Abstract
Background
Detection and removal of adenomas and clinically significant serrated polyps is critical to the effectiveness of colonoscopy in preventing colorectal cancer. While longer withdrawal time has been found to increase polyp detection, this association, and the use of withdrawal time as a quality indicator, remains controversial. Few studies have reported on withdrawal time and serrated polyp detection. Using data from the New Hampshire Colonoscopy Registry, we examined how an endoscopist’s withdrawal time in normal colonoscopies affects adenoma and serrated polyp detection.
Methods
We analyzed 7996 colonoscopies performed in 7972 patients between 2009 and 2011 by 42 endoscopists at 14 hospitals, ambulatory surgery centers, and community practices. Clinically significant serrated polyps (CSSPs) were defined as sessile serrated polyps and hyperplastic polyps proximal to the sigmoid. Adenoma and CSSP detection rates were calculated based on median endoscopist withdrawal time in normal exams. Regression models were used to estimate the association of increased normal withdrawal time and polyp, adenoma, and CSSP detection.
Results
Polyp and adenoma detection rates were highest among endoscopists with 9 minute median normal withdrawal time, while detection of CSSPs reached its highest levels at 8 to 9 minutes. Incident rate ratios for adenoma and CSSP detection increased with each minute of normal withdrawal time above 6 minutes, with maximum benefit at 9 minutes for adenomas (1.50, 95% CI (1.21,1.85)) and CSSPs (1.77, 95% CI (1.15, 2.72)). When modeling was used to set the minimum withdrawal time at 9 minutes, we predicted that adenomas and CSSPs would be detected in 302 (3.8%) and 191 (2.4%) more patients. The increase in detection was most striking for the CSSPs, with nearly a 30% relative increase.
Conclusions
A withdrawal time of 9 minutes resulted in a statistically significant increase in adenoma and serrated polyp detection. Colonoscopy quality may improve with a median normal withdrawal time benchmark of 9 minutes.
Keywords: Colon cancer, cancer early detection, quality indicators, cancer prevention
INTRODUCTION
Colorectal Cancer (CRC), the second most common cause of cancer deaths in the U.S.,1 is one of the few preventable cancers. Polypectomy of adenomatous polyps during colonoscopy has been shown to decrease the incidence of CRC,2–4 and the adenoma detection rate (ADR), which indicates the percent of colonoscopies with one or more adenomatous polyps detected, is a primary quality indicator for colonoscopy.5, 6 Higher ADRs are associated with decreased interval CRC between colonoscopies.7 Sessile serrated polyps are frequently located in the proximal colon, and have been recognized as important precursor lesions for CRC and interval CRC.8 Improved detection of all precursor lesions is critical to colonoscopy quality and CRC prevention. However, Kahi et al demonstrated that a significant proportion of proximal serrated polyps may be missed during colonoscopy,9 and another recent study demonstrated a protective effect for risk of advanced adenomas but not for proximal serrated polyps in patients who had a previous colonoscopy.10 Thus, identification of strategies to improve polyp detection may be particularly important for serrated lesions.
The polyp detection rate (PDR), the percent of colonoscopies with one or more polyps detected, has been shown to be highly correlated with ADR.11, 12 Both PDR and ADR vary substantially among endoscopists,6, 13–18 potentially undermining the effectiveness of colonoscopy. Similarly, detection of proximal serrated polyps has been shown to be highly variable and endoscopist dependent.9 Establishment of quality standards to minimize unwarranted variation in performance is a national and international focus,5, 19–21 particularly since colonoscopy is the most commonly used CRC screening test in the United States,22 and the only one that allows polypectomy.
Withdrawal time is the time spent examining the colon during withdrawal of the colonoscope from the cecum to the anal canal, the phase during which careful inspection occurs. Endoscopists with higher withdrawal time in colonoscopies with no findings have been found to have higher ADRs,6, 14, 15, 23–25 PDRs,24, 26, 27 and serrated polyp detection rates (SDRs).28, 29 As evidence supporting this association has accumulated, recommendations for optimal endoscopist mean withdrawal time in normal colonoscopies (NWT) ranging from 6–10 minutes have been suggested.23–25, 27, 30 However, significant controversy remains concerning those recommendations, as outlined in the comments column of Table 5,31–33 and endoscopists face economic pressures to increase efficiency by decreasing colonoscopy procedure time. Additional evidence to assess the strength of the association between longer NWT and increased adenoma and serrated polyp detection rates is needed.
Table 5.
Prior Studies of Withdrawal Time (WT) Correlation to Adenoma Detection Rate (ADR), Polyp Detection Rate (PDR), and / or Serrated Polyp Detection Rate (SDR)
| Author/Date | N and Setting | Withdrawal Time Measurement | Patient Characteristics | Endoscopist Characteristics | Outcome/Comments |
|---|---|---|---|---|---|
| De Wijkerslooth et al. 201329 | 1354 patients, 5 endoscopists in Norway | WT for all exams but had to be at least 6 minutes | Age, Gender and bowel preparation | Experience (years) and intubation time | Longer WT associated with higher proximal SDR |
| Lee TJ et al. 201325 | 31,088 colonoscopies in England | Endoscopist mean WT for negative colonoscopies | Age, gender, smoking status, alcohol use, bowel prep | None | Increases in mean WT up to 10 minutes associated with increased ADR, mainly small or right sided adenomas. |
| Adler et al. 201232 | 12,134 screening colonoscopies; Germany | Endoscopist mean WT for negative colonoscopies | Age, gender, NSAID use, bowel prep | Volume/year, intubation rate, CME | For WT 6–11 minutes no correlation with ADR; high mean WT (8.7 minutes) in study. |
| Liang et al. 201234 | 18003 exams; by 6 colorectal surgeons; US | WT for normal exams | Age and Gender | None | WT correlated with SDR |
| Moritz et al. 201233 | 4429 colonoscopies; Norway | Endoscopist median WT for negative colonoscopies; <6 min. vs ≥6 min. | Age, Gender | Volume | WT with 6 minute threshold not a strong predictor of PDR |
| Lee RH et al. 201135 | 752 colonoscopies; US | Endoscopist mean WT not including time for polypectomy or | Age, gender | Age | No significant relationship between WT and ADR; study not powered to detect small differences in WT |
| Lee TJ et al. 201131 | 36,460 colonoscopies; UK | Endoscopist mean WT for negative colonoscopies | Age | Sedation practice | Mean WT correlated with higher ADR |
| Benson et al. 201015 | 550 screening colonoscopies; US | Endoscopist mean WT for negative colonoscopies | Age, gender | Years in practice | Significant 5-fold increase in ADR with WT >6 minutes vs. < 6 minutes |
| Gellad et al. 201054 | 304 follow-up colonoscopies in patients with normal baseline colonoscopies; US VA Medical Centers | WT= procedure time minus insertion time for negative colonoscopies | Gender, Age, Family History, CRC, Smoking, ETOH, BMI, diabetes, NSAID use | None | No correlation with ADR seen after center with fastest WT removed; high baseline WT (mean >12 minutes); possible threshold in NWT with no further increase in ADR |
| Overholt et al. 201024 | 15,955 colonoscopies; US | Endoscopist mean WT for negative colonoscopies | Age, gender | Age | Statistically significant increase in PDR and ADR for WT ≥6 minutes vs. < 6 minutes |
| Taber et al. 201052 | 1405 colonoscopies pre and 1387 post intervention; US | Endoscopist mean WT for negative colonoscopies | Age, gender, procedure indication | None | No difference in PDR for endoscopists with mean WT >10 minutes vs ≤ 10 minutes |
| Barclay et al. 200823 | 2053 colonoscopies; US | Endoscopist mean WT for negative colonoscopies | None | None | WT of 8 minutes or more correlated with higher ADR |
| Sawhney et al. 200851 | 23,910 colonoscopies; US | WT for negative colonoscopies recorded as < 7 minutes or ≥7 minutes | Age, gender | Experience performing endoscopy | No increase in PDR with increased compliance with 7 minute min. WT; |
| Barclay et al. 200614 | 7882 colonoscopies; US | Endoscopist mean WT for negative colonoscopies | None | None | Endoscopist mean WT > 6 minutes correlated with more adenomas and advanced adenomas |
| Simmons et al. 200627 | 10,955 colonoscopies; US | Endoscopist mean WT for negative colonoscopies | Age, gender | None | Longer WT correlated with PDR; recommended minimum WT ≥7 minutes |
| Sanchez et al. 200418 | 10,159 colonoscopies; 4312 with polyps; US | Endoscopist mean Procedure Time for negative colonoscopies | Age, Gender | None | Longer procedure time correlated with PDR |
ADR= Adenoma detection rate, ASC= Ambulatory Surgery Center, PDR= Polyp detection rate, SDR = Serrated Polyp Detection Rate, WT= Withdrawal time
Preliminary analysis in the statewide New Hampshire Colonoscopy Registry (NHCR), examining the relationship between endoscopist median NWT and polyp detection, confirmed the positive relationship between increasing NWT and PDR, ADR, and SDR found by others.14, 15, 23–25, 27, 29, 34, 35 In the current analysis, we have used models to allow more detailed examination of the association between NWT and PDR, ADR and SDR, with the aim of identifying an optimal withdrawal time for maximum adenoma and clinically significant sessile serrated polyp detection. Following guideline recommendations,36, 37 we defined clinically significant serrated polyps (CSSPs) to be sessile serrated polyps, and hyperplastic polyps proximal to the sigmoid. Our a priori hypothesis was that increased NWT would be associated with increases in both adenoma and CSSP detection. Our model incorporated patient and endoscopist characteristics which may affect both withdrawal time and rates of adenoma and CSSP detection. Finally, we estimated the potential effect on PDR, ADR, and SDR of increasing the minimum NWT in our cohort.
METHODS
Study Design
The NHCR is a statewide, population-based registry, first piloted in 2004, which collects data from colonoscopy facilities throughout New Hampshire, including urban and rural, academic and community, ambulatory surgery centers and hospital-based practices,38, 39 with participating endoscopists from a variety of specialties (gastroenterology, general surgery, colorectal surgery, and family practice). In 2010, approximately 390,000 New Hampshire residents were between the ages of 50 and 75, and eligible for colorectal cancer screening, and New Hampshire has one of the highest CRC screening rates in the US, at nearly 76%.40The NHCR database is comprised of linked observational data prospectively collected from patients, endoscopists, and pathologists. Patients provide informed consent and complete a Patient Information Form prior to their colonoscopy, providing demographic data, detailed information on family and personal health history, including prior screening information, and reason for exam. A procedure form is used to record exam indication, type of bowel preparation, sedation, completion, withdrawal time, immediate complications, follow-up recommendations, and all findings, including polyps, for which location, size, and treatment are recorded. Quality of bowel preparation is also recorded on the procedure form, which provides a detailed description for each category of prep quality (excellent, good, fair, or poor) and also instructs endoscopists to grade prep quality according to the worst prepped segment of the colon after clearing. Pathology reports for all colonoscopies with findings are abstracted and linked, at the level of the polyp, to findings reported on the procedure form.
The NHCR study protocol, all data collection tools and consent forms were approved by the Dartmouth College Committee for the Protection of Human Subjects (Hanover, New Hampshire) and by all relevant Institutional Review Boards at participating practices.
Study Population: eligible colonoscopies
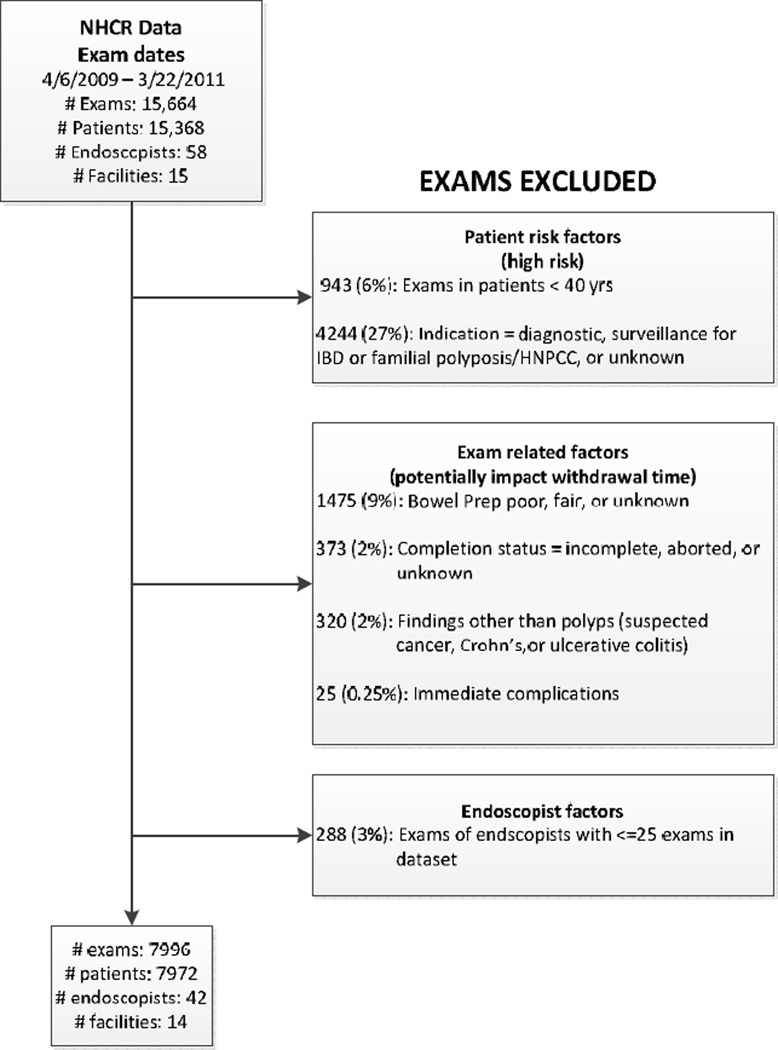
This analysis includes colonoscopies in consenting patients conducted between April 6, 2009 and March 22, 2011. During this time frame, 17,428 patients at the 14 sites included in this study completed both a patient and procedure form and signed an informed consent (72% consent rate), prior to their colonoscopy. Colonoscopies for which the endoscopist was not identified (n=749) and all colonoscopies for two NHCR endoscopists who did not provide withdrawal time (n=1,015) were excluded, leaving 15,664 colonoscopies. After further exclusions (Figure 1), the final data set included 7,996 colonoscopies conducted by 42 endoscopists at 14 facilities. Thirty-one percent of the exams were conducted at a single teaching hospital where residents and fellows are trained. However, because a large proportion of the colonoscopy training for these fellows takes place at a hospital in Vermont which does not participate in the NHCR, the influence of the presence of trainees at colonoscopies in this study is minimal. Individual practices contributed between < 1% and 40% of the exams in the final analysis.
Figure 1.
Exclusion Criteria for withdrawal time analysis
Outcomes
The outcomes of interest were the polyp detection rate (PDR), the adenoma detection rate (ADR), and the serrated polyp detection rate (SDR). The denominator for each outcome was the total number of colonoscopies performed. The numerator for PDR was the number of colonoscopies with one or more polyps detected. The numerator for ADR consisted of the number of colonoscopies in which at least one adenoma (including tubular or villous adenomas, and adenomas with high grade dysplasia or adenocarcinoma) was found. The numerator for SDR included the number of colonoscopies in which any CSSP was detected; following guideline recommendations,36, 37 we considered any serrated adenoma or sessile serrated polyp to be clinically significant, along with those hyperplastic polyps detected proximal to the sigmoid.
Withdrawal Time Measurement
Withdrawal time is recorded on the NHCR procedure form in one minute increments between 2 and 10 minutes, with separate categories for withdrawal times less than 2 minutes or greater than 10 minutes in length. Withdrawal time is recorded immediately after the procedure by either the endoscopist or the nurse present at the procedure. The NHCR did not stipulate the method for measuring withdrawal time, nor did we collect details on how individual practices measured time. All participating endoscopists were aware that their withdrawal times were being recorded.
Covariates
Patient characteristics in this analysis included age at colonoscopy, gender (4% missing), race (4% missing), body mass index (BMI) (6% missing), and a report of a prior colonoscopy. We collapsed race into white and non-white, because of the demographic composition of our population. Endoscopist characteristics included age (2% missing), gender, specialty (gastroenterologist, general or colorectal surgeon), and volume.
Statistical Analysis
Preliminary uni-variate analyses, standard t-tests and chi-squared tests were applied as appropriate. PDR, ADR, SDR, and 95% CI were computed using a nonparametric statistical method for proportions41 for each group, based on median endoscopist NWT (3–5, 6, 7, 8, 9, 10, >10). NWT can only be measured in normal exams (with no findings), because withdrawal time measurements in exams with findings include the time taken for polypectomy. To explore the extent to which patient and endoscopist characteristics affected withdrawal times, a multivariable interval regression on the subset of patients with normal exams modeled NWT as a function of these characteristics. In order to create a complete dataset, we used clinically relevant patient and endoscopist characteristics in normal colonoscopies suggested by this interval regression analysis to impute NWT for the 48% of exams (n = 3,798) in our dataset with findings. We also imputed a small number of missing patient and endoscopist characteristics (sex: 317, race: 304, BMI: 490, first colonoscopy: 220, endoscopist age:1). This allowed us to include all colonoscopies in the second stage of analysis. We employed the multiple imputation by chain equations algorithm (MICE),42 in which a series of regression models are run whereby each variable with missing data (in this case NWT) is modeled conditional upon the other variables in the data, creating 10 complete datasets for use in the second stage of our model. Multiple imputation allows uncertainty to be incorporated in the analysis, by using all available data to preserve sample size and statistical power, and to assure that the resulting estimates are unbiased.
This second stage involved negative binomial regression models for PDR, ADR, and SDR, at the exam level. In these analyses, regression coefficients are expressed as incident rate ratios (IRRs) and 95% confidence intervals (95% CI), using 6 minutes as the reference group for the NWT. To assess the impact of longer NWT on detection rates, a bootstrap technique43 was applied to estimate the percent increase in patients with at least one polyp, adenoma, or CSSP detected that would result from an assumed minimum NWT of 7, 8, 9, or 10 minutes. The percent increase in attributable risk which reflects the influence of NWT on PDR, ADR, and SDR, was also calculated. Attributable risk represents the proportional increase in detection rates with the assumed minimum NWT, and is calculated by subtracting the baseline detection rate from the new estimated detection rate, and then dividing by the baseline detection rate. SAS 9.3 and STATA/SE 12.1 were used for analyses and a p<0.05 indicated statistical significance.
RESULTS
Participant, colonoscopy, and endoscopist characteristics (Table 1)
Table 1.
Patient and Colonoscopy Characteristics
| Characteristics | N | (%) |
|---|---|---|
| Patient Total* | 7972 | 100.0% |
| Age at colonoscopy (years) | ||
| 40–49 | 433 | 5.4% |
| 50–59 | 4141 | 51.9% |
| 60–69 | 2287 | 28.7% |
| 70+ | 1111 | 13.9% |
| Sex | ||
| Male | 3526 | 44.2% |
| Female | 4129 | 51.8% |
| Race | ||
| Non-white | 341 | 4.3% |
| White | 7327 | 91.9% |
| Body Mass Index (kg/m2)** | ||
| <25 (underweight & normal) | 2168 | 27.2% |
| ≥25 to < 30 (overweight) | 2848 | 35.7% |
| ≥30 to <35 (Obesity Class I) | 1600 | 20.1% |
| ≥35 (Obesity Classes II & III) | 866 | 10.9% |
| First colonoscopy** | ||
| No | 4159 | 52.2% |
| Yes | 3593 | 45.1% |
| Colonoscopy Total | 7996 | 100.0% |
| Indication for colonoscopy | ||
| Screening | 5521 | 69.0% |
| Surveillance | 2475 | 31.0% |
| Preparation | ||
| Excellent | 3223 | 40.3% |
| Good | 4773 | 59.7% |
| Withdrawal time recorded (Yes) | 7693 | 96.2% |
| Findings | ||
| Normal colonoscopies (no findings) | 4198 | 52.5% |
| Polyp detected | 3798 | 47.5% |
| Adenoma detected | 2159 | 27.0% |
| CSSP detected*** | 666 | 8.3% |
Missing (N, %): Sex (317,4.0), Race (304, 3.8), BMI (490, 6.2), First colonoscopy (220, 2.8),.
Characteristic pertains to the first exam in the 2 year study analysis period.
CSSP: Clinically significant serrated polyp; includes all serrated adenomas and sessile serrated polyps, and hyperplastic polyps proximal to the sigmoid
Most patients were between 50 and 69 years old (81%), female (52%), white (92%), and overweight or obese (67%).Withdrawal time was reported for 96% of colonoscopies, and polyps, adenomas, and CSSPs were detected in 48%, 27%, and 8.3% of colonoscopies, respectively. Most endoscopists were male (86%), gastroenterologists (69%), with an average of 18 years (SD = 9) performing colonoscopy, and the average age was 52 years (SD = 10). We found endoscopist’s age and number of years performing colonoscopy to be collinear (r=0.94, p<0.0001) and therefore removed number of years performing colonoscopy from the final models.
Polyp, Adenoma, and CSSP Detection Rates by median endoscopist NWT (Table 2)
Table 2.
Polyp, Adenoma, and Serrated Polyp Detection Rates (95% CI*) for Median Endoscopist Normal Withdrawal Time
| Median Endoscopist NWT* |
Endoscopists | Polyp Detection Rate Rate % (95% CI) |
Adenoma Detection Rate Rate % (95% CI) |
Serrated Polyp Detection Rate Rate % (95% CI) |
|
|---|---|---|---|---|---|
| N | % | ||||
| 3–5 minutes | 5 | 11.9 | 38.7 (35.6 – 42.0) | 20.1 (17.5 – 22.8) | 3.9 (2.8 – 5.4) |
| 6 minutes | 5 | 11.9 | 42.6 (39.5 – 45.7) | 23.8 (21.3 – 26.6) | 5.0 (3.7 – 6.5) |
| 7 minutes | 2 | 4.8 | 50.8 (48.2 – 53.5) | 30.2 (27.8 – 32.7) | 8.3 (6.9 – 9.9) |
| 8 minutes | 12 | 28.6 | 52.0 (49.5 – 54.6) | 30.4 (28.1 – 32.8) | 10.2 (8.7 – 11.9) |
| 9 minutes | 8 | 19.0 | 53.1 (50.2 – 56.1) | 33.6 (30.9 – 36.4) | 9.5 (7.9 – 11.4) |
| 10 minutes | 4 | 9.5 | 43.1 (40.3 – 45.9) | 24.5 (22.1 – 27.0) | 8.7 (7.2 – 10.4) |
| >10 minutes | 6 | 14.3 | 47.8 (44.2 – 51.4) | 20.8 (18.0 – 23.8) | 11.8 (9.6 – 14.2) |
NWT: Normal Withdrawal Time; 95% CI: 95 % Confidence Intervals
Detection Rates = the number of exams with findings of polyp / adenoma or serrated polyp divided by the total number of exams
Serrated polyps include any serrated adenoma or sessile serrated polyp or hyperplastic polyps found proximal to the sigmoid.
Endoscopist median NWT varied widely between 3 and >10 minutes. The most common median NWT was 8 minutes (29%), but almost a quarter of endoscopists (24%) had median NWT of 6 minutes or less. Within the range of median NWTs for which we had definite measurements (6 – 10 minutes), PDRs were highest at 9 minutes, at 53.1% (95% CI 50.2 – 56.1) as were ADRs, at 33.6% (95% CI 30.9 – 36.4), while SDRs peaked at 8 minutes, at 10.2% (95% CI 8.7 – 11.9), but remained high at 9 minutes, at 9.5% (95% CI 7.9 – 11.4). Increases in detection rates between endoscopists with 6 minute median NWTs and those with 9 minute median NWTs were substantial: PDRs were 10.5% higher, ADRs were 9.8% higher, and SDRs were 4.5% higher.
PDR, ADR and SDR Regression Analysis (Table 3, Table 4)
Table 3.
Regression analysis of effects of normal withdrawal time and patient characteristics on rates of polyp, adenoma, and serrated polyp detectionΩ
| Polyp Detection Rate | Adenoma Detection Rate | Serrated Polyp Detection Rate | ||||
|---|---|---|---|---|---|---|
| Characteristics | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Exam Characteristics | ||||||
| Withdrawal time | ||||||
| 3–5 min | 1.21 | (0.95, 1.54) | 1.16 | (0.87, 1.56) | 0.87 | (0.53, 1.44) |
| 6 min | 1.00 | (−,−) | 1.00 | (−,−) | 1.00 | (−,−) |
| 7 min | 1.21 | (1.06, 1.38) | 1.23 | (1.01, 1.50) | 1.28 | (0.84, 1.97) |
| 8 min | 1.29 | (1.06, 1.55) | 1.32 | (1.06, 1.64) | 1.39 | (0.86, 2.26) |
| 9 min | 1.46 | (1.22, 1.75) | 1.50 | (1.21, 1.85) | 1.77 | (1.15, 2.72) |
| 10 min | 1.39 | (1.03, 1.87) | 1.41 | (1.03, 1.94) | 1.65 | (0.93, 2.93) |
| >10 min | 1.23 | (0.99, 1.54) | 1.23 | (0.95, 1.59) | 1.73 | (1.07, 2.78) |
| Patient Characteristics | ||||||
| Age at colonoscopy (years) | ||||||
| 40–49 | 1.00 | (−,−) | 1.00 | (−,−) | 1.00 | (−,−) |
| 50–59 | 1.04 | (0.91, 1.19) | 1.04 | (0.85, 1.27) | 1.04 | (0.71, 1.54) |
| 60–69 | 1.21 | (1.08, 1.37) | 1.34 | (1.08, 1.66) | 1.19 | (0.84, 1.69) |
| 70+ | 1.26 | (1.09, 1.45) | 1.53 | (1.22, 1.93) | 1.04 | (0.69, 1.58) |
| Female | 0.78 | (0.75, 0.81) | 0.63 | (0.58, 0.69) | 0.82 | (0.73, 0.92) |
| White | 0.99 | (0.86, 1.13) | 1.02 | (0.84, 1.23) | 0.91 | (0.64, 1.28) |
| Body Mass Index (kg/m2) | ||||||
| <25 (underweight & normal) | 1.00 | (−,−) | 1.00 | (−,−) | 1.00 | (−,−) |
| ≥25 to <30 (overweight) | 1.17 | (1.09, 1.25) | 1.14 | (1.04, 1.26) | 1.06 | (0.85, 1.33) |
| ≥30 to <35 (Obesity Class I) | 1.30 | (1.21, 1.39) | 1.29 | (1.15, 1.44) | 1.22 | (0.97, 1.53) |
| ≥35 (Obesity Classes II/ III) | 1.40 | (1.29, 1.52) | 1.41 | (1.25, 1.59) | 1.45 | (1.08, 1.95) |
| First colonoscopy | 0.96 | (0.92, 1.00) | 0.95 | (0.89, 1.02) | 0.97 | (0.83, 1.13) |
Adjusted for all characteristics listed in the table, as well as for ndoscopist estimated volume, age, gender, and subspecialty.
IRR = Incidence Rate Ratio, 95% CI = 95% Confidence Interval.
Serrated polyps include any serrated adenoma or sessile serrated polyp or hyperplastic polyps found proximal to the sigmoid.
Table 4.
Percent Increase in Patients with a Polyp, Adenoma or Serrated Polyp Detected and in Attributable Risk, Assuming a Minimum Mandated Normal Withdrawal Time (NWT)
| Assumed minimum NWT (minutes)1 |
Percent increase in patients with findings |
95% CI | Percent increase in attributable risk (AR)2 |
95% CI | p-value |
|---|---|---|---|---|---|
| Polyps | |||||
| 7 | 0.96 | (0.32, 2.23) | 2.02 | (0.69, 4.72) | 0.14 |
| 8 | 2.18 | (0.68, 3.68) | 4.59 | (1.42, 7.77) | 0.004 |
| 9 | 6.16 | (3.69, 8.62) | 12.97 | (7.77, 18.17) | <0.0001 |
| 10 | 4.19 | (2.18, 6.20) | 8.82 | (4.53, 13.12) | <0.0001 |
| Adenomas | |||||
| 7 | 0.72 | (0.13, 1.56) | 2.65 | (0.47, 5.77) | 0.10 |
| 8 | 1.53 | (0.62, 2.44) | 5.65 | (2.28, 9.02) | 0.001 |
| 9 | 3.79 | (1.80, 5.78) | 14.01 | (6.59, 21.42) | <0.0001 |
| 10 | 2.51 | (1.08, 3.94) | 9.27 | (4.06, 14.48) | 0.001 |
| Serrated Polyps | |||||
| 7 | 0.71 | (0.11, 1.31) | 8.51 | (1.17, 15.86) | 0.02 |
| 8 | 1.03 | (0.16, 1.91) | 12.40 | (2.48, 22.32) | 0.02 |
| 9 | 2.39 | (0.90, 3.87) | 28.59 | (12.40, 44.78) | 0.002 |
| 10 | 1.90 | (0.52, 3.28) | 22.75 | (6.30, 39.19) | 0.01 |
In this analysis, all NWTs below the ‘assumed minimum NWT’ were replaced with that assumed minimum, and the percentage increase in findings that would result was calculated
Attributable Risk represents the proportional increase in detection rate: the new estimated detection rate based on the assumed minimum NWT minus the baseline detection rate, divided by the baseline detection
In Table 3, we report the results of a multivariable regression model for PDR, ADR, and SDR. In comparison to the 6 minute reference group, PDR, ADR, and SDR incident rate ratios (IRR) steadily increased up to a withdrawal time of 9 minutes. Colonoscopies with 9 minute normal withdrawal times had higher IRRs for all three detection rates (PDR: IRR = 1.46, 95% CI (1.22, 1.75), ADR: IRR = 1.50, 95% CI (1.21, 1.85), SDR: IRR = 1.77, 95% CI (1.15, 2.72)), as compared to colonoscopies with 6 minute normal withdrawal times. Patient characteristics, including male sex (p<0.0001) and increasing BMI also significantly increased PDR, ADR, and SDR (p for trend: <0.0001 for PDR and ADR; 0.01 for SDR). While patient age significantly increased PDR and ADR (p for trend: <0.0001 for PDR and ADR), it was not significantly related to SDR (p for trend: 0.54). Endoscopist gender and specialty significantly affected ADR, but not PDR or SDR, with female endoscopists having lower ADRs than male endoscopists, and surgeons having lower ADRs than gastroenterologists.
The predicted increases in attributable risk that result from increasing the minimum NWT beyond 6 minutes are shown in Table 4. In this analysis, in order to assess the impact of increasing NWT upon attributable risk in our patient cohort, we replaced NWTs below the assumed minimum NWT of 7, 8, 9, or 10 minutes with that assumed minimum NWT. We found that increasing the minimum NWT in our cohort of patients from less than 6 to 9 minutes resulted in a predicted 6.16% (95% CI (3.69, 8.62)) increase in PDR, and increased the attributable risk (AR) by 12.97% (95% CI (7.77, 18.17)). For adenomas, 3.79% (95%CI (1.80, 5.78)) more patients were predicted to have at least one adenoma detected, with an AR of 14.01% (95% CI (6.59, 21.42)), and 2.39% (95% CI (0.90, 3.87)) more patients were predicted to have at least one CSSP detected, with an AR of 28.59% (95% CI (12.40, 44.78)).
DISCUSSION
In our statewide analysis involving 42 endoscopists at 14 facilities, and incorporating patient and endoscopist characteristics, we found a strong and highly significant association between NWT and PDR, ADR, and SDR, which suggests that a NWT of 9 minutes may be associated with an increased yield of adenomas and CSSPs. This finding was present both in our initial comparison of ADR and SDR by endoscopist median NWT, and in our exam-level multivariable regression model. In order to understand the potential impact of a guideline recommendation of a 9 minute rather than 6 minute NWT, we estimated the additional number of patients in the NHCR cohort (n=7972) who would have at least one polyp (491 patients, 6.2%), adenoma (302 patients, 3.8%), or CSSP (191 patients, 2.4%) detected if the minimum withdrawal time were increased from 6 to 9 minutes. This reflects a 13% and 14% relative increase in the number of patients with polyps and adenomas detected, respectively, and a nearly 30% relative increase in the number of patients with CSSPs detected. This finding highlights the potentially important role of sufficient withdrawal time in increasing detection of CSSPs.
While adenomas have long been recognized as potential CRC precursors, a subgroup of serrated lesions have more recently been recognized as such, leading to increased focus on their detection. Sessile serrated lesions have been associated with synchronous advanced neoplasia,44 and the sessile serrated polyp to cancer pathway has been implicated in the development of some interval cancers. Colonoscopy has been shown to have decreased ability to prevent CRC in the proximal, or right, colon,45, 46 and it has been suggested that proximal serrated lesions, which can be more difficult to see than other lesions, may play an important role in this limitation. A recent study demonstrated that patients who had a colonoscopy had a reduced future risk for advanced adenomas but not for proximal serrated polyps.10
Given their potential role in the development of interval cancers, improving the ability to detect proximal serrated lesions is critical to colonoscopy quality. Both the Multi Society Task Force36 and an expert panel37 have issued new recommendations regarding the surveillance of serrated lesions, and it has been suggested that quality of colonoscopy performance is the main factor resulting in variability of proximal SDR.9 The two prior studies which have explored the relationship between SDR and withdrawal time found that longer withdrawal time (either in normal exams34 or not including time spent on polypectomy,29) was associated with higher serrated polyp34 and proximal serrated polyp29 detection. While one study included more total procedures than the current investigation, both studies analyzed data from fewer endoscopists than in the present analysis, and neither provided data to support a potential target withdrawal time which could optimize serrated polyp detection.
Our investigation demonstrates a statistically significant correlation between longer NWT and higher PDR, ADR, and SDR, peaking at 9 minutes, and provides strong evidence to support a 9 minute median NWT as a quality standard. Available evidence to support guideline recommendations has included studies demonstrating higher ADR for endoscopists with NWT greater than 6 to 8 minutes (Table 5). Some of the larger studies such as Barclay (n = 2053 exams), Simmons et al (n = 10,955 exams), Lee et al (n=31,888 exams) and Sawhney et al (n = 23,910 exams) have observed this increase in polyp or adenoma detetion for withdrawal times > 6 minutes. However, despite this evidence, the fact that missed lesions are not uncommon,47–49 and that increased withdrawal time could allow more detailed inspection of the colon, including suctioning of fluid and debris and careful viewing of more difficult areas, a benchmark withdrawal time remains controversial as a colonoscopy quality measure, and current recommendations are based on limited evidence.5
Many prior studies of the relationship between withdrawal time and PDR, ADR, and SDR were based in single practices.6, 14, 15, 23, 26, 27, 34, 50–52 A strength of our study is that it is population based and statewide, reflecting a spectrum of endoscopists and endoscopic environments, rather than mainly high-volume endoscopists within a few sites. Successful CRC prevention must involve effective, high quality screening within community practices, and New Hampshire is likely a reasonable reflection of community practices nationally.
In contrast to our exam-level analysis, prior assessments of withdrawal time and colonoscopy yield have tended to calculate detection rates at the level of the endoscopist, comparing ADRs or PDRs across endoscopists with different median or mean NWTs. Furthermore, detailed prep information collected on all exams allowed us to include only those exams with a good or excellent bowel preparation, thereby avoiding inclusion of exams with sub-optimal preps for which the withdrawal time, PDR, ADR, and SDR could have been influenced by prep quality.
Through careful matching of polyps noted on procedure forms and corresponding pathology reports, the NHCR is able to incorporate polyp location and histology into analyses. This allowed us to include as CSSPs those hyperplastic polyps located proximal to the sigmoid, but not those located within the sigmoid or rectum, as has been outlined in a recent guideline publication.37 To our knowledge only one other study has attempted to estimate the percentage of additional patients who would have adenomas detected if minimum withdrawal times were increased above 6 minutes, and this study did not examine serrated lesions.25 Also of note, we found ADR (but not SDR) to be significantly lower among female endoscopists, and among surgeons as compared to gastroenterologists. The former may be the result of a higher percentage of female patients (with lower ADRs) utilizing female endoscopists (68% of patients of female endoscopists were female, versus 52% of patients of male endoscopists, p<0.0001). Finally, the study highlights the even greater impact of withdrawal time on CSSPs, and adds to the increasing body of work demonstrating the potential use of PDR as a proxy quality measure for ADR.11, 12, 53
Our model results demonstrated a steady increase in withdrawal time IRRs for PDR, ADR, and SDR for each additional minute compared to 6 minutes, leveling off but showing trends of remaining elevated at 10 minutes and beyond (p for trend: PDR, p=0.04, ADR, p=0.06, SDR, p = 0.002). While our results showed elevated IRRs for >10 minutes as compared to 6 minutes, the results were not significant for PDR and ADR. Others have suggested the possibility of a ceiling effect, above which further increases in withdrawal time lead to minimal or no increases in ADR.25, 54 In a recent large English Screening Programme observational study which supported withdrawal time as an important quality indicator, the authors noted that the optimal NWT to maximize ADR may be around 10 minutes, after which the association between increasing ADR and longer mean NWT began to level off.25
Limitations to our study include the fact that withdrawal time was recorded in one minute increments from <2 to >10 minutes; therefore, we do not know the exact NWT for colonoscopies with withdrawal times over 10 minutes. We also do not know the reasons for prolonged withdrawal times in exams with NWT >10 minutes. Withdrawal time was recorded by either the nurse or endoscopist in the procedure room; however, we did not collect data from each endoscopist on specific techniques for measurement. The racial composition of patients in the NHCR reflects that of New Hampshire; therefore, our results describe a predominately white, although ethnically diverse, population. A substantial number of exams (10%) were excluded from analysis because of missing data; this primarily reflects early data collection issues at newly implemented sites. While budgetary constraints limited our ability to track down missing data during the time frame of this study, the NHCR will shortly implement a ‘chase and trace’ protocol developed and used successfully by the NH Mammography Registry, in order to reduce missing data and thus exclusions from future analyses.
Our results suggest that higher rates of ADR and SDR maybe associated with a median colonoscopy withdrawal time recommendation of 9 minutes, not including polypectomy. It is noteworthy that nearly a quarter of the endoscopists in this study had median normal withdrawal times of 6 minutes or less. Benefits of longer withdrawal times might include repeat examination of the right colon, or retroflexing in the cecum, which could contribute to the increase in CSSP detection with longer withdrawal times. These techniques have been suggested to decrease the number of missed proximal colon lesions which could result in subsequent interval cancers.
The primary goal of screening colonoscopy is to find potentially significant polyps before they become cancers; our investigation confirms, through comprehensive evaluation, that withdrawal time is associated with increased polyp, adenoma, and CSSP detection. Adoption of withdrawal time guidelines demonstrated by the evidence presented here could lead to higher quality colonoscopy and improved patient outcomes.
WHAT IS CURRENT KNOWLEDGE.
Detection and removal of adenomas and serrated polyps is critical to the effectiveness of colonoscopy
Longer withdrawal time is associated with an increase in adenoma detection
WHAT IS NEW HERE
A 9 minute withdrawal time resulted in a statistically significant increase in adenoma and clinically significant serrated polyp detection
Serrated polyp detection increased by 30% with a minimum withdrawal time of 9 minutes
Acknowledgments
Grant Support: The project described was supported by Grants # R21CA100553 and R01CA131141 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The National Cancer Institute was not involved in the study design, the collection, analysis, or interpretation of data.
Abbreviations
- ADR
Adenoma Detection Rate
- ASC
Ambulatory Surgery Center
- AR
Attributable Risk
- BMI
Body Mass Index
- CI
Confidence Intervals
- CRC
Colorectal Cancer
- CSSP
Clinically Significant Serrated Polyp
- IRR
Incident Rate Ratios
- MICE
Multiple Imputation by Chain Equations
- NHCR
New Hampshire Colonoscopy Registry
- NWT
Normal Withdrawal Time
- PDR
Polyp Detection Rate
- SD
Standard Deviation
- SDR
Serrated Polyp Detection Rate
- SE
Standard Errors
- WT
Withdrawal Time
Footnotes
Disclosures
No authors have any conflicts of interest to disclose.
Specific Author Contributions
Study concept and design: Lynn Butterly, Julie Weiss, Martha Goodrich, Michael Beach, Tracy Onega
Analysis and interpretation of the data: Julie Weiss, Michael Beach, Lynn Butterly, Christina Robinson, Joseph Anderson, Martha Goodrich, Christopher Amos
Drafting the manuscript: Lynn Butterly, Christina Robinson, Martha Goodrich, Julie Weiss, Michael Beach, Joseph Anderson
Critical revision of the article for important intellectual content: Lynn Butterly, Christina Robinson, Martha Goodrich, Julie Weiss, Michael Beach, Joseph Anderson, Tracy Onega, Christopher Amos
Statistical analysis: Michael Beach, Julie Weiss, Christopher Amos
Obtained funding: Lynn Butterly, Martha Goodrich
Final approval of the article: Lynn Butterly, Christina Robinson, Martha Goodrich, Julie Weiss, Michael Beach, Joseph Anderson, Tracy Onega, Christopher Amos
Contributor Information
Lynn Butterly, Geisel School of Medicine at Dartmouth, Section of Gastroenterology, 46 Centerra Parkway, Evergreen Center, Suite 105, Lebanon, NH 03766, Lynn.Butterly@hitchcock.org, Phone: 603-653-3427, Fax: 603-650-3415.
Christina M. Robinson, Geisel School of Medicine at Dartmouth, Department of Community & Family Medicine.
Joseph Anderson, Geisel School of Medicine at Dartmouth, Department of Medicine and the Dartmouth Institute, VA Medical Center, White River Junction.
Julia E. Weiss, Geisel School of Medicine at Dartmouth, Department of Community & Family Medicine.
Martha Goodrich, Geisel School of Medicine at Dartmouth, Department of Community & Family Medicine.
Tracy L. Onega, Geisel School of Medicine at Dartmouth, Department of Community & Family Medicine and the Dartmouth Institute.
Christopher I. Amos, Geisel School of Medicine at Dartmouth, Department of Community and Family Medicine.
Michael L. Beach, Geisel School of Medicine at Dartmouth, Departments of Anesthesiology and Community & Family Medicine.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 6.Millan MS, Gross P, Manilich E, et al. Adenoma detection rate: the real indicator of quality in colonoscopy. Dis Colon Rectum. 2008;51:1217–1220. doi: 10.1007/s10350-008-9315-3. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 8.Terdiman JP, McQuaid KR. Surveillance guidelines should be updated to recognize the importance of serrated polyps. Gastroenterology. 2010;139:1444–1447. doi: 10.1053/j.gastro.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Burnett-Hartman AN, Newcomb PA, Phipps AI, et al. Colorectal endoscopy, advanced adenomas, and sessile serrated polyps: implications for proximal colon cancer. Am J Gastroenterol. 2012;107:1213–1219. doi: 10.1038/ajg.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: results from a national endoscopy database. Gastrointest Endosc. 2012;75:576–582. doi: 10.1016/j.gie.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73:498–506. doi: 10.1016/j.gie.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247–1256. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 15.Benson ME, Reichelderfer M, Said A, et al. Variation in colonoscopic technique and adenoma detection rates at an academic gastroenterology unit. Dig Dis Sci. 2010;55:166–171. doi: 10.1007/s10620-008-0703-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Imperiale TF, Glowinski EA, Juliar BE, et al. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69:1288–1295. doi: 10.1016/j.gie.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol. 2004;99:1941–1945. doi: 10.1111/j.1572-0241.2004.40569.x. [DOI] [PubMed] [Google Scholar]
- 19.Faigel DO, Cotton PB. The London OMED position statement for credentialing and quality assurance in digestive endoscopy. Endoscopy. 2009;41:1069–1074. doi: 10.1055/s-0029-1215279. [DOI] [PubMed] [Google Scholar]
- 20.Hewett DG, Rex DK. Improving colonoscopy quality through health-care payment reform. Am J Gastroenterol. 2010;105:1925–1933. doi: 10.1038/ajg.2010.247. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Overholt BF, Brooks-Belli L, Grace M, et al. Withdrawal times and associated factors in colonoscopy: a quality assurance multicenter assessment. J Clin Gastroenterol. 2010;44:e80–e86. doi: 10.1097/MCG.0b013e3181bf9b02. [DOI] [PubMed] [Google Scholar]
- 25.Lee TJ, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy. 2013;45:20–26. doi: 10.1055/s-0032-1325803. [DOI] [PubMed] [Google Scholar]
- 26.Munson GW, Harewood GC, Francis DL. Time of day variation in polyp detection rate for colonoscopies performed on a 3-hour shift schedule. Gastrointest Endosc. 2011;73:467–475. doi: 10.1016/j.gie.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Simmons DT, Harewood GC, Baron TH, et al. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965–971. doi: 10.1111/j.1365-2036.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- 28.Liang JJ, Bissett I, Kalady M, et al. Importance of serrated polyps in colorectal carcinogenesis. ANZ J Surg. 2012 doi: 10.1111/j.1445-2197.2012.06269.x. [DOI] [PubMed] [Google Scholar]
- 29.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013 doi: 10.1016/j.gie.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–S28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 32.Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12 134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2012 doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 33.Moritz V, Bretthauer M, Ruud HK, et al. Withdrawal time as a quality indicator for colonoscopy - a nationwide analysis. Endoscopy. 2012;44:476–481. doi: 10.1055/s-0032-1306898. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Kalady MF, Appau K, et al. Serrated polyp detection rate during screening colonoscopy. Colorectal Dis. 2012;14:1323–1327. doi: 10.1111/j.1463-1318.2012.03017.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee RH, Tang RS, Muthusamy VR, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointest Endosc. 2011;74:128–134. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butterly LF, Goodrich M, Onega T, et al. Improving the quality of colorectal cancer screening: assessment of familial risk. Dig Dis Sci. 2010;55:754–760. doi: 10.1007/s10620-009-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney P, Butterly L, Goodrich M, et al. Design and Development of a Population-based Colonoscopy Registry. Journal of Registry Management. 2006;33:91–99. [Google Scholar]
- 40.Laviolette M. Prevalence of screening, current indicator, 2012, by gender, county, age group, and income: Office of Health Statistics and Data Management (HSDM), Bureau of Public Health Statistics and Informatics (BPHSI), Division of Public Health Services, New Hampshire Department of Health and Human Services (DHHS), 2010–2012. 2013 [Google Scholar]
- 41.Conover W. Practical Nonparametric Statistics. 2nd edition ed. John Wiley & Sons, Inc.; 1980. pp. 99–104. [Google Scholar]
- 42.Raghunathan T, Lepkowski J, van Hoewyk J, et al. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 43.Freedman DA. Bootstrapping Regression Models. The Annals of Statistics. 1981;9:1218–1228. [Google Scholar]
- 44.Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol. 2009;104:695–702. doi: 10.1038/ajg.2008.166. [DOI] [PubMed] [Google Scholar]
- 45.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 46.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 47.Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 48.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 49.Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990;82:1769–1772. doi: 10.1093/jnci/82.22.1769. [DOI] [PubMed] [Google Scholar]
- 50.Harewood GC, Petersen BT, Ott BJ. Prospective assessment of the impact of feedback on colonoscopy performance. Aliment Pharmacol Ther. 2006;24:313–318. doi: 10.1111/j.1365-2036.2006.02973.x. [DOI] [PubMed] [Google Scholar]
- 51.Sawhney MS, Cury MS, Neeman N, et al. Effect of institution-wide policy of colonoscopy withdrawal time > or = 7 minutes on polyp detection. Gastroenterology. 2008;135:1892–1898. doi: 10.1053/j.gastro.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71:782–786. doi: 10.1016/j.gie.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Hilsden RJ. Is polyp detection rate a valid proxy for adenoma detection rate for measuring the technical quality of colonoscopy? Gastroenterology. 2010;138:S57. [Google Scholar]
- 54.Gellad ZF, Weiss DG, Ahnen DJ, et al. Colonoscopy withdrawal time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105:1746–1752. doi: 10.1038/ajg.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]