding was explained by correlations that result from the change of the ROI area.
The change of ROI size or shape resulted in significant differences in TA results of most of the ROIs studied among the following TA parameters: angular second moment, difference entropy, entropy and sum entropy. Angular second moment is a measure of homogeneity and gets its largest value when the texture inside the ROI is uniform. Entropy is a measure of complexity and gets its largest value when the ROI is complex.23,24 Among other parameters, no significant differences were found. In further studies, we can also study results of different parameters and further limit the number of calculated parameters based on the repeatability, variation and information content of those parameters.
The weaknesses of the TA method include the huge amount of texture information needed before statistical analysis and the lack of an efficient clinical TA protocol.
This study is a feasibility study to prove the proposed method to be valid in actual muscle studies of airway dilator muscles. To conclude, the TA method for airway dilator muscles is robust for clinical use. All the TA results correlate, and significant differences in the TA results of ROIs of different size and shape can be explained by the variation of the ROI area. We propose a methodology in which the number of co-occurrence parameters is reduced by using mean values from four directions (0°, 45°, 90° and 135°) with pixel spacing of 1 pixel. We recommend that the size of the ROI be selected before the TA process and that circular ROIs be used with sizes as large as possible in particular ROI locations within all the patients.
References
- 1.Svanborg E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir Physiol Neurobiol 2005; 147: 263–72. doi: 10.1016/j.resp.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 2.Mortimore IL, Marhur R, Douglas NJ. Effect of posture, route of respiration, and negative pressure on palatal muscle activity in humans. J Appl Physiol 1995; 79: 448–54. [DOI] [PubMed] [Google Scholar]
- 3.Mortimore IL, Douglas NJ. Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med 1997; 156: 867–73. doi: 10.1164/ajrccm.156.3.9608008 [DOI] [PubMed] [Google Scholar]
- 4.Sunnergren O, Broström A, Svanborg E. Soft palate sensory neuropathy in the pathogenesis of obstructive sleep apnea. Laryngoscope 2011; 121: 451–6. doi: 10.1002/lary.21371 [DOI] [PubMed] [Google Scholar]
- 5.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 1996; 19: 827–53. [DOI] [PubMed] [Google Scholar]
- 6.White DP. Respiration and the human upper airway. In: Altose MD, Kawakami Y, eds. Control of Breathing in Health and Disease. New York, NY: Marcel Dekker; 1999. pp. 163–201. [Google Scholar]
- 7.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, ed. Sleep Apnea: Pathogenesis, Diagnosis and Treatment. New York, NY: Marcel dekker; 2002. pp. 99–154. [Google Scholar]
- 8.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol 2008; 160: 1–7. doi: 10.1016/j.resp.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol 1996; 80: 1595–604. [DOI] [PubMed] [Google Scholar]
- 10.Van de Graaff WB, Gottfried SB, Mitra J, van Lunteren E, Cherniack NS, Strohl KP. Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol 1984; 57: 197–204. [DOI] [PubMed] [Google Scholar]
- 11.Hajek M, Dezortova M, Materka A, Lerski R. Texture Analysis for Magnetic Resonance Imaging. Prague, CZ: Med4Publishing; 2006. [Google Scholar]
- 12.Kassner A. Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol 2010; 31: 809–16. doi: 10.3174/ajnr.A2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellano G, Bonilha LM, Cendes F. Texture analysis of medical images. Clin Radiol 2004; 59: 1061–9. doi: 10.1016/j.crad.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 14.Herlidou S, Rolland Y, Bansard JY, Le Rumeur E, de Certaines JD. Comparison of automated and visual texture analysis in MRI: characterization of normal and diseased skeletal muscle. Magn Reson Imaging 1999; 17: 1393–7. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud-Ghoneim D, Cherel Y, Lemaire L, de Certaines JD, Maniere A. Texture analysis of magnetic resonance images of rat muscles during atrophy and regeneration. Magn Reson Imaging 2006; 24: 167–71. doi: 10.1016/j.mri.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Skoch A, Jirák D, Vyhnanovská P, Dezortová M, Fendrych P, Rolencová E, et al. Classification of calf muscle MR images by texture analysis. MAGMA 2004; 16: 259–67. doi: 10.1007/s10334-004-0032-1 [DOI] [PubMed] [Google Scholar]
- 17.Sikiö M, Harrison LC, Nikander R, Ryymin P, Dastidar P, Ekola HJ, et al. Influence of exercise loading on magnetic resonance image texture of thigh soft tissues. Clin Physiol Funct Imaging 2013; in press. Epub ahead of print November 2013. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Olopade OI, Lan L, Weber BL, Bonta I. Computerized analysis of mammographic parenchymal patterns for assessing breast cancer risk: effect of ROI size and location. Med Phys 2004; 31: 549–55. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Seo JB, Lee JG, Kim SS, Kim N, Kang SH. Performance testing of several classifiers for differentiating obstructive lung diseases based on texture analysis at high-resolution computerized tomography (HRCT). Comput Methods Programs Biomed 2009; 93: 206–15. [DOI] [PubMed] [Google Scholar]
- 20.Szczypinski PM, Strzelecki M, Materka A, Klepaczko A. MaZda—a software package for image texture analysis. Comput Methods Programs Biomed 2009; 94: 66–76. [DOI] [PubMed] [Google Scholar]
- 21.Alanko OME, Svedström-Oristo A-L, Peltomäki T, Kauko T, Tuomisto MT. Psychosocial well-being of prospective orthognathic-surgical patients. Acta Odontol Scand 2014; 22: 1–11. [DOI] [PubMed] [Google Scholar]
- 22.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern 1973; 3: 610–21. [Google Scholar]
- 23.Mathias JM, Tofts PS, Losseff NA. Texture analysis of spinal cord pathology in multiple sclerosis. Magn Reson Med 1999; 42: 929–35. [DOI] [PubMed] [Google Scholar]
- 24.Kassner A, Liu F, Thornhill RE, Tomlinson G, Mikulis DJ. Prediction of hemorrhagic transformation in acute ischemic stroke using texture analysis of postcontrast T1-weighted MR images. J Magn Reson Imaging 2009; 30: 933–41. doi: 10.1002/jmri.21940 [DOI] [PubMed] [Google Scholar]
- 25.Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 2004; 22: 81–91. doi: 10.1016/j.mri.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 26.Cobo T, Bonet-Carne E, Martínez-Terrón M, Perez-Moreno A, Elías N, Luque J, et al. Feasibility and reproducibility of fetal lung texture analysis by automatic quantitative ultrasound analysis and correlation with gestational age. Fetal Diagn Ther 2012; 31: 230–6. doi: 10.1159/000335349 [DOI] [PubMed] [Google Scholar]