Abstract
Although alcohol effects within the liver have been extensively studied, the complex mechanisms by which alcohol causes liver cancer are not well understood. It has been suggested that ethanol (EtOH) metabolism promotes tumor growth by increasing hepatocyte proliferation. In this study, we developed a mouse model of tumor promotion by chronic EtOH consumption in which EtOH feeding began 46 days post-injection of the chemical carcinogen diethylnitrosamine (DEN) and continued for 16 weeks. With a final EtOH concentration of 28% of total calories, we observed a significant increase in the total number of preneoplastic foci and liver tumors per mouse in the EtOH+DEN group compared to corresponding pair-fed (PF)+DEN and chow+DEN control groups. We also observed a 4-fold increase in hepatocyte proliferation (p<0.05) and increased cytoplasmic staining of active-β-catenin in non-tumor liver sections from EtOH+DEN mice compared to PF+DEN controls. In a rat model of alcohol-induced liver disease, we found increased hepatocyte proliferation (p<0.05); depletion of retinol and retinoic acid stores (p<0.05); increased expression of cytosolic and nuclear expression of β-catenin (p<0.05) and phosphorylated-glycogen synthase kinase 3 β (p-GSK3β, p<0.05; significant up-regulation in Wnt7a mRNA expression; and increased expression of several β-catenin targets, including, glutamine synthetase (GS), cyclin D1, Wnt1 inducible signaling pathway protein (WISP1), and matrix metalloproteinase-7 (MMP7), p<0.05. These data suggest that chronic EtOH consumption activates the Wnt/β-catenin signaling pathways to increase hepatocyte proliferation, thus promoting tumorigenesis following an initiating insult to the liver.
Keywords: DEN-induced carcinogenesis, alcohol, β-catenin, tumor promotion, retinoic acids
Introduction
Hepatocellular cancer (HCC) is one of the leading causes of cancer mortality in the world. In the United States, HCC incidence rates have significantly increased in past decades, which corresponds to the increased prevalence of risk factors in the population including excessive alcohol consumption, chronic hepatitis C (HCV) or hepatitis B infections (HBV), and the metabolic syndrome (1–3). Interestingly, of these factors, alcohol consumption is the dominant independent factor associated with increased risk. In a case-control study involving U.S. HCC patients, heavy alcohol consumption (≥ 80 g daily) was the primary contributing factor for a third of the reported cases (2). The authors also reported increased risk (2-fold) in patients with HCV/HBV infections or diabetes mellitus combined with chronic alcohol consumption. Additionally, the synergistic effect between HCV/HBV infection and chronic alcohol abuse has also been reported in other countries where hepatitis infections are more prevalent (4;5).
In rodent models of alcoholic liver disease ethanol (EtOH) is metabolized by alcohol dehydrogenase (ADH) to acetaldehyde, then further reduced to acetate by aldehyde dehydrogenase (ALDH). Acetaldehyde is a potent carcinogen shown to generate DNA damage and strand breaks by interfering with both DNA synthesis and repair mechanisms (6). Alcohol also induces the expression of cytochrome P450 (CYP) CYP 2E1, which not only participates in alcohol metabolism, but also activates environmental pro-carcinogens, i.e. dietary nitrosamines, while simultaneously producing reactive oxygen species and lipid peroxidation products. These reactive products damage DNA through adduct formation, and strand breaks, all of which increases mutagenicity, and initiates hepatocarcinogenesis (6–8).
Apart from the initiation mechanisms, alcohol consumption has proliferative and tumor promoting effects (9–15). Several signaling pathways have been implicated in this process. One of which is decreased retinoic acid receptor (RAR) signaling resulting from vitamin A depletion in alcoholic livers (11). In EtOH-treated rats, down-regulation of RAR signaling resulted in hepatocyte proliferation associated with increased expression of Wnt signaling targets, cyclin D1 and cJun. Moreover, loss of RAR signaling in transgenic mice expressing a dominant-negative form of RAR in the liver also resulted in increased nuclear β-catenin/TCF4 complex formation and tumor incidence (16). Another pathway associated with alcohol exposure is transforming growth factor alpha (TGFα) activation of the epidermal growth factor receptor (EGFR) and p42/p44 mitogen kinase (MAPK) and PI3K/AKT pathways (17,18). Interestingly, in mice overexpressing c-Myc and TGFα in the liver Phenobarbital exposure significantly enhanced proliferation and progression in a subset of tumors with increased nuclear accumulation of β-catenin (18,19).
A significant proportion of HCC patients including those from alcoholics have tumors defined by activated β-catenin (20,21). In these tumors, aberrant activation occurs through mutations found either in the β–catenin gene, primarily in the phosphorylation site for glycogen synthase kinase 3β (GSK3β), or in Axin1/2, a scaffolding protein necessary for targeting β-catenin for degradation (22,23). However, studies using transgenic mice expressing mutant forms of β-catenin have shown that activation through mutations is not sufficient to initiate tumorigenesis, and suggest instead that Wnt/β-catenin signaling participates in tumor promotion (22). In light of the published data showing an association of β-catenin activity with signaling pathways involved in EtOH-mediated proliferation, we developed a mouse model of tumor promotion using diethylnitrosamine (DEN) as the initiation agent to test if long-term EtOH ingestion increases tumor multiplicity through increased β-catenin signaling. To eliminate any possible initiating effects in response to EtOH feeding, primarily through increased CYP 2E1 expression and activity, liquid diets were started 7 wks post DEN injection. A Lieber-DeCarli EtOH liquid diet was maintained until sacrifice (4 mo). At the same time, we utilized liver tissue from a previously published rat model of long-term, chronic alcoholic liver disease to mechanistically validate the hypothesis that chronic EtOH ingestion alone increases hepatocyte proliferation through an up regulation of Wnt signaling, resulting in increased expression of known progression markers of HCC.
Materials and Methods
Animals and experimental design
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Mice were housed in an Association Assessment and Accreditation of Laboratory Animal Care approved animal facility. Forty, time-impregnated C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME) were used to generate male pups (n=51) which received a single i.p. injection of 10 mg/kg DEN on postnatal day (PND)14. Mice were weaned to and maintained on rodent chow (Harlan Laboratories, Houston, TX) until PND60 at which time DEN-injected mice were randomly assigned to three weight-matched diet groups: a chow diet (n=17, chow+DEN), an EtOH-containing Lieber-DeCarli liquid diet (n=15, EtOH+DEN), and a corresponding Pair-fed (PF) diet (n=18, PF+DEN). All groups had access to water ad libitum. EtOH was added to the Lieber-DeCarli liquid diet (35% of energy from fat, 18% from protein, and 47% from carbohydrates) by slowly substituting EtOH for carbohydrate calories (Dyets Inc., Bethlehem, PA) in a stepwise manner until 28% total calories were reached. This dose constitutes a final EtOH concentration of 4.9% (v/v), respectively, and was maintained until sacrifice (4 mo). PF+DEN mice were fed the control diet (Dyets Inc., Bethlehem, PA), that was isocalorically matched to the diet consumptions of the EtOH group. At sacrifice, mouse livers were perfused with 10% neutral buffered formalin (NBF, approximately 10 mls) in situ, removed, and stored in 10%NBF. Blood alcohol concentrations were analyzed using an Analox analyzer as previously reported (24). In a replicate study, 30 male mice were injected with DEN (10 mg/kg, PND 14). At PND60 mice were randomized (n=10) into chow+DEN, PF+DEN, and EtOH+DEN diet groups. Diets were maintained for 4 mo as described above. At sacrifice liver pieces were flash frozen in liquid nitrogen and stored at −70°C. RNA isolated from these livers was transcribed to cDNA according to manufacturer’s instructions (IScript cDNA synthesis, Bio-Rad Laboratories, Hercules, CA) and used in real-time PCR analysis of Tumor necrosis factor α (TNFα), interleukin 6 (IL-6), CD14, and chemokine CXCL-2. Results were quantified using deltaCT method relative to 18s and then to chow+DEN controls. Primer sequences are presented in Supplementary Table 1.
Pathological evaluation
In-situ fixed mouse livers were stored in formalin a minimum of 48h. Each liver lobe was sliced at 3mm intervals paraffin embedded paraffin, sectioned (4µm), stained, and examined in a blinded fashion under a light microscope by a veterinary pathologist (L.Hennings). Lesions were counted at 40× magnification. Preneoplastic foci were defined as follows: a basophilic focus, defined as a non-compressive lesion less than the width of 4 hepatic lobules in which hepatocytes are smaller and stain more basophilic than normal andan eosinophilic focus, defined as a non-compressive lesion less than the width of 4 hepatic lobules in which hepatocytes are slightly larger than normal with more acidophilic cytoplasm. Preneoplastic foci can develop into adenomas, which were defined as a compressive lesion of any size without evidence of invasion or other criteria of malignancy, and may develop into hepatocellular carcinoma, which was defined as a compressive and invasive lesion with criteria of malignancy (25).
Immunohistochemistry
β-catenin expression was assessed in liver sections by immunohistochemistry using standard procedures and a monoclonal β-catenin antibody (1:75) detecting the active, dephosphorylated (Ser37 or Thr41) form (Anti-Active-β-Catenin, clone 8E7, EMD Millipore, Billerica, MA). Quantification of β-catenin staining was achieved by color deconvolution using Aperio Technologies Spectrum analysis algorithm package and Image Scope analysis software. Proliferating cell nuclear antigen (PCNA) staining was assessed by immunohistochemistry following standard procedures and an anti-PCNA antibody (Abcam, Cambridge, MA). Stained slides were evaluated under a light microscope. Nuclei of S-phase cells stained dark brown; a total of 10 observations (100× field counted) were screened per liver sample. Data are expressed as mean ± St.Err of total positive S-phase cells counted per sample in each group. Fibrosis was detected histologically by Sirius Red/Fast Green collagen staining kit (CHnodrex, Redmond, WA) as per manufacturer’s instructions. Fibrosis staging was scored by a pathologist (K. Lai) blinded to the groupings using the Kleiner/Brunt scoring method: 0-no fibrosis, 1-sinusoidal, periportal or portal only, 2-zone 3 and periportal, 3-bridging fibrosis. α-SMA staining was performed using standard procedures and a monoclonal α-SMA antibody (1:100, Clone 1A4, Dako, Carpinteria, CA) and evaluated under a light microscope.
LC/MS/MS analysis of retinoid concentrations in EtOH-treated rat livers
All tissue processing, preparation and extraction occurred in a fume hood, under a red light. Liver tissue (500 mg) was homogenized in 1ml of Dulbecco’s phosphate-buffered saline solution using a Precellys homogenizer (Bertin Technologies, Rockville, MD). Tissue homogenate was added to a glass screw-capped tube covered by aluminum foil. After adding the internal standard (250 µl, 4,4-retinyl acetate) and 0.025 M KOH to the homogenate, retinoids were extracted in hexane, and dried under a nitrogen stream. Retinoid extracts were reconstituted in 200 µl of acetonitrile and separated by HPLC using an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) using Phenomenex Synergi 4 µ Max-RP 80A column (4 µm, 3×150 mm) as previously described (11). Retinol and retinoic acid were identified using a 4000 Q TRAP mass spectrometer (Applied Biosystems) coupled with the HPLC. The mass spectrometer was controlled using Analyst version 1.5.1 software and was operated in a MRM mode. Optimum positive APCI (Atmospheric pressure chemical ionization) conditions included: curtain gas, 30.0; collision gas, medium; nebulizer current, 3; temperature, 500°C; and GS1, 60. Peak area measurements were used to quantify retinoid amounts using standard curves based on retinol or retinoic acid and normalized using the internal standard.
Protein isolation and Western blotting
Frozen liver samples from a previously published study of chronic alcoholic liver disease were also analyzed (14). Nuclear and cytosolic protein fractions were isolated from TEN control and EtOH-treated rat livers using NE-PER Nuclear and Cytoplasmic Extraction reagent kit (Fisher Scientific, Pittsburg, PA). Blotted proteins were incubated with the anti-active β-catenin antibody, or with a polyclonal antibody recognizing the phosphorylated form of GSK3β, (Phospho-GSK-3α/β (Ser21/9), Cell Signaling Technology, Beverly, MA). Expression of the pro- and active-forms of matrix metalloproteinase-7 (MMP7) were determined in blotted total lysate fractions using a polyclonal anti-MMP7 antibody (Sigma-Aldrich, St. Louis, MO). Primary antibodies were diluted 1:1000 and incubated overnight at 4°C. Secondary antibodies were diluted (1:10,000 to 1:50,000) and incubated at room temperature before chemiluminescense detection. Protein bands were quantified using a densitometer and band densities were corrected for total protein loaded by staining with 0.1% amido black.
Gene expression
RNA was isolated from livers acquired from a previously published rat model of alcoholic liver disease (14). Gene expression of cyclin D1,Ki67, glutamine synthetase (GS), and regucalcin were analyzed in duplicate (n=9/group). Results were quantified using deltaCT method relative to B2M and expressed as mean±SEM. Samples from the TEN and TEN+EtOH groups were pooled (n=3) separately, reversed transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA), and analyzed for gene expression using a WNT signaling pathway RT2 profiler PCR array (#PARN 043Z, Sabiosciences,, Valencia, CA). Arrays for each group were performed in triplicate, and results were analyzed using web based analysis software provided by the manufacturer. Array data are presented in Supplementary Table 2. Genes with changes in fold expression greater than 1.5 and genes with fold changes that were significant, p<0.05, were further analyzed by real time RT-PCR using individual cDNA samples from each group (n=9), SYBR green and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). Results were quantified using deltaCT method relative to β2M, and then to TEN controls. Gene specific primers are presented in Supplementary Table 1.
Data and statistical analysis
Data presented as means ± St.Err. Comparisons between two groups were accomplished using either Student’s T-test or Mann-Whitney U rank-sum test. Number of lesions was compared across groups using negative binomial regression which generalizes Poisson regression to account for over-dispersion of the count data. The proportion of new adenomas (incidence) was compared across groups using logistic regression. Both of these models included group membership as a set of indicator (dummy) variables. Continuous outcomes, such as PCNA and β-catenin were compared across groups using non-parametric Kruskal-Wallis rank test, and significant findings were followed by Bonferroni adjusted Wilcoxon/Mann-Whitney U rank-sum test for post hoc comparisons. Statistical analysis was performed using the SigmaPlot software package 11.0 (Systat Software, Inc., San Jose, CA) and Stata statistical software 13.1 (Stata Corporation, College Station, TX). Statistical significance was set at P<0.05.
Results
Study design and observations
In Fig. 1A, DEN-treated male mice were assigned to an EtOH liquid diet, a PF liquid diet or standard chow 46 days post DEN injection. Starting weights for the mice were 23.9 g ± 0.35, 23.2 g ± 0.26 and 23.7 g ± 0.272 for chow-fed, PF and EtOH, respectively. EtOH feeding continued for 16 weeks. On average, the EtOH+DEN group received 21g/kg/day of EtOH, which resulted in a mean (±SEM) blood alcohol concentration of 75± 29 mg/dL (range 13–393) and is consistent with blood alcohol concentrations reported in persons legally intoxicated in the U.S (26). At sacrifice, weight gain was lower in the chow+DEN and EtOH+DEN groups compared to the PF+DEN group, i.e. 29.0g ± 0.3 and 32.4g ± 0.5 vs. 40.2g ± 0.6, respectively (p<0.05). Similar results were observed in the replicate study (data not shown). The difference in weight gain between EtOH and PF groups has been reported previously (24) and has been suggested to be a result of additional disruptive effects of EtOH on endocrine signaling and white adipose tissue differentiation (27,28).
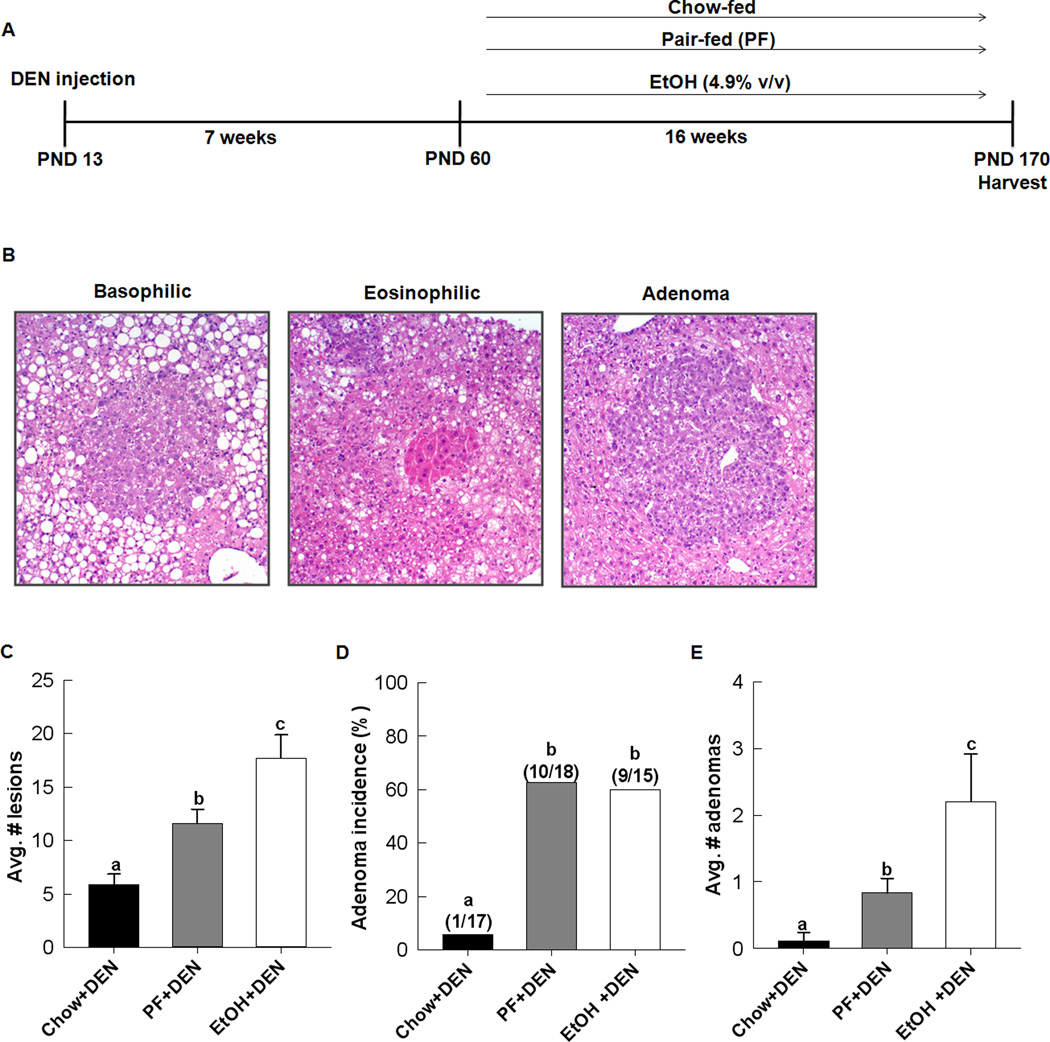
Figure 1.
Chronic EtOH feeding enhances liver tumorigenesis in DEN-treated mice. A, experimental design. B, representative sections of hepatic lesions present in all groups. C, quantification of all lesions per group, D, adenoma incidenc, and E, adenoma multiplicity per mouse for each group, chow+DEN (n=17), PF+DEN (n=18), and EtOH+DEN (n=15). Data are expressed as mean ± St.Err. Significance a<b<c, p<0.05).
EtOH-feeding increases tumor multiplicity but not incidence in DEN-treated mice
Liver lobes taken from chow+DEN, PF+DEN and EtOH+DEN groups were assessed for presence of lesions, which encompassed basophilic and eosinophilic foci and adenomas (Fig. 1B). A small number of mice in the PF+DEN and EtOH+DEN groups had presented carcinomas, 0.22, 4/18 and 0.13 (2/15), respectively, but numbers were insufficient for statistical analysis. Basophilic foci were identified in all DEN-treated mice regardless of diet. Incidence of eosinophilic foci was increased in the DEN-treated mice receiving EtOH, 0.53 (8/15) compared to the PF+DEN, 0.17 (3/18), and chow+DEN, 0.12 (2/17), control groups, p<0.05. Likewise, eosinophilic multiplicity was also greater in the EtOH+DEN group compared to DEN-treated PF and chow fed groups, 0.86 ± 0.27, 0.16 ± 0.09 and 0.17 ± 0.12, respectively, p<0.05. Overall, we observed a significant increase in the total number of lesions present in the EtOH+DEN livers compared to the chow+DEN and PF+DEN groups. (Fig.1C). Adenoma incidence was greater in the PF+DEN and EtOH+DEN groups in comparison to the chow+DEN group (p<0.05). However, we did not observe further increases in adenoma incidence following EtOH feeding, 0.60 vs. 0.55 in the EtOH+DEN and PF+DEN group respectively (Fig. 1D). Adenoma multiplicity was significantly increased in PF+DEN mice compared to chow+DEN mice (p<0.05) and in the EtOH+DEN mice compared to both chow+DEN and PF+DEN groups, P<0.05 (Fig. 1E).
Pro-inflammatory markers are elevated in DEN-treated mice receiving PF and EtOH diets
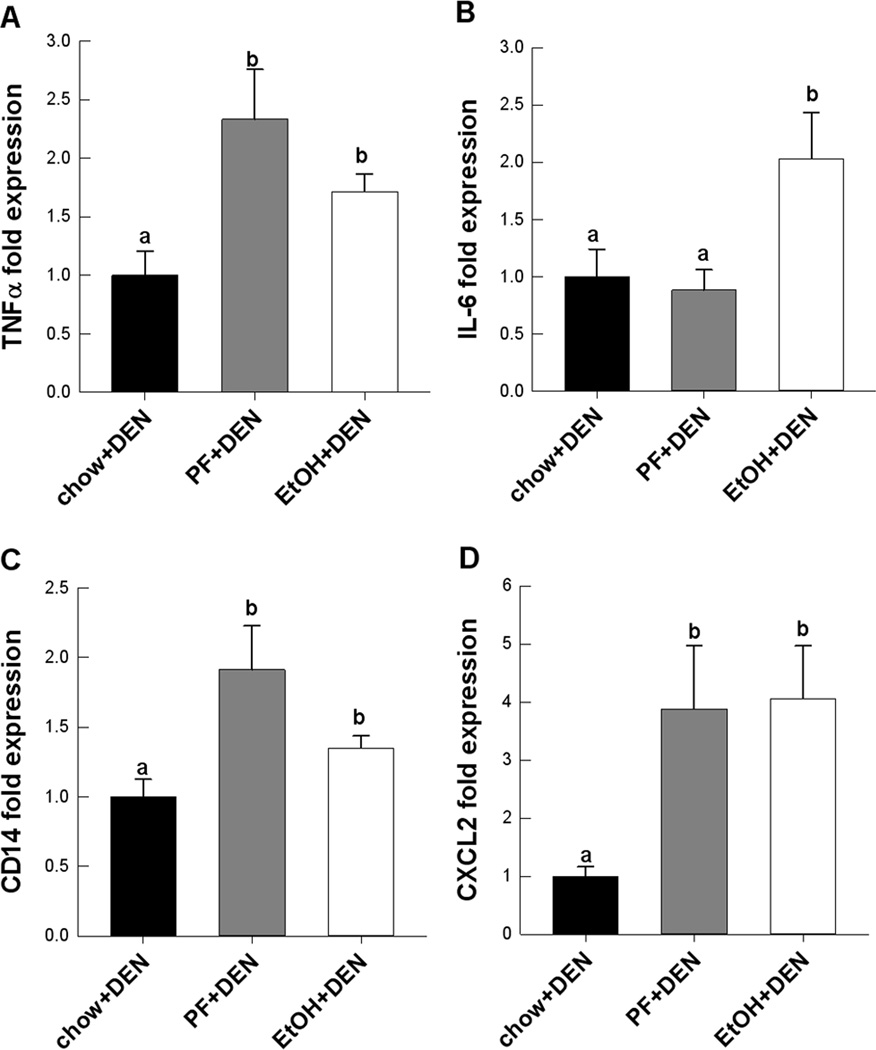
Progression of alcoholic and non-alcoholic fatty liver diseases share several signaling pathways including TNFα-mediated injury (29,30). We measured hepatic expression of TNFα in the different DEN-treated diet groups. TNFα mRNA expression increased significantly in the PF and EtOH groups compared to chow (Fig. 2A). IL-6 expression also increased in the EtOH group compared to both DEN-treated PF and chow fed groups, p<0.05 (Fig. 2B). Corresponding to these findings, we also observed significant increases in CD14 and CXCl-2 expression suggesting increased monocyte infiltration and activation in PF+DEN and EtOH+DEN treated livers compared to the Chow+DEN group (Fig. 2C,D).
Figure 2.
Relative mRNA expression of pro-inflammatory markers, A, TNFα, B, IL-6, and macrophage activation, C, CD14, D, CXCL-2 in DEN-treated mice receiving different diets (n=10/group). Data are expressed as mean ± St. Err. Significance, a<b<c, p<0.05.
Assessment of Fibrosis in DEN-treated PF and ETOH mice
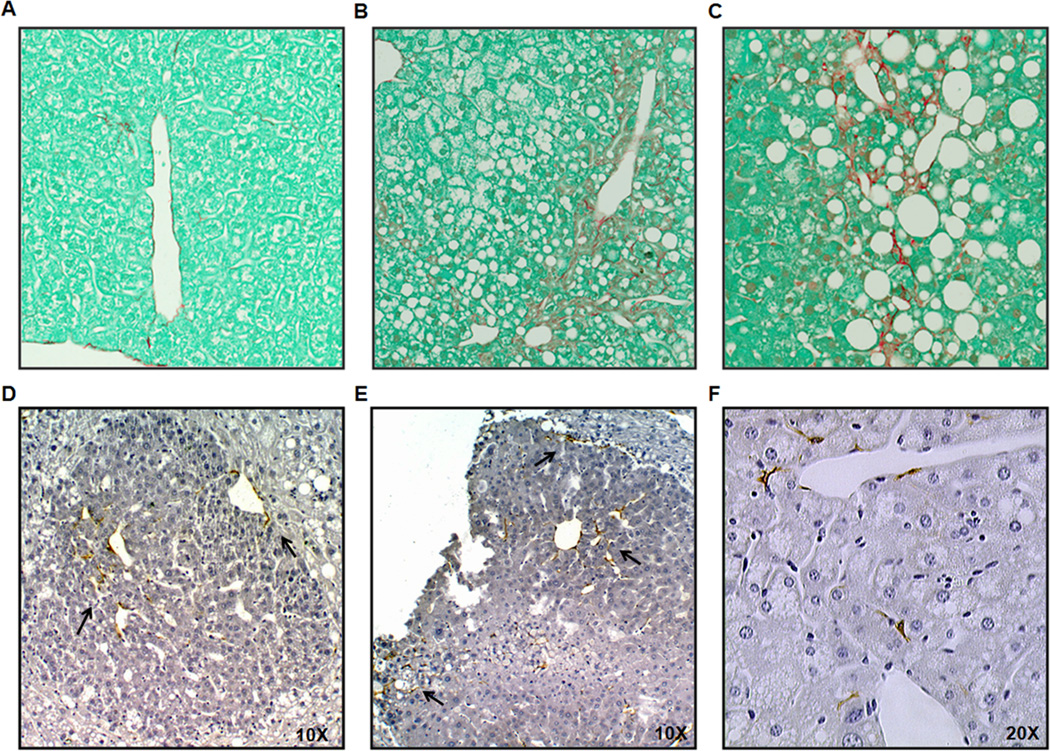
Parenchymal collagen deposition was assessed histologically by Sirius red/fastgreen staining the the DEN-treated groups. A representative section from each group, chow+DEN, PF+DEN and EtOH+DEN is shown in Figs. 3 A–C. In stained sections that were identified as having adenoma tumors fibrosis staging was scored. Overall, fibrosis, when present in the PF+DEN (1/6) and the EtOH+DEN (1/6), was mild (average score: 1). In both the PF+DEN and EtOH+DEN groups, we observed aSMA staining in the smooth muscle cells lining hepatic blood vessels (data not shown). In Figs. 3D, E, we did observed αSMA staining in and surrounding some of the larger adenomas (~0.90 mm × 0.87 mm, W × H) in the EtOH+DEN group. Adenomas of this size were not identified in the PF+DEN sections analyzed for αSMA. We also observed the presence of activated-αSMA-stained stellate cells in the parenchymal tissue in the EtOH+DEN-treated mice (Fig. 3F).
Figure 3.
Sections representing the extent of fibrosis in A, chow+DEN, B, PF+DEN and C, EtOH+DEN lobes identified to have adenomas (× 10 magnification). Immunohistochemical staining of αSMA in D and E, adenomas from the EtOH+DEN group, and F, αSMA-stained stellate cells in nontumorigenic tissue of EtOH+DEN-treated mice (× 20 magnification).
EtOH-mediated proliferation is associated with changes in β-catenin localization
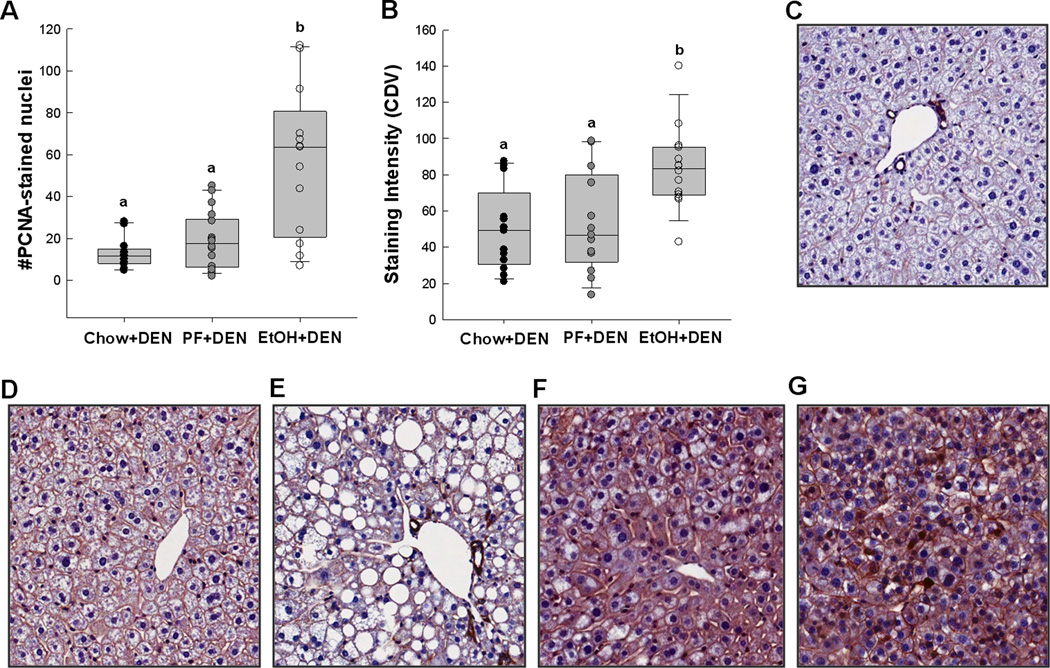
In the EtOH+DEN-treated mice, tumor multiplicity corresponded to a 4-fold increase in the number of PCNA-stained nuclei present in the non-tumorigenic tissue compared to DEN-treated chow and PF mice (Fig.4A), suggesting hepatocyte proliferation, p<0.05. Consistent with this finding, b-catenin staining in the EtOH+DEN –treated mice was significantly increased in comparison to both DEN chow and PF controls (Fig. 4B). Localization of β-catenin expression was also different between diet groups. In comparison to liver taken from an aged-matched, chow-fed mouse (Fig. 4C), DEN treatment alone increased membrane expression of β-catenin in the Chow+DEN group (Fig. 4D). Similar to the chow+DEN group, β-catenin expression remains localized in the membrane of the PF+DEN-treated group (Fig. 4E). Unlike the other groups, in EtOH+DEN-treated mice we observed β-catenin staining in the membrane, cytosol, and in some areas nuclear β-catenin accumulation (Fig. 4F) which is a staining pattern similar to what we observed in tumor tissue (Fig. 4G).
Figure 4.
EtOH increases hepatocyte proliferation and β-catenin expression in non-tumorigenic sections of chow+DEN (n=17), PF+DEN (n=18) and EtOH+DEN (n=15) mice. A, staining intensity of PCNA, B, staining intensity of unphosphorylated (Ser41/33) β-catenin. Representative sections of C, chow control, D, chow+DEN, E, PF+DEN, and F, EtOH+DEN groups in comparsion to G, β-catenin stained adenoma arising in an EtOH+DEN treated mouse (× 20 magnification). Significance, a<b<c, p<0.05.
EtOH feeding alone depletes hepatic retinoid stores in a TEN rodent model of ALD
Using a TEN rat model system of chronic alcohol consumption, we have previously shown that prolonged EtOH feeding (6 mo) resulted in increased hepatocyte proliferation as measured by PCNA staining (14). In Table 1, further analysis of these samples by real time RT-PCR confirmed a 2-fold increase in Ki67 expression in the TEN+EtOH group compared to the TEN controls, p<0.05. Chronic EtOH administration also significantly increased cyclin D1expression, which is also a transcriptional target of β-catenin (31). Additional β-catenin targets, GS and regucalcin were up-regulated in the TEN+EtOH group compared to the TEN control, p<0.05. This EtOH associated-proliferative phenotype was corresponded with depletion of hepatic retinoids, retinol and retinoic acid, p<0.05 in the TEN+EtOH compared to TEN control (Table 1).
Table 1.
Hepatic markers of proliferation, β-catenin activity and retinoid concentrations in a rat TEN model of alcoholic liver disease.
| Group | PCNA, % S-phase |
Gene expression (fold change) | Retinol nmol/g of liver |
Retinoic Acid nmol/g of liver |
|||
|---|---|---|---|---|---|---|---|
| Cyclin D1 | Ki67 | GS | Regucalcin | ||||
| TEN control | 2.16±0.26a | 1.00±0.19a | 1.00±0.13a | 1.00±0.46a | 1.00±0.07a | 165.5±12.5a | 0.12±0.01a |
| TEN+EtOH | 3.64±0.68b | 2.29±0.59b | 2.45±0.46b | 4.47±1.58b | 1.48±0.12b | 113.1± 8.8b | 0.08 ± 0.01b |
Data is expressed as mean ± St.Err.
PCNA staining presented has been published previously (14).
Gene expression and determination of hepatic retinoid concentrations were carried out as described in the Materials and Methods.
Statistical significance determined by Student’s T-Test, a<b, p<0.05.
EtOH feeding alone promotes Wnt/β-catenin signaling in a TEN rodent model of ALD
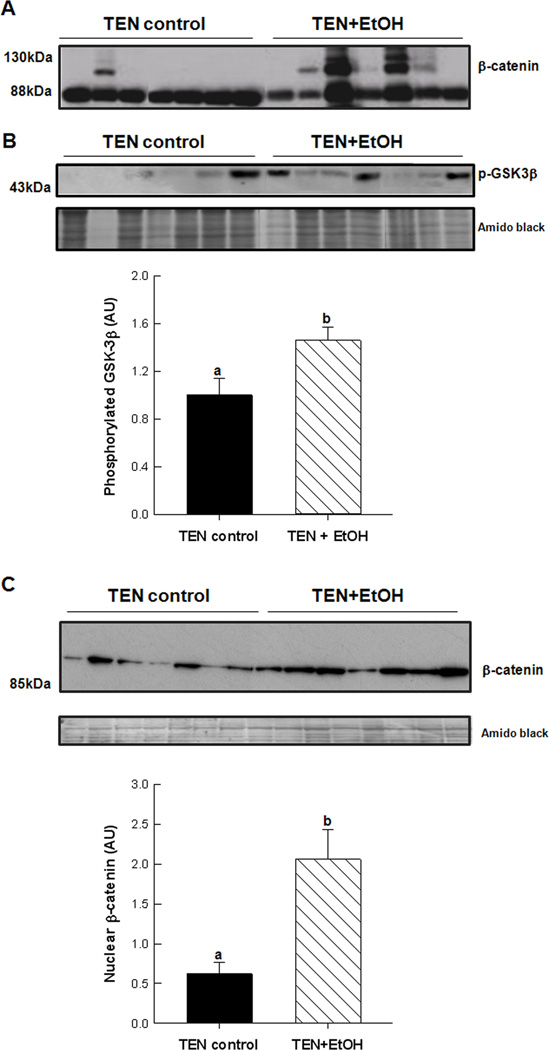
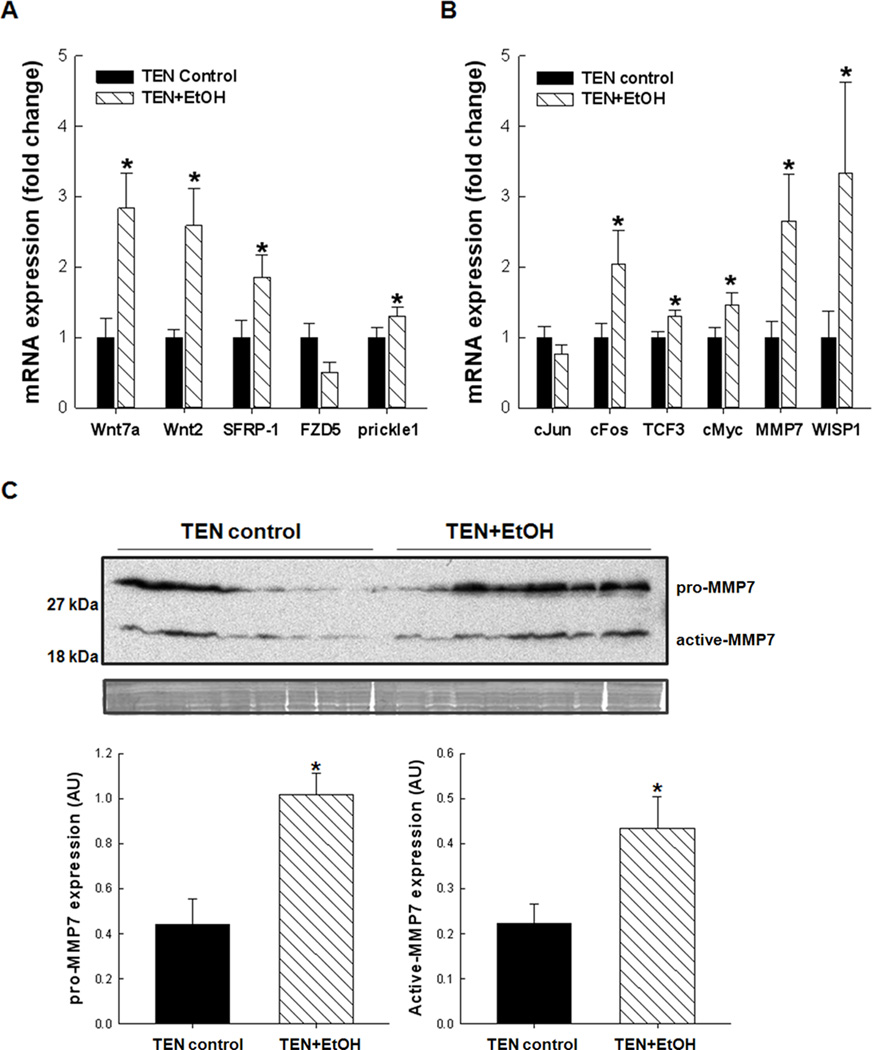
β-catenin expression and localization was assessed in cellular fractions prepared from TEN+EtOH and TEN control livers. In Fig. 5A, cytosolic expression of unphosphorylated β-catenin at serine residues 41 and 33 was increased in EtOH-treated livers compared to TEN controls, which also corresponded to an increase in the inactive, phosphorylated (Ser 21/9) GSK3β in the TEN+EtOH group, p<0.05 (Fig. 5B). In Fig. 5C, β-catenin expression was also significantly increased in the nucleus of in the TEN+EtOH group compared to TEN controls. In these EtOH-treated rats, increased accumulation of b-catenin in the nucleus corresponded to increased expression of genes involved in Wnt signaling pathways as identified by an mRNA expression array and described in the Materials and Methods. In Fig. 6A, EtOH increased expression of Wnt signaling mediators, Wnt2 and Wnt7a, by 2–3 fold, p<0.05. There was also a corresponding increase in negative feedback mechanisms, including secreted frizzled related protein-1 (SFRP-1), and prickle-1 (p<0.05), and a decrease in frizzled family receptor 5 (FZD), p=0.072, in the EtOH+TEN group compared to TEN controls. Transcription factors associated with proliferation and cancer progression, cFos, transcription factor 3(TCF3), and cMyc, were also unregulated in the TEN+EtOH group compared to TEN controls, p<0.05 (Fig. 6B). Likewise, mRNA expression of Wnt1 inducible signaling pathways protein 1 (WISP1), and mRNA and protein expression of MMP7 were significantly increased in EtOH-treated livers compared to their corresponding control group (Fig. 6B,C).
Figure 5.
Western blot analysis of A, active, unphosphorylated (Ser41/33) β-catenin in the cytosol, B, phosphorylated GSK3β in the cytosol and C, nuclear accumulation of active β-catenin in EtOH-treated livers taken from the rat TEN model of chronic alcohol consumption. Data are expressed as mean± St. Err., densitometric values (n=7/group) were corrected for protein loading using Amido black staining of whole lanes. Student’s T-test, a<b, p<0.05.
Figure 6.
Relative mRNA expression of A, Wnt signaling mediators and components and B, β-catenin targets in EtOH-treated rat livers compared to controls. Gene expression (n=9/group) was measured by real-time PCR and normalized to β-actin mRNA and then to TEN controls. C, up regulation of MMP7 mRNA corresponded to increased protein expression of pro-MMP7 (28 kDa) and active-MMP7 (19 kDa) in lysates from ETOH-treated livers. Data represents mean± St. Err., densitometric values (n=9/group) were corrected for protein loading using Amido black staining of whole lanes. Student’s T-test, *p<0.05 EtOH vs. TEN control.
Discussion
Although mechanisms underlying alcohol-induced initiation have been well characterized, alcohol related signaling pathways involved in tumor promotion and progression are not as well delineated. In this study, we developed a mouse model of tumor promotion using DEN as the initiating agent, followed by chronic EtOH feeding. In response to EtOH treatment, we observed enhanced tumor progression in DEN-treated EtOH mice. Increased tumor multiplicity was associated with increased cytosolic and nuclear β-catenin expression and proliferation in hepatic non-tumorigenic tissues. These results are consistent with the findings of Yip-Schneider et al. (15) who reported an increase in tumor multiplicity in male alcohol-preferring rats receiving EtOH in their drinking water compared to pair-matched rats on water alone. Brandon-Warner et al. has also reported increased tumor burden corresponding to a hepatic proliferative response in male DEN-treated mice receiving EtOH in the drinking water, suggestive of an EtOH-related promotional effect (10). However, we did not observe a significant increase in tumor incidence in the EtOH+DEN group compared to the PF+DEN group. In this study, increased tumor incidence was specifically associated with high fat (35%) feeding which corresponded to an increase in TNFα signaling and macrophage recruitment. Recently, Wang et al has demonstrated that an extremely high fat diet (70%) also produces NASH which promoted hepatic carcinogenesis in DEN-treated rats associated with increased TNFα signaling, and increased hepatocyte proliferation (32). In our model, the PF diet (35% fat) alone does not stimulate proliferation, or alter β-catenin cellular localization as observed in the DENtreated EtOH group. Taken together, these findings and those reported by Wang et al. suggest that dietary effects on hepatocyte proliferation and tumorigenesis may be dose-dependent on fat intake, mediated by TNFα signaling, but do not involve direct activation of β-catenin.
In a separate rodent model alcoholic liver disease (the rat TEN model), EtOH feeding inactivates GSK3β activity through phosphorylation, which disrupts sequential phosphorylation of β-catenin necessary for proteosomal degradation, thus increasing β-catenin translocation to the nucleus. As a result, Wnt/β-catenin targets were up regulated, including soluble Wnt mediators and suppressors, indicative of an inhibitory feedback loop in response to chronic activation of canonical Wnt signaling. Previously, we have shown in rats that low dose EtOH exposure (UEC<500 mg/dL) improves hepatic insulin sensitivity, resulting in increased phosphorylation of GSK3β through insulin substrate receptor (IRS1)/ AKT signaling (33). Therefore, the possibility exists that insulin signaling may also be contributing to the overall increase in β-catenin activity. Another potentially important finding which is clinically relevant in alcoholic patients is the subsequent depletion of hepatic retinoic acid stores in rats following chronic EtOH feeding. Basic research has shown that EtOH metabolizing enzymes, ADH and ALDH participate in retinoid metabolism, and in the liver, EtOH inhibits retinol conversion to retinoic acid through substrate competition (34). Additionally, EtOH-mediated induction of CYP 2E1 further increases enzymatic degradation of retinoic acid to polar metabolites (35,36). In mice, loss of retinoid signaling corresponds with increased β-catenin activation, proliferation and tumorigenesis in the liver (37). A potential mechanism may be the suppression of retinoid acid receptor (RAR) inhibitory effect on Wnt/β-catenin signaling through direct binding and removal of β-catenin from transcriptional machinery (38). In animals, low dose supplementation of retinoic acid during EtOH exposure restores hepatic retinoid concentrations, ameliorates alcoholic liver injury and reverses EtOH-mediated hepatocyte proliferation in rats (11,37). Our findings, in light of these reports, suggest a link between increased β-catenin activity and proliferation to EtOH-mediated retinoid depletion.
There are other pathways that may also be involved in increasing β-catenin activity following chronic alcohol exposure. Hepatocyte growth factor (HGF) is a potent mitogen for liver regeneration after injury, which binds to its tyrosine kinase receptor, Met, to stimulate proliferation (39). In animal models of liver regeneration using partial hepatectomy, alcohol exposure inhibits HGF-mediated proliferation and repair (40). However, in acute alcoholic hepatitis patients, hepatic HGF expression correlates with proliferation and patient survival (41). Likewise, there is one report showing increased hepatic HGF expression in male rats receiving liquid EtOH diets (42). Interestingly, Monga et al. has identified a membrane bound Met/β-catenin complex by which β-catenin nuclear translocation and subsequent expression of target genes occurs through tyrosine phosphorylation resulting from HGF/Met activation (43). In addition tyrosine kinase signaling, there is also some evidence to support a role for non-cannonical Wnt signaling through cJun NH2 terminal kinase (JNK) activation in modulating β-catenin translocation and activation (44,45). Many of the Wnt targets we identified as up-regulated following EtOH exposure are also transcriptionally regulated by JNK through cJun. Wnt7a, a proposed mediator for non-canonical Wnt/JNK signaling (46), is also highly expressed in our EtOH-treated rat hepatocytes. Moreover, in some cancer types Wnt7a increases cJun phosphorylation and subsequent proliferation of cancer cells (47,48). At this time it is unknown if tyrosine kinase receptor and/or JNK signaling are involved in EtOH-mediated β-catenin activation in our carcinogenesis model and this issue warrants further investigation.
In conclusion we have developed a new DEN-induced hepatocarcinogenesis mouse model designed to identify EtOH-specific mechanisms involved in tumor promotion and progression. In response to EtOH feeding, DEN-treated mice have increased tumor burden, which corresponded to increased hepatocyte proliferation and increased β-catenin expression and nuclear localization in non tumor hepatic tissue. In a separate study, EtOH feeding alone reduced hepatic retinoid concentrations, increased hepatocyte proliferation and nuclear expression of β-catenin, and increased expression of established markers of HCC progression. Generally speaking, aberrant β-catenin activity in HCC has been associated with gene mutation (22,23). Likewise, tumorigenesis associated with DEN-treated mice receiving phenobarbital is also dependent on mutant-specific β-catenin activation (49,50). However, in these DEN/Phenobarbital mice, mutations within β-catenin occur late (>32 wks) following the development of chromosomal instability (49). In our mouse model, we observed β-catenin activation and translocation at 16 wks, which puts forward the possibility that chronic increases in Wnt/β-catenin signaling apart from acquired mutations may also participate in sensitizing the liver to tumor initiating and promoting insults. Currently, there are no clinical strategies for lowering HCC risk in alcoholics, particularly in recovering alcoholics, whose HCC risk does not decrease with abstinence (7). In the literature, there are several plant derived phytochemicals with anticancer properties, some of which are known to inhibit β-catenin signaling pathways (51). We believe future studies using this animal model will provide valuable information on the molecular mechanisms whereby EtOH acts as a tumor promoter, and insight into potential dietary interventions that may be beneficial in lowering HCC risk in alcoholic populations.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institute of Health (AA0118282) and (CA169389).
Footnotes
The authors of this manuscript do not have any Conflicts of Interest and therefore have nothing to declare.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, Graubard BI, Zeuzem S, El Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- 5.Mohamed AE, Kew MC, Groeneveld HT. Alcohol consumption as a risk factor for hepatocellular carcinoma in urban southern African blacks. Int J Cancer. 1992;51:537–541. doi: 10.1002/ijc.2910510406. [DOI] [PubMed] [Google Scholar]
- 6.Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–512. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 9.Baumgardner JN, Shankar K, Korourian S, Badger TM, Ronis MJ. Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2007;293:G355–G364. doi: 10.1152/ajpgi.00038.2007. [DOI] [PubMed] [Google Scholar]
- 10.Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2012;36:641–653. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung J, Liu C, Smith DE, Seitz HK, Russell RM, Wang XD. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis. 2001;22:1213–1219. doi: 10.1093/carcin/22.8.1213. [DOI] [PubMed] [Google Scholar]
- 12.Isayama F, Froh M, Yin M, Conzelmann LO, Milton RJ, McKim SE, et al. TNF alpha-induced Ras activation due to ethanol promotes hepatocyte proliferation independently of liver injury in the mouse. Hepatology. 2004;39:721–731. doi: 10.1002/hep.20137. [DOI] [PubMed] [Google Scholar]
- 13.Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J, et al. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med (Maywood) 2008;233:344–355. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- 14.Ronis MJ, Hennings L, Stewart B, Basnakian AG, Apostolov EO, Albano E, et al. Effects of long-term ethanol administration in a rat total enteral nutrition model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G109–G119. doi: 10.1152/ajpgi.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip-Schneider MT, Doyle CJ, McKillop IH, Wentz SC, Brandon-Warner E, Matos JM, et al. Alcohol induces liver neoplasia in a novel alcohol-preferring rat model. Alcohol Clin Exp Res. 2011;35:2216–2225. doi: 10.1111/j.1530-0277.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, et al. Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology. 2004;40:366–375. doi: 10.1002/hep.20335. [DOI] [PubMed] [Google Scholar]
- 17.Hennig M, Yip-Schneider MT, Klein P, Wentz S, Matos JM, Doyle C, et al. Ethanol-TGFalpha-MEK signaling promotes growth of human hepatocellular carcinoma. J Surg Res. 2009;154:187–195. doi: 10.1016/j.jss.2008.11.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoresen GH, Guren TK, Sandnes D, Peak M, Agius L, Christoffersen T. Response to transforming growth factor alpha (TGFalpha) and epidermal growth factor (EGF) in hepatocytes: lower EGF receptor affinity of TGFalpha is associated with more sustained activation of p42/p44 mitogen-activated protein kinase and greater efficacy in stimulation of DNA synthesis. J Cell Physiol. 1998;175:10–18. doi: 10.1002/(SICI)1097-4652(199804)175:1<10::AID-JCP2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Calvisi DF, Ladu S, Factor VM, Thorgeirsson SS. Activation of beta-catenin provides proliferative and invasive advantages in c-myc/TGF-alpha hepatocarcinogenesis promoted by phenobarbital. Carcinogenesis. 2004;25:901–908. doi: 10.1093/carcin/bgh083. [DOI] [PubMed] [Google Scholar]
- 20.Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, et al. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334–341. doi: 10.1002/ijc.11254. [DOI] [PubMed] [Google Scholar]
- 21.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 22.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 24.Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, et al. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J Pharmacol Exp Ther. 2012;343:401–412. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardiff RD, Anver MR, Boivin GP, Bosenberg MW, Maronpot RR, Molinolo AA, et al. Precancer in mice: animal models used to understand, prevent, and treat human precancers. Toxicol Pathol. 2006;34:699–707. doi: 10.1080/01926230600930129. [DOI] [PubMed] [Google Scholar]
- 26.Dillner L, Josefson D, Karcher H, Sheldon T, Dorozynski A, Zinn C. Alcohol--pushing the limits. BMJ. 1996;312:7–9. doi: 10.1136/bmj.312.7022.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabb DW, Zeng Y, Liangpunsakul S, Jones R, Considine R. Ethanol impairs differentiation of human adipocyte stromal cells in culture. Alcohol Clin Exp Res. 2011;35:1584–1592. doi: 10.1111/j.1530-0277.2011.01504.x. [DOI] [PubMed] [Google Scholar]
- 28.Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-12. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–546. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He L, Simmen FA, Mehendale HM, Ronis MJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane. Role of TRB3 in inhibition of Akt/protein kinase B activation. J Biol Chem. 2006;281:11126–11134. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- 34.Wang XD. Chronic alcohol intake interferes with retinoid metabolism and signaling. Nutr Rev. 1999;57:51–59. doi: 10.1111/j.1753-4887.1999.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Russell RM, Seitz HK, Wang XD. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–189. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Chung J, Seitz HK, Russell RM, Wang XD. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1703–1709. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 37.Pan Z, Dan Z, Fu Y, Tang W, Lin J. Low-dose ATRA supplementation abolishes PRM formation in rat liver and ameliorates ethanol-induced liver injury. J Huazhong Univ Sci Technolog Med Sci. 2006;26:508–512. doi: 10.1007/s11596-006-0505-8. [DOI] [PubMed] [Google Scholar]
- 38.Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 39.Zarnegar R. Regulation of HGF and HGFR gene expression. EXS. 1995;74:33–49. doi: 10.1007/978-3-0348-9070-0_3. [DOI] [PubMed] [Google Scholar]
- 40.Saso K, Higashi K, Nomura T, Hoshino M, Ito M, Moehren G, et al. Inhibitory effect of ethanol on hepatocyte growth factor-induced DNA synthesis and Ca2+ mobilization in rat hepatocytes. Alcohol Clin Exp Res. 1996;20:330A–334A. [PubMed] [Google Scholar]
- 41.Fang JW, Bird GL, Nakamura T, Davis GL, Lau JY. Hepatocyte proliferation as an indicator of outcome in acute alcoholic hepatitis. Lancet. 1994;343:820–823. doi: 10.1016/s0140-6736(94)92025-7. [DOI] [PubMed] [Google Scholar]
- 42.Tahara M, Matsumoto K, Nukiwa T, Nakamura T. Hepatocyte growth factor leads to recovery from alcohol-induced fatty liver in rats. J Clin Invest. 1999;103:313–320. doi: 10.1172/JCI4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 44.Saadeddin A, Babaei-Jadidi R, Spencer-Dene B, Nateri AS. The links between transcription, beta-catenin/JNK signaling, and carcinogenesis. Mol Cancer Res. 2009;7:1189–1196. doi: 10.1158/1541-7786.MCR-09-0027. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itoh T, Kamiya Y, Okabe M, Tanaka M, Miyajima A. Inducible expression of Wnt genes during adult hepatic stem/progenitor cell response. FEBS Lett. 2009;583:777–781. doi: 10.1016/j.febslet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 48.Heasley LE, Winn RA. Analysis of Wnt7a-stimulated JNK activity and cJun phosphorylation in non-small cell lung cancer cells. Methods Mol Biol. 2008;468:187–196. doi: 10.1007/978-1-59745-249-6_14. [DOI] [PubMed] [Google Scholar]
- 49.Aleksic K, Lackner C, Geigl JB, Schwarz M, Auer M, Ulz P, et al. Evolution of genomic instability in diethylnitrosamine-induced hepatocarcinogenesis in mice. Hepatology. 2011;53:895–904. doi: 10.1002/hep.24133. [DOI] [PubMed] [Google Scholar]
- 50.Stahl S, Ittrich C, Marx-Stoelting P, Kohle C, Altug-Teber O, Riess O, et al. Genotype-phenotype relationships in hepatocellular tumors from mice and man. Hepatology. 2005;42:353–361. doi: 10.1002/hep.20768. [DOI] [PubMed] [Google Scholar]
- 51.Thakur R, Mishra DP. Pharmacological modulation of beta-catenin and its applications in cancer therapy. J Cell Mol Med. 2013;17:449–456. doi: 10.1111/jcmm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.