Abstract
An UHPLC-PDA-ESI/HRMSn profiling method was used to identify the glucosinolates and flavonoids of Rorippa indica (Cruciferae), a wild vegetable and Chinese herb used to treat cough, diarrhea, and rheumatoid arthritis. Thirty-three glucosinolates, more than 40 flavonol glycosides, and 18 other phenolic and common organic compounds were identified. The glucosinolates and polyphenols were separated by UHPLC. High-resolution deprotonated molecules provided high accuracy mass values that were used to determine formulas and provide putative identification of the glucosinolates and flavonoids. The fragments from multistage mass spectrometry were used to elucidate the structures. The concentrations of the main components were based on UV peak areas and molar relative response factors with a single calibration standard. This study found this plant to be a rich source for glucosinolates, containing 24 new glucosinolates, including 14 glucosylated glucosinolates that were previously unidentified.
Keywords: glucosinolates, flavonol glycosides, Rorippa indica (Cruciferae), Chinese wild vegetable, Chinese herb, UHPLC-PDA-ESI/HRMSn, compound identification, main compound quantification
Introduction
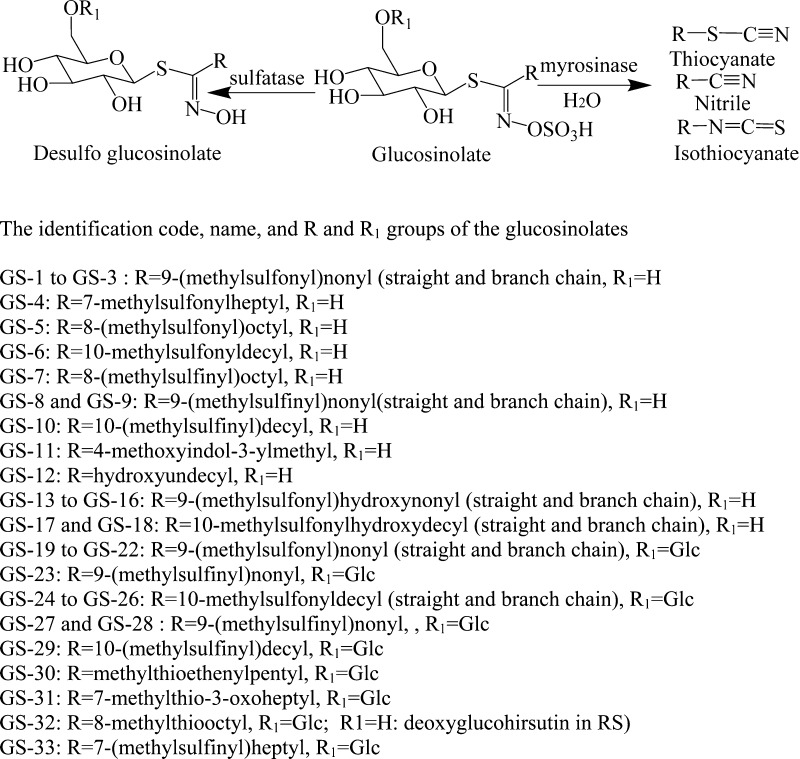
Glucosinolates (GSs) are sulfur- and nitrogen-containing secondary metabolites found in cruciferous plants. A central carbon is connected to a thioglucose, to a sulfate via a nitrogen atom, and to a side chain (R) (Figure 1). The side chain determines whether the glucosinolate is defined as aliphatic (straight- or branched-chain alkyl, alkenyl, and oxo-, hydroxyl forms), aromatic, or indole. Many glucosinolates also contain one or two more sulfur atoms in the side chain to form another sulfur-containing unit, such as methyl-thio- (−S−), methyl-sulfinyl- (−SO−), methyl- or benzyl-sulfonyl- (−SO2−), mercapto- (HS−), glucosyl-disulfanyl (−S–S−), and cysteinyl-thio- [HOOC–CH(NH2)–CH2–S−]. The glucosinolates and their enzyme-hydrolyzed products (thiocyanates, isothiocyanates, and nitriles) have shown important human health benefits, especially with respect to cancer prevention. Thus, many studies have been carried out on the isolation and structural elucidation of glucosinolates and their hydrolyzed products with respect to their biological activities and distribution in the plant kingdom.1−7
Figure 1.
Structures of the glucosinolates of Rorippa indica.
To date, approximately 200 glucosinolates have been reported in plants. An electronic database of the structures, formulas, and accurate masses of 200 knownn and a further 180 predictedn GSs has been established.8 Desulfoglucosinolates, glucosinolate-related nitriles, and isothiocyanates (Figure 1) formed by the loss of SO3 or other functional groups through the action of sulfatase and myrosinase have been isolated from plants.9,10 Glucosinolates, widely distributed in many plants of the Cruciferae family, have also been found in plants from other familiesn although at significantly reduced levels. The glucosinolate profiles of many foods derived from cruciferous plants have been studied qualitatively and quantitatively to establish their dietary intake and relationship to health outcomes in biological, epidemiological, and clinical studies.1−28
The fragmentation patterns of glucosinolates have been studied using multistage mass spectrometry (MSn), primarily in the negative ionization mode. High-performance liquid chromatography and MSn with electrospray ionization (HPLC-ESI/MSn) has been widely used to identify the intact glucosinolate components of plants.8,11−18 Most glucosinolates have an ultraviolet (UV) absorbance maximum wavelength (λmax) between 219 and 228 nm. They can be quantified directly from the UV absorbance at 225 nm when they are well separated.8,14,19,20 However, in most cases, quantification has been based on absorbance of the desulfoglucosinolates following sulfatase hydrolysis. Quantification is based on calibration with a single compound and predetermined relative response factors (RRFs).8,20−26
Rorippa indica (Linn.) Hiern. is a perennial plant of the Cruciferae family that is widely distributed throughout the world. Its fresh spear-shaped leaves and tender seedlings are used as a healthy food, and the whole plant is used as an animal feed in China. The dried whole plant material is used as a Chinese traditional herb with purported diuretic, anti-inflammatory, antifever, and anticough properties and to help with blood circulation and rheumatoid arthritis.27 Some glucosinolates have been reported in R. indica and several other Rorippa plants.4,28 Ronifore (9-methylsulfonylnonyl nitrile) and its related amide (rorifamide) were isolated as the anticough components of Rorippa montana.27,29 Fifteen isothiocyanates and desulfoglucosinolates were isolated from the roots of Rorippa plants, and several flavonol glycosides, phenolic acids, and derivatives, and other organic compounds have been reported.9,28−30 This paper presents a comprehensive study of the glucosinolate and polyphenolic components of R. indica.
Phenolic compounds are widely distributed in plant-derived foods, whereas the glucosinolates are among the biologically active components of Cruciferous plants. Both types of compounds are known to have many potential benefits to human health. As a part of our project to systematically identify and quantify these compounds, both standardized HPLC-PDA-ESI/MS and UHPLC-PDA-ESI/HRMSn methods were developed to identify and quantify the phenolics using their molar relative response factors (MRRFs) for UV absorbance.31−33 In this study, the UHPLC-PDA-ESI/HRMSn method was used to analyze the phenolic and glucosinolate profiles of R. indica and led to the identification of 33 glucosinolates, including 24 for the first time, and more than 40 flavonol glycosides. This study found that R. indica was a rich plant source for glucosinolates on the basis of its structural variety and total concentration of glucosinolates.4,5,8
Materials and Methods
Standards and Other Chemicals
Glucocheirolin potassium salt [3-(methylsulfonyl)propyl glucosinolate-K, MW = 439 + 39 Da], rutin 3H2O (MW = 610 + 54 Da), quercetin, kaempferol, and isorhamnetin were obtained from Chromadex, Inc. (Irvine, CA, USA). Formic acid, hydrochloric acid (∼37%), HPLC grade methanol, and acetonitrile were purchased from VWR International, Inc. (Clarksburg, MD, USA). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA, USA).
Standard Solutions
The stock standard solution consisted of dried rutin (1.27 mg) 34 and glucocheriolin-K (1.43 mg) in 10.0 mL of methanol/water (60:40, v/v). The stock solution was prepared at three concentrations (1, 1/2, and 1/4) to provide a range of signals suitable for the quantification of the main glucosinolates and phenolics of this sample. Each of the solutions (2 μL) was injected in triplicate, and the relative standard deviation (RSD) for each peak was <5.0%. The three standards provided a linear calibration curve for estimating the concentration of the main compounds of the extract.31,32
Plant Materials and Extracts
The dried R. indica plant materials were purchased by the Longjin Pharmaceutical Co., Ltd., from local herb stores in Chongqing, China. The plant materials were ground into powders and passed through 60 mesh sieves prior to extraction. The ground powder (200 mg) was extracted with 5.0 mL of methanol/water (60:40, v/v) using an FS30 ultrasonic sonicator (Fisher Scientific, Pittsburgh, PA, USA) for 60 min at room temperature. The slurry mixture was centrifuged at 2500 rpm for 15 min (IEC Clinical Centrifuge, Damon/IEC Division, Needham, MA, USA), and the supernatant (4.000 mL) was filtered through a 17 mm (0.45 μm) PVDF syringe filter (VWR Scientific, Seattle, WA, USA). Two microliters of the extract was injected in triplicate into the HPLC for analysis.31−33
Acidic Hydrolyzed Extracts
The filtered extracts (0.50 mL) were mixed with concentrated HCl (37%, 0.1 mL) and heated in a capped tube at 85 °C for 2 h. Then, 0.4 mL of methanol was added to the mixture, and the solution was sonicated for 10 min. The solution was refiltered prior to HPLC injection.31
UHPLC-PDA-ESI/HRMSn Conditions
The UHPLC-DAD-ESI/HRMS-MSn system used consisted of an LTQ Orbitrap XL mass spectrometer with an Accela 1250 binary Pump, a PAL HTC Accela TMO autosampler, a diode array detector (DAD) (ThermoScientific, San Jose, CA, USA), and a G1316A column compartment (Agilent, Palo Alto, CA, USA). The separation was carried out on an UHPLC column (200 mm × 2.1 mm i.d., Hypersil Gold AQ RP- C18, 1.9 μm) (ThermoScientific) with an HPLC/UHPLC precolumn filter (UltraShield Analytical Scientific Instruments, Richmond, CA, USA) at a flow rate of 0.3 mL/min. The mobile phase consisted of a combination of A (0.1% formic acid in water, v/v) and B (0.1% formic acid in acetonitrile, v/v). The linear gradient was from 4 to 20% B (v/v) at 40 min, to 35% B at 60 min, to 100% B at 61 min, and held at 100% B to 65 min.32 The PDA recorded spectra from 200 to 700 nm, and peak intensities at 225 and 354 nm were used for the quantification of the main glucosinolates and flavonoids, respectively.33
The MS was operated in the negative ionization mode using the following conditions: sheath gas at 70 (arbitrary units), auxiliary and sweep gas at 15 (arbitrary units), spray voltage at 4.8 kV, capillary temperature at 300 °C, capillary voltage at 15 V, and tube lens at 70 V. The scan range was from m/z 100 to 1500 with a resolution of 15000, FTMS AGC target at 2e5, FT-MS/MS AGC target at 1e5, isolation width of m/z 1.5, and maximum ion injection time of 500 ms. High-accuracy ion mass values were determined for all primary ions (i.e., the deprotonated molecular ions). The most intense ion was selected for the data-dependent scan to provide MS2–MS5 product ions with a normalized collision energy at 35% (CID).33 The product ions were determined with nominal mass accuracy. An additional separate data-dependent scan with high-energy collisional dissociation (HCD) at 75% was also carried out for high-accuracy measurement of some of the MSn ions with the resolution of 7500.
Results and Discussion
Identification of Glucosinolates
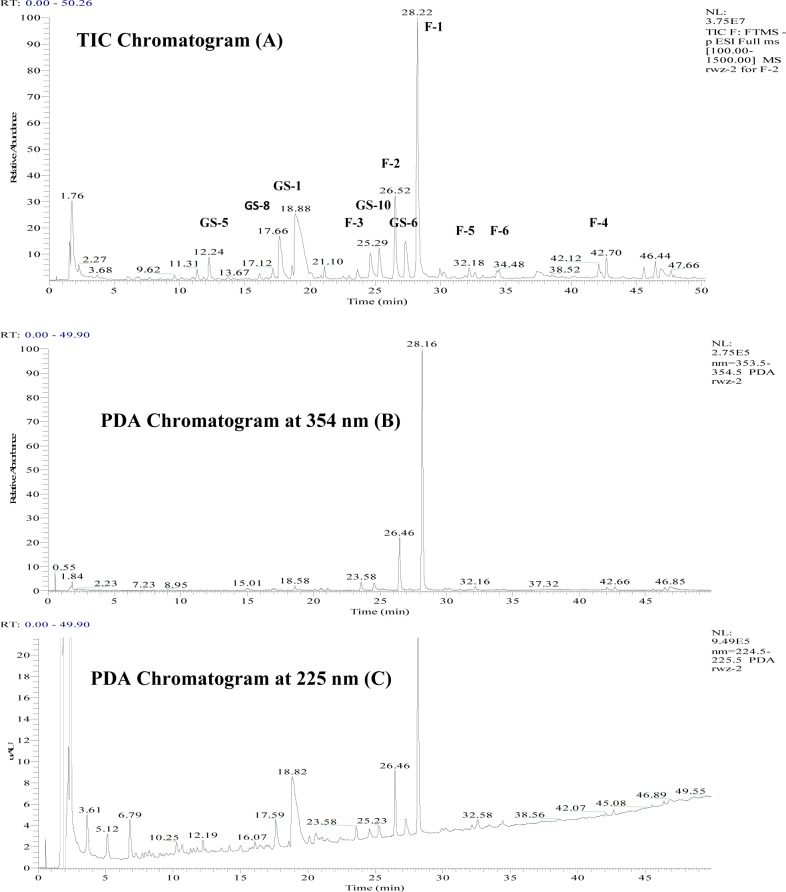
Thirty-three glucosinolates were detected in the plant extract. Figure 1 lists the identification codes, putative identification, retention times (recorded on the TIC chromatogram), deprotonated molecule high-resolution mass, formula, error between the experimental and calculated values, and main and important fragments from MS2, MS3, and MS4 fragmentations for each peak. The listing is in order of molecular weight. The TIC and PDA (at 225 nm) chromatograms are shown in Figure 2. Thirty-three of the compounds were putatively identified, and nine compounds could not be identified.
Figure 2.
Chromatograms (A, TIC; B, PDA at 354 nm; and C, PDA at 225 nm) of Rorippa indica (only the main glucosinolates and flavonoids are labeled).
The main glucosinolates of this plant consisted of methylsulfonyl straight alkyl chain compounds (CH3S(O)2(CH2)n) (Figure 1). The most concentrated glucosinolate (GS-1) had TIC and UV peaks with retention times (tR) at 18.88 and 18.83 min (Figure 2), UV λmax at 225 nm, and a [M – H]− of 522.1134 Da for C17H32O11NS3 (calcd 522.1129 Da, with the error of −1.72 ppm) for 9-(methylsulfonyl)nonylglucosinolate (GS-1). The assignment of this compound as 9-(methylsulfonyl)nonyl glucosinolate (GS-1) was based on the fact that the hydrolyzed products, desulfoglucosinolate, isothiocynate, and nitrile (rofifone), had been previously isolated from this plant.9,27,28 Furthermore, the authors in China isolated the related nitrile from the same plant material and confirmed the structure by nuclear magnetic resonance.
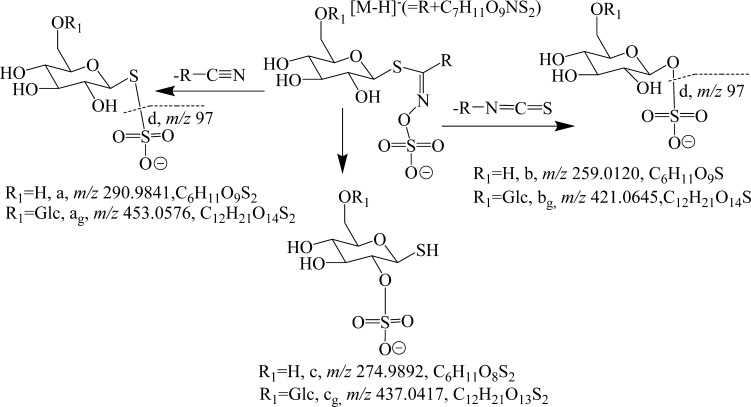
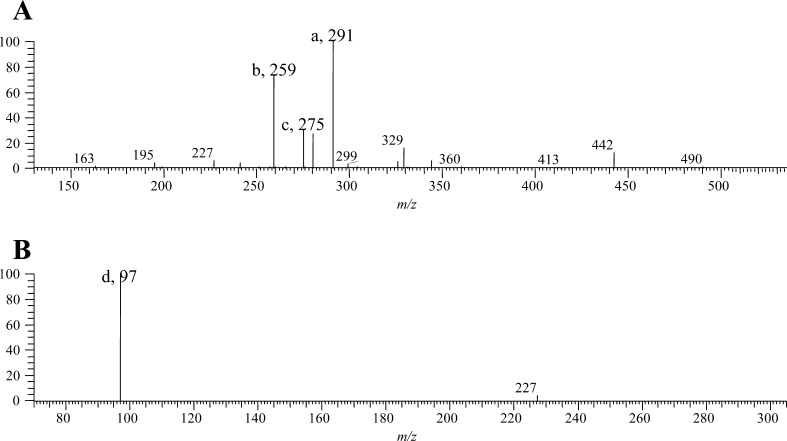
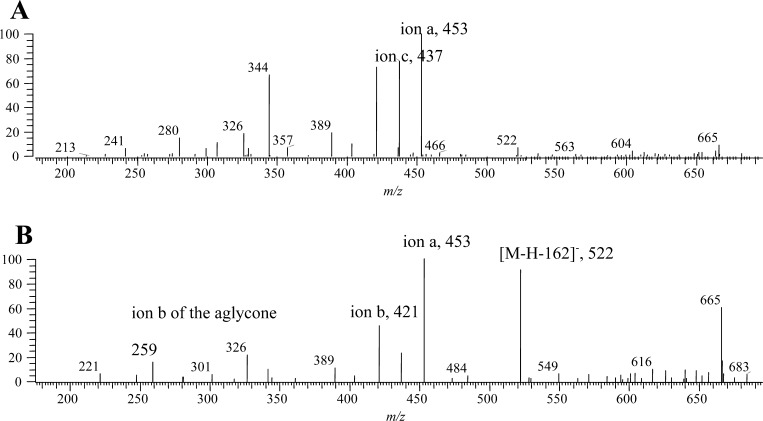
GS-1 had major MS2 fragments at m/z 291 (100%, [C6H11O9S2]−), 275 (28%, [C6H11O8S2]−), and 259 (73%, [C6H11O9S]−), ions a, c, and b in Figures 3 and 4A. MS3 had a major fragment at m/z 97 (100%, [HSO4]−), ion d in Figure 4B. The compositions for ions a, c, and b were confirmed by the experimentally determined masses of 290.9842 (error −2.7 ppm), 274.9890 (error −3.9 ppm), and 259.0117 (error −4.8 ppm, Figure 3), respectively, from the additional data-dependent scan with activation in HCD.
Figure 3.
Molecular and typical fragments for the glucosinolates in Rorippa indica.
Figure 4.
MS2 (A) and MS3 (B) spectra of 9-(methylsulfonyl)nonylglucosinolate (GS-1).
Figure 3 illustrates the fragmentation pathway leading to the four product ions through the loss of RCN and RNCS groups. Some glucosinolates, such as 4-methoxyglucobrassin, glucobrassicin, 3′-hydroxyglucoisatisin, glucoisatinsin, and sinalbin, were reported to present an MS2 fragment (b, 291 Da) with a relative intensity of 6–61%.13,14,22 These diagnostic ions fully supported the identification of this compound as a glucosinolate.
Two minor peaks (GS-2 and GS-3, tR = 10.39 and 21.00 min) had the same high-resolution deprotonated molecular ion masses as GS-1 and similar MS2 and MS3 fragments. Thus, they were determined to be isomers of GS-1. Most likely, the R groups are isomeric.
Three glucosinolates, GS4, GS-5, and GS-6, are analogues of GS-1 and identified as 7-methylsulfonylheptyl- (tR = 6.67 min, [M – H]− = 494.0823 Da), 8-(methylsulfonyl)octyl- (tR = 12.24 min, [M – H]− = 508.0978 Da), and 10-methylsulfonyldecylglucosinolates (tR = 27.25 min, [M – H]− = 536.1301 Da), respectively. Their mass differences arise from the numbers of CH2 units. Their retention times correlate with increasing mass, suggesting that the R groups are straight chains and the increasing number results in increased retention times as the polarity decreases. These data also confirmed the identification of these compounds as glucosinolates.
Four other glucosinolates (GS-7–GS-10) containing methylsulfinyl (CH3SO(CH2)n) were detected. They were putatively identified as 8-(methylsulfinyl)octyl- (tR = 11.39 time, [M – H]− = 492.1031 Da), 9-(methylsulfinyl)nonyl- (tR = 17.66 and 18.71 min, [M – H]− close to 506.1189 Da, two isomers, GS-8 and GS-9), and 10-(methylsulfinyl)decyl glucosinolates (tR = 25.28 min, [M – H]− = 520.1347 Da), respectively. All of them showed the same diagnostic fragments, confirming them as glucosinolates, and their elution time increased with increasing numbers of CH2 units.
GS-11 (tR = 11.85 min) contained an indole function and was putatively identified as 4-methoxyindol-3-ylmethylglucosinolate on the basis of its high-resolution mass value of 477.0635 Da. This compound showed the same fragmentation behavior observed above. Eight of the above glucosinolates (GS-1, GS-4, GS-5, GS-6, GS-7, GS-8, GS-10, and GS-11) were previously reported, and GS-7, GS-8, and GS-11 were found in Rorippa plants.4 Furthermore, the desulfoglucosinolates and/or isothiocyanates of the above glucosinolates (except GS-4) were previously isolated from the roots of this plant.9 However, compounds GS-2, GS-3, and GS-9 were not known glucosinolates.4−8
The remaining 21 peaks were putatively identified as glucosinolates and will be discussed in detail. GS-12 (tR = 33.33 min, [M – H]− = 488.1622 Da) had a deprotonated molecular ion of C18H34O10NS2 (error 0.49 ppm) and showed the classic glucosinolate MS2 fragments at m/z 259 (100%), 275 (44%), and 291 (33%). After subtraction of ion b (m/z 259, C6H11O9S) or ion a (m/z 291, C6H11O9S2) from the deprotonated molecule (Figure 3), the masses for R-NCS or R-CN were obtained. Each showed a formula for R = HO–(CH2)11, that is, C11H23O, the same as that obtained directly from R = C18H34O10NS2 – C7H11O9NS2 (Figure 3). In this way, the structures for the glucosinolates can be figured out easily. Thus, this compound was putatively identified as hydroxyundecyl glucosinolate. This glucosinolate was not among the predicted 180 new glucosinolates in the electronic database of Clarke because the number of CH2 groups (n) is 11 and 10 was the largest considered in the prediction set.8
Two groups of the isomeric alkyl glucosinolates were observed. The first group (GS-13–GS-16) contained four isomers (tR = 5.41, 6.47, 7.74, and 10.55 min, [M – H]− = 538.1081 Da). They showed product ions of m/z 291, 275, 259, and 97 and were identified as 9-(methylsulfonyl)hydroxynonylglucosinolates. They had one more hydroxyl group than that of related 9-(methylsulfonyl)nonylglucosinolate (GS-1) and its isomers (GS-2 and GS-3). The second group (GS-17 and GS-18) consisted of two isomers (tR = 10.97 and 13.34 min, [M − H]− = 552.1236 Da). They showed the same diagnostic GS fragments and were putatively identified as 10-methylsulfonylhydroxydecylglucosinolates and to be the hydroxyl derivatives of 10-methylsulfonyldecylglucosinolate (GS-6) and its isomers. The R groups for the isomers can be determined in the same manner as described previously for GS-12. Among them, GS-13 and GS-17 (with suggested straight chains for the R group) were among the predicted 180 new glucosinolates in the electronic database of Clarke with code numbers 245 and 246, respectively.8
Four minor peaks (GS-19–GS-22) had different retention times (tR = 17.68, 16.08, 16.84, and 20.92 min) but a similar mass ([M – H]− = 684.1658–684.1663 Da) corresponding to a formula of C23H42O16NS3 (errors less than −2.0 ppm). GS-19 had major MS2 fragments at m/z 522 (77%, [M – H] – 162, loss of glucosyl), 453 (100%, 291 + 162, ion ag, the “g” means the fragment contained a glucosyl, C6H10O5, Figure 3), 437 (23%, 275 + 162, ion cg), 421 (94%, 259 + 162, ion bg, Figure 3), and 259 (14%, ion b) (Figures 4 and 5 and Table 1). The detection of ions a, b, and c plus a glucosyl is reasonable, and [M – H]− = 421.0670 (error of 3.0 ppm) for ion b confirmed its composition as C12H21O14S. Thus, the data suggest that these compounds are the glucosides of 9-(methylsulfonyl)nonyl glucosinolate (the glucoside of GS-1).
Figure 5.
MS2 spectra of the glucosides (A, GS-19; and B, GS-22) of 9-(methylsulfonyl)nonylglucosinolate.
Table 1. Compound Code, Putative Identification, Retention Time, High-Resolution Mass of the Deprotonated Molecule, Formula, Error between Calculated and Measured Values, and MSn Data of the Glucosinolates in Rorippa indica.
| code | glucosinolate putative identificationa | tR(min) | [M – H]− (Da) | formula | error (ppm) | MS2 fragments (%) | MS3 fragments (%) | MS4 fragments (%) |
|---|---|---|---|---|---|---|---|---|
| GS-11 | 4-methoxyindol-3-ylmethylglucosinolate | 11.85 | 477.0635 | C17H21Ol0)N2S2 | –1.697 | 459 (61), 291 (39), 275 (61), 259 (100), 235 (28) | ||
| GS-12 | hydroxyundecylglucosinolate | 33.33 | 488.1624 | C18H34O10NS2 | –1.149 | 471 (90), 470 (60), 444 (50), 291 (40), 275 (40), 259 (100), 246 (30), 201 (30), 145 (30) | 470 (89), 291 (33), 275 (44), 259 (100) | |
| GS-7 | 8-(methylsulfinyl)octylglucosinolate (glucohirsutin) | 11.39 | 492.1031 | C16H30O10NS3 | –1.283 | 428 (100, −64) | 275 (36), 259 (100) | |
| GS-4 | 7-(methylsulfonyl)heptylglucosinolate | 6.67 | 494.0823 | C15H28O11NS3 | –1.409 | 476 (21), 291 (100), 275 (32), 259 (79), 252 (21) | ||
| GS-8 | 9-(methylsulfinyl)nonylglucosinolate (glucoarabin) | 17.66 | 506.1188 | C17H32O10NS3 | –0.951 | 442 (100, −64) | 275 (46), 259 (100) | 241 (12), 139 (100), 97 (44), 89 (8) |
| GS-9 | isomer of GS-8 | 18.71 | 506.1189 | C17H32O10NS3 | –0.951 | 442 (100) | ||
| GS-5 | 8-(methylsulfonyl)octylglucosinolate | 12.24 | 508.0978 | C16H30O11NS3 | –1.665 | 291 (100), 275 (21), 259 (45), | 97 (100) | |
| GS-10 | 10-(methylsulfinyl)decylglucosinolate | 25.28 | 520.1347 | C18H34O10NS3 | –0.637 | 456 (100) | 376 (16), 291 (18), 278 (16), 275 (47), 26 3(20), 259 (100) | 241 (12), 223 (10), 139 (100), 97 (38) |
| GS-1 | 9-(methylsulfonyl)nonylglucosinolate | 18.88 | 522.1134 | C17H32O11NS3 | –1.716 | 442 (12), 329 (16), 291 (100), 280 (27), 275 (30), 259 (73), 227 (6) | 97 (100) | |
| GS-2 | isomer of GS-1 | 21.00 | 522.1136 | C17H32O11NS3 | –1.333 | 522 (33), 504 (100), 478 (33), 476 (56), 450 (33), 444 (33), 291 (44), 259 (56) | ||
| GS-3 | isomer of GS-1 | 10.39 | 522.1138 | C17H32O11NS3 | –0.950 | 458 (100) | 378 (39), 332 (35), 280 (26), 275 (30), 259(100), 235 (30) | |
| GS-6 | 10-(methylsulfonyl)decylglucosinolate | 27.25 | 536.1301 | C18H34O11NS3 | 1.125 | 456 (11), 343 (14), 340 (6), 294 (20), 291 (100), 275 (26), 259 (58) | 97 (100) | |
| GS-13 | 9-(methylsulfonyl)hydroxynonylglucosinolate | 5.41 | 538.1082 | C17H32O12NS3 | –1.878 | 520 (53), 494 (24), 458 (24), 291 (100), 275 (41), 259 (59) | ||
| GS-14 | isomer of GS-12 | 6.47 | 538.1082 | C17H32O12NS3 | –2.594 | 520 (45), 494 (36), 450 (27), 448 (27), 362 (27), 296 (45), 291(100), 259 (55) | ||
| GS-15 | isomer of GS-12 | 7.74 | 538.1060 | C17H32O12NS3 | 0.298 | 296 (16), 291 (100), 275 (24), 259 (44) | ||
| GS-16 | isomer of GS-12 | 10.55 | 538.1080 | C17H32O12NS3 | –2.250 | 495 (26), 360 (21), 296 (26), 291 (100), 259 (21) | ||
| GS-17 | 10-(methylsulfonyl)hydroxydecylglucosinolate | 10.97 | 552.1238 | C18H34O12NS3 | –2.761 | 508 (17), 310 (11), 291 (100), 275 (20), 259 (37) | ||
| GS-18 | isomer of GS-16 | 13.34 | 552.1240 | C18H34O12NS3 | –1.559 | 534 (38), 340 (38), 310 (38), 291 (100) | ||
| GS-30 | glucoside of methylthioethylenylpentylglucosinolate | 28.07 | 622.1300 | C21H36O14NS3 | –0.546 | 326 (100, −296), 299 (22) | ||
| GS-31 | glucoside of 7-methylthio-3-oxoheptylglucosinolate | 22.16 | 638.1245 | C21H36O15NS3 | –1.182 | 620 (7), 522 (20), 326 (100, −312), 299 (22), 280 (33) | 494 (14), 442 (11), 423 (7), 407 (25), 391 (100), 264 (7) | |
| GS-32 | glucoside of 8-methylthiooctylglucosinolate | 21.15 | 638.1608 | C22H40O14NS3 | –1.316 | 574 (100, −64) | 423 (11), 407 (37), 391 (100), 191(16) | |
| GS-33 | glucoside of 7-(methylsulfinyl)heptylglucosinolate | 16.51 | 640.1403 | C21H38O15NS3 | –0.945 | 623 (62), 550 (62), 391 (100), 390 (38) | ||
| GS-23 | glucoside of 9-(methylsulfinyl)nonylglucosinolate | 22.55 | 654.1554 | C22H40O15NS3 | –1.765 | 423 (88), 407 (42), 391 (100), 373 (12), 359 (21) | ||
| GS-27 | glucosides of 9-(methylsulfinyl)nonylglucosinolate | 18.58 | 668.1708 | C23H42O15NS3 | –2.102 | 604 (100) | 453 (25), 437 (100), 421 (100), 332 (38), 264 (100) | |
| GS-28 | isomer of GS-27 | 16.70 | 668.1712 | C23H42O15NS3 | –1.504 | 604 (100) | 528 (17), 453 (28), 437 (56), 421 (100), 403 (22) | |
| GS-29 | glucoside of (R)-10-(methylsulfinyl)decylglucosinolate | 22.79 | 682.1868 | C24H44O15NS3 | –1.546 | 618 (100) | ||
| GS-19 | glucosides of 9-(methylsulfonyl)nonylglucosinolate | 17.68 | 684.1663 | C23H42O16NS3 | –1.198 | 666 (59), 522 (91), 453 (100), 437 (23), 421 (45), 326 (23), 259 (14) | ||
| GS-20 | isomer of GS-19 | 16.08 | 684.1658 | C23H42O16NS3 | –1.928 | 453 (81), 437 (29), 421 (100) | ||
| GS-21 | isomer of GS-19 | 16.84 | 684.1630 | C23H42O16NS3 | 2.563 | 643 (21), 453 (100, −231), 437 (42), 421 (96), 389 (42) | ||
| GS-22 | isomer of GS-19 | 20.92 | 684.1658 | C23H42O16NS3 | –1.928 | 453 (100), 437 (83), 421 (72), 389 (18), 344 (66), 326 (18), 307 (11), 280 (15) | ||
| GS-24 | glucosides of 10-(methylsulfonyl)decylglucosinolate | 23.37 | 698.1812 | C24H44O16NS3 | –2.248 | 681 (31), 680 (23), 639 (23), 453 (100), 437 (31), 421 (23) | ||
| GS-25 | isomer of GS-31 | 24.29 | 698.1821 | C24H44O16NS3 | –0.959 | 680 (10), 662 (12), 453 (95), 437 (25), 421 (100), 389 (8), 343 (10) | ||
| GS-26 | isomer of GS-31 | 28.18 | 698.1815 | C24H44O16NS3 | –1.818 | 680 (100), 652 (45), 626 (27), 537 (27), 528 (27), 453 (64), 437 (36), 421 (64), 358 (64) |
GS, glucosinolate.
GS-20, GS-21, and GS-22 also showed the typical glucosinolate glucoside fragments at m/z 453, 437, and 421 (Figures 3 and 5 and Table 1). On the basis of both of the high-resolution mass values and the presence of the gluocosinolate glucoside characteristic fragments, they were identified as the glucosides of 9-(methylsulfonyl)nonylglucosinolates (the glucosides of GS-1–GS-3). Because there was no hydroxyl function on the R group, this glucosyl was most likely connected to the thioglucoside and most likely at the 6-position as that for a monoacylated glucosinolate.6,7
Similarly, four other compounds (GS-23–GS-26) were recognized as the glucosides of glucosinolates on the basis of the fact that they presented the same diagnostic fragments at m/z 453, 437, and 421. GS-23 (tR = 22.55 min, [M – H]− = 654.1554 Da, C22H40O15NS3, error less than −2 ppm) was putatively identified as a glucoside of 9-(methylsulfinyl)nonylglucosinolate (the glucoside of GS-8). GS-24–GS-26 (tR = 23.37, 24.29, and 28.18 min, [M – H]− = 698.1915 Da, C24H44O16NS3, error −1.8 ppm) were putatively identified as the glucosides of 10-methylsulfonyldecylglucosinolates (the glucosides of GS-6 and its isomers).
One pair of isomers, GS-27 and GS-28 (tR = 16.43 and 18.58 min, [M – H]− = 668.1712 Da, C23H42O15NS3, error −1.5 ppm) were putatively identified as glucosides of 9-(methylsulfinyl)nonylglucosinolate (the glucosides of GS-5 or its isomer). GS-29 (tR = 22.79 min, [M – H]− = 682.1868 Da, C23H44O15NS3, error −1.5 ppm) was thought to be the glucoside of 10-(methylsulfinyl)decylglucosinolate (the glucosides of GS-10 or its isomer). The base MS2 fragments (m/z 604 for GS-27 and GS-28 and m/z 618 for GS-29) showed a loss of 64 Da (loss of CH3 – SO – H), indicating that these glucosinolates contained a methylsulfinyl (CH3–SO–) group in the side chain.17,18 Both GS-27and GS-28 had MS3 spectra containing the diagnostic gucosinolate glucoside fragments at m/z 453 (25 and 28%, respectively), 437 (100 and 56%), and 421 (both 100%) to confirm their identification. Similar fragments were not observed for GS-29. However, the MS2 signal was very weak.
Four other compounds (GS-30–GS-33) also appeared to be analogues of the glucosides of glucosinolates on the basis of their masses and formulas. GS-30 (tR = 28.07 min, [M – H]− = 622.1290 Da, C21H36O14NS3, error −0.5 ppm) might be the glucoside of methylthioethenylpentenyl (CH3—S—C=C(CH2)5) or an isomer of methylthioheptenyl. The aglycone with a methylthioethenylpentenyl group is among the 180 predicted new glucosinolates (no. 204) in the electronic database of Clarke.8
GS-31 and GS-32 (tR = 22.16 and 21.15 min) have the same nominal masses, but have different high-accuracy masses. GS-31 ([M – H]− = 638.1245 Da, C21H36O15NS3, error −1.2 ppm) was putatively identified as the glucoside of 7-methylthio-3-oxoheptylglucosinolate or 7-methylsulfinylheptenylglucosinolate. GS-32 ([M – H]− = 638.1608 Da, C22H40O14NS3, error −1.3 ppm) might be the glucoside of 8-methylthiooctylglucosinolate.
GS-33 (tR = 16.51 min, [M – H]− = 640.1403 Da, C21H38O15NS3, error −0.9 ppm) might be the glucoside of 7-(methylsulfinyl)heptyl glucosinolate. Instead of showing the typical glucosinolate glucoside fragment described above, three of them (GS-31–GS-33) had base MS2 or MS3 fragments at m/z 391 like GS-23. Consequently, the lack of definitive MS2 fragments made it impossible to develop a molecular formula for this ion. The putative formula offered in Table 1 was based primarily on the close match of the experimentally measured high-resolution masses with calculated values. The aglycones of these three glucosides were known glucosinolates, but not detected in this plant.
The 14 glucosinolate glucosides mentioned above have not been previously reported in any other plants. They represent a new family of glucosinolates with a glucosyl connected to the thioglucosyl group. All of the observed MSn fragments could be related to the structural elements of the glucosinolate skeleton. For examples, ions a, b, c, and d and thioglucosyl (m/z 195), as well as fragments obtained from further losses of oxygen, sulfur, or parts of the glucosyl were observed. However, not enough fragments were obtained to determine the structure of the R groups.8,11−25 Of course, the R groups for the glucosinolates were not very large (containing fewer than 12 carbon atoms for the aliphatic GSs), but they can be arranged in several different ways to form the isomers. For example, the aliphatic structure can be linear or branched, the oxygen atom can be found in the oxo (keto) or hydroxy form, and the alkenyl double bond can be located at different positions.3−8 As a result, nuclear magnetic resonance (NMR) analysis is still the most accurate way to identify GSs structurally.6,7
The major glucosinolates of R. montana had similar side chains and were well separated, allowing for the measurement of their peak areas at 225 nm (Figure 3). Their concentrations were calculated relative to glucocheirolin using MRRF values of 1.0, on the basis of previous reports that the side chains were similar and did not alter the absorption coefficient of the molecules.8,14,19,20 The relative peak area ratios for GS-1, GS-8, GS-6, GS-5, and GS-10 were 100.0, 15.5, 9.6, 5.1, and 4.6, respectively. The corresponding concentrations for GS-1, GS-8, GS-6, GS-5, and GS-10 were 0.51, 0.079, 0.049, 0.026, and 0.024% on a dry weight basis, and their total contents was 0.69% or 690 mg/100 g of dried plant materials. GS-1 constituted approximately 71% of the total glucosinolate content.
Identification of Phenolic and Other Common Compounds
Table 2 presents the retention times (recorded on the TIC chromatograms), HR masses, and formulas for deprotonated molecules [M – H]−, diagnostic MS2, MS3, MS4, and MS5 product ions, and putative identities of 44 flavonol glycoside, 18 phenolic, and other common compounds. The listing is in order of molecular weight. The typical TIC and PDA (at 354 nm) chromatograms are shown in Figure 2. The strategy for identifying phenolic compounds in food samples has been described previously.31,33 In this study, only the glycosides of flavonols were observed, and most are reported for the first time.
Table 2. Compound Name, Retention Time, High-Resolution Mass of the Deprotonated Molecule, Formula, Error between Calculated and Measured Values, and MSn Data of the Flavonol Glycosides and Other Compounds in Rorippa indica.
| compound putative identification (code)a | tR(min) | [M – H]− (Da) | formula | error (ppm) | MS2 fragments (m/z) (%) | MS3 fragments (m/z) (%) | MS4 fragments (m/z) (%) | MS5 fragments (m/z) (%) |
|---|---|---|---|---|---|---|---|---|
| malic acidb | 2.02 | 133.0137 | C4H5O5 | –4.109 | 115 (100) | 71 (100) | ||
| vanillic acidb | 5.13 | 167.0349 | C8H704 | –0.491 | 152 (100), 123 (65), 108 (16) | |||
| citric acidb | 2.25 | 191.0193 | C6H707 | –3.276 | 173 (26), 111 (100) | 67 (100) | ||
| trans-ferulic acidb | 23.48 | 193.0503 | Cl0H9O4 | –1.720 | 178 (26), 149 (56), 134 (100) | 106 (100) | ||
| cis-feruulic acidb | 25.08 | 193.0503 | C10H9O4 | –1.720 | 178 (26), 149 (56), 134 (100) | 106 (100) | ||
| trans-sinapic acidb | 20.81 | 223.0241 | C10H7O6 | –3.189 | 195 (8), 179 (100) | 135 (100), 109 (66) | ||
| cis -sinapic cidb | 22.00 | 223.0241 | C10H7O6 | –3.189 | 195 (8), 179 (100) | 135 (100), 109 (66) | ||
| dihydroxybenzoylpentoseb | 6.90 | 285.0611 | C12H13O8 | –1.721 | 165 (6), 153 (100), 152 (33), 109 (7) | 109 (100) | ||
| dihydroxybenzoylpentoseb | 8.10 | 285.0611 | C12H13O8 | –1.721 | 153 (100) | 109 (100) | ||
| dihydroxybenzoylpentoseb | 9.23 | 285.0611 | C12H13O8 | –1.721 | 153 (100) | 109 (100) | ||
| p -coumaroylmalic acidb | 20.59 | 279.0505 | C13H11O7 | –1.885 | 163 (100), 133 (70) | |||
| hydroxybenzoylhexoseb | 6.80 | 299.0767 | C13H15O8 | –1.808 | 137 (100) | 93 (100) | ||
| hydroxybenzoylhexoseb | 8.44 | 299.0767 | C13H15O8 | –1.808 | 137 (100) | |||
| dihydroxybenzoylhexoseb | 4.27 | 315.0717 | C13H15O9 | –1.445 | 225 (12), 165 (14), 163 (8), 153 (100), 152 (31) | |||
| dihydroxybenzoylhexoseb | 5.62 | 315.0716 | C13H15O9 | –1.762 | 153 (100) | |||
| dihydroxybenzoylhexoseb | 6.33 | 315.0717 | C13H15O9 | –1.445 | 169 (100), 151 (31) | |||
| galloylquinic acidsb | 4.42 | 343.0665 | C14H15O10 | –1.661 | 299 (28), 153 (100) | |||
| km rhamnoside | 47.97 | 431.0982 | C21H19O10 | –0.394 | 285 (100), 284 (27) | 257 (45), 241 (40), 213 (14), 151 (100) | ||
| km rhamnoside | 46.87 | 431.0984 | C21H19O10 | 0.070 | 285 (100), 284 (30) | 257 (39), 241 (35), 213 (13), 151 (100) | 107 (100), 83 (7) | 65 (100), 63 (41) |
| sinapoylhexose + formic acidb | 16.01 | 431.1919 | C20H31O11 | –0.859 | 385 (100) | 223 (80), 205 (77), 161 (28), 153 (100) | ||
| km hexoside | 32.80 | 447.0927 | C21H19O11 | –1.307 | 327 (22), 301 (49), 300 (11), 285 (100), 284 (93) | |||
| km 3-O-hexoside | 30.58 | 447.0930 | C21H19O11 | –0.636 | 327 (16), 285 (57), 284 (100), 255 (11) | 255 (100), 227 (10) | 255 (24), 227 (88), 211 (100) | |
| km 3-O-hexoside (F-5)b | 32.17 | 447.0931 | C21H19O11 | –0.413 | 327 (20), 285 (56), 284 (100), 255 (15) | 255 (100), 227 (13) | 255 (19), 227 (100), 211 (61) | 227 (23), 199 (56), 183 (100) |
| lariciresinol-4′-glucosideb | 30.91 | 521.2023 | C26H33O11 | –1.026 | 503 (11), 449 (9), 359 (18), 341 (100), 179 (49) | |||
| km 3-O-dirhamnosideb | 33.59 | 577.1555 | C27H29O14 | –1.349 | 431 (100), 285 (25) | 285 (100), 284 (14) | 257 (77), 243 (100), 213 (93) | |
| km rhamnosylhexoside | 17.35 | 593.1505 | C27H29O15 | –1.169 | 503 (43), 473 (100), 383 (25), 353 (30) | 383 (22), 353 (100) | 325 (100), 297 (50) | 297 (100) |
| km 3-O-hexoside-7-O-rhamnoside | 21.04 | 593.1503 | C27H29O15 | –1.506 | 447 (100), 431 (72) | 285 (100), 284 (41) | 257 (50), 241 (33), 151 (100) | 107 (100) |
| km 3-O-rhamnosylhexoside (F-l)b | 28.22 | 593.1491 | C27H29O15 | –3.529 | 447 (100) | 327 (16), 285 (41), 284 (100), 255 (12) | 255 (100) | 227 (100), 211 (63) |
| km 3-O-rhamnosylhexoside (F-2)b | 26.52 | 593.1500 | C27H29O15 | –2.012 | 447 (100), 431 (41), 285 (16) | 327 (22), 285 (20)284 (100), 255 (15) | 255 (100), 227 (11) | 255 (13), 227 (100), 211 (57) |
| km 3-O-rhamnosylhexoside | 30.80 | 593.1504 | C27H29O15 | –1.337 | 473 (6), 447 (100), 43 1(37), 285 (12) | |||
| km 3-O-rhamnosylhexoside | 31.13 | 593.1504 | C27H29O15 | –1.337 | 473 (8), 447 (100), 431 (35), 285 (18) | |||
| km 3-O-hexoside-7-O-hexoside | 16.92 | 609.1469 | C27H29O16 | 1.300 | 489 (14), 447 (100) | 327 (17), 285 (42), 284 (100), 255 (14) | ||
| km 3-O-hexoside-7-O-hexoside | 18.79 | 609.1471 | C27H29O16 | 1.629 | 447 (100) | 327 (20), 285 (49), 284 (100), 255 (17) | 255 (100) | 227 (100), 211 (69) |
| qn 3-O-hexosylrhamnoside | 22.42 | 609.1470 | C27H29O16 | 1.465 | 463 (81), 447 (100), 446 (33), 301 (32) | |||
| qn 3-O-rhamnosylhexoside (rutin)b | 23.42 | 609.1472 | C27H29O16 | 1.793 | 463 (100), 447 (23), 301 (12) | 301 (100), 300 (26) | ||
| qn 3-O-hexosylrhamnoside (F-3)b | 24.54 | 609.1470 | C27H29016 | 1.465 | 463 (67), 447 (100), 446 (34), 301 (31) | 301 (100), 299 (10) | 255 (22), 179 (71), 151 (100) | 107 (100) |
| qn 3-O-hexosylrhamnosideb | 25.82 | 609.1470 | C27H29016 | 1.465 | 463 (64), 447 (100), 446 (25), 301 (31), 285 (11) | 301 (100) | 255 (27), 179 (46), 151 (100) | |
| qn 3-O-hexosylrhamnosideb | 26.17 | 609.1470 | C27H29016 | –1.467 | 463 (56), 447 (100), 301 (29) | 301 (100) | ||
| is 3-O-rhamnosylhexosideb | 30.31 | 623.1611 | C28H29017 | –1.056 | 477 (100), 461 (41), 315 (9) | 357 (19), 314 (100), 285 (8), 271 (6) | 285 (100), 271 (75), 243 (27) | 270 (100) |
| qn 3-O-hexoside-7-O-hexoside | 15.60 | 625.1398 | C29H31O16 | –1.956 | 463 (100), 301 (22) | 301 (100), 300 (32) | ||
| km 3-O-acetyl-hexosylrhamnoside | 31.43 | 635.1605 | C29H31O16 | –1.980 | 489 (57), 431 (100), 430 (12), 285 (73) | |||
| km 3-O-rharnnosylpentasylrhamnoside (F-6) | 34.46 | 709.1978 | C32H37O18 | –1.040 | 563 (100) | 413 (35), 285 (39), 284 (100), 255 (17) | ||
| km 3-O-dirhamnosylpentoside | 33.19 | 709.1972 | C32H37O18 | –1.886 | 665 (14), 547 (8), 489 (7), 447 (100), 285 (20) | |||
| km 3-O-(p-coumaroylhexosyl)rhamnoside | 45.84 | 739.1877 | C36H35O17 | –0.369 | 593 (100), 285 (7) | 447 (6), 285 (100) | 257 (68), 241 (37), 151 (100) | 107 (100), 83 (10) |
| km 3-O-rhamnosylhexosylhexoside | 24.73 | 755.2031 | C33H39O20 | –1.479 | 609 (100) | 285 (100) | 267 (31), 257 (100), 229 (44), 151 (30) | |
| km 3-O-rhamnosylhexosylhexoside | 26.21 | 755.2030 | C33H39O20 | –1.346 | 609 (100) | 285 (100) | 267 (42), 257 (100), 239 (29), 229 (56) | |
| km 3-O-rhamnosylhexosylhexoside | 27.94 | 755.2023 | C33H39O20 | –2.273 | 609 (100), 431 (17), 285 (7) | 285 (100) | 267 (31), 257 (100), 229 (30), 151 (71) | |
| km 3-O-p-coumaroylhexosylhexosideb | 37.65 | 755.1826 | C36H35O18 | –0.380 | 609 (100) | 447 (16), 323 (49), 285 (100), 221 (6) | 257 (100), 241 (42), 229 (45), 151 (74) | |
| km 3-O-p-coumaroylhexosylhexosideb | 39.03 | 755.1814 | C36H35O18 | –1.969 | 609 (100) | 447 (17), 323 (51), 285 (100) | ||
| km 3-O-feruloylhexosylrhamnosideb | 30.24 | 769.1975 | C37H37O18 | –1.349 | 575 (100), 455 (12) | 455 (100) | 335 (100) | 307 (100), 306 (19), 241 (10) |
| km 3-O-feruoylrhamnosylhexoside (F-4) | 42.70 | 769.1974 | C37H37O18 | –1.479 | 623 (100) | 323 (100), 299 (66), 285 (68), 284 (15) | 263 (46), 221 (100), 179 (82), 177 (36) | |
| km 3-O-feruloylrhamnosylhexoside | 46\66 | 769.1972 | C37H37O18 | –1.739 | 653 (7), 623 (100, −146), 285 (7) | 447 (6), 299 (24), 285 (100) | 257 (77), 241 (43), 213 (23), 151 (100) | |
| km 3-O-caffeoyldihexoside | 31.67 | 771.1797 | C36H35O19 | 2.461 | 609 (47), 608 (6), 447 (100), 327 (6), 285 (25) | |||
| qn 3-O-rhamnosyldihexosideb | 22.95 | 771.1971 | C33H39O21 | –2.374 | 625 (100), 609 (68), 463 (40) | |||
| km 3-O -sinapoylhexoside-7-O -rhamnoside | 42.40 | 799.2093 | C38H39O19 | 0.248 | 653 (100) | 353 (100), 339 (22), 299 (50), 285 (39) | 251 (97), 209 (100), 207 (42), 191 (52) | |
| km 3-O -sinapoyIhexosicle-7-O -rhamnoside | 46.26 | 799.2080 | C38H39O19 | –1.379 | 653 (100), 431 (10), 285 (15) | |||
| qn 3-O-sinapoyldihexoside | 41.60 | 831.1974 | C38H39O21 | –1.842 | 787 (100), 641 (42), 625 (57), 607 (7), 479 (74) | |||
| km 3-O-hydroxybenzoylhexoside-7-O-dihexoside | 31.17 | 891.2185 | C40H43O23 | –1.751 | 729 (96), 567 (100) | |||
| qn3-O-hexoside-7-O-methoxybenzoyldihexoside | 33.57 | 921.2290 | C41H45O24 | –1.764 | 593 (25), 463 (100), 457 (37) | |||
| km 3-O -caffeoyldihexoside-7-O -rhamnoside | 32.85 | 917.2340 | C42H45O23 | –1.865 | 771 (100), 755 (29), 609 (31) | 609 (100) | 447 (26), 323 (50), 285 (100), 221 (7) | |
| km 3-O -feruloyldihexoside-7-O -rhamnoside | 34.95 | 931.2505 | C43H47O23 | –0.924 | 785 (100), 623 (10) | 623 (100), 447 (47), 285 (7) | ||
| km 3-O-feruoyldihexoside-7-O-rhamnoside | 38.95 | 931.2503 | C43H47O23 | –1.139 | 785 (100), 769 (38), 623 (54), 447 (37), 431 (6) | 623 (100, −162), 447 (72), 285 (13) | ||
| km 3-O-sinapoyldihexoside-7-O-rhamnoside | 34.46 | 961.2604 | C44H49O24 | –1.587 | 815 (100), 799 (12), 653 (18), 447 (10) | 653 (100), 447 (45), 285 (8) | ||
| qn 3-O-sinapoylhydroxyferuoyl-dihexoside-7-O-hexoside | 39.84 | 1185.2914 | C54H57O30 | –2.205 | 1039 (23), 1023 (100), 893 (12), 877 (18) | 877 (100), 861 (93), 861 (37), 731 (61) |
is, isorhamnetin; km, kaempferol; qn, quercetin.
Identified using in-house database.
Two tetrahydroxyflavone 3-O-rhamnosylhexosides (tR = 28.22 and 26.52 min, [M – H]− = 593.1491) provided the largest peaks (F-1 and F-2 in Figure 2A) in chromatograms of the R. indica extract. They had UV band I maximum absorbances at 265 and 346 nm and base MS2 and MS3 fragments of m/z 447 (by loss of 146) and m/z 284 (the radical anion species of kaempferol from the loss of 162 + 1 rather than the ion at m/z 285), suggesting that they might be kaempferol glycosides containing a rhamnosylhexose. This radical anion had base MS4 and MS5 fragments of m/z 255 (100%) and 221(100%), which suggested that this flavonol was most likely to be kaempferol. This identification was confirmed by the fact that kaempferol was the tetrahydroxyflavone (by direct comparison with kaempferol standards) in the acidic hydrolyzed extract (chromatogram not shown). The close retention times of the flavonol glycoside suggested that they might be kaempferol 3-O-rhamnosylglucosides with the rhamnosyl at the 6-position of hexosyl (glucosyl or galactosyl).33 Their high-resolution mass confirmed their putative identification.
Four other peaks with different retention times (tR = 17.35, 21.04, 30.80, and 32.13 min) had high-resolution masses close to 593.1500 Da, and all had similar fragments, suggesting they were also kaempferol rhamnosylhexosides. However, they were all minor components of the plant and did not provide sufficient UV data or MSn fragments to allow identification of a specific isomeric structure. Thus, their position of attachment to the flavone and the type of hexose were not completely identified.
Similarly, flavonoid F-3 (tR = 24.61 min, [M – H]− = 609.1470 Da) had a MS2 fragment at m/z 447 (loss of 162) and an MS3 fragment at m/z 301 (loss of 146) and was putatively identified as quercetin 3-O-hexosylrhamnoside. Its isomers (tR = 23.42 and 25.84 min) had MS2 fragments at m/z 463 (loss of 146) and MS3 fragments at m/z 301 (loss of 162), suggesting they were quercetin 3-rhamnosylhexosides. The isomer with tR = 23.42 min was further identified as rutin by direct comparison with an authentic standard. Another isomer (tR = 34.50 min) had a MS2 fragment at m/z 301, and its sugar connection could not be determined. The MS4 and MS5 fragments for m/z 301 were 255 (22–27%), 179 (41–71%), 151 (100%), and 107 (100) and suggested the aglycone was quercetin. This was confirmed by the fact that the pentahydroxyflavone in the acidic hydrolyzed extract was identified as quercetin.
One flavonoid (tR = 30.31 min, [M – H]− = 623.1611 Da) had a base MS2 fragment at m/z 477, a base MS3 fragment at m/z 314, and MS4 and MS5 ions at m/z 285 and 270 to suggest it was isorhamnetin 3-O-rhamnosylhexoside. Two rhamnosides and three hexosides of kaempferol were detected. At least one of the rhamnosides and hexosides might have the glycosyl at the 3-position of kaempferol. Another flavonoid (tR = 33.59 min, [M – H]− = 577.1555 Da, C27H29O14) had a base MS2 fragment at m/z 431 and a base MS3 fragment at m/z 285 to suggest it was kaempferol 3-O-rhamnosylrhamnoside.
Similarly, six flavonoids conjugated with three monosaccharides were putatively identified. The first two flavonoids (tR = 34.46 and 33.19 min, C32H37O18) showed one pentosyl more than that of kaempferol 3-O-rhamnosylrhamnoside. The first one (F-6) had a base MS2 fragment at m/z 563 (loss of 146 Da) and a base MS3 fragment at m/z 284 (loss of 278 Da, i.e., 146 + 132) to indicate it was kaempferol 3-O-rhamnosylpentosylrhamnoside. The second one (tR = 33.19) was very similar to F-6 but had a base MS2 fragment at m/z 447 (loss of 294 Da) for a dirhamnosylpentoside. Another three flavonoids (tR = 34.46, 33.19, 24.73, 26.21, and 27.94 min, C33H39O20) had base MS2 fragments at m/z 609 Da (loss of 146 Da), a base MS3 fragment at m/z 285 Da (loss of 324 Da), and a base MS4 fragment at m/z 151, suggesting them to be isomers of kaempferol 3-O-rhamnosyldihexosides. The last flavonoid (tR = 22.95 min [M – H]− = 771.1971 Da, C33H39O21) had a base MS2 fragment at m/z 625 (loss of 146 Da), indicating it might be quercetin 3-O-rhamnosyldihexoside.
Acylation of the flavonol glycosides was determined by mass difference. An increased mass close to 146.0366, 162.0315, 176.0471, or 206.0576 Da was diagnostic of the presence of a p-coumaroyl, caffeoyl, feruloyl, or sinapoyl group. For example, two kaempferol acylglyocosides (tR = 37.65 and 39.03 min, [M – H]− close to 755.1826 Da, C36H35O18) had a base MS2 fragment at m/z 609 Da (calculated as 609.1460, with error of −0.177 ppm for C27H29O16, from loss of 146.0366 Da, C9H6O2 for coumaroyl, instead of loss of 146.0576 Da, C6H10O4, rhamnosyl) and base MS3,4 fragments (m/z 447, 285, 257, 241, 229, 151 Da) identifying them as kaempferol 3-O-p-coumaroyldihexosides. Similarly, three acylated flavonoids (tR = 30.24, 42.70, and 46.66 min, [M – H]− close to 769.1975 Da, C37H37O18) had base MS2 fragments at m/z 575 (loss of around 194.0576 Da for C10H10O4, ferulic acid) suggesting they were kaempferol 3-O- feruloylrhamnosylhexosides. In the same way, four acylated glycosides were putatively identified as the 3-O-caffoeyldihexoside (tR = 31.67 min, [M – H]− = 771.1797 Da), the 3-O-sinapoyldihexoside (tR = 41.60 min, [M – H]− = 831.1974 Da), the 3-O-hexoside-7-O-methoxybenzoyldihexoside (tR = 33.57 min, [M – H]− = 921.2290 Da), and the 3-O-sinapoylhydroxyferuoyldihexoside-7-O-hexoside (tR = 39.84 min, [M – H]− = 1185.2914 Da) of quercetin, respectively. In the same manner, the remaining ones were identified as two 3-O-sinapoylhexoside-7-O-rhamnosides (tR = 42.40 and 46.26 min, [M – H]− close to 799.2093 Da), the 3-O-hydroxybenzoyldihexoside-7-O-hexoside (tR = 31.17 min, [M – H]− = 891.2185 Da), the 3- O-caffeoyldihexoside-7-O-rhamnoside (tR = 32.85 min, [M – H]− = 917.2340 Da), the 3-O-feruloyldihexoside-7-O-rhamnosides (tR = 34.95 and 38.95 min, [M – H]− = 931.2504 Da), two 3-O-sinapoyldihexoside-7-O-rhamnosides (tR = 34.46 min, [M – H]− = 961.2604 Da), and the 3-O-acetylhexosylrhamnoside (tR = 31.43 min, [M – H]− = 635.1605 Da) of kaempferol, respectively.
As mentioned in a previous publication, dried rutin (at 354 nm) was used as the quantification standard and the MRRF values of two main flavonoids were used to determine their concentrations.31 Concentrations of approximately 0.05 and 0.01% (or 50 and 10 mg/100 g of plant material on dry weight basis) were obtained for flavonoids F1 and F2, respectively. The total flavonoid content of this plant is <0.10% because the remaining flavonols were present at very low concentrations and UV peaks were not detectable for absorbance measurements.
In addition to the flavonoids, 1 lignan (lariciresinol 4-O-β-d-glucopyranoside) and 18 other common compounds, including the glucosides of hydroxybenzoic and hydroxycinnamic acids and their derivatives (e.g., bound to sugar, quinic acid, and malic acid) and organic acids, were identified by comparison of the chromatographic and MS data with those in our in-house flavonoid database. Most of the common compounds have previously been detected in other plants analyzed in this laboratory.
The results of this study showed that R. indica is a rich source of glucosinolates.1−5,8 These compounds were reasonably separated using the UHPLC conditions described here and identified by their high-accuracy mass measurement and multistage mass fragments. The UV absorbance of the major glucosinolates was used to compute their concentration. In summary, 33 glucosinolates, over 40 flavonoids, and more than 20 other phenolic and organic compounds were identified.
This research is supported by the Agriculture Research Service of the U.S. Department of Agriculture and an Interagency Agreement with the Office of Dietary Supplements of the National Institutes of Health.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Halkiel B. A.; Gershenzon J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [DOI] [PubMed] [Google Scholar]
- Johnson I. T. Glucosinolates: bioavailability and importance to health. Int. J. Vitam. Nutr. Res. 2002, 72, 26–31. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T.; Rumen V.; Kostov R. T. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [DOI] [PubMed] [Google Scholar]
- Fahey J. W.; Zalcmann A. T.; Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 6, 5–51. [DOI] [PubMed] [Google Scholar]
- Agerbirk N.; Olsen C. E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [DOI] [PubMed] [Google Scholar]
- Reichelt M.; Brown P. D.; Schneider B.; Oldham N. J.; Stauber E.; Tokuhisa J.; Kliebenstein D. J.; Mitchell-Olds T.; Gershenzon J. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 2002, 59, 663–671. [DOI] [PubMed] [Google Scholar]
- Agerbirk N.; Olsen C. E. Isoferuloyl derivatives of five seed glucosinolates in the crucifer genus Barbarea. Phytochemistry 2011, 72, 610–623. [DOI] [PubMed] [Google Scholar]
- Clarke D. B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar]
- Yamane A.; Fujikura J.; Ogawa H.; Mizutani J. Isothiocyanates as alleopathic compounds from Rorippa indica Hiern. (Cruciferae) roots. J. Chem. Ecol. 1992, 18, 1941–1954. [DOI] [PubMed] [Google Scholar]
- Kawabata J.; Fukushi Y.; Hayashi R.; Suzuki K.; Mishima Y.; Yamane A.; Mizutani J. 8-Methylsufinyloctyl isothicyanate as an allelochemical candidate from Rorippa sylvestris Besser. Agric. Biol. Chem. 1989, 53, 3361–3362. [Google Scholar]
- Mellon F. A.; Bennett R. N.; Holst B.; Williamson G. Intact glucosinolate analysis in plant extracts by programmed cone voltage electrospray LC/MS: performance and comparison with LC/MS/MS methods. Anal. Biochem. 2002, 306, 83–91. [DOI] [PubMed] [Google Scholar]
- Fabre N.; Poinsot V.; Debrauwer L.; Vigor C.; Tulliez J.; Fourasté I.; Moulis C. Characterisation of glucosinolates using electrospray ion trap and electrospray quadrupole time-of-flight mass spectrometry. Phytochem. Anal. 2007, 18, 306–319. [DOI] [PubMed] [Google Scholar]
- Rochfort S. J.; Trenerry V. C.; Imsic M.; Panozzo J.; Jones R. Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry 2008, 69, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Francisco M.; Moreno D. A.; Cartea M. E.; Ferreres F.; García-Viguera C.; Velasco P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr., A 2009, 1216, 6611–6619. [DOI] [PubMed] [Google Scholar]
- Cataldi T. R.; Lelario F.; Orlando D.; Bufo S. A. Collision-induced dissociation of the A + 2 isotope ion facilitates glucosinolates structure elucidation by electrospray Ionization-tandem mass spectrometry with a linear quadrupole ion trap. Anal. Chem. 2010, 82, 5686–5696. [DOI] [PubMed] [Google Scholar]
- Bianco G.; Lelario F.; Battista F. G.; Bufob S. A.; Cataldic T. R. I. Identification of glucosinolates in capers by LC-ESI-hybrid linear ion trap with Fourier transform ion cyclotron resonance mass spectrometry (LC-ESI-LTQ-FTICR MS) and infrared multiphoton dissociation. J. Mass Spectrom. 2012, 47, 1160–1169. [DOI] [PubMed] [Google Scholar]
- Glauser G.; Schweizer F.; Turlings T. C.; Reymond P. Rapid profiling of intact glucosinolates in Arabidopsis leaves by UHPLC-QTOFMS using a charged surface hybrid column. Phytochem. Anal. 2012, 23, 520–528. [DOI] [PubMed] [Google Scholar]
- Maldini M.; Baima S.; Morelli G.; Scaccini C.; Natella F. A liquid chromatography-mass spectrometry approach to study “glucosinoloma” in broccoli sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [DOI] [PubMed] [Google Scholar]
- Mohn T.; Cutting B.; Ernst B.; Hamburger M. Extraction and analysis of intact glucosinolates – a validated pressurized liquid extraction/liquid chromatography–mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. J. Chromatogr., A 2007, 1166, 142–151. [DOI] [PubMed] [Google Scholar]
- Kusznierewicz B.; Iori R.; Piekarska A.; Namieśnik J.; Bartoszek A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography-diode array-electrospray ionisation mass spectrometry analysis: method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr., A 2013, 1278, 108–115. [DOI] [PubMed] [Google Scholar]
- Bennett R. N.; Carvalho R.; Mellon F. A.; Eagles J.; Rosa E. A. Identification and quantification of glucosinolates in sprouts derived from seeds of wild Eruca sativa L. (salad rocket) and Diplotaxis tenuifolia L. (wild rocket) from diverse geographical locations. J. Agric. Food Chem. 2007, 55, 67–74. [DOI] [PubMed] [Google Scholar]
- Velasco P.; Francisco M.; Moreno D. A.; Ferreres F.; García-Viguera C.; Cartea M. E. Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem. Anal. 2011, 22, 144–152. [DOI] [PubMed] [Google Scholar]
- Ediage E. N.; Di Mavungu J. D.; Scippo M. L.; Schneider Y. J.; Larondelle Y.; Callebaut A.; Robbens J.; Van Peteghem C.; De Saeger S. Screening, identification and quantification of glucosinolates in black radish (Raphanus sativus L. niger) based dietary supplements using liquid chromatography coupled with a photodiode array and liquid chromatography-mass spectrometry. J. Chromatogr., A 2011, 1218, 4395–4405. [DOI] [PubMed] [Google Scholar]
- Argentieri M. P.; Accogli R.; Fanizzi F. P.; Avato P. Glucosinolates profile of “mugnolo”, a variety of Brassica oleracea L. native to southern Italy (Salento). Planta Med. 2011, 77, 287–292. [DOI] [PubMed] [Google Scholar]
- Hennig K.; Verkerk R.; Bonnema G.; Dekker M. Rapid estimation of glucosinolate thermal degradation rate constants in leaves of Chinese kale and broccoli (Brassica oleracea) in two seasons. J. Agric. Food Chem. 2012, 60, 7859–7865. [DOI] [PubMed] [Google Scholar]
- Márton M. R.; Krumbein A.; Platz S.; Schreiner M.; Rohn S.; Rehmers A.; Lavric V.; Mersch-Sundermann V.; Lamy E. Determination of bioactive, free isothiocyanates from a glucosinolate-containing phytotherapeutic agent: a pilot study with in vitro models and human intervention. Fitoterapia 2013, 85, 25–34. [DOI] [PubMed] [Google Scholar]
- The Editorial Commission of Chinese Materia Medica. Rorippa indica in Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; Vol. 3, pp 731–734. [Google Scholar]
- Lee K. C.; Cheuk M. W.; Chan W.; Lee A. W.; Zhao Z. Z.; Jiang Z. H.; Cai Z. Determination of glucosinolates in traditional Chinese herbs by high-performance liquid chromatography and electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2006, 486, 2225–2232. [DOI] [PubMed] [Google Scholar]
- Tan Z.-G.; Chen Y.; Ji G.-L. The study on the active components of Rorippa indica. Chinese Sci. 1974, No. 1, 15–20. [Google Scholar]
- Ananthi P.; Kumari B. D. R. Gas chromatography-mass spectrometry analysis of methanolic seed and root extracts of Rorippa indica L. Int. J. Chem. Technol. Res. 2013, 5, 299–306. [Google Scholar]
- Lin L.-Z.; Harnly J. M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-Z.; Harnly J. M.; Zhang R.-Z.; Fan X.-E.; Chen H.-J. Quantitation of the hydroxycinnamic acid derivatives and the glycosides of flavonols and flavones by UV absorbance after identification by LC-MS. J. Agric. Food Chem. 2012, 60, 544–553. [DOI] [PubMed] [Google Scholar]
- Lin L.-Z.; Sun J.; Chen P.; Harnly J. M. UHPLC-PDA-EIS/HRMS/MSn analysis of anthocyanins, flavonol glycosides and hydroxycinnamic acid derivatives in red mustard green (Brassica juncea Coss variety). J. Agric. Food Chem. 2011, 59, 12059–12072. [DOI] [PMC free article] [PubMed] [Google Scholar]