Abstract
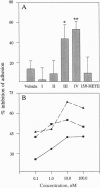
Aspirin [acetylsalicylic acid (ASA)], along with its analgesic-antipyretic uses, is now also being considered for cardiovascular protection and treatments in cancer and human immunodeficiency virus infection. Although many of ASA's pharmacological actions are related to its ability to inhibit prostaglandin and thromboxane biosynthesis, some of its beneficial therapeutic effects are not completely understood. Here, ASA triggered transcellular biosynthesis of a previously unrecognized class of eicosanoids during coincubations of human umbilical vein endothelial cells (HUVEC) and neutrophils [polymorphonuclear leukocytes (PMN)]. These eicosanoids were generated with ASA but not by indomethacin, salicylate, or dexamethasone. Formation was enhanced by cytokines (interleukin 1 beta) that induced the appearance of prostaglandin G/H synthase 2 (PGHS-2) but not 15-lipoxygenase, which initiates their biosynthesis from arachidonic acid in HUVEC. Costimulation of HUVEC/PMN by either thrombin plus the chemotactic peptide fMet-Leu-Phe or phorbol 12-myristate 13-acetate or ionophore A23187 leads to the production of these eicosanoids from endogenous sources. Four of these eicosanoids were also produced when PMN were exposed to 15R-HETE [(15R)-15-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid] and an agonist. Physical methods showed that the class consists of four tetraene-containing products from arachidonic acid that proved to be 15R-epimers of lipoxins. Two of these compounds (III and IV) were potent inhibitors of leukotriene B4-mediated PMN adhesion to HUVEC, with compound IV [(5S,6R,15R)-5,6,15-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoi c acid; 15-epilipoxin A4] active in the nanomolar range. These results demonstrate that ASA evokes a unique class of eicosanoids formed by acetylated PGHS-2 and 5-lipoxygenase interactions, which may contribute to the therapeutic impact of this drug. Moreover, they provide an example of a drug's ability to pirate endogenous biosynthetic mechanisms to trigger new mediators.
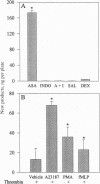
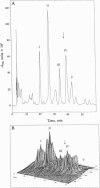
Full text
PDF
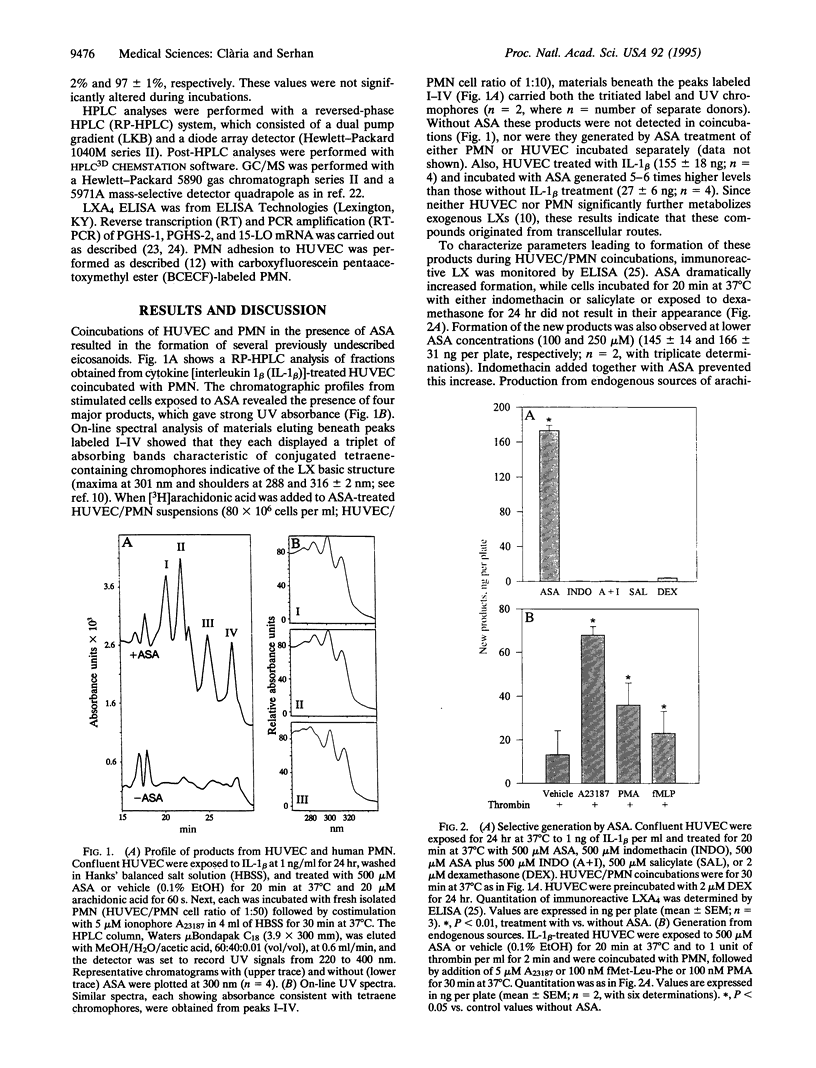

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady H. R., Serhan C. N. Adhesion promotes transcellular leukotriene biosynthesis during neutrophil-glomerular endothelial cell interactions: inhibition by antibodies against CD18 and L-selectin. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1307–1314. doi: 10.1016/s0006-291x(05)81548-7. [DOI] [PubMed] [Google Scholar]
- Brezinski D. A., Nesto R. W., Serhan C. N. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992 Jul;86(1):56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Yadagiri P., Manna S., Falck J. R. Absolute configuration of the hydroxyeicosatetraenoic acids (HETEs) formed during catalytic oxygenation of arachidonic acid by microsomal cytochrome P-450. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1007–1011. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Claesson H. E., Haeggström J. Human endothelial cells stimulate leukotriene synthesis and convert granulocyte released leukotriene A4 into leukotrienes B4, C4, D4 and E4. Eur J Biochem. 1988 Apr 5;173(1):93–100. doi: 10.1111/j.1432-1033.1988.tb13971.x. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Serhan C. N., Parkos C. A., Delp-Archer C., Madara J. L. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993 Jul;92(1):75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart C. E., Coffey R. J., Radhika A., Giardiello F. M., Ferrenbach S., DuBois R. N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994 Oct;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Hempel S. L., Monick M. M., Hunninghake G. W. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest. 1994 Jan;93(1):391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M. J., Turk J., Shornick L. P. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J Biol Chem. 1992 Oct 25;267(30):21438–21445. [PubMed] [Google Scholar]
- Jakobsson P. J., Steinhilber D., Odlander B., Rådmark O., Claesson H. E., Samuelsson B. On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3521–3525. doi: 10.1073/pnas.89.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Lecomte M., Laneuville O., Ji C., DeWitt D. L., Smith W. L. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994 May 6;269(18):13207–13215. [PubMed] [Google Scholar]
- Lee T. H., Horton C. E., Kyan-Aung U., Haskard D., Crea A. E., Spur B. W. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989 Aug;77(2):195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- Levy B. D., Bertram S., Tai H. H., Israel E., Fischer A., Drazen J. M., Serhan C. N. Agonist-induced lipoxin A4 generation: detection by a novel lipoxin A4-ELISA. Lipids. 1993 Dec;28(12):1047–1053. doi: 10.1007/BF02537069. [DOI] [PubMed] [Google Scholar]
- Levy B. D., Romano M., Chapman H. A., Reilly J. J., Drazen J., Serhan C. N. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993 Sep;92(3):1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J. A., O'Neill G. P., Bayly C., Vickers P. J. Mutation of serine-516 in human prostaglandin G/H synthase-2 to methionine or aspirin acetylation of this residue stimulates 15-R-HETE synthesis. FEBS Lett. 1994 Mar 28;342(1):33–37. doi: 10.1016/0014-5793(94)80579-2. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Hajjar D. P. Vascular transcellular signaling. J Lipid Res. 1993 Dec;34(12):2017–2031. [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Nassar G. M., Morrow J. D., Roberts L. J., 2nd, Lakkis F. G., Badr K. F. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994 Nov 4;269(44):27631–27634. [PubMed] [Google Scholar]
- O'Neill G. P., Mancini J. A., Kargman S., Yergey J., Kwan M. Y., Falgueyret J. P., Abramovitz M., Kennedy B. P., Ouellet M., Cromlish W. Overexpression of human prostaglandin G/H synthase-1 and -2 by recombinant vaccinia virus: inhibition by nonsteroidal anti-inflammatory drugs and biosynthesis of 15-hydroxyeicosatetraenoic acid. Mol Pharmacol. 1994 Feb;45(2):245–254. [PubMed] [Google Scholar]
- Raud J., Palmertz U., Dahlén S. E., Hedqvist P. Lipoxins inhibit microvascular inflammatory actions of leukotriene B4. Adv Exp Med Biol. 1991;314:185–192. doi: 10.1007/978-1-4684-6024-7_11. [DOI] [PubMed] [Google Scholar]
- Ristimäki A., Garfinkel S., Wessendorf J., Maciag T., Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994 Apr 22;269(16):11769–11775. [PubMed] [Google Scholar]
- Schreinemachers D. M., Everson R. B. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994 Mar;5(2):138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Fiore S., Brezinski D. A., Lynch S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry. 1993 Jun 29;32(25):6313–6319. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- Serhan C. N. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994 Apr 14;1212(1):1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Soyombo O., Spur B. W., Lee T. H. Effects of lipoxin A4 on chemotaxis and degranulation of human eosinophils stimulated by platelet-activating factor and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Allergy. 1994 Apr;49(4):230–234. doi: 10.1111/j.1398-9995.1994.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Aspirin. Sci Am. 1991 Jan;264(1):84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]