Abstract
Psychostimulant withdrawal leads to depressive symptoms, such as anhedonia and social dysfunction. We determined the effects of withdrawal from chronic exposure to nicotine (9 mg/kg/day salt, 28 days) or amphetamine (10 mg/kg/day salt, 7 days) on the motivated response for a sucrose reward and on social interaction in rats. Both nicotine and amphetamine exposure increased the motivated response for sucrose. However, only spontaneous amphetamine withdrawal led to an immediate and persistent decrease in motivated behavior, which was not correlated with body weight loss. Social interaction was not affected during withdrawal from either drug. These results indicate that withdrawal from chronic amphetamine exposure leads to an immediate and enduring anhedonic state.
Keywords: psychostimulant, dependence, depression, reward, anhedonia, motivation
1. INTRODUCTION
Withdrawal from chronic psychostimulant drug exposure leads to a series of physiological and affective responses. Physiological effects include increased hypothalamic-pituitary-adrenal axis activity, decreased anterior cingulate activity, and increased amygdala activity (Vescovi et al., 1992; London et al., 2004; London et al., 2005). Affective withdrawal effects include dysphoria, anhedonia (i.e., decreased interest in pleasurable or rewarding stimuli), irritability, amotivation, and social dysfunction (American Psychiatric Association, 1994; Brady et al., 2007; Leventhal et al., 2008). These physiological and affective responses are analogous to those exhibited by patients with major depressive disorder. Furthermore, considerable evidence indicates that withdrawal from chronic exposure to psychostimulants may induce a depressive state (for reviews, see Markou et al., 1998; Barr and Markou, 2005). Indeed, the rate of depression among people with substance abuse disorder involving psychostimulants is higher than the general population (Kosten et al., 1998; Leventhal et al., 2008). Nevertheless, because of the high comorbidity of depression and drug dependence, it is unclear whether the drug dependence induces a state of depression or the depression leads to drug dependence. Because of these similarities in drug- and non-drug-induced depressions, pharmacological treatment of withdrawal often includes the use of antidepressants or medications that elevate monoamine levels, an effect hypothesized to be critically involved in the mode of action of many currently available antidepressant medications (for review, see Barr and Markou, 2005; Kampman, 2008).
The similarities between the symptoms of withdrawal and depression in humans have led to the utilization of drug withdrawal as a manipulation to induce depression-like signs, such as anhedonia, in experimental animals (Markou and Kenny, 2002). Withdrawal signs that are similar to depressive symptoms in humans are observed in experimental animals experiencing spontaneous or antagonist-precipitated withdrawal from a number of psychostimulant drugs. For example, during withdrawal from chronic exposure to amphetamine, rats exhibit decreased brain reward function (Paterson et al., 2000; Cryan et al., 2003) and shifts in successive positive and negative contrast effects, whereby changes in the reward value of a stimulus do not elicit appropriate changes in behavior toward that stimulus (e.g., an increase in reward value failing to increase subsequent responding for that reward) (Barr and Phillips, 2002; Vacca and Phillips, 2005). Similarly, withdrawal from chronic exposure to nicotine results in decreased brain reward function (Epping-Jordan et al., 1998) and decreased novelty reward (Besheer and Bevins, 2003).
Anhedonia is also assessed by measuring how motivated an animal is to obtain a rewarding stimulus. Operant responding for a sucrose reward on a progressive-ratio (PR) reinforcement schedule reflects an animal’s motivated behavior toward that reward (Hodos, 1961). In this schedule, the response requirements to receive a single pellet are progressively increased until the rat ceases to respond. The highest ratio the rat attains is called the breakpoint. Decreases in breakpoints reflect diminished interest and decreased motivation to work for an appetitive stimulus (i.e., anhedonia). Few studies have assessed motivated behavior in rats experiencing amphetamine withdrawal. Most reports indicate that amphetamine withdrawal reduces motivated responding for palatable rewards such as food pellets or sucrose solutions (Barr and Phillips, 1999; Orsini et al., 2001; Schwabe and Koch, 2007), although others have failed to find an effect of amphetamine withdrawal on this and other depression-related measures (Russig et al., 2003). Notably, all of these studies utilized a similar administration regimen of multiple daily injections of escalating doses. Only one study demonstrated that nicotine withdrawal decreased motivated behavior for sucrose in rats (LeSage et al., 2006), albeit at a much higher dose than typically reported to induce other depression-like behaviors, such as elevations in intracranial self-stimulation (ICSS) thresholds (Epping-Jordan et al., 1998; Skjei and Markou, 2003). Withdrawal from lower nicotine doses may be sufficient to elicit certain anhedonic responses (e.g., deficits in brain reward function), but not others (e.g., motivated responding for a food reward).
Importantly, few studies conduct PR experiments without exposing the animals to some form of food or water deprivation prior to the test session to increase motivation toward the food (sucrose pellet) or liquid (sucrose solution) reward. All of the studies mentioned above used food or water deprivation for this purpose. Deprivation introduces an additional motivational factor (i.e., hunger or thirst) that may confound or mask deficits in motivation to approach and obtain a stimulus that is rewarding in the absence of any primary survival need. Thus, identifying reward-directed motivated responses without the additional confound introduced by depriving the animal of food or water is important because such measures would have good translational value for the anhedonia experienced by humans undergoing psychostimulant withdrawal or suffering from a major depressive episode.
Drug withdrawal and depression are also both associated with social anxiety disorder, which is defined as a persistent fear of social situations in which the person is exposed to unfamiliar people and the feared situations are either avoided or endured with intense anxiety and distress (American Psychiatric Association, 1994; Brady et al., 2007). Naive rats will readily engage in a series of social behaviors when introduced to a novel rat in a non-threatening environment. These behaviors include sniffing, licking, and grooming. Stressors that induce depression-like behaviors in rats have also been shown to decrease social interaction (Short and Maier, 1993; File and Seth, 2003; Christianson et al., 2008). The effects of drug withdrawal on social interaction have also been investigated, although the results are mixed. The few reports with nicotine indicate that withdrawal from a 7 or 14 day, low dose (0.1 mg/kg/day, salt) nicotine regimen decreases social interaction (Irvine et al., 1999), but 28 day exposure to 0.45 mg/kg/day (salt) fails to alter these behaviors (Irvine et al., 2001b). Previous studies have indicated that withdrawal from both 7 and 28 day exposure to higher doses of nicotine induce depression-like behaviors (Skjei and Markou, 2003). Thus, withdrawal from higher doses of nicotine may also elicit deficits in social interaction. Withdrawal from amphetamine does not affect social interaction when assessed 12 weeks after termination of drug exposure (Morley et al., 2001), which is long after the immediate time point when other consequences of withdrawal (e.g., anhedonia) are pronounced (Paterson et al., 2000). It is unclear whether withdrawal from psychostimulants at doses and exposure times that produce anhedonia will also produce social anxiety.
The aim of the present study was to determine the effect of withdrawal from amphetamine and nicotine, two psychostimulant drugs known to induce depression-like behaviors during withdrawal, on the motivated response for a sucrose reward and on social interaction. To address the concern of the confounding effects of food or water deprivation on motivated responding in the PR test, the present studies were conducted under no food or water deprivation conditions, and the rats responded for a highly palatable sucrose pellet that elicited responding in the absence of food deprivation.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Forty-six adult male Wistar rats (Charles River Laboratories, Raleigh, NC) weighing 275–300 g at the beginning of the experiment were housed in pairs in Plexiglas cages with food and water available ad libitum. The rats were maintained in a climate-controlled colony room at 21°C on a 12 h/12 h reverse light/dark cycle. All experiments were conducted during the dark phase (8:00 h – 20:00 h) under dim red lighting. All rats were naive and allowed a minimum of 1 week adaptation to the housing rooms prior to the initiation of testing. All procedures were conducted in accordance with the guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and were approved by the university Institutional Animal Care and Use Committee.
2.2. Drugs
(−)Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) and D-amphetamine sulfate (Sigma, St. Louis, MO) were dissolved in sterile 0.9% saline and administered subcutaneously via osmotic minipumps (Alzet Osmotic Pumps, Cupertino, CA).
2.3. Apparatus
2.3.1. Progressive-ratio
Progressive-ratio testing was conducted in Plexiglas self-administration chambers with metal grid floors (25 × 31 × 24 cm), each enclosed in a sound-attenuated box (Med Associates, St. Albans, VT). One wall of each chamber was equipped with two levers measuring 3 cm wide and located 3 cm above the floor with a light above each lever and a food tray between the levers. All programs and data collection were controlled by a computer running MED-PC IV software (Med Associates, St. Albans, VT).
2.3.2. Social interaction
Tests for social interaction were conducted in novel Plexiglas housing cages measuring 25.9 × 47.6 × 20.9 cm.
2.4. Procedure
2.4.1. Osmotic minipump surgeries
Rats were anesthetized with isoflurane and prepared for surgery using aseptic procedures. A 2 cm lateral incision was made in either the right or left flank, and each minipump was placed in the subcutaneous space caudal to the incision and parallel to the spine. The incision was then closed using 9 mm stainless steel wound clips (Becton Dickinson Primary Care Diagnostics, Sparks, MD) and treated with topical antibiotic (bacitracin) ointment.
Upon minipump removal, rats were anesthetized again, the wound clips were removed, and an incision was made over the previous incision. The minipumps were removed, and the incision was closed using wound clips and treated with bacitracin.
2.4.2. Progressive-ratio testing
Training sessions were initiated at 8:00 h for half of the rats and at 13:00 h for the other half. Body weights were recorded daily immediately prior to each testing session. Rats were trained on a fixed-ratio 1 schedule of reinforcement with a 1 s time-out period (FR1 TO1 s) to respond for a 45 mg sucrose pellet (Newco Distributors, Rancho Cucamonga, CA). During this training period, rats were limited to 20 g of food per day. After each successful response on the active lever, the light above the lever was illuminated for the duration of the time-out period, and a single pellet was dispensed into the food tray. Training lasted for 1 h per day. After rats had successfully acquired this procedure (defined as 100 pellets earned during each session), training was progressed to an FR2 TO10 s schedule of reinforcement, and rats were returned to an ad libitum feeding schedule. After successful acquisition of this procedure, rats progressed to an FR5 TO20 s schedule and were maintained on this schedule until responding was consistent for 3 consecutive days, defined as < 20% variability in response rates between the three sessions. Rats were then trained on a PR schedule with a 20 s timeout period. The PR was based on the formula (5e[(pellet#+2)/4])−6. That is, the progression of required lever presses to earn one sucrose pellet was 5, 8, 11, 16, 23, 31, 41, 55, 72, 94, 123, 160, 207, 267, 345, etc. Each session lasted until the rat failed to respond a sufficient number of times to receive a sucrose pellet during a 60 min period. The final ratio attained during each session was defined as the breakpoint. The maximum session time was set at 6 h, although no rats reached this time limit.
Once baseline responding (i.e., breakpoints) was stable over 3 consecutive days, rats were randomly assigned to one of two treatment groups, drug or vehicle. Rats were prepared with an osmotic minipump that delivered nicotine solution (9 mg/kg/day, salt, 60 μl/day, 28 days; Alzet minipump model 2ML4, Alzet Osmotic Pumps, Cupertino, CA), amphetamine solution (10 mg/kg/day, salt, 235.2 μl/d, 7 days; Alzet minipump model 2ML1), or saline (28 or 7 days, respectively). The concentration of each solution was adjusted based on each rat’s body weight to equalize the dose administered. Due to the different lengths of drug exposure periods, nicotine-treated rats were tested once every 4 days and amphetamine-treated rats were tested daily for their PR responses, such that both groups were exposed to equal amounts of PR testing (i.e., 7 sessions) prior to the withdrawal period. Minipumps were removed after 28 days of exposure for the nicotine/vehicle-treated rats and after 7 days of exposure for the amphetamine/vehicle-treated rats. Nicotine/vehicle-treated rats were tested 24 h after minipump removal, and amphetamine-treated rats were tested 12 h after minipump removal. Nicotine and amphetamine doses, the duration of exposure to each drug, and the time point for the initial withdrawal test were selected based on prior work indicating that these parameters were effective in producing and detecting peak withdrawal-induced anhedonia measured by somatic signs and elevations in ICSS thresholds (Paterson et al., 2000; Skjei and Markou, 2003). Thereafter, nicotine/vehicle-treated rats were tested daily every 24 h for 9 days, and amphetamine/vehicle-treated rats were tested daily every 24 h for 2 weeks and once every 3 days for an additional 2 weeks. Testing of nicotine/vehicle-treated rats was terminated after 9 days due to the lack of significant differences in ICSS thresholds by this time point (see below).
2.4.3. Social interaction testing
Immediately after each of the last three PR baseline testing sessions, rats were individually placed into a novel housing cage for 15 min to habituate them to this new procedure. A new cage was used for each daily habituation and test sessions to prevent the development of territorial behaviors by the experimental rats. After the final PR baseline session, rats were placed into a novel cage for 15 min, after which a juvenile (28–35 days old), male Wistar rat was placed into the cage for 3 min, and the time engaged in social interaction (i.e., sniffing, licking, and grooming) was manually recorded by an experimenter who was blind to the drug treatment conditions. Only social interactions initiated by the experimental rat were included. Juvenile rats, which typically do not elicit aggression from adult conspecifics, habituation sessions, and testing under dim red light conditions during the rats’ dark period were all used to reduce anxiety and promote maximal exploratory behaviors from the experimental control rats (File and Seth, 2003), thus allowing observation of potential withdrawal-induced anxiety in drug-exposed rats. Because effects of drug withdrawal on social interaction are typically pronounced early during the withdrawal period (File et al., 1992; Irvine et al., 1999; Cheeta et al., 2001), the social interaction test was repeated after each of the first three PR sessions conducted during drug withdrawal. The same experimental and juvenile rats were never paired more than once during the experiment.
2.5. Statistical Analyses
For the PR and social interaction experiments, breakpoints and interaction time, respectively, were analyzed using mixed two-way analysis of variance (ANOVA), with Drug Treatment as the between-subjects factor and Time as the within-subjects factor. Data from the baseline, drug exposure, and withdrawal periods were all included in each ANOVA. Additionally, the average daily breakpoints during the entire drug exposure period, as well as baseline body weights, were analyzed using an unpaired t-test comparing the drug- and saline-treated groups. Body weight was analyzed as a percentage of baseline (weight during the final PR baseline test) using a mixed two-way ANOVA, with Drug Treatment as the between-subjects factor and Time as the within-subjects factor. A Pearson correlation was performed separately within amphetamine-, nicotine-, and both saline-treated groups between absolute body weights and breakpoints during the first withdrawal test to assess potential influences of body weight on breakpoints. Any significant interactions were further analyzed using Newman-Keuls post hoc tests to assess differences between treatment groups at individual time-points. The level of significance was set at 0.05.
3. RESULTS
3.1. Progressive-ratio responding for sucrose during nicotine withdrawal
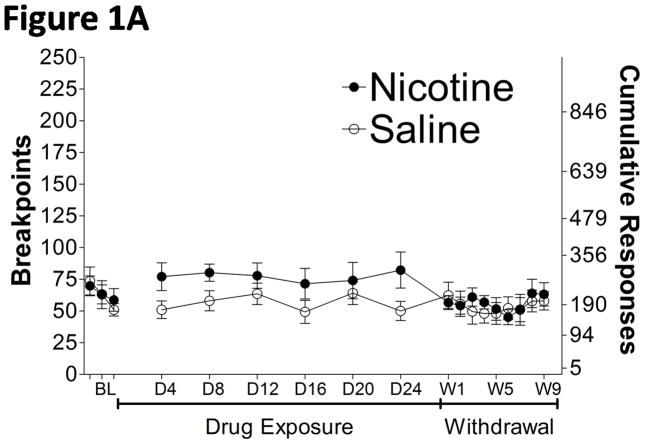
The average baseline breakpoints (± SEM), defined as the mean of the last 3 days of baseline testing, were not significantly different between rats treated with nicotine (63.82 ± 8.566, n = 11) and saline (62.64 ± 7.084, n = 11). The breakpoints for sucrose were slightly increased during nicotine exposure compared with the saline-treated rats (Fig. 1A), reflected by a significant difference in the unpaired t-test comparing the average breakpoints during the entire duration of nicotine/saline exposure (t20 = 2.099, p = 0.0487) (Fig. 1B). A mixed two-way ANOVA analyzing the baseline, exposure, and withdrawal periods indicated a significant main effect of Time (F17,340 = 2.584, p < 0.001), but no significant effect of Drug Treatment and no interaction between the two factors (F17,340 = 1.523, p = 0.0841). However, Newman-Keuls post hoc tests did not reveal differences in breakpoints between the nicotine- and saline-treated groups at any specific “withdrawal” time-point. Breakpoints were not affected by spontaneous nicotine withdrawal compared with saline-treated rats (Fig. 1A).
Figure 1.
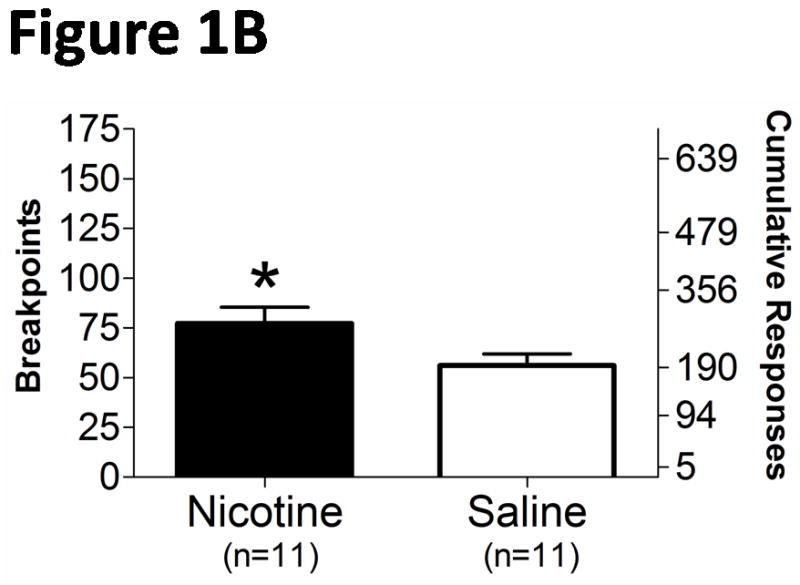
Motivated response for sucrose during exposure to and spontaneous withdrawal from chronic nicotine/saline exposure (9 mg/kg/day, 28 days). A. Daily breakpoints and cumulative responses during nicotine exposure and withdrawal. B. Average (± SEM) breakpoints during the 28 day nicotine exposure period. BL, baseline; D4, drug day 4; W1, withdrawal day 1. *p < 0.05, significantly different from saline-treated controls.
3.2. Social interaction response during nicotine withdrawal
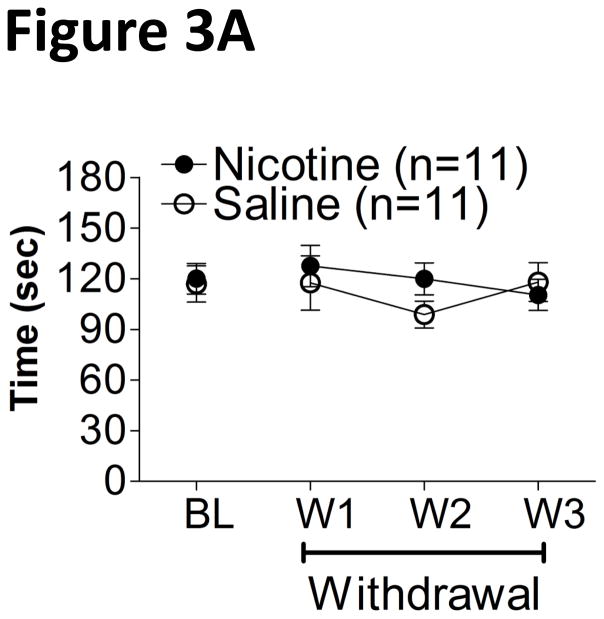
Social interaction behaviors were unaffected during nicotine withdrawal (Fig. 3A), reflected by the absence of any significant main or interaction effects between Drug Treatment and Time in a mixed two-way ANOVA.
Figure 3.
Time (mean ± SEM) engaged in social interaction (e.g., sniffing, licking, and grooming) over a 3 min period during withdrawal from chronic nicotine/saline (A) or amphetamine/saline (B). BL, baseline; W1, withdrawal day 1.
3.3. Progressive-ratio responding for sucrose during amphetamine withdrawal
The average baseline breakpoints (± SEM) were not significantly different between rats treated with amphetamine (43.31 ± 3.952, n = 12) and saline (44.03 ± 6.575, n = 12). Breakpoints for sucrose significantly increased during amphetamine exposure (Fig. 2A), reflected by a significant difference in the unpaired t-test comparing the average breakpoints during the entire duration of amphetamine/saline exposure (t22 = 5.290, p < 0.0001) (Fig. 2B). Breakpoints decreased during spontaneous amphetamine withdrawal and remained below baseline levels for the remainder of the testing period (Fig. 2A). A mixed two-way ANOVA analyzing the baseline, exposure, and withdrawal periods revealed significant main effects of Drug Treatment (F1,616 = 4.349, p < 0.05) and Time (F28,616 = 6.523, p < 0.0001). A significant interaction was also observed between Drug Treatment and Time (F28,616 = 9.076, p < 0.0001). Newman-Keuls post hoc tests revealed significantly elevated breakpoints during each day of amphetamine exposure and significantly decreased breakpoints at 12 h and at 4, 6, 9, 11, 12, 13, 14, 17, 20, 23, and 29 days of withdrawal. Breakpoints were decreased by spontaneous amphetamine withdrawal compared with saline-treated rats (Fig. 2A).
Figure 2.

Motivated response for sucrose during exposure to and spontaneous withdrawal from chronic amphetamine/saline exposure (10 mg/kg/day for 7 days). A. Daily breakpoints and cumulative responses during amphetamine exposure and withdrawal. B. Average (± SEM) breakpoints during the 7 day amphetamine exposure period. BL, baseline; D1, drug day 1; W1, withdrawal day 1. *p < 0.05, *** p < 0.001, significantly different from saline-treated controls.
3.4. Social interaction response during amphetamine withdrawal
Social interaction behaviors were unaffected during amphetamine withdrawal (Fig. 3B). A mixed two-way ANOVA revealed only a significant main effect of Time (F2,44 = 16.31, p < 0.0001), with social interaction decreasing similarly in both amphetamine- and saline-treated rats over time. However, no significant main effect of Drug Treatment and no significant interaction between Drug Treatment and Time were observed.
3.5. Weight gain during exposure to and withdrawal from nicotine or amphetamine
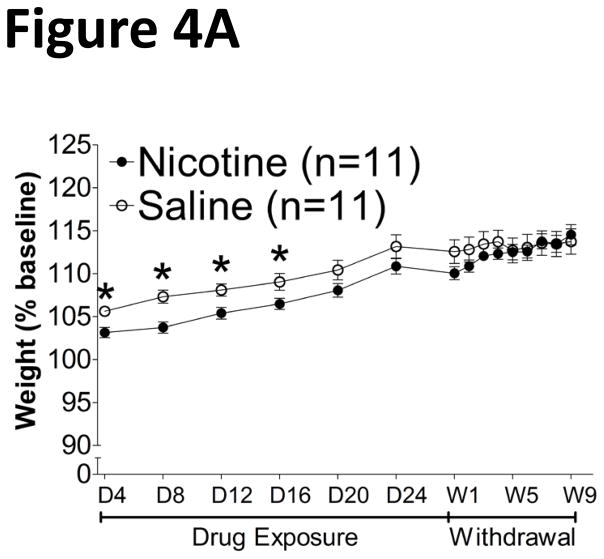
The average baseline body weights (± SEM) were not different between amphetamine- (404.2 g ± 4.584) and saline- (408.8 g ± 7.126) treated rats and between nicotine- (481.4 g ± 9.390) and saline- (460.3 g ± 9.788) treated rats. The rate of weight gain was slightly attenuated in nicotine-treated rats during drug exposure, but returned to control levels upon spontaneous withdrawal (Fig. 4A). A mixed two-way ANOVA revealed no significant main effect of Drug Treatment, but did reveal a significant main effect of Time (F14,280 = 84.99, p < 0.0001) and a significant interaction between the two factors (F14,280 = 3.544, p < 0.0001). Furthermore, Newman-Keuls post hoc tests revealed a decreased rate of weight gain primarily during nicotine exposure, with a return to control levels after spontaneous withdrawal. A Pearson correlation analysis revealed no significant correlation between absolute body weights and breakpoints during the first withdrawal test in either nicotine- or saline-treated rats (data not shown). In contrast, chronic exposure to and withdrawal from amphetamine lowered body weights (Fig. 4B). A mixed two-way ANOVA revealed significant main effects of Time (F25,550 = 464.3, p < 0.0001) and Drug Treatment (F1,550 = 80.97, p < 0.0001) and a significant interaction between the two factors (F25,550 = 23.23, p < 0.0001). Newman-Keuls post hoc tests also revealed decreased weight in the amphetamine-treated group compared with saline-treated controls, an effect that persisted before returning to control levels during the final withdrawal test (day 29). A Pearson correlation analysis revealed no significant correlation between absolute body weights and breakpoints during the first withdrawal test in either amphetamine- (Fig. 5A) or saline-treated rats (Fig. 5B).
Figure 4.
Rate of body weight gain (mean ± SEM) during chronic exposure to and withdrawal from nicotine/saline (A) or amphetamine/saline (B). D1, drug day 1; W1, withdrawal day 1. *p < 0.05, significantly different from saline-treated controls.
Figure 5.
Correlation between absolute body weights and breakpoints during the first withdrawal test in rats previously treated with amphetamine (A) or saline (B).
4. DISCUSSION
Chronic (28 day) exposure to nicotine (9 mg/kg/day) slightly elevated the motivational salience of a sucrose reward, reflected by increased breakpoints when responding on a PR schedule of reinforcement. However, the motivated response for sucrose was not affected during withdrawal from nicotine exposure. Conversely, chronic (7 day) exposure to amphetamine (10 mg/kg/day) increased the motivational salience of sucrose, reflected by increased breakpoints when responding on a PR schedule. The motivated response for sucrose was subsequently attenuated during spontaneous withdrawal from amphetamine exposure and remained attenuated for a prolonged period of time. Twelve hours after removal of the amphetamine minipumps, the motivated response for sucrose was reduced to 45% of pre-drug baseline levels. Twenty-four hours later, responding stabilized at approximately 70% of baseline and remained depressed at this level for the remainder of the testing period, which lasted for 29 days after minipump removal. Social interaction behaviors were not affected during withdrawal from either nicotine or amphetamine.
Withdrawal from the psychostimulants amphetamine and cocaine results in the expression of a number of anhedonic signs, reflected in elevations in ICSS reward thresholds (Markou and Koob, 1991; Cryan et al., 2003), decreased interest in sexual or novel stimuli (Barr et al., 1999; Harris et al., 2007), and altered successive contrast effects (Barr and Phillips, 2002; Vacca and Phillips, 2005). Amphetamine withdrawal also produces deficits (i.e., increased immobility) in the forced swim test (Cryan et al., 2003), an animal model of antidepressant activity. In this test, a rodent placed into a cylinder of water will eventually cease active escape behaviors (e.g., swimming and climbing) and will become immobile; antidepressants tend to decrease immobility. Similarly, withdrawal from cocaine produces motivational deficits toward a sucrose reward indicated in the PR test (Carroll and Lac, 1987). In the present experiments, we found that withdrawal from amphetamine also produced motivational deficits immediately and profoundly following termination of drug exposure. However, this is the first report that we are aware of in which this deficit persisted for such a long period after the initiation of withdrawal (i.e., for at least 29 days), albeit to a lesser extent than at the initial 12 h time-point. In contrast, Russig and colleagues (2003) did not observe any changes in motivated behavior during amphetamine withdrawal indicated in the PR test. The discrepancy between our results and those of Russig and colleagues (2003) may be attributable to the drug administration or withdrawal testing schedule. Our rats were administered amphetamine subcutaneously and consistently throughout the day via osmotic minipumps at a dose of 10 mg/kg/day. In contrast, Russig and colleagues (2003) administered amphetamine intraperitoneally either once daily at a dose of 1.5 mg/kg for 6 days or three times daily for 6 days at doses of 1, 2, and 3 mg/kg on the first day, 4, 5, and 5 mg/kg on the second day, and 5, 5, and 5 mg/kg on the subsequent 4 days. However, similar escalating dose regimens have been used by others reporting deficits in motivation during amphetamine withdrawal (Barr et al., 1999; Orsini et al., 2001; Schwabe and Koch, 2007). More importantly, in the Russig study rats were tested for their PR responses for sucrose either 2 or 4 days after the final amphetamine injection instead of 24 h later, and on a slightly different PR schedule than the one used here. Given the different behavioral assay parameters, Russig and colleagues (2003) may have failed to detect deficits in motivated behavior 2 days after termination of exposure to amphetamine when any potential effects may have dissipated. Indeed, deficits in hedonic capacity have been reported when assessed 24 h after the final amphetamine exposure using amphetamine doses and routes of administration similar to those used by Russig and colleagues (Barr and Phillips, 1999; Lin et al., 1999; Harrison et al., 2001; Orsini et al., 2001; Markou et al., 2005).
Both exposure to and withdrawal from amphetamine were also accompanied by decreased body weights during the drug exposure period followed by a steady increase during the withdrawal period until weights recovered to levels observed in saline-treated controls. Thus, it is possible that lower body weights during the initial days of withdrawal may have contributed to decreased responding for the sucrose reward, such that the lighter amphetamine-treated rats were not as hungry or became quickly satiated compared to the heavier saline-treated rats. However, this interpretation of the data is highly unlikely due to the following reasons. The rate of change in body weights was very stable over time, with a gradual increase during the entire withdrawal period until weights were no longer significantly different from saline-treated controls. This pattern of body weight change differs from the abrupt decrease in breakpoints observed in amphetamine-treated rats during the first withdrawal test, followed by a partial recovery during the second withdrawal test, after which breakpoints remained consistently and significantly depressed throughout the testing period. Further, the difference in the number of sucrose pellets earned during the initial withdrawal test was approximately 6.5 pellets (i.e. 292.5 mg) for saline-treated rats and approximately 3.0 pellets (i.e., 135 mg) for amphetamine-treated rats. This amount of food consumed during the daily session is a very small proportion of the approximately 25 g of food normally ingested on a daily basis by a rat. Thus, it is unlikely that the amphetamine-treated rats that were lighter in weight than saline-treated rats would become satiated after ingesting just 135 mg, or 0.5% of their daily food intake, during the approximately 1 to 4 h of the test session that occurred during their active period. In addition, if quicker satiety in smaller (e.g., amphetamine-treated) rats were to account for the decreased reward strength of the sucrose pellets, and thus decreased breakpoints, one might expect body weight to correlate with sucrose pellet intake in non-food deprived rats within either the amphetamine- or the saline-treated groups. However, no significant correlation was observed between body weights and breakpoints during the initial withdrawal test in amphetamine- or saline-treated rats. Finally, during the initial characterization of the progressive ratio schedule of reinforcement by Hodos (1961), it was determined that increasing the volume of a reward increased, not decreased, motivated responding, indicating that satiation does not factor into this response when relatively small amounts of a palatable reward are used. From a clinical perspective, amphetamine withdrawal is characterized by increased appetite in humans (McGregor et al., 2005), which may explain here the increased rate of weight gain during the 29 day withdrawal period in amphetamine-treated rats relative to saline-treated controls.
In the clinical population, although both depressed and drug-withdrawing individuals display anhedonia, this symptom is often most severe up to 24 h of withdrawal and does not persist in its more severe form as it may during a depressive episode (McGregor et al., 2005). However, mild anhedonic symptoms do persist for at least 3 weeks in patients withdrawing from chronic methamphetamine (McGregor et al., 2005). Additionally, anhedonia, amotivation, and other depressive symptoms are more prevalent in patients who have been in full remission for at least 2 months and up to 5 years from stimulant use disorder compared with control subjects without a history of substance abuse (Rawson et al., 2002; Leventhal et al., 2008). Thus, a behavioral assay of depressive signs in rats would have to be sensitive enough to detect not only the initial severe anhedonia occurring immediately after withdrawal, but also the milder anhedonic signs that persist over time. Few preclinical reports have been able to accurately reflect this persistent effect of drug withdrawal. For example, Zhang and colleagues (2007) reported that rats withdrawing from chronic morphine exposure become anhedonic, reflected by decreased breakpoints for sucrose in a PR task. The greatest deficits were observed shortly after withdrawal at the 24 h time-point, but the anhedonic effect persisted for 10 days, similar to that observed in the present study. Moreover, using the identical amphetamine exposure parameters as in the present study, Paterson and colleagues (2000) assessed ICSS reward thresholds, a sensitive measure of brain reward function, 4, 9, 18, and 24 h after minipump removal and once every 24 h thereafter. Indeed, the peak ICSS reward deficits were observed 18 h after minipump removal and remained significantly elevated for 5 days of withdrawal, indicating a persistent anhedonic response.
Substance use disorders are linked to adverse social consequences, and people with a history of substance use disorder have a high prevalence of social anxiety disorder (Brady et al., 2007). However, based on the available clinical data, it is difficult to evaluate whether repeated drug use per se or withdrawal from repeated use contributes to deficits in social behaviors. Further, to the best of our knowledge, a direct link between amphetamine withdrawal and social anxiety disorder has not been made in the clinical population either. In preclinical assessments, the social interaction test is often used as a measure of social anxiety in which decreased interactions with a naive rat reflect an anxiogenic response (File and Seth, 2003). In the present study, amphetamine withdrawal did not affect social interaction (i.e., sniffing, licking, and grooming) initiated by the withdrawing animal. Saline-treated controls engaged in social interaction at levels similar to controls in other reports (Irvine et al., 2001a; Christianson et al., 2008), and thus absolute social interaction times were likely sufficient to observe any potential withdrawal-induced deficits. Withdrawal from amphetamine has been shown to increase anxiety-like behavior in other behavioral tests, such as the elevated plus maze (Vuong et al., 2010) and the open field test (Russig et al., 2005), and amphetamine withdrawal is associated with increased anxiety in the clinical population as well (Lago and Kosten, 1994). However, Morley and colleagues (2001) also failed to show an effect of amphetamine withdrawal on social interaction, although testing was conducted 12 weeks after amphetamine administration in that study. Thus, while repeated use of amphetamine may indeed elicit general anxiety, as assessed by multiple behavioral tests, amphetamine withdrawal does not appear to elicit social anxiety in either preclinical or clinical assessments. Though withdrawal from many drugs of abuse is strongly associated with social dysfunction, the social consequences of withdrawal cannot be generalized to all drugs of abuse, as it appears that social anxiety is not a consequence of amphetamine withdrawal.
Regarding nicotine, there is a strong association between nicotine dependence and measures of both depression and anxiety (Trosclair and Dube, 2010), though comparison between smokers during withdrawal and those who continue to smoke reveal similar levels of decreased mood and elevated anxiety (Dawkins et al., 2009). These findings again reflect the difficulty in determining whether repeated use or withdrawal from repeated use elicits these behavioral changes. In preclinical studies, spontaneous or antagonist-precipitated withdrawal also results in the expression of depression-like behaviors, reflected by elevations in ICSS reward thresholds (Epping-Jordan et al., 1998; Watkins et al., 2000) or increased conditioned place aversion to a withdrawal-associated environment (Suzuki et al., 1996; Watkins et al., 2000). Much less is known about the effects of nicotine withdrawal on motivated behaviors. In the present study, chronic exposure to nicotine (9 mg/kg/day for 28 days) slightly elevated the motivated response for sucrose assessed on a PR schedule of reinforcement. However, withdrawal from nicotine did not affect the motivated response for sucrose. Similarly, the rate of weight gain was slightly depressed during nicotine exposure and returned to control levels upon spontaneous withdrawal. The testing schedule (i.e., once every 4 days during nicotine exposure versus daily during amphetamine exposure) is not likely to have affected responding during withdrawal, as saline-treated rats tested every 4 days exhibited stable responding throughout the experiment similarly to rats tested daily. Further, it is unlikely that motivational deficits would have appeared more than 9 days after spontaneous withdrawal, as withdrawal effects are typically pronounced earlier rather than later after cessation of drug administration. However, the dose of nicotine used may have accounted for the lack of an effect observed in the present studies. Withdrawal from the same dose of nicotine as that used here and administered for 7 days (Epping-Jordan et al., 1998; Skjei and Markou, 2003), 14 days (Watkins et al., 2000), or 28 days (Skjei and Markou, 2003), has been shown to produce elevations in ICSS reward thresholds 24 h after spontaneous withdrawal. Of these reports, Epping-Jordan and colleagues (1998) also found significantly elevated thresholds 6 to 8 h after spontaneous nicotine withdrawal, and it is possible that we missed the effects on motivated behavior from this earlier time point, which was only measured during amphetamine, but not nicotine, withdrawal. Nonetheless, any potential motivational deficits during this earlier period would have dissipated by 24 h after spontaneous nicotine withdrawal, whereas this motivational deficit persisted for weeks in animals withdrawing from amphetamine treatment. In addition, LeSage and colleagues (2006) demonstrated that withdrawal from nicotine administered at a dose similar to that used here (i.e., 3.2 mg/kg/day free base for 9 days, equivalent to 9 mg/kg/day salt) also did not result in a decrease in breakpoints on a PR schedule. However, withdrawal from a higher dose of nicotine (i.e., 8 mg/kg/day free base for 9 days) decreased breakpoints for sucrose 24 h after minipump removal. Thus, deficits in motivated behavior appear to be only associated with withdrawal from higher doses of nicotine than those used here, whereas withdrawal from more moderate doses may be sufficient to elicit an anhedonic response reflected in increased ICSS reward thresholds.
While considerable evidence indicates that social anxiety is significantly associated with nicotine dependence in humans (Sonntag et al., 2000), nicotine withdrawal in rats has produced mixed results with regard to social interaction depending on the dose and time of administration. Withdrawal from a 7 day treatment of 0.1 mg/kg/day produced deficits in social interaction 72 h after the final nicotine administration (Irvine et al., 1999). However, self-administration of nicotine at a dose of 0.45 mg/kg/day for 4 weeks did not affect social interaction at 24 or 72 h of withdrawal (Irvine et al., 2001a). In the present study, social interaction did not change during withdrawal from a higher dose of nicotine that typically produces anhedonia. Given the lower doses and shorter drug exposure periods used to observe the social interaction deficits in previous studies compared to the present study, withdrawal from less exposure to nicotine appears to be required to produce anxiogenic-like behavior, whereas withdrawal from higher doses of nicotine results in the expression of depression-related behaviors. For example, withdrawal from nicotine administered at 12 or 24 mg/kg/day for 14 days in mice also failed to produce an anxiogenic-like response assessed by activity in the light-dark box, acoustic startle, or prepulse inhibition (Jonkman et al., 2005). To the best of our knowledge, clinical reports do not differentiate between high and low levels of nicotine intake and the subsequent behavioral consequences.
Altogether, these data indicate that withdrawal from chronic amphetamine, but not a low dose of nicotine, leads to an immediate and enduring state of anhedonia reflected by decreased motivation for a sucrose reward assessed in a PR test in non-food-deprived rats. Few other reports have accurately reflected the persistent anhedonic state that is frequently observed in withdrawing or remitted psychostimulant abusers. Thus, the present regimen of amphetamine exposure and assessment of amotivation may offer a preclinical procedure by which to further investigate the comorbidity of substance use disorder and mood disorders that include anhedonia and amotivation as key signs.
Acknowledgments
Funding for this study was provided by NIMH/NIDA Grant U01 MH69062 to AM and a National Research Service Award (NRSA) Individual Postdoctoral Fellowship F32 MH080585 to AD.
The authors wish to thank Mr. Michael Arends for editorial assistance.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. Washington D.C: American Psychiatric Press; 1994. [Google Scholar]
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999;64 (3):597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29 (4–5):675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141 (1):99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Increased successive negative contrast in rats withdrawn from an escalating-dose schedule of D-amphetamine. Pharmacol Biochem Behav. 2002;71 (1–2):293–9. doi: 10.1016/s0091-3057(01)00664-5. [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Impact of nicotine withdrawal on novelty reward and related behaviors. Behav Neurosci. 2003;117 (2):327–40. doi: 10.1037/0735-7044.117.2.327. [DOI] [PubMed] [Google Scholar]
- Brady KT, Verduin ML, Tolliver BK. Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep. 2007;9 (5):374–80. doi: 10.1007/s11920-007-0048-0. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Cocaine withdrawal produces behavioral disruptions in rats. Life Sci. 1987;40 (22):2183–90. doi: 10.1016/0024-3205(87)90009-9. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine’s anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 2001;155 (1):78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193 (1):87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54 (1):49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104 (5):850–8. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393 (6680):76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463 (1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Hitchcott PK. Effects of nitrendipine, chlordiazepoxide, flumazenil and baclofen on the increased anxiety resulting from alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16 (1):87–93. doi: 10.1016/0278-5846(92)90011-3. [DOI] [PubMed] [Google Scholar]
- Fone KC, Beckett SR, Topham IA, Swettenham J, Ball M, Maddocks L. Long-term changes in social interaction and reward following repeated MDMA administration to adolescent rats without accompanying serotonergic neurotoxicity. Psychopharmacology (Berl) 2002;159 (4):437–44. doi: 10.1007/s00213-001-0931-z. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 2007;147 (3):583–91. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25 (1):55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology (Berl) 2001a;153 (3):315–20. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav Pharmacol. 1999;10 (6–7):691–7. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, Marshall M, File SE. Different treatment regimens and the development of tolerance to nicotine’s anxiogenic effects. Pharmacol Biochem Behav. 2001b;68 (4):769–76. doi: 10.1016/s0091-3057(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 2005;516 (1):40–5. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Kampman KM. The search for medications to treat stimulant dependence. Addict Sci Clin Pract. 2008;4 (2):28–35. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and stimulant dependence: neurobiology and pharmacotherapy. J Nerv Ment Dis. 1998;186 (12):737–45. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Lago JA, Kosten TR. Stimulant withdrawal. Addiction. 1994;89 (11):1477–81. doi: 10.1111/j.1360-0443.1994.tb03746.x. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav. 2006;83 (4):585–91. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, et al. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17 (3):218–23. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat--interactions between the two drugs. Psychopharmacology (Berl) 1999;145 (3):283–94. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58 (10):770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61 (1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Markou A, Harrison AA, Chevrette J, Hoyer D. Paroxetine combined with a 5-HT(1A) receptor antagonist reversed reward deficits observed during amphetamine withdrawal in rats. Psychopharmacology (Berl) 2005;178 (2–3):133–42. doi: 10.1007/s00213-004-2008-2. [DOI] [PubMed] [Google Scholar]
- Markou A, Kenny PJ. Neuroadaptations to chronic exposure to drugs of abuse: relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotox Res. 2002;4 (4):297–313. doi: 10.1080/10298420290023963. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4 (1):17–26. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18 (3):135–74. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100 (9):1320–9. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) Eur J Pharmacol. 2001;433 (1):91–9. doi: 10.1016/s0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25 (5):789–92. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152 (4):440–6. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, et al. Status of methamphetamine users 2–5 years after outpatient treatment. J Addict Dis. 2002;21 (1):107–19. doi: 10.1300/j069v21n01_09. [DOI] [PubMed] [Google Scholar]
- Russig H, Murphy CA, Feldon J. Behavioural consequences of withdrawal from three different administration schedules of amphetamine. Behav Brain Res. 2005;165 (1):26–35. doi: 10.1016/j.bbr.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Russig H, Pezze MA, Nanz-Bahr NI, Pryce CR, Feldon J, Murphy CA. Amphetamine withdrawal does not produce a depressive-like state in rats as measured by three behavioral tests. Behav Pharmacol. 2003;14 (1):1–18. doi: 10.1097/00008877-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Koch M. Effects of aripiprazole on operant responding for a natural reward after psychostimulant withdrawal in rats. Psychopharmacology (Berl) 2007;191 (3):759–65. doi: 10.1007/s00213-006-0520-2. [DOI] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol Biochem Behav. 1993;45 (4):827–35. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 2003;168 (3):280–92. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Sonntag H, Wittchen HU, Hofler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur Psychiatry. 2000;15 (1):67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314 (3):281–4. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Trosclair A, Dube SR. Smoking among adults reporting lifetime depression, anxiety, anxiety with depression, and major depressive episode, United States, 2005–2006. Addict Behav. 2010;35 (5):438–43. doi: 10.1016/j.addbeh.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Vacca G, Phillips AG. Inhibition of Successive Positive Contrast in Rats Withdrawn from an Escalating Dose Schedule of D-amphetamine. International Journal of Comparative Psychology. 2005;18 (4):298–306. [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Horm Res. 1992;37 (6):221–4. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 2010;208 (1):278–81. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292 (3):1053–64. [PubMed] [Google Scholar]
- Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W. Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addict Biol. 2007;12 (2):152–7. doi: 10.1111/j.1369-1600.2007.00068.x. [DOI] [PubMed] [Google Scholar]