Abstract
Multiple sclerosis (MS), an inflammatory disease affecting the central nervous system, is considered to exhibit an important neurodegenerative component as well. Laquinimod is an orally administered quinoline-3-carboxamide under development for the treatment of MS. In vitro and animal studies have revealed various mechanisms by which laquinimod may exert its effects on the immune and nervous systems. These include effects on the innate immune system that promote the differentiation of anti-inflammatory/regulatory T cells, the activation of microglia cells, an increase in the expression of brain-derived neurotrophic factor, as well as the prevention of inflammation-induced excitotoxicity. Two phase III studies revealed the clinical benefits of laquinimod in patients with relapsing–remitting MS and exhibited a benign safety profile for this drug. Ongoing clinical trials will help to define the optimal dose and indication for laquinimod in MS. This article reviews current experimental and clinical evidence on the role of laquinimod in patients with this disabling disease.
Keywords: laquinimod, multiple sclerosis, neuroprotection, relapsing–remitting multiple sclerosis
Introduction
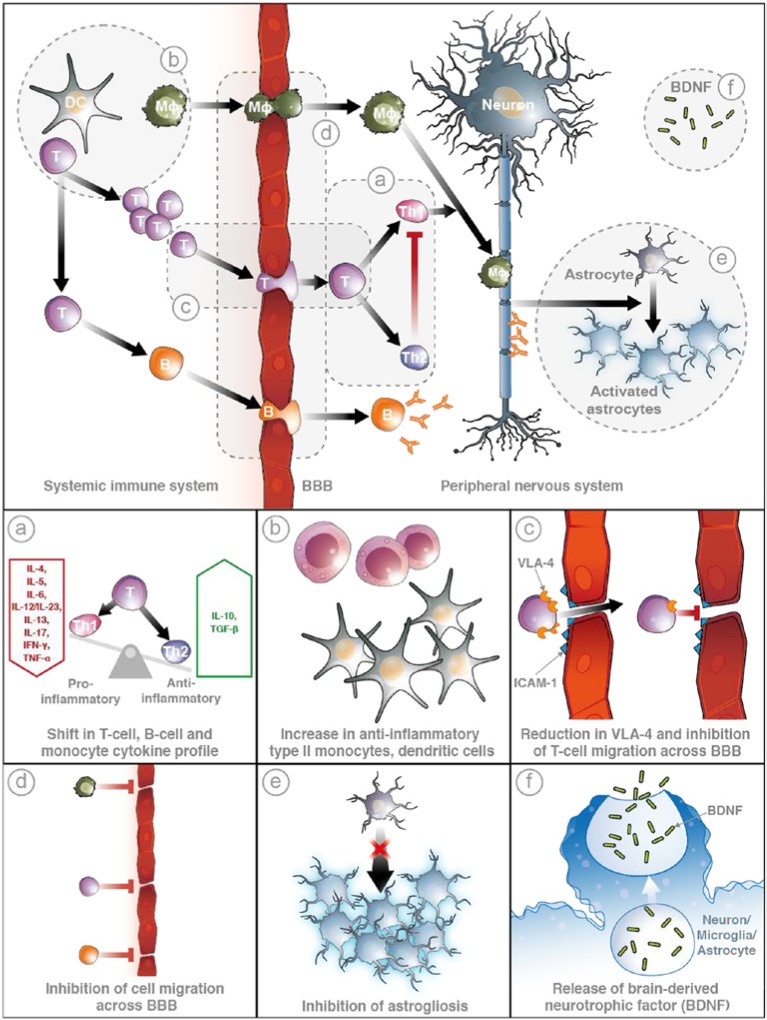
Multiple sclerosis (MS) is a chronic, autoimmune demyelinating disease of the central nervous system (CNS) [Loma and Heyman, 2011]. More than 85% of patients initially present with relapsing remitting MS (RRMS). Histopathologically, MS has been characterized by focal inflammatory infiltrates, demyelination, and in some cases remyelination, astrogliosis, and variable axonal damage within the CNS [Thöne and Gold, 2011]. Autoreactive T cells are considered to infiltrate the CNS and subsequently to release proinflammatory cytokines that activate phagocytic cells, leading to an inflammatory reaction that may be the source of this pathology [Brück and Wegner, 2011]. As a result, treatments for the disease have focused on modulating or reducing the migration of these cells to the CNS and mitigating the inflammatory response. Figure 1 illustrates the cascade of inflammatory events that result in the core symptoms of MS as well as the anti-inflammatory and neuroprotective actions of laquinimod, which will be discussed in this article.
Figure 1.
Upper panel: One hallmark of the pathology of multiple sclerosis (MS) is inflammation involving B cells, T cells, and macrophages, which results in tissue damage within the central nervous system (CNS). Dendritic cells present neural antigens, thereby stimulating the expansion of activated T-cell and B-cell populations that then migrate en masse through the blood–brain barrier (BBB) into the CNS. T cells differentiate into T helper 1 (Th1) and Th2 cells, the former stimulating macrophage activation. Correspondingly, B cells mature and produce immunoglobulin (Ig) G antibodies, which bind to the neuronal membrane, thereby targeting marked cells for phagocytic attack by activated macrophages. These inflammatory events stimulate astrogliosis, demyelination, axonal degeneration, and programmed cell death, and ultimately manifest in tissue damage and brain lesions, core physiologic symptoms of MS. Lower panels: Laquinimod acts at various points within the normal pathology of MS (a–f). Laquinimod promotes an anti-inflammatory system by altering the cytokine profile of T cells, B cells, and monocytes (a) and increasing the population of anti-inflammatory cells (b). Furthermore, laquinimod restricts cell migration by reducing the amount of VLA-4 adhesion molecule on the surface of T cells (c) and increasing the integrity of the BBB (d). Finally, laquinimod demonstrates its role in neuroprotection by inhibiting astrogliosis (e) and triggering the production and release of brain-derived neurotrophic factor (BDNF) (f). ICAM, intercellular adhesion molecule 1; IL, interleukin; IFN, interferon; TGF, transforming growth factor; TNF, tumor necrosis factor; VLA-4, very late antigen 4.
Magnetic resonance imaging (MRI) has shed new light on the pathologic mechanisms of MS; both focal and widespread diffuse damage detected by MRI may occur in normal-appearing white and grey matter, even in the earliest stages of disease [Thöne and Gold, 2011; Filippi and Rocca, 2005; Brück et al. 2012 ]. These discoveries have helped our understanding of MS: we now recognize it to be a diffuse CNS disease, with an important neurodegenerative component and a partially dissociated inflammatory component [Filippi and Rocca, 2005]. The inflammatory process is also thought to contribute, in part, to the neurodegenerative process, and it is likely that, once triggered, neurodegeneration becomes a self-perpetuating process that is responsible for disease progression [Confavreux and Vukusic, 2006]. Thus, to improve MS therapy, agents are needed that effectively protect against both the inflammatory and the neurodegenerative components of this disease.
All currently available agents are approved for relapsing forms of MS and are classified as either immunomodulatory or immunosuppressive in nature. Interferon β (IFNβ), the polypeptide glatiramer acetate, and the monoclonal antibody natalizumab are administered as injections or infusions, which can be problematic in some patients who require chronic treatment [Fernandez, 2011]. The sphingosine 1-phosphate receptor modulator fingolimod is the first oral treatment approved for RRMS [Hemmati et al. 2013], recently followed by teriflunomide [Oh and O’Connor, 2013] and dimethyl fumarate [Gold et al. 2013]. All these agents have differing modes of action, but their efficacy is thought to be related to the restoration of a dysregulated immune response and prevention of autoreactive T-cell migration into the CNS. None of these agents has proven neuroprotective characteristics and none is completely effective in halting the disease. In addition, some side effects of these agents can be severe or life threatening.
Several agents in development hold promise. This review focuses on laquinimod, which is in late-stage clinical development. The studies designed to elucidate laquinimod’s mode of action will be summarized and how this mode of action might translate into clinical benefit in MS will be explored.
Clinical development
Laquinimod is an orally administered quinoline-3-carboxamide small-molecule derivative of the parent compound, the immunomodulator linomide. Laquinimod was developed as a therapy for MS because it lacks the safety concerns seen with the parent compound. In preclinical studies, evidence has accumulated suggesting that laquinimod may exhibit immunomodulatory and potentially neuroprotective properties [Thöne and Gold, 2011]. The clinical development of laquinimod has progressed through one phase II, one phase IIb, and two phase III clinical studies, all of which have provided further evidence for its neuroprotective effects and clinical benefit [Comi et al. 2008, 2010, 2012; Vollmer et al. 2011; Polman et al. 2005].
Mode of action
Pharmacokinetics/pharmacodynamics
Laquinimod has a high level of oral bioavailability, a small distribution volume, and a low rate of total clearance. The maximum plasma concentration is reached within the first hour following its administration and is less than 5 μM after the administration of 0.05–2.4 mg of the drug. Laquinimod is metabolized through one of the cytochrome P450 (CYP) enzymes and is a substrate with low affinity for CYP3A4 in liver microsomes [Fernandez, 2011]. The molar mass of laquinimod is 356.803 g/mol. As a small molecule, laquinimod diffuses freely across the blood–brain barrier without any known active transport by extra- or intracellular receptor [Brück and Wegner, 2011].
Laquinimod in the immune system
Although our understanding of laquinimod’s mechanisms of action is incomplete, studies in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, have provided much information about its immunomodulatory effects. These include decreasing the number of proinflammatory immune cells by decreasing the expression of proinflammatory genes and by activating anti-inflammatory genes. In vitro studies and animal models revealed that laquinimod exhibits anti-inflammatory properties. Laquinimod may inhibit proinflammatory T cells from crossing the blood–brain barrier, thus reducing the extent of damage to the brain and spinal cord (Figure 1). In a rat model, laquinimod reduced the entry of proinflammatory T cells from peripheral blood, spleen, and lymph nodes into the CNS, reducing the relative proportion of proinflammatory cytokines, such as tumor necrosis factor α (TNFα) and interleukin (IL)-12, whereas the relative proportion of the anti-inflammatory cytokines transforming growth factor β (TGFβ) and IL-4 was found to be increased [Brück and Wegner, 2011]. This effect translated in a dose-dependent manner to clinical efficacy [Brück and Wegner, 2011]. An increase in regulatory T cells that suppressed the immune response was also found in EAE mice [Mishra et al. 2012; Schulze-Topphoff et al. 2012].
Another study in EAE mice found that quinolone-3-carboxamides bind to the S100A9 protein, which is expressed on the surface of various monocyte populations in the peripheral blood [Björk et al. 2009]. The quinolone-3-carboxamides inhibited the interaction of S100A9 with two receptors, toll-like receptor 4 and receptor of advanced glycation end products in a dose-dependent manner. This prevented the downstream release of inflammatory cytokines, including TNFα and IL-1 [Björk et al. 2009].
Laquinimod modulates B cells and their regulatory effects on T cells in RRMS. In a study assessing the immunomodulatory effects of laquinimod on B and CD4+ T cells, laquinimod reduced levels of IL-4 while increasing regulatory B-cell markers (CD25, IL-10, and CD86) [Toubi et al. 2012]. In murine models, laquinimod reduced the capacity of human monocyte-derived dendritic cells to induce CD4+ T-cell proliferation and secretion of proinflammatory cytokines [Jolivel et al. 2013]. In immunized mice, laquinimod significantly reduced CD45-positive cellular infiltrates, which reached the same levels as seen in healthy, nonimmunized mice [Brunmark et al. 2002]. At the same time, it increased levels of the anti-inflammatory cytokines IL-10 and TGFβ in both B and T cells, suppressing immune activity and downregulating immunogenicity of dendritic cell response [Toubi et al. 2012]. However, the fact that laquinimod increases the number of T and B cells in the spleen in immunized mice might indicate that it is not generally immunosuppressive but rather acts as an immunomodulator [Brunmark et al. 2002]. Moreover, laquinimod reduced monocyte chemoattraction; this resulted in decreased chemokine production in mature dendritic cells [Jolivel et al. 2013]. In laquinimod-treated patients, reduced chemokine and cytokine secretion by conventional CD1c+ dendritic cells was found upon lipopolysaccharide stimulation, and the number of conventional CD1c+ and plasmacytoid CD303+ dendritic cells was decreased within peripheral blood mononuclear cells [Jolivel et al. 2013].
Laquinimod also exhibits the effects of cell migration: it may reduce the entry of proinflammatory monocytes into the CNS by lowering levels of matrix metalloproteinase 9, which regulates the trafficking of monocytes into inflamed tissues [Mishra et al. 2012]. Furthermore, laquinimod treatment reduced the ability of very late antigen (VLA)-4 to integrate chemokine signaling. Laquinimod has been shown to downregulate VLA-4-mediated adhesiveness in mouse models [Wegner et al. 2010]. The binding affinity of VLA-4 to vascular cell adhesion molecule 1 was also reduced.
Laquinimod in the CNS
Because laquinimod freely diffuses across the blood–brain barrier, it can reach the CNS and may exert direct or indirect neuroprotective effects [Brück and Wegner, 2011; Toubi et al. 2012; Ruffini et al. 2013]. Mechanisms that have been proposed for neuronal and axonal damage in EAE and MS include effects driven by an inflammatory milieu, mitochondrial dysfunction, and glutamate toxicity [Ruffini et al. 2013]. The actual cause of damage may be one or some combination of these. Experimental evidence suggests that laquinimod may be able to inhibit some of these effects.
On the inflammatory level within the CNS, experimental studies have shown that laquinimod decreases the activation of microglia [Brück and Wegner, 2011]. In a toxic model, in which demyelination is induced by cuprizone, laquinimod reduced microglial density within the corpus callosum. Laquinimod-treated mice also displayed significantly fewer T cells than controls [Brück et al. 2012].
Laquinimod may increase expression of neurotrophins, such as brain-derived neurotrophic factor (BDNF), that are necessary for the maintenance of neurons and axons in the CNS (Figure 1) [Thöne et al. 2012]. It has been proposed that laquinimod’s efficacy may even be dependent upon BDNF, as the beneficial effect of laquinimod is reduced in mice with a conditional deficiency in BDNF in immune cells. In mice with EAE, adoptive transfer of laquinimod-stimulated monocytes ameliorated the course of the disease [Thöne et al. 2012]. Moreover, in blood samples from 203 patients with MS treated with laquinimod 0.6 mg/day, 76% showed a significant increase in BDNF serum levels compared with baseline and with samples from placebo-treated patients. Some samples showed up to an 11-fold increase in serum BDNF levels [Thöne et al. 2012].
Modulation of astrocytic activation has been postulated as yet another mechanism of action of laquinimod (Figure 1). Downregulation of the astrocytic proinflammatory response appears to preserve oligodendrocytes, myelin, and axons [Brück et al. 2012]. Animal and in vitro studies on cuprizone-induced demyelination have shown that the density of apoptotic oligodendrocytes in the corpus callosum was significantly lower in laquinimod-treated mice than in controls [Brück et al. 2012].
Pretreatment with 250 nM and 2.5 μM of laquinimod significantly reduced the nuclear factor κB (NFκB) activity induced after TNFα stimulation in primary human astrocytes in vitro compared with stimulated controls [Brück et al. 2012]. Pretreatment with 2.5 μM of laquinimod also significantly reduced NFκB activation after stimulation with the combination of IL-1β and IFNβ compared with stimulated controls. In cuprizone-treated mice, laquinimod attenuated astrocytic NFκB activation by 46%, thereby preventing cuprizone-induced demyelination [Brück et al. 2012].
In EAE, synaptic alterations have been described [Ruffini et al. 2013]. In an experimental study, laquinimod prevented alterations of GABAergic synapses induced by EAE. In addition, laquinimod treatment also preserved cannabinoid receptor type 1, receptor sensitivity normally lost during EAE. Laquinimod was also able to regulate synaptic transmission by increasing inhibitory postsynaptic currents and, at the same time, reducing excitatory postsynaptic currents, pointing to novel, potentially neuroprotective properties of this drug [Ruffini et al. 2013].
From bench to bedside
The clinical effects of laquinimod reflect the reduction of inflammation and demyelination seen in recent studies in animal models. Phase II studies investigating 0.1 mg/day, 0.3 mg/day, and 0.6 mg/day of laquinimod versus placebo found a significant reduction in the cumulative number of active [i.e. MRI gadolinium-enhanced (Gd+)] lesions with laquinimod treatment (Table 1) [Comi et al. 2008, 2010, 2012; Vollmer et al. 2011; Polman et al. 2005]. In each phase II study, relapse rate and disability were also reported on an exploratory/tertiary basis.
Table 1.
Clinical efficacy and safety of laquinimod.
| Polman et al. [2005] | Comi et al. [2008] LAQ/5062 | Comi et al. [2012] ALLEGRO | Vollmer et al. [2014] BRAVO | |
|---|---|---|---|---|
| Study design | Phase II double blind, multicenter | Phase IIb double blind, multicenter | Phase III double blind, multicenter | Phase III multinational, multicenter, randomized, double blind, parallel group, placebo controlled |
| Study period | 24 weeks | 24 weeks and 36 weeks (active extension period) | 24 months | 24 months |
| Patients, n | 67 placebo | 102 placebo | 556 placebo | 450 placebo |
| 68 LAQ 0.1 mg/day | 98 LAQ 0.3 mg/day | 550 LAQ 0.6 mg/day | 434 LAQ 0.6 mg/day | |
| 74 LAQ 0.3 mg/day | 106 LAQ 0.6 mg/day | 447 IFNβ-1a 30 µg/week | ||
| Key efficacy results | Primary endpoint | Primary endpoint | Primary endpoint | Primary endpoint |
| Mean cumulative number of active lesions: 5.24 (0.3 mg LAQ) versus 9.44 (placebo) after 24 weeks of treatment, i.e., 44% reduction by active treatment | 40.4% reduction of baseline adjusted mean number of Gd+ lesions per scan reported with LAQ, 0.6 mg versus placebo (4.2 versus 2.6; p = 0.0048) | Mean ARR significantly reduced in treatment group versus placebo (0.30 ± 0.02 versus 0.39 ± 0.03; p = 0.002) | Mean ARR reduced by 18% with LAQ (0.82; 95% CI 0.66–1.02; p = 0.075) and by 26% with IFNβ-1a, (0.74; 95% CI 0.60–0.92; p = 0.007), versus placebo | |
| 62.9% versus 52.2% in LAQ and placebo group, respectively, relapse free | ||||
| Risk of relapse significantly reduced in LAQ treatment arm (HR 0.72; 95% CI 0.59–0.87; p < 0.001) | ||||
| Secondary endpoints | Secondary endpoints | Secondary endpoints | Exploratory endpoints | |
| Proportion of patients with active scans at weeks 8, 16, and 24: 36.5%, 31.1%, and 20.6% in placebo, 0.1 mg, and 0.3 mg groups | When weeks 12–36 included, 51% reduction in mean number of Gd+ lesions in treatment arm versus placebo arm (2.7 versus 4.4; p < 0.0001). | EDSS scores significantly decreased in LAQ-treated patients versus placebo (11.1% versus 15.7%; HR 0.64; p = 0.01) | PBVC significantly reduced in LAQ group versus placebo at 24 months (treatment effect 0.28%; p < 0.001) | |
| Subgroup of patients with at least one active lesion at baseline: significant difference in number of active MRI scans between 0.3 mg and placebo groups (p = 0.024) | Median number of Gd+ lesions reduced by 60% (6.0 and 15.0, LAQ and placebo groups respectively) | LAQ reduced mean cumulative number of Gd+ lesions versus placebo (rate ratio 0.63; p < 0.001) | PBVC not affected by IFNβ-1a | |
| No significant changes in clinical measures between groups | Cumulative number of new T2 lesions reduced by 44% in LAQ 0.6 mg versus placebo group [simple means 6.4 (14.8) versus 9.4 (12.9); p =0.0013) | 31% and 26% reduction in risk of disability worsening at 3 months with LAQ and IFNβ-1a, respectively, versus placebo | ||
| ARR rate of 0.52 and 0.77 for treatment and placebo groups respectively (p = 0.0978), with 70.8% of LAQ-treated patients relapse-free versus 62.7% in placebo group | EDSS progression significantly reduced with LAQ versus placebo at 6 months (41%; p = 0.042) | |||
| Reductions in the IFNβ-1a group but not significant (28%; p = 0.14) | ||||
| LAQ significantly reduced brain atrophy versus IFNβ-1a (adjusted mean difference 0.42%; 95% CI 0.28–0.56; p < 0.0001) | ||||
| Long-term follow up | Post hoc analyses of efficacy outcomes | |||
| Patients switched from placebo to LAQ 0.3 or 0.6 mg: 52% reduction in mean number of Gd+ lesions from entry values (4.45 ± 6.55 versus 2.12 ± 3.73; p = 0.0006) | Mean ARR reduced significantly with LAQ (0.79; 95% CI 0.66–1.02; p = 0.026) and IFNβ-1a (0.71; 95% CI 0.58–0.89; p = 0.002) versus placebo once adjusted | |||
| Mean value of new T2 and T1 hypointense lesions lower in 0.6 mg versus 0.3 mg LAQ groups | ||||
| Patients switched from placebo to LAQ 0.3 and 0.6 mg: drop in relapse rate (0.54–0.38 in 0.3 mg and 0.55–0.39 in 0.6 mg) | ||||
| No significant changes in clinical measures between groups | ||||
| Key safety results | Four treatment-emergent SAEs: one each in placebo and LAQ 0.1 mg groups, two in LAQ 0.3 mg group | AE frequency similar in all three groups (84.7% LAQ 0.3 mg, 77.4% LAQ 0.6 mg, 82.4% placebo) | No deaths in treatment group; three patients died in placebo group | Safety was evaluable in: |
| Two follow-up SAEs reported in LAQ 0.3 mg group | No deaths during study period | 122 SAEs reported in 11.1% and 9.5% of treatment and placebo groups, respectively | 433 LAQ | |
| Two patients withdrew during 24-week study period | SAEs: 5.1%, 2.8% and 4.9% in LAQ 0.3 mg, 0.6 mg and placebo groups, respectively | More patients in treatment arm versus placebo arm had ALT levels >3 times the ULN but ≤5 times the ULN [18 (3.6%) versus 2 (0.4%) patients] | 499 placebo | |
| Small increase in elevated liver enzyme levels in treatment arms versus placebo (34%, 34%, and 47% in placebo and LAQ 0.1 mg and 0.3 mg groups respectively) | Elevated liver enzymes reported in a dose-dependent manner (LAQ 0.3 mg: 23.4%; LAQ 0.6 mg: 33.0%; placebo: 10.8%) | Frequency of ALT levels >5 times the ULN equal between groups | 442 IFNβ-1a | |
| Liver enzyme elevations appeared to decrease over time in extension study and returned to normal in all three arms | 130 SAEs reported in 7.2%, 8.0%, and 5.7% of LAQ, placebo, and IFNβ-1a groups, respectively | |||
| Discontinuations due to AEs reported in 5%, 4%, and 6% of LAQ, placebo, and IFNβ-1a groups, respectively | ||||
| Abdominal pain (+upper) and headache led to discontinuation more often in LAQ versus placebo groups | ||||
| Influenza-like illness, pyrexia, toxic hepatitis, and myalgia led to discontinuation more often in the IFNβ-1a group | ||||
| Two deaths occurred that were unrelated to study drug: one in LAQ and one in IFNβ-1a group |
AE, adverse event; ALLEGRO, Assessment of Oral Laquinimod in Preventing Progression in Multiple Sclerosis; ALT, alanine aminotransferase; ARR, annualized relapse rate; BRAVO, Benefit–Risk Assessment of AVonex and LaquinimOd; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhanced; HR, hazard ratio; IFN, interferon; LAQ, laquinimod; MRI, magnetic resonance imaging; PBVC, percent brain volume change; SAE, serious adverse event; ULN, upper limit of normal range.
In a multicenter, double-blind, parallel-group study, 209 patients received laquinimod 0.1 mg/day, laquinimod 0.3 mg/day, or placebo for 24 weeks [Polman et al. 2005]. The primary objective was the mean cumulative number of active lesions between week 0 and week 24. Eight weeks after the discontinuation of therapy, brain MRI showed that patients treated with laquinimod 0.3 mg/day had a 44% reduction in the mean cumulative number of active lesions compared with placebo (p = 0.0498), while in a subgroup of patients, the reduction in active lesions reached 52% (p = 0.005). However, in this relatively short treatment period, the mean number of relapses and the mean Expanded Disability Status Scale (EDSS) and Multiple-Sclerosis Functional Composite scores did not differ significantly between treatment groups [Comi et al. 2008].
In a phase IIb study designed to assess the efficacy, tolerability, and safety of laquinimod, 306 patients with RRMS were randomized to receive laquinimod 0.3 or 0.6 mg/day or placebo for 36 weeks [Comi et al. 2008]. Efficacy was determined by the mean cumulative number of Gd+ lesions during the last 12 weeks of the treatment period. Laquinimod 0.6 mg/day significantly reduced the baseline-adjusted mean cumulative number of Gd+ lesions (40.4%; p = 0.005), whereas laquinimod 0.3 mg/day failed to demonstrate a significant effect compared with placebo [Comi et al. 2008]. In a 36-week double-blind extension study, 257 patients received either laquinimod 0.3 or 0.6 mg/day. Patients who were switched from placebo to laquinimod 0.3 or 0.6 mg/day had a 52% reduction in active lesions (p < 0.0006); however, the effect on clinical scores, a secondary endpoint, was not statistically significant [Comi et al. 2010]. With the 0.6 mg/day dose, the effect on different parameters of disease activity was sustained for a further 2 years.
These encouraging results from the phase II program prompted two large phase III studies that investigated the clinical efficacy and safety of laquinimod in larger patient cohorts: Assessment of Oral Laquinimod in Preventing Progression in Multiple Sclerosis (ALLEGRO) and Benefit–Risk Assessment of AVonex and LaquinimOd (BRAVO). Because the phase II studies showed a more rapid onset of action and greater efficacy for 0.6 mg compared with 0.3 mg, with equivalent safety and tolerability, in these phase III trials, laquinimod 0.6 mg/day was compared with placebo (ALLEGRO [Comi et al. 2012] and BRAVO [Vollmer et al. 2011]) and INFβ-1a (BRAVO) in patients with RRMS.
ALLEGRO
This randomized, double-blind trial assessed the safety, efficacy, and tolerability of laquinimod 0.6 mg/day versus placebo [Comi et al. 2012]. The study population comprised 1106 patients at 139 sites in 24 countries. The primary endpoint was the annualized relapse rate during the 24-month follow-up period; secondary outcomes were disability progression, defined as increase in EDSS score sustained for at least 3 months, and the number of Gd+ lesions, as well as new or enlarging lesions on T2-weighted MRI at 12 and 24 months. Laquinimod significantly reduced the mean annualized relapse rate compared with placebo (0.30 ± 0.02 versus 0.30 ± 0.03; p = 0.002). Of laquinimod-treated patients, 62.9% were relapse free compared with 52.2% of those receiving placebo (p < 0.001). Risk of confirmed disability progression was also modestly but significantly reduced compared with placebo [11.1% versus 15.7%; hazard ratio 0.64; 95% confidence interval (CI) 0.45–0.91; p = 0.01]. The mean cumulative numbers of Gd+ lesions and new or enlarging lesions on T2-weighted MRI were lower for laquinimod-treated patients compared with placebo (1.33 ± 0.14 versus 2.12 ± 0.22 and 5.03 ± 0.08 versus 7.14 ± 0.07 respectively) [Comi et al. 2012].
Potential neuroprotective effects of laquinimod in RRMS were investigated as part of the ALLEGRO study extension using 3D T1-weighted images, magnetization transfer ratio (MTR) of white matter, grey matter, normal-appearing brain tissue, and T2 lesions [Filippi et al. 2012]. White matter N-acetylaspartate and creatine (NAA/Cr) levels were also assessed using proton 1H-magnetic resonance spectroscopy. Compared with placebo, patients treated with laquinimod showed lower percentages of white and grey matter and thalamic volume loss (both p < 0.01 at month 12) and a reduction in the number of persistent black holes at 12 and 24 months (p < 0.01). The white matter NAA/Cr ratio tended to increase with laquinimod and decrease with placebo (p = 0.17); MTR decreased significantly in white matter (p = 0.04) and normal-appearing brain tissue (p = 0.05) with placebo but not with laquinimod. These results from a variety of MRI measures suggest a neuroprotective effect of oral laquinimod in patients with RRMS and are consistent with the drug’s beneficial effects on the progression of disability [Filippi et al. 2012].
BRAVO
This phase III study compared the efficacy, safety, and tolerability of laquinimod 0.6 mg/day with placebo in patients with RRMS, and the benefit–risk profile of laquinimod was descriptively compared with intramuscular IFNβ-1a. In this 24-month study, 1331 patients from 153 sites in 18 countries were randomized to oral laquinimod 0.6 mg/day, matching oral placebo, or intramuscular IFNβ-1a 30 µg/week [Vollmer et al. 2011]. Patients on laquinimod or oral placebo were evaluated in a double-blind manner, whereas only the neurological rater was blinded to treatment with IFNβ-1a. The primary endpoint was the annualized relapse rate. At 24 months, the reduction in the annualized relapse rate was statistically nonsignificant (risk ratio = 0.823, 95% CI 0.664–1.020; p = 0.075). According to the sponsor, in a prespecified analysis adjusted for an imbalance between groups in the volume of T2 lesions and the number of Gd+ lesions on MRI at baseline, the reduction in the annualized relapse rate compared with placebo became statistically significant with laquinimod treatment (p = 0.026), as did the risk of disability progression as measured by the EDSS (p = 0.044) and a brain atrophy measure on MRI (p < 0.001) [Vollmer et al. 2011]. The results of the BRAVO study were recently published [Vollmer et al. 2014].
A disproportionate effect on disability and brain atrophy was evident with laquinimod in both the ALLEGRO and BRAVO studies, with greater improvements in outcomes in the latter. Laqunimod’s effect on EDSS scores may possibly be due to its ability to affect the CNS directly, thereby reducing the diffuse neurodegenerative effects of MS, which are more linked to long-term disability progression than are the peripherally initiated, T-cell-mediated focal lesions that are linked to relapses.
Safety and tolerability
Clinical data show laquinimod to be well tolerated in patients with RRMS. Only 5% of study patients withdrew from laquinimod 0.6 mg treatment in the phase II study [Comi et al. 2008]. The safety profile was also favorable in the phase III ALLEGRO trial, in which serious adverse reactions occurred in 9.5% of laquinimod participants (61/550) and 9.5% of placebo participants (53/556) [Comi et al. 2012]. The most common adverse events (AEs) associated with laquinimod were dose-dependent elevations of alanine aminotransferase, which occurred twice as frequently in the laquinimod group versus placebo; however, these elevations were transient and not associated with liver failure. Moreover, elevated liver enzymes predominantly occurred within the first month of treatment and normalized without discontinuation of the drug. No cases of fatal liver failure or concomitant elevation of bilirubin or coagulation values were reported. Other common AEs in the laquinimod group included abdominal pain, back pain, cough, respiratory tract infections, headache, asthenic conditions, insomnia, nausea and vomiting, dizziness, arthralgia, and diarrhea [Comi et al. 2012]. Across all trials, only one death was reported in the laquinimod group; it was assessed by the investigator as unrelated to the study medication.
Laquinimod is metabolized primarily through CYP450 3A4, and therefore concomitant systemic use of CYP3A4 inhibitors or inducers should be avoided. No evidence of cardiac AEs has been seen in previous laquinimod studies [Comi et al. 2012]. There is no current evidence of teratogenicity, though currently, the use of contraceptives is necessary in women of childbearing age.
Laquinimod’s place in the therapeutic armamentarium
Laquinimod has been compared in a clinical trial setting only with IFNβ-1a, in BRAVO, in which both agents reduced cumulative numbers of GdE lesions and new or newly enlarging T2 lesions at 12 and 24 months compared with placebo, and both showed equivalent reductions in annualized relapse rate, EDSS, and Multiple Sclerosis Functional Composite scores. In that context, these two agents are discussed in greater detail below. Natalizumab, a parenteral agent, cannot be self administered, and carries a risk of progressive multifocal leukoencephalopathy. Therefore, its use is generally limited to patients who cannot tolerate or have responded inadequately to other MS therapy. All of the currently available oral agents have safety or tolerability considerations: use of fingolimod and teriflunomide is associated with cardiovascular, hepatic, and immunosuppressive effects. Dimethyl fumarate reduces the lymphocyte count by around 30% in the first year of treatment. Thus there is still an unmet need for an agent that can be self administered, is as efficacious as IFNβ-1a, and has good safety and tolerability; based on available evidence, laquinimod appears to meet those criteria.
What’s next for laquinimod?
In response to a request from the US Food and Drug Administration to explore the efficacy of a higher dose of laquinimod, Teva is recruiting patients for the CONCERTO study, which will compare the efficacy, safety, and tolerability of laquinimod 0.6 versus 1.2 mg/day in subjects with RRMS. This higher dose (1.2 mg/day) was evaluated in a recent phase II dose tolerability study (EudraCT #2009-011234-99); results will be forthcoming. CONCERTO is a multinational, multicenter, randomized, double-blind, parallel-group, placebo-controlled study followed by an active treatment phase. The primary outcome measure is time to confirmed disability progression as measured by EDSS. The study will also examine the impact of laquinimod on endpoints such as percent change in brain volume, as well as other clinical and MRI markers of disease activity [ClinicalTrials.gov identifier: NCT01707992]. The anticipated completion date is May 2018. In addition, three open-label 36-month extension studies assessing the long-term safety and efficacy of laquinimod 0.6 mg/day are ongoing [ClinicalTrials.gov identifier: NCT00745615, NCT00988052, NCT01047319]. Finally, in light of laquinimod’s proven neuroprotective effects, the manufacturer is planning to investigate its use in a population with primary progressive MS.
Summary
Laquinimod may be considered an immunomodulatory drug. However, murine studies in EAE and results from MRI suggest that this drug may also exhibit indirect and potentially direct neuroprotective effects. In adults with RRMS, laquinimod has demonstrated the ability to slow disease progression; its beneficial effects have been demonstrated on both clinical endpoints and MRI surrogate markers. It has a favorable safety profile. Its unique profile, coupled with the convenience of an orally administered drug, make laquinimod an attractive agent for patients with MS. Evaluating the efficacy of laquinimod at higher doses could help to further define the role of this promising agent in the clinical arena.
Acknowledgments
The authors would like to thank Ruth Sussman, PhD, who provided editorial support with funding from Teva Pharmaceuticals Industries Ltd, Petach Tikva, Israel. Teva was given the opportunity to provide a review for medical accuracy; however, suggestions from the review were not considered mandatory, and the content was written entirely independently of Teva. The topic was chosen by the lead author, and all conclusions are expressions of the lead author’s scientific viewpoint.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors other than as indicated in the Acknowledgements.
Conflict of interest statement: Prof. Dr Kieseier has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Health Care, Biogen Idec, Genzyme/Sanofi Aventis, Grifols, Merck Serono, Mitsubishi Europe, Novartis, Roche, Talecris, and Teva.
References
- Björk P., Björk A., Vogl T., Stenström M., Liberg D., Olsson A., et al. (2009) Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 7: e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W., Pförtner R., Pham T., Zhang J., Hayardeny L., Piryatinsky V., et al. (2012) Reduced astrocytic NF-kappaB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol 124: 411–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W., Wegner C. (2011) Insight into the mechanism of laquinimod action. J Neurol Sci 306: 173–179 [DOI] [PubMed] [Google Scholar]
- Brunmark C., Runström A., Ohlsson L., Sparre B., Brodin T., Aström M., et al. (2002) The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis. J Neuroimmunol 130: 163–172 [DOI] [PubMed] [Google Scholar]
- Comi G., Abramsky O., Arbizu T., Boyko A., Gold R., Havrdová E., et al. (2010) Oral laquinimod in patients with relapsing-remitting multiple sclerosis: 36-week double-blind active extension of the multi-centre, randomized, double-blind, parallel-group placebo-controlled study. Mult Scler 16: 1360–1366 [DOI] [PubMed] [Google Scholar]
- Comi G., Jeffery D., Kappos L., Montalban X., Boyko A., Rocca M., et al. ; ALLEGRO Study Group (2012) Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 366: 1000–1009 [DOI] [PubMed] [Google Scholar]
- Comi G., Pulizzi A., Rovaris M., Abramsky O., Arbizu T., Boiko A., et al. ; LAQ/5062 Study Group (2008) Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 371: 2085–2092 [DOI] [PubMed] [Google Scholar]
- Confavreux C., Vukusic S. (2006) Accumulation of irreversible disability in multiple sclerosis: from epidemiology to treatment. Clin Neurol Neurosurg 108: 327–332 [DOI] [PubMed] [Google Scholar]
- Fernandez O. (2011) Oral laquinimod treatment in multiple sclerosis. Neurologia 26: 111–117 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M. (2005) MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol 252(Suppl. 5): v16–v24 [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A., De Stefano N. (2012) Evidence for a neuroprotective effect of oral laquinimod in relapsing-remitting multiple sclerosis. Poster presented at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); Lyon, France; 10–13 October [Google Scholar]
- Gold R., Kappos L., Arnold D., Bar-Or A., Giovannoni G., Selmaj K., et al. ; DEFINE study investigators (2013) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367: 1098–1107 [DOI] [PubMed] [Google Scholar]
- Hemmati F., Dargahi L., Nasoohi S., Omidbakhsh R., Mohamed Z., Chik Z., et al. (2013) Neurorestorative effect of FTY720 in a rat model of Alzheimer’s disease: comparison with memantine. Behav Brain Res 252: 415–421 [DOI] [PubMed] [Google Scholar]
- Jolivel V., Luessi F., Masri J., Kraus S., Hubo M., Poisa-Beiro L., et al. (2013) Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 136: 1048–1066 [DOI] [PubMed] [Google Scholar]
- Loma I., Heyman R. (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9: 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Wang J., Silva C., Mack M., Yong V. (2012) Kinetics of proinflammatory monocytes in a model of multiple sclerosis and its perturbation by laquinimod. Am J Pathol 181: 642–651 [DOI] [PubMed] [Google Scholar]
- Oh J., O’Connor P. (2013) Teriflunomide for the treatment of multiple sclerosis. Semin Neurol 33: 45–55 [DOI] [PubMed] [Google Scholar]
- Polman C., Barkhof F., Sandberg-Wollheim M., Linde A., Nordle O., Nederman T. (2005) Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 64: 987–991 [DOI] [PubMed] [Google Scholar]
- Ruffini F., Rossi S., Bergamaschi A., Brambilla E., Finardi A., Motta C., et al. (2013) Laquinimod prevents inflammation-induced synaptic alterations occurring in experimental autoimmune encephalomyelitis. Mult Scler 19: 1084–1094 [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U., Shetty A., Varrin-Doyer M., Molnarfi N., Sagan S., Sobel R., et al. (2012) Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PloS One 7: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöne J., Ellrichmann G., Seubert S., Peruga I., Lee D., Conrad R., et al. (2012) Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol 180: 267–274 [DOI] [PubMed] [Google Scholar]
- Thöne J., Gold R. (2011) Laquinimod: a promising oral medication for the treatment of relapsing–remitting multiple sclerosis. Expert Opin Drug Metab Toxicol 7: 365–370 [DOI] [PubMed] [Google Scholar]
- Toubi E., Nussbaum S., Staun-Ram E., Snir A., Melamed D., Hayardeny L., et al. (2012) Laquinimod modulates B cells and their regulatory effects on T cells in multiple sclerosis. J Neuroimmunol 251: 45–54 [DOI] [PubMed] [Google Scholar]
- Vollmer T., Sorensen P., Arnold D.; on behalf of the BRAVO Study Group (2011) A placebo-controlled and active comparator phase III trial (BRAVO) for relapsing-remitting multiple sclerosis [abstract 148]. Presented at Fifth Joint Triennial Congress of the European and Americas Committee for Treatment and Research in Multiple Sclerosis; Amsterdam, Netherlands; October 19–22, 2011 Mult Scler 17: S507 [Google Scholar]
- Vollmer T., Sorensen P., Selmaj K., Zipp F., Havrdova E., Cohen J., et al. ; on behalf of the BRAVO Study Group (2014) A randomized, placebo-controlled, phase III trial of oral laquinimod for multiple sclerosis. J Neurol 18 February (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Wegner C., Stadelmann C., Pförtner R., Raymond E., Feigelson S., Alon R., et al. (2010) Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 227: 133–143 [DOI] [PubMed] [Google Scholar]