Abstract
Objective
To determine the prevalence of nonconvulsive seizures in children with abusive head trauma.
Design
Retrospective study of children with abusive head trauma undergoing clinically indicated continuous electroencephalographic monitoring.
Setting
PICU of a tertiary care hospital.
Subjects
Children less than or equal to 2 years old with evidence of abusive head trauma determined by neuroimaging, physical examination, and determination of abuse by the Child Protection Team.
Interventions
None.
Measurements and Main Results
Thirty-two children with abusive head trauma were identified with a median age of 4 months (interquartile range 3, 5.5 months). Twenty-one of 32 children (66%) underwent electroencephalographic monitoring. Those monitored were more likely to have a lower admission Glasgow Coma Scale (8 vs 15, p = 0.05) and be intubated (16 vs 2, p = 0.002). Electrographic seizures occurred in 12 of 21 children (57%) and constituted electrographic status epilepticus in 8 of 12 children (67%). Electrographic seizures were entirely nonconvulsive in 8 of 12 children (67%). Electroencephalographic background category (discontinuous and slow-disorganized) (p = 0.02) and neuroimaging evidence of ischemia were associated with the presence of electrographic seizures (p = 0.05). Subjects who had electrographic seizures were no more likely to have clinical seizures at admission (67% electrographic seizures vs 33% none, p = 0.6), parenchymal imaging abnormalities (61% electrographic seizures vs 39% none, p = 0.40), or extra-axial imaging abnormalities (56% electrographic seizures vs 44% none, p = 0.72). Four of 21 (19%) children died prior to discharge; none had electrographic seizures, but all had attenuated-featureless electroencephalographic backgrounds. Follow-up outcome data were available for 16 of 17 survivors at a median duration of 9.5 months following PICU admission, and the presence of electrographic seizures or electrographic status epilepticus was not associated with the Glasgow Outcome Scale score (p = 0.10).
Conclusions
Electrographic seizures and electrographic status epilepticus are common in children with abusive head trauma. Most seizures have no clinical correlate. Further study is needed to determine whether seizure identification and management improves outcome.
Keywords: abusive head trauma, electroencephalographic monitoring, electroencephalography, seizure, traumatic brain injury
Electrographic seizures (ES) are common (1–14) and associated with worse short-term neurodevelopmental outcome in critically ill children (11, 12, 15). The majority of ES have no clinical correlate (3, 8, 10–12, 14, 16, 17) and are therefore referred to as nonconvulsive seizures (NCS) (18). Importantly, these NCS would not be identified without continuous electroencephalographic (EEG) monitoring. Acute structural brain injury (11, 17), including traumatic brain injury (10), has been described as a risk factor for ES, and ES may occur more often with abusive head trauma (AHT) than accidental traumatic brain injury (14). Although studies have demonstrated that children with AHT often have clinical seizures at presentation or during hospitalization (13, 19–24), they have not evaluated the epidemiology of ES in children with AHT. We aimed to determine the prevalence of ES in children with AHT and to explore EEG monitoring indications, clinical and radiographic risk factors for ES, and clinical outcomes.
METHODS
We conducted a retrospective study of children with AHT treated in the PICU at a tertiary care pediatric hospital from July 2009 to January 2012. This study was approved by the hospital institutional review board, and the requirement for obtaining informed consent was waived.
Institutional pathways require consultation of the Child Protection Team in any child in whom abuse is suspected. Subjects were identified by screening consult logs from the Child Protection Team. Inclusion criteria included: 1) age less than or equal to 2 years old at hospital admission, 2) evidence of head trauma by imaging or physical exam, 3) PICU admission, and 4) determination of abuse by the Child Protection Team. All patients with intracranial injuries are admitted to the PICU.
Data collection was guided by the National Institutes of Health core pediatric traumatic brain injury common data elements (25). Demographic variables included age and sex. Presenting symptoms and signs were categorized as acute symptomatic seizures (26), altered mental status, cardiac arrest, or emesis/choke/gag events. Extracerebral injuries indicative of trauma were categorized as skull fractures, extracranial fractures, skin injury (bruising or bite marks), neck injury, or other soft tissue injury. Other clinical variables included preexisting neurodevelopmental diagnosis, initial Glasgow Coma Scale (GCS) score, intubation status, paralytic administration, cardiac arrest occurrence, and PICU and hospital lengths of stay. Paralytics are intermittently administered for procedures, endotracheal tube repositioning, and periodic life-threatening movements. Neuroradiological variables included parenchymal injury (contusion, hemorrhage, edema, or ischemic injury), extra-axial abnormalities, and skull fractures. Ischemia was defined as evidence of restricted diffusion on MRI or cerebral hypoattenuation on CT. Survival to hospital discharge was documented. Functional outcome was assessed at the latest neurology clinic follow-up note, with additional data from physical therapy, feeding team, and primary care notes when available. Follow-up outcome was quantified using the Glasgow Outcome Scale (GOS) (27), which is composed of five categories, including: 1) death, 2) persistent vegetative state, 3) severe disability (conscious but disabled, dependent for daily support), 4) moderate disability, and 5) good recovery.
EEG data were obtained from neurophysiology databases, which included EEG tracings. An institutional clinical pathway recommends that EEG monitoring be considered for children with acute brain injury and encephalopathy, but EEG monitoring is not mandated. EEG monitoring was performed using a Grass-Telefactor (West Warwick, RI) video-EEG system using standard EEG procedures. Data were acquired on a portable bedside computer networked to the hospital’s EEG server. EEG tracings were reviewed by a pediatric electroencephalographer to ensure consistent description within this study. EEG background categories previously shown to have substantial interrater agreement were used (28), including slow-disorganized, discontinuous, or attenuated-featureless. Seizures were classified as present or absent, and if present were classified as clinical or nonconvulsive (25). ES were defined as abnormal paroxysmal events that were different from the background; lasted longer than 10 seconds (or shorter if associated with a clinical change); and had a temporal-spatial evolution in morphology, frequency, and amplitude with a plausible electrographic field. ES with a clinical correlate were designated as clinical seizures, whereas those without any clinical correlate were designated as NCS. Electrographic status epilepticus (ESE) was defined as a single seizure lasting more than 30 minutes or repetitive seizures totaling more than 30 minutes of any 1 hour epoch (50% seizure burden) (18).
Summary statistics are presented as median with inter-quartile ranges [IQR] and proportions with percentages. The association between covariates and seizure category was evaluated using Wilcoxon signed rank test for continuous data and chi-square or Fisher exact test for proportions. A p value of less than or equal to 0.05 indicated statistical significance. Data analysis was performed using Stata 10 (Stata Corporation, College Station, TX).
RESULTS
Subjects With AHT
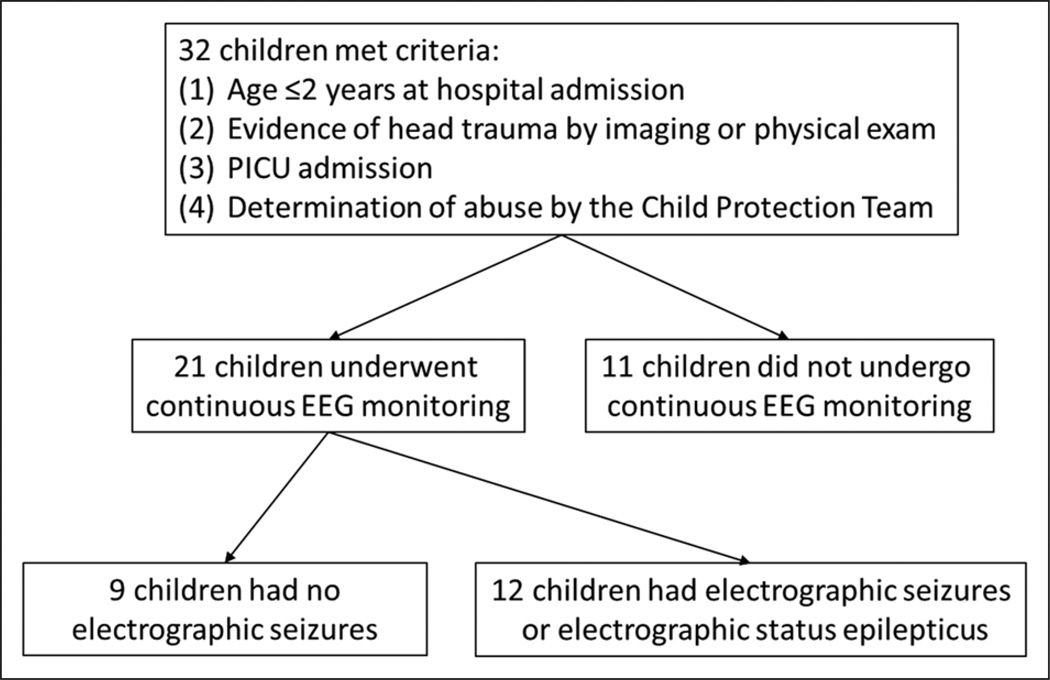
Thirty-two children were identified (Fig. 1). The median age was 4 months [3, 5.5]. Twenty-one of 32 children (66%) underwent EEG monitoring. Extracerebral injuries were present in 27 of 32 subjects (84%) and included skull fractures in 13 (41%), extracranial fractures in 21 (66%), skin injuries in 11 (34%), neck injury in 6 (19%), and other injuries in 3 (9%). Five subjects presented with an out-of-hospital cardiac arrest, and an additional two subjects had cardiac arrest in the emergency department shortly after presentation. Subjects who underwent EEG monitoring were more likely to have a lower admission GCS score (8 [3, 14] vs 15 [11, 15], p = 0.05) and more likely to be intubated (89% vs 11%, p = 0.002). Subjects who underwent EEG monitoring had longer lengths of stay in the PICU (6 [4, 9] d vs 1 [1,3] d, p = 0.001) and hospital (15 [8, 25] d vs 6 [2, 7] d, p = 0.002) (Table 1).
Figure 1.
Overview of study design. EEG = electroencephalography.
Table 1.
Description of Subjects With Abusive Head Trauma (n = 32)
| Variable | No EEG Monitoring (n = 11) n (%) |
EEG Monitoring (n = 21) n (%) |
p |
|---|---|---|---|
| Age (mo) (median [IQR]) | 4 [3,11] | 4 [3,4] | 0.23 |
| Sex | 0.71 | ||
| Male | 6 (38) | 10 (62) | |
| Female | 5 (31) | 11 (69) | |
| Pre-existing neurodevelopmental diagnosis | 0 (0) | 1 (100) | 1 |
| Presenting symptoms/Signsa | |||
| Seizure | 0 (0) | 4 (100) | 0.27 |
| Altered mental status | 8 (35) | 15 (65) | 1 |
| Cardiac arrest | 0 (0) | 5 (100) | 0.14 |
| Emesis/choke/gag | 4 (31) | 9 (69) | 1 |
| Initial Glasgow Coma Scale (median [IQR])b | 15 [11,15] | 8 [3,14] | 0.05 |
| Intubated | 2 (11) | 16 (89) | 0.003 |
| Mortality | 1 (20) | 4 (80) | 0.64 |
| Length of stay (d) (median [IQR]) | |||
| PICU | 1 [1,3] | 6 [4,9] | 0.001 |
| Hospital | 6 [2,7] | 15 [8,25] | 0.002 |
EEG = electroencephalography, IQR = interquartile range.
Coexisting presenting symptoms/signs were possible.
Glasgow Coma Scale was not documented for nine subjects.
Values in bold font indicate significant value.
Subjects With AHT and EEG Monitoring
The median duration from admission to EEG monitoring onset was 6 hour [5, 8]. EEG monitoring was performed for a median of 53.8 hours [16.5, 79]. ES occurred in 12 of 21 subjects (57%) who underwent EEG monitoring. The first ES was identified at a median of 16 hours [5.3, 27.3] after admission and at a median of 0.5 hours [0.2, 1.1] after EEG onset. ESE occurred in 8 of 12 subjects (67%) with ES. ES were only nonconvulsive in 8 of 12 subjects (67%). Paralytics were administered during EEG monitoring in 14 subjects, including 10 of 12 subjects (83%) with seizures and six of eight subjects (75%) with only NCS. Thus, although paralytics may have masked clinical seizures in many subjects, two of eight subjects (25%) with NCS had not received any paralytics.
Table 2 compares subjects with and without ES. ES occurrence was associated with EEG background category (discontinuous and slow-disorganized) (p = 0.02) and neuroimaging evidence of ischemia (p = 0.05). There was no difference in clinical seizures at admission (67% ES vs 33% no ES, p = 0.60). All subjects had a head CT on presentation. MRI was performed prior to EEG monitoring in 17 subjects and after EEG monitoring in 14 subjects. The four subjects without MRI presented with cardiac arrest had an attenuated-featureless EEG background and with-drawal of technological support. There was no difference in parenchymal (61% ES vs 39% no ES, p = 0.40) or extra-axial imaging abnormalities (56% ES vs 44% no ES, p = 0.72) between subjects with ES and those without ES.
Table 2.
Characteristics by Seizure Classification Among Subjects Who Underwent Electroencephalography Monitoring (n = 21)
| Variable | No Seizures (n = 9) n (%) |
Seizures or Status Epilepticus (n = 12) n (%) |
p | |
|---|---|---|---|---|
| Acute care | Initial Glasgow Coma Scale (median [IQR]) | 5 [3,12.5] | 10.5 [3,14] | 0.58 |
| Clinical seizures prior to EEG monitoring | ||||
| Yes | 2 (33) | 4 (67) | 0.60 | |
| No | 7 (47) | 8 (53) | ||
| Cardiac arrest | 5 (71) | 2 (29) | 0.16 | |
| Imaging | ||||
| Skull fracture | 5 (56) | 4 (44) | 0.40 | |
| Extra-axial injury | 8 (44) | 10 (56) | 0.72 | |
| Parenchymal injury | 7 (39) | 11 (61) | 0.37 | |
| Ischemia | 4 (27) | 11 (73) | 0.05 | |
| Medications prior to EEG monitoring | ||||
| Benzodiazepine—dose(s) | 7 (47) | 8 (53) | 0.66 | |
| Benzodiazepine—continuous infusion | 1 (20) | 4 (80) | 0.34 | |
| Anticonvulsant | 1 (12) | 7 (88) | 0.07 | |
| Medications during EEG monitoring | ||||
| Benzodiazepine—dose(s) | 7 (37) | 12 (63) | 0.17 | |
| Benzodiazepine—continuous infusion | 2 (22) | 7 (78) | 0.18 | |
| Anticonvulsant | 2 (14) | 12 (86) | < 0.001 | |
| EEG background | 0.02 | |||
| Normal or sedated sleep | 4 (80) | 1 (20) | ||
| Slow-disorganized | 0 (0) | 6 (100) | ||
| Discontinuous | 0 (0) | 1 (100) | ||
| Attenuated-featureless | 5 (56) | 4 (44) | ||
| Mortality in PICU | 4 (100) | 0 (0) | 0.02 | |
| Outcome | Glasgow Outcome Scale (n = 16)a | 0.10 | ||
| Good recovery | 2 (50) | 2 (50) | ||
| Moderately disabled | 1 (50) | 1 (50) | ||
| Severely disabled | 1 (11) | 8 (89) | ||
| Vegetative state | 1 (100) | 0 (0) | ||
| Follow-up outcomes (n = 16) | ||||
| Developmental delay/intellectual disability | 3 (25) | 9 (75) | 0.52 | |
| Feeding tube | 2 (33) | 4 (67) | 1 | |
| Receiving anticonvulsants | 1 (17) | 5 (83) | 0.60 | |
EEG = electroencephalography, IQR = interquartile range, GCS = Glasgow Coma Scale.
Outcome is assessed at the latest neurological follow-up visit.
Values in bold font indicate significant value.
There was no significant difference in benzodiazepine or anticonvulsant use prior to EEG monitoring in subjects with and without ES (Table 2). During EEG monitoring, anticonvulsant administration was higher among subjects with ES than those without ES (86% vs 14%, p < 0.001). Fourteen subjects were treated with anticonvulsants. The first-line anticonvulsants were phenobarbital in eight subjects, levetiracetam in four subjects, and phenytoin in two subjects. These same anticonvulsants were used in varying orders as second-line (in 12 subjects) and third-line (in 6 subjects) medications when needed.
Five of seven subjects with cardiac arrest died. One subject had determination of brain death upon arrival (including a routine EEG performed with brain death protocol which demonstrated electrocerebral inactivity), and EEG monitoring was not performed. Four of the six who underwent EEG monitoring had attenuated-featureless EEG backgrounds without seizures and had eventual withdrawal of technological support. Thus, 17 of 21 who underwent EEG monitoring survived to hospital discharge. Follow-up outcome was assessed for 16 of 17 (94%) surviving subjects who underwent EEG monitoring at the latest available neurology clinic visit, which occurred at a median duration of 9.5 months [5.5, 16.5] from PICU admission. Among the 16 survivors with follow-up data, GOS score was Good Recovery for 4 (25%), Moderate Disability for 1 (13%), Severe Disability for 9 (56%), and Vegetative State for 1 (6%). There was no difference in follow-up GOS score between subjects with and without ES (p = 0.10). At follow-up, 6 (38%) were feeding tube dependent, 12 (75%) had developmental delay, and 6 (38%) were being treated with an anticonvulsant.
DISCUSSION
This small retrospective study demonstrates that ES are common in children with AHT. EEG monitoring was performed in 66% of 32 children with AHT. Monitored children may have had more severe injury as indicated by lower GCS scores, a higher proportion that were intubated, and longer PICU and hospital lengths of stay. This would be consistent with our institution’s EEG monitoring pathway, which suggests monitoring in children with brain injury and altered mental status but generally does not call for monitoring of children with normal mental status. ES occurred in 57% of the 21 children who underwent EEG monitoring. Even if none of the 11 unmonitored children had seizures, at least 38% (12 of 32) of the full cohort experienced ES. Among children with ES, 67% had seizures that constituted ESE, indicating that the seizure burden is often high. ES had no clinical correlate in 67% of subjects, indicating these seizures would have been missed without EEG monitoring. Although 75% of subjects with NCS had received paralytics which potentially masked the clinical correlates of seizures, 25% of subjects with NCS had not received paralytics.
Clinical seizures were common as a presenting symptom and during the early hospitalization in children with AHT. Clinical seizures occurred at presentation in 22% of the full cohort and in 47% of those who underwent EEG monitoring, consistent with previously published data (13, 14, 19–24, 29). In our cohort, seizures at presentation did not predict initiation of the EEG monitoring, but this likely reflects that our institution’s EEG monitoring pathway suggests EEG monitoring in children with an acute brain injury and encephalopathy regardless of clinical seizure occurrence. Even among those subjects who underwent EEG monitoring, the presence of clinical seizures at presentation did not predict ES occurrence. This is consistent with a prior study that reported one third of children with AHT had seizures during the first week of hospitalization, and these later seizures were not predicted by seizures on presentation (13). In contrast, studies of critically ill subjects with acute encephalopathy due to varied etiologies have found that acute clinical seizures predict ES occurrence (10, 11, 17). In our cohort of 12 subjects with ES, 7 had received anticonvulsants for clinical seizures prior to EEG monitoring and 7 were even receiving benzodiazepine infusions during EEG monitoring. These data indicate that neither anticonvulsant administration nor benzodiazepine infusions preclude subsequent seizures. The retrospective study design did not enable valid comments about anticonvulsant efficacy.
ES are common in critically ill children although the incidence varies according to EEG monitoring indications and study design (1–13). A prospective study in our PICU identified ES in 46% of critically ill children with acute brain injury and encephalopathy (16). Several studies have indicated that younger age (10, 16) and acute structural brain injury (11, 17), including traumatic brain injury (10), are risk factors for ES, and this is consistent with the high seizure occurrence found in our current cohort of children with AHT. Previous studies have reported that 14–38% of children with AHT undergoing EEG monitoring have ES (13, 14, 22, 24).
In subjects with ES, ESE was common. This is consistent with prior studies that focused on clinically evident seizures in children with AHT and described that the majority of patients with seizures had multiple seizures at admission (13, 29). In the current cohort, the majority of ES were NCS, and this is consistent with prior studies of EEG monitoring in cohorts of critically ill children with heterogeneous etiologies for their acute encephalopathy (3, 8, 10–14, 16, 17). Most seizures occurred in the first 1–2 days of the admission and within the initial several hours of EEG monitoring. This is consistent with prior studies of children with heterogeneous etiologies for their acute encephalopathy, which demonstrate that 80–100% of seizures occur within 24 hours of monitoring (3, 8, 10, 11, 14, 16, 17, 30), studies of early post-traumatic seizures indicating most occur in the first 12 hours (29), and a prior study of AHT, which demonstrated that ES were common in routine or continuous EEGs that were generally performed 2–3 days after admission (13). The timing of seizures relative to injury is difficult to determine since children with AHT may have a delay in presenting for medical attention or may present following multiple episodes of AHT, making any assessment of timing difficult in this population.
The only risk factors for ES were a moderate abnormal EEG background and neuroimaging evidence of ischemia. ES occurred in all children with EEG background classifications of slow-disorganized or discontinuous, whereas they occurred only in some children with normal or severely abnormal (attenuated-featureless) backgrounds. This suggests that a moderately injured brain may be more likely to generate seizures than a less injured or extremely injured brain. However, since seizures occurred in some children with all background categories and other risk factors were not identified, data from this study cannot help guide optimal use of limited EEG monitoring resources. Neuroimaging evidence of ischemia was also associated with seizure occurrence, and this is consistent with other studies that have reported that seizures are common in patients with other forms of hypoxic-ischemic brain injury, such as following cardiac arrest resuscitation (9). Future studies with larger numbers and EEG monitoring in all consecutive patients might better identify risk factors.
Unexpectedly, lack of seizures was associated with higher mortality. However, all subjects who died also had cardiac arrest and severely abnormal EEG backgrounds without seizures, perhaps indicating that a severely injured brain is less likely to generate seizures and also to have a more unfavorable outcome. The majority of published evidence indicate that ES are associated with worse outcome in children with AHT (13, 22) and etiologically heterogeneous cohorts of critically ill children (11, 12, 14, 15). In one study of 54 children with AHT, there was a nonsignificant trend toward worse outcome, defined as mortality or need for inpatient rehabilitation, in those with seizures during hospitalization (61% vs 31%, odds ratio, 2.2 [0.6–7.7]) (13). A study of 44 children with AHT reported early post-traumatic seizures in 33 (75%) subjects and found that with a median follow-up length of 3 years, neurodevelopmental outcome correlated significantly with the presence and severity of early post-traumatic seizures (22). Mechanisms by which ES could worsen outcome include elevations in intracranial pressure and lactate-pyruvate ratios (suggesting metabolic dysfunction) during ES in adults with traumatic brain injury (31). In adults with traumatic brain injury, ES are also associated with later hippocampal atrophy (32). If ES during the acute period in children with AHT predispose to epileptogenic changes in the brain, this might increase the risk for subsequent epilepsy, which is common in children following AHT (22, 24, 33). Even if ES are associated with worse outcome, further study is needed to determine whether ES identification and management improves clinical outcome or whether seizures are just a marker of more severe brain injury.
This study has limitations. First, this was a small retrospective study and not all children with AHT underwent EEG monitoring. Second, although misclassification of abuse is possible, the overwhelming majority of the children in this study had additional extracerebral injury, further supporting the diagnosis (34–37).
CONCLUSIONS
Although clinically evident seizures are known to occur in children with AHT, there has been little study of ES in this population. Our data were obtained in a cohort of children with AHT who underwent EEG monitoring soon after admission. These data indicate that children with AHT are at high risk for ES that usually have no clinical correlate and therefore would not be identified without EEG monitoring. Anticonvulsant administration for clinically evident seizures and benzodiazepine administration did not preclude ES on subsequent EEG monitoring, and lack of paralytic administration did not preclude the occurrence of seizures without any clinical correlate. Most ES are identified in the initial 1–2 days of EEG monitoring. Further study is needed to determine whether ES identification and management improves outcome.
Acknowledgments
Supported, in part, by grants U01HL094345 and K23NS075363 (Dr. Topjian), K08NS064051 (to Dr. Friess), R01HD061963 (to Dr. Huh), and K23NS076550 (to Dr. Abend) from the NIH.
Dr. Topjian received grant support from the National Institutes of Health (NIH). Dr. Friess received grant support from NIH. Dr. Christian provided expert opinions and testimony in child abuse cases.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 4.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–170. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol. 2001;16:484–487. doi: 10.1177/088307380101600704. [DOI] [PubMed] [Google Scholar]
- 6.Tay SK, Hirsch LJ, Leary L, et al. Nonconvulsive status epilepticus in children: Clinical and EEG characteristics. Epilepsia. 2006;47:1504–1509. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: Etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–1518. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 8.Shahwan A, Bailey C, Shekerdemian L, et al. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 9.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- 11.Greiner HM, Holland K, Leach JL, et al. Nonconvulsive status epilepticus: The encephalopathic pediatric patient. Pediatrics. 2012;129:e748–e755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: Incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein JL, Leonhardt D, Kmytyuk N, et al. Abnormal neuroimaging is associated with early in-hospital seizures in pediatric abusive head trauma. Neurocrit Care. 2011;15:63–69. doi: 10.1007/s12028-010-9468-5. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber JM, Zelleke T, Gaillard WD, et al. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31–38. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- 15.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- 18.Abend NS, Chapman KE, Gallentine WB, et al. Electroencephalographic monitoring in the pediatric intensive care unit. Curr Neurol Neurosci Rep. 2013;13:330. doi: 10.1007/s11910-012-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiwara T, Okuyama M, Miyasaka M. Characteristics that distinguish abusive from nonabusive head trauma among young children who underwent head computed tomography in Japan. Pediatrics. 2008;122:e841–e847. doi: 10.1542/peds.2008-0387. [DOI] [PubMed] [Google Scholar]
- 20.Keenan HT, Runyan DK, Marshall SW, et al. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114:633–639. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechtel K, Stoessel K, Leventhal JM, et al. Characteristics that distinguish accidental from abusive injury in hospitalized young children with head trauma. Pediatrics. 2004;114:165–168. doi: 10.1542/peds.114.1.165. [DOI] [PubMed] [Google Scholar]
- 22.Barlow KM, Spowart JJ, Minns RA. Early posttraumatic seizures in non-accidental head injury: Relation to outcome. Dev Med Child Neurol. 2000;42:591–594. doi: 10.1017/s0012162200001110. [DOI] [PubMed] [Google Scholar]
- 23.King WJ, MacKay M, Sirnick A, et al. Shaken baby syndrome in Canada: Clinical characteristics and outcomes of hospital cases. CMAJ. 2003;168:155–159. [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois M, Di Rocco F, Garnett M, et al. Epilepsy associated with shaken baby syndrome. Childs Nerv Syst. 2008;24:169–172. doi: 10.1007/s00381-007-0493-4. [DOI] [PubMed] [Google Scholar]
- 25.Adelson PD, Pineda J, Bell MJ, et al. Common data elements for pediatric traumatic brain injury: Recommendations from the working group on demographics and clinical assessment. J Neurotrauma. 2012;29:639–653. doi: 10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 27.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 28.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liesemer K, Bratton SL, Zebrack CM, et al. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: Rates, risk factors, and clinical features. J Neurotrauma. 2011;28:755–762. doi: 10.1089/neu.2010.1518. [DOI] [PubMed] [Google Scholar]
- 30.Hyllienmark L, Amark P. Continuous EEG monitoring in a paediatric intensive care unit. Eur J Paediatr Neurol. 2007;11:70–75. doi: 10.1016/j.ejpn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 32.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow KM, Thomson E, Johnson D, et al. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:e174–e185. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- 34.Piteau SJ, Ward MG, Barrowman NJ, et al. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: A systematic review. Pediatrics. 2012;130:315–323. doi: 10.1542/peds.2011-1545. [DOI] [PubMed] [Google Scholar]
- 35.Maguire S, Pickerd N, Farewell D, et al. Which clinical features distinguish inflicted from non-inflicted brain injury? A systematic review. Arch Dis Child. 2009;94:860–867. doi: 10.1136/adc.2008.150110. [DOI] [PubMed] [Google Scholar]
- 36.Maguire SA, Kemp AM, Lumb RC, et al. Estimating the probability of abusive head trauma: A pooled analysis. Pediatrics. 2011;128:e550–e564. doi: 10.1542/peds.2010-2949. [DOI] [PubMed] [Google Scholar]
- 37.Kemp AM, Jaspan T, Griffiths J, et al. Neuroimaging: What neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011;96:1103–1112. doi: 10.1136/archdischild-2011-300630. [DOI] [PubMed] [Google Scholar]