Abstract
Pancreatic ductal adenocarcinoma (PDAC) is considered a “non-Immunogenic” neoplasm. Single agent immunotherapies have failed to demonstrate significant clinical activity in PDAC and other “non-immunogenic” tumors, in part due to a complex tumor microenvironment (TME) that provides a formidable barrier to immune infiltration and function. We designed a neo-adjuvant and adjuvant clinical trial comparing an irradiated, granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting, allogeneic PDAC vaccine (GVAX) given as single agent, or in combination with low dose cyclophosphamide (Cy) to deplete regulatory T cells (Tregs), to study how the TME is altered by immunotherapy. Examination of resected PDACs revealed the formation of vaccine-induced intratumoral tertiary lymphoid aggregates in 33/39 patients two weeks following vaccine treatment. Immunohistochemical analysis showed these aggregates to be regulatory structures of adaptive immunity. Microarray analysis of microdissected aggregates identified gene-expression signatures in five signaling pathways involved in regulating immune cell activation and trafficking that were associated with improved post-vaccination responses. A suppressed Treg pathway and an enhanced Th17 pathway within these aggregates were associated with improved survival, enhanced post-vaccination mesothelin-specific T-cell responses and increased intratumoral Teffector/Treg ratios. This study provides the first example of immune-based therapy converting a “non-immunogenic” neoplasm into an “immunogenic” neoplasm by inducing infiltration of T cells and development of tertiary lymphoid structures in the TME. Post-GVAX T-cell infiltration and aggregate formation resulted in the upregulation of immunosuppressive regulatory mechanisms including the PD-1/PD-L1 pathway, suggesting that vaccine-primed PDAC patients may be better candidates than vaccine–naïve patients for immune checkpoint and other immunomodulatory therapies.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a lethal malignancy with less than 5% of patients alive at 5 years (1). Standard therapies provide only short-term benefit before chemoresistance develops. Immunotherapy, vaccines, and immune modulating agents, have shown progress against chemotherapy-sensitive and chemoresistant “immunogenic” cancers such as renal cell carcinoma (RCC) and melanoma that naturally attract tumor-infiltrating effector T cells (2–4). However, PDAC and other malignancies that are considered “non-immunogenic” neoplasms typically lack tumor-infiltrating effector lymphocytes (5–8), and are less responsive to immunotherapy (9). Thus, single-agent inhibitors of T-cell regulatory signals such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) receptor, which demonstrate significant clinical activity against melanoma, RCC, and non-small cell lung cancer (NSCLC), do not have activity in PDAC (2, 10, 11). However, we recently reported tumor regressions and improved survival in patients with advanced metastatic PDAC, who were treated with PDAC GVAX combined with ipilimumab, which targets the inhibitory molecule CTLA-4 on T cells (12), as compared with patients treated with ipilimumab alone. These data suggest that T cells first need to be induced to provide available cells for the activation by T-cell modulating agents like ipilimumab and nivolumab.
Antigen-specific T-cell responses have been observed in some PDAC patients treated with vaccines (13). We reported the induction of systemic mesothelin-specific T-cell responses following treatment with PDAC GVAX in patients with resected and metastatic PDAC (12, 14–18). Mesothelin is an antigen expressed by virtually all PDACs, and post-treatment detection of enhanced mesothelin-specific T-cell responses in peripheral blood lymphocytes (PBL) is associated with improved disease-free (DFS) and overall survival (OS) in GVAX-treated patients (12, 16–18). Despite evidence of peripheral immune activation and antitumor activity in some patients, immune tolerance mechanisms within the tumor microenvironment (TME) likely inhibit the full potential of vaccines alone (13). Thus, measures of peripheral immune activation following treatment with immunotherapy may not represent the immune activation status within the TME.
Tumors evolve numerous mechanisms to escape immune recognition (19). For PDAC, suppressive monocytes including dendritic cells (DCs), neutrophils, and myeloid-derived suppressor cells (MDSCs), immune checkpoints (CTLA-4 and PD-1), and CD4+CD25+FoxP3+ Tregs have been reported in preclinical and clinical studies (13). Tregs have been found infiltrating the TME of many human tumors, including PDAC, and elevated Treg numbers are generally associated with shorter patient survival (6, 20–23). Previous studies have suggested that Tregs can be depleted with immune-modulating doses of Cy to enhance immunotherapies (24–28). We previously reported the induction of higher avidity mesothelin-specific T-cell responses in the periphery of metastatic PDAC patients when low dose Cy is given 1 day prior to vaccination (16). Furthermore, our preclinical studies suggest that Cy primarily affects subsets of Tregs found infiltrating tumors, and that studying peripheral Tregs does not provide insight into the mechanisms by which Tregs regulate immune responses within the TME (29). However, the effect of Cy on intratumoral Tregs and other immune-cell populations within human cancers has not been well studied.
In this study, we tested the hypothesis that vaccine-based immunotherapy can convert PDACs from “non-immunogenic” into “immunogenic” tumors with infiltrating effector lymphocytes. We evaluated the effects of GVAX, given alone or in combination with Treg-modulating doses of Cy, on lymphocytes infiltrating PDAC tumors. Treatment was initiated as neo-adjuvant therapy two weeks prior to surgical resection to enable the direct assessment of the TME following treatment. Here we show for the first time that an immune-based therapy induces the development of tertiary lymphoid aggregates within this “non-immunogenic” neoplasm that resemble ectopic lymph node-like structures observed in subsets of immunotherapy-naïve patients with more “immunogenic” cancers such as melanoma and NSCLC (30–33). The development of intratumoral tertiary lymphoid aggregates in PDAC tumors was dependent on vaccination, supporting GVAX as a trigger of antigen-specific immune responses at the tumor site. Microdissection and gene array analyses demonstrated that decreased Treg and increased Th17 immune effector signatures within the vaccine-induced intratumoral lymphoid aggregates was associated with: enhanced systemic post-vaccination mesothelin-specific T-cell responses, higher intratumoral Teffector/Treg ratios, and longer patient survival. However, as has been observed for melanomas (34, 35) that are naturally infiltrated with T cells, the T-cell infiltration following treatment with GVAX was also associated with the upregulation of immunosuppressive regulatory mechanisms within the PDAC TME that can be targeted by immune modulation. This process has been termed adaptive resistance. Thus, these data support a new model for developing immunotherapy approaches in tumors lacking natural T-cell infiltration.
Materials and Methods
Study Subjects and Tissue Specimens
Between July 2008 and September 2012, 59 patients were enrolled into an ongoing study (NCT00727441) of an irradiated, allogeneic GM-CSF-secreting pancreatic tumor vaccine (GVAX) administered intradermally either alone or in combination with immune modulatory doses of cyclophophamide (Cy) as neoadjuvant and adjuvant treatment for patients with resectable PDAC. Patients were randomized 1:1:1 into 3 treatment arms (Figure S1, study schema). In Arm A, patients received GVAX alone; in Arm B, patients received GVAX plus a single intravenous dose of Cy at 200 mg/m2 1 day prior to each vaccination; in Arm C, patients received GVAX plus oral Cy at 100 mg once daily for 1 week on and 1 week off (36). Up to 6 GVAX treatments were administered and all of the patients remained in their initial treatment arms throughout the duration of the study. All 59 patients received the 1st GVAX treatment 2 weeks +/− 4 days prior to surgery. Fifty-four patients successfully underwent pancreaticoduodenectomy (the Whipple surgery) and received the 2nd GVAX treatment. Five patients were found during surgery to have liver metastases, which were not radiographically identified prior to surgery, and instead underwent a bypass surgery. Among the 54 patients that had pancreaticoduodenectomy, 1 patient was found to have ampullary cancer, 1 to have neuroendocrine tumor, 2 to have undifferentiated carcinoma, and 1 to have autoimmune pancreatitis. These patients’ preoperative CT scans did not distinguish their disease process from PDAC. In addition, 1 patient had grossly residual tumors and another 11 patients had recurrence immediately following surgery. All of these patients were taken off the study postoperatively. The 39 patients remaining on the study received standard adjuvant chemotherapy and radiation therapy. Patients remaining disease-free following chemoradiation therapy received up to 4 additional PDAC GVAX treatments every 4 weeks. A full clinical report, including survival analyses, will be submitted at the completion of the study.
Tumor specimens from the 39 patients that completed 2 vaccine treatments and continued onto adjuvant chemotherapy were analyzed in this study. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were obtained from our pathology archive. For most tumors, a piece was also stored frozen at −80° C in OCT freezing medium. PDAC tumor specimens collected prior to vaccine exposure from the 54 subjects treated in a previous adjuvant vaccine study (17) and from four unvaccinated patients that underwent surgery concurrently at our institution were obtained from an IRB-approved retrospective database and used as controls in this study.
Isolation of PDAC tumor-infiltrating lymphocytes (TILs)
After keeping adequate tissue for surgical pathology diagnosis, the majority of the remaining tissue was processed for isolating tumor-infiltrating lymphocytes (TILs). The spared portion of each tumor was digested with enzymes into single-cell suspensions as described previously (15). Lymphocytes were isolated from these tumor-digested single-cell suspensions by Percoll density gradient centrifugation and stored frozen at −140° C. To isolate TIL sub-populations, TIL were thawed and labeled with anti-human CD3-PE, anti-human CD4-PECy5, anti-human CD8-APC and anti-human CD19-FITC (all monoclonal antibodies were from BD Pharmingen). CD3+CD4+ cells (CD4+ T cells), CD3+CD8+ cells (CD8+ T cells), CD3−CD19+ cells (B cells) and CD3−CD4−CD8−CD19− cells (non-T or -B cells) were isolated by fluorescence-assisted cell sorting (FACS) using a Beckman Coulter MoFlo FACS sorter. Dead cells were excluded using LIVE/DEAD Fixable Blue Dead Cell Stain (Invitrogen).
Immunohistochemistry
Immunohistochemistry (IHC) was performed on FFPE 5µm sections of resected PDAC. Immunostaining for CD1a, CD3, CD4, CD8, CD20, CD56, CD68, and CD163 was performed on Ventana autostainer using Ultra-view detection (Ventana Medical Systems, Inc. Tucson, AZ). The protocols and sources of antibodies are summarized in Table S1. Staining for IL17A and RORγt were performed on Leica auto stainers using Bond Refine detection (Leica microsystems, Bannockburn, IL). For double staining of IL17A and RORγt, Chromplex detection was used per manufacturer’s guidelines (Bond Leica, Leica microsystems, Bannockburn, IL). For some protocols, antigen-retrieval was performed manually as indicated. PD-L1 staining was performed as previously described (37). Following standard protocol, slides were deparaffinized, hydrated, and heat-induced antigen-retrieval was performed. Incubation with the primary antibody using optimal conditions was followed by development of immunostaining. Counterstaining with hematoxylin (H&E) was applied, and slides were dehydrated and cover slipped.
Microarray Analysis
Approximately 20 3-µm sections of FFPE tissue from each subject were anonymized and stained with H&E immediately before the lymphoid aggregates were microdissected by a pathologist (JW) using a dissection microscope. The microdissected FFPE tissue was stored in RNALater solution (Ambion) until RNA was purified using the RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (Ambion) and amplified by the WT-Ovation™ FFPE System (NuGEN Technologies) per manufacturers' protocols. Gene expression microarray analysis was performed using the Affymetrix Human U133 Plus 2.0 array chips. The microarray data have been submitted to the Gene Expression Omnibus (Accession number: GSE52171).
Immunohistochemistry (IHC) Image analysis
All slides were anonymized, scanned, and whole-slide images were analyzed using the Image Analysis Software (Aperio Technologies, Vista, CA). The area of tumor to be analyzed was circled by a pathologist (RAA) on the anonymized H&E stained slides, with attention to the inclusion of the largest area possible of continuous neoplastic tissue while excluding, to the extent possible, normal pancreatic, intestinal and smooth muscle tissue. The Positive Pixel Count algorithm was used to quantify IL17 expression. The Cell Surface Count algorithm and the Nuclear Count algorithm were used to quantify the number of cells positive for other immune cell surface or nuclear markers, respectively. The automatic quantification results were anonymized and validated first by manual quantification by a pathologist (RAA).
Detection of Mesothelin-specific T-cell responses by IFNγ ELISPOT
The Mesothelin peptide sequences and ELISPOT assay used in this study have been described previously (17, 18). Peripheral blood lymphocytes (PBL) were isolated by density gradient centrifugation using Ficoll-Hypaque from peripheral blood collected at baseline, prior to, and following each Panc GVAX treatment. The post-vaccination blood after the 1st vaccination was obtained either the day before surgery, or on the day of surgery prior to anesthesia. Post-vaccination bloods for all subsequent vaccinations were obtained one month following each vaccination (Figure S1). Only PBLs from 27 of the 39 patients expressed the HLA-A*0101 and/or HLA-A*0201 alleles and were analyzed by ELISPOT. T-cell responses to mesothelin peptides were adjusted for background measured against irrelevant melanoma or renal cell carcinoma control peptides. Responses were measured to 8 HLA-A*0101 and 6 HLAA*0201 mesothelin peptides. Post-vaccination responses were considered enhanced when the frequency of mesothelin-specific T cells increased by at least 2-fold when compared to pre-treatment baseline frequencies. Mesothelin-specific T-cell levels measured in 16 patients (eight responders and eight nonresponders) whose microdissected lymphoid aggregates and/or TIL were used for gene-expression or FACS analysis are shown in Figure S2A&B.
Quantitative real-time PCR analysis
Quantitative real-time PCR was performed with the SYBR green system (SABiosciences) per manufacturer's protocol. Pre-validated PCR primers for each gene listed in Table S2 were obtained from Real Time Primer, LLC or SABiosciences. The expression levels of each gene were normalized to the same scale (1 to 4) as previously described (38) (Table S3). The average score for the set of genes representing each pathway were used to compare T-helper pathway gene expression between grouped patients’ TIL specimens.
Intracellular cytokine and FoxP3 staining analysis of TIL
Cryopreserved TIL isolated from ten vaccinated patients treated in this study and four unvaccinated control subjects were analyzed. TIL were thawed and incubated at 37 °C overnight either alone (“unstimulated”) or at a 1:1 ratio with human T-cell Activator CD3/CD28 Dynabeads (Invitrogen) (“stimulated”) in IMDM medium supplemented with 5% FBS. After 16 hrs, Protein Transport Inhibitor Cocktail (eBioscience) was added and the TIL were cultured for an additional 4 hrs. Unstimulated and stimulated TIL were then harvested and surface-stained with anti-CD3-PECy7 (BD Pharmingen), anti-CD4-PECF594 (BD Horizon), and anti-CD8-APCEF780 (eBioscience), and intracellular-staining were performed following permeabilization and fixation using eBioscience FoxP3/transcription Factor Staining Buffers with anti-IFNγ-FITC (BD Pharmingen), anti-IL17A-PE (eBioscience), and anti-FoxP3-V450 (BD Horizon). Dead cells were excluded using LIVE/DEAD fixable aqua dead-cell stain (Invitrogen). Stained samples were analyzed using a Beckman Coulter Galios flow cytometer and Flowjo software (Tree Star, Inc.).
Statistical analysis
Overall survival (OS) is defined as the time from surgery to death from any cause. Comparisons between groups were made using two-tailed unpaired Student’s T tests or Wilcoxon signed rank tests. All graphing and statistical analyses were performed using either GraphPad Prism software (GraphPad Software, SanDiego, CA) or SAS version 9.3 statistical software (SAS Inc, Cary, NC). Gene Set Enrichment Analysis (GSEA) was performed according to the algorithm described by Subramanian et al. (39), using the analysis software provided by the Broad Institute (Cambridge, MA).
Results
Vaccine-based immunotherapy induces the neogenesis of intratumoral tertiary lymphoid aggregates that resemble ectopic lymph node-like structures
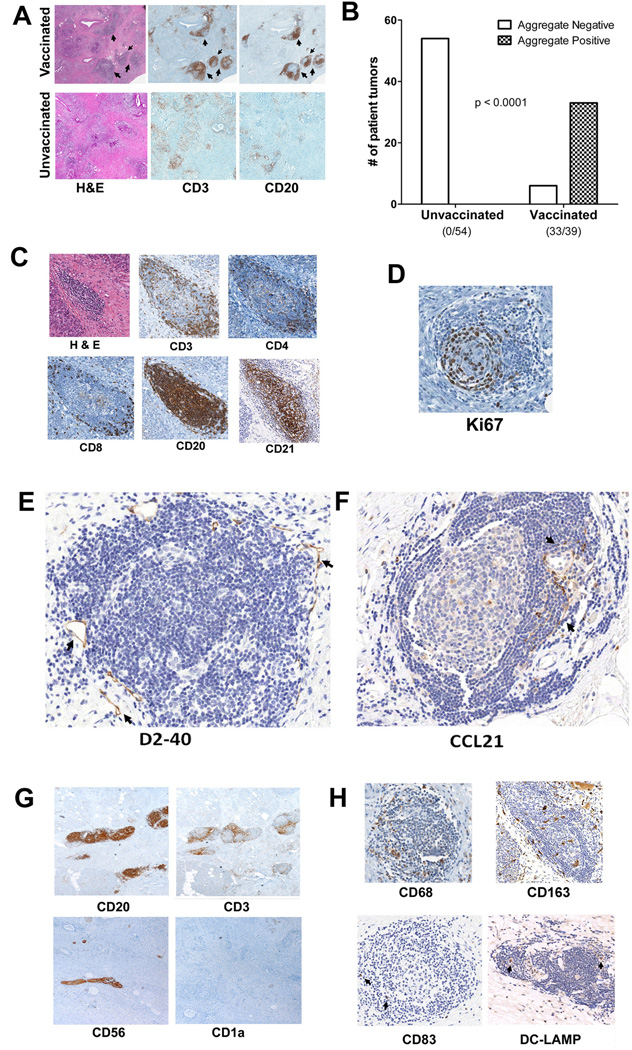
Thirty-nine patients with resectable PDAC were randomized to receive either GVAX alone, GVAX with one dose of intravenous Cy one day prior to vaccine, or GVAX with daily oral Cy for one week on and one week off (Figure S1). Patients underwent pancreaticoduodenectomy two weeks following GVAX treatment and the resected PDAC tumor specimens were analyzed histologically. The majority (33/39 or 85%) of tumors contained intratumoral lymphoid aggregates (Figure 1A&B). In contrast, even though lymphocytes were sometimes present, organized intratumoral lymphoid aggregates like the ones observed in tumors from GVAX-treated patients were not detected in 54 PDAC specimens collected prior to vaccine exposure from patients treated in a previous study (17) (Figure 1A&B). Thus, the formation of intratumoral aggregates was dependent on GVAX treatment (P < 0.001).
Figure 1. Intratumoral tertiary lymphoid aggregates observed in PDACs from Panc GVAX-treated patients.
A. Lymphoid aggregates (indicated by arrows) observed in PDACs from Panc GVAX-treated (vaccinated) patients are not seen in Panc GVAX naïve (unvaccinated) patients. Lymphoid aggregates are composed of T cells (anti-CD3 staining) and B cells (anti-CD20 staining). B. Numbers of PDACs with (TLA+) or without (TLA−) at least one lymphoid aggregate present in the intratumoral area in 54 unvaccinated patients and 39 vaccinated patients. Intratumoral lymphoid aggregates are observed specifically in PDACs from vaccinated patients (Fisher’s exact test p<0.001). C. IHC analysis with T-cell markers (CD3, CD4 and CD8), B-cell marker (CD20) and follicular dendritic cell marker (CD21). D. IHC analysis of the cell-proliferation marker Ki67. E. IHC analysis of the lymphatic-vessel (arrows) marker D2-40. F. IHC analysis of the chemokine ligand CCL21 (arrows). G. IHC analysis of CD20+ B cells, CD3+ T cells, CD56+ NK cells, and CD1a+ immature dendritic cells. Note that anti-CD56 staining also marks neural tissues (arrow). H. IHC analysis of CD68+ and CD163+ macrophages and CD83+ and DC-LAMP+ mature dendritic cells (arrows). All positive IHC signals are in brown.
IHC analysis demonstrated that these vaccine-induced aggregates were organized tertiary lymphoid structures (Figures 1A&C) and not random clusters of lymphocytes (Figure S3). They were composed of organized T- and B-cell zones, and contained germinal center-like structures marked by CD21+ follicular dendritic cells (40) (Figure 1C) and Ki67+ actively proliferating cells (Figure 1D). In most, the B-cell zones were in the center while the T-cell zones were in the periphery of the aggregate. There was also evidence of lymphoid neogenesis including the presence of lymphatic vessels marked by D2-40 (Figure 1E) and infiltration of CCL21-expressing cells, a chemokine known to be involved in lymphoid neogenesis (41) (Figure 1F).
Tertiary lymphoid structures develop in response to antigen stimulation. Thus, it is not surprising that, innate immune cells capable of presenting antigens were also present in these aggregates. Although CD56+ (NK cell marker) and CD1a+ cells (immature DC marker) were not present (Figure 1G), CD83+ and DC-LAMP+ mature DCs were present within most aggregates (Figure 1H). CD68+ and CD163+ cells with morphologies consistent with monocyte/macrophages were also commonly present in these aggregates (Figure 1H). Thus, these vaccine-induced lymphoid aggregates are organized structures of adaptive immunity.
The lymphoid aggregates that formed in GVAX-treated PDAC patients resemble naturally induced ectopic lymph node-like structures observed in tumors from immunotherapy-naïve patients with other cancers that are associated with longer survival (30–33). Consistent with these prior studies, there was an association between the formation of post-vaccination lymphoid aggregates and longer survival (Figure S4). Since lymphoid aggregates formed in most (85%) vaccinated patients’ tumors (Figure 1B), not all PDAC tumors from patients that survived < 1.5 years lacked intratumoral lymphoid aggregates (Figure S4). Thus, the induction of lymphoid aggregates alone did not accurately predict the post-vaccination induction of a clinically effective antitumor response.
Post-vaccination effector T-cell trafficking into the TME to form intratumoral lymphoid aggregates is associated with evidence of early T-cell activation, increased recruitment of Tregs, and upregulation of IFNγ and the PD-1/PD-L1 T-cell suppressive pathway
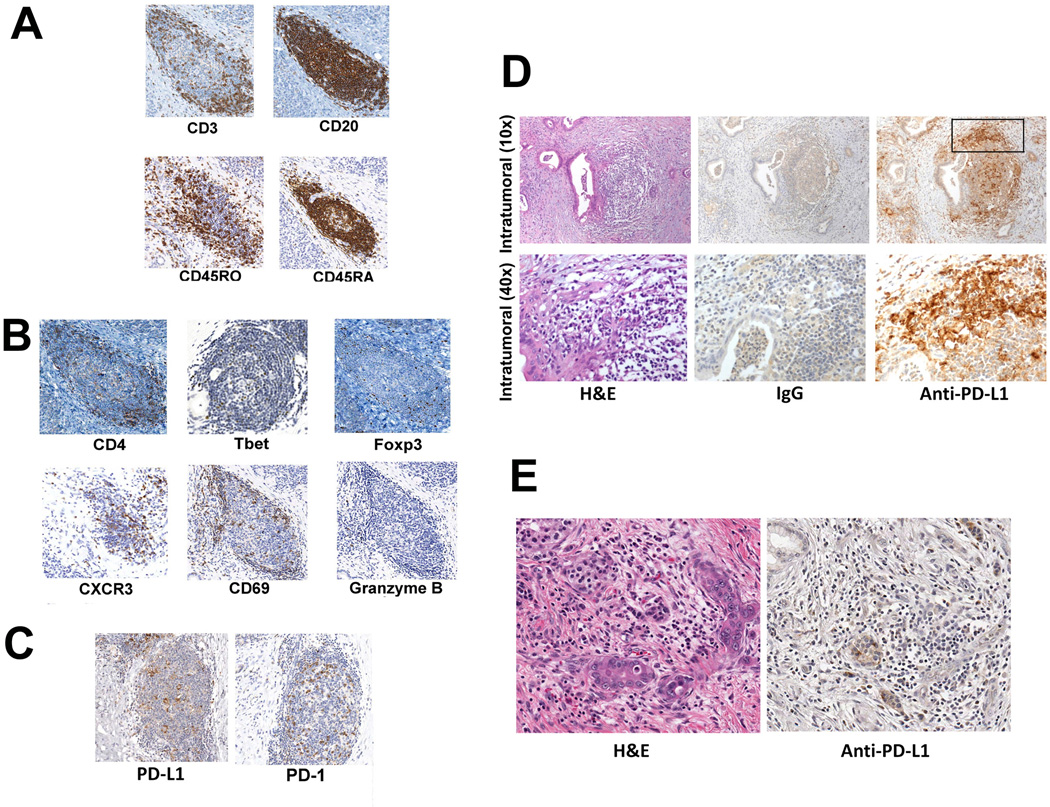
The post-vaccination formation of intratumoral lymphoid aggregates suggests that treatment with GVAX triggers an adaptive T-cell response at the tumor site. To assess the activation status and functional polarity of T cells within these aggregates, we evaluated the expression of a number of T-cell markers by IHC. The lymphoid aggregates contained both CD45RA+ naïve, and CD45RO+ antigen-experienced T cells (Figure 2A). CD45RA+ T cells were clustered in B-cell zones whereas CD45RO+ T cells were more enriched in T-cell zones located in the periphery of the aggregates. These aggregates did not contain many granzyme B-expressing T cells (Figure 2B), which were found frequently in lymphocytes outside of the aggregates (Figure S5). By contrast, the early T-cell activation marker CD69 and the activated T-cell trafficking chemokine receptor CXCR3 were abundantly expressed in the lymphoid aggregates (Figure 2B). The aggregates also contained T-bet+ cells consistent with the induction of IFNγ-expressing effector T-cell responses (Figure 2B). These data suggest that vaccine-induced aggregates are not major sites for cytotoxic T-cell lysis of target cells, but more likely are sites of initial T-cell activation within the TME.
Figure 2. Intratumoral lymphoid aggregates are sites of post-vaccination T-cell activation and regulation.
A. IHC analysis of CD3-, CD20-, CD45RO-, and CD45RA-expressing cells. B. IHC analysis of CD4-, T-bet-, Foxp3-, CXCR3-, CD69-, and Granzyme B-expressing cells (Granzyme B+ cells are indicated by arrows). C. IHC analysis of B7-H1/PD-L1- and PD-1-expressing cells. D. PD-L1 expression in a representative intratumoral lymphoid aggregate from a vaccinated patient. The square indicates the edge of the intratumoral lymphoid aggregate that is shown in the bottom panels at a higher magnification. Brown staining indicates expression of the indicated markers. E. Absence of PD-L1 expression in a representative PDAC specimen from an unvaccinated patient.
Along with signals of effector T-cell trafficking and early activation, negative regulatory signals were also present in these aggregates. FoxP3-expressing Tregs were present in most aggregates (Figure 2B). In addition, PD-L1, an inhibitory ligand that signals through the negative regulatory receptor PD-1 on activated T cells (42), was commonly expressed by cells in aggregates with morphologies consistent with monocytes/macrophages (Figure 2C). These PD-L1+ cells were surrounded by lymphocytes that expressed PD-1. Clusters of PD-L1+ cells were also seen at the edges of intratumoral lymphoid aggregates where T cells tended to be located (Figure 2D). In contrast, PD-L1-expressing cells were rarely detected in PDAC tumors from vaccine naïve patients (Figure 2E), supporting our hypothesis that PD-L1 expression is induced by vaccine treatment.
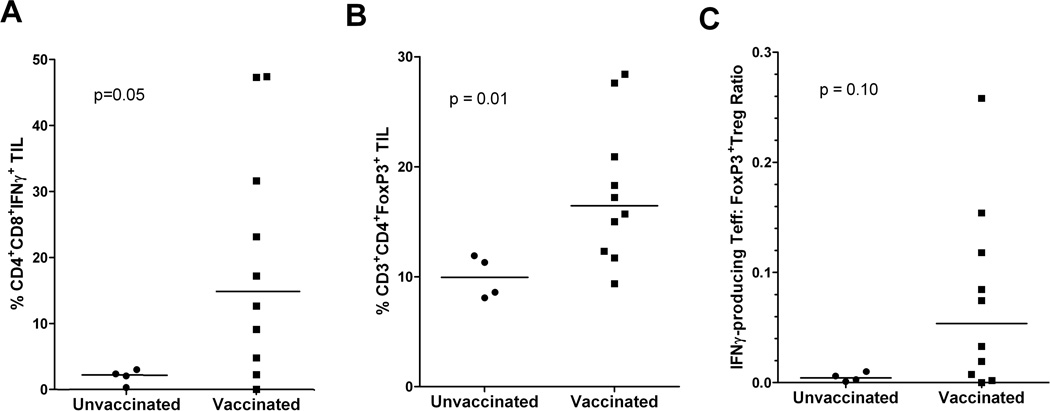
Some functional markers are difficult to assess by IHC analysis. To explore the functional activity of these aggregates, we next studied post-vaccination TIL isolated from portions of the resected tumors. We compared IFNγ production by unstimulated TIL from vaccinated and unvaccinated patients by FACS. Analysis was limited to specimens from ten vaccinated patients and four unvaccinated patients that had enough TIL recovered for the assays. Elevated numbers of IFNγ-producing T cells were detected in TIL from vaccinated patients (Figures 3A). To evaluate whether Treg infiltration into tumors was affected by GVAX, we compared the levels of PDAC tumor-infiltrating Tregs between the same ten vaccinated and four unvaccinated patients. As shown in figure 3B, the levels of CD3+CD4+FoxP3+Tregs in TIL, quantified by FACS, were higher in vaccinated patients compared to that in unvaccinated patients. We observed a similar GVAX-induced increase in tumor-residing Tregs in a murine PDAC model (Soares et al. manuscript submitted). Thus, the activities of GVAX included both the recruitment of effector T cells into the TME, and the upregulation of immunosuppressive regulatory mechanisms, specifically the expression of PD-L1 and Treg infiltration.
Figure 3. Post-vaccination effector T-cell activation in intratumoral lymphoid aggregates is associated with Treg infiltration into the TME.
Percentages of (A) IFNγ-producing CD4+CD8+ effector T cells and (B) CD3+CD4+FoxP3+ Tregs quantified by FACS in unstimulated TIL isolated from 10 vaccinated patients compared to those isolated from 3 unvaccinated patients. C. IFNγ-producing Teffector:Treg ratios measured in unstimulated TIL from the same sets of vaccinated and unvaccinated patients. Comparisons between vaccinated and unvaccinated TIL were made using Wilcoxon signed-rank tests and the calculated p values are shown.
In order to estimate the net impact of the simultaneous infiltration of both effector T cells and Tregs in the TME, we compared the ratios between the number of IFNγ-producing effector T cells to the number of CD4+FoxP3+ Tregs, both measured by FACS directly in TIL without stimulation. Higher T effector/Treg ratios measured by CD8+/Foxp3+ T-cell ratio in TIL in untreated patients have been reported to correlate with better prognosis in multiple human cancers including PDAC (3, 43–45). We found that the ratios of IFNγ-producing effector T cells:Tregs were higher in 7 of the 10 vaccinated patients with over a log-fold increase in 5 of these 7 vaccinated patients when compared with unvaccinated patients (Figure 3C). These data suggest that GVAX can alter the balance of T effector to Tregs in favor of an antitumor response.
Specific gene expression signatures in intratumoral lymphoid aggregates define regulatory structures that correlate with immune responses and OS
To evaluate possible mechanisms regulating vaccine-induced lymphoid aggregates, and to assess whether particular functional immune signatures within these aggregates were associated with response to treatment, we first performed gene microarray analysis on RNA isolated from microdissected lymphoid aggregates (Figure S6). Gene expression was compared among samples grouped according to three different correlates of vaccine response: patient OS, post-vaccination mesothelin-specific T-cell responses in PBL (17), and the intratumoral CD8+ T effector to FoxP3+ Treg ratio. Mesothelin-specific CD8+ T cells specific for six HLA-A2-binding and eight HLA-A1-binding mesothelin epitopes were measured using IFNγ ELISPOT assays as described previously (17). Mesothelin-specific T-cell levels measured before and 12-14 days following the first vaccination just prior to surgery are shown for 16 patients who were either HLA-A1+ or -A2+, eight responders who developed enhanced (at least 2-fold higher than baseline) post-vaccination mesothelin-specific T-cell responses, and eight non-responders who did not, in Figure S2A&B, respectively. The post-vaccination levels of mesothelin-specific T cells were 2.6 – 54 fold higher than baseline in responders compared to 0.97 - 57 fold lower than baseline in non-responders.
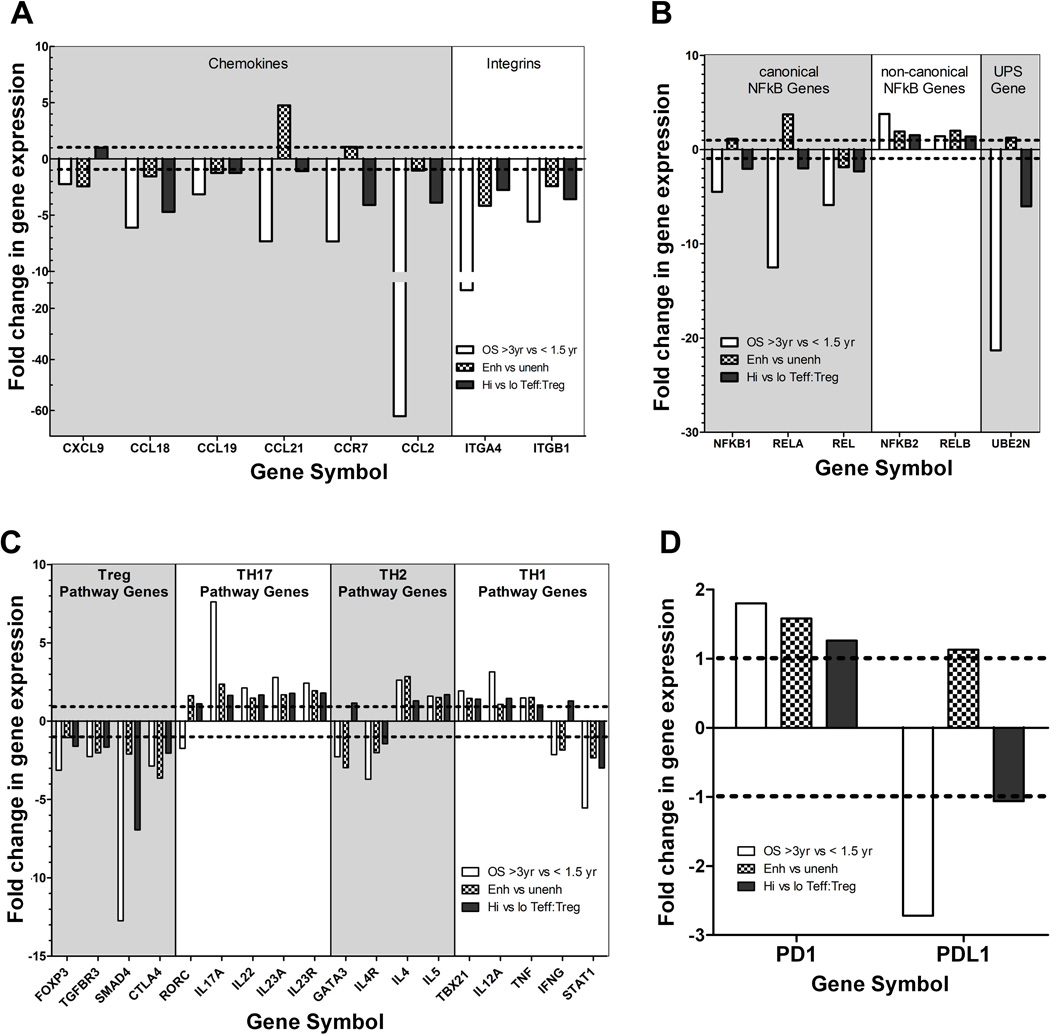
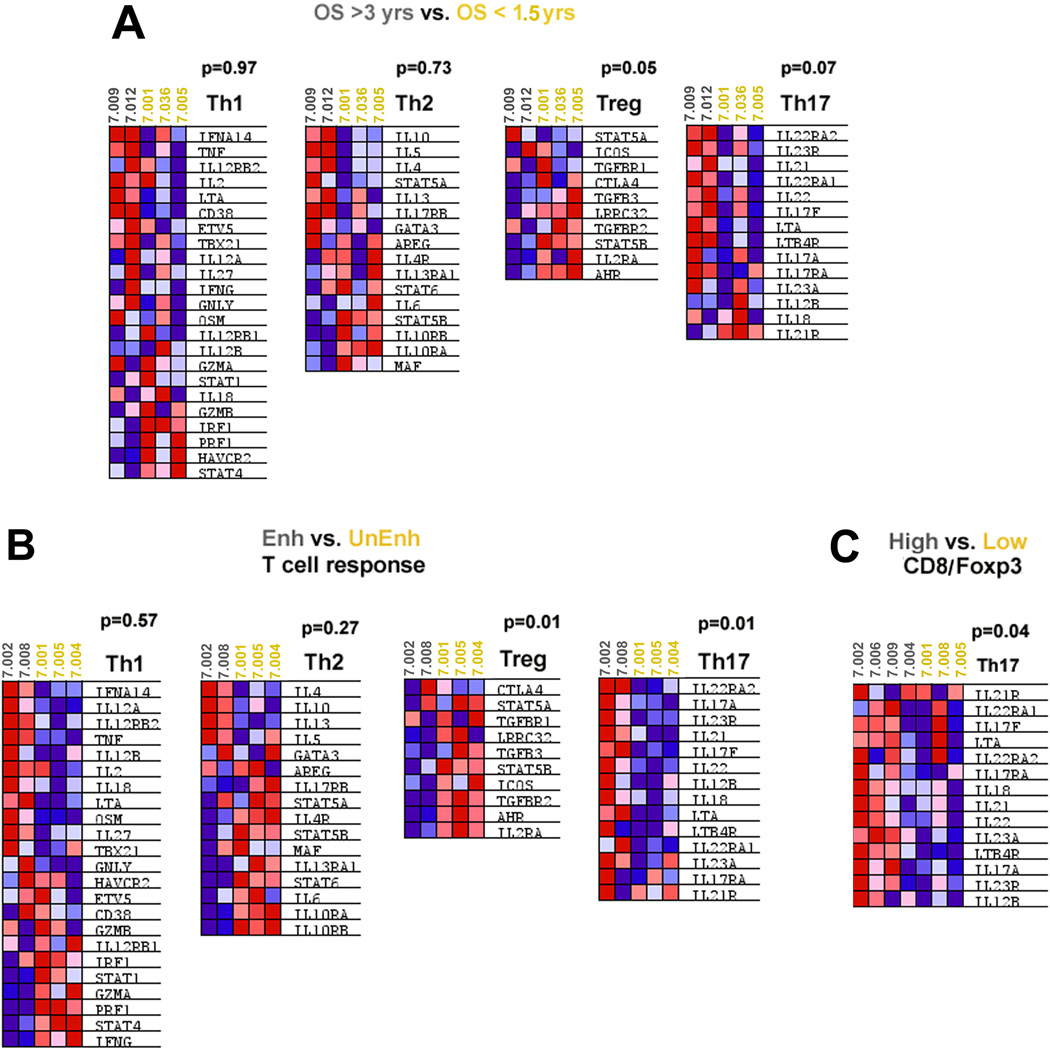
Gene ontology analysis of the microarray data revealed that genes involved in regulating immune cell trafficking and function were differentially expressed, including genes encoding multiple integrins, chemokines, and chemokine-receptors, and members of the NF-κB pathway, and the ubiquitin-proteasome system (UPS) (Figures S7A-D, respectively). These pathways are involved in regulating both adaptive and innate immune cells. A few themes among the signatures were present. Specifically, chemokine and integrin signals involved in recruiting immunosuppressive populations were downregulated in patients who had longer survival. These include: the chemokines CXCL9, CCL18, CCL19, and CCL21, the chemokine receptor CCR7 involved in recruiting Tregs (34, 46–48), chemokine CCL2 involved in recruiting immunosuppressive populations of monocytes/macrophages (49, 50), and the integrins ITGA4 and ITGB1 that are expressed by the suppressive MDSCs (51) (Figure 4A). A specific NF-κB signature defined by the downregulation of canonical NF-κB genes (NFKB1, RELA, REL) combined with the upregulation of non-canonical NF-κB genes (NFKB2 and RELB) was associated with improved post-vaccination responses (Figure 4B). Although NF-κB is a known regulator of all immune subsets, this signature is consistent with polarization toward Th17 and away from Tregs (52, 53). The UPS signature may also represent both adaptive and innate immune signals but the underlying mechanisms of this signature are less clear. However it is interesting to note that the downregulation of the UBE2N gene that encodes an E2 ubiquitin-conjugating enzyme important for maintaining Treg cell function and preventing Tregs from acquiring effector T cell-like function (54) was specifically associated with longer survival and higher intratumoral effector T cell:Treg ratios (Figure 4B). In addition, multiple genes involved in the Treg pathway were downregulated while multiple genes in the Th17 pathway were upregulated in lymphoid aggregates from patients who survived longer and developed enhanced immune responses (Figure 4C & Figure S8). In contrast, the expression of Th1 and Th2 effector pathway genes was not uniformly upregulated or downregulated (Figure 4C). Gene signature enrichment analysis (39) (GSEA) of the microarray data further supported the hypothesis that the differences in gene expression in the Treg and Th17 pathways, but not in the Th1 or Th2 pathways, in these aggregates may predict clinical and immune responses (Figure 5).
Figure 4. Gene expression signatures in lymphoid aggregates associated with immune responses and overall survival.
Gene expression measured by microarray was compared between microdissected lymphoid aggregates from 14 tumors grouped according to overall patient survival (OS > 3 years vs. < 1.5 years), post-vaccination induction of enhanced mesothelin-specific T-cell responses in PBL (Enh vs. Unenh), and the intratumoral CD8+ T effector/FoxP3+ Treg ratio (High vs. Low CD8/Foxp3 ratio). Gene ontology analysis revealed the enrichment of differentially expressed genes encoding numerous chemokine and chemokine receptor, integrin and adhesion molecule, NFkB pathway, and UPS components (Figure S6). Differences in Treg and Th17 pathway but not the Th1 or Th2 pathway genes were also associated with improved post-vaccination responses and survival (Figure S7). The linear fold-change in expression for each comparison is shown for representative genes in: (A) Chemokine, chemokine receptor, and integrin genes; (B) NFKB pathway genes; (C) T-helper pathway genes; and (D) PD-1 and PD-L1.
Figure 5. GSEA analyses of Th1, Th2, Th17 and Treg pathways.
GSEA analyses were performed on the microarray data. A, Comparison between specimens from patients with OS > 3 years (subject numbers in grey) and patients with OS < 1.5 years (subject numbers in yellow); B, Comparison between specimens from patients who developed enhanced T-cell responses (in grey) and patients who did not (in yellow); C, Comparison between specimens from patients whose tumors were infiltrated with a high CD8/FoxP3 ratio (in grey) and whose tumors were infiltrated with a low CD8/FoxP3 ratio (in yellow). Heat maps of the microarray gene expression data are shown. Blue shading represents downregulation and red represents upregulation of gene expression. P values are shown for each GSEA comparison.
Since PD-L1 protein expression was shown by IHC to be induced by vaccination, we next compared PD-L1 and PD-1 gene expression with respect to OS, enhanced T-cell responses, and Effector:Treg ratios (Figure 4D). PD-L1 expression was downregulated in patients who survived > 3 years whereas there was no correlation with enhanced T-cell responses and Effector:Treg ratios, In contrast, PD-1 expression was increased in lymphoid aggregates from patients who survived > 3 years and who demonstrated enhanced mesothelin-specific T-cell responses. Since PD-1 upregulation was associated with improved survival and immune responses, it may be a marker of post-vaccination T-cell activation in these aggregates, independent of its role in downregulating antitumor T-cell responses when engaged by its ligand, PD-L1.
Decreased Tregs are associated with enhanced Th1/Th17 signals in lymphoid aggregates and prolonged survival following vaccine therapy
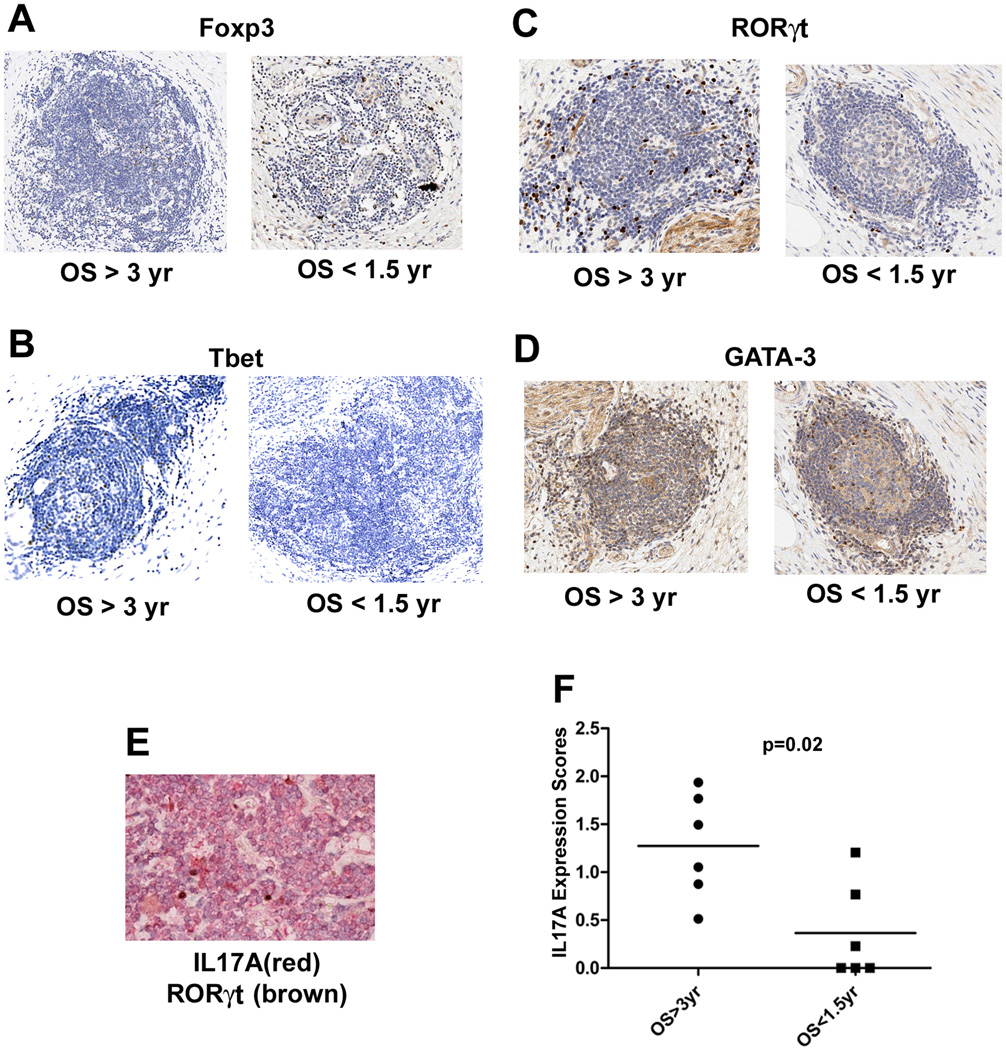
Microarray analysis of microdissected intratumoral lymphoid aggregates was performed in a subset of vaccinated patients and IHC was used to further evaluate the in situ expression of T-helper pathway genes in a larger cohort of patients. We examined the expression of representative transcription factors known to drive Th1 (T-bet), Th2 (GATA-3), Treg (Foxp3), and Th17 (RORγt) differentiation. As shown in Figures 6A-D, T-bet and RORγt protein expression was increased, whereas Foxp3 expression was decreased in the immune aggregates from patients who survived longer. No difference in GATA3 expression was observed. Co-staining of RORγt and IL17A (Figure 6E) confirmed that RORγt+ cells also expressed IL17A, although many IL17A+ T cells did not express RORγt, likely because Th17 cytokine expression is also regulated by other transcription factors (55). Depending on the cytokine milieu, Th17 cells can also express T-bet and possess Th1-like effector functions (56), but it is not yet clear whether T-bet+ cells in these lymphoid aggregates were producing Th1-like or Th17-like cytokines, or both. Regardless of the specific transcription factor(s) driving IL17A expression, we found that IL17A protein expression increased significantly in lymphoid aggregates from patients who survived >3 years compared to those who survived <1.5 years (Figure 6F).
Figure 6. In situ expression of signature genes in the Th1, Th2, Th17 and Treg pathways.
A. Anti-Foxp3 antibody staining for Tregs. B. Anti-T-bet staining for Th1 cells. C. Anti-RORγt staining for Th17 cells. D. Anti-GATA-3 staining for Th2 cells. For each target, one representative lymphoid aggregate from a patient with OS > 3 years and one from a patient with OS < 1.5 years is shown. All positive signals are in brown. E. Co-staining of IL17A (in red) and RORγt (in brown) in a representative lymphoid aggregate. F. Cumulative IL17 signals for all intratumoral lymphoid aggregates for each patient’s tumor section analyzed were quantified using Image Analysis Software (Aperio) and the expression levels were compared between patients with OS > 3 years (n=6) and OS < 1.5 years (n=6). Medians and the p value calculated using the Wilcoxon signed rank test are shown.
Signatures within TIL reveal reduced Tregs but no differences in Th1/Th2/Th17 gene expression in association with survival
We next evaluated gene expression in TIL isolated from PDAC tumor specimens to determine if the same T-cell signatures in the aggregates were also present in TIL. TIL were sorted from 20 PDAC TIL specimens for the analysis of gene expression in individual CD4+CD8+ and non-T cell sub-populations. Consistent with signatures in the aggregates, Th1 and Th2 pathway gene expression was not associated with any parameters of response (Table S3), and Treg pathway gene expression was decreased in CD4+ TIL from patients with longer survival (Table S3 & Figure S9A). However, unlike signatures in the aggregates, Th17 pathway gene expression in CD4+ TIL or CD8+ TIL was not different between patients who survived > 3 years and those who survived < 1.5 years (Figures S9B & C respectively & Table S3). This may not be surprising since some key Th17 pathway cytokines, such as IL23A, are not expressed by T cells but by other cell types (Table S4) that are abundant in the aggregates. However, the majority of TIL specimens also did not have detectable levels of IL17 expression (Table S4). These data suggest that gene expression signatures in the total population of T cells that infiltrate tumors are similar, but not identical to gene signatures in T cells within the lymphoid aggregates. Furthermore, these data suggest that IL17A-producing T cells represent a relatively small population of T cells within the TME that are enriched in lymphoid aggregates of vaccine responders. In support of this notion, only 0.124 - 2.27% of CD4+ TIL and 0 - 0.410% of CD8+ TIL from vaccinated patients produced IL17A following overnight stimulation with anti-CD3- and anti-CD28-coated beads (Figure S10). Taken together, these data suggest that vaccine-induced Th17 signatures reside mainly within the lymphoid aggregates.
Low Treg numbers in lymphoid aggregates enable enhanced T-cell activation and trafficking within PDAC tumors
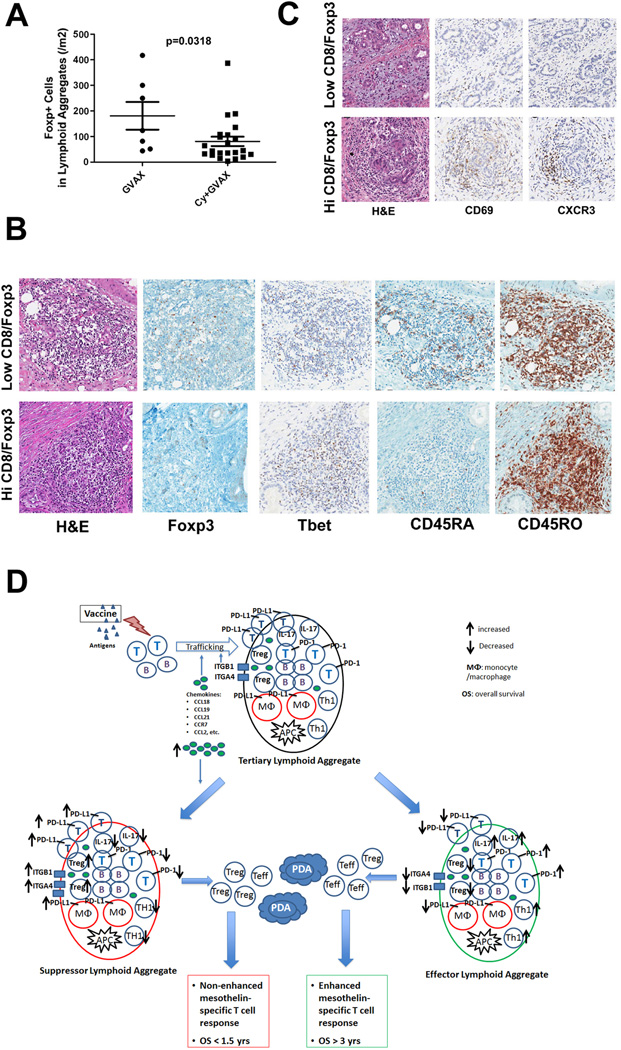
Since decreased Treg signatures in the aggregates and TIL are associated with improved post-vaccination responses, and the intended purpose of low-dose Cy is Treg-depletion, we next evaluated by IHC the effects of Cy on the number of Tregs in vaccine-induced aggregates. A trend toward increased numbers of aggregates infiltrating PDACs was observed in patients treated with GVAX plus Cy (Figure S11A). Although the overall numbers of CD4+ T cells were not different (Figure S12), fewer Foxp3+ Tregs were detected in the aggregates from patients treated with GVAX plus Cy versus with GVAX alone (Figure 7A). Since both forms of Cy used to deplete Tregs showed similar results, the two Cy groups were combined for the analyses. Even though we only evaluated TIL from ten treated patients (Figure 3C), consistent with the IHC data, CD3+CD4+FoxP3+Treg levels measured in TIL by FACS were also lower in patients who received GVAX plus Cy versus GVAX alone (Figure S11B). These data suggest that Cy can lower Treg levels in the TME, and Tregs may inhibit lymphoid aggregate formation.
Figure 7. Low-dose cyclophosphamide reduces intratumoral Treg numbers and promotes enhanced T-cell trafficking and activation within the tertiary lymphoid aggregates.
A. Comparison of the densities of Foxp3+ T cells in lymphoid aggregates between patients receiving GVAX alone (GVAX) and patients receiving GVAX plus Treg-modulating doses of Cy (CAX+Cy). B. IHC staining of Foxp3, T-bet, CD45RA, and CD45RO in representative lymphoid aggregates in PDACs from patients with high Teffector:Treg ratios in the TME compared to those with low Teffector:Treg ratios in the TME. C. IHC staining of CD69 and CXCR3 in representative PDAC tumor areas from patients with high versus low Teffector:Treg ratios in the TME. All positive IHC signals are in brown. D. Proposed model of vaccine-induced T-cell infiltration into the PDAC TME. At baseline, the “non-immunogenic” PDAC TME is primarily immunosuppressed and infiltrated with Tregs and other immunosuppressive populations of innate immune cells, and low numbers of effector T cells. Vaccination induces antigen-specific T cells systemically that traffic to the TME and initiate an inflammatory reaction. Chemokines including CCL2, CCL18, CCL19 and CCL21, and other signals produced during this inflammatory reaction recruit additional immune effector and regulatory cells, and induce the neogenesis of tertiary lymphoid aggregates within the TME, converting the PDAC TME into an “immunogenic” environment. These lymphoid aggregates are composed of effector T cells, B cells, Tregs and antigen-presenting cells (APCs), and serve as nodal sites for immune activation and regulation within the TME. Paradoxically, higher expression of the chemokines associated with the development of these aggregates favors the recruitment of immunosuppressive immune cells into the lymphoid aggregates in the TME. Lymphoid aggregates dominated by effector T cells and immune activation signals (“Effector Lymphoid Aggregates”) including: increased CD8+ effector T cells and effector T cells with Th17 signatures, increased PD-1 expression on effector T cells, decreased Tregs, and lower expression of PD-L1, are associated with higher Teffector:Treg ratios in TIL and enhanced post-vaccination T-cell responses in PBL, and generate productive antitumor responses that can prolong survival. In contrast, lymphoid aggregates dominated by immunosuppressive immune cells and signals (“Suppressor Lymphoid Aggregates”) including: decreased CD8+ effector T cells and effector T cells with Th17 signatures, decreased PD-1 expression on effector T cells, increased Tregs, and higher expression of PD-L1, are associated with decreased T effector:Treg ratios in TIL and unenhanced T-cell responses in PBL, do not generate productive antitumor responses, and cannot prolong survival. Use of targeted immune modulators may tip the balance between these signals in favor of the development of “effector lymphoid aggregates” and enhance the intratumoral immune responses induced by vaccines. However, without a vaccine, an “immunogenic” TME containing effector T cells and lymphoid aggregates is not available for immune modulators to act on.
To evaluate the impact of Teffector:Treg ratios on T-cell activation within the TME, we evaluated the expression of T-cell activation markers in tumors with low and high T effector:Treg ratios defined by IHC. Teffector:Treg ratios were considered high if they were above 10, and low if they were below 5. Increased numbers of CD45RO+ antigen-experienced and T-bet+ T cells, and decreased numbers of CD45RA+ naïve T cells were detected in lymphoid aggregates from vaccinated patients that had higher Teffector:Treg ratios in their TME (Figure 7B). In patients with higher Teffector:Treg ratios, greater numbers of activated lymphocytes expressing CD69 and CXCR3 were also found trafficking outside of lymphoid aggregates into the stroma surrounding neoplastic cells (Figure 7C). Thus, these data suggest that treatment with Cy skews the post-vaccination intratumoral Teffector:Treg ratio, and promotes effector T-cell activation and trafficking within the TME.
Discussion
Here we report the first description of tertiary lymphoid aggregates infiltrating human PDAC. These aggregates develop two weeks after a single treatment with a PDAC vaccine. These novel structures express TME signatures that: describe previously unrecognized foci of immune regulation within PDAC; uncover PDAC signaling pathways associated with long-term survival; and identify potential targets for collaborative immune modulation in the setting of a PDAC vaccination. Taken together, these lymphoid aggregates provide the first demonstration that an immune-based therapy can convert a “non-immunogenic” neoplasm into one that is similarly “immunogenic” to melanomas and RCCs.
Tertiary lymphoid structures play a critical role in numerous autoimmune and inflammatory diseases (57). Different from primary and secondary lymphoid structures, their formation depends on antigen stimulation and represents an ongoing adaptive immune response. However, their role in cancer development and cancer therapy is not clear. In immunotherapy-naïve patients with colon cancer, NSCLC, and melanoma, increased numbers of lymphoid structures have correlated with better prognoses (31–33) and may be useful for selecting patients that are better candidates for immunotherapy (31). However, whether these structures are formed in response to tumor-antigen stimulation or to the pathogens and inflammatory conditions to which they are often exposed is unknown. Furthermore, the anti-cancer role of these structures is not established. In fact, under chronic inflammatory conditions, similar lymphoid structures are also associated with cancer development (58).
We have established for the first time that GVAX can induce similar intratumoral tertiary lymphoid structures not naturally formed in PDAC. Our data support the notion that these structures are newly formed based on the expression of neogenesis markers within these structures together with antigen-experienced and activated T cells not commonly found in untreated PDACs. Whether these tertiary lymphoid structures are unique to GVAX or can be elicited by other forms of immune-based therapy is not yet known. Similar to reports in the literature (32, 33), these structures were associated with prolonged survival in some but not all GVAX-treated patients. This is not surprising since our analyses support their role as organized structures of immune regulation. Evidence supporting their role as regulators includes the expression of early markers of T-cell activation, IFNγ, as well as the recruitment of suppressive cell populations and the upregulation of T-cell suppressing regulatory pathways. It is possible that the formation of lymphoid aggregate results from the activation and re-organization of immune cells present in the TME prior to vaccination. Since GVAX is delivered intradermally and not at the tumor site, presumably additional immune cells may traffic into the tumors following vaccination. In this study, we did not obtain pre-vaccination tumor biopsies because it is not standard of care at our institution. For future studies, collection of pre-treatment tumor biopsies will be necessary to evaluate changes in the TME following vaccination and to elucidate the mechanisms driving the development of post-vaccination lymphoid aggregates. Additional studies will be required to determine whether T cells that are activated within the lymphoid aggregates migrate directly into the tumor tissue, or re-enter the circulation first. We have assumed that mesothelin-specific T cells measured in the PBL are activated in the vaccine-draining lymph nodes. However, it is possible that some mesothelin-specific T cells are actually activated within the aggregates. In fact, it is possible that different routes can be used to enter the tumor depending on whether a T cell is activated in the periphery, or within a lymphoid aggregate.
In some PDACs, the lymphoid aggregates were dominated by immunosuppressive signals such as IL6, STAT3, and TGFβ, while others had proinflammatory signals such as IL-12, IFNγ, and IL-17, which are associated with activated T-cell responses. The immunosuppressive signaling proteins PD-1 and PD-L1 were present in all intratumoral lymphoid aggregates. Since IFNγ can induce PD-L1 expression (34, 35), it is likely that the PD-L1 expressed in the intratumoral lymphoid aggregates was induced by the IFNγ produced by the lymphoid aggregate-residing CD4+ and CD8+ T cells. This could explain partially why IFNγ gene expression was lower in lymphoid aggregates in patients who demonstrated improved post-vaccination responses and longer survival. In contrast, PD-1 was upregulated in the lymphoid aggregates from patients who demonstrated improved survival and enhanced post-vaccination peripheral T-cell responses. PD-1 is a marker of T-cell activation and likely requires the engagement with PD-L1 to confer a downregulatory signal to the T cell. However, PD-L1 was not naturally upregulated in treatment-naïve patients, or in untreated mice bearing implanted PDAC tumors (Soares et al. manuscript submitted). Our data support prior reports that PD-L1 is upregulated by IFNγ-secreting T cells recruited into the TME (34, 35). However, patients with lower levels of PD-L1 within their lymphoid aggregates demonstrated longer OS, which is consistent with the increased antitumor effect observed in PDAC-bearing mice treated with GVAX in combination with PD-1/PD-L1-targeted immune modulation (Soares et al. manuscript submitted).
This study also aimed to address the role of Tregs in altering T-cell responses within the TME. Prior reports from our group and others have shown that Treg infiltration in PDAC and other cancers is associated with T-cell suppression within the TME in human patients and in mouse models (16, 21–29). Some reports have also correlated baseline Treg-infiltration with poorer prognosis in patients with PDAC (20, 43). Preclinical data have indicated that the Teffector:Treg ratio, rather than the absolute numbers of Tregs infiltrating the TME, correlates with antitumor immunity (59). This is the first study conducted in human patients confirming that it is the balance between the infiltrating effector T cells and Tregs that correlates with treatment outcomes. This is also the first study to show in patients that Cy given with vaccination does indeed alter the balance in favor of an effector T-cell response. However, not all patients that did not receive Cy had a lower Teffector to Treg ratio, supporting differences in the natural inflammatory composition of PDACs among patients, which will require further exploration.
The complex nature of the TME is demonstrated by the functional and signaling differences between the lymphoid aggregates and the TIL that were evaluated in a smaller subset of patients. The lymphoid aggregates expressed signals of T-cell activation and regulation; TIL expressed signals of activation predominantly. Notably, the aggregates expressed mixed Th1 and Th17 signals whereas TIL mostly expressed Th1-like signals. In fact, differentially expressed genes in the five signaling pathways (Th17/Treg, NFkB, Ubiquitin-proteasome, Chemokines/chemokine receptors, and Integrins/adhesion molecules) within the intratumoral lymphoid aggregates were associated with improved post-vaccination responses. These pathways have known effects on immune responses and the development of lymphoid structures. In particular, the activation of the non-canonical NF-kB pathway following lymphotoxin/lymphotoxin receptor engagement is critical for lymphoid structure development (60). Our results are consistent with prior studies showing that Tregs can suppress the development of tertiary lymphoid structures (61, 62) whereas cells with Th17 sub-signatures can promote their development (63, 64). Pro-cancer inflammatory signals, including IL-6 and STAT3 (65) that are also positive regulators of Th17 responses (55, 66), were not upregulated in the vaccine responders. Our data suggest that the upregulation of the Th17 pathway is associated with improved responses to GVAX, but the exact role of IL17-producing cells in the post-vaccination TME is not yet known. Given that Th17 cells can possess both Treg and Th1-like effector functions (56, 67–70), it is possible that particular Th17 sub-signatures are correlated more closely with improved post-vaccination immune responses (65, 71–73). Although it is not clear how the vaccine-induced lymphoid aggregates identified in this study compare to naturally occurring tertiary lymphoid structures, several of the twelve chemokines (including CCL2, CCL18, CCL19, CCL21 and CXCL9) that are associated with melanoma (31), were expressed but downregulated in post-vaccination aggregates in patients who demonstrated prolonged survival and elevated intratumoral Teffector: Treg ratios. These chemokines may affect the formation of vaccine-induced lymphoid aggregates by attracting both effector T cells and Tregs; our data suggest that at higher levels, these chemokines may favor Tregs and a more immunosuppressive TME. Additional studies are required to refine these signatures and determine which members of these pathways are critical for regulating the trafficking of lymphocytes into the tumor, the formation of lymphoid aggregates, and the evolution of the PDAC TME following vaccination.
Based on our findings, we propose a new model in which traditionally “non-immunogenic” tumors can be converted into “immunogenic” tumors responsive to combination immune-based therapies. In this model (Figure 7D) a systemically administered vaccine is required to initiate the induction of immune responses that can traffic into tumors and create an inflammatory environment. This environment has the potential to support an anticancer immune response, but it contains numerous immunosuppressive components, such as infiltrating Tregs and other suppressive cell populations, and the upregulation of PD-L1, which is a natural response to the initial inflammatory “invasion” of the TME. At this stage, the organized inflammatory infiltrates are sites of immune regulation. In some patients, this initial inflammation is enough to shift the balance from immune suppression to immune activation, particularly when a vaccine is given with Treg-depleting therapy. However, in most patients, additional immune modulation is needed, which may include targeting MDSC, tumor-associated macrophages (TAM), and tumor-associated neutrophils (TAN), to more effectively shift the balance from predominantly cumulative T-cell downregulatory signals toward a majority of T-cell activating signals. Additional studies are required to determine the role of these immunosuppressive cell subsets in modulating the post-vaccination TME.
Based on the evidence available, this model explains why vaccination and immune modulation as single-agent therapies have failed in PDAC patients. This model is further supported by our recent study demonstrating objective responses in metastatic PDAC patients treated with the combination of GVAX + ipilimumab but not with ipilimumab alone (12). Our model provides the strongest rationale so far for combining immune modulating agents with vaccination in patients whose tumors do not naturally contain abundant effector T cells.
Supplementary Material
Acknowledgements
A full clinical and immune correlates report will be submitted at the completion of the clinical trial. A.A.W and E.B. contribute equally. We thank Dr. Katie Bever, who provided technical assistance in the beginning of this study. We thank Lee Shu-Fune Wu, Connie Talbot, Soonweng Cho, and Donger Zhou for their advice on microarray data analyses. This work was supported by the NIH K23 CA148964-01 (L.Z.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), an American Society of Clinical Oncology Young Investigator Award (L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (D.L., E.M.J., E.R.L. and L.Z), The National Pancreas Foundation (L.Z.), Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M.J., L.Z., and E.R.L.), Lustgarten Foundation (E.M.J. and L.Z.), and the Sol Goldman Pancreatic Cancer Center (E.R.L. and L.Z.), AACR-FNAB Fellows Grant for Translational Pancreatic Cancer Research Award 09-30-14-LUTZ (E.R.L.). Dr Jaffee is the first recipient of the Dana and Albert “Cubby” Broccoli Endowed Professorship. Under a licensing agreement between Aduro BioTech, Inc. and the Johns Hopkins University and Dr. Elizabeth Jaffee, the University is entitled to milestone payments and royalty on sales of the vaccine product described in this manuscript.
RAA is supported by a commercial research grant from Bristol-Meyers Squibb.
Footnotes
Conflict of interest:
The other authors have no conflict to disclose.
References
- 1.Society AC. Cancer Facts & Figures. Atlanta: 2012. [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Letters. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 7.Melief CJ, Kast WM. T-cell immunotherapy of cancer. Res Immunol. 1991;142:425–429. doi: 10.1016/0923-2494(91)90042-h. [DOI] [PubMed] [Google Scholar]
- 8.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–932s. [PubMed] [Google Scholar]
- 9.Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, et al. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol. 2011;2011:267539. doi: 10.1155/2011/267539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares KC, Zheng L, Edil B, Jaffee EM. Vaccines for pancreatic cancer. Cancer J. 2012;18:642–652. doi: 10.1097/PPO.0b013e3182756903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, et al. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci. Am. 1998;4:194–203. [PubMed] [Google Scholar]
- 16.Laheru D, Lutz E, Burke J, Bierdrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz E, Yeo CJ, Lillemoe KD, Bierdrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, HUang LQ, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 22.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Hermann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 23.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 24.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. . J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 26.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–8413. [PubMed] [Google Scholar]
- 27.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss VL, Lee TH, Jaffee EM, Armstrong TD. Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology. 2012;1:1191–1193. doi: 10.4161/onci.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PloS One. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 31.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mule JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179:37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wisiez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 34.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-Regulation of PD-L1 IDO Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigelow E, Bever KM, Xu H, Yager A, Wu A, Taube J, et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013;71:4059. doi: 10.3791/4059. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubbers J, Brink M, van de Stadt LA, Vosslamber S, Wesseling JG, van Schaardenburg D, et al. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann Rheum Dis. 2013;72:776–780. doi: 10.1136/annrheumdis-2012-202753. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Nierop K, de Groot C. Human follicular dendritic cells: function, origin and development. Sem Immunol. 2002;14:251–257. doi: 10.1016/s1044-5323(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 41.Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- 42.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs. 2010;11:1354–1359. [PubMed] [Google Scholar]
- 43.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PubMed] [Google Scholar]
- 45.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 Conditioned Regulatory T Cells Induce Allotolerance through Enhanced Homing to Lymphoid Tissue. J Immunol. 2014;192:817–823. doi: 10.4049/jimmunol.1203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B, Zhang D, Zhou J, Li Q, Zhou L, Li SM, et al. High CCR6/CCR7 expression and Foxp3+ Treg cell number are positively related to the progression of laryngeal squamous cell carcinoma. Oncol Rep. 2013;30:1380–1390. doi: 10.3892/or.2013.2603. [DOI] [PubMed] [Google Scholar]
- 48.Chenivesse C, Chang Y, Azzaoui I, Ait Yahia S, Morales O, Ple C, et al. Pulmonary CCL18 recruits human regulatory T cells. J Immunol. 2012;189:128–137. doi: 10.4049/jimmunol.1003616. [DOI] [PubMed] [Google Scholar]
- 49.Porta C, Subhra Kumar B, Larghi P, Rubino L, Mancino A, Sica A. Tumor promotion by tumor-associated macrophages. Adv Exp Med Biol. 2007;604:67–86. doi: 10.1007/978-0-387-69116-9_5. [DOI] [PubMed] [Google Scholar]
- 50.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 51.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–210. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 52.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruan Q, Chen YH. Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol. 2012;946:207–221. doi: 10.1007/978-1-4614-0106-3_12. [DOI] [PubMed] [Google Scholar]
- 54.Chang JH, Xiao Y, Hu H, Jin J, Zhou X, Wu X, et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol. 2012;13:481–490. doi: 10.1038/ni.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 58.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leuk Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- 59.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 61.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal immunol. 2010;3:537–544. doi: 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 62.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grogan JL, Ouyang W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur J Immunol. 2012;42:2255–2262. doi: 10.1002/eji.201242656. [DOI] [PubMed] [Google Scholar]
- 64.Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 67.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J immunol. 2012;42:2215–2220. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 70.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gottorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 71.Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-beta-dependent immunosuppressive activity? Trends in Mol Med. 2012;18:742–749. doi: 10.1016/j.molmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182:10–20. doi: 10.1016/j.ajpath.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.