Abstract
The initial pathological changes of diffuse axonal injury following traumatic brain injury (TBI) include membrane disruption and loss of ionic homeostasis, which further lead to dysfunction of axonal conduction and axon disconnection. Resealing the axolemma is therefore a potential therapeutic strategy for the early treatment of TBI. Monomethoxy poly (ethylene glycol)-poly (D, L–lactic acid) di-block copolymer micelles (mPEG-PDLLA) have been shown to restore depressed compound action potentials (CAPs) of spinal axons and promote functional recovery after spinal cord injury. Here, we evaluate the effect of the micelles on repairing the injured cortical axons following TBI. Adult mice subjected to controlled cortical impact (CCI) were treated with intravenous injection of the micelles at 0 h or 4 h after injury. Evoked CAPs were recorded from the corpus callosum of coronal cortical slices at 2 days after injury. The CCI caused significant decreases in the amplitudes of two CAP peaks that were respectively generated by the faster myelinated axons and slower unmyelinated axons. Micelle treatment at both 0 h and 4 h after CCI resulted in significant increases in both CAP peak amplitudes. Injection of fluorescent dye-labeled micelles revealed high fluorescent staining in cortical gray and white matters underneath the impact site. Labeling membrane-perforated neurons by injecting a membrane impermeable dye Texas Red-labeled dextran into lateral ventricles at 2 h post-CCI revealed that immediate micelle injection after CCI did not reduce the number of dye-stained cortical neurons and dentate granule cells of the hippocampus, indicating its ineffectiveness in repairing plasma membrane of neuronal somata. We conclude that intravenous administration of mPEG-PDLLA micelles immediately or at 4 h after TBI allows brain penetration via the compromised blood brain–barrier, and thereby improves the function of both myelinated and unmyelinated axons of the corpus callosum.
Key words: : axon, CAP, cerebral cortex, micelles, TBI
Introduction
Traumatic brain injury (TBI) often leads to the impairment of sensory, motor, and cognitive function of the brain, and is the leading cause of death and disability in children and adults.1–4 Axons are one of the most vulnerable components of the central nervous system (CNS) that are commonly injured in brain traumas of various severities.5–7 Traumatic axonal injury (TAI) is regarded as one of the most important causes of morbidity and mortality in TBI patients.8,9 Although TAI has been shown to involve a series of pathological changes, including proteolysis, ionic dysregulation, and mitochondrial failure, formation of membrane pores caused by mechanical deformation, termed “mechanoporation,” is thought to be the initial pathological mechanism that may result in membrane failure, loss of ionic homeostasis, and axon degeneration and disconnection.10–13 Membrane sealing has, therefore, been hypothesized to be a promising strategy that may stop the initiation of the pathological cascade and rescue axons following spinal cord and brain injuries. Administration of polymers such as Poloxamer 188 and polyethylene glycol (PEG) has been demonstrated to seal broken membranes, promote axon conductance, and recover lost brain functions.14–17
A previous study demonstrated that micelles consisting of self-assembled monomethoxy poly (ethylene glycol)-poly (D, L-lactic acid) di-block copolymer micelles (mPEG-PDLLA) are effective in restoring the compound action potentials (CAPs) of the injured spinal cord, decreasing calcium influx into axons, and promoting the recovery of locomotion function in a rat model of spinal cord injury.18 Intravenously administered PEG-PDLLA micelles can penetrate into the injured spinal tissue to exert its membrane-repairing effect without causing any detectable adverse effects. Additionally, it may be used as a carrier for efficient delivery of drugs.18 These unique properties make the PEG-PDLLA micelles a valuable candidate for the early treatment of TBI.
The purpose of the current study is to use the micelles as a membrane sealant for repairing the injured cortical axons following TBI by evaluating their effect on axon conductance using extracellular field recording of CAPs from the corpus callosum. The corpus callosum contains myelinated axons and unmyelinated axons,19,20 which can be separately evaluated from the first and second peaks of the CAP waveform from field potential recordings.21–23 CAPs evoked in the corpus callosum are dramatically suppressed in the fluid percussion model of TBI,21,24 and are rescued by treatments with the immunophilin ligands cyclosporin-A (CsA) and FK506, or the calpain inhibitor MDL 28170.25–27 In the controlled cortical impact model of TBI, our results indicated that micelles treatment improved the function of both the myelinated and unmyelinated axons in the corpus callosum in up to 4 h after TBI.
Methods
Animals
Male CD1 mice 30–35 days old were used for this experiment. The mice were housed five per cage in a temperature- and humidity-controlled animal facility on a 12 h light/dark cycle, with food and water supplied ad libitum. All procedures were approved by the Animal Care and Use Committee of the Institutional Guide for the Care and Use of Laboratory Animals at Indiana University School of Medicine.
Controlled cortical impact (CCI) model and micelles administration
The CCI model was prepared similarly to published experiments.28,29 Mice were anaesthetized with ketamine/xylazine (87.7/12.3 mg/kg, i.p.) and fixed on a stereotaxic apparatus. Following the exposure of the skull with a midline incision, a 4 mm diameter cranial window was made between the lambda and bregma sutures of the left hemisphere, with the medial edge being 1 mm lateral from the midline. CCI was induced on the exposed cortex by using a 3 mm diameter impacting rod tip to compress the cortex at a velocity of 3.0 m/sec to a depth of 1.0 mm. We kept the consistency of physical impact in all animals that underwent CCI by using constant velocity (3.0±0.04 m/sec) and depth (1.0±0.1 mm). Mice with impacting parameters out of the range were excluded from further experiment. The cranial window was covered with a small piece of plastic sheet and the incision was sutured. The mice in the sham group received only craniotomy without CCI.
A single dose of mPEG-PDLLA micelles (200 μL at 10 mg/kg) or vehicle (200 μL saline) was injected via tail vein immediately (CCI+0 h micelles group) or 4 h (CCI+4 h micelles group) after CCI surgery. Our previous study successfully revealed the fusion of micelles and plasma membrane by using micelles that were labeled by a fluorescent dye DiIC18(3), a hydrophobic fluorescence probe.30 To trace the distribution of the micelles in brain tissues, two naïve mice and two CCI-injured mice were injected with fluorescently labeled PEG-PDLLA micelles (200 μl) immediately after CCI. The mice were perfused 2 h later, and the brains were postfixed and sectioned for confocal imaging.
Field potential recording
Two days after CCI, each mouse was anesthetized with 65 mg/kg pentobarbital (i.p.) and was decapitated, and the brain was rapidly removed. Coronal slices of 400 μm thick were cut in ice cold (4°C) artificial cerebrospinal fluid (ACSF) with a vibratome (LEICA VT1200, Buffalo Grove, IL). The ACSF consisted of (in mM): NaCl 126, KCl 2.5, NaH2PO4 1.4, CaCl2 2, MgSO4 2, NaHCO3 26, and glucose 10, pH 7.4, saturated with a mixture of 95% O2 and 5% CO2. Slices that contained midline-crossing segments of the corpus callosum overlying the mid-dorsal hippocampus were transferred to a holding chamber that contained oxygenated ACSF at room temperature for at least 1 h before recording.
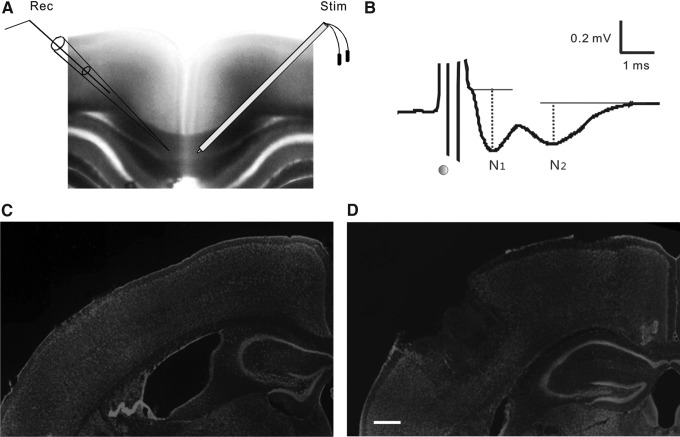
Extracellular CAPs were recorded according to techniques previously described.21,31 Individual slices were transferred to an interface chamber and perfused with oxygenated ACSF at a rate of 1–2 mL/min. Recordings were performed at 22–23°C, at which the two components of CAP were more discernible. CAPs were evoked with stimuli delivered via a bipolar tungsten electrode (FHC Inc., Bowdoin, ME) placed in the corpus callosum of the uninjured hemisphere, at ∼0.5 mm lateral to the midline. The responses were recorded with a recording electrode consisting of a glass pipette with 3–5 MΩ resistance when filled with 4M NaCl. The tip of the pipette was placed in the corpus callosum of the injured hemisphere, at a distance of ∼1.0 mm from the stimulating electrode (Fig. 1A). The depth of the stimulating and recording electrodes in the slice was adjusted so as to evoke maximal responses.
FIG. 1.
Extracellular field recording of compound action potentials (CAPs) for evaluating axonal function in cortical slices of sham and controlled cortical impact (CCI)-injured mice. (A) For CAP recording, a tungsten bipolar electrode (right, “Stim”) was placed in the corpus callosum of one hemisphere of a coronal slice (400 μm thick), and a glass pipette (left, “Rec”) was placed in the corpus callosum of the other hemisphere, ∼1 mm from the stimulating electrode. (B) A CAP waveform evoked in the corpus callosum usually consisted of two sequential negative peaks (N1 and N2), which were generated by myelinated and unmyelinated axons, respectively. The time of electrical stimulation is marked with a black dot. (C,D) Two-photon images of fluorescence Nissl staining of coronal cortical slices from a sham-injured mouse (C) and a CCI-injured mouse at 2 days after injury (D). Note the impact site and lost cortical tissue of the injured slice. Scale bar: 250 μm.
Current pulses of electrical stimulation were generated by a stimulus isolator (Isolated Pulse Stimulator Model 2100, A-M Systems, WA) and delivered once every 20 sec. For analyses of TBI-related changes in CAP amplitude, standardized input-output functions were generated by varying the intensity of stimulus pulses (50–1500 μA, 200 μs) from threshold level to an asymptotic maximum for CAP components. Signals were filtered (DC to 10 kHz), recorded using a differential amplifier (DP-304, Warner Instruments, CT), and digitized with PowerLab digitizer (ADInstruments, Colorado Springs, CO). Data were stored and analyzed with LabChart 7.0 software.
Intra-ventricular injection of Texas Red-labeled dextran dye
The mice in the treatment or vehicle groups first received CCI surgery and immediate intravenous injection of the micelles or saline as described. Two hours later, a small burr hole was drilled on the contralateral hemisphere. A glass pipette with a sharpened tip (∼35–50 μm) was lowered into the lateral ventricle (0.5 mm caudal and 1 mm lateral from bregma, depth 2.0 mm), and 6 μL Texas Red-labeled dextran (10,000 kDa, Invitrogen, CA) at a concentration of 25 mg/mL was injected at a rate of 0.5 μL/min using a Pneumatic PicoPump (PV 820, World Precision Instruments, Sarasota, FL). The pipette was kept in position for 3–5 min and then withdrawn slowly. Two hours later, the animals were perfused with 4% paraformaldehyde. The brains were removed for overnight postfixation and transferred to a 30% sucrose solution until the brains sank. Coronal brain sections were cut at a thickness of 25 μm using a cryostat (Leica CM1950). Mice from a normal control group (n=3) received no CCI and intravenous micelles injection, but they received an intraventricular injection of dextran dye, and were processed identically.
The sections were examined under an invert microscopy system (Zeiss, Axiovert 200M equipped with Apotome) interfaced with a digital camera (Zeiss, Axio Cam MRc5). Dextran dye-labeled neurons in the ipsilateral hemisphere of the cortex and the dentate gyrus of the hippocampus were counted at 40× magnification. In brain sections that contained the lesion cavity, all fluorescent cortical and hippocampal neurons were separately counted from a set of sections 250 μm apart. The total number of fluorescent cortical or hippocampal neurons in each mouse were estimated by multiplying the total counted number of cortical or hippocampal neurons by the total set number of sections.
Statistical analysis
Electrophysiological data were analyzed similarly to previous studies.21,31 From average traces of four successive sweeps at each stimulation intensity, the peak amplitudes of N1 and N2 waves were determined by manually measuring the vertical distance from the individual negative peaks to the baseline (Fig. 1B). In each mouse, measurements from recordings of 3–5 slices were averaged. The significances in the shifts of the input-output curves of the CAP were evaluated with the Mann–Whitney U test. Because of the inhomogeneity of variance of the data sets, a nonparametric statistical test was used (SPSS v19) (Fig. 2).25 Dextran dye-labeled cortical and hippocampal neurons (Fig. 4) were analyzed by using Student's t test. Values are presented as mean±SEM.
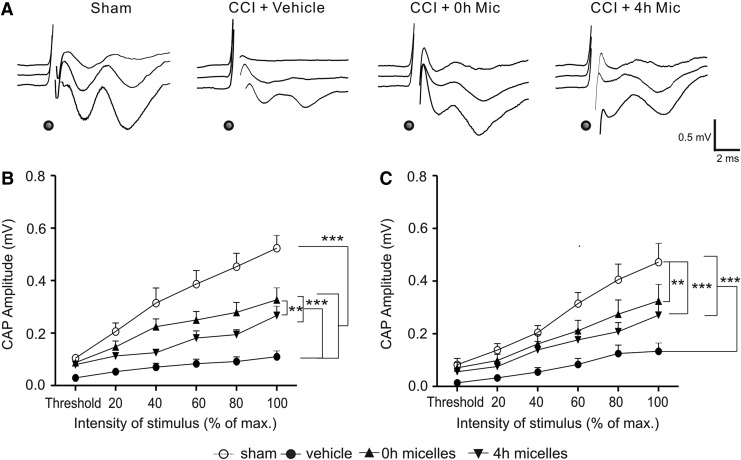
FIG. 2.
Intravenous injection of the micelles within 4 h after controlled cortical impact (CCI) improved the function of myelinated and unmyelinated axons of the corpus callosum. (A) Representative traces of compound action potentials (CAPs) evoked at threshold (top traces), 2× threshold (middle traces), and maximum (bottom traces) stimulation intensities in the sham, CCI+vehicle, CCI+0h micelles, and CCI+4h micelles groups. The times of stimulation are marked with black dots. (B,C) Input-output curves of mean N1 (B) and N2 (C) peak amplitudes evoked at increasing stimulation intensities in the four groups. CCI resulted in significant decreases in both N1 and N2 amplitudes. Injection of the micelles at 0 h or 4 h after injury improved the recovery of N1 and N2 amplitudes. **p<0.01, ***p<0.001. Group sizes (mice): Sham=14; CCI+vehicle=11; CCI+0 h micelles=13; CCI+4 h micelles=12.
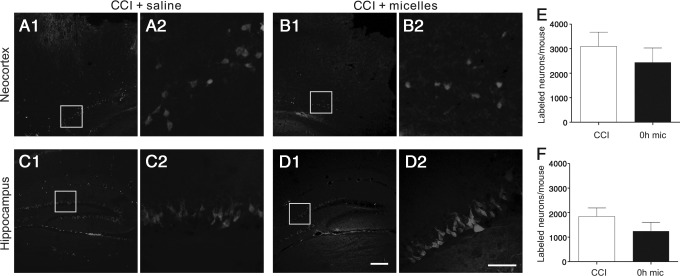
FIG. 4.
Micelles treatment did not improve resealing of neuronal plasma membrane following controlled cortical impact CCI. (A–D) Two-photon images of cortical (A,B) and hippocampal (C,D) neurons after CCI, which were labeled by ventricular injection of 10 kDa Texas Red-labeled dextran dye. The moderate CCI resulted in membrane disruption and Texas Red-dextran labeling of cortical neurons (A,B) in the vicinity of the injury sites and the dentate granule cells of the hippocampus (C,D). (E,F) There were no significant differences in the number of dye-labeled neurons in either the cortex (E) or hippocampus (F) between the saline+CCI (white bars) and micelles+CCI (black bars) groups (p>0.05). Scale bars: 200 μm in D1 for A1, B1, C1, and D1; 50 μm in D2 for A2, B2, C2, and D2. Group sizes=10–12 mice.
Results
Electrophysiological recordings were made from 11–14 mice in each of the sham, CCI+vehicle, CCI+0 h micelles, and CCI+4 h micelles groups. We recorded extracellular CAPs to evaluate the effect of moderate CCI on axonal function, and the efficacy of intravenously administered mPEG-PDLLA micelles at 0 h or 4 h after TBI. In coronal slices from uninjured neocortex, CAPs were recorded at room temperature by separately placing a stimulating electrode and a recording electrode in the corpus callosum as shown in Figure 1A. The evoked responses usually exhibited two well-separated negative peaks (Fig. 1B), representing action potential conduction of the fast myelinated axons (N1) and slow unmyelinated axons (N2), respectively, which were similar to previously reported results.21–22,27,31 The mean peak amplitudes of the N1 and N2 waves at the maximal stimulus were 0.52±0.05 mV and 0.47±0.07 mV, respectively.
In cortical slices prepared from CCI mice at 2 days after injury, the impact sites of the neocortex were clearly visible under a 5× objective. A portion of the injured superficial cortex was usually missing in the slices. The medial edges of the impact sites were ∼ 2 mm from the midline of the cortex (Fig. 1C and D). Recordings at 2 days after CCI revealed that both N1 and N2 components of the CAPs were significantly suppressed in CCI-vehicle group when compared with the sham group. Statistical analysis of the input-output curves showed significant decreases in the peak amplitudes of N1 (Z=−8.421, p<0.001) as well as N2 (Z=−6.811, p<0.001) components in CCI-vehicle animals when compared with the sham group (Fig. 2), with the maximal amplitude of N1 being 21.0% of the sham group (p<0.001), and that of N2 being 28.4% of the sham control group (p<0.001). The result indicated that the CCI caused severe functional loss in both myelinated and unmyelinated axons.
Immediate intravenous injection of the micelles after CCI resulted in highly significant increases in the N1 (Z=−7.388, p<0.001) and N2 amplitudes (Z=−5.146, p<0.001) when compared with the CCI-vehicle group, although they were still significantly lower than the sham group (Z=−3.32, p<0.001 for N1 and Z=−2.686, p<0.01 for N2, respectively) (Fig. 2). Similarly, treatment with the micelles at 4 h after CCI also resulted in highly significant increases in N1 (Z=−6.343, p<0.001) and N2 amplitudes (Z=−4.920, p<0.001) when compared with the CCI-vehicle group, although they were still significantly lower than the sham group (Z=−5.143, p<0.001 for N1 and Z=−3.446, p<0.001 for N2, respectively) (Fig. 2). Additionally, the recovery of N1 amplitude in the 0 h micelle group was better than that of the 4 h micelle group (Z=−2.717, p<0.01) (Fig. 2B), but this difference was not statistically significant in the recovery of N2 amplitude (Z=−0.772, p>0.05) (Fig. 2C). Together, the results indicated that intravenous injection of the micelles at either 0 h or 4 h post-CCI was effective in improving the function of both myelinated and unmyelinated axons of the corpus callosum following TBI.
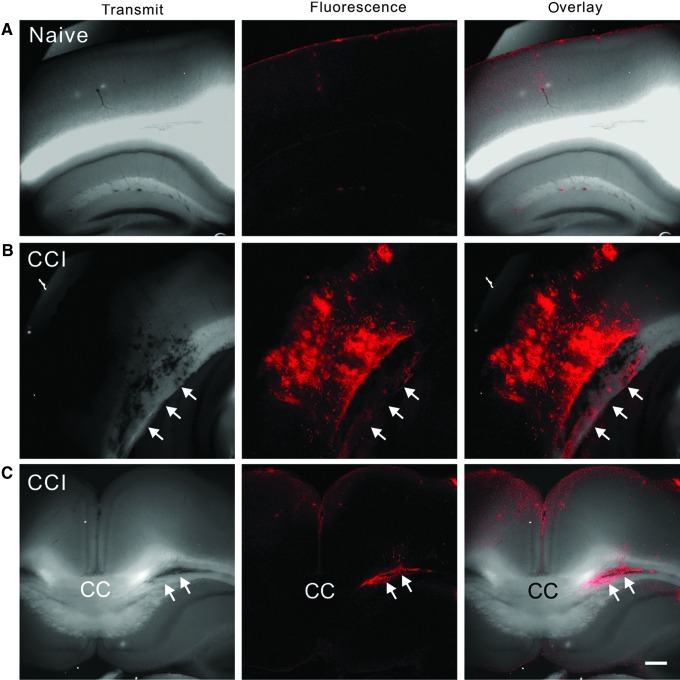
A focal contusion lesion induced by CCI is usually accompanied by dramatic damage of the blood–brain barrier, which may allow the micelles in the bloodstream to diffuse into brain tissue and exert their membrane repairing effect. To trace the distribution of the micelles in the brain tissue, we injected DiI-labeled micelles into the tail vein of naïve and injured mice immediately after CCI. In coronal cortical sections from the naïve mice, the micelles only lightly stained scattered spots of the brain tissue, with some spots surrounding vessels (Fig. 3A). In the CCI injured mice, the micelles heavily stained cortical tissues, including the gray and white matters underneath the impact site (Fig. 3B and C). In some sections, the corpus callosum was also strongly stained (Fig. 3C). The results indicated that micelles were able to leak through the compromised blood–brain barrier and bind with the injured gray and white matters of the impact site.
FIG. 3.
Penetration of DiI-labeled micelles into injured cortical gray and white matters. (A–C) Confocal images of coronal cortical sections under transmitted light (left column), red fluorescence (middle column), and the combined channel (right column) show that the dye-containing micelles only minimally stained uninjured brain tissues of the naïve mice (A, n=2), but they heavily stained not only cortical gray matter directly underneath the impact site (B), but also the white matter including the corpus callosum (white arrows in B and C) of the controlled cortical impact (CCI) mice (n=2). Scale bar in C: 250 μm. Color image is available online at www.liebertpub.com/neu
TBI has been shown to cause disruption of the neuronal plasma membrane and subsequent cell death,13,32 which leads to the idea that the micelles may work as a membrane sealant to repair the damaged plasma membrane and to rescue the injured neurons. To determine whether intravenous injection of the micelles promoted membrane resealing in neurons after CCI, we injected 10 kDa of Texas Red-labeled dextran into the lateral ventricle of the mice at 2 h after CCI, and immediately administered an intravenous injection of the micelles or saline.32 Similar to previously reported results,32 uninjured cell membrane was impermeable to fluorescent-conjugated dextran dyes, and no neurons in the neocortex or hippocampus were labeled by the fluorescent dye (data not shown). The CCI resulted in membrane disruption and fluorescence labeling of cortical and hippocampal neurons (Fig. 4). In the neocortex, neuronal somata beneath and on the edge of the impact sites were frequently labeled (Fig. 4A). In the hippocampus, neuronal somata in the ipsilateral dentate gyrus, CA3, and CA1 were also frequently labeled (Fig. 4C). In some mice, a smaller number of neurons in the contralateral dentate gyrus were also labeled (data not shown). In mice that received the micelles treatment, fluorescent neurons were still visible in the neocortex and hippocampus (Fig. 4B and D). Quantitative analysis of the dye- stained neurons showed that there were no significant differences between the CCI+saline and CCI+micelles groups in fluorescently-labeled neocortical neurons (Fig. 4E) (3096±580 and 2440±588 neurons per mouse in the CCI+saline and CCI+micelles groups respectively, n=12 mice in each group) and fluorescently-labeled hippocampal neurons (Fig. 4F) (1846±347 and 1246±358 neurons per mouse in the CCI+saline [11 mice] and CCI+micelle [10 mice] groups respectively) (p>0.05 in both comparisons). Additionally, we did not find dye staining in axons in the corpus callosum or white matter of the CCI injured brain slices (data not shown).
Discussion
Perturbation of the axolemma in TBI often results in the entry of ionic species and large molecules,10 which is commonly followed by progressive abnormality of neurofilaments and microtubules, and eventual disconnection of axons.11,33,34 Therefore, sealing the damaged axolemma at the acute stage of TBI may be a valid strategy for the early treatment of TBI. Consistent with the effective results of the mPEG-PDLLA micelles on axonal damage following spinal cord injury in an earlier study,18 here we demonstrated that intravenous injection of the mPEG-PDLLA micelles within 4 h is effective in improving the function of both myelinated and unmyelinated axons in the CCI model of TBI in mice. Additionally, we also found that immediate administration of the micelles following CCI did not have a significant effect on resealing the ruptured plasma membrane of neuronal somata in the neocortex and hippocampus, suggesting that the effect of the micelles on axonal function is independent of resealing plasma membrane.
The corpus callosum is the largest bundle of axonal fibers in the mammalian brain that connects the right and left hemispheres of the cerebral cortex. Recording CAPs of the corpus callosum is an efficient method for separately evaluating changes in electrophysiological properties of the fast-conducting myelinated axons and the slow-conducting unmyelinated axons under pathological conditions such as TBI and demyelination.21,24,31 Depressed CAPs of the corpus callosum have been demonstrated in the lateral fluid percussion and CCI models of TBI.21,24,27,35 In the current study, we found that the peak CAP amplitudes of both N1 and N2 of the corpus callosum were significantly lower in the CCI group than in the sham group, suggesting an impaired function of both myelinated and unmyelinated axons in this model. The maximal amplitudes of the N1 and N2 were 21.0% and 28.4% of the values of the sham group, indicating that CCI caused slightly more severe damage to the myelinated axons than to the unmyelinated axons. This result is different from previous morphological and electrophysiological studies in the fluid percussion model showing that unmyelinated axons of the corpus callosum are more vulnerable to TBI than myelinated axons.19,21 This difference in the severity of axonal injury may result from a difference in biomechanical features between the CCI and lateral fluid percussion models.36 Alterations that may potentially contribute to a decrease in CAP amplitude include a decreased number of axons that are capable of conducting action potentials, poor action potential conductions of axons caused by axonal dysfunction, damaged myelin, or changed gating and conductance of ionic channels (e.g., voltage-gated sodium channels). Given the spectrum of axonal alterations such as abnormal axonal cytoskeleton, transport interruption, mitochondria dysfunction, and structure disconnection after TBI,6,7 it is likely that several of these pathophysiological mechanisms are involved in the TBI-induced depression of CAP amplitude. Although determining the exact mechanisms of CAP change is out of the scope of the current study, the result of CAP recording does provide an efficient and comprehensive measurement of axonal function.
Polyethylene glycol (PEG) is a well-known fusogen that has been shown to induce a rapid recovery of axonal integrity after spinal cord injury and promote its functional recovery.37,38 The polymeric mPEG-PDLLA micelles are recently developed nanoscale particles that contain a hydrophilic PEG (monomethoxy polyethylene glycol) shell and a hydrophobic PDLLA (poly D, L-lactic acid) core. This structural feature may allow them to quickly fuse with plasma membrane for repairing damaged membrane and for delivering hydrophobic drugs.30 The use of these types of nanoparticles provides several advantages over traditional membrane sealing agents, including better tissue penetration, high efficacy at a very low concentration, minimal toxicity, and the capability of carrying hydrophobic drugs.18 Here, we demonstrated that the DiI-labeled micelles penetrated the compromised blood–brain barrier and entered the gray and white matters underneath the impact site of cortical focal contusion. The presence of the micelles in the white matter, including the corpus callosum, may allow them to reseal the injured axolemma and improve axonal function, although additional benefits of the micelles on cerebral vasculature or gray matter still cannot be excluded.
The mPEG-PDLLA micelles have been shown to restore CAPs and diminish calcium influx into the spinal axons after compression injury.18 Similarly, we found that the micelles significantly increased the amplitudes of both myelinated and unmyelinated axons of the corpus callosum following TBI. This nonselective repairing effect of the micelles is in contrast to certain therapeutic agents reported earlier, which have a more prominent effect in repairing either myelinated or unmyelinated axons. For example, immunophilin ligand tacrolimus (FK506) has a much greater effect on increasing the N2 CAP amplitude than on increasing the N1 amplitude.27 In contrast, early administration of CsA, another immunophilin ligand, is shown to significantly improve the N1, but not the N2, component of the CAP in the corpus callosum after fluid percussion injury.25 The efficacy of the micelles in improving the function of both types of axons may be explained by their ability to physically reseal the damaged axolemma of both myelinated and unmyelinated axons.30
TBI also causes almost immediate membrane perturbation of neuronal somata in the brain, which is followed by abnormal ultrastructural changes such as plasma and nuclear membrane damages, nuclear chromatin condensation, and eventual necrosis.13,32 In the CCI model, the number of neurons that have a damaged plasma membrane increase dramatically between 5 min and 1 h after injury.13 This progressive membrane damage may provide a therapeutic window for early neuroprotection through resealing the leaky membrane. Unfortunately, immediate micelles treatment following CCI did not result in a significant reduction in the number of neurons that were labeled by the membrane impermeable fluorescent dextran dye. The different effect of the micelles on resealing plasma membrane and axolemma may indicate that the mechanism, severity, and consequence of plasma membrane damage of neuronal somata are different from those of axolemma damage, and, therefore, that their response to a therapeutic treatment may also be different. In support of this idea, persistent elevation of intracranial pressure after TBI exacerbates poration of the plasma membrane but not axonal injury.39 Furthermore, it seems that neurons with plasmalemma damage will inevitably degenerate and die by 7 days after CCI, even though some neurons are found to undergo spontaneous resealing of the damaged plasma membrane, suggesting that permeable plasmalemma after TBI is probably fatal to neurons.13,40 Similarly, although the ruptured somata membrane could be repaired by using a membrane-resealing agent Kollidon VA64, such effective repairing of the membrane does not rescue the injured neurons.40
Repairing the injured CNS remains a major challenge in neurotrauma research. Rescuing and preserving the injured axons at the early stage of injury may be a more efficient and practical strategy than promoting axonal regeneration at a later time period. The restoration of depressed CAP in axons by the micelles suggests a better axonal function and potentially better preservation of axonal structure, which allows more efficient conduction of electrical signals. The improved functional recovery of the corpus callosum in this study may not only promote the communication between the two hemispheres of the cerebral cortex, but also be indicative of a repairing effect on other axonal fibers in the brain, such as the equally vulnerable white matter and the internal capsule.41,42
Acknowledgments
This research was supported by National Institutes of Health Grant NS057940 and by the Indiana Spinal Cord and Brain Injury Research Fund from the Indiana State Department of Health (A70-0-079212 to X.J.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Salmond C.H., and Sahakian B.J. (2005). Cognitive outcome in traumatic brain injury survivors. Curr. Opin. Crit. Care 11, 111–11615758589 [Google Scholar]
- 2.Willemse-van Son A.H., Ribbers G.M., Verhagen A.P., and Stam H.J. (2007). Prognostic factors of long-term functioning and productivity after traumatic brain injury: a systematic review of prospective cohort studies. Clin. Rehabil. 21, 1024–103717984154 [Google Scholar]
- 3.Langlois J.A., Rutland–Brown W., and Thomas K.E. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 20, 229–23815908823 [Google Scholar]
- 4.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., and Hemphill J.D. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill Summ 60, 1–3221544045 [Google Scholar]
- 5.Newcombe V., Chatfield D., Outtrim J., Vowler S., Manktelow A., Cross J., Scoffings D., Coleman M., Hutchinson P., Coles J., Carpenter T.A., Pickard J., Williams G., and Menon D. (2011). Mapping traumatic axonal injury using diffusion tensor imaging: correlations with functional outcome. PloS One 6, e1921421573228 [Google Scholar]
- 6.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–4322285252 [Google Scholar]
- 7.Buki A., and Povlishock J.T. (2006). All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. 148, 181–19316362181 [Google Scholar]
- 8.Cordobes F., Lobato R.D., Rivas J.J., Cabrera A., Sarabia M., Castro S., Cisneros C., Torres I.D., and Lamas E. (1986). Post-traumatic diffuse axonal brain injury. Analysis of 78 patients studied with computed tomography. Acta Neurochir. (Wien) 81, 27–353728087 [Google Scholar]
- 9.Graham D.I., McIntosh T.K., Maxwell W.L., and Nicoll J.A. (2000). Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 59, 641–65110952055 [Google Scholar]
- 10.Pettus E.H., Christman C.W., Giebel M.L., and Povlishock J.T. (1994). Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J. Neurotrauma 11, 507–5227861444 [Google Scholar]
- 11.Povlishock J.T., and Pettus E.H. (1996). Traumatically induced axonal damage: evidence for enduring changes in axolemmal permeability with associated cytoskeletal change. Acta Neurochir. Suppl. 66, 81–868780803 [Google Scholar]
- 12.Maxwell W.L., Povlishock J.T., and Graham D.L. (1997). A mechanistic analysis of nondisruptive axonal injury: a review. J. Neurotrauma 14, 419–4409257661 [Google Scholar]
- 13.Whalen M.J., Dalkara T., You Z., Qiu J., Bermpohl D., Mehta N., Suter B., Bhide P.G., Lo E.H., Ericsson M., and Moskowitz M.A. (2008). Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 490–50517713463 [Google Scholar]
- 14.Krause T.L., and Bittner G.D. (1990). Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc. Natl. Acad. Sci. U.S.A. 87, 1471–14752304913 [Google Scholar]
- 15.Shi R., Borgens R.B., and Blight A.R. (1999). Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J. Neurotrauma 16, 727–73810511246 [Google Scholar]
- 16.Cho Y., and Borgens R.B. (2012). Polymer and nano-technology applications for repair and reconstruction of the central nervous system. Exp. Neurol. 233, 126–14421985867 [Google Scholar]
- 17.Kilinc D., Gallo G., and Barbee K.A. (2008). Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp. Neurol. 212, 422–43018572167 [Google Scholar]
- 18.Shi Y., Kim S., Huff T.B., Borgens R.B., Park K., Shi R., and Cheng J.X. (2010). Effective repair of traumatically injured spinal cord by nanoscale block copolymer micelles. Nat. Nanotechnol. 5, 80–8719898498 [Google Scholar]
- 19.Reeves T.M., Smith T.L., Williamson J.C., and Phillips L.L. (2012). Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J. Neuropathol. Exp. Neurol. 71, 198–21022318124 [Google Scholar]
- 20.Gravel C., Sasseville R., and Hawkes R. (1990). Maturation of the corpus callosum of the rat: II. Influence of thyroid hormones on the number and maturation of axons. J. Comp. Neurol. 291, 147–1612298928 [Google Scholar]
- 21.Reeves T.M., Phillips L.L., and Povlishock J.T. (2005). Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 196, 126–13716109409 [Google Scholar]
- 22.Preston R.J., Waxman S.G., and Kocsis J.D. (1983). Effects of 4-aminopyridine on rapidly and slowly conducting axons of rat corpus callosum. Exp. Neurol. 79, 808–8206825765 [Google Scholar]
- 23.Swanson T.H., Krahl S.E., Liu Y.Z., Drazba J.A., and Rivkees S.A. (1998). Evidence for physiologically active axonal adenosine receptors in the rat corpus callosum. Brain Res. 784, 188–1989518606 [Google Scholar]
- 24.Baker A.J., Phan N., Moulton R.J., Fehlings M.G., Yucel Y., Zhao M., Liu E., and Tian G.F. (2002). Attenuation of the electrophysiological function of the corpus callosum after fluid percussion injury in the rat. J. Neurotrauma 19, 587–59912042094 [Google Scholar]
- 25.Colley B.S., Phillips L.L., and Reeves T.M. (2010). The effects of cyclosporin-A on axonal conduction deficits following traumatic brain injury in adult rats. Exp. Neurol. 224, 241–25120362574 [Google Scholar]
- 26.Ai J., Liu E., Wang J., Chen Y., Yu J., and Baker A.J. (2007). Calpain inhibitor MDL-28170 reduces the functional and structural deterioration of corpus callosum following fluid percussion injury. J. Neurotrauma 24, 960–97817600513 [Google Scholar]
- 27.Reeves T.M., Phillips L.L., Lee N.N., and Povlishock J.T. (2007). Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury. Brain Res. 1154, 225–23617481596 [Google Scholar]
- 28.Gao X., and Chen J. (2011). Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 70, 183–19121293299 [Google Scholar]
- 29.Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and McIntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–1787629863 [Google Scholar]
- 30.Chen H., Kim S., Li L., Wang S., Park K., and Cheng J.X. (2008). Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc. Natl. Acad. Sci. U. S. A. 105, 6596–660118445654 [Google Scholar]
- 31.Crawford D.K., Mangiardi M., and Tiwari–Woodruff S.K. (2009). Assaying the functional effects of demyelination and remyelination: revisiting field potential recordings. J. Neurosci. Methods 182, 25–3319481113 [Google Scholar]
- 32.Farkas O., Lifshitz J., and Povlishock J.T. (2006). Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J. Neurosci. 26, 3130–314016554464 [Google Scholar]
- 33.Christman C.W., Grady M.S., Walker S.A., Holloway K.L., and Povlishock J.T. (1994). Ultrastructural studies of diffuse axonal injury in humans. J. Neurotrauma 11, 173–1867523685 [Google Scholar]
- 34.Povlishock J.T., Becker D.P., Cheng C.L., and Vaughan G.W. (1983). Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 42, 225–2426188807 [Google Scholar]
- 35.Ajao D.O., Pop V., Kamper J.E., Adami A., Rudobeck E., Huang L., Vlkolinsky R., Hartman R.E., Ashwal S., Obenaus A., and Badaut J. (2012). Traumatic brain injury in young rats leads to progressive behavioral deficits coincident with altered tissue properties in adulthood. J. Neurotrauma 29, 2060–207422697253 [Google Scholar]
- 36.Clausen F., and Hillered L. (2005). Intracranial pressure changes during fluid percussion, controlled cortical impact and weight drop injury in rats. Acta Neurochir. 147, 775–78015900397 [Google Scholar]
- 37.Shi R., and Borgens R.B. (1999). Acute repair of crushed guinea pig spinal cord by polyethylene glycol. J. Neurophysiol. 81, 2406–241410322076 [Google Scholar]
- 38.Borgens R.B., and Shi R. (2000). Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 14, 27–3510627277 [Google Scholar]
- 39.Lafrenaye A.D., McGinn M.J., and Povlishock J.T. (2012). Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation. J. Cereb. Blood Flow Metab. 32, 1919–193222781336 [Google Scholar]
- 40.Mbye L.H., Keles E., Tao L., Zhang J., Chung J., Larvie M., Koppula R., Lo E.H., and Whalen M.J. (2012). Kollidon VA64, a membrane-resealing agent, reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 32, 515–52422086196 [Google Scholar]
- 41.Koliatsos V.E., Cernak I., Xu L., Song Y., Savonenko A., Crain B.J., Eberhart C.G., Frangakis C.E., Melnikova T., Kim H., and Lee D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–41621487304 [Google Scholar]
- 42.Xu J., Rasmussen I.A., Lagopoulos J., and Haberg A. (2007). Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma 24, 753–76517518531 [Google Scholar]