Abstract
To date, the most frequently used Parkinson’s disease (PD) biomarkers are the brain imaging measures of dopaminergic dysfunction using positron emission tomography and single photon emission computed tomography. However, major advances have occurred in the development of magnetic resonance imaging (MRI) biomarkers for PD in the past decade. Although conventional structural imaging remains normal in PD, advanced techniques have shown changes in the substantia nigra and the cortex. The most well-developed MRI markers in PD include diffusion imaging and iron load using T2/T2* relaxometry techniques. Other quantitative biomarkers such as susceptibility-weighted imaging for iron load, magnetization transfer and ultra-high-field MRI have shown great potential. More sophisticated techniques such as tractography and resting state functional connectivity give access to anatomical and functional connectivity changes in the brain, respectively. Brain perfusion can be assessed using non-contrast-agent techniques such as arterial spin labelling and spectroscopy gives access to metabolites concentrations. However, to date these techniques are not yet fully validated and standardized quantitative metrics for PD are still lacking. This review presents an overview of new structural, perfusion, metabolic and anatomo-functional connectivity biomarkers, their use in PD and their potential applications to improve the clinical diagnosis of Parkinsonian syndromes and the quality of clinical trials.
Keywords: diffusion tensor imaging, magnetization transfer, relaxometry, resting state fMRI
Introduction
The diagnosis of Parkinson’s disease (PD) is mainly based on clinical features and does not rely on imaging biomarkers. Commonly used imaging techniques in PD include positron emission tomography (PET) and single photon emission computed tomography (SPECT), but conventional magnetic resonance imaging (MRI) is of little use in clinical practice. However, advances in structural and functional imaging have improved the capacity of MRI to detect changes in PD as well as to differentiate between PD and other Parkinsonian syndromes. MRI has provided several candidate biomarkers that have a potential to inform on the disease process. Biomarkers are quantitative characteristics which are used as indicators of biological or pathological states. In neuroimaging, biomarkers are measures derived from images that reflect the presence of diseases or their severity and that can be used for early diagnosis, prognosis or to monitor responses to therapeutic interventions. Biomarkers are expected to detect early neuropathological features and mechanisms underlying neurodegeneration in PD and to correlate with disease progression in order to allow the monitoring of disease status. Ideally, they should be able to detect preclinical changes. Reliable biomarkers need to be confirmed by independent studies.
Promising candidate biomarkers were able to detect changes at various levels of the central nervous system, including the substantia nigra (SN), the brainstem, the basal ganglia and the cortex. New MR contrasts and image analysis techniques have improved the visualization of the SN and other brainstem nuclei. Cortical lesions can be followed using various techniques that can detect changes in the thickness, volume and density of grey matter in the cortex. Quantitative imaging includes techniques such as relaxometry, susceptibility-weighted, diffusion and magnetization transfer (MT) imaging. Perfusion can be assessed using non-contrast-agent technique MRI and brain metabolism using magnetic resonance spectroscopy. Tractography was used to investigate anatomical connectivity and resting state functional MRI (rsfMRI) provides new information on functional connectivity changes in the brain of PD patients. Task-related activation functional MRI (fMRI) will not be reviewed here. In the SN, although most MRI biomarker candidates were not validated in experimental models and require confirmation in independent studies, it is clear that they have the potential to quantify the pathology and follow the disease progression of PD.
This article reviews new MRI techniques and their use in PD and explains how these techniques may be used to detect changes in the brain of PD patients and their relationships with Parkinsonian symptoms. A summary of these techniques is presented in Table 1 and Figure 1. Each imaging technique and their respective contribution to the study of PD will be presented in the following sections. They include structural imaging, techniques that can assess iron load, other quantitative techniques (e.g. MT, diffusion imaging), perfusion imaging, magnetic resonance spectroscopy and techniques that can assess anatomical and functional connectivity. For the review, the authors searched PubMed from 1990 until early 2013 by using the terms corresponding to each technique and the terms corresponding to the pathological conditions, i.e. Parkinson’s disease and related atypical Parkinsonian conditions.
Table 1.
MRI techniques.
| Method | Techniques | Information | Changes in PD |
|---|---|---|---|
| Structural | T1-w, T2-w, IR, MT | Morphometry | SN: variable volume changes Cortex: mild reduction in volume and thickness |
| Neuromelanin | Spin echo T1-w | Presence of melanin-containing cells | Reduced signal intensity |
| Magnetization transfer | Images with (MT) and without (M0) MT pulse | Degree of myelination, axonal density | Reduced MT ratio (MTR = M0 – MT/M0) |
| Relaxometry | T2/T2* measurements | Brain iron | Reduced T2/T2* |
| Increased R2/R2* | |||
| Susceptibility-weighted | Phase images | Brain iron | Increased susceptibility due to iron load |
| Diffusion | DTI | Diffusion of water in biological tissues | Reduced FA |
| Tractography | DTI | Fibre tract-specific reconstruction | Reduced probability of connections |
| Functional | Resting state BOLD fMRI | Functional connectivity within brain networks | Reduced FC in sensorimotor |
| Increased FC in associative | |||
| MR spectroscopy | 1H MRS | NAA, Cho, mIns, GABA, Glx, GSH, lactate | Trend for metabolite reduction |
| 31P MRS | Energy metabolism: ADP, PCr/ATP | Decreased ATP in midbrain | |
| Perfusion | ASL | rCBF | Reduced in cortex, Variable in basal ganglia |
1H MRS, proton magnetic resonance spectroscopy; 31P MRS, phosphorus magnetic resonance spectroscopy; ADP, adenosine diphosphate; ASL, arterial spin labelling; ATP, adenosine triphosphate; BOLD, blood oxygen level-dependent contrast; Cho, choline-containing compounds; DTI, diffusion tensor imaging; FA, fractional anisotropy; FC, functional connectivity; fMRI, functional magnetic resonance imaging; Glx, glutamate/glutamine; GSH, glutathione; IR, inversion recovery; mIns, myo-inositol; MT, magnetization transfer; MTR, magnetization transfer ratio; NAA, N-acetylaspartate; rCBF, regional cerebral blood flow; PCr, phosphocreatine; SN, substantia nigra; T2, T2 relaxation time; T2*, gradient echo T2 relaxation time; T1-w, T1-weighted; T2-w, T2-weighted; R2, T2 relaxation rate; R2*, T2* relaxation rate.
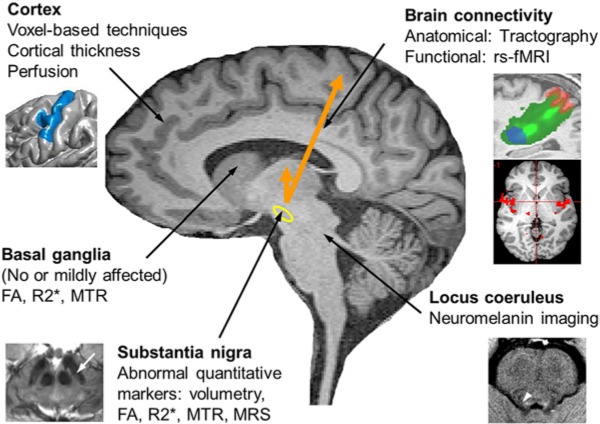
Figure 1.
Overview of MRI methods used to study PD. Cortex: changes were detected using voxel-based techniques, cortical thickness measurements and perfusion imaging. Brain connectivity was investigated using resting-state functional MRI (rs-fMRI) for functional connectivity and tractography for structural connectivity. Substantia nigra (yellow contour): changes were detected using diffusion imaging (reduced fractional anisotropy - FA), relaxometry (increased R2* indicating increased iron load and more recently susceptibility-weighted imaging), magnetization transfer ratio (MTR reduced) and spectroscopy. Basal ganglia: studies showed no or mild changes in FA, R2* or MTR. Locus coeruleus area (white arroxw head): reduced signal intensity was detected using neuromelanin imaging.
Structural imaging
Techniques for visualization and segmentation of brain regions
MRI sequences for visualization of the basal ganglia and the cortex
Conventional three-dimensional T1-weighted (T1-w) sequences present high grey/white matter contrast for cortical and some basal ganglia structures, but poor contrast in many structures of interest in PD (SN, subthalamic nucleus [STN], globus pallidus [GP], red nucleus [RN]). These latter structures contain high iron levels leading to shortened T1 and reduced contrast [Hardy et al. 2005]. Iron load is an advantage in T2-w and T2*-w sequences that use the T2 shortening effects of iron providing enhanced contrast. Susceptibility-weighted imaging (SWI) is promising in PD due to its improved sensitivity to brain mineralization [Haacke et al. 2005]. SWI uses the magnetic susceptibility differences in brain tissues [Haacke et al. 2005; Lotfipour et al. 2012; Schafer et al. 2012; Wang et al. 2012]. SWI images are sensitive to iron concentration and improve visualization of the SN [Haacke et al. 2005; Lotfipour et al. 2012; Schafer et al. 2012; Wang et al. 2012]. Proton-density and short tau inversion recovery (STIR) images were suggested as good alternatives. Other contrasts, such as the MT [Helms et al. 2009; Menke et al. 2010] and inversion recovery sequences [Hutchinson et al. 2003] also improved visualization of the SN. MT pulses increase contrast between the SN and the surrounding white matter, resulting in more accurate delineation and improved visualization of these structures [Helms et al. 2009; Menke et al. 2010]. Lastly, the increase in magnetic field provided by 7T human MRI, provided not only increased spatial resolution but also improved contrast, which allowed better visualization of basal ganglia contours and shapes. The volume and contours of the SN depend on the MRI contrast that was used [Oikawa et al. 2002]. For instance, contours that were shown using the T2-w sequences, which are sensitive to iron load, probably differed from those detected using inversion recovery and neuromelanin imaging.
The anatomy of the cortex is usually better depicted using conventional three-dimensional T1-weighted sequences which present high grey/white matter contrast.
Segmentation and volume calculation
Small structures such as the SN are usually outlined and segmented manually. Some cortical regions (e.g. the hippocampus, the striatum and the thalamus) can be segmented manually, but automated techniques are preferred as exploratory tools to detect grey and white matters changes in the cortex [Destrieux et al. 2010; Patenaude et al. 2011]. These techniques are designed for group studies, are automated, whole brain and rater independent. They include voxel-based, deformation-based and tensor-based morphometry, cortical thickness and sulci measurements. Voxel-based morphometry (VBM) is now a standard technique, sensitive to differences in grey and white matter [Ashburner and Friston, 2000]. VBM provides metrics such as the concentration, density and volume of grey matter. A major limitation of VBM is the nonspecificity with respect to the underlying tissue changes. Deformation-based and tensor-based morphometry provide information about global or local differences in shape, respectively [Apostolova and Thompson, 2007; Good et al. 2001]. Other software allows measurements of topographic differences in thickness, surface area and curvature of the cortex [Dale et al. 1999; Fischl et al. 1999; Makris et al. 2005].
Volume and shape changes in Parkinson disease
Substantia nigra
The volume of the SN is usually measured using manual segmentation. Results in PD are discordant with normal [Oikawa et al. 2002; Peran et al. 2010], reduced [Hutchinson et al. 2003; Menke et al. 2009; Minati et al. 2007] and even increased volumes [Cho et al. 2011; Kwon et al. 2012] (Table 1). The origin of this variability is probably due to differences in methodology between studies. First, the variability of volume measurements is large, given the small size of the structure. Second, different contrasts were used resulting in changes in SN contours. Third, some methods relied on tractography and anatomical connectivity changes which are completely different in nature as compared with other structural methods. Lastly, increased SN volume was obtained using 7T imaging and may be explained by the increased volume of regions with iron load. Inversion recovery methods were able to detect a predominant involvement of the lateral segments of the SN [Hutchinson et al. 2003; Minati et al. 2007] in line with the preferential degeneration of dopaminergic neurons in the caudal and lateral SN in histological studies [Redgrave et al. 2010]. Overall, although volume changes were detected in the SN of PD patients, changes varied between studies and the reproducibility was low. Ultra-high-field MRI may provide improved results due to increased spatial resolution.
Other basal ganglia and brainstem nuclei
Global measurements of brainstem volumes [Longoni et al. 2011; Oba et al. 2005] and rate of atrophy [Paviour et al. 2006] were usually normal in PD patients. Similarly, most studies did not find any global volumetric changes in the basal ganglia [Bonneville et al. 2005; Oba et al. 2005; Paviour et al. 2006]. Neuromelanin-sensitive imaging showed reduced signal intensity in the locus coeruleus in PD patients compared with controls, probably reflecting loss of neuromelanin-containing neurons [Sasaki et al. 2006] (Table 1). A reduction in signal intensity in the locus coeruleus/subcoeruleus area correlated with the percentage of atonia during rapid eye movement (REM) sleep suggesting that damage to this region is implicated in the pathophysiology of REM sleep behaviour disorder [Garcia-Lorenzo et al. 2013].
Cortex
The understanding of brain mechanisms underlying cognitive impairment is of particular interest in PD. Mild cognitive impairment is common in nondemented PD patients and is a risk factor of progression to dementia, which develops in about 30% of PD patients [Litvan et al. 2011; Svenningsson et al. 2012]. Impaired cognition in PD has a heterogeneous profile including attention, executive, visuospatial and memory deficits [Svenningsson et al. 2012]. Different lesions may explain the different cognitive deficits [Brooks and Pavese, 2010]. Executive dysfunction may be related to dopaminergic fronto-subcortical deficits whereas dementia may be associated with widespread atrophy in posterior parietal and temporal cortical areas, associated with Lewy body deposition or Alzheimer plaques, and with nondopaminergic neurotransmitter dysfunctions such as cholinergic deficiency [Brooks and Pavese, 2010; Ibarretxe-Bilbao et al. 2009, 2011a; Svenningsson et al. 2012]. Structural MRI is a powerful technique to detect cortical changes in PD and evidence for cortical damage will be presented below.
Nondemented cognitively intact PD
Cortical morphology in cognitively intact PD patients may be normal [Cerasa et al. 2011; Feldmann et al. 2008; Messina et al. 2011; Paviour et al. 2006; Rizzo et al. 2008; Seppi and Poewe, 2010; Tessitore et al. 2012a] or altered in frontal regions [Biundo et al. 2011; Burton et al. 2004; Karagulle Kendi et al. 2008] or in more widespread cortical regions including parietal, temporal and occipital areas [Jubault et al. 2011; Lyoo et al. 2010; Meppelink et al. 2010; Tinaz et al. 2011]. Variations may be related to changes in methodology or disease stage but changes were most often mild. Cortical atrophy in fronto-temporal regions accelerates with disease progression [Ibarretxe-Bilbao et al. 2012].
Mild cognitive impairment and dementia
In contrast, impaired cognition and dementia in PD were associated with more severe patterns of cortical atrophy [Beyer et al. 2007; Burton et al. 2004] as reviewed by Ibarretxe-Bilbao and colleagues [Ibarretxe-Bilbao et al. 2009, 2011a]. Regions predictive of cognitive decline included the hippocampus and parieto-temporal regions [Weintraub et al. 2012]. The Alzheimer’s disease pattern of brain atrophy may thus be a preclinical biomarker of impaired cognition in PD patients.
Cortical correlates of other nonmotor dysfunctions
Apart from impaired cognition and dementia, other nonmotor symptoms in PD include mood disorders, psychotic symptoms, hyposmia and sleep disorders [Brooks and Pavese, 2010]. Depression in PD patients was associated with structural changes in the limbic system including medial, orbitofrontal and temporal regions [Feldmann et al. 2008; Kostic et al. 2010] and the mediodorsal thalamus [Cardoso et al. 2009]. Visual hallucinations were associated with atrophy in limbic, paralimbic and neocortical areas in some studies [Ibarretxe-Bilbao et al. 2010, 2011b], but not others [Meppelink et al. 2010]. Olfactory dysfunction was related to grey matter atrophy in olfactory regions including the piriform cortex and amygdala [Wattendorf et al. 2009]. Nonmotor symptoms were thus associated with structural changes in specific related brain networks.
Morphometric and signal changes in other Parkinsonian syndromes
Volumetric MRI was efficient for differentiating Parkinsonian disorders such as progressive supranuclear palsy (PSP) and the Parkinson variant of multiple system atrophy (MSA-P) from PD. There changes were reviewed extensively [Seppi and Poewe, 2010]. In contrast to PD, where structural changes are subtle or absent, PSP patients present with extensive atrophy in the brainstem, basal ganglia and cortex. Midbrain atrophy in PSP has a characteristic shape described as the ‘penguin’ or ‘hummingbird’ sign [Kato et al. 2003; Righini et al. 2004] and can be quantified using various indexes [Oba et al. 2005; Paviour et al. 2006; Quattrone et al. 2008; Righini et al. 2004]. Using VBM, grey and white matter reductions have been constantly reported in PSP in the midbrain and more variably in the basal ganglia, the frontal cortex, the insula and the thalamus [Boxer et al. 2006; Brenneis et al. 2004; Cordato et al. 2005; Josephs et al. 2008; Lehericy et al. 2010; Messina et al. 2011; Padovani et al. 2006]. In MSA-P, atrophy and signal changes were observed in the putamen (hypointensity in the dorsolateral part and hyperintense rim around the putamen), the pons (cross sign), the middle cerebellar peduncle (T2 hyperintensity) and the cerebellum [Seppi and Poewe, 2010]. PSP patients had greater midbrain and superior cerebellar atrophy whereas MSA-P patients had greater pontine and middle cerebellar atrophy [Oba et al. 2005; Paviour et al. 2006; Quattrone et al. 2008; Righini et al. 2004]. In MSA-P patients atrophy was also evidenced in the cortex using VBM [Brenneis et al. 2003; Minnerop et al. 2007]. Quantitative volumetric measures of pontine and midbrain areas [Oba et al. 2005; Quattrone et al. 2008] were able to discriminate accurately PSP and MSA-P from PD patients or healthy controls.
Iron load
Iron load may be estimated using measurements of T2 and T2* relaxation times and more recently, using SWI (Table 1). T2 relaxation times characterize how fast water magnetization returns to equilibrium after perturbation by a radiofrequency pulse during an MRI sequence. R2 relaxation rates correspond to 1/T2. T2 relaxation times are characteristic of tissue composition and depend on its molecular structure. The apparent transverse relaxation time T2* (T2 star) is sensitive to the macroscopic and microscopic inhomogeneities of the magnetic field. Macroscopic inhomogeneities are linked to hardware imperfections or to the presence of tissue interfaces characterized by a significant gradient of susceptibility. Microscopic inhomogeneities are linked to the tissue microstructure. Many experiments have shown that T2/R2 and T2*/R2* relaxation rates are noninvasive estimates of iron content either in primate or postmortem studies in humans, with better results obtained using R2* [Hardy et al. 2005; Langkammer et al. 2010; Ordidge et al. 1994]. Improved relaxation measurement techniques have recently been proposed based on adiabatic pulse sequences (T1ρ and T2ρ) [Menke et al. 2010; Michaeli et al. 2005, 2007]. Changes in iron concentration also result in changes in the phase of the tissue relative to its surroundings, a property which is used in SWI to quantify iron load [Haacke et al. 2005; Lotfipour et al. 2012; Schafer et al. 2012; Wang et al. 2012] (Table 1).
In PD iron load is increased [Dexter et al. 1989]. T2 relaxometry has provided largely consistent and reproducible results with decreased T2/T2*/adiabatic T2ρ relaxation times and increased R2/R2* relaxation rates [Baudrexel et al. 2010; Dexter et al. 1989; Du et al. 2011; Graham et al. 2000; Martin et al. 2008; Michaeli et al. 2007; Ordidge et al. 1994; Peran et al. 2010; Wang et al. 2012; Zhang et al. 2010]. However, some studies observed a change in T2 only in a particular subgroup [Ordidge et al. 1994] or no change at all. A predominance of R2* changes in the lateral SNc [Martin et al. 2008], in line with the location of predominant cell loss reported in histological studies [Damier et al. 1999]. Changes in R2* in the SN correlated with the motor score of the UPDRS [Martin et al. 2008] or with the levodopa equivalent daily dose scores [Peran et al. 2010].
In PSP [Boelmans et al. 2012] and MSA-P, changes in T2 relaxation times affected more widespread regions including the striatum and the GP as shown using phase-contrast susceptibility imaging [Wang et al. 2012] or relaxometry [Arabia et al. 2010; Boelmans et al. 2012].
Other quantitative biomarkers
In addition to relaxation times and rates, other quantitative markers include magnetization transfer ratio (MTR) and diffusion metrics such as fractional anisotropy (FA) and mean diffusivity (MD).
Magnetization transfer
MT imaging is a technique which relies on the transfer of energy between highly bound protons, e.g. linked to macromolecules, and mobile protons, i.e. in free water [Wolff and Balaban, 1989] (Table 1). Highly bound protons linked to macromolecules are present within structures such as myelin. The amount of MT is thus considered to correlate with the degree of myelination [Rademacher et al. 1999] and axonal density [van Waesberghe et al. 1999]. MT is therefore used in diseases such as multiple sclerosis [Dousset et al. 1992]. MT provides a quantitative parameter, the MTR [Dousset et al. 1992]. The MTR was reduced in the SN as well as in other basal ganglia (GP, the putamen, caudate nucleus) in PD patients [Eckert et al. 2004; Tambasco et al. 2011].
Water diffusion
Diffusion MRI is technique that is sensitive to water diffusion in biological tissues [Le Bihan, 2003]. Diffusion MRI provides several indexes that characterize the overall displacement of molecules (apparent diffusion coefficient [ADC] and MD), the orientation of diffusion (FA) and the characteristics of diffusion along the main direction of diffusion (axial or longitudinal diffusivity) and perpendicular to it (radial or transverse diffusivity) [Le Bihan, 2003]. At the cellular level, anisotropy may result from the presence of obstacles to diffusion due to oriented structures such as the membrane, myelin, longitudinal filaments and cytoskeleton [Le Bihan, 2003]. Changes in axial diffusivity may reflect axonal damage whereas changes in radial diffusivity may reflect myelin damage. Although efforts are pursued to develop methods that better characterize brain changes [Alexander, 2008; Assaf et al. 2008; Jensen et al. 2005; Zhang et al. 2012], to date diffusion parameters suffer from a lack of specificity and poor knowledge of the underlying neuronal substrates.
In the SN of PD patients, changes in the orientation of diffusion, i.e. anisotropy, appeared more consistent than changes in diffusivity (Table 1). Most studies reported reduced anisotropy in early PD patients [Chan et al. 2007; Du et al. 2011; Peran et al. 2010; Vaillancourt et al. 2009; Yoshikawa et al. 2004; Zhan et al. 2012], however others did not [Menke et al. 2009, 2010]. Changes in diffusion kurtosis, a method that quantifies non-Gaussian water diffusion [Jensen et al. 2005] were also reported in PD patients [Wang et al. 2011]. In contrast, diffusivity was mostly unchanged in PD patients [Seppi and Poewe, 2010; Seppi and Schocke, 2005]. Reduction in FA in the SN inversely correlated with clinical severity in some studies [Chan et al. 2007; Zhan et al. 2012] but not in other studies [Du et al. 2011].
In the striatum, diffusion measures were most often normal [Cochrane and Ebmeier, 2013; Nicoletti et al. 2006; Paviour et al. 2007] although few studies reported increased diffusivity in the striatum and thalamus in PD [Peran et al. 2010; Zhan et al. 2012].
Diffusion imaging is also useful to discriminate PD from other Parkinsonian syndromes [Cochrane and Ebmeier, 2013]. In PSP, diffusivity was increased in the basal ganglia [Rizzo et al. 2008; Seppi et al. 2003], the midbrain [Blain et al. 2006; Seppi and Poewe, 2010; Seppi and Schocke, 2005], the superior cerebellar peduncle [Nicoletti et al. 2008], the precentral white matter [Ohshita et al. 2000] and the total white matter [Padovani et al. 2006]. In MSA-P, diffusivity was increased and anisotropy was decreased in the putamen, pons and middle cerebellar peduncle [Blain et al. 2006; Nicoletti et al. 2006; Paviour et al. 2007; Schocke et al. 2004] and MTR was reduced in the putamen [Eckert et al. 2004].
Differential diagnosis of Parkinsonian syndromes using quantitative biomarkers
Several studies have suggested that the combination of R2* and FA markers may better differentiate people with PD from healthy aged subjects with greater than 95% global accuracy [Du et al. 2011; Peran et al. 2010].
In PSP, increased diffusivity and reduction in T2 relaxation times were widespread involving the basal ganglia [Boelmans et al. 2012; Rizzo et al. 2008; Seppi et al. 2003], the midbrain [Blain et al. 2006; Seppi and Poewe, 2010; Seppi and Schocke, 2005], the superior cerebellar peduncle [Nicoletti et al. 2008] and the white matter [Ohshita et al. 2000; Padovani et al. 2006].
In MSA-P, increased diffusivity and reduced anisotropy was found in the putamen, pons and middle cerebellar peduncle [Blain et al. 2006; Nicoletti et al. 2006; Paviour et al. 2007; Schocke et al. 2004] and greater iron deposition in the putamen, using phase-contrast susceptibility imaging [Wang et al. 2012] or relaxometry [Arabia et al. 2010; Boelmans et al. 2012].
Tractography and anatomical connectivity
Tractography is a technique based on the anisotropy of water diffusion that allows reconstructing fibre tracts in the brain. Tracks are reconstructed by assuming that the main direction of diffusion in a voxel indicates the local orientation of white matter fibres [Dell’acqua and Catani, 2012; Mori et al. 1999]. Using tractography, diffusion measures (MD, FA) and connectivity measures (e.g. the number of tracks or connection probability between brain regions) can be calculated within the specific fibre tracks. Tractography has also been successfully used to parcel out the SN [Menke et al. 2009] and the basal ganglia into specific territories [Draganski et al. 2008; Lehericy et al. 2004].
In PD, reduced connectivity was observed between the SN and ipsilateral putamen and thalamus [Menke et al. 2009; Sharman et al. 2012] as well as in the sensorimotor circuit of the basal ganglia [Sharman et al. 2012] (Table 1). Automated diffusion-based parcellation of SN subregions showed that the SNr and SNc in PD patients showed a general atrophy [Menke et al. 2010]. Tractography is therefore used to investigate changes in anatomical connectivity in PD patients [Menke et al. 2009, 2010; Sharman et al. 2012].
Perfusion
Perfusion can be assessed noninvasively with MRI without contrast agent administration using arterial spin labelled (ASL) techniques [Detre et al. 1992]. ASL techniques have been recently introduced as a noninvasive alternative to PET or SPECT imaging for perfusion measurements in PD [Fernandez-Seara et al. 2012; Kamagata et al. 2011; Ma et al. 2010; Melzer et al. 2011]. In ASL, the tracer is endogenous arterial blood water that is labelled electromagnetically using radiofrequency (RF) irradiation [Detre et al. 2012]. ASL perfusion imaging provides quantitative measurements of cerebral blood flow [Detre et al. 2012].
Compared with healthy controls, perfusion in PD was decreased in the cortex [Fernandez-Seara et al. 2012; Kamagata et al. 2011; Melzer et al. 2011] and either preserved [Melzer et al. 2011] or decreased [Fernandez-Seara et al. 2012] in the basal ganglia and preserved in the sensorimotor areas [Melzer et al. 2011] (Table 1). Perfusion patterns were correlated with metabolic changes using fluorodeoxyglucose PET [Ma et al. 2010].
Metabolic biomarkers
Proton magnetic resonance spectroscopy (1H MRS) provides information about the levels of metabolites in the brain (Table 1). Metabolites that are evaluated include N-acetylaspartate (NAA; considered as a marker of neuronal number and health), choline-containing compounds (Cho; markers of demyelination and cell proliferation), myo-inositol (mIns; considered as a marker for osmotic stress or astrogliosis), lactate (frequently associated brain pathologies), glutathione (GSH) as well as neurotransmitters such as glutamate/glutamine or GABA (using specific editing techniques). Metabolite levels may be reported as their ratios over sum of creatine and phosphocreatine (Cr; which used a concentration reference) or as absolute concentrations. MRS is highly sensitive to magnetic field homogeneity and lipid artefact signals when the regions examined are too close to the skull. MRS of the SN is therefore very challenging in humans due to the location, small size and high iron content of the structure. Regions studied present partial voluming with the rest of the midbrain.
Using 1H-MRS, two groups reported changes in metabolites at 3T [Groger et al. 2011; Hattingen et al. 2009]. This was not confirmed at higher field as MRS at 4T [Oz et al. 2006] or 7T [Emir et al. 2012] did not show significant changes in the SN of PD patients. The authors only report a trend for Glu, NAA and GSH to decrease, and for Cho to increase [Oz et al. 2006].
Phosphorus magnetic resonance spectroscopy (31P MRS) allows direct monitoring of the energy metabolism of the brain [Ross and Bluml, 2001] (Table 1). PD patients showed abnormal phosphate metabolisms [Barbiroli et al. 1999; Hattingen et al. 2009; Hu et al. 2000] that suggested mitochondrial dysfunction of mesostriatal neurons [Barbiroli et al. 1999; Hattingen et al. 2009; Hu et al. 2000; Rango et al. 2006].
Functional connectivity
Initially designed to detect task-related signal changes in the brain, blood oxygen level-dependent (BOLD) fMRI is also increasingly used to study functional connectivity between distant brain regions. Spatially remote regions forming distributed cortical and subcortical networks can present temporal correlations of the fluctuations of their fMRI signal at rest [Biswal et al. 1995; Friston et al. 1993]. These networks are extracted from rsfMRI data using various mathematical approaches [Bullmore and Sporns, 2009; Ramnani et al. 2004] (Table 1).
Functional interactions in resting brain networks are abnormal in PD [Helmich et al. 2009; Hacker et al. 2012; Sharman et al. 2012; Skidmore et al. 2011b; Wu et al. 2011]. rsfMRI studies have shown that PD is associated with changes in cerebral connectivity between the basal ganglia and the cortex or cerebellum [Helmich et al. 2009] and between the STN and cortical motor and premotor areas [Baudrexel et al. 2011]. Changes predominate in the sensorimotor circuit with decreased functional coupling whereas functional connectivity was increased in the associative territory [Sharman et al. 2012; Hacker et al. 2012; Helmich et al. 2009]. Abnormal functional connectivity was also evidenced in the default-mode network in cognitively unimpaired PD patients that correlated with cognitive performances in memory and visuospatial tests [Tessitore et al. 2012b]. In drug-naïve PD patients, the supplementary motor area showed reduced signal fluctuations in PD whereas levodopa enhanced functional connectivity in this region [Esposito et al. 2013]. Changes were observed in a specific frequency band [Esposito et al. 2013]. Changes in resting state BOLD fluctuations were successful in predicting the presence of PD [Long et al. 2012; Skidmore et al. 2011b].
Changes in functional connectivity were differently associated with symptoms in PD. Tremor has been related to increased functional connectivity between the STN and the primary sensorimotor area [Baudrexel et al. 2011] and to abnormal functional interactions between the basal ganglia and the cerebello-thalamic circuit [Helmich et al. 2011]. Apathy was related to abnormal functional connectivity in the orbitofrontal, supplementary motor and middle frontal areas [Skidmore et al. 2011a]. In contrast, depression was associated with functional connectivity changes in the subgenual cingulate cortex [Skidmore et al. 2011a]. Increased amplitude of low-frequency BOLD signal oscillations in the premotor cortex predicted motor performances [Kwak et al. 2012].
In PSP, rsfMRI showed connectivity disruptions between the dorsal midbrain tegmentum and the cerebellum, diencephalum, basal ganglia and cortex that were associated with more severe functional impairment [Gardner et al. 2013]. Another study found disconnection between the thalamus and the striatum, supplementary motor area and cerebellum [Whitwell et al. 2011].
Overall, findings with resting state fMRI suggest that dopamine depletion in PD leads to a remapping of cerebral connectivity that affects predominantly the sensorimotor circuit and sensorimotor integration, that is influenced by levodopa and differently associated with motor and nonmotor symptoms. rsfMRI methods also showed reduced brainstem and thalamic connectivity in PSP that correlated with clinical deficits.
Conclusion
Over the last decade and a half, research in PD imaging has focused on developing quantitative techniques in the aim of providing measurements that can be used a markers of the disease process. In the SN, several techniques including diffusion imaging, T2* relaxometry and MT have shown great promises for detecting pathological changes. Investigators have developed computerized neuroanatomical techniques to study cortical changes that have been related to specific motor and nonmotor symptoms in the disease. MRI gives also access to the anatomical and functional connectivity changes in the brain of PD patients. MRI techniques could be used to monitor disease progression and to detect brain changes in preclinical patients or in patients at risk of developing PD, such as gait REM sleep behaviour disorder patients. However, to date these techniques suffer from the lack of standardization, particularly methods for extracting quantitative information from images, and of validation in large cohorts of subjects in longitudinal studies. It is expected that future efforts in imaging research will provide significant improvement in this respect.
Footnotes
Funding: This work was supported by ‘Investissements d’avenir’ (grant number ANR-10-IAIHU-06).
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributor Information
Nadya Pyatigorskaya, Institut du Cerveau et de la Moelle épinière, Centre de Neuroimagerie de Recherche, Paris, France; Service de neuroradiologie, Groupe Hospitalier Pitié-Salpêtrière, Paris, France.
Cécile Gallea, Institut du Cerveau et de la Moelle épinière, Centre de Neuroimagerie de Recherche, Paris, France; Université Pierre et Marie Curie (UPMC Univ Paris 6), Centre de Recherche de l’Institut du Cerveau et de la Moelle epiniere, Paris, France.
Daniel Garcia-Lorenzo, Institut du Cerveau et de la Moelle épinière, Centre de Neuroimagerie de Recherche, Paris, France; Université Pierre et Marie Curie (UPMC Univ Paris 6), Centre de Recherche de l’Institut du Cerveau et de la Moelle epiniere, Paris, France.
Marie Vidailhet, Université Pierre et Marie Curie (UPMC Univ Paris 6), Centre de Recherche de l’Institut du Cerveau et de la Moelle epiniere, Paris, France; Federation de Neurologie, Groupe Hospitalier Pitié-Salpêtrière, Paris, France.
Stéphane Lehericy, Service de neuroradiologie, Groupe Hospitalier Pitié-Salpêtrière, 47 boulevard de l’hopital, 75651 Paris cedex 13, France.
References
- Alexander D. (2008) A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magn Reson Med 60: 439–448 [DOI] [PubMed] [Google Scholar]
- Apostolova L., Thompson P. (2007) Brain mapping as a tool to study neurodegeneration. Neurotherapeutics 4: 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabia G., Morelli M., Paglionico S., Novellino F., Salsone M., Giofre L., et al. (2010) An magnetic resonance imaging T2*-weighted sequence at short echo time to detect putaminal hypointensity in Parkinsonisms. Mov Disord 25: 2728–2734 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K. (2000) Voxel-based morphometry - the methods. Neuroimage 11: 805–821 [DOI] [PubMed] [Google Scholar]
- Assaf Y., Blumenfeld-Katzir T., Yovel Y., Basser P. (2008) AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med 59: 1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli B., Martinelli P., Patuelli A., Lodi R., Iotti S., Cortelli P., et al. (1999) Phosphorus magnetic resonance spectroscopy in multiple system atrophy and Parkinson’s disease. Mov Disord 14: 430–435 [DOI] [PubMed] [Google Scholar]
- Baudrexel S., Nurnberger L., Rub U., Seifried C., Klein J., Deller T., et al. (2010) Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. Neuroimage 51: 512–520 [DOI] [PubMed] [Google Scholar]
- Baudrexel S., Witte T., Seifried C., von Wegner F., Beissner F., Klein J., et al. (2011) Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage 55: 1728–1738 [DOI] [PubMed] [Google Scholar]
- Beyer M., Janvin C., Larsen J., Aarsland D. (2007) A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 78: 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F., Haughton V., Hyde J. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541 [DOI] [PubMed] [Google Scholar]
- Biundo R., Formento-Dojot P., Facchini S., Vallelunga A., Ghezzo L., Foscolo L., et al. (2011) Brain volume changes in Parkinson’s disease and their relationship with cognitive and behavioural abnormalities. J Neurol Sci 310: 64–69 [DOI] [PubMed] [Google Scholar]
- Blain C., Barker G., Jarosz J., Coyle N., Landau S., Brown R., et al. (2006) Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology 67: 2199–2205 [DOI] [PubMed] [Google Scholar]
- Boelmans K., Holst B., Hackius M., Finsterbusch J., Gerloff C., Fiehler J., et al. (2012) Brain iron deposition fingerprints in Parkinson’s disease and progressive supranuclear palsy. Mov Disord 27: 421–427 [DOI] [PubMed] [Google Scholar]
- Bonneville F., Welter M., Elie C., du Montcel S., Hasboun D., Menuel C., et al. (2005) Parkinson disease, brain volumes, and subthalamic nucleus stimulation. Neurology 64: 1598–1604 [DOI] [PubMed] [Google Scholar]
- Boxer A., Geschwind M., Belfor N., Gorno-Tempini M., Schauer G., Miller B., et al. (2006) Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol 63: 81–86 [DOI] [PubMed] [Google Scholar]
- Brenneis C., Seppi K., Schocke M., Benke T., Wenning G., Poewe W. (2004) Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 75: 246–249 [PMC free article] [PubMed] [Google Scholar]
- Brenneis C., Seppi K., Schocke M., Muller J., Luginger E., Bosch S., et al. (2003) Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord 18: 1132–1138 [DOI] [PubMed] [Google Scholar]
- Brooks D., Pavese N. (2010) Imaging non-motor aspects of Parkinson’s disease. Prog Brain Res 184: 205–218 [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198 [DOI] [PubMed] [Google Scholar]
- Burton E., McKeith I., Burn D., Williams E., O’Brien J. (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127: 791–800 [DOI] [PubMed] [Google Scholar]
- Cardoso E., Maia F., Fregni F., Myczkowski M., Melo L., Sato J., et al. (2009) Depression in Parkinson’s disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage 47: 467–472 [DOI] [PubMed] [Google Scholar]
- Cerasa A., Messina D., Pugliese P., Morelli M., Lanza P., Salsone M., et al. (2011) Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord 26: 807–812 [DOI] [PubMed] [Google Scholar]
- Chan L., Rumpel H., Yap K., Lee E., Loo H., Ho G., et al. (2007) Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78: 1383–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Z., Oh S., Kim J., Park S., Kwon D., Jeong H., et al. (2011) Direct visualization of Parkinson’s disease by in vivo human brain imaging using 7.0T magnetic resonance imaging. Mov Disord 26: 713–718 [DOI] [PubMed] [Google Scholar]
- Cochrane C., Ebmeier K. (2013) Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology 80: 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordato N., Duggins A., Halliday G., Morris J., Pantelis C. (2005) Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 128: 1259–1266 [DOI] [PubMed] [Google Scholar]
- Dale A., Fischl B., Sereno M. (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194 [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E., Agid Y., Graybiel A. (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122: 1437–1448 [DOI] [PubMed] [Google Scholar]
- Dell’acqua F., Catani M. (2012) Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol 25: 375–383 [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre J., Leigh J., Williams D., Koretsky A. (1992) Perfusion imaging. Magn Reson Med 23: 37–45 [DOI] [PubMed] [Google Scholar]
- Detre J., Rao H., Wang D., Chen Y., Wang Z. (2012) Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging 35: 1026–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter D., Wells F., Lees A., Agid F., Agid Y., Jenner P., et al. (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52: 1830–1836 [DOI] [PubMed] [Google Scholar]
- Dousset V., Grossman R., Ramer K., Schnall M., Young L., Gonzalez-Scarano F., et al. (1992) Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 182: 483–491 [DOI] [PubMed] [Google Scholar]
- Draganski B., Kherif F., Kloppel S., Cook P., Alexander D., Parker G., et al. (2008) Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28: 7143–7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M., Styner M., Shaffer M., Sen S., Yang Q., et al. (2011) Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord 26: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T., Sailer M., Kaufmann J., Schrader C., Peschel T., Bodammer N., et al. (2004) Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 21: 229–235 [DOI] [PubMed] [Google Scholar]
- Emir U., Tuite P., Oz G. (2012) Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 Tesla proton MRS. PLoS One 7(1): e30918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Tessitore A., Giordano A., De Micco R., Paccone A., Conforti R., et al. (2013) Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain 136: 710–725 [DOI] [PubMed] [Google Scholar]
- Feldmann A., Illes Z., Kosztolanyi P., Illes E., Mike A., Kover F., et al. (2008) Morphometric changes of gray matter in Parkinson’s disease with depression: a voxel-based morphometry study. Mov Disord 23: 42–46 [DOI] [PubMed] [Google Scholar]
- Fernandez-Seara M., Mengual E., Vidorreta M., Aznarez-Sanado M., Loayza F., Villagra F., et al. (2012) Cortical hypoperfusion in Parkinson’s disease assessed using arterial spin labeled perfusion MRI. Neuroimage 59: 2743–2750 [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M., Dale A. (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195–207 [DOI] [PubMed] [Google Scholar]
- Friston K., Frith C., Liddle P., Frackowiak R. (1993) Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14 [DOI] [PubMed] [Google Scholar]
- Garcia-Lorenzo D., Longo-Dos Santos C., Ewenczyk C., Leu-Semenescu S., Gallea C., Quattrocchi G., et al. (2013) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136: 2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R., Boxer A., Trujillo A., Mirsky J., Guo C., Gennatas E., et al. (2013) Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 73: 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C., Ashburner J., Frackowiak R. (2001) Computational neuroanatomy: new perspectives for neuroradiology. Rev Neurol (Paris) 157: 797–806 [PubMed] [Google Scholar]
- Graham J., Paley M., Grunewald R., Hoggard N., Griffiths P. (2000) Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 123: 2423–2431 [DOI] [PubMed] [Google Scholar]
- Groger A., Chadzynski G., Godau J., Berg D., Klose U. (2011) Three-dimensional magnetic resonance spectroscopic imaging in the substantia nigra of healthy controls and patients with Parkinson’s disease. Eur Radiol 21: 1962–1969 [DOI] [PubMed] [Google Scholar]
- Haacke E., Cheng N., House M., Liu Q., Neelavalli J., Ogg R., et al. (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23: 1–25 [DOI] [PubMed] [Google Scholar]
- Hacker C., Perlmutter J., Criswell S., Ances B., Snyder A. (2012) Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 135: 3699–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P., Gash D., Yokel R., Andersen A., Ai Y., Zhang Z. (2005) Correlation of R2 with total iron concentration in the brains of rhesus monkeys. J Magn Reson Imaging 21: 118–127 [DOI] [PubMed] [Google Scholar]
- Hattingen E., Magerkurth J., Pilatus U., Mozer A., Seifried C., Steinmetz H., et al. (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132: 3285–3297 [DOI] [PubMed] [Google Scholar]
- Helmich R., Derikx L., Bakker M., Scheeringa R., Bloem B., Toni I. (2009) Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex 20: 1175–1186 [DOI] [PubMed] [Google Scholar]
- Helmich R., Janssen M., Oyen W., Bloem B., Toni I. (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69: 269–281 [DOI] [PubMed] [Google Scholar]
- Helms G., Draganski B., Frackowiak R., Ashburner J., Weiskopf N. (2009) Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. Neuroimage 47: 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Taylor-Robinson S., Chaudhuri K., Bell J., Labbe C., Cunningham V., et al. (2000) Cortical dysfunction in non-demented Parkinson’s disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain 123: 340–352 [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Raff U., Lebedev S. (2003) MRI correlates of pathology in Parkinsonism: segmented inversion recovery ratio imaging (SIRRIM). Neuroimage 20: 1899–1902 [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Marti M., Tolosa E. (2011a) Brain structural MRI correlates of cognitive dysfunctions in Parkinson’s disease. J Neurol Sci 310: 70–74 [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Marti M., Tolosa E. (2011b) Cerebral basis of visual hallucinations in Parkinson’s disease: structural and functional MRI studies. J Neurol Sci 310: 79–81 [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Segura B., Baggio H., Marti M., Valldeoriola F., et al. (2012) Progression of cortical thinning in early Parkinson’s disease. Mov Disord 27: 1746–1753 [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Ramirez-Ruiz B., Junque C., Marti M., Valldeoriola F., Bargallo N., et al. (2010) Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry 81: 650–657 [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Tolosa E., Junque C., Marti M. (2009) MRI and cognitive impairment in Parkinson’s disease. Mov Disord 24(Suppl. 2): S748–S753 [DOI] [PubMed] [Google Scholar]
- Jensen J., Helpern J., Ramani A., Lu H., Kaczynski K. (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53: 1432–1440 [DOI] [PubMed] [Google Scholar]
- Josephs K., Whitwell J., Dickson D., Boeve B., Knopman D., Petersen R., et al. (2008) Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 29: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubault T., Gagnon J., Karama S., Ptito A., Lafontaine A., Evans A., et al. (2011) Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage 55: 462–467 [DOI] [PubMed] [Google Scholar]
- Kamagata K., Motoi Y., Hori M., Suzuki M., Nakanishi A., Shimoji K., et al. (2011) Posterior hypoperfusion in Parkinson’s disease with and without dementia measured with arterial spin labeling MRI. J Magn Reson Imaging 33: 803–807 [DOI] [PubMed] [Google Scholar]
- Karagulle Kendi A., Lehericy S., Luciana M., Ugurbil K., Tuite P. (2008) Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol 29: 501–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Arai K., Hattori T. (2003) Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 210: 57–60 [DOI] [PubMed] [Google Scholar]
- Kostic V., Agosta F., Petrovic I., Galantucci S., Spica V., Jecmenica-Lukic M., et al. (2010) Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology 75: 857–863 [DOI] [PubMed] [Google Scholar]
- Kwak Y., Peltier S., Bohnen N., Muller M., Dayalu P., Seidler R. (2012) L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson’s disease: a resting state fMRI study. Front Syst Neurosci 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D., Kim J., Oh S., Jeong H., Park S., Oh E., et al. (2012) Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol 71: 267–277 [DOI] [PubMed] [Google Scholar]
- Langkammer C., Krebs N., Goessler W., Scheurer E., Ebner F., Yen K., et al. (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257: 455–462 [DOI] [PubMed] [Google Scholar]
- Le Bihan D. (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4: 469–480 [DOI] [PubMed] [Google Scholar]
- Lehericy S., Ducros M., Van de, Moortele P., Francois C., Thivard L., Poupon C., et al. (2004) Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55: 522–529 [DOI] [PubMed] [Google Scholar]
- Lehericy S., Hartmann A., Lannuzel A., Galanaud D., Delmaire C., Bienaimee M., et al. (2010) Magnetic resonance imaging lesion pattern in Guadeloupean Parkinsonism is distinct from progressive supranuclear palsy. Brain 133: 2410–2425 [DOI] [PubMed] [Google Scholar]
- Litvan I., Aarsland D., Adler C., Goldman J., Kulisevsky J., Mollenhauer B., et al. (2011) MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26: 1814–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Wang J., Xuan M., Gu Q., Xu X., Kong D., et al. (2012) Automatic classification of early Parkinson’s disease with multi-modal MR imaging. PLoS One 7(11): e47714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni G., Agosta F., Kostic V., Stojkovic T., Pagani E., Stosic-Opincal T., et al. (2011) MRI measurements of brainstem structures in patients with Richardson’s syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson’s disease. Mov Disord 26: 247–255 [DOI] [PubMed] [Google Scholar]
- Lotfipour A., Wharton S., Schwarz S., Gontu V., Schafer A., Peters A., et al. (2012) High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J Magn Reson Imaging 35: 48–55 [DOI] [PubMed] [Google Scholar]
- Lyoo C., Ryu Y., Lee M. (2010) Topographical distribution of cerebral cortical thinning in patients with mild Parkinson’s disease without dementia. Mov Disord 25: 496–499 [DOI] [PubMed] [Google Scholar]
- Ma Y., Huang C., Dyke J., Pan H., Alsop D., Feigin A., et al. (2010) Parkinson’s disease spatial covariance pattern: noninvasive quantification with perfusion MRI. J Cereb Blood Flow Metab 30: 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Schlerf J., Hodge S., Haselgrove C., Albaugh M., Seidman L., et al. (2005) MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage 25: 1146–1160 [DOI] [PubMed] [Google Scholar]
- Martin W., Wieler M., Gee M. (2008) Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70: 1411–1417 [DOI] [PubMed] [Google Scholar]
- Melzer T., Watts R., MacAskill M., Pearson J., Rueger S., Pitcher T., et al. (2011) Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain 134: 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke R., Jbabdi S., Miller K., Matthews P., Zarei M. (2010) Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson’s disease. Neuroimage 52: 1175–1180 [DOI] [PubMed] [Google Scholar]
- Menke R., Scholz J., Miller K., Deoni S., Jbabdi S., Matthews P., et al. (2009) MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage 47: 435–441 [DOI] [PubMed] [Google Scholar]
- Meppelink A., de Jong B., Teune L., van Laar T. (2010) Regional cortical grey matter loss in Parkinson’s disease without dementia is independent from visual hallucinations. Mov Disord 26: 142–147 [DOI] [PubMed] [Google Scholar]
- Messina D., Cerasa A., Condino F., Arabia G., Novellino F., Nicoletti G., et al. (2011) Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord 17: 172–176 [DOI] [PubMed] [Google Scholar]
- Michaeli S., Grohn H., Grohn O., Sorce D., Kauppinen R., Springer C., Jr, et al. (2005) Exchange-influenced T2rho contrast in human brain images measured with adiabatic radio frequency pulses. Magn Reson Med 53: 823–829 [DOI] [PubMed] [Google Scholar]
- Michaeli S., Oz G., Sorce D., Garwood M., Ugurbil K., Majestic S., et al. (2007) Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov Disord 22: 334–340 [DOI] [PubMed] [Google Scholar]
- Minati L., Grisoli M., Carella F., De Simone T., Bruzzone M., Savoiardo M. (2007) Imaging degeneration of the substantia nigra in Parkinson disease with inversion-recovery MR imaging. AJNR Am J Neuroradiol 28: 309–313 [PMC free article] [PubMed] [Google Scholar]
- Minnerop M., Specht K., Ruhlmann J., Schimke N., Abele M., Weyer A., et al. (2007) Voxel-based morphometry and voxel-based relaxometry in multiple system atrophy - a comparison between clinical subtypes and correlations with clinical parameters. Neuroimage 36: 1086–1095 [DOI] [PubMed] [Google Scholar]
- Mori S., Crain B., Chacko V., van Zijl P. (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45: 265–269 [DOI] [PubMed] [Google Scholar]
- Nicoletti G., Lodi R., Condino F., Tonon C., Fera F., Malucelli E., et al. (2006) Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain 129: 2679–2687 [DOI] [PubMed] [Google Scholar]
- Nicoletti G., Tonon C., Lodi R., Condino F., Manners D., Malucelli E., et al. (2008) Apparent diffusion coefficient of the superior cerebellar peduncle differentiates progressive supranuclear palsy from Parkinson’s disease. Mov Disord 23: 2370–2376 [DOI] [PubMed] [Google Scholar]
- Oba H., Yagishita A., Terada H., Barkovich A., Kutomi K., Yamauchi T., et al. (2005) New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 64: 2050–2055 [DOI] [PubMed] [Google Scholar]
- Ohshita T., Oka M., Imon Y., Yamaguchi S., Mimori Y., Nakamura S. (2000) Apparent diffusion coefficient measurements in progressive supranuclear palsy. Neuroradiology 42: 643–647 [DOI] [PubMed] [Google Scholar]
- Oikawa H., Sasaki M., Tamakawa Y., Ehara S., Tohyama K. (2002) The substantia nigra in Parkinson disease: proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR Am J Neuroradiol 23: 1747–1756 [PMC free article] [PubMed] [Google Scholar]
- Ordidge R., Gorell J., Deniau J., Knight R., Helpern J. (1994) Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 Tesla. Magn Reson Med 32: 335–341 [DOI] [PubMed] [Google Scholar]
- Oz G., Terpstra M., Tkac I., Aia P., Lowary J., Tuite P., et al. (2006) Proton MRS of the unilateral substantia nigra in the human brain at 4 Tesla: detection of high GABA concentrations. Magn Reson Med 55: 296–301 [DOI] [PubMed] [Google Scholar]
- Padovani A., Borroni B., Brambati S., Agosti C., Broli M., Alonso R., et al. (2006) Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 77: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S., Kennedy D., Jenkinson M. (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56: 907–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paviour D., Price S., Jahanshahi M., Lees A., Fox N. (2006) Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain 129: 1040–1049 [DOI] [PubMed] [Google Scholar]
- Paviour D., Thornton J., Lees A., Jager H. (2007) Diffusion-weighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord 22: 68–74 [DOI] [PubMed] [Google Scholar]
- Peran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., et al. (2010) Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133: 3423–3433 [DOI] [PubMed] [Google Scholar]
- Quattrone A., Nicoletti G., Messina D., Fera F., Condino F., Pugliese P., et al. (2008) MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 246: 214–221 [DOI] [PubMed] [Google Scholar]
- Rademacher J., Engelbrecht V., Burgel U., Freund H., Zilles K. (1999) Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. Neuroimage 9: 393–406 [DOI] [PubMed] [Google Scholar]
- Ramnani N., Behrens T., Penny W., Matthews P. (2004) New approaches for exploring anatomical and functional connectivity in the human brain. Biol Psychiatry 56: 613–619 [DOI] [PubMed] [Google Scholar]
- Rango M., Bonifati C., Bresolin N. (2006) Parkinson’s disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab 26: 283–290 [DOI] [PubMed] [Google Scholar]
- Redgrave P., Rodriguez M., Smith Y., Rodriguez-Oroz M., Lehericy S., Bergman H., et al. (2010) Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 11: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righini A., Antonini A., De Notaris R., Bianchini E., Meucci N., Sacilotto G., et al. (2004) MR imaging of the superior profile of the midbrain: differential diagnosis between progressive supranuclear palsy and Parkinson disease. AJNR Am J Neuroradiol 25: 927–932 [PMC free article] [PubMed] [Google Scholar]
- Rizzo G., Martinelli P., Manners D., Scaglione C., Tonon C., Cortelli P., et al. (2008) Diffusion-weighted brain imaging study of patients with clinical diagnosis of corticobasal degeneration, progressive supranuclear palsy and Parkinson’s disease. Brain 131: 2690–2700 [DOI] [PubMed] [Google Scholar]
- Ross B., Bluml S. (2001) Magnetic resonance spectroscopy of the human brain. Anat Rec 265(2): 54–84 [DOI] [PubMed] [Google Scholar]
- Sasaki M., Shibata E., Tohyama K., Takahashi J., Otsuka K., Tsuchiya K., et al. (2006) Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 17: 1215–1218 [DOI] [PubMed] [Google Scholar]
- Schafer A., Forstmann B., Neumann J., Wharton S., Mietke A., Bowtell R., et al. (2012) Direct visualization of the subthalamic nucleus and its iron distribution using high-resolution susceptibility mapping. Hum Brain Mapp 33: 2831–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schocke M., Seppi K., Esterhammer R., Kremser C., Mair K., Czermak B., et al. (2004) Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson’s disease. Neuroimage 21: 1443–1451 [DOI] [PubMed] [Google Scholar]
- Seppi K., Poewe W. (2010) Brain magnetic resonance imaging techniques in the diagnosis of parkinsonian syndromes. Neuroimaging Clin N Am 20: 29–55 [DOI] [PubMed] [Google Scholar]
- Seppi K., Schocke M. (2005) An update on conventional and advanced magnetic resonance imaging techniques in the differential diagnosis of neurodegenerative parkinsonism. Curr Opin Neurol 18: 370–375 [DOI] [PubMed] [Google Scholar]
- Seppi K., Schocke M., Esterhammer R., Kremser C., Brenneis C., Mueller J., et al. (2003) Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the Parkinson variant of multiple system atrophy. Neurology 60: 922–927 [DOI] [PubMed] [Google Scholar]
- Sharman M., Valabregue R., Perlbarg V., Marrakchi-Kacem L., Vidailhet M., Benali H., et al. (2012) Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord, in press. [DOI] [PubMed] [Google Scholar]
- Skidmore F., Yang M., Baxter L., von Deneen K., Collingwood J., He G., et al. (2011a) Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage, in press. [DOI] [PubMed] [Google Scholar]
- Skidmore F., Yang M., Baxter L., von Deneen K., Collingwood J., He G., et al. (2011b) Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage, in press. [DOI] [PubMed] [Google Scholar]
- Svenningsson P., Westman E., Ballard C., Aarsland D. (2012) Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol 11: 697–707 [DOI] [PubMed] [Google Scholar]
- Tambasco N., Belcastro V., Sarchielli P., Floridi P., Pierguidi L., Menichetti C., et al. (2011) A magnetization transfer study of mild and advanced Parkinson’s disease. Eur J Neurol 18: 471–477 [DOI] [PubMed] [Google Scholar]
- Tessitore A., Amboni M., Cirillo G., Corbo D., Picillo M., Russo A., et al. (2012a) Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Vitale C., Santangelo G., Amboni M., Russo A., et al. (2012b) Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79: 2226–2232 [DOI] [PubMed] [Google Scholar]
- Tinaz S., Courtney M., Stern C. (2011) Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord 26: 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt D., Spraker M., Prodoehl J., Abraham I., Corcos D., Zhou X., et al. (2009) High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 72: 1378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waesberghe J., Kamphorst W., De Groot C., van Walderveen M., Castelijns J., Ravid R., et al. (1999) Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 46: 747–754 [DOI] [PubMed] [Google Scholar]
- Wang J., Lin W., Lu C., Weng Y., Ng S., Wang C., et al. (2011) Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology 261: 210–217 [DOI] [PubMed] [Google Scholar]
- Wang Y., Butros S., Shuai X., Dai Y., Chen C., Liu M., et al. (2012) Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR Am J Neuroradiol 33: 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattendorf E., Welge-Lussen A., Fiedler K., Bilecen D., Wolfensberger M., Fuhr P., et al. (2009) Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci 29: 15410–15413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D., Dietz N., Duda J., Wolk D., Doshi J., Xie S., et al. (2012) Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 135: 170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J., Avula R., Master A., Vemuri P., Senjem M., Jones D., et al. (2011) Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord 17: 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S., Balaban R. (1989) Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 10: 135–144 [DOI] [PubMed] [Google Scholar]
- Wu T., Long X., Wang L., Hallett M., Zang Y., Li K., et al. (2011) Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp 32: 1443–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Nakata Y., Yamada K., Nakagawa M. (2004) Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry 75: 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W., Kang G., Glass G., Zhang Y., Shirley C., Millin R., et al. (2012) Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord 27: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C., Alexander D. (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61: 1000–1016 [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang Y., Wang J., Cai P., Luo C., Qian Z., et al. (2010) Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: an in vivo MR study. Brain Res 1330: 124–130 [DOI] [PubMed] [Google Scholar]