Introduction
Low-grade gliomas (LGGs) are the most common brain tumor of childhood accounting for 35% of all pediatric central nervous system tumors1,2. Pediatric LGGs (PLGGs), classified as World Health Organization (WHO) grade I or II3 represent a heterogeneous group of tumors. PLGGs are classified according to the cellular aspect of the most important constitutive cell type, including astrocytic, oligodendroglial, mixed oligoastrocytic, neuronal, or mixed glioneuronal morphology (Table 1). Although this classification aims to encompass every tumor, a significant number of tumors do not meet the typical criteria for WHO categories or have overlapping histology for multiple categories. In clinical practice, these tumors are often given non-categorical diagnoses with varied and confusing terminology. As such the category of ‘low grade glioma, non-otherwise specified’ (LGG-NOS) has been formally utilized by several groups including ours as a clinical and research diagnosis for these histologically difficult to classify tumors.
Table 1.
Major different subtypes of pediatric low-grade gliomas according to the latest WHO classification
| Astrocytic tumors | Grade (WHO) |
|---|---|
|
|
|
| Pilocytic astrocytoma (PA) | I |
| Pilomyxoid astrocytoma (PMA) | II |
| Diffuse astrocytoma (DA) | II |
| Pleiomorphic xanthoastrocytoma (PXA) | II |
|
|
|
| Oligodendroglial oligoastrocytic tumors | |
|
|
|
| Oligodendroglioma (OD) | II |
| Oligoastrocytoma (OA) | II |
|
|
|
| Neuronal, mixed neuro-glial neuroepithelial tumors | |
|
|
|
| Ganglioglioma (GG) | I |
| Desmoplastic infantile tumors | I |
| Dysembryoplastic neuroepithelial tumor (DNT) | I |
| Angiocentric glioma (AG) | I |
Despite having a similar histological appearance to adult LGG, PLGGs have a distinct and more favorable course and should be considered a different disease entity. Indeed, the majority of children diagnosed with a PLGGs are long-term survivors well into adulthood (Bandopadhayay et al, in press, Pediatric Blood and Cancer 2014), imploring treatment strategies that minimize long-term morbidities4,5. Therefore, it is crucial to understand the biology of PLGGs to allow the development of targeted therapies with less toxicity.
The explosion of novel technologies and multi-platform integrative genomics in recent years has yielded new insights into the oncogenesis of PLGGs. These findings not only bring a paradigm shift to the traditional histological classification of PLGGs but also reveal new therapeutic targets.
In this review, we highlight the biologic complexity of PLGGs, present current diagnostic and management dilemmas, and propose the natural evolution and augmentation of microscopic histological diagnoses with modern genomic profiles. Increased understanding of the molecular identity of these tumors will help drive the development of target-driven therapies.
Histopathologic classification
The WHO classifies low-grade gliomas according their morphological features3. Tumors that do not meet the typical criteria of any single category are commonly labeled LGG-NOS for ‘not-otherwise specified’, which comprise more than a third of all PLGGs6. This sometimes results from small biopsy samples that lack sufficient material on which to assign a WHO grade, and at other times, as a result of pathologic features that do not fit any one category. PLGGs typically have a low proliferative index, with MIB-1 scores between 0.1–10%7–11. This index is often higher in younger children where MIB-1 index higher than 10% can be seen in true PLGGs. However, correlation to either overall or progression-free survival in most studies has been variable and it remains unclear as to whether there is any prognostic significance7,12–20.
Grade I pilocytic astrocytomas (PAs) are classically characterized by the presence of Rosenthal fibers, biphasic architecture, vascular proliferation, and eosinophilic granular bodies3. Eosinophilic granular bodies are often located near cystic areas and may be implicated in cyst formation21. Less commonly, PAs contain regions of calcification22. Useful positive immunohistochemical markers include oligodendroglial markers OLIG2, myelin basic protein (MBP), platelet-derived growth factor (PDGF)23–26 as well as the astrocytic marker Glial Fibrillary Acid Protein (GFAP), which is also considered a stem cell marker 27,28. Gangliogliomas (GGs) are also grade I, and are characterized by perivascular chronic inflammation, granular bodies, binucleated neurons, calcification, and cystic degeneration29. DNTs and AGs are recently described subtypes also defined as grade I tumors. Dysembryoplastic neuroepithelial tumors (DNTs) include a specific entity characterized by GFAP-negative oligodendroglia-like cells and floating neurons with a mucinous eosinophilic background 30. Angiocentric gliomas (AGs), initially described by Tubiana et al., also named angiocentric neuroepithelial tumors (ANET), encompasses classically fusiform and bipolar astrocytic cells which stain positively for GFAP and S-100 arranged around blood vessels creating palisade-like structures 31,32. Microcalcifications are infrequently present.
WHO grade II lesions include diffuse astrocytomas (DAs), pilomyxoid astrocytoma (PMAs), pleomorphic xanthroastrocyoma (PXAs), and oligodendroglial tumors. DAs are characterized by the presence of nuclear atypia, a low mitotic rate, and absence of vascular proliferation or palisading necrosis. PMAs are characterized by astrocytic pleomorphism, significant cellular atypia, and multinucleated giant cells with intracellular lipid accumulation. PXAs consist of pleomorphic and lipidized cells and tend to follow a more aggressive course with an increased frequency of leptomeningeal disease33,34. Oligodendroglial tumors contain monomorphic cells with uniform round nuclei and perinuclear halos, microcalcifications and network of capillaries.
While the WHO classification remains the standard of care in clinical practice for determining management and prognosis, use of histopathological classification alone has significant limitations in PLGG.
In this group of diseases the criteria are naturally limited by the overlap in histologic and clinical features in patients, inter-observer diagnostic variability, and the intrinsic challenge of tumor heterogeneity. As such the current approach provides little information on prognosis and treatment recommendations for individual patients. A more effective and predictive approach integrating pathology and molecular data emerging from recent genomic profiling is greatly needed. Such an integrative classification system based on the molecular signature of individual tumors is likely to be more accurate and reproducible in guiding diagnostic, prognostic, and management decisions.
Epidemiology
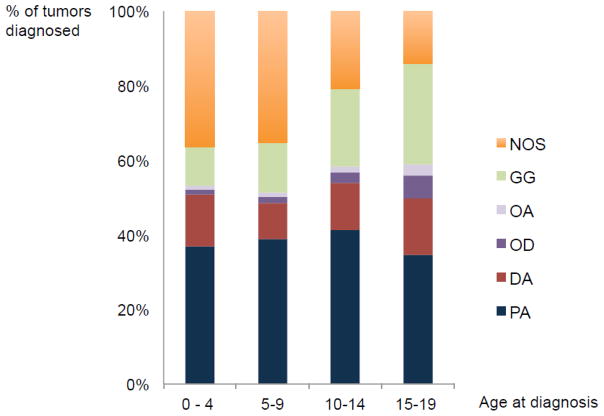
Brain tumors represent the most common solid tumor of childhood, of which PLGGs are the most frequent35. The annual incidence of PLGGs is 2.1 per 100,000 persons in the United States36,37, accounting for 1600 new diagnosis each year. The relative incidence of each LGG histological subtype varies with age, with clear differences in distribution between pediatric and adult LGGs (Figure 1). PAs most frequently develop during childhood and are extremely rare in adults. They represent the most common PLGG, accounting for 15% of all pediatric brain tumors1,6,38–40 (Figure 2). DAs, oligodendrogliomas and oligoastrocytomas are more common in adults but extremely rare in children, representing less than 5% of PLGG 10,6,27–29. Similarly, neuronal and mixed glial-neuronal tumors occur more commonly in the pediatric population. Table 2 summarizes the frequency of the major PLGG subtypes reported in recent epidemiologic studies6,38,39,41–43.
Figure 1.
Comparison of the distribution of histological subtypes developping during childhood (0–19 years) and adulthood, according to the CBTRUS Statistical Report, 2012
Figure 2.
Distribution of PLGGs histological subtypes during 4 stages of development, according to the CBTRUS Statistical Report, 2012
Table 2.
Overview of six epidemiological studies including PLGGs performed in various countries around the world
| GRADE (WHO) | WHO International Classification of Diseases | USA | Germany | France | Denmark | Brazil | |
|---|---|---|---|---|---|---|---|
| Reference | [41] | [6] | [39] | [38] | [42] | [43] | |
| Number of total CNS tumors | 5200 | 20,709 | 3268 | 1017 | 911 | 1058 | |
| Type of study | Retrospective | Retrospective CBTRUS Statistical Report | Retrospective German Childhood Cancer Registry | Prospective French Brain Tumor Database | Retrospective Multi-institution | Retrospective Single-institution Hospital das Clinicas of Sao Paulo | |
| Age | 0–19 years | 0–19 years | 1–15 years | 0–19 years | 0–15 years | 0–21 years | |
| Follow-up period | 1980–1999 | 2005–2009 | 1990–1999 | 2004–2006 | 1960–1984 | 1974–2003 | |
| Frequency of tumors (%) | |||||||
|
| |||||||
| Astrocytic tumors | 16.4 | ||||||
| PA | I | 14.8 | 15.5 | 23.1 | 16.5 | 18.2 | |
| DA | II | 1.8 | 5.2 | NA | 1 | ||
| PXA | II | 0.4 | NA | 0.3 | 13 | 6.2 | |
|
| |||||||
| Oligodendroglial tumors | |||||||
| Oligodendroglioma | II | 1.4 | 1.1 | 1.1 | 4 | 1.6 | 0.9 |
|
| |||||||
| Oligoastrocytic tumors | |||||||
| Oligoastrocytoma | II | 0.6 | 0.7 | NA | 1.1 | NA | NA |
|
| |||||||
| Neural and mixed neuro-glial tumors | |||||||
| GG | I | 2.5 | 3.2 | 4.6 | 2.2 | 3.6 | |
| Desmoplastic infantile astrocytoma | I | NA | 7 | NA | 0.1 | NA | 0.3 |
| DNT | I | NA | NA | 3.1 | NA | 1.3 | |
|
| |||||||
| LGG-NOS tumors | I, II | 0.1–6 | 11.3 | NA | 1.8 | 0.4 | NA |
In addition to the defined groups of tumors, LGG-NOS tumors represent the second most prevalent diagnosis and have been reported to account for at least 17% of all PLGGs 6. This is despite the fact that in most historical studies and governmental databases, this catagory is not included. This highlights the increasing need for integration of histology with molecular data to improve categorization of PLGG tumors.
Although PLGG tumors can occur anywhere throughout the CNS, different subtypes demonstrate predilection for specific sites within the brain or the spine44. Pediatric DAs, AGs, PXAs and oligodendrogliomas are most frequently supratentorial 45–47,48, GGs occur most frequently within the temporal lobes17,18,49 while PAs tend to localize to the cerebellum or the brainstem50. A small fraction of PLGG can arise in the optical pathway as well as in the diencephalic/hypothalamic region; the incidence of those tumors is significantly higher in patients with neurofibromatosis type 1 (NF1). Five percent of all PLGGs primary tumors are located in the spine and these are most frequently PAs51. PLGG can also develop in the cervicomedullary region52 as well as in the tectum.
Genetic predisposition syndromes
Initial insight into the molecular characteristics of PLGGs was derived from the subset of non-sporadic tumors associated with genetic syndromes. Among these, the most frequent association is with NF1, also known as von Recklinghausen disease. PAs and DAs are the most common subtypes associated with NF153 and most commonly involve the optic pathway and hypothalamus54–56. NF1 is characterized by a germline mutation of neurofibromin 1 (NF1), located on chromosome 17q, which results in activation of the RAS/MAPK signaling pathway. Importantly, only 30% of the tumors become symptomatic and require treatment, which suggests a unique biology underlying these tumors57,58.
Tuberous Sclerosis (TS) is another neuro-cutaneous disorder with increased predilection for LGG, with brain tumors found in 5–14% of patients59. The most frequent brain tumor associated with TS is sub-ependymal giant cell astrocytoma (SEGA)60. TS is caused by mutations in two tumor suppressor genes, TSC1 (hamartin, on chromosome 9q34) and TSC2 (tuberin, on chromosome 16p13)61. These genes are part of the Rheb-mTOR pathways that function in regulation of cell proliferation.
These genetic syndromes contributed to our understanding of the importance of the ras/mTOR pathway in the oncogenesis of PLGGs. Additional findings from recent genomic studies have added further insights into the vital role of this pathway in the pathogenesis of PLGGs.
Clinical presentation
The clinical presentation of PLGGs is dictated by their location. Tumors in the posterior fossa typically present with acute signs and symptoms of elevated intracranial pressure secondary to obstructive hydrocephalus, as well as cerebellar signs62, whereas LGGs of the optic pathway impair vision. PLGGs affecting the cerebral cortex typically present with focal neurological manifestations such as seizures or behavioral changes. Seizures are particularly associated with temporal, frontal, or parietal localization and oligodendrogliomas, GGs, DNTs or AGs subtypes63–67. Tumors involving the hypothalamus manifest with endocrinopathies or the diencephalic syndrome68–70. Tectal gliomas are often associated to hydrocephalus due to their expansion to the periaqueductal space. Compared to sporadic PLGGs, the clinical spectrum of NF1-related PLGGs diverges. NF1 patients more commonly present with multifocal tumors compared to sporadic cases71.
PLGGs are most frequently localized at diagnosis, although they can present with disseminated disease. Leptomeningeal dissemination is reported in approximately 3–5% of children at presentation, especially in the setting of spinal cord or diencephalic/hypothalamic lesions72–74, and may be associated with inferior overall survival compared to those who present with localized disease75–77.
Radiological features of PLGGs are variable. These neoplasms are usually hypodense on CT compared to more malignant neoplasms. Grade I PLGGs are typically well-circumscribed tumors, with T1-hypointensity and T2-hyperintensity on MRI imaging. Following gadolinium administration, grade I astrocytomas usually demonstrate homogeneous enhancement. In contrast, grade II gliomas, especially DAs, are typically non-enhancing and may be less circumscribed78–82. PMAs express usually heterogeneous enhancement82. PLGGs are not usually associated with peri-tumoral edema or restricted diffusion on diffusion-weighted MRI sequences83. Magnetic resonance spectrometry (MRS), diffusion-weighted MRI (DWI) and diffusion tensor imaging (DTI) serve as useful adjuncts in further characterizing PLGGs. PET-scan and single-photon emission CT (SPECT) may also aid assessment of treatment efficiency and tumor recurrence. GGs typically exhibit contrast enhancement on CT scans and can have variable gadolinium enhancements on MRI – from absence of contrast enhancement to nodular or circumferential. Similar to astrocytomas and oligodendroglial tumors, they appear T1-hypointense and T2-hyperintense on MRIs. The contrast enhancement for oligodendroglial tumors is variable and is related to the infiltrative aspect of the tumors with a higher gadolinium contrast enhancement in solid and non-invasive tumors. DNTs do not displace brain structures but tend to infiltrate and usually have low or no contrast enhancement. They appear as bright T2-weighted and hypointense T1 tumors with typically neither mass effect nor peritumoral edema. Their slow growth may be associated with skull deformation when located in the cortex.
Natural history
The natural history of pediatric LGGs is distinct from that of adult LGGs. On the whole, PLGGs exhibit slow rates of growth. Thus, the majority of children are diagnosed at least six months after symptom onset84. PLGG have been reported to spontaneously regress, especially in patients with NF185–89, who have been reported to have superior outcomes compared to sporadic cases90–92. Tumors that can be completely resected often require no further therapy highlighting the importance of location on outcome. In a recent prospective population-based study of a large cohort of 639 PLGGs, the 5-year PFS (progression free survival) was 69.4%93, which is comparable to other studies84,94–99. Given the fact that two thirds of NF1 patients never progress, the recurrence rate of sporadic PLGGs is near 55%, as reported in the recent COG study100. The most significant risk factors for progression identified on multivariate analysis were young age at diagnosis (<1 year), subtotal resection, and DA histology101. Due in part to a better chance of complete resection, tumors involving the optic nerve or cerebellum have better progression-free survival (PFS) compared to those involving the chiasm and hypothalamus. Even if progression occurs, children diagnosed with PLGG have an excellent overall survival long-term, as described in a recent analysis of the SEER (Surveillance Epidemiology and End Results) database showing a 20 year overall survival of 87% (Bandopadhayay et al, in press, Pediatric Blood and Cancer 2014). In contrast to adults, PLGGs are characterized by a low incidence of malignant transformation102–104. Importantly, adult survivors of PLGG have low glioma related mortality, suggesting a very low propensity for malignant transformation of PLGG (Bandopadhayay et al, in press, Pediatric Blood and Cancer 2014).
Treatment strategies
Given the excellent overall survival for the majority of PLGG patients, the treatment goal is to achieve tumor control while minimizing long-term tumor and treatment related morbidity105. Most patients require only surveillance after surgery. If progression, recurrence and/or symptoms occur, then treatment modalities including surgery, chemotherapy (including biologic therapy), or less frequently, radiation therapy are indicated.
Surgical resection remains the cornerstone of PLGG management. Patients with gross total resection of tumor typically do not need further treatment. However, gross total resection is not always achievable without significant neurological impairment for some tumor locations, such as the optic pathway, hypothalamus, diencephalon, and brainstem. In these instances, the goal of surgery is to achieve maximal resection without risking severe neurologic deficits. Even in the event of a subtotal resection, the overall survival of patients remains excellent (Bandopadhayay et al, in press, Pediatric Blood and Cancer 2014)106–111.
Chemotherapy is usually initiated for radiological and/or symptomatic progression. Over the last few decades, many protocols using either monotherapy or poly-chemotherapy have been tried for PLGG, as shown in Table 3. Platinum-based chemotherapy such as carboplatin112–114, cisplatin115, oxaliplatin116, ibroplatine117 alone or in combination with vincristine118–126 or etoposide (VP16)127 have been widely utilized and evaluated. The combination of vincristine and carboplatin is commonly used as first-line therapy, with 5-year overall and progression-free survival rates of 86 to 97% and 39 to 61%(Table 3). Carboplatin hypersensitivity is the most frequent adverse event128–130, which can be effectively managed with pre-medication35. Ototoxicity is another issue that is important to monitor during treatment with platinum compounds. A combination of thioguanine, procarbazine, lomustine, and vincristine (TPCV) is another well-established chemotherapy regimen for progressive PLGG126,131–133. A prospective randomized clinical trial comparing outcomes of vincristine/carboplatin versus TPCV revealed that treatment with TPCV had a trend towards superior 5-year event-free survival (EFS) compared to vincristine/carboplatin (52% vs 39%, respectively), although this did not reach statistical significance126. However, the potential long-term morbidity associated with alkylating agents such as infertility and increased risk of secondary malignancy has led most oncologists to use vincristine/carboplatin as a first-line therapy over TPCV. Hematologic dyscrasias are other potential complications, especially of alkylating agents.
Table 3.
Summary of the different chemotherapy strategies evaluated in PLGGs
| Regimen course | Reference | Study length | Number of patients | Eligbility | Response | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First line | Recurrence/progression | CR (%) | PR (%) | MR/SD (%) | PD (%) | year | % | year | % | ||||
| CBP-VCR | Packer et al 1993 | NA | 60 | yes | yes | 2 | 37 | 50 | 11 | NA | NA | ||
| Packer et al 1997 | 1989–1993 | 78 | yes | yes | 5 | 28 | 60 | 6 | 3 | 97 | 3 | 68 | |
| Demaerel et al 2002 | NA | 9 | yes | no | 0 | 55 | 45 | 0 | NA | NA | |||
| Gnekow et al 2004 | 1996–2004 | 123 | yes | no | 2 | 6 | 76 | 7 | 5 | 87 | 5 | 61 | |
| Ronghe M et al 2010 | 1996–2006 | 16 | yes | yes | 6 | 50 | 38 | 6 | NA | NA | |||
| Ater et al 2012 | 1997–2000 | 137 | yes | yes | CR/PR: 35 | 32 | 33 | 5 | 86 | 5 | 39 | ||
|
| |||||||||||||
| CBP/VP16 CPP/VCR lomustine/PC/VCR |
Bruggers et al 2007 | NA | 10 | yes | yes | 20 | 10 | 70 | 0 | NA | NA | ||
|
| |||||||||||||
| PC/CBP VP16/CisP VCR/CCP |
Laithier et al 2003 | 1990–1998 | 85 | no | yes | 0 | 42 | 45 | 13 | 5 | 89 | 5 | 34 |
|
| |||||||||||||
| CBP/VP16 | Castello et al 1995 | NA | 13 | yes | no | 8 | 0 | 70 | 22 | NA | NA | ||
|
| |||||||||||||
| CBP/tamoxifene | Walther et al 2011 | NA | 13 | yes | no | 0 | 15 | 69 | 15 | 3 | 69 | 3 | 47 |
|
| |||||||||||||
| CBP/vinblastine | Jakackl et al 2011 | 2006–2008 | 26 | yes | yes | 0 | 5 | 81 | 14 | NA | NA | ||
|
| |||||||||||||
| CBP or Ibroplatine | Friedman et al 1992 | 1986–1990 | 12 | yes | yes | 0 | 0 | 75 | 25 | NA | NA | ||
|
| |||||||||||||
| CisP/VCR | Massimino 2002 | 1991–2000 | 34 | yes | no | 3 | 32 | 65 | 0 | 3 | 100 | 3 | 78 |
| Massimino 2010 | 2001–2007 | 37 | yes | no | 0 | 47 | 20 | 33 | 3 | 97 | 3 | 65 | |
|
| |||||||||||||
| CBP | Moghrabl et al 1993 | NA | 6 | no | yes | 0 | 0 | 100 | 0 | NA | NA | ||
| Aquino et al 1999 | 1992–1996 | 12 | no | yes | 0 | 33 | 50 | 17 | 3 | 83 | NA | ||
| Gururangan et al 2002 | 1993–2000 | 81 | no | yes | 2 | 21 | 82 | 15 | 3 | 84 | 3 | 64 | |
|
| |||||||||||||
| CisP | Hsu et al 2008 | 1992–2007 | 16 | no | yes | 0 | 25 | 31 | 44 | 5 | 94 | 5 | 56 |
|
| |||||||||||||
| oxaliplatin | Beaty et al 2010 | 2004–2006 | 9 | no | yes | 0 | 0 | 38 | 62 | NA | NA | ||
|
| |||||||||||||
| CisP/VCR | Sawamura et al 2009 | 1992–2008 | 15 | no | yes | 7 | 53 | 40 | 0 | NA | NA | ||
|
| |||||||||||||
| VP16-VCR | Pons et al 1992 | NA | 20 | yes | yes | 0 | 0 | 71 | 29 | NA | NA | ||
|
| |||||||||||||
| VCR/VP16/CPP/5-FU | Lee et al 2006 | 1999–2004 | 13 | no | yes | 8 | 38 | 23 | 31 | 6 | 100 | 6 | 67 |
|
| |||||||||||||
| TMZ | Nicholson et al 2007 | 1998–1999 | 22 | no | yes | 0 | 5 | 95 | 0 | NA | NA | ||
| Gururangan et al 2007 | 1999–2005 | 30 | no | yes | 0 | 10 | 43 | 47 | 4 | 71 | 4 | 17 | |
| Khaw et al 2007 | 1999–2005 | 13 | no | yes | 15 | 23 | 23 | 38 | NA | 3 | 57 | ||
| Bartels et al 2011 | 2000–2006 | 28 | no | yes | NA | NA | NA | NA | 2 | 71 | NA | ||
|
| |||||||||||||
| TPDCV | Prados et al 1997 | 1984–1992 | 42 | yes | yes | 0 | 36 | 59 | 5 | 5 | 78 | NA | |
| Mishra et al 2010 | 1984–1992 | 33 | yes | yes | 0 | NA | NA | 76 | 15 | 71 | 15 | 23 | |
|
| |||||||||||||
| TPCV | Lancaster et al 2003 | NA | 10 | no | yes | NA | NA | 78 | NA | NA | NA | ||
| Ater et al 2012 | 1997–2000 | 137 | yes | yes | CR/PR: 30 | 36 | 34 | 5 | 87 | 5 | 52 | ||
|
| |||||||||||||
| BCNU/VCR/MTX-IT | Sumer et al 1978 | NA | 6 | NA | NA | NA | NA | 50 | NA | NA | NA | ||
|
| |||||||||||||
| vinblastin | Lafaye-Cousin et al 2005 | NA | 9 | no | yes | 11 | 56 | 22 | 11 | NA | NA | ||
| Bouffet et al 2012 | 2002–2009 | 51 | no | yes | 2 | 34 | 38 | 26 | 5 | 93 | 5 | 43 | |
|
| |||||||||||||
| CPP | Kadota et al 1999 | 1996–1997 | 15 | no | yes | 7 | 0 | 57 | 36 | NA | NA | ||
|
| |||||||||||||
| CBP/VCR VCR/VP16 TPCV vinblastine |
Scheinemann et al 2011 | 1985–2009 | 38 | no | yes | NA | NA | NA | NA | 5 | 86 | ; | 37 |
|
| |||||||||||||
| bevacizumab/irinotecan | Packer et al 2009 | 2006–2008 | 10 | no | yes | 11 | 44 | 45 | 0 | NA | NA | ||
| Coucec et al 2012 | 2007–2010 | 11 | no | yes | 0 | 63 | 0 | 37 | NA | NA | |||
|
| |||||||||||||
| nimotuzumab | Saurez et al 2009 | 2005–2007 | 4 | no | yes | 0 | 0 | 50 | 50 | NA | NA | ||
|
| |||||||||||||
| erlotinib/rapamycin | Yalon et al 2013 | 2007–2010 | 21 | no | yes | 0 | 6 | 35 | 59 | NA | NA | ||
|
| |||||||||||||
| everolimus | Krueger et al 2010 | 2009–2010 | 28 | no | yes | 0 | 75 | 25 | 0 | NA | NA | ||
CBP: carboplatin, VCR: vincristine, VP16: etoposide, CPP: cyclophosphamide, PC: procarbazine, CisP: cisplatin, 5-FU: 5-fluorouracile, TMZ: temozolomide, TPDCV: five-drug regimen consisting of 6- thioguanine, procarbazine, dibromodulcitol, 1-(2-chloro- ethyl)-3-cyclohexyl-1-nitrosourea (CCNU), and vincristine, TPCV: thioguanine, procarbazine, lomustine, and vincristine, BCNU: carmustine, MTX-IT: intrathecal injection of methotrexate, CR: complete response, PR: partial response, MR/SD: minor response/stable disease, PD: progressive disease, OS: overall survival, PFS: progression free survival
Alkylating agents have also been tested in combination with tamoxifene 134 or vinblastine 135 as well as in polychemotherapy regimens with other agents including procarbazine, cyclophosphamide, lomustine, vincristine, VP16 or 5-fluorouracile 136–139. Monotherapy using temozolomide 140–143, vinblastine 144–146 or cyclophosphamide 147 have been used in progressive PLGGs with variable results in terms of outcome, depending on the ages of the children and the tumor locations enrolled in the studies.
Other protocols including vincristine/VP16 148 or vincristine/carmustine 149 associated with intrathecal injection of methotrexate have shown 50–70% tumor control (defined as radiologic response or stable disease) in progressive PLGGs. Other chemotherapy regimens tested include vincristine alone 150, vincristine in combination with actinomycin 151, high dose ifosfamide 152, high dose cyclophosphamide 153, bleomycine 154, topotecan 155, idarubicin 156 or lenalinomide 157.
The anti-VEGF agent bevacizumab has recently been evaluated in combination with irinotecan for PLGG disease progression158–160. A recent phase II study which included 35 recurrent PLGGs reported at 2-year PFS of 47.8% using this treatment strategy 160. Bevacizumab is generally well tolerated, however, patients need close monitoring for the development of hypertension or proteinuria and there are concerns for premature ovarian failure.
Radiation therapy was once standard-of-care for PLGG, however its use has decreased in PLGGs with increased awareness of its devastating long-term morbidities including cognitive deficits, increased risk of secondary high-grade malignancies, vasculopathy, endocrinopathy, and effects on growth 161–163,216,217. Given the excellent overall survival of children with PLGG and the numerous available chemotherapy regimens, the use of radiation therapy for PLGG is generally avoided to minimize long-term and irreversible morbidity, and is used for those in whom disease control cannot be achieved with either surgery or chemotherapy (including targeted therapies). Several protocols using conformal external beam radiotherapy at doses between 50–59 Gy have been reported in the treatment of non-operable or progressive PLGGs with 5-year PFS ranging from 74%–88% 121,164–166. Over last decade, through the advances in radiotherapy techniques, significant progress has been made in minimizing scatter doses to normal brain. These techniques include stereotactic conformational external radiotherapy 167–169, gamma-knife stereotactic radiotherapy techniques 170–172 and proton beam radiotherapy 173,174.
While numerous treatment options for PLGG patients are available, all of these current approaches have acute and/or long-term toxicity, have frequent recurrences and are based on non-tumor specific mechanisms of action. With the development of new molecular technologies, the opportunity to dissect the molecular basis of PLGGs might assist in the improved classification of these lesions. More importantly, the identification of specific pathways also provides for the potential institution of tumor specific targeted therapy.
Genomic alterations in pediatric low-grade gliomas
General genomic features
Recent advances in high-throughput genetic sequencing and gene expression profiling have shed important insights into the genomic alterations of PLGGs175. Table 4 summarizes the major mutations and chromosomal rearrangements that have been described in different cohorts of PLGGs. One important limitation to these studies is the lack of sufficient tumor tissue from rarer subtypes of PLGGs such as tectal gliomas, thalamic and optic pathway tumors.
Table 4.
Summary of all the major mutations described in PLGGs
| Reference | Number of tumors analyzed | Mutation | Histology |
|---|---|---|---|
| Sharma et al 2005 | 21 | KRAS | 5% PA |
|
| |||
| Janzarik et al 2007 | 25 | KRAS | 4% LGA |
|
| |||
| Jones et al 2008 | 44 | KIAA-BRAF dup (3 fusion types) | 66% PA |
|
| |||
| Jones et al 2009 | 44 | SRGAPS-RAF1 dup | 7% PA |
| BRAF V600E | 2% PA | ||
|
| |||
| Jacob et al 2009 | 36 | BRAF V600E | 20% GG |
|
| |||
| Forshew et al 2009 | 50 | KIAA-BRAF dup (6 fusion types) | 94% PA |
| 9% DA | |||
| 22% PM | |||
| SRGAP3-RAF1 dup | 3% PM | ||
| BRAF V600E | 9% DA | ||
| 100% PXA | |||
| KRAS | 3% PA | ||
|
| |||
| Sievert et al 2009 | 28 | KIAA-BRAF dup | 77% PA |
| 50% DA | |||
|
| |||
| McConail et al 2009 | 117 | BRAF V600E | 57% GG |
| 23%NDS | |||
| 2% PA | |||
| MYC | 2% PA | ||
| 7%GG | |||
| PIK3CA | 2% PA | ||
| 2% NOS | |||
| CUBN | 4% PA | ||
| CTNNB1 | 2% PA | ||
| TP53+PKHD1 | 2% NOS | ||
| PDGFRA | 2% NOS | ||
|
| |||
| Yu et al 2010 | 79 | KIAA-BRAF dup (3 fusion types) | 60% PA |
|
| |||
| Dougherty et al 2010 | 27 | BRAF V600E | 25% PXA |
| 50% GG | |||
|
| |||
| Schindler et al 2011 | 133 | BRAF V600E | 9% PA |
| 69% PXA | |||
| 13% GG | |||
|
| |||
| Dias-Santagata et al 2011 | 11 | BRAF V600E | 64% PXA |
|
| |||
| Lin et al 2012 | 106 | KIAA-BRAF dupl (5 fusion types) | 60% PA |
| 24% DOS | |||
| 36% GN | |||
| 33% PMA | |||
|
| |||
| Padovani et al 2012 | 24 | IDH1 mutation | 1 OA |
|
| |||
| Ramkissoon et al 2013 | 45 | KIAA-BRAF dup | 22% GG |
| 10% NOS | |||
| BRAF V600E | 75% GG | ||
| 71% NOS | |||
| MYBL1 rearrangement | 36% DA | ||
| 28% DA | |||
|
| |||
| Zhang et al 2013 | 148 | KIAA-BRAF dup | 76% PA |
| 11% GG | |||
| BRAF-MACF1 dup | 11 %GG | ||
| RAF fusions | 2% PA | ||
| NF1 mutation | 2% PA | ||
| 3% DA | |||
| V600E mutation | 5.5% PA | ||
| 70% PXA | |||
| 33% GG | |||
| 12% DA | |||
| FGFR1 duplication (TDK) | 24% DA | ||
| 3% PA | |||
| 100% DNT | |||
| FGFR1-TACC1 translocation | 1% PA | ||
| 9% DA | |||
| FGFR1 mutation | 2% PA | ||
| 3% DA | |||
| KRAS mutation | 1%PA | ||
| 3% DA | |||
| MYB/MYBL1 rearrangement | 21% DA | ||
| 100% AG | |||
| IDH1 mutation | 3% DA | ||
| H3F3A mutation | 9% DA | ||
| NTRK2 fusion -NAV1 | 3% DA | ||
|
| |||
| Jones DT et al 2013 | 96 PA | KIAA-BRAF dup | 70% |
| BRAF other rearrangements (-FAM131B, -RNF130, -CLCN6, -MKRN1, -GNAI1) | 5.5% | ||
| BRAF ins599T | 1% | ||
| BRAF p.R506 insVLR | 1% | ||
| V600E mutation | 4% | ||
| KRAS point mutations | 2% | ||
| NTRK2 fusions (QKI or NACC2) | 3% | ||
| FGFR1 mutations | 5% | ||
| FGFR1 tandem duplication | 1% | ||
| PTPN11 mutation | 2% | ||
| H3F3A mutation | 1% | ||
A striking finding of PLGGs is the low number of genetic alterations present in the tumors. Early cytogenetic studies revealed almost normal diploid karyotypes across multiple subtypes of PLGGs176–178. The most frequent recurrent chromosomal alteration identified was a gain of chromosome 7, especially in PAs 176,179–181. Other chromosomal structural abnormalities included gains of chromosome 4, 5, 6, 8, and 11 and deletion of 17p in a subset of PAs, inversion in chromosome 8, and loss of chromosome 1q176,179–187.
Genetic alterations in pediatric LGGs differ from adult LGGs. Concomitant deletion of chromosome 1p and 19q is one of the most frequent recurrent genetic alterations in adult oligodendrogliomas, aiding in diagnosis as well as serving as a favorable prognostic marker188,189. In contrast, concomitant deletion of chromosome 1p and 19q is rare in children with oligodendrogliomas190,191, and does not confer similar chemosensitivity when present192. Similarly, mutations in TP53, a tumor suppressor gene that codes for a nuclear phosphoprotein and regulates cycle cell arrest, apoptosis, and genetic stability, are frequently found in adult but rarely in pediatric LGGs193,194–198. IDH1 and IDH2 mutations are also rarely observed in PLGGs while they are frequent in adults. In a recent study examining IDH1 and IDH2 in 445 CNS tumors and 494 non-CNS tumors, IDH1/2 mutations were described to occur with a frequency of more than 70% in adult patients across a variety of glial tumors including low-grade astrocytomas, anaplastic astrocytomas, oligodendrogliomas and oligoastrocytomas and secondary glioblastomas derived from the lower-grade gliomas199. In contrast, IDH1/2 mutations are rare in children, although when found in adolescent patients they may be a harbinger of the adult form of the disease, meriting concordant treatment recommendations200,201.
NF1
The increased risk of LGGs in children with NF1 was one of the first clues that dysregulation of the mitogen-activated protein kinases (MAPK) pathway may be important in the pathogenesis of PLGGs. NF1 encodes neurofibromin, which is ubiquitously expressed at variable levels in different tissue types during development. Structurally, neurofibromin contains a central domain homologous to Ras-GTPase-activating (Ras-GAP) proteins and acts as a negative regulator of the Ras-Raf-MEK-ERK pathway202. In neurofibromatosis, NF1 mutations produce a loss of function of neurofibromin that leads to the constitutive activation of the Ras pathway and results in proliferation of astrocytes35, among other phenotypes. Thus, MAPK pathway activation has long been known to contribute to the pathogenesis of LGGs in NF1 patients203. In addition, constitutive expression of MEK1 causes an increase in astrocytic proliferation.
BRAF duplication-fusions
Genetic rearrangements of the oncogene BRAF are the most common genomic alterations found in sporadic PLGGs. Early studies utilizing comparative genomic hybridization (CGH) identified a gain of the specific chromosomal region 7q34 containing the BRAF locus as the most frequent copy number alteration in PLGGs35, involving 50–100% of pediatric PAs204–206. The BRAF duplication is found more frequently in cerebellar and hypothalamic-chiasmatic tumors206.
The 7q34 gain has been characterized to represent a duplication of BRAF with a tandem insertion in the KIAA1549 gene35. This BRAF duplication results in the activation of the downstream effectors of the MAPK pathway, MEK and ERK207,208. Subsequently, variants of the fusion transcript involving BRAF gene have been described, involving not only KIAA1549 but also other fusion partners, SRGAP3, FAM131B, MACF1, RNF130, CLCN6, MKRN1 and GNAI1 (Table 5)209–212,213,214. RAF1, which encodes a protein that leads to the stabilization and activation of BRAF, has also been described to harbor gene fusions with SRGAP3 and QK1, leading to the constitutive activation of MAPK pathway209,211,213. These BRAF rearrangements tend to occur frequently in cerebellar lesions, Strikingly, all of the fusion protein variants are characterized by loss of the N-terminal inhibitory domains of BRAF, resulting in constitutive activation of the BRAF kinase and downstream activation of MAPK and its effectors, MEK and ERK.
Table 5.
Summary of all the different fusions types of BRAF and RAF1 described in PLGGs
| KIAA – BRAF duplication-translocation | |
| KIAA Ex 15 - BRAF Ex 9 KIAA Ex 16 - BRAF Ex 11 KIAA Ex 16 - BRAF Ex 9 KIAA Ex 15 - BRAF Ex 11 KIAA Ex 17 - BRAF Ex 10 KIAA Ex 16 - BRAF Ex 10 |
Jones et al 2008&2009 Forshew et al 2009 Sievert et al 2009 Yu et al 2010 Lin et al 2012 Lin et al 2012 Dahiya et al 2012 |
| RAF1 duplication-translocation | |
| SRGAP3 Ex 11-RAF1 Ex8 SRGAP3 Ex 12-RAF1 Ex10 SRGAP3-RAF1 QK1 - RAF1 |
Forshew et al 2009 Jones et al 2009 Jones et al 2009 Zhang et al 2013 Zhang et al 2013 |
| Other fusion types | |
| FAM131B-BRAF MACF1 - BRAF RNF130 Ex 3 - BRAF Ex 9 CLCN6 – BRAF (intrachromosomal) MKRN1 - BRAF GNAI1 - BRAF |
Cin et al 2011 Jones et al 2013 Zhang et al 2013 Jones et al 2013 |
Although the BRAF fusion protein has been shown to result in a tandem duplication of the BRAF locus, further studies are necessary to explain the precise mechanism by which the fusions contribute to the formation of tumor and the specific role of KIAA1549 and SRGAP3 segments within the BRAF fusion transcripts. One recent study reported that regions flanking the breakpoints of the RAF gene fusion are enriched with microhomologous sequences. This has led to the hypothesis that tandem duplications of the RAF gene might be generated by microhomology-mediated break-induced replication215. In vitro evaluation of the effect of the BRAF fusion protein has suggested that this protein has oncogenic properties and is able to activate the MAPK pathway. The short form of KIAA1549-BRAF fusion induces anchorage-independent growth in multiple cell lines211,216. Furthermore, pharmacologic inhibition of MEK1/2 in short-term cultured PLGG cell lines significantly diminishes cell proliferation207, supporting a role of the MAPK pathway in promoting proliferation. Taken together, BRAF and RAF1 fusion transcripts, leading to constitutive activation of MAPK pathway, may play a crucial role in the pathogenesis of sporadic PAs and may also present potential therapeutic targets for PLGGs.
BRAF V600E and other less frequent mutations
Another frequent genomic alteration in PLGGs is the BRAF V600E mutation169, which also results in deregulation of the MAPK pathway35. This mutation has been described in other cancer subtypes, including melanoma217, colorectal cancer218, leukemia219, and high-grade gliomas220. BRAF is one of the most mutated genes in cancer221. The BRAF V600E point mutation occurs most commonly in PXAs, GGs, DAs, and PMAs196,209,213,214,222–225 and is only rarely detected in PAs226. Thus BRAF duplications and V600E point mutation are almost always mutually exclusive. The BRAF V600E alteration confers constitutive BRAF kinase activation, and transforms NIH3T3 fibroblasts in vitro211. Other rare forms of small amino-acid insertions in BRAF have been identified in PAs214. The BRAF V600E mutation has been shown to promote transformation of human neural stem cells, followed by senescence227. However, it remains unclear whether this recurrent alteration is sufficient to drive the development of PAs.
Other mutations and rearrangements involving the MAPK pathway
Recent landmark sequencing projects including large cohorts of PLGGs identified recurrent genomic alterations in fibroblast growth factor receptor type 1 (FGFR1) 213,214. FGFR1 genomic alterations have also been described in breast cancer, lung cancer, and glioblastomas. FGFR1 point mutations (N546K and K656E) were found in 5% of supra-tentorial PAs. Both mutations have been described to transform cells in vitro. In 2% of cases, FGFR1 mutations were associated with the presence of a PTPN11 mutation, another downstream effector of FGFR1214. In the same study, one PA possessed a tandem duplication of FGFR1. Importantly, gene expression analysis revealed that FGF2, a ligand of FGFR1, was significantly over-expressed in PAs compared to other astrocytic tumors, suggesting that the FGF/FGFR pathway alteration plays an important role in tumorigenesis of PLGGs. Additionally, FGFR1 mutations and duplication of its tyrosine kinase domain have also been described in PAs, DAs, and DNTs213.
Alterations of other MAPK members have also been described in PLGG. These include genomic alterations affecting the kinase domain of neurotrophic tyrosine kinase type 2 (NTRK2), which have been described in pediatric PAs214. Finally, KRAS activating mutations have also been described in 3–5% of sporadic PAs209,213,214,228,229 (Table 2).
PI3K and RTK Signaling
After the MAPK pathway, the other most frequently altered pathways in PLGGs include the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, the epidermal growth factor receptor (EGFR) pathway, sonic hedgehog (SHH) signaling, and the vascular endothelial growth factor (VEGF) signaling pathway.
PI3K is an intracellular protein that is recruited to the cell membrane after stimulation of a transmembrane growth receptor such as EGFR or platelet derived growth factor receptor A (PDGFRA - which also signals along the Ras-Raf-MEK-MAPK pathway), resulting in activation of downstream effectors, such as AKT and mTOR, to induce cell proliferation and inhibition of apoptosis. As initially suggested by early studies of tuberous sclerosis, activation of mTOR through mutations of its upstream inhibitor result in increased predisposition for PLGGs, in particular the SEGA subtype. In a series of PLGG, 44% of tumors were demonstrated to have evidence of PI3K/Akt/mTOR pathway activation35. Over-expression of the BRAF-fusion transcript in neural stem cells results in activation of mTOR pathway, leading to the formation of glioma-like lesions and further supports the cross communication between these two pathways230. Additionally, the deregulation of Rheb and further mTOR activity in TS patients is another important insight for the role of MAPK pathway in PLGGs as mTOR pathway is connected to the MAPK pathway. In contrast, MEK1/2 knockdown in mice results in the absence of glial cell differentiation and proliferation231.
Activation of the EGFR pathway has been shown in a small series of PLGGs. Comparative genomic hybridization and fluorescent in situ hybridization (FISH) studies of six disseminated PLGGs demonstrated EGFR amplification, while none was observed in a cohort of localized tumors. This led to speculation that deregulation of the EGFR pathway may play a role in the pathogenesis of disseminated PLGGs232. Additionally, rare mutations of PDGFRA have been reported in PAs, GGs, and LGG-NOS tumors196.
Although the sonic hedgehog pathway is most commonly associated with tumorigenesis of medulloblastoma and high-grade gliomas233,234, a recent study suggests that this pathway could play a role in a subset of pediatric PAs via the over-activity of PTCH235. In this series of 20 pediatric PAs, 45% of tumors demonstrated over-expression of PTCH mRNA. Interestingly, a significant inverse correlation between PTCH expression level and patient age suggests that the SHH pathway is more frequently activated in young patients.
Finally, the potential role of angiogenesis is highlighted through studies involving the VEGF pathway, one of the major signaling pathways in cancer biology, contributing to neovascularization which is essential for tumor growth35. Comparative analysis of vessel architecture in 59 pediatric PAs and adult high-grade gliomas showed that vessel immaturity and instability are present in both tumor types236. Another study of 17 pediatric PAs demonstrated immunohistochemical reactivity for activated VEGF receptors. However, further validation studies are necessary to confirm altered VEGF signaling in pediatric PAs.
Transcription factors
Genomic alterations affecting key transcription factors have been described in PLGGs. These include MYB amplification in DAs and focal deletions of MYB in AGs237. MYB is an oncogene that is mutated or altered in T-ALL238,239, breast cancer, pancreatic cancer, and CNS tumors, including primitive neuroectodermal tumors and medulloblastoma240,241. In PLGGs, MYB expression has been shown to be up-regulated in a proportion of diffuse LGGs (60%) and PAs (41%). Its role in the normal development of the CNS and tumorigenesis remains unknown.
More recently, a novel recurrent genetic rearrangement involving another member of the MYB transcription factor family, MYBL1, was identified in a cohort of grade II DAs and AGs213,242. Importantly, this specific duplication-truncation of MYBL1 has demonstrated tumorigenic properties in vitro.
Epigenetic analysis of pediatric low-grade gliomas
Aberrant epigenetic regulation has been increasingly described in human cancers and has become a major focus in a number of pediatric cancers 243. Epigenetic regulation of the genome can be defined as heritable modifications in gene expression that do not directly affect the DNA sequence244. Epigenetic modifications include multiple mechanisms affecting the chemical properties of DNA, histones, or other proteins involved in DNA packaging245. The frequency of alterations in epigenetic modifiers in cancer has been shown in multiple cancer types including hematologic tumors246,247, Wilm’s tumors248, retinoblastoma249, neuroblastoma, thyroid carcinoma, hepatocellular carcinoma, sarcoma250, and brain tumors such as medulloblastoma251 and Atypical teratoid rhabdoid tumors (ATRTs) with SMARCB1 mutations252,253.
The evidence that epigenetics is a major factor in pediatric glioma biology is extremely strong. Direct mutations in the chromatin modifier H3F3A have been described in pediatric GBMs254 as well as DAs and PAs213,214. This suggests that dysregulation of chromatin remodeling effectors are also acting with genomic alterations in the tumorigenesis of a subset of PLGGs. Other genomic alterations include HIPK2 genomic gains and increased mRNA expression level in a subset of sporadic PAs arising from the cerebellum204,255 and BCR gene rearrangement in one PMA256.
The role of epigenetic dysregulation of tumor suppressor gene expression has been described in multiple adult LGGs. Several lines of evidence also support a role of epigenetics in PLGGs. First, the spectrum and frequency of mutations in PLGGs is limited, compared to adult tumors242,252. Moreover, most of these mutations are not oncogenic independently. Recent in vivo studies suggest that BRAF alterations in gliomas are not sufficient to induce tumor formation. Additionally, the natural history of PLGGs suggests regulation in addition to somatic DNA mutations that controls PLGG tumor behavior. PLGGs appear to enter growth arrest after the teenage years, which are unlikely to be driven by somatic changes. These mechanisms remain to be characterized in PLGGs. Thus, epigenetic profiling of PLGGs presents great potential to further the understanding of the pathophysiology underlying these heterogenic and poorly understood tumors.
Prognostic implications
Recently attempts have been made to correlate specific genomic alterations to clinical outcome with controversial results. A multivariate analysis of 146 patients reported that the presence of KIAA1549-BRAF fusion protein was the most significant favorable prognostic factor in pediatric PAs following subtotal resection257. Another study including 106 PLGGs, most of which were sporadic PAs, showed no statistical superior progression-free survival rates among tumors with the BRAF-duplication compared to the wild-type tumors258. The observation that BRAF duplicated tumors behave differently that the others remains an open question, especially with the recent discovery of new BRAF fusion types that might have biased the previous studies. Further larger and controlled or prospective analyses are needed to address this question. It has been hypothesized that improved outcome in PAs conferred by the BRAF duplication may be due to oncogene-induced senescence (OIS), which occurs through the activation of p16Ink4a pathway259. OIS is a mechanism of tumor suppression that has been implicated in other cancer subtypes260. In contrast, p16 deletion has been identified as a negative prognosticator in 198 PLGG261. This remains to be further validated. Similarly, a recent study performed on GGs has showed that the presence of the V600E point mutation was associated with significant lower recurrence-free survival262. The recent discovery of other genomic alterations such as FGFR1 mutations will also enlarge the field of exploration between clinical outcome and biology.
Towards new therapeutic approaches
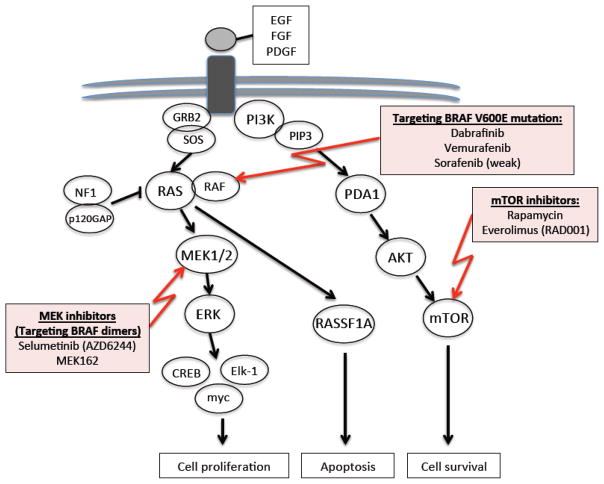
Our recent increase in understanding the genomic alterations of PLGGs has expanded standard therapeutic approaches into targeted therapies. Identification of frequent and recurrent alterations of BRAF resulting in MAPK pathway activation across many PLGGs offers great potential as a therapeutic target. There are currently three drugs, which target various members of the MAPK pathway undergoing evaluation for a potential role in PLGG treatment (Figure 3). The first two agents, vemurafenib and dabrafinib are BRAF inhibitors currently in early phase clinical trials for PLGGs that harbor the BRAFV600E mutation. The BRAF inhibitor sorafenib is another commercially available albeit weak BRAF inhibitor. Based on the known MAPK feedback loops that regulate BRAF inhibition, patients with the V600E mutation, which signal as monomers, should be very sensitive to BRAF inhibitors. By contrast, when these same compounds are used to down-regulate BRAF dimers, they cause a paradoxical amplification in signaling due to these feedback loops and thus would be expected to stimulate tumor growth rather than inhibit it263,264. Treating PLGG patients with BRAF inhibitors should therefore not be undertaken until the tumor has been profiled and the appropriate targets identified. The second group, MEK1/2 inhibitor, which prevent the feedback inhibitory loop that results from BRAF targeted agents as discussed above, are currently being evaluated in early phase clinical trials for PLGGs with the BRAF duplication. The third group include the mTOR inhibitors rapamycin and everolimus which have also been used in PLGGs. Rapamycin has also been used in combination with erlotinib, an anti-EGFR agent, in a cohort of 21 progressive PLGGs, with limited clinical benefit with only 6% partial response (PR) and 35% residual disease/stable disease (RD/SD)265. Single-drug therapy using everolimus, an mTOR targeted agent, has recently been successfully used in the treatment of pediatric subependymal giant-cell astrocytomas in TSC and is now approved for this indication 266,267.
Figure 3.
Targeted therapies currently in evaluation for PLGG treatment
EGFR pathway activation in a subset of PLGG has also brought insights to evaluate anti-EGFR targeted agents in those tumors. A pilot study using Nimotozumab in 4 PLGGs reported partial responses268. The recent discovery of FGFR1 alterations in PAs and other PLGG subtypes represents another potential target in the treatment of those tumors. Preclinical and early phase trials using a FGFR1 targeted agent, dovitinib (TKI258) in FGFR1 amplified breast cancer models has already shown antitumor activity 269. Functional validation in PLGG models or in early clinical trials are needed to support the role of these genomic alterations in PLGG tumorigenesis.
Although the identification of genomic alterations represents a major milestone in the biology of PLGGs, many unanswered questions remain. Further investigation is needed to unveil the mechanisms that govern the unique clinical course of PLGGs, notably their lack of malignant transformation and quiescence after attaining adulthood. In addition to genomic alterations, epigenetic mechanisms, which vary with development, may potentially influence the growth of PLGGs. One major caveat to move forward is the lack of relevant preclinical model.
Conclusions
Low-grade gliomas, the most common brain tumor of childhood, encompass a heterogeneous group of WHO grade I and II tumors. Although they are associated with excellent overall survival rates, children can suffer morbidity from both the tumor and therapy. The striking predominance of the RAS/RAF/MAPK pathway alteration in PLGG tumorigenesis may help redefine traditional histopathological classifications and also represents exciting new avenues for the development of novel targeted therapies. Many unanswered questions remain regarding the biology of these tumors. Further analysis of the interplay between genetic, epigenetic alterations, and clinical behaviour across a larger number of PLGGs will hopefully fill some of these remaining gaps.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–11. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SHFD, Look TA, Lux S, Ginsburg D, Nathan D. Oncology of Infancy and Childhood. Philidelphia: Elseviers; 2009. [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajjar A, SR, Heideman R, Jenkins JJ, Walter A, Li Y, Langston JW, Muhlbauer M, Boyett JM, Kun LE. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15:2792–9. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 5.Qaddoumi ISI, Broniscer A. Pediatric low-grade gliomas and the need for new options for therapy: Why and how? Cancer Biol Ther. 2009;8:4–10. doi: 10.4161/cbt.8.1.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14 (Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machen SK, Prayson RA. Cyclin D1 and MIB-1 immunohistochemistry in pilocytic astrocytomas: a study of 48 cases. Hum Pathol. 1998;29:1511–6. doi: 10.1016/s0046-8177(98)90023-5. [DOI] [PubMed] [Google Scholar]
- 8.Prayson RA, Khajavi K, Comair YG. Cortical architectural abnormalities and MIB1 immunoreactivity in gangliogliomas: a study of 60 patients with intracranial tumors. J Neuropathol Exp Neurol. 1995;54:513–20. doi: 10.1097/00005072-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Prayson RA, Morris HH, Estes ML, Comair YG. Dysembryoplastic neuroepithelial tumor: a clinicopathologic and immunohistochemical study of 11 tumors including MIB1 immunoreactivity. Clin Neuropathol. 1996;15:47–53. [PubMed] [Google Scholar]
- 10.Johannessen AL, Torp SH. The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res. 2006;12:143–7. doi: 10.1007/BF02893360. [DOI] [PubMed] [Google Scholar]
- 11.Neder L, Colli BO, Machado HR, Carlotti CG, Jr, Santos AC, Chimelli L. MIB-1 labeling index in astrocytic tumors--a clinicopathologic study. Clin Neuropathol. 2004;23:262–70. [PubMed] [Google Scholar]
- 12.Roessler KBA, Jezan H, Ba-Ssalamah A, Slavc I, Czech T, Budka H. Proliferative activity as measured by MIB-1 labeling index and long-term outcome of cerebellar juvenile pilocytic astrocytomas. J Neurooncol. 2002;58:141–6. doi: 10.1023/a:1016053229688. [DOI] [PubMed] [Google Scholar]
- 13.Kayaselcuk F, Zorludemir S, Gumurduhu D, Zeren H, Erman T. PCNA and Ki-67 in central nervous system tumors: correlation with the histological type and grade. Journal of neuro-oncology. 2002;57:115–21. doi: 10.1023/a:1015739130208. [DOI] [PubMed] [Google Scholar]
- 14.Paixao Becker A, de Oliveira RS, Saggioro FP, Neder L, Chimelli LM, Machado HR. In pursuit of prognostic factors in children with pilocytic astrocytomas. Childs Nerv Syst. 2010;26:19–28. doi: 10.1007/s00381-009-0990-8. [DOI] [PubMed] [Google Scholar]
- 15.Bowers DCGL, Kapur P, Reisch JS, Mulne AF, Shapiro KN, Elterman RD, Winick NJ, Margraf LR. Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol. 2003;21:2968–73. doi: 10.1200/JCO.2003.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Margraf LRGL, Butt Y, Raghunathan N, Bowers DC. Proliferative and metabolic markers in incompletely excised pediatric pilocytic astrocytomas--an assessment of 3 new variables in predicting clinical outcome. Neuro Oncol. 2011;13:767–74. doi: 10.1093/neuonc/nor041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings TJ, Provenzale JM, Hunter SB, et al. Gliomas of the optic nerve: histological, immunohistochemical (MIB-1 and p53), and MRI analysis. Acta Neuropathol. 2000;99:563–70. doi: 10.1007/s004010051161. [DOI] [PubMed] [Google Scholar]
- 18.Faria C, Miguens J, Antunes JL, et al. Genetic alterations in a papillary glioneuronal tumor. Journal of neurosurgery Pediatrics. 2008;1:99–102. doi: 10.3171/PED-08/01/099. [DOI] [PubMed] [Google Scholar]
- 19.Czech T, Slavc I, Aichholzer M, et al. Proliferative activity as measured by MIB-1 labeling index and long-term outcome of visual pathway astrocytomas in children. J Neurooncol. 1999;42:143–50. doi: 10.1023/a:1006244724476. [DOI] [PubMed] [Google Scholar]
- 20.Dirven CMKJ, Mooij JJ, Molenaar WM. The proliferative potential of the pilocytic astrocytoma: the relation between MIB-1 labeling and clinical and neuro-radiological follow-up. J Neurooncol. 1998;37:9–16. doi: 10.1023/a:1005905009449. [DOI] [PubMed] [Google Scholar]
- 21.Tung JN, Tsao TY, Tai CJ, Yeh KT, Cheng YW, Jiang MC. Distribution of lysosome-associated membrane proteins-1 and -2, and cathepsin D in eosinophilic granular bodies: possible relationship to cyst development in pilocytic astrocytomas. J Int Med Res. 2010;38:1354–64. doi: 10.1177/147323001003800417. [DOI] [PubMed] [Google Scholar]
- 22.Berhouma M, Jemel H, Kchir N. Calcified pilocytic astrocytoma of the medulla mimicking a brainstem “:stone”. Pathologica. 2008;100:408–10. [PubMed] [Google Scholar]
- 23.Takei HYS, Wong KK, Mehta V, Chintagumpala M, Dauser RC, Lau CC, Adesina AM. Expression of oligodendroglial differentiation markers in pilocytic astrocytomas identifies two clinical subsets and shows a significant correlation with proliferation index and progression free survival. J Neurooncol. 2008;86:183–90. doi: 10.1007/s11060-007-9455-7. [DOI] [PubMed] [Google Scholar]
- 24.Otero JJRD, Vandenberg S. OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. J Neurooncol. 2011;104:423–38. doi: 10.1007/s11060-010-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka YSA, Ishiuchi S, Nakazato Y. Diversity of glial cell components in pilocytic astrocytoma. Neuropathology. 2008 Aug;28(4):399–407. doi: 10.1111/j.1440-1789.2008.00896.x. Epub 2008 Feb 26. [DOI] [PubMed] [Google Scholar]
- 26.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gullotta F, Schindler F, Schmutzler R, Weeks-Seifert A. GFAP in brain tumor diagnosis: possibilities and limitations. Pathology, research and practice. 1985;180:54–60. doi: 10.1016/S0344-0338(85)80075-3. [DOI] [PubMed] [Google Scholar]
- 28.Pfister S, Witt O. Pediatric gliomas. Recent Results Cancer Res. 2009;171:67–81. doi: 10.1007/978-3-540-31206-2_4. [DOI] [PubMed] [Google Scholar]
- 29.Zentner J, Wolf HK, Ostertun B, et al. Gangliogliomas: clinical, radiological, and histopathological findings in 51 patients. Journal of neurology, neurosurgery, and psychiatry. 1994;57:1497–502. doi: 10.1136/jnnp.57.12.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JY, Suh YL, Han J. Dysembryoplastic neuroepithelial tumor. Features distinguishing it from oligodendroglioma on cytologic squash preparations. Acta cytologica. 2003;47:624–9. doi: 10.1159/000326579. [DOI] [PubMed] [Google Scholar]
- 31.Lellouch-Tubiana A, Boddaert N, Bourgeois M, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol. 2005;15:281–6. doi: 10.1111/j.1750-3639.2005.tb00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buccoliero AM, Castiglione F, Degl’innocenti DR, et al. Angiocentric glioma: clinical, morphological, immunohistochemical and molecular features in three pediatric cases. Clin Neuropathol. 2013;32:107–13. doi: 10.5414/NP300500. [DOI] [PubMed] [Google Scholar]
- 33.Forbes JA, Mobley BC, O’Lynnger TM, et al. Pediatric cerebellar pilomyxoid-spectrum astrocytomas. Journal of neurosurgery Pediatrics. 2011;8:90–6. doi: 10.3171/2011.4.PEDS1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amatya VJ, Akazawa R, Sumimoto Y, Takeshima Y, Inai K. Clinicopathological and immunohistochemical features of three pilomyxoid astrocytomas: comparative study with 11 pilocytic astrocytomas. Pathol Int. 2009;59:80–5. doi: 10.1111/j.1440-1827.2008.02332.x. [DOI] [PubMed] [Google Scholar]
- 35.Hemmati HDNI, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–20082012. 2012. [Google Scholar]
- 37.Bleyer WA. Epidemiologic impact of children with brain tumors. Childs Nerv Syst. 1999;15:758–63. doi: 10.1007/s003810050467. [DOI] [PubMed] [Google Scholar]
- 38.Bauchet L, Rigau V, Mathieu-Daude H, et al. Clinical epidemiology for childhood primary central nervous system tumors. J Neurooncol. 2009;92:87–98. doi: 10.1007/s11060-008-9740-0. [DOI] [PubMed] [Google Scholar]
- 39.Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92:3155–64. doi: 10.1002/1097-0142(20011215)92:12<3155::aid-cncr10158>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 40.Radner H, Blumcke I, Reifenberger G, Wiestler OD. The new WHO classification of tumors of the nervous system 2000. Pathology and genetics. Der Pathologe. 2002;23:260–83. doi: 10.1007/s00292-002-0530-8. [DOI] [PubMed] [Google Scholar]
- 41.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gjerris F, Agerlin N, Borgesen SE, et al. Epidemiology and prognosis in children treated for intracranial tumours in Denmark 1960–1984. Childs Nerv Syst. 1998;14:302–11. doi: 10.1007/s003810050231. [DOI] [PubMed] [Google Scholar]
- 43.Pinho RS, Andreoni S, Silva NS, et al. Pediatric central nervous system tumors: a single-center experience from 1989 to 2009. Journal of pediatric hematology/oncology. 2011;33:605–9. doi: 10.1097/MPH.0b013e31822031d9. [DOI] [PubMed] [Google Scholar]
- 44.Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622–6. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 45.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman CR, Farmer JP, Montes J. Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys. 1998;41:979–87. doi: 10.1016/s0360-3016(98)00163-1. [DOI] [PubMed] [Google Scholar]
- 47.Rosemberg S, Fujiwara D. Epidemiology of pediatric tumors of the nervous system according to the WHO 2000 classification: a report of 1,195 cases from a single institution. Childs Nerv Syst. 2005;21:940–4. doi: 10.1007/s00381-005-1181-x. [DOI] [PubMed] [Google Scholar]
- 48.Peters O, Gnekow AK, Rating D, Wolff JE. Impact of location on outcome in children with low-grade oligodendroglioma. Pediatr Blood Cancer. 2004;43:250–6. doi: 10.1002/pbc.20111. [DOI] [PubMed] [Google Scholar]
- 49.Luyken C, Blumcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101:146–55. doi: 10.1002/cncr.20332. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery. 2003;53:544–53. doi: 10.1227/01.neu.0000079330.01541.6e. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 51.Scheinemann K, Bartels U, Huang A, et al. Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. Journal of neurosurgery Pediatrics. 2009;4:254–61. doi: 10.3171/2009.4.PEDS08411. [DOI] [PubMed] [Google Scholar]
- 52.Robertson PL, Allen JC, Abbott IR, Miller DC, Fidel J, Epstein FJ. Cervicomedullary tumors in children: a distinct subset of brainstem gliomas. Neurology. 1994;44:1798–803. doi: 10.1212/wnl.44.10.1798. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez FJ, Perry A, Gutmann DH, et al. Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–9. doi: 10.1097/NEN.0b013e318165eb75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guillamo JS, Creange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain : a journal of neurology. 2003;126:152–60. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 55.Korf BR. Malignancy in neurofibromatosis type 1. The oncologist. 2000;5:477–85. doi: 10.1634/theoncologist.5-6-477. [DOI] [PubMed] [Google Scholar]
- 56.Hernaiz Driever P, von Hornstein S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. Journal of neuro-oncology. 2010;100:199–207. doi: 10.1007/s11060-010-0159-z. [DOI] [PubMed] [Google Scholar]
- 57.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Annals of neurology. 1997;41:143–9. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 58.Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. American journal of medical genetics Part A. 2004;127A:224–9. doi: 10.1002/ajmg.a.20650. [DOI] [PubMed] [Google Scholar]
- 59.Al-Saleem T, Wessner LL, Scheithauer BW, et al. Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer. 1998;83:2208–16. [PubMed] [Google Scholar]
- 60.Hirose T, Scheithauer BW, Lopes MB, et al. Tuber and subependymal giant cell astrocytoma associated with tuberous sclerosis: an immunohistochemical, ultrastructural, and immunoelectron and microscopic study. Acta Neuropathol. 1995;90:387–99. doi: 10.1007/BF00315012. [DOI] [PubMed] [Google Scholar]
- 61.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–8. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 62.Bilginer B, Narin F, Oguz KK, Uzun S, Soylemezoglu F, Akalan N. Benign cerebellar pilocytic astrocytomas in children. Turk Neurosurg. 2011;21:22–6. [PubMed] [Google Scholar]
- 63.Gelabert-Gonzalez M, Amo JM, Arcos Algaba A, et al. Intracranial gangliogliomas. A review of a series of 20 patients. Neurologia. 2011;26:405–15. doi: 10.1016/j.nrl.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Prayson RA. Tumours arising in the setting of paediatric chronic epilepsy. Pathology. 2010;42:426–31. doi: 10.3109/00313025.2010.493870. [DOI] [PubMed] [Google Scholar]
- 65.Alexiou GA, Varela M, Sfakianos G, Prodromou N. Benign lesions accompanied by intractable epilepsy in children. J Child Neurol. 2009;24:697–700. doi: 10.1177/0883073808331079. [DOI] [PubMed] [Google Scholar]
- 66.De Rose M, Luzi M, Trignani R, et al. Cingulate epilepsy in a child with a low-grade glioma. Childs Nerv Syst. 2009;25:1507–11. doi: 10.1007/s00381-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 67.Gaggero R, Consales A, Fazzini F, et al. Epilepsy associated with supratentorial brain tumors under 3 years of life. Epilepsy research. 2009;87:184–9. doi: 10.1016/j.eplepsyres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Huber J, Sovinz P, Lackner H, Mokry M, Eder H, Urban C. Diencephalic syndrome: a frequently delayed diagnosis in failure to thrive. Klinische Padiatrie. 2007;219:91–4. doi: 10.1055/s-2007-921559. [DOI] [PubMed] [Google Scholar]
- 69.Waga S, Shimizu T, Sakakura M. Diencephalic syndrome of emaciation (Russell’s syndrome) Surgical neurology. 1982;17:141–6. doi: 10.1016/s0090-3019(82)80043-8. [DOI] [PubMed] [Google Scholar]
- 70.Perilongo G, Carollo C, Salviati L, et al. Diencephalic syndrome and disseminated juvenile pilocytic astrocytomas of the hypothalamic-optic chiasm region. Cancer. 1997;80:142–6. doi: 10.1002/(sici)1097-0142(19970701)80:1<142::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 71.Czyzyk E, Jozwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18:471–8. doi: 10.1177/08830738030180070401. [DOI] [PubMed] [Google Scholar]
- 72.Rondinelli PI, Osorio CA, Cohen MP, Novaes PE. Unusual dissemination patterns of low-grade astrocytomas in childhood. Arquivos de neuro-psiquiatria. 2008;66:45–9. doi: 10.1590/s0004-282x2008000100011. [DOI] [PubMed] [Google Scholar]
- 73.Civitello LA, Packer RJ, Rorke LB, Siegel K, Sutton LN, Schut L. Leptomeningeal dissemination of low-grade gliomas in childhood. Neurology. 1988;38:562–6. doi: 10.1212/wnl.38.4.562. [DOI] [PubMed] [Google Scholar]
- 74.Perilongo G, Garre ML, Giangaspero F. Low-grade gliomas and leptomeningeal dissemination: a poorly understood phenomenon. Childs Nerv Syst. 2003;19:197–203. doi: 10.1007/s00381-003-0733-1. [DOI] [PubMed] [Google Scholar]
- 75.Hukin JSJ, Cohen H, Velasquez L, Zagzag D, Allen J. Leptomeningeal dissemination at diagnosis of pediatric low-grade neuroepithelial tumors. Neuro Oncol. 2003;5:188–96. doi: 10.1215/S1152-8517-02-00029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazloom A, Hodges JC, Teh BS, Chintagumpala M, Paulino AC. Outcome of patients with pilocytic astrocytoma and leptomeningeal dissemination. Int J Radiat Oncol Biol Phys. 2012;84:350–4. doi: 10.1016/j.ijrobp.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 77.von Hornstein S, Kortmann RD, Pietsch T, et al. Impact of chemotherapy on disseminated low-grade glioma in children and adolescents: Report from the HIT-LGG 1996 trial. Pediatr Blood Cancer. 2011;56:1046–54. doi: 10.1002/pbc.23006. [DOI] [PubMed] [Google Scholar]
- 78.Porto L, Kieslich M, Franz K, et al. Spectroscopy of untreated pilocytic astrocytomas: do children and adults share some metabolic features in addition to their morphologic similarities? Childs Nerv Syst. 2010;26:801–6. doi: 10.1007/s00381-009-1062-9. [DOI] [PubMed] [Google Scholar]
- 79.Strong JAHHJ, Brown MT, Debatin JF, Friedman HS, Oakes WJ, Tien R. Pilocytic astrocytoma: correlation between the initial imaging features and clinical aggressiveness. AJR Am J Roentgenol. 1993;161:369–72. doi: 10.2214/ajr.161.2.8333380. [DOI] [PubMed] [Google Scholar]
- 80.Lee YY, Van Tassel P, Bruner JM, Moser RP, Share JC. Juvenile pilocytic astrocytomas: CT and MR characteristics. AJR Am J Roentgenol. 1989;152:1263–70. doi: 10.2214/ajr.152.6.1263. [DOI] [PubMed] [Google Scholar]
- 81.Seo HS, Kim JH, Lee DH, et al. Nonenhancing intramedullary astrocytomas and other MR imaging features: a retrospective study and systematic review. AJNR American journal of neuroradiology. 2010;31:498–503. doi: 10.3174/ajnr.A1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komotar RJ, Zacharia BE, Sughrue ME, et al. Magnetic resonance imaging characteristics of pilomyxoid astrocytoma. Neurological research. 2008;30:945–51. doi: 10.1179/174313208X322806. [DOI] [PubMed] [Google Scholar]
- 83.Chang SC, Lai PH, Chen WL, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clinical imaging. 2002;26:227–36. doi: 10.1016/s0899-7071(02)00436-9. [DOI] [PubMed] [Google Scholar]
- 84.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–50. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 85.Perilongo G, Moras P, Carollo C, et al. Spontaneous partial regression of low-grade glioma in children with neurofibromatosis-1: a real possibility. J Child Neurol. 1999;14:352–6. doi: 10.1177/088307389901400602. [DOI] [PubMed] [Google Scholar]
- 86.Rozen WMJS, Lo PA. Spontaneous regression of low-grade gliomas in pediatric patients without neurofibromatosis. Pediatric neurosurgery. 2008;44:324–8. doi: 10.1159/000134925. [DOI] [PubMed] [Google Scholar]
- 87.Gunny RS, Hayward RD, Phipps KP, Harding BN, Saunders DE. Spontaneous regression of residual low-grade cerebellar pilocytic astrocytomas in children. Pediatric radiology. 2005;35:1086–91. doi: 10.1007/s00247-005-1546-z. [DOI] [PubMed] [Google Scholar]
- 88.Schmandt SM, Packer RJ, Vezina LG, Jane J. Spontaneous regression of low-grade astrocytomas in childhood. Pediatric neurosurgery. 2000;32:132–6. doi: 10.1159/000028917. [DOI] [PubMed] [Google Scholar]
- 89.Kernan JC, Horgan MA, Piatt JH, D’Agostino A. Spontaneous involution of a diencephalic astrocytoma. Pediatric neurosurgery. 1998;29:149–53. doi: 10.1159/000028710. [DOI] [PubMed] [Google Scholar]
- 90.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR American journal of neuroradiology. 2001;22:1963–9. [PMC free article] [PubMed] [Google Scholar]
- 91.Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46:1652–60. doi: 10.1212/wnl.46.6.1652. [DOI] [PubMed] [Google Scholar]
- 92.Milstein JM, Geyer JR, Berger MS, Bleyer WA. Favorable prognosis for brainstem gliomas in neurofibromatosis. J Neurooncol. 1989;7:367–71. doi: 10.1007/BF02147094. [DOI] [PubMed] [Google Scholar]
- 93.Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702) Neuro Oncol. 2010;12:1257–68. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82:536–47. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- 95.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–52. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 96.Smoots DW, Geyer JR, Lieberman DM, Berger MS. Predicting disease progression in childhood cerebellar astrocytoma. Childs Nerv Syst. 1998;14:636–48. doi: 10.1007/s003810050290. [DOI] [PubMed] [Google Scholar]
- 97.El Khashab MGL, Margraf L, Koral K, Nejat F, Swift D, Weprin B, Bowers DC. Predictors of tumor progression among children with gangliogliomas. Clinical article. Journal of neurosurgery Pediatrics. 2009;3:461–6. doi: 10.3171/2009.2.PEDS0861. [DOI] [PubMed] [Google Scholar]
- 98.Bowers DC, Mulne AF, Weprin B, Bruce DA, Shapiro K, Margraf LR. Prognostic factors in children and adolescents with low-grade oligodendrogliomas. Pediatric neurosurgery. 2002;37:57–63. doi: 10.1159/000065106. [DOI] [PubMed] [Google Scholar]
- 99.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. European journal of cancer. 2006;42:1807–16. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 100.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2641–7. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42:1807–16. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Parsa CFGS. Juvenile pilocytic astrocytomas do not undergo spontaneous malignant transformation: grounds for designation as hamartomas. Br J Ophthalmol. 2008;92:40–6. doi: 10.1136/bjo.2007.125567. [DOI] [PubMed] [Google Scholar]
- 103.Zoeller GK, Brathwaite CD, Sandberg DI. Malignant transformation of an optic pathway glioma without prior radiation therapy. Journal of neurosurgery Pediatrics. 2010;5:507–10. doi: 10.3171/2009.12.PEDS09173. [DOI] [PubMed] [Google Scholar]
- 104.Unal E, Koksal Y, Cimen O, Paksoy Y, Tavli L. Malignant glioblastomatous transformation of a low-grade glioma in a child. Childs Nerv Syst. 2008;24:1385–9. doi: 10.1007/s00381-008-0716-3. [DOI] [PubMed] [Google Scholar]
- 105.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115:5761–70. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery. 2011;68:1548–54. doi: 10.1227/NEU.0b013e318214a66e. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 107.Benesch MEH, Sovinz P, Raith J, Lackner H, Moser A, Urban C. Residual or recurrent cerebellar low-grade glioma in children after tumor resection: is re-treatment needed? A single center experience from 1983 to 2003. Pediatric neurosurgery. 2006;42:159–64. doi: 10.1159/000091859. [DOI] [PubMed] [Google Scholar]
- 108.Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665–73. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 109.Uliel-Sibony S, Kramer U, Fried I, Fattal-Valevski A, Constantini S. Pediatric temporal low-grade glial tumors: epilepsy outcome following resection in 48 children. Childs Nerv Syst. 2011;27:1413–8. doi: 10.1007/s00381-011-1454-5. [DOI] [PubMed] [Google Scholar]
- 110.Teo C, Siu TL. Radical resection of focal brainstem gliomas: is it worth doing? Childs Nerv Syst. 2008;24:1307–14. doi: 10.1007/s00381-008-0647-z. [DOI] [PubMed] [Google Scholar]
- 111.Ramina RCNM, Fernandes YB, Borges G, Honorato DC, Arruda WO. Intrinsic tectal low grade astrocytomas: is surgical removal an alternative treatment? Long-term outcome of eight cases. Arquivos de neuro-psiquiatria. 2005;63:40–5. doi: 10.1590/s0004-282x2005000100008. [DOI] [PubMed] [Google Scholar]
- 112.Moghrabi A, Friedman HS, Burger PC, Tien R, Oakes WJ. Carboplatin treatment of progressive optic pathway gliomas to delay radiotherapy. J Neurosurg. 1993;79:223–7. doi: 10.3171/jns.1993.79.2.0223. [DOI] [PubMed] [Google Scholar]
- 113.Aquino VM, Fort DW, Kamen BA. Carboplatin for the treatment of children with newly diagnosed optic chiasm gliomas: a phase II study. J Neurooncol. 1999;41:255–9. doi: 10.1023/a:1006149809479. [DOI] [PubMed] [Google Scholar]
- 114.Gururangan S, Cavazos CM, Ashley D, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–8. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Hsu TR, Wong TT, Chang FC, et al. Responsiveness of progressive optic pathway tumors to cisplatin-based chemotherapy in children. Childs Nerv Syst. 2008;24:1457–61. doi: 10.1007/s00381-008-0707-4. [DOI] [PubMed] [Google Scholar]
- 116.Beaty O, 3rd, Berg S, Blaney S, et al. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55:440–5. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Friedman HS, Krischer JP, Burger P, et al. Treatment of children with progressive or recurrent brain tumors with carboplatin or iproplatin: a Pediatric Oncology Group randomized phase II study. J Clin Oncol. 1992;10:249–56. doi: 10.1200/JCO.1992.10.2.249. [DOI] [PubMed] [Google Scholar]
- 118.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–6. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 119.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–54. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 120.Demaerel P, de Ruyter N, Casteels I, Renard M, Uyttebroeck A, van Gool S. Visual pathway glioma in children treated with chemotherapy. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2002;6:207–12. doi: 10.1053/ejpn.2002.0595. [DOI] [PubMed] [Google Scholar]
- 121.Gnekow AKKR, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy -- report from the multicenter treatment study for children and adolescents with a low grade glioma -- HIT-LGG 1996 -- of the Society of Pediatric Oncology and Hematology (GPOH) Klinische Padiatrie. 2004;216:331–42. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 122.Ronghe M, Hargrave D, Bartels U, et al. Vincristine and carboplatin chemotherapy for unresectable and/or recurrent low-grade astrocytoma of the brainstem. Pediatr Blood Cancer. 2010;55:471–7. doi: 10.1002/pbc.22557. [DOI] [PubMed] [Google Scholar]
- 123.Sawamura Y, Kamoshima Y, Kato T, Tajima T, Tsubaki J. Chemotherapy with cisplatin and vincristine for optic pathway/hypothalamic astrocytoma in young children. Japanese journal of clinical oncology. 2009;39:277–83. doi: 10.1093/jjco/hyp012. [DOI] [PubMed] [Google Scholar]
- 124.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–16. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 125.Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100:65–71. doi: 10.1007/s11060-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 126.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2641–7. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Castello MASA, Padula A, Varrasso G, Properzi E, Trasimeni G, Operamolla P, Gualdi GF, Clerico A. Does chemotherapy have a role in low-grade astrocytoma management? A report of 13 cases. Med Pediatr Oncol. 1995;25:102–8. doi: 10.1002/mpo.2950250210. [DOI] [PubMed] [Google Scholar]
- 128.Yu DY, Dahl GV, Shames RS, Fisher PG. Weekly dosing of carboplatin increases risk of allergy in children. Journal of pediatric hematology/oncology. 2001;23:349–52. doi: 10.1097/00043426-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 129.Lazzareschi I, Ruggiero A, Riccardi R, Attina G, Colosimo C, Lasorella A. Hypersensitivity reactions to carboplatin in children. J Neurooncol. 2002;58:33–7. doi: 10.1023/a:1015853200090. [DOI] [PubMed] [Google Scholar]
- 130.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112:892–9. doi: 10.1002/cncr.23249. [DOI] [PubMed] [Google Scholar]
- 131.Lancaster DL, Hoddes JA, Michalski A. Tolerance of nitrosurea-based multiagent chemotherapy regime for low-grade pediatric gliomas. J Neurooncol. 2003;63:289–94. doi: 10.1023/a:1024278925822. [DOI] [PubMed] [Google Scholar]
- 132.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. Journal of neuro-oncology. 1997;32:235–41. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 133.Mishra KK, Squire S, Lamborn K, et al. Phase II TPDCV protocol for pediatric low-grade hypothalamic/chiasmatic gliomas: 15-year update. J Neurooncol. 2010;100:121–7. doi: 10.1007/s11060-010-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walter AW, Gajjar A, Reardon DA, et al. Tamoxifen and carboplatin for children with low-grade gliomas: a pilot study at St. Jude Children’s Research Hospital Journal of pediatric hematology/oncology. 2000;22:247–51. doi: 10.1097/00043426-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 135.Jakacki RI, Bouffet E, Adamson PC, et al. A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children’s Oncology Group phase 1 consortium study. Neuro Oncol. 2011;13:910–5. doi: 10.1093/neuonc/nor090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–8. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 137.Bruggers CS, Greene D. A phase 2 feasibility study of sequential, dose intensive chemotherapy to treat progressive low-grade gliomas in children. Journal of pediatric hematology/oncology. 2007;29:602–7. doi: 10.1097/MPH.0b013e318142b585. [DOI] [PubMed] [Google Scholar]
- 138.Lee MJ, Ra YS, Park JB, et al. Effectiveness of novel combination chemotherapy, consisting of 5-fluorouracil, vincristine, cyclophosphamide and etoposide, in the treatment of low-grade gliomas in children. J Neurooncol. 2006;80:277–84. doi: 10.1007/s11060-006-9185-2. [DOI] [PubMed] [Google Scholar]
- 139.Scheinemann K, Bartels U, Tsangaris E, et al. Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22917. [DOI] [PubMed] [Google Scholar]
- 140.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110:1542–50. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 141.Gururangan S, Fisher MJ, Allen JC, et al. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9:161–8. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khaw SL, Coleman LT, Downie PA, Heath JA, Ashley DM. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49:808–11. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 143.Bartels UBS, Carret AS, Crooks B, Hukin J, Johnston D, Silva M, Strother D, Wilson B, Zelcer S, Eisenstat D, Sung L, Bouffet E. The use and effectiveness of temozolomide in children with central nervous system tumours: a survey from the Canadian Paediatric Brain Tumour Consortium. Curr Oncol. 2011;18:e19–24. doi: 10.3747/co.v18i1.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636–42. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 145.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–63. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 146.Singh G, Wei XC, Hader W, Chan JA, Bouffet E, Lafay-Cousin L. Sustained Response to Weekly Vinblastine in 2 Children With Pilomyxoid Astrocytoma Associated With Diencephalic Syndrome. Journal of pediatric hematology/oncology. 2012 doi: 10.1097/MPH.0b013e3182707e67. [DOI] [PubMed] [Google Scholar]
- 147.Kadota RP, Kun LE, Langston JW, et al. Cyclophosphamide for the treatment of progressive low-grade astrocytoma: a Pediatric Oncology Group phase II Study. Journal of pediatric hematology/oncology. 1999;21:198–202. doi: 10.1097/00043426-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 148.Pons MA, Finlay JL, Walker RW, Puccetti D, Packer RJ, McElwain M. Chemotherapy with vincristine (VCR) and etoposide (VP-16) in children with low-grade astrocytoma. J Neurooncol. 1992;14:151–8. doi: 10.1007/BF00177619. [DOI] [PubMed] [Google Scholar]
- 149.Sumer T, Freeman AI, Cohen M, Bremer AM, Thomas PR, Sinks LF. Chemotherapy in recurrent noncystic low-grade astrocytomas of the cerebrum in children. Journal of surgical oncology. 1978;10:45–54. doi: 10.1002/jso.2930100108. [DOI] [PubMed] [Google Scholar]
- 150.Rosenstock JG, Evans AE, Schut L. Response to vincristine of recurrent brain tumors in children. J Neurosurg. 1976;45:135–40. doi: 10.3171/jns.1976.45.2.0135. [DOI] [PubMed] [Google Scholar]
- 151.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83:583–9. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 152.Heideman RL, Douglass EC, Langston JA, et al. A phase II study of every other day high-dose ifosfamide in pediatric brain tumors: a Pediatric Oncology Group Study. J Neurooncol. 1995;25:77–84. doi: 10.1007/BF01054726. [DOI] [PubMed] [Google Scholar]
- 153.McCowage G, Tien R, McLendon R, et al. Successful treatment of childhood pilocytic astrocytomas metastatic to the leptomeninges with high-dose cyclophosphamide. Med Pediatr Oncol. 1996;27:32–9. doi: 10.1002/(SICI)1096-911X(199607)27:1<32::AID-MPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 154.Disabato JAHM, Strain JD, Fleitz JM, Foreman NK. Successful use of intracavitary bleomycin for low-grade astrocytoma tumor cyst. Pediatric neurosurgery. 1999;31:246–50. doi: 10.1159/000028871. [DOI] [PubMed] [Google Scholar]
- 155.Blaney SM, Phillips PC, Packer RJ, et al. Phase II evaluation of topotecan for pediatric central nervous system tumors. Cancer. 1996;78:527–31. doi: 10.1002/(SICI)1097-0142(19960801)78:3<527::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 156.Dreyer ZE, Kadota RP, Stewart CF, et al. Phase 2 study of idarubicin in pediatric brain tumors: Pediatric Oncology Group study POG 9237. Neuro Oncol. 2003;5:261–7. doi: 10.1215/S115285170200056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Warren KE, Goldman S, Pollack IF, et al. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol. 2011;29:324–9. doi: 10.1200/JCO.2010.31.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791–5. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 159.Couec ML, Andre N, Thebaud E, et al. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: toxicity and efficacy trends. Pediatr Blood Cancer. 2012;59:34–8. doi: 10.1002/pbc.24066. [DOI] [PubMed] [Google Scholar]
- 160.Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2013 doi: 10.1093/neuonc/not154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Annals of neurology. 1999;45:393–6. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 162.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–34. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68–78. [PubMed] [Google Scholar]
- 164.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–7. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nishihori T, Shirato H, Aoyama H, et al. Three-dimensional conformal radiotherapy for astrocytic tumors involving the eloquent area in children and young adults. J Neurooncol. 2002;60:177–83. doi: 10.1023/a:1020617717664. [DOI] [PubMed] [Google Scholar]
- 167.Saran FH, Baumert BG, Khoo VS, et al. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys. 2002;53:43–51. doi: 10.1016/s0360-3016(02)02734-7. [DOI] [PubMed] [Google Scholar]
- 168.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61:374–9. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 169.Roberge DSL, Olivier A, Leblanc R, Podgorsak E. Hypofractionated stereotactic radiotherapy for low grade glioma at McGill University: long-term follow-up. Technol Cancer Res Treat. 2006;5:1–8. doi: 10.1177/153303460600500101. [DOI] [PubMed] [Google Scholar]
- 170.Henderson MA, Fakiris AJ, Timmerman RD, Worth RM, Lo SS, Witt TC. Gamma knife stereotactic radiosurgery for low-grade astrocytomas. Stereotactic and functional neurosurgery. 2009;87:161–7. doi: 10.1159/000209297. [DOI] [PubMed] [Google Scholar]
- 171.Wang LW, Shiau CY, Chung WY, et al. Gamma Knife surgery for low-grade astrocytomas: evaluation of long-term outcome based on a 10-year experience. J Neurosurg. 2006;105 (Suppl):127–32. doi: 10.3171/sup.2006.105.7.127. [DOI] [PubMed] [Google Scholar]
- 172.Kida Y, Kobayashi T, Mori Y. Gamma knife radiosurgery for low-grade astrocytomas: results of long-term follow up. J Neurosurg. 2000;93 (Suppl 3):42–6. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 173.Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2002;178:10–7. doi: 10.1007/s00066-002-0874-2. [DOI] [PubMed] [Google Scholar]
- 174.Kuhlthau KA, Pulsifer MB, Yeap BY, et al. Prospective study of health-related quality of life for children with brain tumors treated with proton radiotherapy. J Clin Oncol. 2012;30:2079–86. doi: 10.1200/JCO.2011.37.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nature reviews Genetics. 2010;11:685–96. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 176.Bigner SH, McLendon RE, Fuchs H, McKeever PE, Friedman HS. Chromosomal characteristics of childhood brain tumors. Cancer Genet Cytogenet. 1997;97:125–34. doi: 10.1016/s0165-4608(96)00404-9. [DOI] [PubMed] [Google Scholar]
- 177.Rey JA, Bello MJ, de Campos JM, Kusak ME, Moreno S. Chromosomal composition of a series of 22 human low-grade gliomas. Cancer Genet Cytogenet. 1987;29:223–37. doi: 10.1016/0165-4608(87)90233-0. [DOI] [PubMed] [Google Scholar]
- 178.Bhattacharjee MB, Armstrong DD, Vogel H, Cooley LD. Cytogenetic analysis of 120 primary pediatric brain tumors and literature review. Cancer Genet Cytogenet. 1997;97:39–53. doi: 10.1016/s0165-4608(96)00330-5. [DOI] [PubMed] [Google Scholar]
- 179.Griffin CA, Long PP, Carson BS, Brem H. Chromosome abnormalities in low-grade central nervous system tumors. Cancer Genet Cytogenet. 1992;60:67–73. doi: 10.1016/0165-4608(92)90235-z. [DOI] [PubMed] [Google Scholar]
- 180.Neumann EKD, Norman MG, Steinbok P, Cochrane DD, Goddard K. Cytogenetic analysis of 109 pediatric central nervous system tumors. Cancer Genet Cytogenet. 1993;71:40–9. doi: 10.1016/0165-4608(93)90200-6. [DOI] [PubMed] [Google Scholar]
- 181.White FV, Anthony DC, Yunis EJ, Tarbell NJ, Scott RM, Schofield DE. Nonrandom chromosomal gains in pilocytic astrocytomas of childhood. Hum Pathol. 1995;26:979–86. doi: 10.1016/0046-8177(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 182.Zattara-Cannoni HGD, Lena G, Dufour H, Choux M, Grisoli F, Vagner-Capodano AM. Are juvenile pilocytic astrocytomas benign tumors? A cytogenetic study in 24 cases. Cancer Genet Cytogenet. 1998;104:157–60. doi: 10.1016/s0165-4608(97)00455-x. [DOI] [PubMed] [Google Scholar]
- 183.Karnes PS, Tran TN, Cui MY, et al. Cytogenetic analysis of 39 pediatric central nervous system tumors. Cancer Genet Cytogenet. 1992;59:12–9. doi: 10.1016/0165-4608(92)90150-7. [DOI] [PubMed] [Google Scholar]
- 184.Neumann E, Kalousek DK, Norman MG, Steinbok P, Cochrane DD, Goddard K. Cytogenetic analysis of 109 pediatric central nervous system tumors. Cancer Genet Cytogenet. 1993;71:40–9. doi: 10.1016/0165-4608(93)90200-6. [DOI] [PubMed] [Google Scholar]
- 185.Willert JR, Daneshvar L, Sheffield VC, Cogen PH. Deletion of chromosome arm 17p DNA sequences in pediatric high-grade and juvenile pilocytic astrocytomas. Genes, chromosomes & cancer. 1995;12:165–72. doi: 10.1002/gcc.2870120303. [DOI] [PubMed] [Google Scholar]
- 186.Sawyer JR, Thomas JR, Teo C. Low-grade astrocytoma with a complex four-breakpoint inversion of chromosome 8 as the sole cytogenetic aberration. Cancer Genet Cytogenet. 1995;83:168–71. doi: 10.1016/0165-4608(95)00074-y. [DOI] [PubMed] [Google Scholar]
- 187.Orr LC, Fleitz J, McGavran L, Wyatt-Ashmead J, Handler M, Foreman NK. Cytogenetics in pediatric low-grade astrocytomas. Med Pediatr Oncol. 2002;38:173–7. doi: 10.1002/mpo.1305. [DOI] [PubMed] [Google Scholar]
- 188.Makar AB, McMartin KE, Palese M, Tephly TR. Formate assay in body fluids: application in methanol poisoning. Biochemical medicine. 1975;13:117–26. doi: 10.1016/0006-2944(75)90147-7. [DOI] [PubMed] [Google Scholar]
- 189.Smith JSPA, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–45. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 190.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neuro Oncol. 2007;9:113–23. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Suri VJP, Agarwal S, Pathak P, Sharma MC, Sharma V, Shukla S, Somasundaram K, Mahapatra AK, Kale SS, Sarkar C. Molecular profile of oligodendrogliomas in young patients. Neuro Oncol. 2011;13:1099–106. doi: 10.1093/neuonc/nor146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kreiger PA, Okada Y, Simon S, Rorke LB, Louis DN, Golden JA. Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol. 2005;109:387–92. doi: 10.1007/s00401-004-0976-2. [DOI] [PubMed] [Google Scholar]
- 193.Huang H, Okamoto Y, Yokoo H, et al. Gene expression profiling and subgroup identification of oligodendrogliomas. Oncogene. 2004;23:6012–22. doi: 10.1038/sj.onc.1207781. [DOI] [PubMed] [Google Scholar]
- 194.Pollack IF, Finkelstein SD, Burnham J, et al. Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res. 2001;61:7404–7. [PubMed] [Google Scholar]
- 195.Litofsky NS, Hinton D, Raffel C. The lack of a role for p53 in astrocytomas in pediatric patients. Neurosurgery. 1994;34:967–72. doi: 10.1227/00006123-199406000-00003. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 196.MacConaill LE, Campbell CD, Kehoe SM, et al. Profiling critical cancer gene mutations in clinical tumor samples. PloS one. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patt S, Gries H, Giraldo M, et al. p53 gene mutations in human astrocytic brain tumors including pilocytic astrocytomas. Hum Pathol. 1996;27:586–9. doi: 10.1016/s0046-8177(96)90166-5. [DOI] [PubMed] [Google Scholar]
- 198.Paulus W, Lisle DK, Tonn JC, et al. Molecular genetic alterations in pleomorphic xanthoastrocytoma. Acta Neuropathol. 1996;91:293–7. doi: 10.1007/s004010050428. [DOI] [PubMed] [Google Scholar]
- 199.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miwa THY, Sasaki H, Ezaki T, Yoshida K, Kawase T. Single-copy gain of chromosome 1q is a negative prognostic marker in pediatric nonependymal, nonpilocytic gliomas. Neurosurgery. 2011;68:206–12. doi: 10.1227/NEU.0b013e3181fd2c2e. [DOI] [PubMed] [Google Scholar]
- 201.Padovani L, Colin C, Fernandez C, et al. Search for distinctive markers in DNT and cortical grade II glioma in children: same clinicopathological and molecular entities? Current topics in medicinal chemistry. 2012;12:1683–92. doi: 10.2174/156802612803531450. [DOI] [PubMed] [Google Scholar]
- 202.Ballester R, Marchuk D, Boguski M, et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63:851–9. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- 203.Yunoue S, Tokuo H, Fukunaga K, et al. Neurofibromatosis type I tumor suppressor neurofibromin regulates neuronal differentiation via its GTPase-activating protein function toward Ras. J Biol Chem. 2003;278:26958–69. doi: 10.1074/jbc.M209413200. [DOI] [PubMed] [Google Scholar]
- 204.Deshmukh H, Yeh TH, Yu J, et al. High-resolution, dual-platform aCGH analysis reveals frequent HIPK2 amplification and increased expression in pilocytic astrocytomas. Oncogene. 2008;27:4745–51. doi: 10.1038/onc.2008.110. [DOI] [PubMed] [Google Scholar]
- 205.Hoischen A, Ehrler M, Fassunke J, et al. Comprehensive characterization of genomic aberrations in gangliogliomas by CGH, array-based CGH and interphase FISH. Brain Pathol. 2008;18:326–37. doi: 10.1111/j.1750-3639.2008.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jacob KAS, Sollier C, Faury D, Sader E, Montpetit A, Serre D, Hauser P, Garami M, Bognar L, Hanzely Z, Montes JL, Atkinson J, Farmer JP, Bouffet E, Hawkins C, Tabori U, Jabado N. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–33. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–49. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–58. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. The Journal of pathology. 2009;218:172–81. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 210.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jones DTKS, Liu L, Pearson DM, Ichimura K, Collins VP. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–23. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121:763–74. doi: 10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 213.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nature genetics. 2013;45:602–12. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jones DT, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature genetics. 2013;45:927–32. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lawson AR, Hindley GF, Forshew T, et al. RAF gene fusion breakpoints in pediatric brain tumors are characterized by significant enrichment of sequence microhomology. Genome research. 2011;21:505–14. doi: 10.1101/gr.115782.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–7. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–90. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 218.Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Molecular cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takahashi Y, Mori J, Kami M. BRAF mutations in hairy-cell leukemia. The New England journal of medicine. 2011;365:960–1. doi: 10.1056/NEJMc1108130. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 220.Yeo YH, Byrne NP, Counelis GJ, Perry A. Adult with cerebellar anaplastic pilocytic astrocytoma associated with BRAF V600E mutation and p16 loss. Clin Neuropathol. 2013;32:159–64. doi: 10.5414/NP300564. [DOI] [PubMed] [Google Scholar]
- 221.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014 doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schiffman JDHJ, VandenBerg SR, Flaherty P, Polley MY, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger MS, Ji H, Gutmann DH, James CD. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–9. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12:621–30. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dias-Santagata D, Lam Q, Vernovsky K, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PloS one. 2011;6:e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 226.Craig Horbinski MNN, Hagenkord Jill M, Hamilton Ronald L, Pollack aIF. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol. 2012 doi: 10.1093/neuonc/nos077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Raabe EHLK, Kim JM, Meeker A, Mao XG, Nikkhah G, Maciaczyk J, Kahlert U, Jain D, Bar E, Cohen KJ, Eberhart CG. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:3590–9. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sharma MK, Zehnbauer BA, Watson MA, Gutmann DH. RAS pathway activation and an oncogenic RAS mutation in sporadic pilocytic astrocytoma. Neurology. 2005;65:1335–6. doi: 10.1212/01.wnl.0000180409.78098.d7. [DOI] [PubMed] [Google Scholar]
- 229.Janzarik WGKC, Loges NT, Olbrich H, Klein C, Schäfer T, Scheurlen W, Roggendorf W, Weiller C, Niemeyer C, Korinthenberg R, Pfister S, Omran H. Further evidence for a somatic KRAS mutation in a pilocytic astrocytoma. Neuropediatrics. 2007;38:61–3. doi: 10.1055/s-2007-984451. [DOI] [PubMed] [Google Scholar]
- 230.Kaul A, Chen YH, Emnett RJ, Dahiya S, Gutmann DH. Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26:2561–6. doi: 10.1101/gad.200907.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li X, Newbern JM, Wu Y, et al. MEK Is a Key Regulator of Gliogenesis in the Developing Brain. Neuron. 2012;75:1035–50. doi: 10.1016/j.neuron.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tabori U, Rienstein S, Dromi Y, et al. Epidermal growth factor receptor gene amplification and expression in disseminated pediatric low-grade gliomas. J Neurosurg. 2005;103:357–61. doi: 10.3171/ped.2005.103.4.0357. [DOI] [PubMed] [Google Scholar]
- 233.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 234.Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009;28:3468–76. doi: 10.1038/onc.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rush SZ, Abel TW, Valadez JG, Pearson M, Cooper MK. Activation of the Hedgehog pathway in pilocytic astrocytomas. Neuro Oncol. 2010;12:790–8. doi: 10.1093/neuonc/noq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sie M, de Bont ES, Scherpen FJ, Hoving EW, den Dunnen WF. Tumour vasculature and angiogenic profile of paediatric pilocytic astrocytoma; is it much different from glioblastoma? Neuropathol Appl Neurobiol. 2010;36:636–47. doi: 10.1111/j.1365-2990.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 237.Tatevossian RG, Tang B, Dalton J, et al. MYB upregulation and genetic aberrations in a subset of pediatric low-grade gliomas. Acta Neuropathol. 2010;120:731–43. doi: 10.1007/s00401-010-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Clappier ECW, Kalota A, Crinquette A, Cayuela JM, Dik WA, Langerak AW, Montpellier B, Nadel B, Walrafen P, Delattre O, Aurias A, Leblanc T, Dombret H, Gewirtz AM, Baruchel A, Sigaux F, Soulier J. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110:1251–61. doi: 10.1182/blood-2006-12-064683. [DOI] [PubMed] [Google Scholar]
- 239.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nature genetics. 2007;39:593–5. doi: 10.1038/ng2025. [DOI] [PubMed] [Google Scholar]
- 240.Kauraniemi P, Hedenfalk I, Persson K, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60:5323–8. [PubMed] [Google Scholar]
- 241.Wallrapp CM-PF, Solinas-Toldo S, Lichter P, Friess H, Büchler M, Fink T, Adler G, Gress TM. Characterization of a high copy number amplification at 6q24 in pancreatic cancer identifies c-myb as a candidate oncogene. Cancer Res. 1997;57:3135–9. [PubMed] [Google Scholar]
- 242.Ramkissoon LA, Horowitz PM, Craig JM, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8188–93. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nature medicine. 2011;17:330–9. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 244.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 245.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 246.Corn PGKS, van Noesel MM, Esteller M, Compitello N, Baylin SB, Herman JG. Transcriptional silencing of the p73 gene in acute lymphoblastic leukemia and Burkitt’s lymphoma is associated with 5′ CpG island methylation. Cancer Res. 1999;59:3352–6. [PubMed] [Google Scholar]
- 247.Kuerbitz SJ, Pahys J, Wilson A, Compitello N, Gray TA. Hypermethylation of the imprinted NNAT locus occurs frequently in pediatric acute leukemia. Carcinogenesis. 2002;23:559–64. doi: 10.1093/carcin/23.4.559. [DOI] [PubMed] [Google Scholar]
- 248.Wagner KJ, Cooper WN, Grundy RG, et al. Frequent RASSF1A tumour suppressor gene promoter methylation in Wilms’ tumour and colorectal cancer. Oncogene. 2002;21:7277–82. doi: 10.1038/sj.onc.1205922. [DOI] [PubMed] [Google Scholar]
- 249.Choy KW, Pang CP, To KF, Yu CB, Ng JS, Lam DS. Impaired expression and promotor hypermethylation of O6-methylguanine-DNA methyltransferase in retinoblastoma tissues. Investigative ophthalmology & visual science. 2002;43:1344–9. [PubMed] [Google Scholar]
- 250.Wong IH, Chan J, Wong J, Tam PK. Ubiquitous aberrant RASSF1A promoter methylation in childhood neoplasia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:994–1002. doi: 10.1158/1078-0432.ccr-0378-3. [DOI] [PubMed] [Google Scholar]
- 251.Jones DT, Jager N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kieran MW, Roberts CW, Chi SN, et al. Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59:1155–7. doi: 10.1002/pbc.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hasselblatt M, Isken S, Linge A, et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes, chromosomes & cancer. 2013;52:185–90. doi: 10.1002/gcc.22018. [DOI] [PubMed] [Google Scholar]
- 254.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3. 3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 255.Yu J, Deshmukh H, Gutmann RJ, et al. Alterations of BRAF and HIPK2 loci predominate in sporadic pilocytic astrocytoma. Neurology. 2009;73:1526–31. doi: 10.1212/WNL.0b013e3181c0664a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Melendez B, Fiano C, Ruano Y, Hernandez-Moneo JL, Mollejo M, Martinez P. BCR gene disruption in a pilomyxoid astrocytoma. Neuropathology. 2006;26:442–6. doi: 10.1111/j.1440-1789.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 257.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4790–8. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 258.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549: BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jacob K, Quang-Khuong DA, Jones DT, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4650–60. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 260.Reddy JP, Li Y. Oncogene-induced senescence and its role in tumor suppression. Journal of mammary gland biology and neoplasia. 2011;16:247–56. doi: 10.1007/s10911-011-9221-5. [DOI] [PubMed] [Google Scholar]
- 261.Horbinski CNM, Hagenkord JM, Hamilton RL, Pollack IF. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol. 2012;14:777–89. doi: 10.1093/neuonc/nos077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Dahiya S, Haydon DH, Alvarado D, Gurnett CA, Gutmann DH, Leonard JR. BRAF(V600E) mutation is a negative prognosticator in pediatric ganglioglioma. Acta Neuropathol. 2013;125:901–10. doi: 10.1007/s00401-013-1120-y. [DOI] [PubMed] [Google Scholar]
- 263.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5957–62. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yalon M, Rood B, MacDonald TJ, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr Blood Cancer. 2013;60:71–6. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- 266.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. The New England journal of medicine. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 267.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–32. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 268.Saurez G, Cabanas R, Zaldivar M, et al. Clinical experience with nimotuzumab in cuban pediatric patients with brain tumors, 2005 to 2007. MEDICC review. 2009;11:27–33. doi: 10.37757/MR2009V11.N3.7. [DOI] [PubMed] [Google Scholar]
- 269.Andre F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3693–702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]